Abstract
The purpose of this study was to characterize rat adipose-derived stem cells, induce adipose-derived stem cell tenogenesis, and analyze adipose-derived stem cell effects on tendon repair in vivo. Adipose-derived stem cells demonstrated an immunomodulatory, pro-angiogenic, and pro-proliferatory profile in vitro. Tenogenesis was induced for 1, 7, 14, and 21 days with 24 combinations of growth differentiation factor-5, 6, and 7 and platelet-derived growth factor–BB. Adipose-derived stem cells expression of scleraxis and collagen type I increased the most after 14 days of induction with growth differentiation factor-6 and platelet-derived growth factor–BB. Achilles excision defects injected with hydrogel alone (Gp2), with undifferentiated (Gp3) adipose-derived stem cells, or tenogenically differentiated (Gp4) adipose-derived stem cells exhibited improved tissue repair compared with untreated tendons (Gp1). Addition of adipose-derived stem cells improved tissue cytoarchitecture and increased expression of collagen type I and III, scleraxis, and tenomodulin. Adipose-derived stem cells significantly improved biomechanical properties (ultimate load and elastic toughness) over time more than hydrogel alone, while tenogenically differentiated adipose-derived stem cells improved the mean histological score and collagen fiber dispersion range closest to normal tendon. In addition, tendon sections treated with GFP-adipose-derived stem cells exhibited green fluorescence and positive GFP immunostaining on microscopy confirming the in vivo survival of adipose-derived stem cells that were injected into tendon defects to support the effects of adipose-derived stem cells on tissue up to 4.5 weeks post injury.
Keywords: Achilles tendon, adipose-derived stem cells, hydrogel, tenogenesis, growth factors
Introduction
Tendinopathy is the general clinical descriptor of tendon injury.1 Although tendon damage can occur near any joint, heel, elbow, and shoulder injuries are the most common and can range from simple micro-tears with inflammation to complete tears and ruptures.2 Tendon injuries are among the most common orthopedic injuries in soldiers, athletes, and the general public with over 800,000 patients needing surgical treatment annually.3 It affects over 50% of athletes in jumping sports and up to 80% of runners.4–6
Most tendon injury repair is an active topic of debate in orthopedics, especially due to lack of understanding of the pathologies involved.7 Some physicians advocate for surgical repair, while others favor conservative treatment with anti-inflammatory medication.8–10 As reviewed by Chan, Fu, and Yung, current treatments are empirical and symptom based, ranging from physiotherapy with non-steroidal anti-inflammatory drugs (NSAIDs) and steroid injections to shock wave therapy or surgical repair with reattachment or excision and reconstruction.8 Different treatment modalities depend on the extent of tendon injury with a poor clinical outcome for most approaches.10 Bioengineered materials have not been able to overcome these obstacles.3,8–13 No treatment guarantees 100% return to function, especially for athletes, thus novel therapeutic approaches are needed.14
Injuries to tendon are difficult to heal due to low cellularity and blood supply.7 The study of tendon repair is complicated by the dependence of the healing process on anatomical location and load type, tendon interface, and tendon type with respect to the affected joint.15 Achilles tendon injuries are mostly ruptures occurring in the midportion of the tendon. Our study focused on Achilles tendon excision injury, a type of extra-synovial injury at the tendon-to-tendon interface.2,15
Mesenchymal stem cells (MSCs) are multipotent stromal cells in the connective tissue of most organs, with adipose-derived stem cells (ADSCs) being an adult MSC subtype easily harvested from fat of most animals and humans.16,17 Tendon-derived stem cells (TDSCs) have also been discovered; however, the use of TDSCs requires harvest of healthy tendon tissue from the patient.18–21
Recently, ADSCs have been successfully differentiated into multiple mesodermal cell types demonstrating promise for regenerative medicine and tissue engineering strategies.13,16,22,23 As reviewed by Melief et al.,24 ADSCs are known to possess higher immunomodulatory capacity than other stem cells. Moreover, ADSCs possess the same self-renewal capacity and multilineage differentiation potential as bone marrow-derived mesenchymal stem cells (BMSCs), with lower morbidity during harvest and higher cell count, colony frequency, and proliferation in vitro.24 Tendon tissue engineering involving ADSCs has shown less ectopic bone formation and higher COL1 and COL3 expression than BMSCs.16,24,25
Multiple reviews of various studies indicate that musculoskeletal repair with ADSC adjuncts might improve the histological, biomechanical, and molecular properties of tendon.22,26,27 ADSCs with or without tenocytic differentiation can be applied to repair tendon defects as an autologous source of tissue repair that avoids the complications of graft versus host disease, the need for medications, and immunosuppression as in other tissue transplant strategies.19,28,29 There still remain questions about the pathway of tendon development, and thus, genes that are expressed less in other musculoskeletal tissues, like scleraxis (SCX), tenomodulin (TNMD), decorin (DCN), or tenascin C (TNC), are used to identify a tendon lineage.18,25,30–34 Various methods and growth factors (GFs) have been used to differentiate stem cells into tendon-like cells in vitro, including growth differentiation factor-5 (GDF-5), GDF-6, GDF-7, platelet-derived growth factor (PDGF), culture with three-dimensional scaffolds or other cells, adenoviral GF transfections, and mechanical stretch.2,8,11–14,31,35–37 However, no one method has been successful in translating to an in vivo tendon repair model.
The goal of this study was to characterize rat ADSCs and the paracrine factors they release in vitro to induce ADSC tenogenesis and to analyze ADSC effects on tendon repair in vivo. We hypothesized that ADSCs release anti-inflammatory and pro-immunomodulatory factors in vitro that could be useful for tissue repair in vivo.
Differentiation toward a tenogenic lineage with GDF-5, GDF-6, GDF-7, and PDGF-BB in vitro, GFs known to induce the expression of tendon-lineage genes have not yet been explored as a combination cocktail with ADSCs. We hypothesized that naïve ADSCs could be differentiated into tendon-like cells following treatment with combinations of GFs not examined previously. The tenogenic GF combination that would achieve the highest expression of SCX and COL1 after induction would be used with ADSCs for the in vivo application. Our goal was to achieve tenogenic differentiation of ADSCs for an in vivo tendon repair application and to examine the effects of ADSCs (both undifferentiated and tenogenically differentiated) on the repair quality of Achilles tendon that underwent excision injury. We hypothesized that administration of ADSCs within a hydrogel would enhance the histological, molecular, and biomechanical quality of tendons after excision injury in a rat Achilles model and that tenogenically differentiated ADSCs would enhance tissue repair better than undifferentiated ADSCs when compared with the unrepaired tendons.
Materials and methods
Study design (Level of Evidence): Basic science study (Level V). Approval from our Institutional Animal Care and Use Committee was obtained prior to performing the study (protocol 2015–007).
Tissue harvest and fat isolation
Fat was isolated from inguinal regions of six adult (12-week-old) male Sprague Dawley rats (SDRs). The harvested tissue was combined and placed in Dulbecco’s Modified Eagle’s Medium with Ham’s F-12 (DMEM/F-12) and digested for 1 h with 0.075% collagenase/DNase mixture while agitated in a 21% O2, 5% CO2 37°C incubator. The resulting stromal vascular fraction (SVF) was filtered through a 100 µm NYTEC filter, centrifuged at 24°C and 1500 r/min for 5 min, and washed twice in phosphate-buffered saline (PBS) containing 1% (v/v) penicillin/streptomycin/amphotericin (PSA; Corning). The cells from SVF were cultured in vitro in T150 cell culture flasks in ADSC culture medium (DMEM/F-12, 10% (v/v) fetal bovine serum (FBS; Crystalgen), 1% PSA, at 37°C, 21% O2, and 5% CO2 with media change every 3 days to obtain ADSCs after the first passage. Cells were passaged at 95% confluency after a PBS wash and detachment with 0.05% trypsin–ethylenediaminetetraacetic acid (EDTA; Gibco).
Cell characterization
Undifferentiated ADSCs were culture expanded in vitro using standard cell culture flasks and ADSC culture medium (as described above) at 37°C, 21% O2, and 5% CO2 with media change every 3 days. ADSCs at passage 3 were then characterized as stem cells with the following criteria: adherence to plastic confirmed by cell culture, spindle-shaped morphology confirmed by light microscopy, specific cell surface antigen expression confirmed by flow cytometry, and multilineage differentiation potential confirmed by induction into multiple mesodermal lineages in culture.16,18 To determine paracrine factor synthesis of ADSCs at passage 3 in vitro, ADSC culture supernatant was tested with rat-specific enzyme-linked immunosorbent assays (ELISAs) after 48 h growth in culture and included mouse anti-rat interferon (IFN)-γ; interleukin (IL)-10 and IL-8; vasculoendothelial growth factor (VEGF)-A, B, and C; fibroblast growth factor (FGF)-1 and -2; stromal cell-derived factor (SDF)-1; and insulin-like growth factor (IGF)-1 and 2 (BosterBio and MyBioSource). Cell analyses were done in quadruplicate.
Flow cytometry
Undifferentiated ADSCs were analyzed at passage 3 by flow cytometry to determine specific cell surface antigen expression. Briefly, cells were detached from tissue culture flasks with AccutaseTM Cell Detachment Solution (BD Biosciences), washed twice with PBS, and resuspended in Stain Buffer (bovine serum albumin (BSA); BD Pharmingen) at 2 × 106 cells/mL. Cells were incubated on ice with mouse anti-rat CD106-PE, CD90-APC-Cy7, purified CD73, CD45-PE-Cy5 (BD Pharmingen), and CD31-BB515 (BD Horizon) antibodies for 30 min in the dark at room temperature. Following incubation with purified mouse anti-rat CD73, cells were also incubated with goat anti-mouse Ig-BV421 antibody (BD Horizon) for 30 min on ice in the dark at room temperature. All cells were washed twice, resuspended in washing buffer, and analyzed using FACS Fortessa with FACSDiva software (BD Biosciences). All data were collected for non-specific binding using isotype-matched negative controls, and fresh non-conditioned media was used as a negative control.
Multilineage differentiation potential
Induction of cells into multiple mesodermal cell lineages was performed at passage 3 (n = 4) and included differentiation into osteocytic and adipocytic lineages in monolayer cultures or chondrocytic lineages in pellet culture for 3 weeks with media change every other day, followed by analysis of specific gene expression (messenger RNA (mRNA)) by quantitative real-time polymerase chain reaction (qPCR) and morphology by histology. The osteogenic induction medium included DMEM-high glucose media with l-glutamine, 10% FBS, 1% PSA, 100 nM dexamethasone, 10 mM β-glycerophosphate, and 200 µM ascorbic acid. Cells were stained with Alizarin Red for the presence of calcium phosphate crystals found in osteocyte extracellular matrix (ECM). The adipogenic medium included DMEM/F-12 with l-glutamine, 15 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 3% FBS, 1% PSA, 0.1–1 µM dexamethasone, 500 µM 3-Isobutyl-1-methylxanthine (IBMX), 10 µg/mL insulin, 200 µM indomethacin, and 1 µM rosiglitazone. Cells were stained with Oil Red O soluble in fat vesicles of adipocytes. The chondrogenic medium included DMEM/F-12 with l-glutamine, 15 mM HEPES, 1% FBS, 1% PSA, 100 nM dexamethasone, 100 µM ascorbic acid, 1% insulin–transferring–selenium (ITS) Premix, and 10 ng/mL transforming growth factor (TGF)-β. Cell pellets were embedded in paraffin, sliced at 5 µm in thickness, and stained with Safranin-O/Fast Green for the presence of proteoglycans found in chondrocytes.
Tenogenic differentiation
ADSCs were differentiated into a tenocytic lineage in vitro using known tenogenic GFs after ADSCs reached at least 80% confluence per flask at passage 3 (n = 4). To induce tenogenic differentiation, monolayer cell cultures were incubated in standard ADSC culture media supplemented with different GFs, which included GDF-7,38,39 GDF-6,40,41 GDF-5,11,12 and PDGF-BB.2,3 Cells without GF supplementation served as negative controls at each time point. Based on previous studies,38,39 in the first phase of the induction experiments, culture media were supplemented with each single GF at one of three concentrations (GDF-5, 6, and 7 at 80, 100, or 120 ng/mL; PDGF-BB at 10, 30, or 60 ng/mL) with media change every 3 days. During the second phase, supplementation included GF combinations not previously tested experimentally (one GDF at 50 ng/mL with PDGF-BB at 10 ng/mL; two GDFs alone at 50 ng/mL each; two GDFs at 50 ng/mL each, with PDGF-BB at 10 ng/mL; three GDFs at 35 ng/mL each; or all four GFs at 25 or 35 ng/mL each of GDF-5, 6, and 7 with 10 ng/mL of PDGF-BB). In total, we tested 24 different tenogenic cocktails incubated for four time periods (1, 7, 14, and 21 days). Cells were then examined microscopically for appropriate cell phenotype, stained with rabbit anti-rat TNMD and SCX antibodies, and assessed for tenocyte-like characteristic gene expression. The GF cocktail that would produce the highest increase in the expression of SCX and COL1 after 2 weeks of ADSC differentiation in culture would be used for the in vivo application of ADSCs as an intra-operative adjunct to Achilles excision defect repair.
Animal study and sample harvest
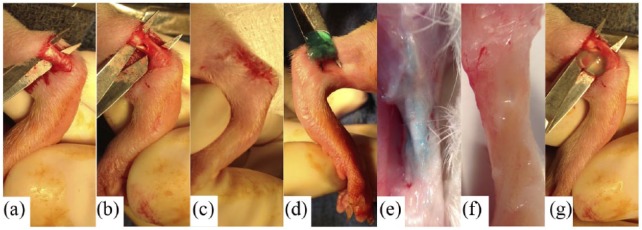
Adult male (12-week-old) SDRs (weight: 350–400 g, N = 180) underwent randomized unilateral tendon surgery to create excision defects in Achilles tendon with contralateral ankle serving as negative control. Intraperitoneal injection of ketamine (80 mg/kg) and xylazine (5 mg/kg) was used for anesthesia, which was maintained with 2% isoflurane (Baxter) with 1 L/min O2 flow rate. To access the tendon, a longitudinal 1–1.5 cm incision in the overlying skin of the ankle exposed the tendon by blunt dissection under the skin. A variation in the excision model was used,42,43 with an intratendinous cylindrical defect of 2 mm diameter (63 ± 18% of original size) made with a surgical tendon punch (Fine Science Tools, Inc.) through the midportion of the tendon with sterile plastic material as a guide and to protect adjacent tissue (Figure 1). All defects were identical in size and made in the same location using appropriately engineered guides. The control group received no injection (Gp1). In treatment groups, 250 μL of hydrogel solution (with or without ADSCs, Gp2–Gp4) was injected into the defect and surrounding area of the tendon. The skin overlying the Achilles tendon was closed with interrupted 4–0 Vicryl sutures (Ethicon). Animals were allowed to recover with weight-bearing motion without restraint. Buprenorphine (0.05 mg/kg) was administered subcutaneously for post-operative analgesia.
Figure 1.
Representative images of Achilles tendon defects in the (a–c) unrepaired (Gp1 - defect only) and (d–g) repaired groups (Gp2 - hydrogel only, Gp3 - hydrogel + undifferentiated ADSCs, Gp4 - hydrogel + tenogenically differentiated ADSCs).
A combination of collagen and alginate gels was chosen as a biodegradable hydrogel/scaffold to prevent clearance and provide controlled release of ADSCs in tendon tissue.30,41,43 The following gel solutions (3 × 106 ADSCs per milliliter of gel) were prepared for injection into rat tendons during surgery: gel alone (Gp2), gel with undifferentiated ADSCs (Gp3), and gel with tenogenically differentiated ADSCs (Gp4), which were induced with GDF-6 + PDGF-BB for 14 days prior to injection. Gels were crosslinked by an addition of 100 mM CaSO4 followed by 30 min incubation at 37°C. Gels were also crosslinked with 500 mM CaSO4 immediately following the intra-surgical application and before the skin closure.
At 1.5, 3, and 4.5 weeks post injury, animals (n = 15 at each time point; n = 45 total per group) were euthanized with CO2 inhalation and tendons were harvested for analysis. Achilles tendons were examined visually and documented photographically for repair extent and presence of adhesions. For histological analysis (n = 4 per group at each time point), the entire leg halfway up the femur (including the foot) was removed, fixed in plantar flexion in 10% buffered formalin in large tissue cassettes, and stored at room temperature until further processing. Fixation in flexion was performed to ensure tendon fibers were fixed in their tensed position. Dissection of tendon free of muscle and bone before fixation contributes to loss of normal tensed physiological architecture of tendon fibers, which could later alter the histological grading of samples. For biomechanical analysis (n = 7 per group at each time point), each sample was harvested with a bone and muscle margin, preserving tendon sheaths to maintain orientation of tendon bundles, wrapped in PBS-soaked gauze and frozen in a specimen cup at −20°C. For gene expression analysis (n = 4 per group at each time point), samples were harvested free of muscle and bone, submerged in RNAlater (Ambion, Thermo Fisher Scientific), and stored at −20°C.
Gene expression analysis
For multilineage differentiation potential and tenogenesis, each cell line was seeded at 2.5 × 105 cells for qPCR. The midportion of each tendon sample was excised and homogenized by Gentle MACS Dissociator with M Tubes (Miltenyi Biotec). Cell harvest and RNA extraction/purification was completed with RNeasy Mini Kit (Qiagen) and RNase-Free DNase Set (Qiagen). Complementary DNA (cDNA) was synthesized with iScript cDNA Synthesis Kit (Bio-Rad) and T100 Thermal Cycler (Bio-Rad). Gene expression analysis was performed using the Light Cycler 480 (Roche) with iQTM SYBR Green Supermix (Bio-Rad) and protocol. Fold change of gene expression (mRNA) was normalized to the housekeeping gene, ribosomal protein 13A (RPL13A)44 for in vitro studies, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH)16 for in vivo studies and calculated relative to the controls using the Pfaffl method (ΔΔCt method).45
To confirm multilineage differentiation potential, gene expression (mRNA) analysis was completed with the following primers:22,46 alkaline phosphatase (ALP; basic phosphatase found in bone), osteocalcin (OCN; secreted by osteoblasts, marker of bone formation), and runt-related transcription factor 2 (RUNX2; transcription factor associated with osteoblast differentiation) following osteogenesis; lipoprotein lipase (LPL; hydrolase of lipoproteins found in adipocytes), peroxisome proliferator–activated receptor γ2 (PPAR-γ2; transcription factor associated with adipocyte differentiation), and fatty acid binding protein-4 (FABP4; carrier protein for fatty acids expressed in adipocytes) following adipogenesis; collagen type-II (COL2; cartilage ECM component) and AGCAN (cartilage ECM component that withstands compression) following chondrogenesis; and SCX (neotendon formation marker), COL1 and COL3 (tendon ECM components),7,14,36 TNMD (tendon differentiation and mature tendon marker), and TNC (collagen fibrillogenesis regulator)16,36 following tenogenesis.30 Gene expression (mRNA) analysis of tendon samples was completed with the following primers: SCX, COL1 and COL3, TNMD, TNC, matrix metalloproteinase-3 (MMP-3), MMP-9, and MMP-13 (ECM digestion enzymes), and tissue inhibitor of MMPs 1 (TIMP1) and TIMP2.
Histological analysis
Fixed tendons were excised from muscle tissue and bone, processed for histology, embedded in paraffin, and cut in a coronal plane 7 μm in thickness in a serial fashion. Upon mounting onto slides and rehydration, slides were stained with picrosirius red and Mallory’s Trichrome (MT) staining kits (American MasterTech). Micrographs of slides were acquired with BH-2 microscope and DP72 camera (Olympus). Evaluation of quality of repair and grading was performed by blind review using our validated scoring system. Briefly, the samples were graded in the following categories:47 cellularity (inflammatory cell presence), collagen fiber organization, vascularity/angiogenesis, cell shape (fibroblastic changes), presence of ectopic cartilage, and granulation tissue. Each variable received a score of 0–3, with 0 being normal and having no abnormalities, 1 having less than 25% abnormalities, 2 having less than 50% abnormalities, and 3 being the most disorganized tissue having more than 50% abnormalities and classified as poorly healed. Two graders analyzed the slides to obtain the average grade for each sample and experimental group.
Images of picrosirius red–stained tendon sections were taken using polarized light microscopy (BH-2 microscope and DP72 camera) with tendon fibers aligned vertically to quantitatively evaluate collagen fiber organization with fast Fourier transform (FFT).48 Briefly, FFT transforms the original image from real space into frequency space from which a pixel intensity plot against the angle of acquisition is generated. Fiber alignment in the original image is indicated by the height and width of the intensity frequency plot. Position of the peak in the plot determines the principal axis of orientation. The lower and upper limits of fiber angles are defined as the angles at which intensity drops to 50% of peak intensity. The range between the lower and upper limit of fiber angles is computed as the range of fiber dispersion.
ADSCs have been tracked in vivo with the use of GFP-ADSCs harvested from transgenic GFP-expressing rats. GFP-ADSCs were confirmed for positive GFP expression post tissue harvest and maintenance of expression in culture by flow cytometry analysis of unstained GFP-ADSCs (four preparations at passage 3, data not shown). GFP-ADSCs were visualized with light/fluorescent microscopy and immunostaining of tendon sections. GFP-ADSC pellet was used as a positive control, while non-GFP ADSC pellet as a negative control.
Structural and biomechanical analysis
Biomechanical testing was performed on samples from normal (uninjured) tendons and four experimental groups repaired with (Gp1) no gel and no cells, (Gp2) gel, no cells, (Gp3) gel + undifferentiated ADSCs, and (Gp4) gel + tenogenically differentiated ADSCs. All mechanical testing was performed in uniaxial tension (Instron Model #5566) in a blinded fashion with respect to the treatment group. Frozen samples were thawed in PBS at room temperature and tested while submersed in a PBS + propidium iodide (PI) bath at 37°C. Load was measured by a 100 N load cell (load accuracy ±0.5%) with data acquisition and device control by Blue Hill software (v 2.15) at 10 Hz. Specimens were processed before testing by embedding the bone insertion in bone cement and then stripping the muscle insertion fibers away exposing the intramuscular tendon fibers in order to affix them to sand paper soaked in liquid adhesive (Loctite 495; Henkel Corp). Sand paper was then clamped with a hemostat until dry to prevent slippage and allow for proper mounting in hydraulic grips between two roughened surface plates.49 Briefly, after 0.5 N preload was applied to the specimens, digital calipers were used to measure specimen length and mid-segment cross-sectional area (CSA). The tendon samples were then subjected to tensile extension at a strain rate of 0.25 mm/s until failure and the resultant load was recorded. Sample stiffness was assessed from the best fit of the linear portion of the load–displacement curve. The ultimate tensile strength, corresponding to maximum load at failure, and stiffness were also computed.
Statistical analysis
Data from the experimental manipulations were averaged and expressed as the mean ± standard deviation followed by a two-way analysis of variance (ANOVA) with Tukey–Kramer adjustment using GraphPad Prism software (version 6; GraphPad Software, Inc.). The differences were considered statistically significant at p < 0.05, and trends were noted at p < 0.1. Each experimental analysis was performed in quadruplicate.
Results
Cell characterization
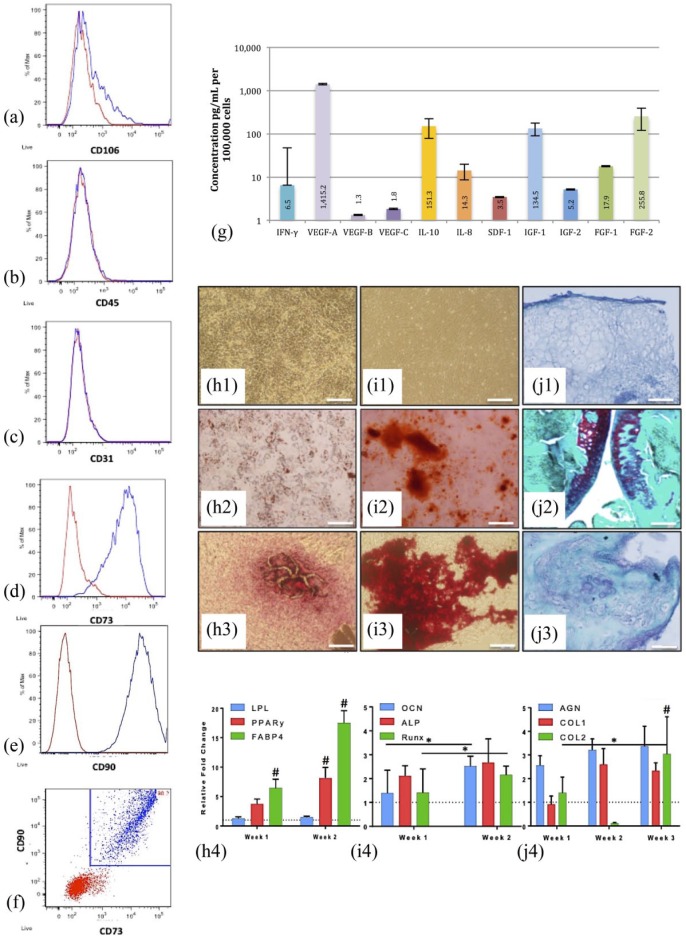
ADSCs expressed CD73 (>89.8%) and CD90 (>99.1%), but not CD31 (<0.5%), CD45 (<1.3%), or CD106 (<11.9%) on flow cytometry classifying them as stem cells unique from endothelial, leukocytic, or hematopoietic lineages (Figure 2(a)–(f)). Rat ADSCs showed adherence to plastic in culture and spindle-shaped morphology as visualized by light microscopy (Figure 2(f)–(i1)). ELISAs of cell culture supernatant detected high levels (>100 pg/mL) of IL-10, IGF-1, FGF-2, and VEGF-A (>1000 pg/mL) supporting an immunomodulatory, pro-angiogenic, and pro-proliferatory paracrine profile of ADSCs grown in vitro (Figure 2(g)). Primary ADSCs were successfully differentiated into multiple mesodermal lineages including fat, bone, and cartilage. Following adipogenic induction, ADSCs showed an increase in LPL (1.3-fold at 1 week, 1.5-fold at 2 weeks, p > 0.05), PPAR-γ2 (3.7-fold at 1 week, p = 0.088; 8-fold at 2 weeks, p < 0.001), and FABP4 expression (6.5-fold at 1 week, p = 0.004; 17.5-fold at 2 weeks, p < 0.001), as well as positive Oil Red O staining on histology (Figure 2(h1)–(h4)). Following osteogenic induction, ADSCs exhibited increased ALP (2-fold at 1 week, 2.7-fold at 2 weeks, p > 0.05), OCN (1.4-fold at week 1, 2.5-fold at week 2, p > 0.05), and RUNX2 expression (1.4-fold at week 1, 2.2-fold at week 2, p > 0.05), as well as positive Alizarin Red staining on histology (Figure 2(i1)–(i4)). Following chondrogenic induction, ADSCs showed an increase in COL1 (2.6-fold at 2 weeks, 2.3-fold at 3 weeks; p > 0.05), COL2 (3-fold at 3 weeks, p = 0.009), and AGCAN expression (2.6-fold at 1 week, 3.2-fold at 2 weeks, 3.4 at 3 weeks, p > 0.05) and positive Safranin-O staining on histology (Figure 2(j1)–(j4)).
Figure 2.
Characterization of ADSCs and their multilineage differentiation potential: flow cytometry analysis of rat ADSCs (n = 4, at passage 3) (a-f): (Red) Unstained and (Blue) ADSCs stained with anti- (a) CD106, (b) CD45, (c) CD31, (d) CD73, and (e) CD90 antibodies; (f) Dot plot of combined CD73 and CD90-stained cells; Concentration of paracrine factors (protein) present in the media of rat ADSCs after 48 hours in culture (g): (From left) IFN-γ, VEGF-A, VEGF-B, VEGF-C, IL-10, IL-8, SDF-1, IGF1, IGF-2, FGF-1, and FGF-2; [pg/ml] per 105 cells; Multilineage differentiation potential determination (h–j): adipogenesis (h1–4), osteogenesis (i1–4), and chondrogenesis (j1–4). The negative control (h1–J1), positive control (h2–J2), and treatment (h3–J3) stained with Oil Red O (h1–3), Alizarin Red (i1–3), and Safranin-O (j1–3); The relative fold change of gene expression (mRNA) (h4–j4), n = 4 at each time point: Dashed lines indicate the expression of ADSC controls, *p < 0.05 denotes significance with time and #p < 0.01 denotes significance at the same time point when compared with the control; 100x, scale bar = 0.2 mm
Tenogenic differentiation
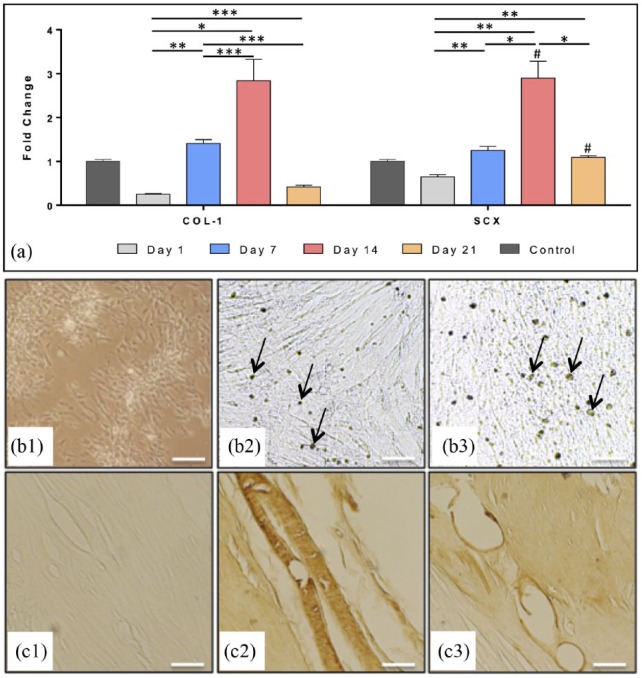
Effects of dose and time of GDF-5, 6, and 7, PDGF-BB, and their combinations were investigated in ADSCs using qPCR. Data show that use of single GFs in ADSC culture media increases the expression of tenogenic markers, but that combining these GFs has significantly more profound effect. Supplementation of ADSC culture media with GDF-6 and PDGF significantly increased the expression of SCX and COL1, which were the characteristics we were looking to increase in cells used in our in vivo tendon injury model. GDF-6 and PDGF produced significant increases in COL1 expression (3-fold at 14 days, p < 0.0001), as well as SCX expression over time (p < 0.0001), yielding best results (3.3-fold, p < 0.01) at 14 days (Figure 3(a)).
Figure 3.
Tenogenic differentiation of rat ADSCs at passage 3 (n = 4) in monolayer at 14 days: (a) Relative fold change of gene expression (mRNA) measured by quantitative real-time PCR of collagen type-I (COL1) and scleraxis (SCX) after tenogenic differentiation with GDF-6 (50 ng/ml) + PDGF-BB (10 ng/ml). The dashed line indicates the expression of ADSC controls, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 denote significance with time when compared with the same treatment; #p < 0.05 denotes significance when compared with the ADSC control at the same time point; Immunostaining of ADSCs (b1–b3) and rat Achilles tendon sections (c1–c3): unstained (negative) controls (b1, c1), samples stained brown with anti-scleraxis (b2, c2, arrows pointing to the dark nuclei), and anti-tenomodulin antibody (b3, c3, arrows pointing to the lighter-stained cell membranes); 100x, Scale bar = 0.2 mm.
Tenogenically differentiated ADSCs also exhibited elongated cell phenotype on microscopy and positive staining with rabbit anti-rat TNMD and SCX antibodies on immunohistochemistry (Figure 3(b2) and (b3). The negative controls (ADSCs without GF supplementation and unstained rat tendon tissue) did not stain, while the positive controls (tendon tissue stained with the same antibodies) also stained positively (Figure 3(b1) and (c1)–(c3)). Although this was not a quantitative method of measuring protein expression, it verified the presence of tendon-lineage proteins in ADSC cultures undergoing tenogenic induction.
Gene expression analysis of tendon tissue
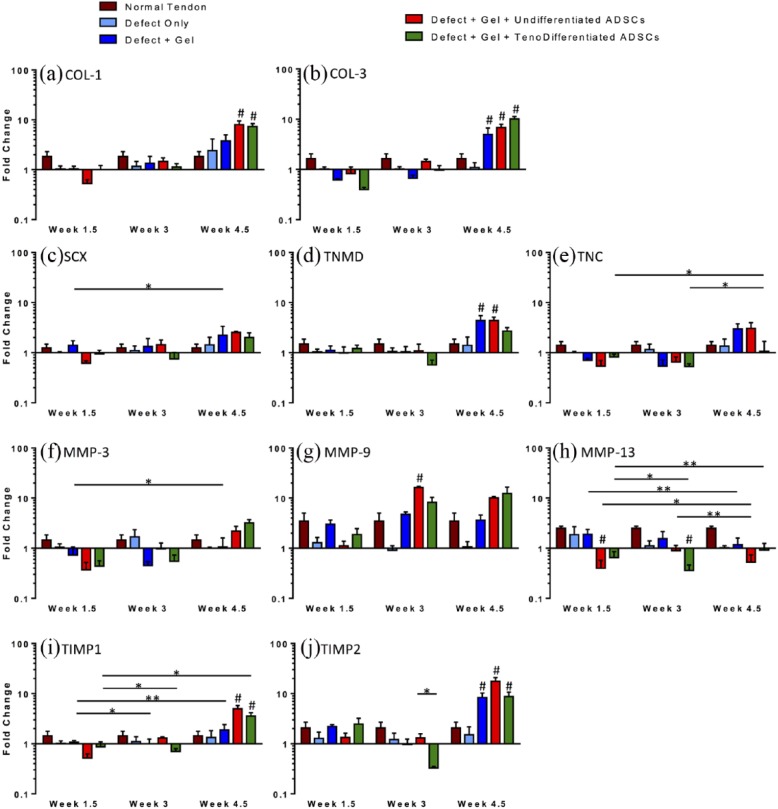
The gene expression (mRNA) of tendons from in vivo experimental groups is reported as fold change relative to the expression levels measured in the control (Gp1; untreated) tendons (n = 4 per group at each time point; Figure 4). The expression levels of normal (uninjured) tendons were also analyzed and graphed for reference. Tendons treated with gel and cells (Gp3 and Gp4) exhibited a trend of increasing both COL1 and COL3 expression over time, with significant increases at 4.5 weeks (COL1 in Gp3: p = 0.0003, Gp4: p = 0.0004 and COL3 in Gp3: p = 0.0004 and Gp4: p < 0.0001; Figure 4(a) and (b)). COL3 expression increased over time also with gel-only treatment (Gp2), with a significant increase at 4.5 weeks (5-fold, p = 0.023; Figure 4b). Tendons treated with tenogenically differentiated ADSCs (Gp4) exhibited a higher expression of COL3 relative to COL1 at 4.5 weeks (10- and 7-fold, respectively; Figure 4(a) and (b)), while those treated with undifferentiated ADSCs (Gp3) exhibited a higher expression of COL1 relative to COL3 (8- and 7-fold, respectively; Figure 4(a) and (b)). There was a trend of increasing SCX, TNMD, and TNC expression with time in Gp3 (at 4.5 weeks SCX: p = 0.064 and TNC: p = 0.089; Figure 4(c)–(e)), with a significant increase in TNMD expression at 4.5 weeks (4-fold, p = 0.013; Figure 4(d)). Groups receiving gel only (Gp2) or gel with tenogenically differentiated ADSCs (Gp4) exhibited a trend of decreasing SCX, TNMD, and TNC expression between 1.5 and 3 weeks and increasing expression from 3 to 4.5 weeks (Figure 4(c)–(e)). There was a significant increase in the expression of SCX in Gp2 from 1.5 to 4.5 weeks (p = 0.001; Figure 4(c)). The expression of TNC increased significantly in Gp4 between 1.5 and 4.5 weeks and then from 3 to 4.5 weeks (p < 0.0001 and p = 0.02; Figure 4(e)). There was an increase in TIMP1 and TIMP2 expression between 1.5 and 4.5 weeks regardless of treatment, with a significant increase in TIMP1 expression in Gp2 and Gp4 (p = 0.0001 and p = 0.024, respectively; Figure 4(i) and (j)). However, at 1.5 and 3 weeks, tendons treated with gel and cells (Gp3 and Gp4) exhibited a decrease in the expression of TIMPs below the levels of the uninjured (normal) tendons. Compared with the untreated tendons (Gp1), at 1.5 weeks, TIMP1 expression was decreased in Gp3, while tendons treated with tenogenically differentiated ADSCs (Gp4) exhibited decreased expression of TIMP1 at both 1.5 and 3 weeks and a significant decrease in TIMP1 expression from 1.5 to 3 weeks (p = 0.029). Although not significant (p = 0.113), at 3 weeks, TIMP2 expression in Gp4 was also decreased when compared with the untreated control (Gp1). There was an increase in MMP-3 and MMP-9 expression with time regardless of treatment (Figure 4(f) and (g)), with a significant increase in MMP-3 expression from 1.5 to 4.5 weeks in Gp2 (p = 0.023) and MMP-9 expression in Gp3 at 3 weeks (p = 0.015). Treatment with cells (Gp3 and Gp4) decreased the expression of MMP-3 below those of the untreated tendons (Gp1) at the early time points (at 1.5 weeks, Gp3: p = 0.072, Gp4: p = 0.066; at 3 weeks, Gp3: p = 0.785, Gp4: p = 0.358; Figure 4(f)) and significantly decreased MMP-13 expression over time (between 1.5 and 3 weeks, Gp4: p = 0.010; between 1.5 and 4.5 weeks, Gp3: p = 0.017, Gp4: p = 0.0006; between 3 and 4.5 weeks, Gp3: p = 0.0003; Figure 4(h)). Expression levels of MMP-1 were undetectable in many experimental samples and thus could not be further evaluated.
Figure 4.
Relative fold change of gene expression measured by quantitative real-time PCR of (a) collagen type-I (COL-1) (b) collagen type-III (COL-3), (c) scleraxis (SCX), (d) tenomodulin (TNMD), (E) tenascin-C (TNC), (f) matrix metalloproteinase-3 (MMP-3), (g) MMP-9, (h) MMP-13, (i) tissue inhibitor of MMP 1 (TIMP1), and (j) TIMP2; *p < 0.05 and **p < 0.01 denote significance with time or when compared with another group at the same time point; #p < 0.05 denotes significance when compared with the untreated control (Gp1) at the same time point.
Histological analysis
Representative images of Achilles tendon sections stained with MT are presented in Figure 5. Tissue organization improved over time in all groups, with tendons evaluated at 4.5 weeks exhibiting the most organized tissue structure (and thus lowest mean histological grading scores) at the excision site (Figure 5(d)). Compared to groups that received hydrogel (Gp2) or hydrogel with tenogenically differentiated ADSCs (Gp4) as treatment, tendons from the unrepaired group (Gp1) exhibited a more disorganized tissue structure at all time points, with increased vascularity, granulation tissue formation, and calcifications.
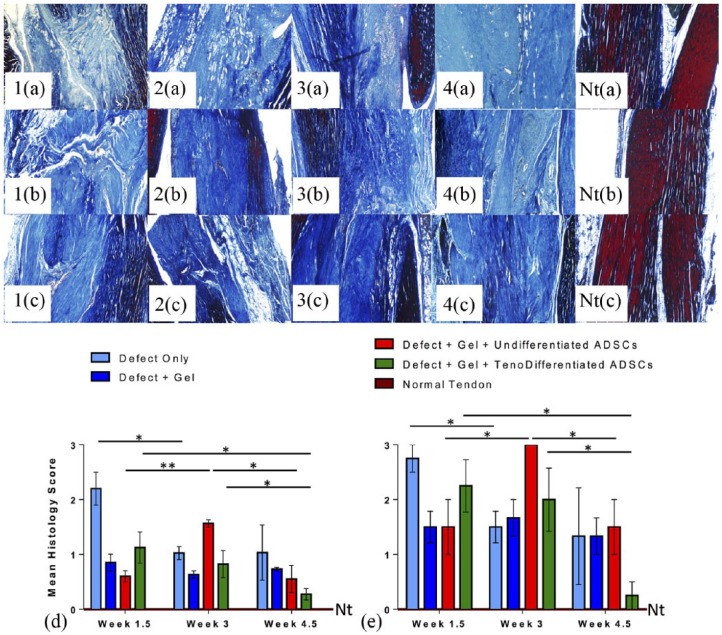
Figure 5.
Achilles tendon sections (n = 4 per group and per time point) stained with Mallory’s Trichrome: representative images of tendon sections showing matrix organization at (a) 1.5, (b) 3, and (c) 4.5 weeks post excision injury: (1a–c) Gp1 (Defect Only), (2A–C) Gp2 (Defect + Gel), (3a–c) Gp3 (Defect + Gel + Undifferentiated ADSCs), (4a–c) Gp4 (Defect + Gel + Tenogenically Differentiated ADSCs), (Nta–c) normal tendon; 100x, Scale bar = 0.2 mm; the mean histological grading scores (d) and the mean collagen fiber organization scores (e) of tendon sections; normal tendon (Nt); *p < 0.05 and **p < 0.01 denote significance with time.
Groups that received hydrogel with undifferentiated ADSCs (Gp3) exhibited more improved tissue organization when compared to unrepaired tendons at 1.5 and 4.5 weeks (p < 0.0244 and p < 0.0405; Figure 5(d)). Tendons from the group that received hydrogel with tenogenically differentiated ADSCs (Gp4) showed the most significant improvement in mean histologic grading scores with time when compared to the other groups (p < 0.0306 from 1.5 to 4.5 weeks and p < 0.0878 from 3 to 4.5 weeks; Figure 5(d)). The highest mean grading scores were observed in the unrepaired group (Gp1) at 1.5 and 4.5 weeks indicating poor healing and disorganized matrix (Figure 5(d)). The biggest changes in mean histology scores were observed at 3 weeks in tendons treated with gel and undifferentiated ADSCs (Gp3, Figure 5(d)). The scores increased significantly from 1.5 to 3 weeks (p < 0.0034) and then significantly decreased from 3 to 4.5 weeks (p < 0.0158). Treatment of tendon injury with hydrogel only (Gp2) significantly improved the histologic grading scores of tendons when compared to unrepaired tendons (Gp1) at 1.5 and 3 weeks (p < 0.0072 and p < 0.0481, respectively; Figure 5(d)). This was further improved in the presence of undifferentiated ADSCs (Gp3) at 1.5 and 4.5 weeks (p < 0.0244 and p < 0.0527). Treatment of tendon injury with tenogenically differentiated ADSCs (Gp4) resulted in a significant trend of increasing tissue organization and repair with time, demonstrated by decreasing mean histologic grading scores (Figure 5(d)). The lowest mean grading scores at 1.5 and 3 weeks were observed in the hydrogel-only group (Gp2, Figure 5(d)). However, at 4.5 weeks, the lowest scores were observed in tendons treated with hydrogel and tenogenically differentiated ADSCs (Gp4) when compared to any other treatment group, which were also significantly lower than in the hydrogel-only group (Gp2; p < 0.0145; Figure 5(d)) and lowest overall across all groups and time points, demonstrating superior tendon repair. Tendon tissue had poor collagen fiber organization at earlier time points (1.5 and 3 weeks; Figure 5(e)), but we observed significant improvement by 4.5 weeks in the group that received gel with tenogenically differentiated ADSCs (Gp4, p < 0.01) as treatment (Figure 5(e)). There was no difference in collagen fiber organization in tendons treated with gel and undifferentiated ADSCs (Gp3) between 1.5 and 4.5 weeks (p = 1.0; Figure 5(e)). There was also no difference in mean collagen fiber organization scores at 4.5 weeks between untreated tendons (Gp1) and tendons treated with gel only (Gp2, p = 1.0; Figure 5(e)).
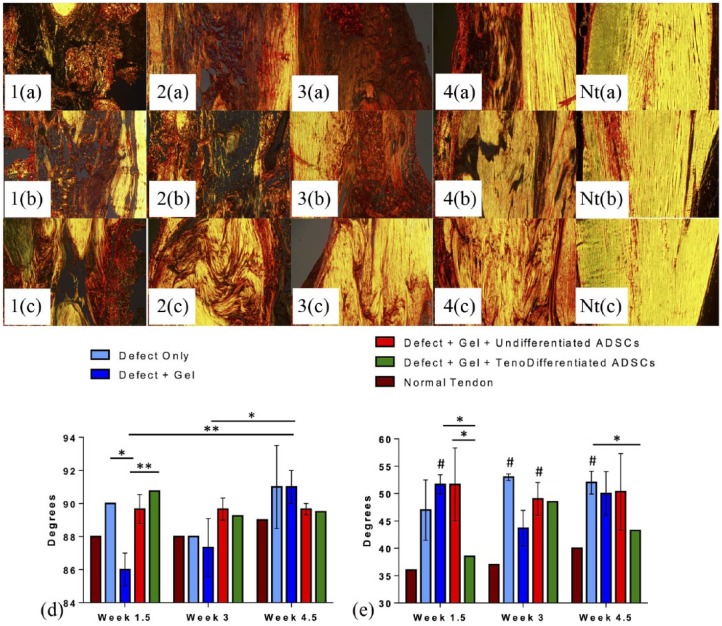
Representative images of Achilles tendon sections stained with picrosirius red are presented in Figure 6. The stained tendon sections of all groups were analyzed for collagen fiber orientation. Uninjured tendons exhibited a tall and narrow shape distribution on the frequency plot of collagen fiber angles, with a principal axis direction of 88°–89° (where x-axis = 0° or 180° and y-axis = 90°) and a dispersion range of 36° to 40° (Figure 6(d) and (e)). Injured tendons exhibited greater principal direction of collagen fibers (87.3° ± 3.1° to 91° ± 1.7°) and a broader fiber dispersion range (38.5° ± 5.7° to 53° ± 1.4°). Principal fiber orientation of samples of all groups was within 3°–4° of normal fiber orientation (88°). The mean principal fiber angle in untreated tendons (Gp1) decreased from 90° ± 0.1° at 1.5 weeks to 88° ± 0.1° at 3 weeks and then increased to 91° ± 4.4° at 4.5 weeks (Figure 6(d)). In injured tendons that received gel treatment with no cells (Gp2), we observed a trend of increasing principal fiber angle with time from 86° ± 1.7° at 1.5 weeks to 91° ± 1.7° at 4.5 weeks (Figure 6(d)). Although not significant, the principal fiber direction that was the nearest to normal tendon (89°) at the 4.5 week time point was observed in Gp3 and Gp4 samples (89.7° ± 0.6° and 89.5° ± 1.5°, respectively; Figure 6(d)). Fiber dispersion range in untreated tendons (Gp1) increased from 47° ± 9.5° at 1.5 weeks to 53° ± 1.4° at 3 weeks and then decreased to 52° ± 3.6° at 4.5 weeks (ns, Figure 6(e)) and was significantly higher than the normal tendons at 3 and 4.5 weeks (p < 0.00001 and p < 0.004 respectively). Groups that received gel only or gel and undifferentiated cells (Gp2 and Gp3) exhibited an initial decrease in dispersion range from 1.5 to 3 weeks (51.7° ± 3.1° to 43.7° ± 5.7° and 51.7° ± 11.5° to 49° ± 5.2° respectively), with a followed increase at 4.5 weeks (50° ± 7.0° and 50.3° ± 12.1°, ns; Figure 6(e)). Treatment with gel and tenogenically differentiated ADSCs (Gp4) improved fiber dispersion, with levels closest to normal tendon at 1.5 and 4.5 week (38.5° ± 5.7° vs 36° and 43.3° ± 1.7° vs 40°, ns; Figure 6(e)) and significantly lower than in the gel-only (Gp2) group at 1.5 weeks (p < 0.004) and the untreated group (Gp1) at 4.5 weeks (p < 0.021; Figure 6(e)).
Figure 6.
Achilles tendon sections (n = 4 per group and per time point) stained with Picro-sirius Red: representative images of tendon sections showing collagen fiber organization at (a) 1.5, (b) 3, and (c) 4.5 weeks post excision injury, (1a–c) Gp1 (Defect Only), (2a–c) Gp2 (Defect + Gel), (3a–c) Gp3 (Defect + Gel + Undifferentiated ADSCs), (4a–c) Gp4 (Defect + Gel + Tenogenically Differentiated ADSCs), (Nta–c) normal tendon; 100x, Scale bar = 0.2 mm; the mean peak angle of primary collagen fiber orientation (d) and fiber dispersion range (e) were calculated for tendon samples in each group; Treatment with tenodifferentiated ADSCs resulted in smallest dispersion range at 4.5 weeks, closest to normal tendon (p < 0.1). *p < 0.05 and **p < 0.01 denote significance with time or when compared with another group at the same time point; #p < 0.05 denotes significance when compared with the normal tendon at the same time point.
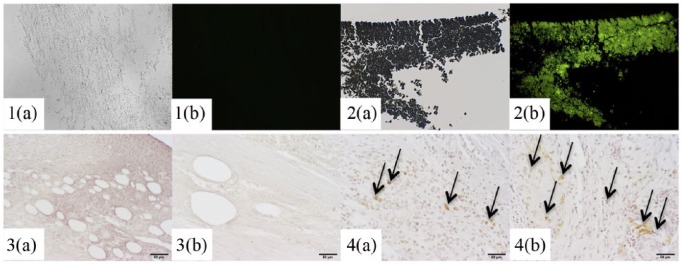
Representative images of Achilles tendon tissue stained for GFP protein are presented in Figure 7. Tendons that were injected with non-GFP ADSCs exhibited no green fluorescence or brown immunostaining of GFP on light microscopy, while tendons injected with GFP-ADSCs exhibited green fluorescence and positive staining. GFP-ADSCs were present at the defect site, as round cells, as well as on the periphery of tendon sections and throughout the tendon substance as elongated, tenocyte-like looking cells. The images confirm the in vivo survival of ADSCs that were injected into tendon defects to support the effects of ADSCs on tissue up to 4.5 weeks post injury.
Figure 7.
Light/fluorescent microscopy (1–2) and immunostaining (3–4) of ADSCs and Achilles tendons: GFP-ADSC pellet (1a) light microscopy, (1b) fluorescent microscopy, non-GFP-ADSCs (2a) light microscopy, (2b) fluorescent microscopy; tendons defects injected with non-GFP ADSCs (3a) at 1.5 and (3b) 4.5 weeks, with GFP-ADSCs (4a) at 1.5 and (4b) 4.5 weeks; arrows point to brown staining of GFP; GFP-ADSCs were present at the defect site at 1.5 and 4.5 weeks, as round cells, as well as on the periphery of tendon sections and throughout the tendon substance as elongated, tenocyte-like looking cells, confirming their survival after injection in vivo; 200x, Scale bar = 0.05 mm.
Structural and biomechanical analyses
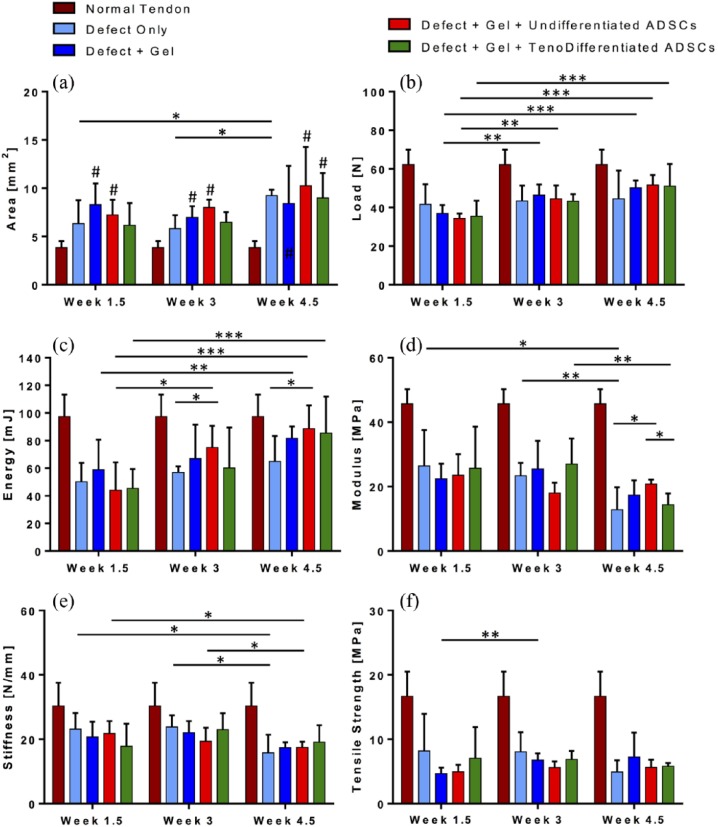
The results from biomechanical analysis of tendons from in vivo experimental groups (n = 7 per group per time point) are reported in Figure 8(a)–(f) and compared to normal uninjured tendons. The CSA (Figure 8(a)) of the repaired tendons in animals treated with gel and tenogenically differentiated ADSCs (Gp4) was comparable to that in the untreated group (Gp1) at all time points (Gp4 6.15 ± 2.30 mm2 vs Gp1 6.33 ± 2.42 mm2 at 1.5 weeks, Gp4 6.48 ± 1.17 mm2 vs Gp1 5.83 ± 1.54 mm2 at 3 weeks, and Gp4 8.99 ± 3.34 mm2 vs Gp1 9.24 ± 0.80 mm2 at 4.5 weeks). At 1.5 weeks, tendons treated with gel only (Gp2) had the highest CSA, significantly higher than the uninjured tendons (8.31 ± 2.43 mm2 vs 3.87 ± 0.85 mm2, p < 0.0007). Compared to normal tendons (3.87 ± 0.85 mm2), tendons from experimental groups had a higher CSA at any time point (ns in Gp3 at 1.5 and 3 weeks). At 1.5 weeks, tendons from Gp2 had the highest CSA; at 3 and 4.5 weeks, Gp3 had the overall highest CSA, but not significantly higher than tendons from any other experimental group. The only significant difference in CSA over time was observed in the untreated group (Gp1), an increase from 1.5 and 3 to 4.5 weeks (p < 0.017 and p < 0.0002).
Figure 8.
Biomechanical properties of Achilles tendon samples (n = 7) post excision injury compared to normal tendon (brown bars): cross sectional area (a), ultimate load at failure (b), elastic toughness (c), Young’s Modulus (d), stiffness (e), and ultimate tensile strength (f) measured at 1.5, 3, and 4.5 weeks; Treatment with tenodifferentiated ADSCs (Gp4) resulted in the most improved maximum load at 4.5 weeks, closest to normal tendon (p < 0.08); *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 denote significance with time or when compared with another group at the same time point; #p < 0.05 denotes significance when compared with the normal tendon at the same time point.
The maximum load at failure (Figure 8(b)) was significantly higher in normal tendons versus tendons from any experimental group at 1.5 and 3 weeks, but not significantly higher than tendons treated with tenogenically differentiated ADSCs (Gp4) at 4.5 weeks (p < 0.078). We observed a significant increase in ultimate load from 1.5 to 4.5 weeks in tendons treated with gel only (37.0 N to 50.2 N Gp2, p < 0.0002) or gel and cells (34.4 N to 51.7 N Gp3, p < 0.00003 and 35.5 N to 51.2 N Gp4, p < 0.02), but not in the untreated tendons (41.7 N to 44.5 N Gp1, ns). In addition, at 4.5 weeks, tendons treated with undifferentiated ADSCs (Gp3) or tenogenically differentiated ADSCs (Gp4) exhibited maximum load at failure closest to that of normal tendons (51.7 N Gp3 and 51.2 N Gp4 vs 62.2 N).
The elastic toughness of analyzed tendons exhibited similar trends observed with maximum load at failure (Figure 8(c)). Energy at maximum tensile stress was significantly higher in normal tendons (97.3 mJ) than in tendons from any experimental group at 1.5 weeks (50.1 mJ Gp1, p < 0.0003; 58.9 mJ Gp2, p < 0.006; 44.1 mJ Gp3, p < 0.0005; 45.4 mJ Gp4, p < 0.0001), but not significantly higher than tendons treated with undifferentiated ADSCs (Gp3) or tenogenically differentiated ADSCs (Gp4) at 4.5 weeks. Untreated tendons (Gp1) had significantly lower toughness than the normal tendons at 3 and 4.5 weeks (57.2 mJ, p < 0.0001 and 64.9 mJ, p < 0.009, respectively). We observed a significant increase in elastic toughness from 1.5 to 4.5 weeks in tendons treated with gel only (58.9–81.8 mJ Gp2, p < 0.038) or gel and cells (44.1–88.6 mJ Gp3, p < 0.002 and 45.4–85.4 mJ Gp4, p < 0.008), but not in the untreated tendons (Figure 8(c)). At 4.5 weeks, tendons treated with undifferentiated ADSCs (Gp3) or tenogenically differentiated ADSCs (Gp4) exhibited the highest elastic toughness, closest to that of normal tendons (97.3 mJ). In addition, toughness of Gp3 tendons was significantly higher than that of the untreated tendons (Gp1) at 3 and 4.5 weeks (p < 0.021 and p < 0.044, respectively).
The elastic modulus of normal tendons was significantly higher than tendons from any experimental group at any time point, and although there was a trend of decreasing modulus in experimental groups over time, this trend was not statistically significant (p < 0.1; Figure 8(d)). At 3 weeks, tendons treated with undifferentiated ADSCs (Gp3) exhibited significantly lower modulus than the untreated tendons (Gp1) or tendons treated with tenogenically differentiated ADSCs (18.0 MPa vs Gp1 23.4 MPa, p < 0.028; Gp4 26.9 MPa, p < 0.028), but at 4.5 weeks, the modulus of Gp3 was significantly higher (20.8 MPa vs Gp1 14.5 MPa, p < 0.021; Gp4 14.4 MPa, p < 0.002). Treatment of Achilles tendon defects with tenogenically differentiated ADSCs (Gp4) resulted in a significant decrease in elastic modulus from 26.9 MPa at 3 weeks to 14.4 MPa at 4.5 weeks (p < 0.005). Stiffness (Figure 8(e)) of tendons from any experimental group followed the same trend of decreasing with time and was significantly lower than that of normal tendons at 4.5 weeks (p < 0.05). In addition, we observed a significant decrease in stiffness from 21.8 N/mm at 1.5 weeks to 17.4 N/mm at 4.5 weeks in tendons treated with undifferentiated ADSCs (Gp3, p < 0.001). The tensile strength of normal tendons (16.7 MPa) was significantly higher than tendons from any experimental group at any time point. Although not significantly, the ultimate tensile strength (Figure 8(f)) of the untreated tendons (Gp1) or tendons treated with tenogenically differentiated ADSCs (Gp4) decreased with time (8.2–4.9 MPa and 7.1–5.8 MPa, respectively), while the tensile strength of tendons treated with gel only (Gp2) or gel with undifferentiated ADSCs (Gp3) increased over time (4.7–7.3 MPa and 5.0–5.6 MPa, respectively).
Discussion
The aim of this study was to investigate the effects of GDF-5, 6, and 7 and PDGF-BB, GFs known to induce tenocytic gene expression in ADSCs in vitro, through media supplementation with novel combinations of these GFs, in order to investigate the effects of undifferentiated and tenogenically differentiated ADSCs on the healing of Achilles tendon excision defects in vivo. We found that combinations of GFs produced significantly better tenogenic effects on rat ADSCs in vitro than single GFs. Our results indicate that the use of ADSCs improves Achilles tendon quality and biomechanical properties at the early stages of tissue repair. Compared with the untreated tendons (Gp1), Achilles defects injected with hydrogel alone (Gp2) or hydrogel with ADSCs (Gp3 and Gp4) exhibited improved tissue repair on histology, collagen fiber alignment closer to normal tendon, and increased expression of COL1, COL3, SCX, and TNMD. In addition, treatment of tendon defects with gel or gel with cells significantly improved biomechanical properties (ultimate load and elastic toughness) of tendons over time. Addition of ADSCs improved tissue architecture and gene expression better than hydrogel alone, while tenogenically differentiated ADSCs showed the most improved tissue repair based on histology and collagen fiber dispersion. Tendons treated with tenogenically differentiated ADSCs exhibited collagen fiber dispersion range closest to normal tendon based on quantitative analysis of picrosirius red–stained samples. We speculate that the improvement in tissue repair may be due to increased localization of neotendon-like cells at the injury site as well as the anti-inflammatory, pro-angiogenic, and pro-proliferatory mediators released by the ADSCs.
We observed an increase in expression of tendon-specific genes over time with the use of combinations of GDF-5, 6, and 7 and PDGF-BB (p < 0.0001). COL1 expression increased over time regardless of treatment, with overall highest levels of expression after 14 days of combined GF treatments, which were significantly higher than treatment with single GFs. Interestingly, we observed a trend of decreased COL1 expression in all groups by the 21-day time point. We postulate that this may be caused by molecular changes within the cell. As differentiation into a tendon-like cell progresses, different molecules may have priority in synthesis. Once the cell has been fully reprogramed into lineage change and matures into a tendon-like cell, the synthesis of tendon’s ECM proteins, such as COL1, would increase after the 21 days time point. SCX expression also increased over time, with overall highest levels of expression observed in groups receiving GF combinations for 21 days. In groups treated with single GFs, SCX expression increased with time, with highest levels achieved by 14 days, after which we observed a decrease in expression. These data suggest that single GF supplementation stimulates an increase in SCX expression at an earlier time point; however, combination of the same GFs achieves significantly higher levels of expression.
Specific markers of most stages of tendon development have yet to be identified; however, we know that the process involves the initial emergence of tendon progenitor cells followed by differentiation and maturation. SCX is a crucial transcription factor expressed at early stages of tendon formation and known to stimulate the formation of tendon progenitors. TNMD and COL1 are downstream molecules positively affected by SCX. TNMD (type II transmembrane protein) is expressed at late stages of tendon differentiation and maturation. Considering what is known about these molecular mechanisms and trends of SCX and TNMD expression observed in this study, we hypothesize that these changes mirror the molecular mechanisms in ADSCs undergoing tenodifferentiation and are therefore indicative of successful tenogenesis. Early in the process of tenoinduction, we observed an increase in both SCX and TNMD expression, followed by continued increase in SCX and decrease in TNMD expression. Since SCX is required to stimulate the cell to become a tendon progenitor, and TNMD is highly expressed in mature tendons, we postulate that an ADSC undergoing differentiation into a tendon-like cell exhibits an increase in most tenogenic markers early on. Once a certain threshold is reached, the cell commits to the tenocytic lineage further increasing SCX expression and becoming a tendon-like progenitor cell. TNMD expression would be expected to increase later, once the cell has completed lineage switch and is stimulated to undergo maturation, a process of which the induction signal is currently unknown. It might be difficult to achieve such maturation in an adherent cell culture without some sort of an additional stimulus, whether by architectural (aligned fibers or scaffold), biomechanical (uniaxial tensile stretching), or further biologic (GFs) cues for actual tendon tissue engineering.
Results from this study confirm and further expand those from previous work published on effects of GDF-5, 6, and 7 or PDGF on tenogenic differentiation.3,11,35,37,43 It has been demonstrated that the use of GDF-5 or GDF-6 in conjunction with an ECM for ADSCs or BMSC tenogenesis in vitro increases SCX, COL1, and TNC expression.11,12,50 The use of GDF-7 with ADSCs, TDSCs, or umbilical cord blood (UCB)-MSCs achieved similar results, albeit with lesser increases in the expression of tendon-specific genes (SCX, TNMD, and TNC).39,51,52 PDGF has also been used in tenodifferentiation of ADSCs with increases in SCX and TNMD expression when PDGF was gradually released into cell media from a porous membrane. The use of aligned collagen fibers has shown the most successful increases in SCX and TNMD expression; however, variation in positive results was large.2,3 GDF-5 and GDF-7 were also tested, both separately or in combination, with best results in COL1, COL3, and TNMD expression after 14 days with the use of a single GF (100 ng/mL of G5 or 1000 ng/mL of G7). A variety of combinations of GDF-5, 6, and 7 were tested in combination with tensile stimulation of ADSCs; however, no detailed gene expression data were provided.37 We demonstrated comparable results with our approach, with significant increases in expression of COL1, COL3, SCX, and TNC after 14 days of treatment, further supporting our data.
One major limitation of this study was that using GFs as media supplementation for induction of tenodifferentiation was done without the use of additional interventions that are known to stimulate the tenocytic phenotype, that is, growing cells in culture with GF supplementation additionally enhanced with a cellular matrix or scaffold or subjecting cells to uniaxial tensile stimulation in culture. Such biomechanical, biochemical, or architectural cues are known to stimulate MSC differentiation.12,37,50,53,54 However, since such cues used alone have not been enough to achieve successful differentiation into tendon-lineage or engineered tendon tissue, we propose that once optimal GF supplementation has been determined successful for use in a specific in vivo or clinical application that the GF combinations be then used together with other available induction techniques to further benefit the tendon tissue engineering field and possibly the study of tendon development and disease.
Taken together, our results demonstrate that the use of GF combinations as media supplementation investigated in this study is more effective at inducing tenocyte-like characteristics in rat ADSCs than the use of single GFs or other GF combinations. Our results indicate that the combination of GDF-6 and PDGF-BB is the most successful at induction of tendon-lineage characteristics in rat ADSCs, and thus, this was the GF combination used to tenogenically induce rat ADSCs for use in the in vivo phases of our study.
These findings can benefit a variety of other studies. There are three animal models commonly used to investigate tendons and include basic mechanisms of chronic tendon injury, process of tendon healing, and translation to clinical care. Application of tenoinduction cocktails from this study in any of the aforementioned models may shed new insights on the subject matter with novel therapeutic strategies possibly being developed. In addition, since tendon injuries range from simple tears to ruptures, as well as chronic pain from tendinopathy, treatments are mostly symptomatic. Future clinical applications may include injections of cells into tendon defects after stimulation with tenogenic cocktails or using them as adjuncts to surgical management. Furthermore, the use of tenoinduction supplementation from this study might be an additional resource for tendon tissue engineering or stem cell reprogramming investigations.
The results from an in vivo part of study further expand on those from previous work on effects of MSC treatment of musculoskeletal and cutaneous injuries.9,25,33,42,55 Although the collagen/alginate hydrogel was used as a vehicle for stem cells, the addition of hydrogel alone improved the repair of Achilles tendon over time, as indicated by better histological grading scores and higher elastic toughness and ultimate tensile load at failure at 4.5 weeks post injury. The unique tensile strength of tendons is derived from the high ratio and parallel arrangement of COL1 fibers. The improvements observed in gel-treated tendons (Gp2) might be due to the increased concentration of COL1 at the injury site, since the hydrogel was observed at the site of tendon defects grossly and microscopically on histology. Tendons treated with gel exhibited both more alignment of fibers histologically as well as better tensile properties than the controls. Connective tissue injury exposes COL1 receptors, which might bind the collagen particles from the injected hydrogel. This might be the potential mechanism explaining continued presence of the gel at the site of tendon injury (and not throughout the surrounding injected tissue) and thus also the improvements in the histological and biomechanical properties of gel-treated tendons.
The addition of ADSCs further enhanced tissue repair and was superior over both the untreated tendons and tendons treated with gel only. There was an improvement in the mean histological as well as mean collagen fiber organization scores in all experimental groups over time, with Gp4, which received tenogenically differentiated ADSCs, exhibiting the best (closest to normal tendon) scores at 4.5 weeks. Although Gp4 did not exhibit the best scores at all time points compared with all the other groups, there was a statistically significant improvement in both scores in Gp4 between 1.5 and 4.5 weeks, which was not observed in other groups. Gp3, which received undifferentiated ADSCs, exhibited a mean histological score close to that of Gp4 at 4.5 weeks, but did not demonstrate fiber dispersion close to normal tendon and exhibited a decline, rather than improvement, in the mean histological score at 3 weeks. The collagen fiber organization scoring obtained by histological grading was consistent with the FFT analysis of picrosirius red–stained samples, validating the precision of that analysis. Although the hydrogel was not the superior treatment, the presence of a viscous liquid at the site of injury and paratenon may have affected the migratory as well as secretory abilities of both resident and injected cells. Further investigations into combinations of collagen hydrogels with cells and their mechanisms of action are needed.
Although injured tendons exhibited CSA greater than normal uninjured tendons, this was not a statistically significant difference. The only statistically significant increase in CSA over time was observed in the untreated group (Gp1). In addition, the control group (Gp1) did not demonstrate significant improvements in repair over time and exhibited inferior biomechanical and histological characteristics of tendons when compared with other treatments. Tendons treated with undifferentiated ADSCs (Gp3) had the largest CSA out of all the groups, but demonstrated improved histological grading scores, collagen fiber alignment, and biomechanical properties over time. Our results are similar to those reported by another study where tendon excision repair was examined at 2 and 4 weeks between sham, gel, and ADSC-treated groups.42 The authors did not include a comparison with normal uninjured tendons or tenogenically differentiated ADSCs, used a lower strain rate of 10 mm/min (our rate 0.25 mm/s = 15 mm/min) and a higher preload of 0.1 N.42 Thus, for a more accurate comparison, we averaged the results between the three time points of our experiments to obtain values at a halfway point. Similar to our study, there was no change in CSA over time in gel-only group and a significant increase in CSA in the untreated tendons. Authors also reported an increase in ultimate load over time in all experimental groups, with tendons treated with gel and cells having a higher load at failure than the untreated tendons and although not significant, higher stiffness in ADSCs treated tendons than in the controls. Results from another study that examined tendon repair at the 2 and 4 week time points with Achilles tendon transection and BMSCs also correlate with our findings.56 Although in the aforementioned study, the load cell capacity was lower than in our instrument (10 N vs 100 N) and the strain rate (50 mm/min) and preload (1 N) higher, the ultimate tensile strength at 2 and 4 weeks was also higher in cell-treated tendons than in the controls but still significantly less than the normal uninjured tendons. We recorded higher tensile strength testing. This could be explained by the difference in injury model we employed, which was an excision defect and not a full tendon transection.
There was an increase in expression (mRNA) of MMPs and TIMPs with increasing time post injury, which was consistent with other studies of Achilles tendon rupture and repair.57,58 Relative to the untreated tendons or tendons treated with hydrogel only (Gp2), treatment with ADSCs decreased the expression of MMPs and TIMPs at the early time points after the injury. The expression of MMP-3, TIMP1, and TIMP2 was decreased with ADSC treatment at 1.5 and 3 weeks, while MMP-13 expression was significantly decreased at all the time points. Since both MMPs and TIMPs are upregulated during the process of remodeling, this might suggest that ADSCs, regardless of differentiation status, stimulate tissue remodeling. Treatment with ADSCs, but not hydrogel alone, also stimulated a significant increase in expression of COL1 and COL3 in injured tendons as early as 3 weeks after the injury. Undifferentiated ADSCs stimulated an equal increase in the expression of both types of collagen, while tenogenically differentiated ADSCs stimulated higher expression of COL3, although the increase in COL1 expression was comparable to that of undifferentiated ADSCs. These results may explain how both cell treatments improved the tensile strength of healing tendons, as COL1 being composed of inelastic fibers, contributes to the tensile properties of tendons. We expected to observe the highest expression of SCX in tendons treated with tenogenically differentiated ADSCs. However, although when compared with the untreated controls, tendons treated with cells demonstrated increased expression of SCX, TNMD, and TNC after 3 weeks, their expression was higher in tendons treated with undifferentiated ADSCs, while TNMD expression was higher in tendons treated with gel only (Gp2). We speculate that the addition of gel may have enhanced the migration of tendon’s intrinsic cells to the site of injury, resulting in the increase of the mature tendon marker, TNMD. The increase in expression of mediators known to be involved in tendon tissue remodeling, as well as ECM components, might be the underlying reason for the improved histological scores, collagen fiber organization, and biomechanical properties in groups treated with ADSCs, as opposed to gel-only and untreated groups.
The logical next step in our experiments is to investigate the effects of combining undifferentiated and tenogenically differentiated ADSCs together to treat Achilles tendon defects. This could be achieved through a combination of both cell types for injection intra-operatively or by introducing two injections as a treatment option—first with undifferentiated ADSCs to explore the possible benefits of ADSC anti-inflammatory and pro-proliferatory paracrine modulation and second with tenogenically differentiated ADSCs to increase the number of tendon-like cells at the injury site and improve the tissue organization properties. Another possible application of this approach is tendon tissue engineering, using both cell types within a hydrogel as well as uniaxial tensile stretching to build tendon tissue in vitro for use in animal models of tendon injury in vivo.
One major limitation of this study was using a rat model of tendon injury as a vehicle for translation into a clinical human injury. Although rat Achilles tendon injury models are among the standard animal models used in orthopedic research, it is unknown how well they can translate into clinical practice. One solution would be to investigate this treatment a larger animal model, for example, a rabbit or goat model of injury. However, it has been shown that some mechanical properties of Achilles tendons are species specific, and although investigations with animals provide insight into processes that occur in tendons, species-specific geometries may have an effect on mechanical behavior of tendons and should be taken into account when translating results from in vivo animal studies.59
Clinically, Achilles tendon ruptures occur during acute trauma resulting in “mop end” morphology of injured tissue, whereas we used a surgical model resulting in a midsubstance excision defect. It is unknown whether a more severe clinical injury would benefit from ADSC administration to the same level as in our induced excision model. In addition, our study demonstrated observable benefits of ADSC treatment on the mechanical properties (improved elastic toughness and ultimate load at failure) of Achilles tendon at three time points early during healing. However, although not significant, we observed a decrease in elastic modulus and ultimate tensile strength in all experimental groups. We expect that an improvement in both properties will occur at a later time point. Tendon repair is not complete at the 4.5-week time point, and healing tissue is undergoing the process of remodeling between 3 and 6 weeks post injury15 during which loose, unorganized COL3 is replaced by tough, parallel fibers of COL1 with the aid of MMPs and TIMPs. Even though there was a significant increase in COL1 expression at 4.5 weeks post injury in Gp3 and Gp4, it might not correlate ideally to actual COL1 protein levels in the tendon. In addition, at 4.5 weeks, the collagen might still be immature and the cross-linking between the fibers might not be complete. Maturity and cross-linkage of collagen determine tendon’s mechanical functionality and material properties, providing a most likely explanation for the decrease in modulus and strength between 3 and 4.5 weeks. In addition, another picrosirius red analysis can be used in future studies to quantify the cross-linking and maturity of collagen fibers to determine any temporal changes that might be affecting the biomechanical properties of healing tendons.
In summary, our findings indicate that treatment of Achilles tendon injury with ADSCs improved tissue repair and functional properties of tendon. Addition of ADSCs improved tissue architecture on histology, increased (mRNA) expression of COL1, COL3, SCX, and TNMD, and significantly improved mechanical properties (ultimate load and elastic toughness) over time more than hydrogel alone, while tenogenically differentiated ADSCs improved the mean histological score and collagen fiber dispersion range closest to normal tendon. While both treatments with undifferentiated ADSCs and tenogenically differentiated ADSCs significantly improved recovery of elastic toughness and ultimate tensile load of tendons over time, treatment with undifferentiated ADSCs improved those biomechanical properties at an earlier time point. These findings suggest that a combination treatment of both undifferentiated and tenogenically differentiated ADSCs may be optimal solution for decreasing scar tissue formation during healing and improving the quality of repair after Achilles tendon injury.
Acknowledgments
The authors would like to thank Melissa Duran, BS, for her contributions with biomechanical testing and Josephine R Coury, BA, for her contributions with ADSC paracrine characterization. J.B.N. conceived the study, collected and analyzed the data, and wrote the manuscript. D.P.P., D.S., A.V., and H.L. contributed to data collection and analysis. D.A.G. conceived the study and edited the manuscript. All the authors approved the manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was funded by the Feinstein Institute for Medical Research and the Orthopedic Surgery Department at Northwell Health. None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
References
- 1. Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med 2003; 22: 675–692. [DOI] [PubMed] [Google Scholar]
- 2. Min HK, Oh SH, Lee JM, et al. Porous membrane with reverse gradients of PDGF-BB and BMP-2 for tendon-to-bone repair: in vitro evaluation on adipose-derived stem cell differentiation. Acta Biomater 2014; 10(3): 1272–1279. [DOI] [PubMed] [Google Scholar]
- 3. Cheng X, Tsao C, Sylvia VL, et al. Platelet-derived growth-factor-releasing aligned collagen-nanoparticle fibers promote the proliferation and tenogenic differentiation of adipose-derived stem cells. Acta Biomater 2014; 10(3): 1360–1369. [DOI] [PubMed] [Google Scholar]
- 4. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med 2005; 33(4): 561–567. [DOI] [PubMed] [Google Scholar]
- 5. Peers KH, Lysens RJ. Patellar tendinopathy in athletes: current diagnostic and therapeutic recommendations. Sports Med 2005; 35(1): 71–87. [DOI] [PubMed] [Google Scholar]
- 6. Tonoli C, Cumps E, Aerts I, et al. Incidence, risk factors and prevention of running related injuries in long distance running: a systematic review. Sport Geneeskunde 2010; 43: 12–18. [Google Scholar]
- 7. Liu H, Zhu S, Zhang C, et al. Crucial transcription factors in tendon development and differentiation: their potential for tendon regeneration. Cell Tissue Res 2014; 356(2): 287–298. [DOI] [PubMed] [Google Scholar]
- 8. Chan K-M, Fu S-C, Yung S-H. Functional tissue engineering for tendinopathies: what’s new on the horizon? In: Doral MN, Karlsson J. (eds) Sports injuries. Berlin; Heidelberg: Springer, 2014, pp. 1–10. [Google Scholar]
- 9. Deng D, Wang W, Wang B, et al. Repair of Achilles tendon defect with autologous ASCs engineered tendon in a rabbit model. Biomaterials 2014; 35(31): 8801–8809. [DOI] [PubMed] [Google Scholar]
- 10. Turner NJ, Badylak SF. Biologic scaffolds for musculotendinous tissue repair. Eur Cell Mater 2013; 25: 130–143. [DOI] [PubMed] [Google Scholar]
- 11. Zhao C, Ozasa Y, Reisdorf RL, et al. CORR® ORS Richard A. Brand Award for outstanding orthopaedic research: engineering flexor tendon repair with lubricant, cells, and cytokines in a canine model. Clin Orthop Relat Res 2014; 472(9): 2569–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. James R, Kumbar SG, Laurencin CT, et al. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed Mater 2011; 6(2): 025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young M, Doran M. Mesenchymal stem cell therapies for bone and tendon conditions. In: Chase LG, Vemuri MC. (eds) Mesenchymal stem cell therapy. New York: Humana Press, 2013, pp. 117–144. [Google Scholar]
- 14. Zi Y, Xiao C, Heng B, et al. Tendon injury: role of differentiation of adult and embryonic derived stem cells. In: Hayat MA. (ed.) Stem cells and cancer stem cells, vol. 4 Dordrecht: Springer, 2012, pp. 87–95. [Google Scholar]
- 15. Thomopoulos S, Parks WC, Rifkin DB, et al. Mechanisms of tendon injury and repair. J Orthop Res 2015; 33: 832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burk J, Gittel C, Heller S, et al. Gene expression of tendon markers in mesenchymal stromal cells derived from different sources. BMC Res Notes 2014; 7: 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu X, Ren J, Li J. Fibrin glue as the cell-delivery vehicle for mesenchymal stromal cells in regenerative medicine. Cytotherapy 2012; 14(5): 555–562. [DOI] [PubMed] [Google Scholar]
- 18. Filomeno P, Dayan V, Tourino C. Stem cell research and clinical development in tendon repair. Muscles Ligaments Tendons J 2012; 2(3): 204–211. [PMC free article] [PubMed] [Google Scholar]
- 19. Chen L, Dong SW, Liu JP, et al. Synergy of tendon stem cells and platelet-rich plasma in tendon healing. J Orthop Res 2012; 30(6): 991–997. [DOI] [PubMed] [Google Scholar]
- 20. Xu K, Al-Ani MK, Sun Y, et al. Platelet-rich plasma activates tendon-derived stem cells to promote regeneration of Achilles tendon rupture in rats. J Tissue Eng Regen Med 2015; 11: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 21. Chen L, Liu JP, Tang KL, et al. Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat Achilles tendinopathy. Cell Physiol Biochem 2014; 34(6): 2153–2168. [DOI] [PubMed] [Google Scholar]
- 22. Tapp H, Hanley EN, Jr, Patt JC, et al. Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp Biol Med (Maywood) 2009; 234(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 23. Vindigni V, Tonello C, Lancerotto L, et al. Preliminary report of in vitro reconstruction of a vascularized tendonlike structure: a novel application for adipose-derived stem cells. Ann Plast Surg 2013; 71(6): 664–670. [DOI] [PubMed] [Google Scholar]
- 24. Melief SM, Zwaginga JJ, Fibbe WE, et al. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med 2013; 2(6): 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grange S. Current issues and regulations in tendon regeneration and musculoskeletal repair with mesenchymal stem cells. Curr Stem Cell Res Ther 2012; 7(2): 110–114. [DOI] [PubMed] [Google Scholar]
- 26. Brehm W, Burk J, Delling U, et al. Stem cell-based tissue engineering in veterinary orthopaedics. Cell Tissue Res 2012; 347(3): 677–688. [DOI] [PubMed] [Google Scholar]
- 27. Chen L, Lu X, Li S, et al. Sustained delivery of BMP-2 and platelet-rich plasma-released growth factors contributes to osteogenesis of human adipose-derived stem cells. Orthopedics 2012; 35(9): e1402–e1409. [DOI] [PubMed] [Google Scholar]
- 28. Del Bue M, Ricco S, Ramoni R, et al. Equine adipose-tissue derived mesenchymal stem cells and platelet concentrates: their association in vitro and in vivo. Vet Res Commun 2008; 32(Suppl. 1): S51–S55. [DOI] [PubMed] [Google Scholar]
- 29. Atashi F, Jaconi ME, Pittet-Cuenod B, et al. Autologous platelet-rich plasma: a biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue Eng Part C Methods 2015; 21: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nam HY, Pingguan-Murphy B, Amir Abbas A, et al. The proliferation and tenogenic differentiation potential of bone marrow-derived mesenchymal stromal cell are influenced by specific uniaxial cyclic tensile loading conditions. Biomech Model Mechanobiol 2015; 14(3): 649–663. [DOI] [PubMed] [Google Scholar]
- 31. Gordon S, Pittenger M, McIntosh K, et al. Tendon regeneration using mesenchymal stem cells. In: Maffulli N, Renström P, Leadbetter W. (eds) Tendon injuries. London: Springer, 2005, pp. 313–320. [Google Scholar]
- 32. Uysal CA, Tobita M, Hyakusoku H, et al. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg 2012; 65(12): 1712–1719. [DOI] [PubMed] [Google Scholar]
- 33. Reed SA, Leahy ER. Growth and development symposium: stem cell therapy in equine tendon injury. J Anim Sci 2013; 91(1): 59–65. [DOI] [PubMed] [Google Scholar]
- 34. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007; 213(2): 341–347. [DOI] [PubMed] [Google Scholar]
- 35. Lamplot JD, Angeline M, Angeles J, et al. Distinct effects of platelet-rich plasma and BMP13 on rotator cuff tendon injury healing in a rat model. Am J Sports Med 2014; 42(12): 2877–2887. [DOI] [PubMed] [Google Scholar]
- 36. Luo Q, Song G, Song Y, et al. Indirect co-culture with tenocytes promotes proliferation and mRNA expression of tendon/ligament related genes in rat bone marrow mesenchymal stem cells. Cytotechnology 2009; 61(1–2): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raabe O, Shell K, Fietz D, et al. Tenogenic differentiation of equine adipose-tissue-derived stem cells under the influence of tensile strain, growth differentiation factors and various oxygen tensions. Cell Tissue Res 2013; 352(3): 509–521. [DOI] [PubMed] [Google Scholar]
- 38. Ruschke K, Hiepen C, Becker J, et al. BMPs are mediators in tissue crosstalk of the regenerating musculoskeletal system. Cell Tissue Res 2012; 347(3): 521–544. [DOI] [PubMed] [Google Scholar]
- 39. Mohanty N, Gulati BR, Kumar R, et al. Immunophenotypic characterization and tenogenic differentiation of mesenchymal stromal cells isolated from equine umbilical cord blood. In Vitro Cell Dev Biol Anim 2014; 50(6): 538–548. [DOI] [PubMed] [Google Scholar]
- 40. Gomiero C, Bertolutti G, Martinello T, et al. Tenogenic induction of equine mesenchymal stem cells by means of growth factors and low-level laser technology. Vet Res Commun 2016; 40(1): 39–48. [DOI] [PubMed] [Google Scholar]
- 41. Theiss F, Mirsaidi A, Mhanna R, et al. Use of biomimetic microtissue spheroids and specific growth factor supplementation to improve tenocyte differentiation and adaptation to a collagen-based scaffold in vitro. Biomaterials 2015; 69: 99–109. [DOI] [PubMed] [Google Scholar]
- 42. Lee SY, Kwon B, Lee K, et al. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med 2017; 45(6): 1429–1439. [DOI] [PubMed] [Google Scholar]
- 43. Chiou GJ, Crowe C, McGoldrick R, et al. Optimization of an injectable tendon hydrogel: the effects of platelet-rich plasma and adipose-derived stem cells on tendon healing in vivo. Tissue Eng Part A 2015; 21(9–10): 1579–1586. [DOI] [PubMed] [Google Scholar]
- 44. Amable PR, Teixeira MV, Carias RB, et al. Identification of appropriate reference genes for human mesenchymal cells during expansion and differentiation. PLoS ONE 2013; 8(9): e73792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25(4): 402–408. [DOI] [PubMed] [Google Scholar]
- 46. Bunnell BA, Estes BT, Guilak F, et al. Differentiation of adipose stem cells. Methods Mol Biol 2008; 456: 155–171. [DOI] [PubMed] [Google Scholar]
- 47. Nguyen Q, Norelli JB, Graver A, et al. Therapeutic effects of doxycycline on the quality of repaired and unrepaired Achilles tendons. Am J Sports Med 2017; 45: 2872–2881. [DOI] [PubMed] [Google Scholar]
- 48. Ayres C, Bowlin GL, Henderson SC, et al. Modulation of anisotropy in electrospun tissue-engineering scaffolds: analysis of fiber alignment by the fast Fourier transform. Biomaterials 2006; 27(32): 5524–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pelled G, Snedeker JG, Ben-Arav A, et al. Smad8/BMP2-engineered mesenchymal stem cells induce accelerated recovery of the biomechanical properties of the Achilles tendon. J Orthop Res 2012; 30(12): 1932–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jiang D, Gao P, Zhang Y, et al. Combined effects of engineered tendon matrix and GDF-6 on bone marrow mesenchymal stem cell-based tendon regeneration. Biotechnol Lett 2016; 38(5): 885–892. [DOI] [PubMed] [Google Scholar]
- 51. Zarychta-Wisniewska W, Burdzinska A, Kulesza A, et al. Bmp-12 activates tenogenic pathway in human adipose stem cells and affects their immunomodulatory and secretory properties. BMC Cell Biol 2017; 18(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu J, Tao X, Chen L, et al. CTGF positively regulates BMP12 induced tenogenic differentiation of tendon stem cells and signaling. Cell Physiol Biochem 2015; 35(5): 1831–1845. [DOI] [PubMed] [Google Scholar]
- 53. Wang W, Li J, Wang K, et al. Induction of predominant tenogenic phenotype in human dermal fibroblasts via synergistic effect of TGF-β and elongated cell shape. Am J Physiol Cell Physiol 2016; 310: C357–C372. [DOI] [PubMed] [Google Scholar]
- 54. Yang G, Rothrauff BB, Lin H, et al. Tendon-derived extracellular matrix enhances transforming growth factor-β3-induced tenogenic differentiation of human adipose-derived stem cells. Tissue Eng Part A 2017; 23: 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ellera Gomes JL, da Silva RC, Silla LM, et al. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc 2012; 20(2): 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang TF, Yew TL, Chiang ER, et al. Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. Am J Sports Med 2013; 41(5): 1117–1125. [DOI] [PubMed] [Google Scholar]
- 57. Jones GC, Corps AN, Pennington CJ, et al. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human Achilles tendon. Arthritis Rheum 2006; 54(3): 832–842. [DOI] [PubMed] [Google Scholar]
- 58. Karousou E, Ronga M, Vigetti D, et al. Collagens, proteoglycans, MMP-2, MMP-9 and TIMPs in human Achilles tendon rupture. Clin Orthop Relat Res 2008; 466(7): 1577–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gatt R, Vella Wood M, Gatt A, et al. Negative Poisson’s ratios in tendons: an unexpected mechanical response. Acta Biomater 2015; 24: 201–208. [DOI] [PubMed] [Google Scholar]