Abstract
Objective:
Type 2 diabetes is a risk factor for the development of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction. Our aim was to provide a summary estimate of the prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in type 2 diabetes patients and to investigate sex disparities.
Methods and results:
A systematic search of the databases Medline and Embase was conducted for studies reporting the prevalence of left ventricular diastolic dysfunction or heart failure with preserved ejection fraction among type 2 diabetes patients. Studies were only included if echocardiography was performed. Prevalence estimates were pooled using random-effects meta-analysis. A total of 28 studies were included. Data on the prevalence of left ventricular diastolic dysfunction were available in 27 studies. The pooled prevalence for left ventricular diastolic dysfunction in the hospital population (2959 type 2 diabetes participants) and in the general population (2813 type 2 diabetes participants) was 48% [95% confidence interval: 38%–59%] and 35% (95% confidence interval: 24%–46%), respectively. Heterogeneity was high in both populations, with estimates ranging from 19% to 81% in the hospital population and from 23% to 54% in the general population. For women and men, the pooled prevalence estimates of left ventricular diastolic dysfunction were 47% (95% confidence interval: 37%–58%) and 46% (95% confidence interval: 37%–55%), respectively. Only two studies presented the prevalence of heart failure with preserved ejection fraction; 8% (95% confidence interval: 5%–14%) in a hospital population and 25% (95% confidence interval: 21%–28%) in the general population [18% in men (mean age: 73.8; standard deviation: 8.6) and 28% in women (mean age: 74.9; standard deviation: 6.9)].
Conclusion:
The prevalence of left ventricular diastolic dysfunction among type 2 diabetes patients is similarly high in men and women, while heart failure with preserved ejection fraction seems to be more common in women than men, at least in community people with type 2 diabetes.
Keywords: Diabetes, left ventricular diastolic dysfunction, prevalence, heart failure with preserved ejection fraction
Introduction
Heart failure (HF) and type 2 diabetes are both major public health concerns and impose a considerable burden on the health budget for Western societies. Mortality and hospitalization rates are much higher among individuals with both type 2 diabetes and HF than in individuals suffering from HF alone.1,2 It is well recognized that type 2 diabetes is a significant risk factor for HF. In the Framingham Heart Study, it was shown that HF was twice as common among men and five times as common among women with diabetes as among those without diabetes.3
Until recently, HF was most often categorized into heart failure with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF) with the single left ventricular cut-point 45%, but currently three categories are used, and the cut-points changed; HFrEF (left ventricular ejection fraction (LVEF) < 40%), HFpEF (EF ⩾ 50%) and a grey area in between (EF: 40%–49%) now categorized as mid-range (HFmrEF).4 A recent systematic review showed that in the general Western population aged 60 years or over, HFpEF with a prevalence of 4.9% is now more common than HFrEF with a prevalence of 3.3%.5 Longitudinal data from the United States suggest that over the last 10 years the incidence of HFrEF seems to be decreasing, while the incidence of HFpEF is increasing.6,7 A reduction in myocardial infarction, notably ST-segment elevation myocardial infarction, over the last decades may be the major cause behind the relative reduction of HFrEF, while the worsening epidemic of overweight and type 2 diabetes affecting Western societies may be one of the major explanations behind the increasing trend in HFpEF.8–10 As such, type 2 diabetes seems to be more strongly associated with the development of HFpEF than with HFrEF.11,12 In line with these findings, left ventricular diastolic dysfunction (LVDD), the preclinical stage of HFpEF, is also more prevalent among type 2 diabetes patients than in those without diabetes.13–15 Although type 2 diabetes is a known risk factor of LVDD and HFpEF, the use of echocardiography is in general not considered in existing type 2 diabetes primary care disease management programmes. Sex differences in the prevalence of LVDD and HFpEF in patients with type 2 diabetes are generally unclear so far. Although some studies suggest that women more often have LVDD and HFpEF than men, some argue this to be related to an average older age of women.6,16 A systematic review and meta-analysis could help clarify whether differences in the prevalence of HFpEF or LVDD exist between women and men with type 2 diabetes.
Given the large impact of both type 2 diabetes and HFpEF for patients, but also for the community, it is important to know the exact prevalence of LVDD in patients with type 2 diabetes as this can be helpful to target prevention and intervention strategies for both LVDD and early stages of HFpEF. The prevalence of both HF and LVDD in type 2 diabetes patients has been studied previously.3,17,18 However, most of these studies did not distinguish between HFrEF and HFpEF, nor assessed LVDD adequately with echocardiography.17,19 Moreover, many studies on LVDD were exclusively performed in type 2 diabetes patients managed in secondary care and thus are not representative of type 2 diabetes patients from the population at large.20,21 A systematic review of studies on the prevalence of LVDD and/or HFpEF in type 2 diabetes patients is lacking. Therefore, we reviewed the existing literature to estimate the prevalence of LVDD and HFpEF in type 2 diabetes patients in both the hospital setting and the general population. Furthermore, we examined whether these prevalence estimates differed between men and women.
Methods
Data sources and searches
A search using the Medline and Embase databases was conducted up to and including May 2016. We used the search terms and synonyms of ‘heart failure’, ‘diastolic ventricular dysfunction’, ‘systolic ventricular dysfunction’, ‘diabetes mellitus, type 2’, ‘prevalence’ and ‘incidence’. For the exact search strategy, see Supplementary Table S1. Of the studies retrieved for full-text assessment, reference lists were screened for other relevant studies.
Study selection
Only studies published in English were considered. Letters, editorials, case reports, practical guidelines and animal or in vitro studies were excluded. The following predefined inclusion criteria were applied: (1) the study reported the prevalence of HFpEF and/or LVDD in patients with type 2 diabetes; (2) the study population was derived from the population at large or from the hospital population; (3) Only studies that used echocardiography to establish or confirm the diagnosis of previously undetected HFpEF and/or LVDD were included; (4) type 2 diabetes defined by one of the following criteria: documentation in medical record, physician’s diagnosis, self-reported history, use of anti-diabetic agents and random serum glucose ⩾ 200 mg/dL (or ⩾11.1 mmol/L) or serum fasting glucose ⩾ 126 mg/dL (or ⩾7.0 mmol/L).
LVDD was defined as an ejection fraction of ⩾45% and diastolic abnormalities on echocardiography such as an E/A ratio < 0.75 or >1.50, E/é ratio > 13 and left atrial (LA) volume index > 34 mL/m2. HFpEF was defined as having an ejection fraction of ⩾45% and clinical symptoms and signs suggestive of HF (i.e. shortness of breath, fatigue, pulmonary congestion and/or peripheral oedema) and objective evidence of diastolic dysfunction measured with echocardiography.
If multiple studies were based on the same study population, we selected the study with the largest population for data extraction. Selection of publications and data extraction was done independently by two reviewers (S.B. and G.B.V.). Consensus was used to resolve disagreement. If consensus could not be reached, a third reviewer (F.H.R.) was consulted.
Data extraction and quality assessment
A methodological quality assessment of each of the included studies was performed independently by two authors (S.B. and G.B.V.). In case of discrepancies, consensus was reached after discussion between the two assessors. If disagreement remained, a third assessor was asked and the majority of votes counted. As there is no formal checklist available specifically designed to appraise risk of bias in prevalence studies, we based our assessment on the risk of bias tool of Hoy et al.22 This is a new risk of bias tool for prevalence studies based on a modification of an existing tool and on the approach of the QUADAS-2 (tool for the Quality Assessment of Diagnostic Accuracy Studies).23 Signalling questions were used to identify potential problems in the design, conduct and analysis of a study that might introduce bias or raise concerns about the applicability of the findings. The following signalling questions were used:
(a) Do the included patients and setting match what is intended by the review question (type 2 diabetes patients from the general population, referral centres and hospital centre)?
(b) Is the sampling frame a true or close representation of the population intended by the review question?
(c) Is an unselected (random/consecutive) sample of patients invited to participate?
(d) Is the response rate ⩾ 75% or did a non-response analysis show no difference between participants and non-participants?
(e) Is an acceptable case definition for LVDD and/or HFpEF used in the study?
(f) Is the instrument to measure LVDD and/or HFpEF valid?
(g) Is the same mode of data collection used for all subjects?
(h) Is it unlikely that the handling of missing (endpoint) data introduced bias?
(i) Were the numerator(s) and denominator(s) for the parameter of interest appropriate?
All signalling questions were scored with either low or high risk of bias. Studies had an overall risk of bias which was classified as low if ⩽1 question had a risk of bias, a medium risk of bias if 2–3 questions had a high risk bias or finally a high risk of bias if >3 questions had a high risk of bias.
Data synthesis and analysis
Information on study characteristics was collected with a data extraction form and comprised the first author’s name, publication year, source population and setting, age, number of participants, duration of type 2 diabetes, exclusion criteria, echocardiographic measurements used, LVEF threshold used and prevalence estimates of HFpEF and/or LVDD. Prevalence numerators and denominators were extracted from the studies.
Individual study prevalence and the corresponding 95% confidence intervals (CIs) were calculated for all the included studies. To perform meta-analysis, the prevalence data were logit transformed so that the data followed a normal distribution. A random-effects model was used to obtain pooled estimates (with the corresponding 95% CI) of the logit-transformed prevalence data, as this model takes the between-study heterogeneity into account better than a fixed-effects model. Heterogeneity was assessed using Cochrane’s Q test and the I2 statistic.24 The pooled prevalence estimate was calculated for all the included studies and separately for studies concerning the general population and hospital population. If we could not recalculate prevalence estimates, because of missing information on the number of individuals suffering from LVDD or HFpEF, they were not included in the meta-analysis. Results of the meta-analysis are presented as Forest plots showing prevalence proportions with the corresponding 95% CIs for each study and the overall random-effects pooled estimate. Publication bias was first assessed by visually inspecting the distribution of the observed studies on a funnel plot. To quantify the degree of bias illustrated in the funnel plot, Begg’s rank correlation test and Egger’s linear regression were used.25,26 A p-value < 0.05 was considered significant. All statistical analyses were performed in R using the ‘metafor’ package.27
Results
Search results and characteristics
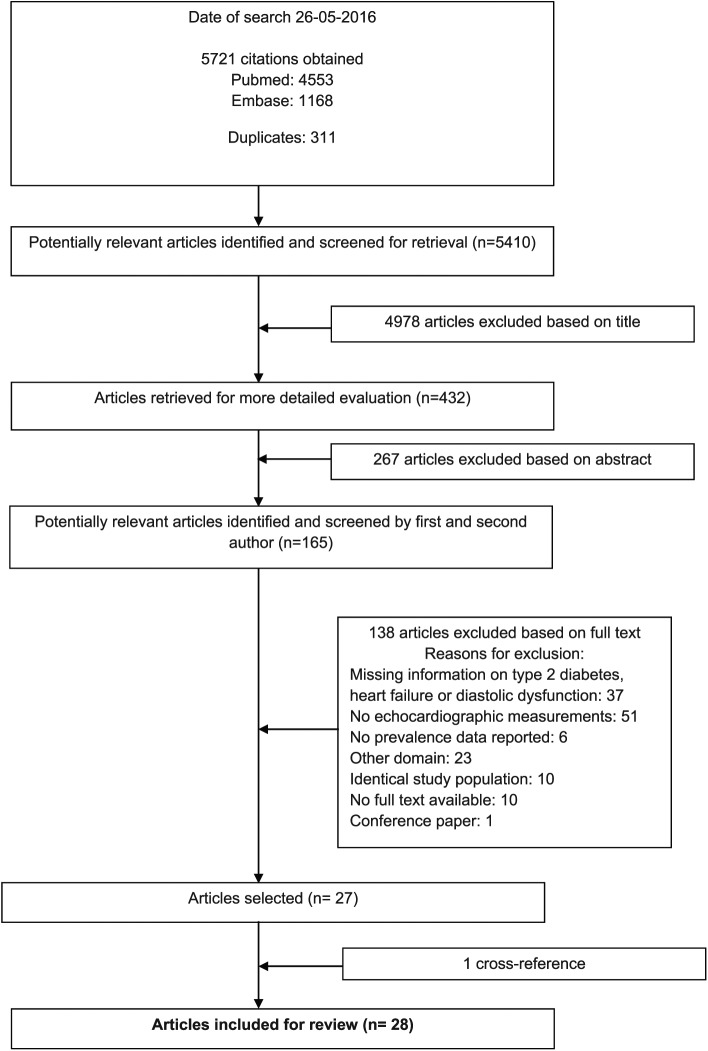
In total, our search resulted in 5410 unique studies. These studies were first screened on title and then on abstract for eligibility. We additionally screened the full-text article of 165 studies for more detailed information. The main reasons for exclusion included the following: no echocardiographic measurements, missing information on type 2 diabetes, HFpEF or diastolic dysfunction, or studies had another domain of interest, for instance, hypertensive patients with diabetes.28 Finally, 28 studies were included in this review. Details of the selection process are provided in Figure 1.
Figure 1.
Flow chart of the process for selection of relevant articles.
Study characteristics and quality assessment of all the 28 included studies are shown in Table 1. Of all the included studies, the majority included participants derived from a hospital setting (n = 18),13–15,20,21,29–41 six studies recruited their participants from the population at large18,42–46 and four studies failed to report where they had selected their participants from.47–50 Data on the prevalence of LVDD were available in 27 studies and data on HFpEF in two studies (Table 1). Data on prevalence numbers were available from 16 different countries: 4 from Africa, 2 from Australia, 11 from Europe, 4 from the United States and 3 from Asia (Table 1). Of the 28 studies analysed, 24 reported the age of their participants, with only 5 studies reporting sex-specific mean age. The mean age ranged from 44 ± 6 years (in an American cohort with an upper age limit of 65) to 71.5 ± 7.5 years in a European cohort. Duration of type 2 diabetes was reported in 19 of the 28 studies and ranged from new-onset diabetes to a mean duration of more than 18 years. Different parameters were used to assess LVDD including the ratio between early (E) and late (A) ventricular filling velocity over the mitral valve (E/A ratio), E-wave deceleration time (DT), isovolumetric relaxation time (IVRT) and the ratio of mitral early diastolic inflow velocity to mitral early annular lengthening velocity (E/é ratio). See Table 1 for the exact cut-off values of the different parameters and the classification of LVDD used in the included studies. The LVEF cut-point ranged from 45% to 55%, with most studies using 50% (n = 15). Most articles had a medium risk of bias (n = 19), five had a high risk of bias and four had a low risk of bias. Most studies scored a high risk of bias on item (b) concerning the sampling frame.
Table 1.
General characteristics and quality assessment of the included studies.
| Author (year of publication) | Source population and setting | Age in yearsa | No. of participants (male) | Type 2 diabetes duration (years), [means ± SD or median (range)] | Exclusion criteria | Echocardiographic measurements
(methods) |
Heart failure (yes/no) | Risk of bias (low/high) |
Overall risk (low/medium/high) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-point LVEF to separate LVSD from LVDD | Classification of LVDD | (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | ||||||||
| Poirier (2001) | Consecutive Caucasian sedentary men, setting not reported | 38–67 | 46 (100%) | Normal LVDD: 4 (1–10) Impaired relaxation: 2.5 (0.25–32) Pseudonormalized pattern: 6.5 (1.5–30) |
Cardiovascular or respiratory disease, hypertension, not well-controlled DM during 3 months before enrolment, retinopathy, neuropathy and macro-albuminuria | Normal LVEF, no cut-point reported | Classified according to Canadian consensus on LVDD
(impaired, pseudonormal, restrictive) Pseudonormal: two of the three criteria: E/A < 1 after Valsalva manoeuvre E/A ratio decrease ⩾ 25% Pulmonary A-wave duration longer than mitral A-wave duration |
No | H | H | L | H | L | L | L | L | L | Medium |
| Zabalgoitia (2001) | Source population and setting not reported | 38–59 46 ± NA |
86 (57%) | Normal LVDD: 4.6 (1–10) Impaired relaxation: 4.5 (0.5–12) Pseudonormalized pattern: 6.5 (1–10) |
Ischemic heart disease, congestive heart failure, hypertension, insulin therapy, uncontrolled diabetes < 1 month before enrolment, retinopathy, neuropathy and nephropathy | Not reported | Divided into impaired, pseudonormal and
restrictive Pseudonormal: E/A ratio > 1 and DT 160–240, but E/A ratio < 1 after Valsalva manoeuvre |
No | H | H | H | H | H | L | L | L | L | High |
| Annonu (2001) | Patients attending the Diabetic Centre of Cairo University hospital, Egypt | 39–64 57 ± 6.8 |
66 (53%) | Not reported | Insulin use, alcoholism, clinical or electrocardiographic evidence of heart diseases and hypertension | 50% | E/A ratio < 1 | No | L | H | H | H | L | L | L | L | L | Medium |
| Boyer (2004) | Consecutive asymptomatic, normotensive patients, setting not reported | 49 (31–59) | 57 (47%) | Normal LVDD: 4.7 ± 3.3 Abnormal LVDD: 5.8 ± 5.5 |
Hypertension, coronary artery disease, valvular heart disease and congestive heart failure, > 60 years of age | Not reported | One of the following findings by conventional
echo: E/A ratio < 1 or >2 DT < 150 or >220 IVRT < 60 or >100 Pseudonormal: change in E/A ratio > 40% after Valsalva manoeuvre TDI: septal and lateral walls < 8 cm/s Colour M-mode propagation velocity < 45 |
No | H | H | L | H | L | L | L | L | L | Medium |
| Fang (2005) | Asymptomatic patients from the ambulatory diabetes clinic at Princess Alexandra Hospital, Australia | No age range or overall mean age reported | 101 (not reported) | Not reported | History of complaints of cardiac disease, history of coronary artery disease, valvular disease, atrial fibrillation, severe arrhythmias and congenital heart disease | 50% | Resting basal segmental myocardial peak diastolic velocity (Em) | No | L | H | L | H | H | L | L | H | H | High |
| Bajraktari (2005) | Consecutive patients from the Clinic of Internal Medicine, University Clinical Centre, Kosovo | 56 ± 8.3 | 228 (114 cases, 33% male) | Not reported | Arterial hypertension, ischemic heart disease, cardiac arrhythmias, congenital or acquired valvular heart disease, chronic renal failure, age > 75, insulin therapy and poor echocardiographic window | Not reported |
E/A
ratio < 1 Pseudonormal: E/A ratio ⩾ 1 and Vp > 55 |
No | L | H | L | H | L | L | L | L | L | Medium |
| Dawson (2005) | Random volunteers from the Diabetes Centre, Ninewells Hospital, Scotland | 63.8 ± 10.6 | 500 (61.6%) | 6.0 ± 5.5 | Frailty and inability to give written informed consent | 45% |
E/A ratio, E-wave DT, IVRT
according to European Study Group on Diastolic Heart
Failure: LVEF ⩾ 45% and E/A ratio < 50 years < 1.0 and DT < 50 years > 220 ms, E/A ratio > 50 years < 0.5 and DT > 50 years > 280 ms and/or IVRT < 30 years > 92 ms, IVRT 30–50 years > 100 ms, IVRT > 50 years > 105 ms |
No | L | L | L | L | L | L | L | H | H | Medium |
| Albertini (2008) | Consecutive asymptomatic patients admitted at the Avicenne Hospital endocrinology unit, France | 59.8 ± 1.5 Male: 60 ± 14 Female: 61 ± 15 |
91 (54%) | 13 ± 1.1 | Previous or suspected history of heart disease, intrinsic lung or overt renal disease, incomplete echocardiographic data or poor echogenicity | 50% | Impaired: E/A ratio < 1
or >1, E/é
ratio > 10 Restrictive: E/A ratio > 2 or EA ratio 1–2 with DT ⩽ 130 Pseudonormal: E/A ratio 1–2 with DT 150–220 |
No | L | H | L | H | L | L | L | L | L | Medium |
| Henry (2008) | Participants from the Hoorn Study and Hoorn Screening Study, both population-based studies, the Netherlands | 66.9 ± 8.2 | 746 (298 DMII patients, 54%) | Not reported | None | 55% | One of the following criteria: Peak A velocity ⩾ 97 Difference between Apv and Amv duration ⩾ 41 LA volume ⩾ 57 |
No | L | L | L | L | L | L | L | L | H | High |
| Srivastava (2008) | Patients referred for echocardiography as part of a routine complications surveillance programme, mainly by general practitioners (80%) and 20% from the hospital, at the Diabetic Clinic at Austin Health, Australia. | 62 ± 1 | 229 (58%) | 10 ± 1 | None | 50% | Divided into impaired, pseudonormal and
restrictive Pseudonormal: evidence of increased LV filling pressures with three of four of the following criteria: E/é ratio > 10 Depressed Vp (< 50) Pulmonary A duration > mitral A duration PulAVmax > 0.35 and positive Valsalva manoeuvre |
No | L | L | H | H | L | L | L | H | L | Medium |
| From (2010) | Participants from Olmsted County population, USA | 60 ± 14 | 1760 (49%) | Not reported | Diagnosis of HF before echocardiogram or made within 30 days after echocardiogram | Not reported | E/é ratio > 15 | No | L | L | H | L | L | L | L | L | L | Low |
| Poulsen (2010) | Patients referred, for the first time, for diabetes education or poorly regulated diabetes to the Diabetes Clinic at Odense University Hospital, Denmark | 58.6 ± 11.3 | 305 (54%) | 4.5 ± 5.3 | History of CVD, malignancy or end-stage kidney disease, pregnancy, body weight > 150 kg, physical or mental disability, not able to provide inform consent | 50% | Grade I: DT > 240, E/A
ratio < 0.7, Vp ⩽ 45 and
eseptum’ < 8 Grade II: DT 140–240, E/A ratio 0.7–1.5, Vp ⩽ 45 and eseptum’ < 8 Grade III: DT < 140, E/A ratio > 1.5, Vp ⩽ 45 and eseptum’ < 8 |
No | L | H | L | H | L | L | L | L | L | Medium |
| Kazlauskaite (2010) | Consecutive adults from ethnic minority groups (African-American, Hispanic, other immigrant) with newly diagnosed type 2 diabetes attending a diabetes clinic at a large urban public hospital in Chicago, USA | No age range or overall mean age reported | 126 (48%) | Not reported | History of cardiovascular diseases, creatinine > 141 µmol/L, current or chronic infectious disease, prolonged cocaine or heroin use or alcoholism | Not reported | Grade I: DT > 240, E/A
ratio ⩽ 0.75, IVRT > 90 Grade II (pseudonormal filling): DT > 140, 0.75 < E/A ratio < 1.5, E/é ratio ⩾ 10 Grades III and IV: not reported |
No | L | H | L | H | L | L | L | L | L | Medium |
| Patil (2011) | Normotensive patients with >5 years DMII at the Krishna Institute of Medical Sciences, Karad, India | Male: 51 ± 9 Female: 49 ± 10 |
227 (127 cases, 55% male) | Male: 11 ± 5 Female: 10 ± 4 |
Evidence of coronary artery disease, valvular disease, hypertension or anti-hypertensive medication, poor transthoracic echo window | 50% | One of the following
findings: E/A ratio < 1 or >2 DT < 150 or >220 IVRT < 60 or >100 E/é ratio > 15 |
No | L | H | H | H | L | L | L | L | L | Medium |
| Ernande (2011) | Consecutive patients referred to the outpatient clinical department of diabetology of Louis Pradel Hospital, Lyon, France | 52 ± 4.5 | 200 (114 cases, 61% male) | 11 ± 7 | Absence of sinus rhythm, coronary and valvular heart diseases, severe renal failure, severely uncontrolled DM and uncontrolled blood pressure (SBP > 180 mm Hg and/or DBP > 100 mm Hg), DM I, echo images unsuitable for quantification | 55% | Septal é < 8 and lateral é < 10 and LA volume > 34, then they were further categorized into grade I, II or III according to ASE and EAE recommendations using E/A ratio, mDT, E/é ratio and Ar-A time interval | No | L | H | L | H | L | L | L | L | L | Medium |
| Aigbe (2012) | Randomly selected patients at the University Teaching Hospital, Nigeria | 26–80 55.4 ± 11.6 |
300 (150 cases, 43% male) | 4.5 ± 4.5 | Hypertension, pregnancy, sickle cell disease and structural heart disease | 50% | Impaired: E/A ratio < 1
or >1, DT > 120 Pseudonormalization: E/A ratio 1–2, IVRT 80–110, DT 150–220 and S/D < 1 Restrictive: E/A ratio > 2 and DT < 150 |
No | L | H | L | H | L | L | L | L | H | Medium |
| Boonman-de Winter (2012) | Patients enrolled in the Diabetes Care programme of the Centre for Diagnostic Support in Primary Care, the Netherlands | 71.5 ± 7.5 Male: 71.9 ± 7.5 Female: 71.4 ± 7.4 |
605 (54%) | Not reported | none | 45% |
E/é ratio ⩾ 15 or
E/é ratio 8–15 and
septal é < 8. Then further categorized
as follows: Grade I: E/A ratio ⩽ 0.75, DT ⩾ 180, S/D ⩾ 1 Grade II: 0.75 < E/A ratio < 1.5, 140 < DT < 320, S/D < 1 Grade III: E/A ratio > 1.5, DT < 140, S/D < 1 |
Yes | L | L | L | H | L | L | L | L | L | Low |
| Cioffi (2012) | Non-institutionalized subjects > 45 years of age participating in the Dysfunction in DiAbetes’ (DYDA) study recruited in 37 diabetes referral centres, Italy | 61 ± 7 | 751 (61%) | 7 (3–13) | Myocardial infarction, myocarditis, HF, coronary heart disease, alcoholic cardiomyopathy, primary hypertrophic cardiomyopathy, asymptomatic known LVD, prior myocardial revascularization, valvular heart disease, atrial fibrillation, electrocardiographic findings of myocardial ischaemia, DMI and severe systematic disease with life expectancy < 2 years | 50% | All conditions different from normal LVDD defined as
follows: E/A ratio 0.75–1.5 and DT of E wave > 140 ms according to Redfield |
No | L | H | L | L | L | L | L | L | L | Low |
| Utrera-Lagunas (2013) | Patients attending the Internal Medicine Service at Nacional de Ciencias Medicas y Nutricion, Mexico | No age range or overall mean age reported | 160 (45%) | With HF: 17.4 ± 8.5 Without HF: 19.4 ± 9.7 |
Pancreatitis, liver failure, end-stage renal failure, recent (<3 months) acute coronary syndrome and/or myocardial revascularization, congenital heart disease, myocarditis, valvular heart disease, myocardial dysfunction secondary to radio-chemotherapy | 45% | LAD > 45 mm, ventricular septal thickness > 12 mm, posterior wall thickness > 12 and characteristic pattern of transmitral Doppler flow (slow, inverted, pseudonormal or restrictive) | Yes | L | H | H | H | L | L | L | L | H | High |
| Faden (2013) | Consecutive non-institutionalized subjects > 18 years of age attending a prospective, multicentre study, (SHORTWAVE) in cardiology and diabetes referral centres in 4 hospitals, Italy | 69 ± 10 | 386 (57%) | 5 (2–10) | Myocardial infarction, dilated cardiomyopathy or HF, primary hypertrophic cardiomyopathy, prior myocardial revascularization, valvular disease, atrial fibrillation, chronic pulmonary disease, DMI | Not reported | Mild: E/A ratio ⩽ 0.75,
ΔE/A ratio < 0.5.
E/é ratio < 10,
S > D
Moderate: E/A ratio 0.75–1.5, DT > 140, ΔE/A ⩾ 0.5. E/é ratio ⩾ 10, S < D Severe: E/A ratio > 1.5, DT < 140, 0.5 < ΔE/A ⩾ 0.5. E/é ratio ⩾ 10, S < D According to Redfield (2003) |
No | L | H | L | H | L | L | L | L | L | Medium |
| Chillo (2013) | Patients who participated in a survey to determine prevalence of microalbuminuria attending the outpatient clinic of Muhimbili National Hospital in Dar es Salaam, Tanzania | 55 ± 9 | 180 (122 DMII patients, sex not reported) | 11 ± 6 | None (reported) | 50% | E/é ratio ⩾ 15 | No | L | H | H | L | L | L | L | L | L | Medium |
| Dodiyi-Manuel (2013) | Patients attending the Medical Outpatient Department of the University of Port Harcourt Teaching Hospital, Nigeria | 36–65 50.8 ± 9.1 |
180 (90 DMII patients, 43% male) | 3.4 ± 2.9 | Hypertension (>140/90 mm Hg), anti-hypertensive medications, valvular abnormalities and wall motion abnormalities | 55% | Impaired relaxation: E/A
ratio < 1 Pseudonormal using Valsalva method Restrictive: E/A ratio > 2 |
No | L | H | H | H | L | L | L | L | L | Medium |
| Akiyama (2014) | Asymptomatic outpatients, setting not reported | 61.6 ± 9.7 | 100 (55%) | Not reported | Overt heart failure, LVEF < 50, history of CAD, severe valvulopathy and chronic atrial fibrillation | 50% |
E/A ratio < 0.75, or
⩽0.75 and E/é
ratio ⩾ 10 According to the definition of Redfield |
No | L | H | H | H | L | L | L | L | L | Medium |
| Chen (2014) | Consecutive patients treated with stable hypoglycaemic medication for at least 3 months recruited from the medical outpatient clinic of Queen Mary Hospital, Hong Kong, China | 62 ± 9 | 95 (39%) | 10 ± 8 | History or clinical symptoms of cardiovascular disease, including CAD, MI, stroke or peripheral vascular disease, renal impairment (eGFR < 30 mL/min/1.73 m2), liver failure, SLE, rheumatoid arthritis, systemic sclerosis | 50% | Septal é < 8 and lateral
é < 10 and LA volume > 34, then
further categorized as follows: Grade I: E/A ratio < 0.8, DT > 200, E/é ratio ⩽ 8, Ar-A < 0 Grade II: E/A ratio 0.8–1.5, DT 160–200, E/é ratio 9–12, Ar-A ⩾ 30 Grade III: E/A ratio ⩾ 2, DT < 160, E/é ratio ⩾ 13, Ar-A ⩾ 30 According to Nagueh (2009) |
No | L | H | L | H | L | L | L | L | L | Medium |
| Dandamundi (2014) | Random sample of residents participating in the Rochester Epidemiology Project, Olmsted County, USA | No age range or overall mean age reported | 2042 (136 DMII patients, 60% male) | Not reported | Missing data on systolic or diastolic assessments | 50% | Mild (impaired relaxation without increased filling
pressures): E/A
ratio ⩽ 0.75 and E/é
ratio < 10 Moderate (impaired relaxation with moderately elevated filling pressures of pseudonormal filling): 0.75 < E/A ratio < 1.5 and E/é ratio ⩾ 10 Severe (reversible of fixed restrictive filling): E/A ratio > 1.5 and E/é ratio ⩾ 10 |
No | L | L | L | H | L | L | L | L | L | Low |
| Habek (2014) | Patients were recruited from the Centre for Diabetes Clinic of Internal Medicine, University Hospital Osijek, and the cardiac and diabetic outpatient ‘Sunce’ policlinic, Zagreb, Croatia | 60.2 ± NA Male: 63 ± NA Female: 58.5 ± NA |
202 (61%) | 8.9 ± NA | LVEF < 50%, AF, pacemaker or ICD, history of MI, AP, left bundle branch block, chronic congestive HF, serious valvular of congenital cardiac disease, active myocarditis, severe hepatic of renal disease and type I DM | 50% | Septal é < 8 and lateral
é < 10 and LA volume > 34, then
further categorized as follows: Grade I: E/A ratio < 0.8, DT > 200, E/é ratio ⩽ 8, Ar-A < 0 Grade II: E/A ratio 0.8–1.5, DT 160–200, E/é ratio 9–12, Ar-A ⩾ 30 Grade III: E/A ratio ⩾ 2, DT < 160, E/é ratio ⩾ 13, Ar-A ⩾ 30 According to Nagueh (2009) |
No | L | H | H | H | L | L | L | L | H | High |
| Pareek (2015) | Subjects derived from a population-based cohort study (Mälmo Preventive Project), Sweden | 66 (IQR 60–70) | 691 (107 with DMII, 79% male) | New onset | Cardiovascular disease and/or cardiovascular, anti-diabetic or lipid-lowering therapy | 50% | Grade I (mild): septal é < 8, lateral
é < 10, DT ⩾ 240,
E/A ratio < 0.8,
E/é
ratio ⩽ 12 Grade II (moderate): septal é < 8, lateral é < 10, DT 140–240, E/A ratio 0.8–1.5, E/é ratio ⩾ 9 Grade III (severe): septal é < 8, lateral é < 10, DT < 140, E/A ratio > 1.5, E/é ratio ⩾ 13 |
No | L | H | L | H | L | L | L | L | H | Medium |
| Chaudhary (2015) | Normotensive patients with newly diagnosed (within 1 month) DMII recruited from the SVBP Hospital, LLRM Medical College, Meerut, India | 30–60 50.1 ± 6.3 Male: 50.7 ± 5.7 Female: 48.9 ± 7.0 |
100 (65%) | New onset | Hypertension > 130/80, abnormal ECG, already diagnosed DMII, anti-diabetic treatment, valvular heart disease, ischemic and hypertensive heart disease, congestive HF, cardiomyopathy, renal failure, COPD, severe anaemia and haemoglobinopathies | 50% | Any of the following
criteria: E/A ratio < 1 or > 2 DT < 150 or > 220 IVRT < 60 or > 100 E/é ratio > 15 |
No | L | H | H | H | L | L | L | L | L | Medium |
SD: standard deviation; LVEF: left ventricular ejection fraction; LVSD: left ventricular systolic dysfunction; LVDD: left ventricular diastolic dysfunction; DM: diabetes mellitus; DT: E-wave deceleration time; IVRT: isovolumetric relaxation time; TDI: tissue Doppler imaging; Vp: flow propagation velocity; PulAVmax: pulmonary venous atrial reversal maximal velocity; CVD: cardiovascular disease; LVD: left ventricular dysfunction; HF: heart failure; LAD: left atrial diameter; CAD: coronary artery disease; MI: myocardial infarction; eGFR: estimated glomerular filtration ratio; SLE: systemic lupus erythematosus; ECG: electrocardiogram; COPD: chronic obstructive pulmonary disease; LA: left atrial; AF: atrial fibrillation; ICD: internal cardiac defibrillator; AP: angina pectoris; NA: not available; E (wave): peak early diastolic mitral inflow velocity; A (wave): peak late mitral inflow velocity; E/A (ratio): ratio of peak early and peak late mitral inflow velocities: M-mode: motion mode; E/é (ratio): peak early mitral inflow velocity divided by peak mitral annular velocity; Apv: pulmonary vein flow A-wave duration; Amv: mitral valve A-wave duration; eseptum’: peak septal mitral annular velocity; mDT: E-wave deceleration time; Ar-A: time difference between atrial reversal velocity waveform and mitral late filling duration; S/D ratio: peak systolic velocity divided by peak anterograde diastolic velocity in the pulmonary vein: ASE: American Society of Echocardiography; EAE: European Association of Echocardiography.
Values indicate the age range, mean ± standard deviation or median (range).
Prevalence of LVDD and HFpEF
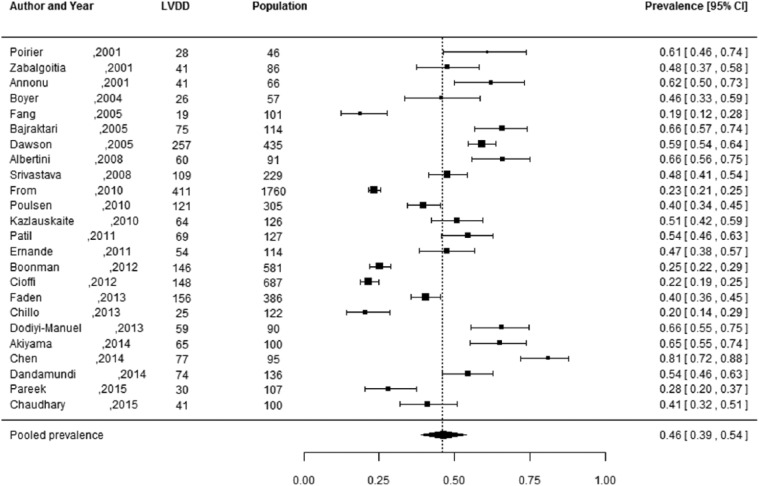
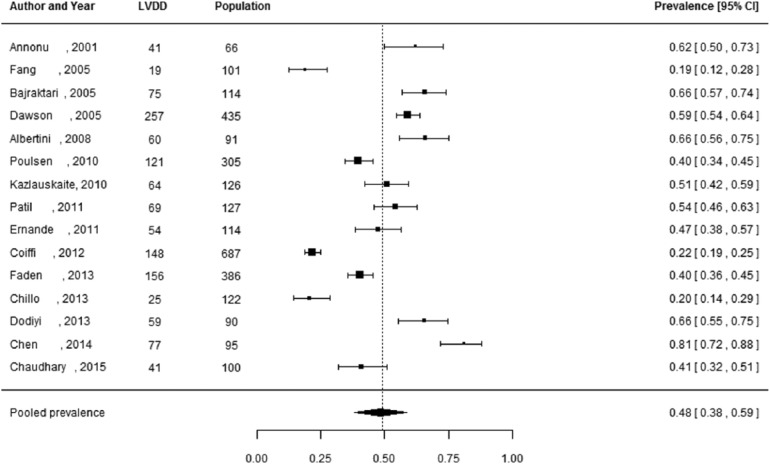
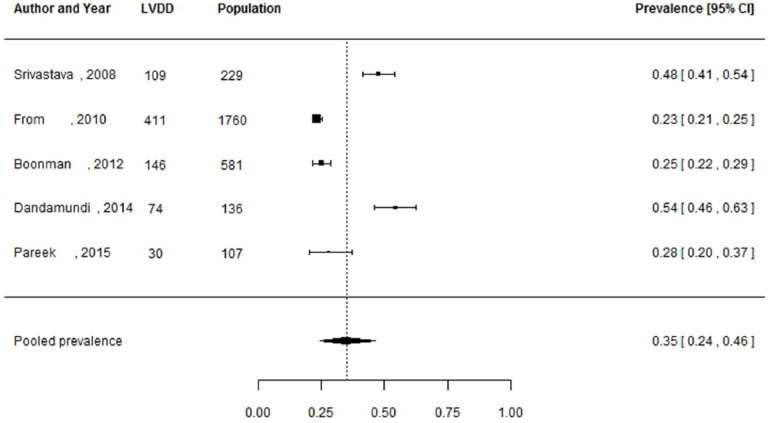
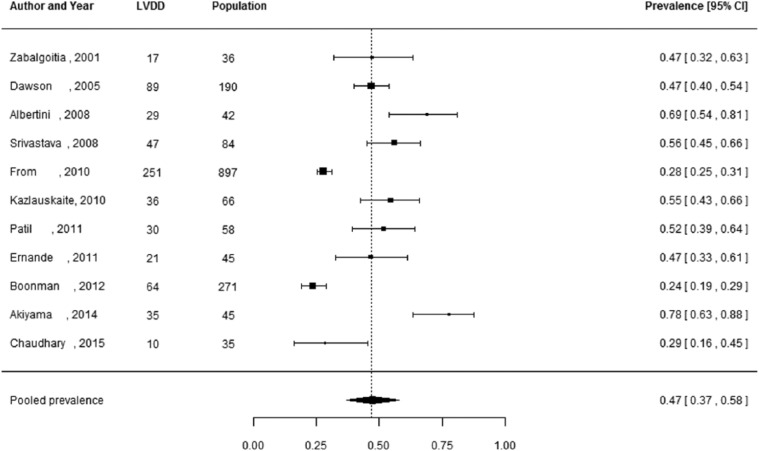
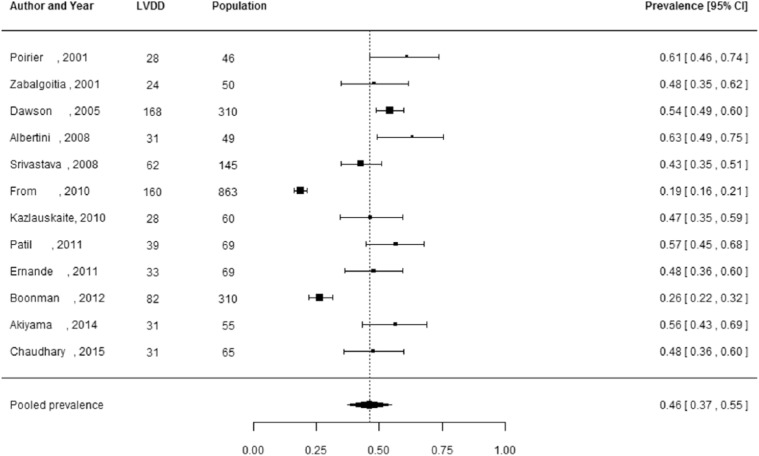
Of the 27 studies, 3 studies did not report the number of individuals diagnosed with LVDD, but only reported the prevalence estimates.29,37,44 These studies were not included in the meta-analysis, as these prevalence estimates could not be manually verified and 95% CIs could not be reliably calculated. Pooled prevalence estimates for LVDD are presented for all of the included studies (n = 24 including a total of 6061 individuals) and separately for studies including the hospital population (n = 15) with 2959 participants and the general population (n = 5) with 2813 participants (Figures 2 to 4). These meta-analyses yielded a summary prevalence of LVDD of 46% (95% CI: 39%–54%), 48% (95% CI: 38%–59%) and 35% (95% CI: 24%–46%), respectively. Estimates ranged from 23% to 54% in the general population and from 19% to 81% in the hospital population (Figures 3 and 4) and there was a high level of study heterogeneity (hospital population: Q = 326.87, p < 0.001, I2 = 96.3%; general population: Q = 104.58, p < 0.001, I2 = 96.7%). The pooled prevalence estimate of the four studies with an unknown setting was 55% (95% CI: 46%–63%). Two funnel plots were constructed: one for the general population studies and one for the hospital population studies (Supplementary Figures S1 and S2). Although visual inspection revealed slight asymmetry, both Begg’s test (p = 0.48 and p = 0.56, respectively) and Egger’s test (p = 0.39 and p = 0.30, respectively) showed no potential risk of publication bias. Sex-specific data were available in 12 studies (including 3609 individuals), and separately for studies including the hospital population (n = 3) with 2570 participants and the general population (n = 7) with 1039 participants. For two studies the settings were unknown. One study had only information about the prevalence in men. Sex-specific pooled prevalence estimates of LVDD revealed a prevalence of 47% (95% CI: 37%–58%) for women and 46% (95% CI: 37%–55%) for men (Figures 5 and 6) and there was a high level of heterogeneity (men: Q = 224.87, p < 0.001, I2 = 91.5%; women: Q = 128.89, p < 0.001, I2 = 92.5%), with prevalence estimates ranges from 24% to 78% in women and 19% to 63% in men. Only five studies reported sex-specific mean ages with differences between men and women of 1–3 years. This did not explain differences in sex-specific prevalence in those studies.
Figure 2.
Prevalence of left ventricular diastolic dysfunction among type 2 diabetes patients in both general and hospital populations.
Prevalence proportions with 95% confidence interval of left ventricular diastolic dysfunction among type 2 diabetes patients in both general and hospital populations and pooled prevalence estimate with 95% confidence interval.
Figure 4.
Prevalence of left ventricular diastolic dysfunction among type 2 diabetes patients in the hospital.
Prevalence proportions with 95% confidence interval of left ventricular diastolic dysfunction among type II diabetes patients in the hospital population and pooled prevalence estimate with 95% confidence interval.
Figure 3.
Prevalence of left ventricular diastolic dysfunction among type 2 diabetes patients in the general population.
Prevalence proportions with 95% confidence interval of left ventricular diastolic dysfunction among type 2 diabetes patients in the general population and pooled prevalence estimate with 95% confidence interval.
Figure 5.
Prevalence of left ventricular diastolic dysfunction among women with type 2 diabetes.
Prevalence proportions with 95% confidence interval of left ventricular diastolic dysfunction among women with type 2 diabetes and pooled prevalence estimate with 95% confidence interval.
Figure 6.
Prevalence of left ventricular diastolic dysfunction among men with type 2 diabetes.
Prevalence proportions with 95% confidence interval of left ventricular diastolic dysfunction among men with type 2 diabetes and pooled prevalence estimate with 95% confidence interval.
The prevalence of HFpEF was only available in two studies including a total of 765 individuals, one from the general population and one from the hospital population, and was therefore not pooled. The prevalence of HFpEF found in the general population (605 individuals with type 2 diabetes) was 25% (95% CI: 21%–28%) and 8% (95% CI: 5%–14%) in the hospital population (160 individuals with type 2 diabetes).18,41 The general population study by Boonman-Winter et al.18 was the only study presenting also sex-specific prevalence of (previously undetected) HFpEF: 18% in men [mean age: 73.8 years; standard deviation (SD): 8.6 years] and 28% in women (mean age: 74.9 years; SD 6.9 years).
Discussion
Our review is the first to provide pooled estimates of the prevalence of LVDD among type 2 diabetes patients and demonstrates that LVDD is an important problem among men and women with type 2 diabetes, affecting on average 35% (95% CI: 24%–46%) of type 2 diabetes patients in the community and 48% (95% CI: 38%–59%) of type 2 diabetes patients in the hospital population. This review, however, demonstrates a wide variation in the prevalence of LVDD among type 2 diabetes patients and therefore the pooled prevalence estimates need to be interpreted with caution. Only two studies provided prevalence estimates of HFpEF among type 2 diabetes patients; among 605 type 2 diabetes patients from the general population, aged 60 years or over, the prevalence of HFpEF was 24.8% (95% CI: 21%–28%), and in a hospital population among 160 type 2 diabetes patients the prevalence was 8% (95% CI: 5%–14%). The prevalence estimates of HFpEF in type 2 diabetes from the general population are high compared to a prevalence of 4.9% of HFpEF in community dwellers 60 years or over, as presented in a recent review.5
By definition, the denominator of the prevalence is (a sample of) the population at large. As such, studies investigating type 2 diabetes patients from the general population provide better estimates than studies that calculate a prevalence in a hospital population with only a selection of patients with type 2 diabetes, in generally more diseased patients. Nevertheless, for clinical practice prevalence data from the hospital setting are especially useful for specialists, while the prevalence data from the community are of interest for the general practitioner.
LVDD is a risk factor for developing HF, notably HFpEF, but likely HFrEF as well, and it is associated with an increase in all-cause mortality compared to people (age and gender adjusted) without LVDD.51,52 HFpEF is increasingly considered to be important and is known for its high mortality rates.6,51 Studies reporting comparisons in mortality rates between HFrEF and HFpEF are conflicting, with some studies showing that HFpEF patients have a somewhat lower mortality rate than HFrEF patients, while others suggest similar mortality rates.53–55 Unfortunately though, as compared with HFrEF, clear mortality-reducing therapies for HFpEF have not yet been identified.56 Furthermore, debate remains ongoing regarding the criteria of LVDD and the cut-points to be used for echocardiographic parameters. Also the exact pathophysiology underlying LVDD and HFpEF has not yet been unravelled.11,12 It has been well recognized that HFpEF typically occurs in patients with comorbidities including type 2 diabetes, which is in line with our findings in this review showing very high prevalence rates of LVDD among type 2 diabetes patients.5,11,12
Only one study so far has shown, with longitudinal data, that 9% of patients with LVDD improve to a better diastolic function in 4 years in contrast to 23% worsening and the remainder having a similar grade of diastolic dysfunction.57 It is currently unknown who with LVDD will eventually become symptomatic, that is, develop HFpEF, and after how many years. For that, longitudinal studies need to be performed, also among patients with type 2 diabetes. Such studies could help focus identifying those type 2 diabetes patients with LVDD at high risk of developing HFpEF, and in order to optimize cardiovascular risk prevention, including optimal blood pressure control.58,59 Another important prospective research area lies in the development of prognostically effective treatment strategies of HFpEF. There have been some suggestions for targeting specific subgroups of HFpEF; however, these treatment strategies need to be further developed.60,61
Previous research suggested that women are more likely to develop HFpEF than men based on a bimodal distribution for sex and ejection fraction in HF, with female sex as a risk factor for HFpEF.53,54,62 One study in our review clearly showed in a general population setting that women with type 2 diabetes [mean age: 74.9 (SD: 6.9) years] had a higher prevalence of HFpEF than men with type 2 diabetes [mean age: 73.8 (SD: 8.6) years]: 28% versus 18%.18 Interestingly, however, in this same population study, the prevalence rates of LVDD were similar among women and men (24% vs 26%).18 Also in our systematic review, based on 12 studies providing such data, the prevalence of LVDD was similar between women and men (47% vs 46%). An explanation may be that women with LVDD develop HFpEF more easily than men do. However, we could not completely account for the effect of age, as only six studies reported sex-specific mean age.
Many studies included in this review used a relatively young and healthy study population by excluding several comorbidities. However, the pathophysiology of HFpEF and diastolic dysfunction is complicated by a host of comorbidities, as well as by age and sex, with a different impact on cardiac function and remodelling.63 As has been recently proposed in a review by Dunlay et al.,64 part of the explanation for the female predominance for developing HFpEF could lie in their older age at time of detection. The general lack of studies examining the natural progression of diastolic dysfunction to HFpEF makes it difficult to state if the difference in HFpEF prevalence between men and women is largely attributable to ageing or a combination of sex differences in cardiac remodelling and ageing. Given, however, the results of Boonman-de Winter et al.,18 it seems that the difference between men and women with type 2 diabetes in the prevalence of HFpEF is not driven by differences in age because they were of similar age.
The higher prevalence of diastolic dysfunction and HFpEF in type 2 diabetes patients seems to show the impact of diabetes in the development of these conditions. Diabetes is associated with changes in cardiac metabolism, structure and function. Mechanisms contributing to myocardial dysfunction in diabetes include hyperglycaemia, lipotoxicity and insulin resistance.11,12 Perhaps, these factors differed between men and women in the studies included and may thus impact the underlying pathophysiology of diastolic dysfunction and hence the prevalence rates. However, further research is necessary to confirm this finding and to unravel possible underlying pathways.
A number of limitations of this review need to be addressed. First of all, we noted significant heterogeneity between the included studies, a common finding in meta-analyses concerning prevalence estimates.65–67 Many hospital population studies in this review excluded patients with a history of cardiovascular diseases, hypertension, atrial fibrillation, valvular diseases and renal diseases, which was in contrast to the general population studies (except for the study by Pareek et al.), and resulted in very select study populations.45 So it is highly plausible that the prevalence of LVDD (and HFpEF) is much higher among unselected hospitalized type 2 diabetes patients than what the results from this review suggest.68 Other important reasons for different prevalence rates in our review are differences in case definition and echocardiographic criteria for diastolic dysfunction. There is no uniform agreement on the definition of diastolic dysfunction, and it has only been agreed upon that multiple echocardiographic measurements should be used. However, because a reference standard is lacking, an algorithm of echocardiographic parameters variables is not generally accepted nor could be validated.51,69 Tissue Doppler imaging (TDI), widely available since 2002, is considered crucial in the diagnosis of diastolic dysfunction, notably the use of the parameter E/é, but we also included studies performed after 2002 that did not incorporate the use of TDI. Importantly, we conducted sensitivity analyses by only including studies using similar case definitions for LVDD, but again prevalence rates largely varied (data not shown), suggesting that more factors may have influenced the results, including differences in source population, study setting, variation in age and gender distribution, duration of type 2 diabetes and different cut-off points for ejection fraction. In addition, survey year, study design and sample size may have had an influence on the prevalence of LVDD. Unfortunately, we only identified five studies conducted in the community at large. They were of reasonable quality, ranging from low (n = 2) to medium (n = 3) risk of bias. The quality of the 15 hospital population studies included in this review was of a moderate standard with the majority having a medium risk of bias, but one study had a low risk of bias and another a high risk of bias.
Conclusion
The prevalence of LVDD among type 2 diabetes patients is similarly high in men and women, while HFpEF seems to be much more common in women than men in community people with type 2 diabetes. More general population studies should be performed for an improved understanding of the prevalence of undetected LVDD and HFpEF. In addition, there is a need for more longitudinal studies to identify who with type 2 diabetes and LVDD will develop HFpEF, after how much time, and whether this differs between men and women, so strategies for better management of these at-risk groups can be developed.
Supplemental Material
Supplemental material, dvdres-jan-2018-00008-File002 for The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis by Selma Bouthoorn, Gideon B Valstar, Aisha Gohar, Hester M den Ruijter, Hans B Reitsma, Arno W Hoes and Frans H Rutten in Diabetes & Vascular Disease Research
Footnotes
Author’s contributions: S.B. conceived of the study, performed the systematic search, screened articles, performed the meta-analysis and wrote the manuscript. G.B.V. screened the articles and wrote the manuscript. A.G. contributed to the results and reviewed/edited the manuscript. H.M.d.R. reviewed/edited the manuscript. H.B.R. contributed to the methods. A.W.H. reviewed/edited the manuscript. F.H.R. conceived of the study, contributed to the methods and results and reviewed/edited the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: G.B.V was supported by the Netherlands Cardiovascular Research Initiative, supported by the Dutch Heart Foundation Grant CVON [2014-11] RECONNECT. A.G. was supported by the Queen of Hearts Consortium that had been supported by a grant from the Netherlands Heart Foundation [2013/T084].
References
- 1. Bertoni AG, Hundley WG, Massing MW, et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004; 27: 699–703. [DOI] [PubMed] [Google Scholar]
- 2. MacDonald MR, Petrie MC, Varyani F, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) programme. Eur Heart J 2008; 29: 1377–1385. [DOI] [PubMed] [Google Scholar]
- 3. Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979; 241: 2035–2038. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 5. Van Riet EES, Hoes AW, Wagenaar KP, et al. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016; 18: 242–252. [DOI] [PubMed] [Google Scholar]
- 6. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 7. Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015; 175: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pr 2011; 94: 311–321. [DOI] [PubMed] [Google Scholar]
- 9. Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res 2016; 118: 1723–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zimmet PZ, Magliano DJ, Herman WH, et al. Diabetes: a 21st century challenge. Lancet Diabetes Endo 2014; 2: 56–64. [DOI] [PubMed] [Google Scholar]
- 11. Seferovic PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J 2015; 36: 1718–1727, 1727a–1727c. [DOI] [PubMed] [Google Scholar]
- 12. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 13. Bajraktari G, Qirko S, Rexhepaj N, et al. Non-insulin dependent diabetes as an independent predictor of asymptomatic left ventricular diastolic dysfunction. Croat Med J 2005; 46: 225–231. [PubMed] [Google Scholar]
- 14. Annonu AK, Fattah AA, Mokhtar MS, et al. Left ventricular systolic and diastolic functional abnormalities in asymptomatic patients with non-insulin-dependent diabetes mellitus. J Am Soc Echocardiog 2001; 14: 885–891. [DOI] [PubMed] [Google Scholar]
- 15. Dodiyi-Manuel ST, Akpa MR, Odia OJ. Left ventricular dysfunction in normotensive type II diabetic patients in Port Harcourt, Nigeria. Vascular Health Risk Manage 2013; 9: 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho JE, Gona P, Pencina MJ, et al. Discriminating clinical features of heart failure with preserved vs reduced ejection fraction in the community. Eur Heart J 2012; 33: 1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nichols GA, Hillier TA, Erbey JR, et al. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care 2001; 24: 1614–1619. [DOI] [PubMed] [Google Scholar]
- 18. Boonman-de Winter LJ, Rutten FH, Cramer MJ, et al. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 2012; 55: 2154–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barzilay JI, Kronmal RA, Gottdiener JS, et al. The association of fasting glucose levels with congestive heart failure in diabetic adults > or =65 years: The Cardiovascular Health Study. J Am Coll Cardiol 2004; 43: 2236–2241. [DOI] [PubMed] [Google Scholar]
- 20. Albertini JP, Cohen R, Valensi P, et al. B-type natriuretic peptide, a marker of asymptomatic left ventricular dysfunction in type 2 diabetic patients. Diabetes Metab 2008; 34: 355–362. [DOI] [PubMed] [Google Scholar]
- 21. Fang ZY, Schull-Meade R, Leano R, et al. Screening for heart disease in diabetic subjects. Am Heart J 2005; 149: 349–354. [DOI] [PubMed] [Google Scholar]
- 22. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012; 65: 934–939. [DOI] [PubMed] [Google Scholar]
- 23. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 25. Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Brit Med J 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Begg CB, Mazumdar M. Operating characteristics of a bank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 27. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. [Google Scholar]
- 28. Andersen NH, Poulsen SH, Poulsen PL, et al. Left ventricular dysfunction in hypertensive patients with type 2 diabetes mellitus. Diabet Med 2005; 22: 1218–1225. [DOI] [PubMed] [Google Scholar]
- 29. Aigbe IF, Kolo PM, Omotoso AB. Left ventricular structure and function in black normotensive type 2 diabetes mellitus patients. Ann Afr Med 2012; 11: 84–90. [DOI] [PubMed] [Google Scholar]
- 30. Chaudhary AK, Aneja GK, Shukla S, et al. Study on diastolic dysfunction in newly diagnosed type 2 diabetes mellitus and its correlation with glycosylated haemoglobin (HbA1C). J Clin Diagn Res 2015; 9: OC20–OC22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Y, Zhao CT, Zhen Z, et al. Association of myocardial dysfunction with vitamin D deficiency in patients with type 2 diabetes mellitus. J Diabetes Complicat 2014; 28: 286–290. [DOI] [PubMed] [Google Scholar]
- 32. Chillo P, Rieck AE, Lwakatare J, et al. Left atrial volume index as a marker of left ventricular diastolic dysfunction in asymptomatic Tanzanian diabetic patients. Blood Pressure 2013; 22: 86–93. [DOI] [PubMed] [Google Scholar]
- 33. Cioffi G, Giorda CB, Chinali M, et al. Analysis of midwall shortening reveals high prevalence of left ventricular myocardial dysfunction in patients with diabetes mellitus: the DYDA study. Eur J Prev Cardiol 2012; 19: 935–943. [DOI] [PubMed] [Google Scholar]
- 34. Dawson A, Morris AD, Struthers AD. The epidemiology of left ventricular hypertrophy in type 2 diabetes mellitus. Diabetologia 2005; 48: 1971–1979. [DOI] [PubMed] [Google Scholar]
- 35. Ernande L, Bergerot C, Rietzschel ER, et al. Diastolic dysfunction in patients with type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J Am Soc Echocardiog 2011; 24: 1268–1275.e1. [DOI] [PubMed] [Google Scholar]
- 36. Faden G, Faganello G, De Feo S, et al. The increasing detection of asymptomatic left ventricular dysfunction in patients with type 2 diabetes mellitus without overt cardiac disease: data from the SHORTWAVE study. Diabetes Res Clin Pr 2013; 101: 309–316. [DOI] [PubMed] [Google Scholar]
- 37. Habek JC, Lakusic N, Kruzliak P, et al. Left ventricular diastolic function in diabetes mellitus type 2 patients: correlation with heart rate and its variability. Acta Diabetologica 2014; 51: 999–1005. [DOI] [PubMed] [Google Scholar]
- 38. Kazlauskaite R, Doukky R, Evans A, et al. Predictors of diastolic dysfunction among minority patients with newly diagnosed type 2 diabetes. Diabetes Res Clin Pr 2010; 88: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patil V, Patil H, Shah K, et al. Diastolic dysfunction in asymptomatic type 2 diabetes mellitus with normal systolic function. J Cardiovasc Dis Res 2011; 2: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poulsen MK, Henriksen JE, Dahl J, et al. Left ventricular diastolic function in type 2 diabetes mellitus: prevalence and association with myocardial and vascular disease. Circ: Cardiovasc Imag 2010; 3: 24–31. [DOI] [PubMed] [Google Scholar]
- 41. Utrera-Lagunas M, Orea-Tejeda A, Castillo-Martínez L, et al. Abnormal myocardial perfusion and risk of heart failure in patients with type 2 diabetes mellitus. Exp Clin Cardiol 2013; 18: e44–e46. [PMC free article] [PubMed] [Google Scholar]
- 42. Dandamudi S, Slusser J, Mahoney DW, et al. The prevalence of diabetic cardiomyopathy: a population-based study in Olmsted County, Minnesota. J Card Fail 2014; 20: 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol 2010; 55: 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Henry RM, Paulus WJ, Kamp O, et al. Deteriorating glucose tolerance status is associated with left ventricular dysfunction – The Hoorn Study. Neth J Med 2008; 66: 110–117. [PubMed] [Google Scholar]
- 45. Pareek M, Nielsen ML, Gerke O, et al. Worsening diastolic function is associated with elevated fasting plasma glucose and increased left ventricular mass in a supra-additive fashion in an elderly, healthy, Swedish population. Int J Cardiol 2015; 184: 466–472. [DOI] [PubMed] [Google Scholar]
- 46. Srivastava PM, Calafiore P, Macisaac RJ, et al. Prevalence and predictors of cardiac hypertrophy and dysfunction in patients with type 2 diabetes. Clin Sci 2008; 114: 313–320. [DOI] [PubMed] [Google Scholar]
- 47. Akiyama T, Eto Y, Matsuda H, et al. Albuminuria and left ventricular mass index are associated with left ventricular diastolic dysfunction in type 2 diabetes mellitus patients. Diabetol Int 2014; 5: 129–133. [Google Scholar]
- 48. Poirier P, Bogaty P, Garneau C, et al. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care 2001; 24: 5–10. [DOI] [PubMed] [Google Scholar]
- 49. Zabalgoitia M, Ismaeil MF, Anderson L, et al. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol 2001; 87: 320–323. [DOI] [PubMed] [Google Scholar]
- 50. Boyer JK, Thanigaraj S, Schechtman KB, et al. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 2004; 93: 870–875. [DOI] [PubMed] [Google Scholar]
- 51. Redfield MM, Jacobsen SJ, Burnett JC, Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003; 289: 194–202. [DOI] [PubMed] [Google Scholar]
- 52. Aurigemma GP, Gottdiener JS, Shemanski L, et al. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol 2001; 37: 1042–1048. [DOI] [PubMed] [Google Scholar]
- 53. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 2011; 32: 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 55. Meta-Analysis Global Group in Chronic Heart Failure. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 2012; 33: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 56. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 57. Kane GC, Karon BL, Mahoney DW, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011; 306: 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013; 62: 1365–1372. [DOI] [PubMed] [Google Scholar]
- 59. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide–based screening and collaborative care for heart failure. JAMA 2013; 310: 66. [DOI] [PubMed] [Google Scholar]
- 60. Shah SJ, Kitzman DW, Ba B, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction. Circulation 2016; 134: 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tschöpe C, Birner C, Böhm M, et al. Heart failure with preserved ejection fraction: current management and future strategies: expert opinion on the behalf of the nucleus of the ‘Heart Failure Working Group’ of the German Society of Cardiology (DKG). Clin Res Cardiol 2017; 107: 1–19. [DOI] [PubMed] [Google Scholar]
- 62. Fa M, Havranek EP, Smith G, et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol 2003; 41: 217–223. [DOI] [PubMed] [Google Scholar]
- 63. Gori M, Lam CS, Gupta DK, et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail 2014; 16: 535–542. [DOI] [PubMed] [Google Scholar]
- 64. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017; 14: 591–602. [DOI] [PubMed] [Google Scholar]
- 65. Lange S, Shield K, Rehm J, et al. Prevalence of fetal alcohol spectrum disorders in child care settings: a meta-analysis. Pediatrics 2013; 132: e980–e995. [DOI] [PubMed] [Google Scholar]
- 66. Jones L, Bellis MA, Wood S, et al. Prevalence and risk of violence against children with disabilities: a systematic review and meta-analysis of observational studies. Lancet 2012; 380: 899–907. [DOI] [PubMed] [Google Scholar]
- 67. Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians: a systematic review and meta-analysis. JAMA 2015; 314: 2373–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 2013; 34: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 69. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiog 2009; 22: 107–133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, dvdres-jan-2018-00008-File002 for The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis by Selma Bouthoorn, Gideon B Valstar, Aisha Gohar, Hester M den Ruijter, Hans B Reitsma, Arno W Hoes and Frans H Rutten in Diabetes & Vascular Disease Research