Abstract
The 2014 follow-up of the Anniston Community Health Survey (ACHS II) consisted of 338 surviving participants from the 2005–2007 baseline study (ACHS) who had previous polychlorinated biphenyl (PCB) measurements, were not pregnant, and were not institutionalized. Questionnaires and blood samples provided the demographic, personal history, and chemical concentration data of the Anniston residents. Approximately 51% of participants were African American, 72% were female, and the mean age was 63 years old. The objectives of this study were to provide an exposure assessment of dioxin-like chemicals in the ACHS II participants and compare the measurements with the general United States (U.S.) population via the National Health and Nutrition Examination Survey (NHANES). Stratified analyses revealed significantly higher average total dioxin toxic equivalencies (TEQs) among African Americans compared to Whites (33.1 vs. 19.2 pg/g lipid), and in females compared to males (29.8 vs. 17.0 pg/g lipid). When adjusting for age, sex, and race in linear regression, we found ACHS II participants to have significantly higher total dioxin TEQ than the general 2014 U.S. population that we estimated for using half-life and NHANES 2003/04 data (most recent NHANES individual samples data), by 16.7 pg/g lipid. Principal component analyses showed that non-ortho and mono-ortho PCBs were separated from the other dioxin-like chemicals among the Anniston residents, whereas the chemicals were all clustered together for estimated NHANES 2014. The concentrations of dioxin-like chemicals, especially non-ortho and mono-ortho PCBs, in Anniston residents who resided near the former PCB production plant were higher than those in the general U.S. population. Although data strongly supported this difference, these inferences are limited because NHANES 2013/14 data were unavailable and we used estimated NHANES 2014 levels that we imputed from NHANES 2003/04 data in conjunction with half-life values estimated from Milbrath et al., 2009.
Keywords: Anniston, Blood levels, Polychlorinated dibenzo-p-dioxins, Polychlorinated dibenzofurans, Polychlorinated biphenyls, Total dioxin toxic equivalent
1. Introduction
Anniston, Alabama was the site of a production facility where approximately half of the total United States (U.S.) production of polychlorinated biphenyls (PCBs) occurred, from the 1930s to 1970s. Earlier ATSDR investigations detailing the extent of exposure to PCBs in Anniston communities found high concentrations of PCBs present in the environment and in local residents (ATSDR, 2000). We conducted the Anniston Community Health Survey (baseline ACHS 2005–2007) to investigate further PCB exposure and potential health effects in 765 participants (Pavuk et al., 2014a, 2014b). Several studies conducted in the ACHS cohort found positive associations between PCBs and diabetes, hypertension, and lipids (Silverstone et al., 2012, Goncharov et al., 2010, Aminov et al., 2013).
The follow-up of ACHS (ACHS II) was conducted in 2014 to expand on the chemical and health studies. Polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and non-ortho PCBs were added to ACHS II in addition to mono-ortho PCBs, which were also measured during ACHS, to provide a more extensive exposure profile. The inclusion of these dioxin-like chemicals was supported by the results of a small, nested pilot study (subset, n=65) conducted within the ACHS baseline population. We found significantly higher concentrations of the non-ortho PCBs 126 and 169 when compared to NHANES 2001/02 (Pavuk et al., 2014c); this finding was the impetus for the current analyses of samples collected in 2014 for ACHS II. Another reason for including dioxin-like chemicals in ACHS II was that commercially produced PCBs have been shown to be contaminated with small amounts (10–1,000 ng/g) of PCDFs. The heating or burning of PCBs is also known to produce PCDFs, small amounts of polychlorinated terphenyls, polychlorinated quaterphenyls, and traces of PCDDs (Kannan et al., 1987; Kodavanti et al., 2001). Today, uncontrolled burning of residential waste (outdoor/backyard trash burning) is considered to be the single largest source for releasing dioxin-like chemicals, such as PCB 169, in the U.S., in contrast to a larger contribution of historical releases by various industrial operations in the past (EPA, 2013; Brown et al., 1995; NIH, 2016).
The concentration of dioxin-like chemicals in the environment and humans has declined over time in most industrial countries (Lakind et al., 2009; Schecter, 2003; Consonni et al., 2012) because of improved environmental controls and reduction in emissions. Over the past three decades, exposure to dioxin-like chemicals has been assessed in various background adult populations across the world. Meta-analyses performed on the concentration levels revealed a significant decrease over time from 1985 to 2008 among PCDDs and PCDFs (Consonni et al., 2012). However, no significant decreases were found for non-ortho PCBs, while only a few mono-ortho PCB congeners exhibited clear, significant declines (Consonni et al., 2012). The National Health and Nutrition Examination Survey (NHANES) has also measured dioxin-like chemicals since 1999. NHANES found PCDDs, PCDFs, non-ortho PCBs, mono-ortho PCBs, and total dioxin toxic equivalencies (TEQs) to have decreased from 2001 to 2008 (Ferriby et al., 2007; Patterson et al., 2009).
There is a strong need to continue studying and monitoring dioxin-like chemical concentrations (to prevent background exposure from reaching hazardous levels), especially in localities where environmental deposits of organochlorine chemicals may lead to bioaccumulation and associated health consequences. We present here the concentrations of individual dioxin-like chemicals and the TEQs of PCDD, PCDF, non-ortho PCB, and mono-ortho PCB groups measured from the human sera of ACHS II participants. We also compared ACHS II chemical concentrations to those of NHANES. The objectives of this study were to measure the concentrations of dioxin-like chemicals in the Anniston residents for providing internal comparisons and making external comparisons with the general U.S. population.
2. Methods
2.1. Data Collection
ACHS II in 2014 accounted for the follow-up of the residential cohort of Anniston, Alabama from baseline ACHS in 2005–2007. The methods on how ACHS participants were followed over time are described by Birnbaum et al., 2016. All surviving participants with valid PCB results, who were neither pregnant nor incarcerated individuals were eligible to participate in the follow-up. From the initial 765 people, we attained the mortality status of 114 via the Social Security Index (Birnbaum et al., 2016). Another 69 participants were not re-contacted because they had moved to new locations outside the study area, which was confirmed through site visits and phone calls (Birnbaum et al., 2016). Out of 438 participants successfully reached, 359 were enrolled in ACHS II. Health questionnaires, medications, demographic information, lifestyle factors, and occupational and family medical history were collected to attain covariate data for regression analyses. We had 338 participants with available chemical concentration data and covariate information to study in ACHS II.
2.2. Laboratory Analysis
The sera were isolated by centrifugation using red top vacutainer tubes from each participant and shipped on dry ice to the Division of Laboratory Sciences at the Centers for Disease Control and Prevention (CDC). Until measurements of persistent organic pollutants (POPs) were conducted on the samples, they were stored at −70°C. The serum samples were measured first for ortho-substituted PCBs, persistent pesticides, and polybrominated diphenyl ethers (PBDEs) according to previously published methodology (Sjödin et al., 2004; Jones et al., 2012) using 2 grams of serum (median 2.0 grams; 1.0 – 2.0 grams; 10th percentile was 2.0 grams). The samples were then measured for PCDDs, PCDFs, and non-ortho PCBs based on published methodology (Turner et al., 1997) using 20 grams of serum (median 20 grams; 2.5–20.7 grams; 10th percentile was 14.0 grams). Each analytical batch for ortho-substituted PCBs, persistent pesticides, and PBDEs was defined as twenty-four unknowns, three quality controls, and three method blanks while in the case of PCDDs, PCDFs, and non-ortho PCBs, each analytical batch was defined as eight unknowns, two quality controls, and two method blanks. Measurements of target organohalogens were made by gas chromatography isotope dilution high-resolution mass spectrometry (GC/ID-HRMS). In HRMS, the accurate mass of a labeled or unlabeled compound is known and calculated. The limit of detection (LOD) was defined as the higher value calculated by two methods, (i) the lowest point of the calibration curve having a signal-to-noise ratio of greater than 10:1 and (ii) three times the standard deviation of method blanks analyzed in parallel with the unknown samples after subtracting the median blank value. A few criteria had to be fulfilled for quality assurance and quality control: the target analyte’s measurement in the sample had to be three standard deviations or less away from the mean of the quality assurance/quality control (QA/QC) samples; at least ten consecutive measurements of the QA/QC sample could not be outside the range of the mean of the QA/QC samples if one QA/QC sample failed the previous criterion. Regarding criteria for each measurement of a set of samples, the ratio of the two ions monitored for each analyte and 13C-labeled internal standard could not be less than or greater than 20% of the theoretical value; the ratio of the retention time of the analyte and corresponding 13C-labeled internal standard needed to fall within 0.99 and 1.01.
A total of 28 dioxin-like chemicals with the World Health Organization’s (WHO) assigned toxic equivalency factors (TEFs, Van den Berg et al., 2006) were selected for further statistical evaluation in ACHS II. These included seven PCDD congeners (2,3,7,8-TCDD, 1,2,3,7,8-PeCDD, 1,2,3,4,7,8-HxCDD, 1,2,3,6,7,8-HxCDD, 1,2,3,7,8,9-HxCDD, 1,2,3,4,6,7,8-HpCDD, and OCDD), ten PCDFs (2,3,7,8-TCDF, 1,2,3,7,8-PeCDF, 2,3,4,7,8-PeCDF, 1,2,3,4,7,8-HxCDF, 1,2,3,6,7,8-HxCDF, 1,2,3,7,8,9-HxCDF, 2,3,4,6,7,8-HxCDF, 1,2,3,4,6,7,8-HpCDF, 1,2,3,4,7,8,9-HpCDF, and OCDF), three non-ortho PCBs (PCBs 81, 126, and 169), and eight mono-ortho-PCBs (PCBs 105, 118, 156, 157, 167, 189, 114, and 123).
2.3. Statistical Analysis
Based on the inclusion criteria and having covariate data, 338 participants remained for statistical analyses. Serum total lipids were attained from triglyceride and total cholesterol measurements using the enzymatic “summation” method (Bernert et al., 2007). We stratified by race when analyzing the various demographic variables to be consistent with the baseline ACHS study (Pavuk et al., 2014a). Regarding missing demographic variable data, there were less than four missing (<1.2%) for waist size, years residing in Anniston, and high school graduation status. 29 people did not have data for fish consumption. Multiple imputation methods as described in Pavuk et al., 2014b were used to account for the missing demographic variable data. Numerical variables were compared between African Americans and Whites race using the two-tailed t-test. Categorical variables were analyzed by race using the chi-square test. Statistical significance was determined by a p-value of less than 0.05.
We provided statistics on PCDD, PCDF, non-ortho PCB, and mono-ortho PCB congeners in lipid weight form as opposed to wet weight because the majority of studies present results using lipid weight (Fierens et al., 2005; Garabrant et al., 2009; Sjödin et al., 2014). We also did not find any notable differences in the analyses when using wet weight data (Supplemental Table 1). Each lipid weight congener was assigned a potency relative to 2,3,7,8-TCDD (TEF). Based on the WHO 2005 guidelines, we multiplied the TEF values by the associated congener concentration to attain specific TEQs (Van den Berg et al., 2006). PCDD, PCDF, non-ortho PCB, and mono-ortho PCB TEQs were the sum of the individual congener-specific classifications. Total Dioxin TEQ was the sum of all individual dioxin-like chemical TEQs. For internal ACHS II analyses, we did not impute for the non-detects in order to provide the most representative chemical concentrations in the population. Their arithmetic means and percentiles were stratified by race and sex. In addition to keeping analyses consistent with the baseline ACHS study, which revealed substantial differences in chemical concentrations by race, and also sex to a lesser extent (Pavuk et al., 2014), our use of these strata was further justified by Sjödin et al., 2014 who found significant differences in various POPs such as PBDEs, PCBs, and pesticides between various combinations of age, sex, and race groups. Fierens et al., 2005 and Garabrant et al., 2009 analyzed dioxin-like chemicals specifically and also found a link to sex, race, and age. In our analyses, we chose not to stratify by age groups specifically because the age distribution was heavily skewed towards the older population (>60 years old). To test statistical significance (p-value<0.05) when comparing the dioxin-like chemical levels by race and sex, we used linear regression with the concentrations as the outcome and age, race, and sex as the predictors.
When making external comparisons with the general U.S. population, measured by NHANES, we imputed for the non-detect chemical concentrations in the ACHS II participants. For ACHS II participants with analyte values below the detection limits, we used congener-specific LOD values and divided by the square root of 2 (LOD/sqrt(2)) in order to approximate those levels (Hornung et al., 1990). Each participant had a congener-specific LOD based on the serum volume they were able to provide. NHANES used this same method to impute for samples below the detection limits and provided data to the public in this form (Sjödin et al., 2014). Newer methods include reverse Kaplan-Meier estimator (Gillespie et al., 2010) and multiple imputation and trending approach (Bichteler et al., 2017). However, we continued the use the LOD/sqrt(2) approach of NHANES in our analyses in order to be consistent when making comparisons to NHANES data.
The last cohort of NHANES that was measured using an individual sampling approach was from 2003/04 (n=1546); we restricted the NHANES 2003/04 population to individuals at least 20 years old to better match the age range of ACHS II. In order to properly compare with the period of the ACHS II participants (2014), we used adult reference half-life values of dioxin-like chemicals provided by Milbrath et al., 2009 to estimate the 2014 chemical measurements of NHANES 2003/04 participants. They compared congener-specific elimination rates from over 30 studies and extracted the data to develop predictive equations based on the assumed linear relationship between half-life of dioxin-like chemicals and age, adjusting for body fat, smoking, and breast-feeding (Milbrath et al., 2009). With half-life values calculated from their models, we estimated NHANES 2014 levels using the exponential decay formula, N(t)=N0e−λt, where N0 is the NHANES 2003/04 congener-specific concentration, e is the Euler’s number (~2.72), λ is the congener-specific half-life from Milbrath et al., 2009, and t is 11 for the number of years from 2003 to 2014. We assumed that the elimination rate used in this formula was stable over time and did not account for background exposure.
We provided ACHS II arithmetic means to compare with the estimated NHANES 2014 survey means; we accounted for weights, primary sampling units, and strata (Curtin et al., 2012) for the estimated NHANES 2014 data in R 3.3.0. Regarding statistical variance, we provided 95% confidence intervals (CIs) and ranges for all the individual dioxin-like chemicals and TEQs. To directly compare ACHS II and the estimated NHANES 2014 levels in a statistical test, we performed linear regression, adjusting for age (years), sex (reference group=male), and race (reference group=people who are not African American or White). Each dioxin-like chemical and TEQ was the outcome in its individual models. The main predictor was “population group”, with NHANES participants as the reference group. We provided the slope, 95% CI, and p-value of the “population group” variable from each model. The slope shows the pg/g lipid difference between the ACHS II and NHANES populations for each dioxin-like chemical. All direct comparisons between ACHS II and estimated NHANES 2014 levels were conducted in R 3.3.0.
Principal component analyses (PCA) were used to analyze potential clustering patterns of the dioxin-like chemicals in both ACHS II and estimated NHANES 2014 (Johnson, 2002; Joliffe, 2002; Megson et al., 2013). For ACHS II PCA, we removed the dioxin-like chemicals confirmed to have less than 60% of measurements above the LOD. The variables were centered and scaled by subtracting the means (Kuhn and Johnson, 2013). The loadings, which correspond to each dioxin-like chemical variable, of the first two principal components were plotted, one plot for ACHS II and another for the estimated NHANES 2014. R 3.3.0 was used for PCA and for creating all the plots.
3. Results
Demographics of ACHS II, stratified by race, are exhibited in Table 1. The participants consisted of 172 (50.9%) African Americans; females accounted for 245 (72.5%). The mean age was 61.3 (range of 26 to 86) among African Americans and 64.1 (range of 27 to 87) among Whites. 59.5% of the participants were at least 60 years or older. Body mass index (BMI) average and the proportion of current smokers were similar between the two race groups; both had an average BMI above 30 (obese) and had about 20% who currently smoked. Approximately 76.2% were high school graduates. Both African Americans and Whites resided in Anniston, Alabama for approximately 52 years. There were significantly more (p-value<0.05) African Americans who resided in west Anniston and had ever eaten fish from local waterways (Snow Creek, Choccolocco Creek, or Lake Logan Martin under Alabama PCB fish consumption advisories), than Whites.
Table 1.
Demographic characteristics (mean ± SE or n (percent)) of the participants of ACHS II.
| Characteristic | African Americans (n=172) |
Whites (n=166) |
Total (n=338) |
|---|---|---|---|
| Mean ± Standard Error | |||
| Age in years | 61.3 ± 0.91 | 64.1 ± 1.1 | 62.7 ± 0.71 |
| BMI – kg/m2 | 32.4 ± 0.59 | 30.8 ± 0.66 | 31.6 ± 0.44 |
| Total lipid (mg/dL) | 612.2 ± 12.3 | 634.0 ± 11.5 | 622.9 ± 8.4 |
| Waist size (inches) | 42.1 ± 0.47 | 41.6 ± 0.49 | 41.9 ± 0.34 |
| Years residing in Anniston | 51.8 ± 1.2 | 51.9 ± 1.4 | 51.8 ± 0.92 |
| Count (Percentage non-missing) | |||
| Female | 132 (76.7%) | 113 (68.1%) | 245 (72.5%) |
| Age groups (years) | |||
| 18–39 | 8 (4.7%) | 11 (6.6%) | 19 (5.6%) |
| 40–59 | 68 (39.5%) | 50 (30.1%) | 118 (34.9%) |
| ≥60 | 96 (55.8%) | 105 (63.3%) | 201 (59.5%) |
| BMI classification | |||
| Normal < 25 | 30 (17.4%) | 38 (22.9%) | 68 (20.1%) |
| Overweight 25–29 | 45 (26.2%) | 51 (30.7%) | 96 (28.4%) |
| Obese ≥ 30 | 97 (56.4%) | 77 (46.4%) | 174 (51.5%) |
| Smoking status | 37 (21.6%) | 34 (20.5%) | 71 (21.1%) |
| Reside in west Anniston | 162 (94.2%)a | 132 (79.5%) | 294 (87.0%) |
| High school graduate | 135 (79.0%) | 121 (73.3%) | 256 (76.2%) |
| Ever eaten fish from Snow Creek, Choccolocco Creek, or Lake Logan Martin | 124 (78.0%)a | 90 (60.0%) | 214 (69.3%) |
| Ever exposed at a job to PCBs | 37 (21.5%) | 46 (27.7%) | 83 (24.6%) |
p < 0.05 for African Americans compared to Whites using Chi-square test.
Regarding internal ACHS II comparisons, we did not impute for the non-detect dioxin-like chemical concentrations. Tables 2a–b exhibit the summary TEQs stratified by race and sex. African Americans and females had higher TEQs than Whites and males. The African American/White and female/male arithmetic mean ratios were higher for the mono-ortho and non-ortho PCB TEQs than they were for PCDD/F TEQs (1.9–2.9 vs. 1.2–1.5). All TEQ groups were significantly higher (p-value<0.05) among African Americans and females when compared to Whites and males, which were tested using linear regression adjusted for age, race, and sex. Tables 3a–b show arithmetic means and selected percentile distributions of the individual dioxin-like chemicals, stratified by race and sex. Of the 28 lipid adjusted dioxins, arithmetic means were calculated for 21 in which over 60% of the samples were above the LOD. The seven congeners in which <60% of the samples exceeded the LOD were 2,3,7,8 TCDF, 1,2,3,7,8 PeCDF, 1,2,3,7,8,9 HxCDF, 1,2,3,4,7,8,9 HpCDF, OCDF, PCB 81, and PCB 123. When comparing the congener levels by race and sex, we used linear regression adjusting for age, sex, and race to determine statistical significance (p-value<0.05). In general, we found PCBs to have significantly higher concentrations (p-value<0.05) in African Americans and females than in Whites and males. All PCDDs were significantly higher (p-value<0.05) in females than in males. PCDFs however, showed little difference by race and sex. Among PCDDs, OCDD had the highest mean concentration (358.9 pg/g lipid in African Americans and 304.6 in Whites; 377.6 in females and 212.7 in males). Among PCDFs, the congener with the highest mean concentration was 2, 3,4,7,8 PeCDF (7.1 pg/g lipid in African Americans and 5.6 in Whites; 6.8 in females and 5.1 in males). Of the non-ortho PCBs measured, PCB 126 had the highest average concentration in participants (114.5 pg/g lipid in African Americans and 35.4 in Whites; 92.5 in females and 31.2 in males). The mono-ortho PCB with the highest average concentration was PCB 118 (80510 pg/g lipid in African Americans and 24550 in Whites; 63290 in females and 25990 in males).
Table 2a.
Arithmetic means (AMs) and selected percentiles for the summary TEQs (pg/g lipid) of the ACHS II participants stratified by race.
| TEQ | Race | n | AM | AM Race Ratio | Min. | 10th% | 50th% | 90th% | 95th% | Max. |
|---|---|---|---|---|---|---|---|---|---|---|
| PCDDa | AA | 172 | 12.5 | 1.2 | 1.8 | 4.6 | 9.4 | 21.6 | 31.1 | 76.5 |
| W | 166 | 10.5 | 1.4 | 4.2 | 9.4 | 16.8 | 22.6 | 39.0 | ||
| PCDFa | AA | 172 | 3.3 | 1.3 | 0.57 | 1.3 | 2.5 | 5.6 | 7.4 | 26.4 |
| W | 166 | 2.6 | 0.24 | 1.3 | 2.4 | 4.1 | 5.2 | 8.5 | ||
| Mono-ortho PCBa | AA | 172 | 4.5 | 2.8 | 0.15 | 0.60 | 2.5 | 11.8 | 13.5 | 29.4 |
| W | 166 | 1.6 | 0.076 | 0.25 | 0.88 | 3.3 | 4.6 | 20.2 | ||
| Non-ortho PCBa | AA | 172 | 12.8 | 2.9 | 0.14 | 0.93 | 5.8 | 32.4 | 48.4 | 124.8 |
| W | 166 | 4.4 | 0.11 | 0.42 | 2.3 | 10.3 | 13.2 | 80.2 | ||
| Total Dioxina | AA | 172 | 33.1 | 1.7 | 2.9 | 8.4 | 21.7 | 74.3 | 99.5 | 177.4 |
| W | 166 | 19.2 | 1.9 | 6.6 | 14.7 | 32.9 | 43.2 | 131.5 |
Statistically significant difference (p < 0.05) in TEQ by race in a linear regression model adjusting for age and sex.
Table 2b.
Arithmetic means (AMs) and selected percentiles for the summary TEQs (pg/g lipid) of the ACHS II participants stratified by sex.
| TEQ | Race | n | AM | AM Sex Ratio | Min. | 10th% | 50th% | 90th% | 95th% | Max. |
|---|---|---|---|---|---|---|---|---|---|---|
| PCDDa | F | 245 | 12.6 | 1.5 | 1.4 | 4.9 | 10.4 | 22 | 29.8 | 76.5 |
| M | 93 | 8.6 | 2.7 | 3.6 | 7.4 | 14.4 | 17.7 | 42.4 | ||
| PCDFa | F | 245 | 3.1 | 1.3 | 0.24 | 1.3 | 2.6 | 5.4 | 6.9 | 26.4 |
| M | 93 | 2.4 | 0.57 | 1.2 | 2 | 3.9 | 4.5 | 20.1 | ||
| Mono-ortho PCBa | F | 245 | 3.6 | 1.9 | 0.076 | 0.48 | 2 | 10 | 12.6 | 29.4 |
| M | 93 | 1.9 | 0.097 | 0.29 | 0.84 | 4.4 | 8.9 | 19.8 | ||
| Non-ortho PCBa | F | 245 | 10.5 | 2.5 | 0.11 | 0.73 | 4.5 | 29.5 | 39.9 | 124.8 |
| M | 93 | 4.2 | 0.11 | 0.54 | 1.7 | 6.1 | 20.9 | 58.2 | ||
| Total Dioxina | F | 245 | 29.8 | 1.8 | 1.9 | 8.3 | 20.5 | 69.6 | 83.2 | 177.4 |
| M | 93 | 17.0 | 4.2 | 6.2 | 12.5 | 26 | 53.7 | 90 |
Statistically significant difference (p < 0.05) in TEQ by sex in a linear regression model adjusting for age and race.
Table 3a.
Dioxin-like chemicals (pg/g lipid) in ACHS II participants stratified by race.
| Dioxin Congener | Race | n | % > LOD | AM | AM Race Ratio |
Min | 10th % | 50th % | 90th % | 95th % | Max |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2,3,7,8 TCDDa | AA | 171 | 82.5 | 1.7 | 1.2 | <LOD | <LOD | 1.3 | 3.3 | 4.8 | 15.6 |
| W | 166 | 86.1 | 1.4 | <LOD | <LOD | 1.2 | 2.7 | 3.3 | 7.1 | ||
| 1,2,3,7,8 PeCDDa | AA | 172 | 96.5 | 5.8 | 1.2 | <LOD | 2.1 | 4.8 | 10.2 | 13.7 | 25.3 |
| W | 166 | 98.2 | 5.0 | <LOD | 1.9 | 4.5 | 8.9 | 11.2 | 17.5 | ||
| 1,2,3,4,7,8 HxCDDa | AA | 172 | 97.7 | 4.4 | 1.1 | <LOD | 1.3 | 3.2 | 9.2 | 11.6 | 19.1 |
| W | 166 | 99.4 | 3.9 | <LOD | 1.4 | 3.2 | 7.2 | 8.9 | 22.3 | ||
| 1,2,3,6,7,8 HxCDDa | AA | 172 | 99.4 | 31.6 | 1.1 | <LOD | 13.3 | 25.2 | 56.1 | 73.6 | 148.3 |
| W | 166 | 100 | 28.7 | 3.1 | 11.7 | 26.8 | 48.5 | 56.7 | 96.9 | ||
| 1,2,3,7,8,9 HxCDD | AA | 172 | 75.6 | 4.2 | 1.1 | <LOD | <LOD | 3.7 | 8.8 | 13.5 | 20.0 |
| W | 166 | 79.5 | 4.0 | <LOD | <LOD | 3.7 | 7.3 | 9.6 | 21.1 | ||
| 1,2,3,4,6,7,8 HpCDD | AA | 172 | 76.2 | 28.9 | 1.1 | <LOD | <LOD | 22.1 | 61.9 | 91.9 | 176.9 |
| W | 166 | 77.7 | 26.2 | <LOD | <LOD | 19.5 | 52.3 | 71.2 | 299.7 | ||
| OCDDa | AA | 172 | 87.2 | 358.9 | 1.2 | <LOD | <LOD | 298.6 | 693.8 | 945.9 | 1557 |
| W | 166 | 86.8 | 304.6 | <LOD | <LOD | 263.3 | 602.7 | 704.9 | 1962 | ||
| 2,3,7,8 TCDF | AA | 172 | 26.2 | -- | <LOD | <LOD | <LOD | 0.9 | 1.3 | 19.3 | |
| W | 166 | 18.1 | -- | <LOD | <LOD | <LOD | 0.7 | 1.1 | 2.7 | ||
| 1,2,3,7,8 PeCDF | AA | 172 | 7.6 | -- | <LOD | <LOD | <LOD | <LOD | 0.5 | 19.0 | |
| W | 166 | 10.8 | -- | <LOD | <LOD | <LOD | 0.5 | 1.1 | 3.1 | ||
| 2,3,4,7,8 PeCDFa | AA | 171 | 98.3 | 7.1 | 1.3 | <LOD | 2.2 | 5.2 | 12.6 | 16.8 | 78.6 |
| W | 166 | 99.4 | 5.6 | <LOD | 2.4 | 5.1 | 9.6 | 11.9 | 22.7 | ||
| 1,2,3,4,7,8 HxCDFa | AA | 172 | 98.8 | 4.1 | 1.2 | <LOD | 1.9 | 3.5 | 7.4 | 9.5 | 15.8 |
| W | 166 | 99.4 | 3.5 | <LOD | 1.8 | 3.2 | 5.7 | 6.5 | 10.1 | ||
| 1,2,3,6,7,8 HxCDF | AA | 172 | 98.3 | 3.9 | 1.0 | <LOD | 1.7 | 3.5 | 6.1 | 8.3 | 16.2 |
| W | 166 | 99.4 | 3.8 | <LOD | 1.8 | 3.3 | 6.2 | 8.0 | 11.1 | ||
| 1,2,3,7,8,9 HxCDF | AA | 172 | 1.2 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 17.4 | |
| W | 166 | 0.6 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 0.7 | ||
| 2,3,4,6,7,8 HxCDF | AA | 172 | 64.5 | 0.76 | 1.0 | <LOD | <LOD | 0.7 | 1.6 | 1.9 | 17.4 |
| W | 166 | 72.3 | 0.79 | <LOD | <LOD | 0.7 | 1.8 | 2.4 | 3.0 | ||
| 1,2,3,4,6,7,8 HpCDFa | AA | 172 | 89.5 | 5.4 | 1.4 | <LOD | <LOD | 4.6 | 8.7 | 10.8 | 57.8 |
| W | 166 | 82.5 | 3.8 | <LOD | <LOD | 3.7 | 6.9 | 8.5 | 20.0 | ||
| 1,2,3,4,7,8,9 HpCDF | AA | 172 | 5.8 | -- | <LOD | <LOD | <LOD | <LOD | 0.3 | 15.3 | |
| W | 166 | 3.6 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 0.6 | ||
| OCDF | AA | 172 | 1.2 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 18.5 | |
| W | 166 | 0.6 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 2.2 | ||
| PCB 81 | AA | 172 | 5.2 | -- | <LOD | <LOD | <LOD | <LOD | 4.7 | 71.4 | |
| W | 166 | 1.8 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 9.9 | ||
| PCB 126a | AA | 172 | 86.6 | 114.5 | 3.2 | <LOD | <LOD | 44.9 | 293.2 | 449 | 1196 |
| W | 166 | 71.1 | 35.4 | <LOD | <LOD | 15.7 | 81.7 | 121.7 | 760.1 | ||
| PCB 169a | AA | 172 | 89.5 | 43.4 | 1.5 | <LOD | <LOD | 31.1 | 94.9 | 122.6 | 363.6 |
| W | 166 | 86.8 | 28.7 | <LOD | <LOD | 23.7 | 53.9 | 74.4 | 175.5 | ||
| PCB 105a | AA | 172 | 97.7 | 14700 | 3.7 | <LOD | 1000 | 7000 | 35700 | 56800 | 101700 |
| W | 166 | 89.2 | 3920 | <LOD | <LOD | 1364.5 | 10500 | 14300 | 60430 | ||
| PCB 118a | AA | 172 | 100 | 80510 | 3.3 | 1694 | 6200 | 40200 | 202900 | 277500 | 532600 |
| W | 166 | 100 | 24550 | 716.9 | 2400 | 10475 | 53800 | 83700 | 382200 | ||
| PCB 156a | AA | 172 | 100 | 28120 | 2.0 | 700 | 4900 | 19285 | 64100 | 88400 | 170600 |
| W | 166 | 98.2 | 13930 | <LOD | 1817 | 9885 | 29810 | 40500 | 94600 | ||
| PCB 157a | AA | 172 | 97.1 | 7570 | 2.2 | <LOD | 1100 | 5003.5 | 17300 | 23100 | 44800 |
| W | 166 | 91.0 | 3510 | <LOD | 483.4 | 2500 | 8000 | 10600 | 30200 | ||
| PCB 167a | AA | 172 | 98.8 | 12700 | 2.7 | <LOD | 1300 | 7816 | 32600 | 39800 | 73310 |
| W | 164 | 92.7 | 4660 | <LOD | 633.1 | 2433.5 | 10810 | 14500 | 70900 | ||
| PCB 189a | AA | 168 | 92.3 | 2900 | 2.2 | <LOD | 500 | 2000.5 | 5800 | 9700 | 21650 |
| W | 164 | 84.2 | 1300 | <LOD | <LOD | 1000 | 2700 | 3800 | 8900 | ||
| PCB 114a | AA | 162 | 92.6 | 4430 | 2.2 | <LOD | 700 | 2622 | 12700 | 14300 | 31850 |
| W | 151 | 88.1 | 1990 | <LOD | <LOD | 1300 | 3700 | 6107 | 18600 | ||
| PCB 123 | AA | 151 | 32.5 | -- | <LOD | <LOD | <LOD | 2300 | 3400 | 9086 | |
| W | 138 | 15.2 | -- | <LOD | <LOD | <LOD | 793.3 | 1264 | 11400 |
% > LOD—percent over the limit of detection
AA—African American
W—White
AM—arithmetic mean
Arithmetic means were only calculated for congeners with 60% >LOD
Statistically significant (p < 0.05) by race when adjusted for age and sex in linear regression.
Table 3b.
Dioxin-like chemicals (pg/g lipid) in ACHS II participants stratified by sex.
| Dioxin Congener | Sex | n | % > LOD | AM | AM Sex Ratio |
Min | 10th % | 50th % | 90th % | 95th % | Max |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2,3,7,8 TCDDa | F | 244 | 88.1 | 1.7 | 1.8 | <LOD | <LOD | 1.4 | 3.3 | 4.3 | 12.7 |
| M | 93 | 74.2 | 0.94 | <LOD | <LOD | 0.72 | 1.6 | 2.3 | 15.6 | ||
| 1,2,3,7,8 PeCDDa | F | 245 | 97.1 | 5.9 | 1.4 | <LOD | 2.1 | 5.0 | 10.3 | 13.1 | 25.3 |
| M | 93 | 97.9 | 4.3 | <LOD | 1.9 | 3.9 | 7.2 | 9.0 | 17.0 | ||
| 1,2,3,4,7,8 HxCDDa | F | 245 | 98.8 | 4.6 | 1.5 | <LOD | 1.5 | 3.7 | 8.9 | 11.6 | 22.3 |
| M | 93 | 97.9 | 3.1 | <LOD | 1.1 | 2.5 | 5.3 | 8.1 | 14.7 | ||
| 1,2,3,6,7,8 HxCDDa | F | 245 | 99.6 | 32.7 | 1.4 | <LOD | 12.9 | 28.5 | 56.1 | 74.5 | 148.3 |
| M | 93 | 100 | 23.4 | 4.9 | 9.4 | 21.5 | 42.8 | 49.1 | 69.3 | ||
| 1,2,3,7,8,9 HxCDDa | F | 245 | 84.5 | 4.8 | 2.0 | <LOD | <LOD | 4.3 | 8.9 | 12.9 | 21.1 |
| M | 93 | 59.1 | 2.4 | <LOD | <LOD | 2.4 | 5.6 | 6.3 | 16.0 | ||
| 1,2,3,4,6,7,8 HpCDDa | F | 245 | 78.8 | 31.4 | 1.8 | <LOD | <LOD | 24.4 | 63.7 | 91.9 | 299.7 |
| M | 93 | 72.0 | 17.6 | <LOD | <LOD | 16.5 | 35.4 | 46.2 | 94.9 | ||
| OCDDa | F | 245 | 91.0 | 377.6 | 1.8 | <LOD | 112.9 | 317.1 | 702.7 | 920.6 | 1962.0 |
| M | 93 | 76.3 | 212.7 | <LOD | <LOD | 200.4 | 424.1 | 626.5 | 765.4 | ||
| 2,3,7,8 TCDF | F | 245 | 24.1 | -- | <LOD | <LOD | <LOD | 0.88 | 1.3 | 5.1 | |
| M | 93 | 17.2 | -- | <LOD | <LOD | <LOD | 0.60 | 1.0 | 19.3 | ||
| 1,2,3,7,8 PeCDF | F | 245 | 8.2 | -- | <LOD | <LOD | <LOD | <LOD | 0.69 | 3.1 | |
| M | 93 | 11.8 | -- | <LOD | <LOD | <LOD | 0.40 | 0.79 | 19.0 | ||
| 2,3,4,7,8 PeCDFa | F | 245 | 98.8 | 6.8 | 1.3 | <LOD | 2.6 | 5.6 | 12.3 | 14.3 | 78.6 |
| M | 92 | 98.9 | 5.1 | <LOD | 2.2 | 4.1 | 9.1 | 10.7 | 34.0 | ||
| 1,2,3,4,7,8 HxCDFa | F | 245 | 99.2 | 4.1 | 1.3 | <LOD | 1.9 | 3.6 | 7.2 | 8.8 | 14.1 |
| M | 93 | 98.9 | 3.2 | <LOD | 1.8 | 2.8 | 4.8 | 5.7 | 15.8 | ||
| 1,2,3,6,7,8 HxCDFa | F | 245 | 98.8 | 4.0 | 1.2 | <LOD | 1.8 | 3.6 | 6.5 | 8.5 | 13.0 |
| M | 93 | 98.9 | 3.3 | <LOD | 1.8 | 3.0 | 5.0 | 5.8 | 16.2 | ||
| 1,2,3,7,8,9 HxCDF | F | 245 | 0.82 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 0.74 | |
| M | 93 | 1.1 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 17.4 | ||
| 2,3,4,6,7,8 HxCDF | F | 245 | 69.8 | 0.76 | 0.92 | <LOD | <LOD | 0.70 | 1.8 | 2.0 | 3.0 |
| M | 93 | 64.5 | 0.83 | <LOD | <LOD | 0.69 | 1.4 | 1.6 | 17.4 | ||
| 1,2,3,4,6,7,8 HpCDF | F | 245 | 86.9 | 4.6 | 1.0 | <LOD | <LOD | 4.1 | 8.2 | 9.9 | 23.9 |
| M | 93 | 83.9 | 4.6 | <LOD | <LOD | 3.9 | 6.9 | 9.2 | 57.8 | ||
| 1,2,3,4,7,8,9 HpCDF | F | 245 | 3.7 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 2.0 | |
| M | 93 | 7.5 | -- | <LOD | <LOD | <LOD | <LOD | 0.43 | 15.3 | ||
| OCDF | F | 245 | 0.41 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 17.9 | |
| M | 93 | 2.2 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 18.5 | ||
| PCB 81 | F | 245 | 4.1 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 40.5 | |
| M | 93 | 2.2 | -- | <LOD | <LOD | <LOD | <LOD | <LOD | 71.4 | ||
| PCB 126*a | F | 245 | 84.5 | 92.5 | 3.0 | <LOD | <LOD | 36.4 | 271.6 | 382.0 | 1196.0 |
| M | 93 | 64.5 | 31.2 | <LOD | <LOD | 12.1 | 51.2 | 141.1 | 559.7 | ||
| PCB 169 | F | 245 | 88.2 | 37.7 | 1.2 | <LOD | <LOD | 28.7 | 76.9 | 102.7 | 363.0 |
| M | 93 | 88.2 | 32.3 | <LOD | <LOD | 23.9 | 69.1 | 94.9 | 235.9 | ||
| PCB 105a | F | 245 | 95.5 | 11300 | 2.6 | <LOD | 700 | 5000 | 33800 | 43500 | 101700 |
| M | 93 | 88.2 | 4370 | <LOD | <LOD | 1219 | 6900 | 25560 | 81200 | ||
| PCB 118a | F | 245 | 100 | 63290 | 2.4 | 716.9 | 4800 | 29500 | 178700 | 235500 | 532600 |
| M | 93 | 100 | 25990 | 900 | 2117 | 8242 | 55640 | 144900 | 409900 | ||
| PCB 156 | F | 245 | 98.8 | 22600 | 1.3 | <LOD | 3300 | 14490 | 52700 | 67300 | 170600 |
| M | 93 | 100 | 17300 | 400 | 3300 | 9600 | 42700 | 61100 | 129100 | ||
| PCB 157 | F | 245 | 94.7 | 6040 | 1.4 | <LOD | 700 | 2500 | 10720 | 16000 | 32200 |
| M | 93 | 92.5 | 4350 | <LOD | 900 | 3900 | 13100 | 18900 | 44800 | ||
| PCB 167a | F | 244 | 96.7 | 9920 | 1.7 | <LOD | 1100 | 5250 | 27610 | 36200 | 73310 |
| M | 92 | 93.5 | 5680 | <LOD | 668.5 | 2370.5 | 16710 | 26300 | 56500 | ||
| PCB 189 | F | 241 | 87.6 | 2100 | 1.0 | <LOD | <LOD | 1400 | 4606 | 5800 | 21650 |
| M | 91 | 90.1 | 2100 | <LOD | 399.8 | 1300 | 4185 | 6200 | 16800 | ||
| PCB 114a | F | 229 | 93.5 | 3680 | 1.8 | <LOD | 600 | 2300 | 9700 | 13400 | 31850 |
| M | 84 | 82.1 | 2080 | <LOD | <LOD | 1080.5 | 6498 | 9000 | 19400 | ||
| PCB 123 | F | 212 | 29.7 | -- | <LOD | <LOD | <LOD | 2300 | 3300 | 11400 | |
| M | 77 | 9.1 | -- | <LOD | <LOD | <LOD | <LOD | 844.9 | 2312 |
% > LOD—percent over the limit of detection
F—Female
M—Male
AM—arithmetic mean
Arithmetic means were only calculated for congeners with 60% >LOD
Statistically significant (p < 0.05) by sex when adjusted for age and race in linear regression.
Table 4 shows comparisons of ACHS II arithmetic means and estimated NHANES 2014 survey means of dioxin-like chemicals and TEQ groups. ACHS II had considerably higher concentrations than estimated NHANES 2014. Total dioxin TEQ was 26.3 pg/g lipid for ACHS II and 6.2 pg/g lipid for estimated NHANES 2014. PCDD and PCDF TEQs in ACHS II were approximately twice as high (PCDD TEQ (pg/g lipid): 11.5 in ACHS II vs. 5.0 in estimated NHANES 2014; PCDF TEQ (pg/g lipid): 2.9 in ACHS II vs. 0.93 in estimated NHANES 2014). Moreover, non-ortho PCB TEQ and mono-ortho PCB TEQ were 45.8 times (8.7 pg/g lipid in ACHS II vs. 0.19 pg/g lipid in estimated NHANES 2014) and 14.8 times (3.1 pg/g lipid in ACHS II vs. 0.21 pg/g lipid in estimated NHANES 2014) higher in ACHS II, respectively. PCDD, PCDF, non-ortho PCB, and mono-ortho PCB congener concentrations for ACHS II ranged from being 1.9 to 4.3, 2.8 to 6.5, 6.4 to 346.4 (PCB 126 highest), and 5.9 to 102.6 (PCB 105 highest) times higher than those of estimated NHANES 2014, respectively.
Table 4.
Dioxin-like chemical concentration and summary TEQ (mean (95% confidence interval) and range) comparisons between ACHS II and estimated NHANES 2014 (imputed from NHANES 2003/04 using half-life estimates of Milbrath et al., 2009).
| Dioxin-like Chemicals (pg/g lipid) | ACHS II (2014) 26–87 years old (n=338) |
Estimated NHANES (2014) 31–96 years old (n=1546) |
||
|---|---|---|---|---|
| Arithmetic Mean (95% CI) | Range | Survey Mean (95% CI) |
Range | |
| 2,3,7,8 TCDD | 1.7 (1.5, 1.9) | 0.23–15.6 | 0.69 (0.61, 0.77) | 0.10–9.9 |
| 1,2,3,7,8 PeCDD | 5.6 (5.2, 6.0) | 0.72–25.3 | 2.3 (2.1, 2.5) | 0.20–18.4 |
| 1,2,3,4,7,8 HxCDD | 4.2 (3.9, 4.5) | 0.58–22.3 | 1.9 (1.8, 2.1) | 0.46–15.2 |
| 1,2,3,6,7,8 HxCDD | 30.2 (28.1, 32.3) | 3.1–148.3 | 15.9 (14.5, 17.3) | 0.61–115.1 |
| 1,2,3,7,8,9 HxCDD | 4.2 (3.8, 4.5) | 1.9–21.1 | 0.98 (0.91, 1.1) | 0.16–7.6 |
| 1,2,3,4,6,7,8 HpCDD | 28.8 (25.5, 32.1) | 5.7–299.7 | 7.8 (6.9, 8.6) | 0.27–96.2 |
| OCDD | 337.1 (306.7, 367.5) | 71.0–1962 | 94.7 (82.2, 107.2) | 10.0–1051 |
| 2,3,7,8 TCDF | -- | -- | 0.043 (0.041, 0.045) | 0.011–0.33 |
| 1,2,3,7,8 PeCDF | -- | -- | 0.19 (0.19, 0.20) | 0.057–2.2 |
| 2,3,4,7,8 PeCDF | 6.5 (5.8, 7.1) | 0.77–78.6 | 1.9 (1.8, 2.0) | 0.24–22.0 |
| 1,2,3,4,7,8 HxCDF | 3.9 (3.6, 4.2) | 0.66–15.8 | 1.3 (1.2, 1.4) | 0.18–9.8 |
| 1,2,3,6,7,8 HxCDF | 3.9 (3.6, 4.1) | 0.87–16.2 | 1.4 (1.3, 1.5) | 0.17–12.5 |
| 1,2,3,7,8,9 HxCDF | -- | -- | 0.68 (0.64, 0.72) | 0.14–9.3) |
| 2,3,4,6,7,8 HxCDF | 0.84 (0.71, 0.96) | 0.35–17.4 | 0.13 (0.13, 0.14) | 0.033–0.76 |
| 1,2,3,4,6,7,8 HpCDF | 5.0 (4.4, 5.6) | 1.8–57.8 | 0.79 (0.69, 0.90) | 0.068–31.4 |
| 1,2,3,4,7,8,9 HpCDF | -- | -- | 0.42 (0.38, 0.46) | 0.095–10.2 |
| OCDF | -- | -- | 0.020 (0.013, 0.027) | 0.0026–1.9 |
| PCB 81 | -- | -- | 0.00011 (0.000094, 0.00012) | 0.00002–0.013 |
| PCB 126 | 76.2 (61.1, 91.3) | 6.3–1196 | 0.22 (0.19, 0.25) | 0.016–6.1 |
| PCB 169 | 36.7 (32.6, 40.7) | 5.1–363.6 | 5.7 (5.3, 6.1) | 0.67–69.0 |
| PCB 105 | 9410 (7760, 11100) | 400–101700 | 91.7 (81.0, 102.5) | 1.7–3204 |
| PCB 114 | 3280 (2830, 3730) | 300–31850 | -- | -- |
| PCB 118 | 53030 (44270, 61780) | 716.9–532600 | 1480 (1330, 1630) | 94.1–47600 |
| PCB 123 | -- | -- | -- | -- |
| PCB 156 | 21150 (18570, 23740) | 400–170600 | 3610 (3310, 3910) | 24.8–227900 |
| PCB 157 | 5590 (4890, 6290) | 368.3–44800 | 906 (828.4, 983.6) | 26.2–54990 |
| PCB 167 | 8770 (7500, 10040) | 255.6–73310 | 726.3 (670.0, 782.6) | 15.9–26960 |
| PCB 189 | 2130 (1860, 2400) | 387.9–21650 | 260.2 (193.0, 327.4) | 14.1–8485 |
| Summary TEQs | ||||
| PCDD | 11.5 (10.6, 12.4) | 1.4–76.5 | 5.0 (4.5, 5.4) | 0.55–38.8 |
| PCDF | 2.9 (2.7, 3.2) | 0.24–26.4 | 0.93 (0.89, 0.98) | 0.011–9.1 |
| Non-ortho PCB | 8.7 (7.1, 10.3) | 0.11–124.8 | 0.19 (0.18, 0.21) | 0–2.1 |
| Mono-ortho PCB | 3.1 (2.7, 3.6) | 0.076–29.4 | 0.21 (0.20, 0.22) | 0.0081–9.8 |
| Total Dioxin | 26.3 (23.6, 29.0) | 1.9–177.4 | 6.2 (5.7, 6.6) | 0.016–48.0 |
Table 5 provides a direct statistical test to compare the two cohort groups while adjusting for age, sex, and race. ACHS II consistently had significantly higher levels (p-value<0.0001) compared to estimated NHANES 2014. After controlling for age, sex, and race, we found PCDD, PCDF, non-ortho PCB, mono-ortho PCB, and total dioxin TEQs to be 4.7, 1.8, 7.4, 2.5, and 16.7 pg/g lipid higher in ACHS II than in estimated NHANES 2014.
Table 5.
βs, 95% Confidence Intervals (CIs), and p-values of linear regression comparing ACHS II and estimated NHANES 2014 (imputed from NHANES 2003/04 using half-life estimates from Milbrath et al., 2009).
| Dioxin-like Chemicals | β | 95% CI | p-value |
|---|---|---|---|
| 2,3,7,8 TCDD | 0.65 | 0.50, 0.80 | <0.0001 |
| 1,2,3,7,8 PeCDD | 2.4 | 2.1, 2.7 | <0.0001 |
| 1,2,3,4,7,8 HxCDD | 1.7 | 1.5, 2.0 | <0.0001 |
| 1,2,3,6,7,8 HxCDD | 9.4 | 7.8, 11.0 | <0.0001 |
| 1,2,3,7,8,9 HxCDD | 2.9 | 2.7, 3.1 | <0.0001 |
| 1,2,3,4,6,7,8 HpCDD | 18.7 | 16.8, 20.7 | <0.0001 |
| OCDD | 204.7 | 186.0, 223.5 | <0.0001 |
| 2,3,7,8 TCDF | -- | -- | -- |
| 1,2,3,7,8 PeCDF | -- | -- | -- |
| 2,3,4,7,8 PeCDF | 4.0 | 3.6, 4.4 | <0.0001 |
| 1,2,3,4,7,8 HxCDF | 2.2 | 2.1, 2.4 | <0.0001 |
| 1,2,3,6,7,8 HxCDF | 2.3 | 2.1, 2.5 | <0.0001 |
| 1,2,3,7,8,9 HxCDF | -- | -- | -- |
| 2,3,4,6,7,8 HxCDF | 0.71 | 0.64, 0.78 | <0.0001 |
| 1,2,3,4,6,7,8 HpCDF | 4.0 | 3.7, 4.4 | <0.0001 |
| 1,2,3,4,7,8,9 HpCDF | -- | -- | -- |
| OCDF | -- | -- | -- |
| PCB 81 | -- | -- | -- |
| PCB 126 | 66.2 | 58.0, 74.4 | <0.0001 |
| PCB 169 | 27.6 | 25.4, 29.9 | <0.0001 |
| PCB 105 | 7990 | 7100, 8880 | <0.0001 |
| PCB 118 | 44100 | 39400, 48800 | <0.0001 |
| PCB 156 | 14600 | 13000, 16170 | <0.0001 |
| PCB 157 | 3870 | 3450, 4290 | <0.0001 |
| PCB 167 | 6700 | 6020, 7390 | <0.0001 |
| PCB 189 | 1660 | 1500, 1820 | <0.0001 |
| Summary TEQs | |||
| PCDD | 4.7 | 4.1, 5.3 | <0.0001 |
| PCDF | 1.8 | 1.6, 1.9 | <0.0001 |
| Non-ortho PCB | 7.4 | 6.6, 8.3 | <0.0001 |
| Mono-ortho PCB | 2.5 | 2.2, 2.7 | <0.0001 |
| Total Dioxin | 16.7 | 15.2, 18.1 | <0.0001 |
The outcomes are lipid adjusted dioxin-like chemicals (pg/g lipid). The main predictor is cohort group, with estimated NHANES 2014 as the reference group. The βs show the number of pg/g lipid higher the concentration is in ACHS II than in NHANES 2014. Each model is adjusted for age, sex, and race.
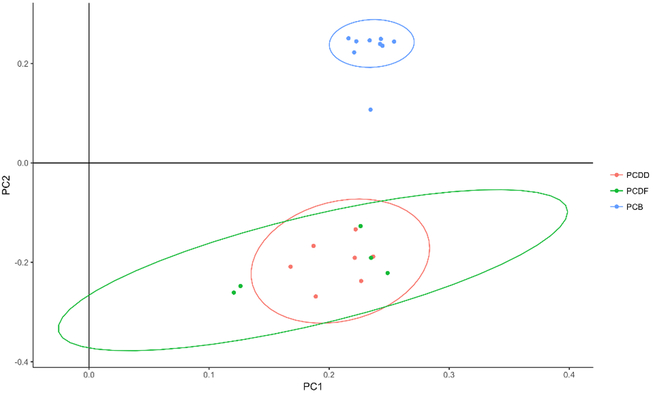
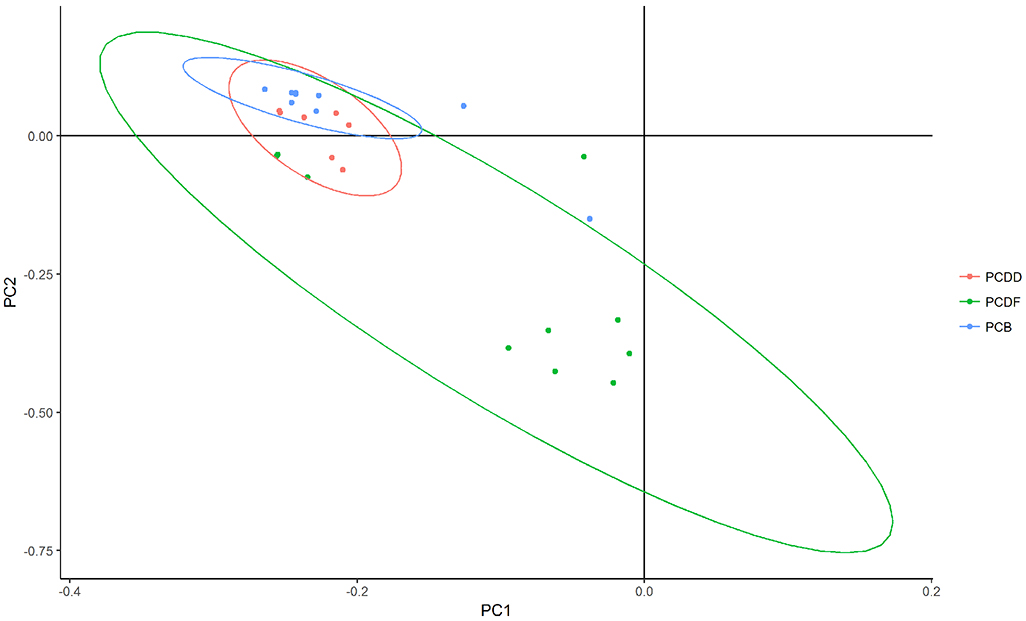
Figure 1a shows PCA loading plot of the individual congeners in ACHS II. The first principal component accounted for about 54.1% variance and the second one accounted for 19.0%. PCDDs and PCDFs were close together, with the latter showing greater variance. PCBs were all clustered near each other, with the exception of PCB 169, indicative of congener 169 possibly stemming from a different source than the other PCBs. There was a clear separation between PCBs (non-ortho and mono-ortho) and the other dioxin-like chemicals, which is indicative of the two classes of compounds coming from different sources. Within the PCDD/F cluster, 2, 3, 4,6,7,8 HxCDF and 1,2,3,4,6,7,8 HpCDF were the only congeners that were outside the grouping. Figure 1b shows the PCA plot for estimated NHANES 2014. The first principal component accounted for 46.9% variance while the second component accounted for 14.2%. Distinct from ACHS II data, no clear separation of clusters was visible in estimated NHANES 2014; PCDDs and PCBs were more clustered together while PCDFs showed the greatest variance.
Figure 1a.
PCA loading plot for ACHS II showing groupings of PCDD, PCDF, non-ortho PCB, and mono-ortho PCB congeners based on type. PC1 accounts for 54.1% of variance and PC2 accounts for 19.0% of variance.
Figure 1b.
PCA loading plot for estimated NHANES 2014 showing groupings of PCDD, PCDF, non-ortho PCB, and mono-ortho PCB congeners based on type. PC1 accounts for 46.9% of variance and PC2 accounts for 14.2% of variance.
4. Discussion
4.1. Summary of Findings
This study provides an extensive exposure profile of the Anniston residents, building on the baseline ACHS study from 2005–2007. In addition to ortho-substituted PCBs, which were previously analyzed in ACHS, we added dioxin-like chemicals in the follow-up study (Pavuk et al., 2014c; Birnbaum et al., 2016). In ACHS II, we found that concentrations of dioxin-like chemicals were significantly higher in African Americans than in Whites, adjusting for age and sex. African Americans had about 3 times higher non-ortho PCB and mono-ortho PCB TEQs than Whites; PCDD and PCDF TEQs were only higher among African Americans by 1.2 and 1.3 times, respectively. We also found significantly higher levels of dioxin-like chemicals in females compared to males, when adjusted for age and race. Females had higher non-ortho PCB and mono-ortho PCB TEQs than males by 1.9 and 2.5 times, respectively, while PCDD and PCDF TEQs were higher by 1.5 and 1.3 times, respectively. PCA showed non-ortho and mono-ortho PCBs to be in a separate cluster from PCDD/Fs, indicating that they could potentially come from a different source. When comparing to the estimated 2014 levels of NHANES, ACHS II showed significantly higher concentrations of dioxin-like chemicals. After adjusting for age, race, and sex, we found PCDD, PCDF, non-ortho PCB, mono-ortho PCB, and total dioxin TEQs in ACHS II to be 4.7, 1.8, 7.4, 2.5, and 16.7 pg/g lipid higher than those of estimated NHANES 2014.
4.2. Internal Comparisons
Regarding internal ACHS II comparisons, similar to ACHS findings, ACHS II revealed higher concentrations of dioxin-like chemicals, especially for non-ortho and mono-ortho PCBs, in African Americans after adjusting for age and sex. This could be due to the significantly higher proportion of African Americans than Whites who resided in west Anniston, where the former PCB plant operated. We also found a significantly higher proportion of African Americans who have ever eaten fish from Snow Creek, Choccolocco Creek, or Lake Logan Martin, which was consistent with ACHS (Pavuk et al., 2014a, and 2014b). Potential pollution of PCBs in the environment could have led to bioaccumulation in the fish over time (Durfee, 1976). Past consumption of locally produced foods could also have potentially contributed to the racial difference in PCB concentrations (Pavuk et al., 2014b). The significantly higher levels of PCDDs and PCDFs found in African Americans could be directly associated with the PCB trends, because PCDDs and PCDFs can form from the burning of PCBs (Kannan et al., 1987; Kodavanti et al., 2001). Garabrant et al., 2009 found that living in the Midland and Saginaw counties near a Dow Chemical Company discharge site, along with hunting and fishing in contaminated areas, were significantly associated with higher levels of dioxin-like chemicals, consistent with our findings regarding close proximity and conducting activities near the contamination sites. Contrary to ACHS findings, which showed no consistent pattern in mono-ortho PCB concentrations when stratified by sex (Pavuk et al., 2014a), ACHS II showed significantly higher chemical concentrations in females than in males, even when adjusting for age and race. Previous research showed that among people who have never smoked, women reported higher levels of PCDDs, PCDFs, and non-ortho PCBs than men (Fierens et al., 2005). Patterson et al., 2008 found higher TEQs for all dioxin-like chemicals when comparing females and males using NHANES 2001/02. Overall, race appears to be a stronger indicator for dioxin-like chemical concentrations than sex, particularly with higher levels being present in African Americans (Pavuk et al., 2014a; Patterson et al., 2008).
ACHS dioxin-like chemical concentrations of the participants from the pilot study compared with ACHS II suggested that they were similar for the most part, with a slight decrease in the follow-up (Supplemental Table 2). There were several congeners that showed a slight increase from baseline to follow-up. They consisted of 2,3,4,7,8 PeCDF from 7.9 to 8.6 pg/g lipid, PCB 169 from 42.3 to 55.2 pg/g lipid, PCB 156 from 31800 to 34200 pg/g lipid, PCB 167 from 13270 to 13800 pg/g lipid, and PCB 189 from 2960 to 3170 pg/g lipid. We were unable to definitively explain what could have caused these increases for some congeners but decreases for others. The small sample size of Anniston participants with both baseline and follow-up dioxin-like chemical data (n=35), however, could explain some of these occurrences. We could not exclude ongoing exposures in Anniston; this will be examined in further detail in a follow-up paper on changes in PCB concentrations (Pavuk et al., 2015). Dioxin TEQs from the pilot study showed a pattern of slight (<10%) decrease from 2007 to 2014; PCDD TEQ decreased from 16.5 to 15.9 pg/g lipid, PCDF TEQ remained the same around 3.7–3.8 pg/g lipid, non-ortho PCB TEQ decreased from 15.8 to 13.4 pg/g lipid, mono-ortho PCB TEQ decreased from 5.4 to 5.1 pg/g lipid, and total dioxin TEQ decreased from 41.4 to 38.1 pg/g lipid, indicating that the few congeners that showed increases over time could have occurred by chance. The general temporal trend shown in this study was consistent with previous literature documenting a decrease in dioxin-like chemical exposure around the world over time (Consonni et al., 2012; Fang et al., 2013; Lakind et al., 2009).
4.3. External Comparisons
Analyses showed that dioxin-like chemical concentrations were significantly higher in ACHS II than in the general U.S. population measured by estimated NHANES 2014, which were imputed from NHANES 2003/04 individual data and adult reference half-lives calculated by Milbrath et al., 2009. This was supported from our comparison of means, 95% CIs, range, and from linear regression analyses, which adjusted for age, sex, and race. The results of higher dioxin-like chemicals were consistent with previous findings from the ACHS, where we found significantly higher levels of the sum of mono-ortho and other ortho-substituted PCBs when comparing the Anniston cohort (2005–2007) with NHANES 2003/04 (Pavuk et al., 2014a, 2014c; Sjödin et al., 2014). Although concentrations of dioxin-like chemicals in Anniston residents appear higher than those of the general U.S. population, we cannot definitively provide a quantitative measure at this point in time. NHANES 2013/14 data was not available for analyses. When it becomes available, it would likely be pooled data, making direct comparisons with individual samples complicated at the least (Bichteler et al., 2017; Heffernan et al., 2014; Caudill, 2011). Our measures were estimates based on half-life curves, which could very likely underestimate the actual concentrations because the general U.S. population should already be on the lower end of the half-life exponential decay curve since they should be from background exposure (Lakind et al., 2009). Temporal trends found in Anniston residents (7–9 years) suggested that any decrease in concentrations of dioxin-like chemicals should be relatively small. The Milbrath et al., 2009 half-life formula assumed that the NHANES 2003/04 concentrations would start the elimination on the beginning, steep part of the curve. What made the differences between ACHS II and estimated NHANES 2014 levels substantially large were the short half-lives of various dioxin-like chemicals used to impute for estimated NHANES 2014 values, such as 2,3,7,8 TCDF (2.1 years), 2,3,4,6,7,8 HxCDF (2.8 years), 1,2,3,4,6,7,8 HpCDF (3.1 years), OCDF (1.4 years), PCB 77 (0.1 years), PCB 81 (0.7 years), PCB 126 (1.6 years), PCB 105 (2.4 years), and PCB 118 (3.8 years) (Milbrath et al., 2009). Supplemental table 3 shows the dioxin-like chemical concentrations of NHANES from 1999 to 2010. The decreases in chemical concentrations were more modest on the population level and did not clearly follow the half-life values of Milbrath et al., 2009. However, each NHANES year used different participants, making any conclusions about the half-life curves difficult to support. Another concern was that NHANES 2005–2010 used pooled samples (Bichteler et al., 2017). Ongoing or current exposures in Anniston cannot be completely excluded but would likely be limited to a few individuals (Franzblau et al., 2009). Although the inference of ACHS II having higher levels than estimated NHANES 2014 is limited due to the latter being calculated from half-life estimates, there is still a strong likelihood that ACHS II levels were higher because NHANES measurements showed background levels (Lakind et al., 2009) and previous literature supported Anniston residents having higher PCB levels in earlier years (Pavuk et al., 2014a).
PCA revealed that PCDD, PCDF, non-ortho PCB, and mono-ortho PCB congeners within estimated NHANES 2014 were all clustered together indicating that they potentially come from a similar source, most likely from consumption of food contaminated with background concentrations of dioxin-like chemicals (Health Canada, 2006a; Health Canada, 2006b; Startin, 2003; Sjödin et al., 2014). On the other hand, the ACHS II plot revealed a distinct separation of PCDD and PCDFs in one cluster and mono-ortho PCBs and non-ortho PCBs in the other, indicating that the PCBs potentially originated from a different source. However, among the PCBs, non-ortho PCB 169 was distinctly farther away from the PCB cluster. Most Aroclor® mixtures contained relatively large amounts of mono-ortho PCBs in percent weight (Frame et al., 1996; ATSDR, 2000), so the contamination of environmental and local food sources historically represent a likely pathway which resulted in elevated mono-ortho PCB concentrations in Anniston residents. Small amounts of PCB 126 and to a lesser extent PCB 169 have only been detected in Aroclor® products by applying more sensitive analytical methods (Johnson et al., 2008; Kodavanti et al., 2001; Rushneck et al., 2004). Some increases in non-ortho PCB 169 could also be partly due to the burning of residential waste in Anniston, which is still a common practice, in neighborhoods close to the plant (Brown et al., 1995; EPA, 2013, 2016). However, no major increases in PCDFs were observed in ACHS II, which can also be formed during burning waste (EPA, 2013). The cause for the clustering patterns for the ACHS II PCA plot could stem from the Anniston residents being exposed to elevated PCBs in the food and environment while living in close proximity to the former PCB production plant, which may also explain the considerable difference in non-ortho PCB and mono-ortho PCB TEQ levels comparing ACHS II and NHANES data, much larger than the difference in PCDD and PCDF levels.
4.4. Strengths and Limitations
This study had a balanced racial distribution, approximately equal between African Americans and Whites. It also contained in-depth, congener-specific analyses and comparisons between the PCB-exposed population of Anniston and the general U.S. population. Race and sex stratified comparisons of ACHS II dioxin-like chemical concentrations were adjusted for either race or sex, and age. When comparing ACHS II participants’ demographics from phase I with those ACHS participants who did not participate in the follow-up, we found the sex distribution and averages of age, total lipids, and mono-ortho PCB concentrations to be similar. ACHS II had very low LODs compared to NHANES 2003/04 (Supplemental Table 4), enabling us to quantify dioxin-like chemical concentrations for most of the participants without imputing for non-detects; over 60% of our samples for 21 out of 28 dioxin-like chemicals were above the LOD. This study also provided a direct, statistical comparison with estimated NHANES 2014 values, using linear regression adjusted for age, sex, and race.
Limitations included some small sample bias in sex-stratified analyses from the internal ACHS II comparisons; males made up only 27.5% of the sample. From comparisons between ACHS II participants’ demographics at baseline and demographics of ACHS participants who did not participate in the follow-up, there was a higher proportion of African Americans and a lower proportion of smokers among ACHS II participants than the ACHS participants who were not part of the follow-up study. Over half of the ACHS II population was obese and over 60 years old; with this issue, comparisons between different cohorts can be complicated. Regarding BMI, some ACHS II individuals who had dropped in BMI from baseline to follow-up showed an increase in dioxin-like chemical concentrations due to the decrease in lipids releasing the stored chemicals; the overall trend however, still remained a decrease over time. The initial study design intentionally oversampled areas closer to the plant with higher potential for PCB exposure and was not intended to represent the city population. Half of the PCDFs had over 60% of measurements below LOD. One of the non-ortho PCBs, congener 77, could not be measured in this study, and we had only 3% of measurements of PCB 81 above the LOD. This made summary TEQ comparisons for non-ortho PCB TEQs and total dioxin TEQs more difficult with studies that included PCBs 77 or 81. However, these rapidly eliminated congeners with low concentrations contributed little to the summary non-ortho PCB TEQ. Most of the non-ortho PCB TEQ was comprised of PCBs 126 and 169, which were measured in over 79% and 88% of ACHS II samples, respectively.
5. Conclusion
This is the first report from a large population sample on measurements of dioxin-like chemicals from Anniston, Alabama. African Americans had higher levels of exposure than Whites, after adjusting for age and sex. The reason for this was likely because a higher proportion of African Americans lived in west Anniston, where the former PCB plant operated, and a higher proportion of them had eaten contaminated local foods including fish. Females had higher concentrations of dioxin-like chemicals when compared to males after adjusting for race and sex. However, the sex difference was not as pronounced as the race comparisons.
The concentrations of dioxin-like chemicals, especially non-ortho and mono-ortho PCBs, in Anniston residents who lived in close proximity to the former PCB production plant were higher than those in the general U.S. population. However, this conclusion is limited due to the fact that the NHANES 2013/14 data were unavailable and we estimated NHANES 2014 levels from NHANES 2003/04 data in conjunction with half-life values estimated from Milbrath et al., 2009.
Supplementary Material
Supplemental Table 1. Dioxin-like chemicals (pg/g wet weight) in ACHS II participants stratified by race.
Supplemental Table 2. Comparison of ACHS (2007–2007) and ACHS II (2014) PCDDs, PCDFs, non-ortho PCBs, mono-ortho PCBs, and TEQs (pg/g lipid) of individuals from the pilot study (n=35)
Supplemental Table 3. Arithmetic mean concentrations of NHANES dioxin-like chemicals (1999–2010) among participants at least 20 years old. PCDDs, PCDFs, and non-ortho PCBs are in pg/g lipid. Mono-ortho PCBs are in ng/g lipid.
Supplemental Table 4. Limit of detection values (pg/g lipid) for ACHS II and NHANES 2003/04.
Acknowledgements
We would like to thank Dr. Stephen T. Mennemeyer of University of Alabama Birmingham for all his contributions to the study. We would also like to thank all of the study participants. The data used for the baseline study (ACHS) were collected using a grant from ATSDR to Jacksonville State University, #5U50TS473215. Data for the follow up study (ACHS II) were funded by the National Cancer Institute through interagency agreements with the Centers for Disease Control and Prevention (CDC) (IAA#: 11-AT1-001-00; IAA#: 12-AT-12-ANNISTON) and by ATSDR. Data collection for this study was funded via contract from ATSDR to the University of Alabama at Birmingham (UAB) (CDC Contract No. 200-2011-40834). This research was also supported in part by an appointment to the Research Participation Program at the CDC administered by the Oak Ridge Institute for Science and Education. The contents of this publication are solely the responsibility of the authors and do not necessarily represent ATSDR/CDC official views.
Abbreviations
- PCB
polychlorinated biphenyls
- PCDD
polychlorinated dibenzo-p-dioxins
- PCDF
polychlorinated dibenzofurans
- LOD
limit of detection
- PCA
principal component analysis
- ACHS II
Anniston Community Health Survey follow-up
Footnotes
Conflicts of Interest Statement
J.R. Olson served as an expert witness for the plaintiffs in legal actions regarding the residents of Anniston, Alabama being exposed to PCBs. The other authors declare that they have no competing interests.
References
- ATSDR (Agency for Toxic Substances and Disease Registry). (2000). Health consultation: evaluation of soil, blood & air data from Anniston, Alabama Monsanto Company, Anniston, Calhoun County, Alabama. CERCLIS No. ALD004019048. Atlanta: U.S. Department of Health and Human Services, 2000. [Google Scholar]
- Aminov Z, Haase RF, Pavuk M, Carpenter DO. Analysis of the effects of exposure to polychlorinated biphenyls and chlorinated pesticides on serum lipid levels in residents of Anniston, Alabama. J. Environ Health 2013; 12(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert JT, Turner WE, Patterson DG Jr, Needham LL. Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere 2007;68:824–31. [DOI] [PubMed] [Google Scholar]
- Bichteler A, Wikoff DS, Loko F, Harris MA. Estimating serum concentrations of dioxin-like compounds in the U.S. population effective 2005–2006 and 2007–2008: A multiple imputation and trending approach incorporating NHANES pooled sample data. Environ Int 2017; 105:112–25. [DOI] [PubMed] [Google Scholar]
- Brown JF, Frame GM, Olson DR, Webb JL. The sources of the coplanar PCBs. Organohalogen Compd 1995; 26:427–30. [Google Scholar]
- Caudill SP. Important issues related to using pooled samples for environmental chemical biomonitoring. Stat Med 2011; 30(5):515–21. [DOI] [PubMed] [Google Scholar]
- Consonni D, Sindaco R, Bertazzi PA. Blood levels of dioxins, furans, dioxin-like PCBs, and TEQs in general populations: a review, 1989–2010. Environ Int 2012; 44:151–62. [DOI] [PubMed] [Google Scholar]
- Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, Carroll MD, Hirsch R, Schober S, Johnson CL. The National Health and Nutrition Examination Survey: sample design, 1999–2006. Vital Health Stat 2 Data Eval Meth Res 2012; 155:1–39. [PubMed] [Google Scholar]
- Durfee RL. Production and usage of PCBs in the United States Proceedings of the National Conference on Polychlorinated Biphenyls, Chicago, 1975. EPA-560/6-75-004. Washington, DC; U.S. Environmental Protection Agency; 1976. p. 103–7. [Google Scholar]
- EPA (Environmental Protection Agency). “Human Health.” Wastes - Non-Hazardous Waste - Municipal Solid Waste. http://www.epa.gov/waste/nonhaz/municipal/backyard/health.htm; 2013. [Accessed 12/01/2016].
- EPA (Environmental Protection Agency). Learn about Dioxins; 2016. [Google Scholar]
- Fierens S, Eppe G, De Pauw E, Bernard A. Gender dependent accumulation of dioxins in smokers. Occup Environ Med 2005; 62(1):61–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame GM, Cochran JW, Bowadt SS. Complete PCB congener distributions for 17 Aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. J High Resolut Chromatogr 1996;19(12):657–68. [Google Scholar]
- Franzblau A, Hedgeman E, Jiang X, Chen Q, Hong B, Knutson K, Towey T, Adriaens P, Demond A, Gillespie B, Jolliet O, Lepkowski J, Garabrant D. The University of Michigan dioxin exposure study an investigation of serum outliers for TEQ, 2,3,7,8-TCDD, 2,3,4,7,8-PeCDF and PCB-126. Slides 2–18, 2009. [Google Scholar]
- Garabrant DH, Franzblau A, Lepkowski J, Gillespie BW, Adriaens P, Demond A, Hedgeman E, Knutson K, Zwica L, Olson K, Towey T, Chen Q, Hong B, Chang CW, Lee SY, Ward B, Ladronka K, Luksemburg W, Maier M. The University of Michigan Dioxin Exposure Study: predictors of human serum dioxin concentrations in Midland and Saginaw, Michigan. Environ Health Perspect 2009;117(5):818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin RK. Production, distribution, and fate of polychlorinated dibenzo-p-dioxins, dibenzofurans, and related organohalogens in the environment In: Schecter A, editor. Dioxins and health. Hoboken, NJ: John Wiley & Sons, Inc; 2003. P. 55–88. [Google Scholar]
- Goncharov A, Bloom M, Pavuk M, Birman I, Carpenter DO. Blood pressure and hypertension in relation to levels of serum polychlorinated biphenyls in residents of Anniston, Alabama. J Hypertens 2010;28(10):2053–60. [DOI] [PubMed] [Google Scholar]
- Canada Health. Dioxins and furans. http://www.hc-sc.gc.ca/hl-vs/iyh-vsv/environ/dioxin-eng.php; 2006a.
- Canada Health. PCBs. http://www.hc-sc.gc.ca/hl-vs/iyh-vsv/environ/pcb-bpc-eng.php; 2006b.
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Applied occupational and environmental hygiene. 1990;5(1):46–51. [Google Scholar]
- Johnson GW, Ehrlich R, Full W. Principal component analysis and receptor models in environmental forensics In: Murphy BL, Morrison RD, editors. Introduction to environmental forensics. Academic Press; 2002. [Google Scholar]
- Johnson GW, Hansen LG, Hamilton MC, Fowler B, Hermanson MH. PCB, PCDD and PCDF congener profiles in two types of aroclor 1254. Environ Toxicol Pharmacol 2008;25(2):156–63. [DOI] [PubMed] [Google Scholar]
- Joliffe IT. Principal component analysis. 2nd ed. Springer; 2002. [Google Scholar]
- Jones R, Edenfield E, Anderson S, Zhang Y, Sjödin A. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Compd 2012;74:97–8. [Google Scholar]
- Kannan N, Tanabe S, Wakimoto T, Tatsukawa R. Coplanar polychlorinated biphenyls in aroclor and kanechlor mixtures. J Assoc Off Anal Chem 1987;70(3):451–4. [PubMed] [Google Scholar]
- Kodavanti PR, Kannan N, Yamashita N, Derr-Yellin EC, Ward TR, Burgin DE, Tilson HA, Birnbaum LS. Differential effects of two lots of aroclor 1254: congener-specific analysis and neurochemical end points. Environ Health Perspect 2001;109(11):1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M, Johnson K. Applied Predictive Modeling. New York, NY: Springer; 2013. P. 35–40. [Google Scholar]
- Lakind JS, Hays SM, Aylward, LL, Naiman DQ. Perspective on serum dioxin levels in the United States: an evaluation of the NHANES data. J Expo Sci Environ Epidemiol 2009;19(4):435–41. [DOI] [PubMed] [Google Scholar]
- Megson D, O’Sullivan G, Comber S,Worsfold PJ, Lohan MC, Edwards MR, et al. Elucidating the structural properties that influence the persistence of PCBs in humans using the National Health and Nutrition Examination Survey (NHANES) dataset. Sci Total Environ 2013;461–462: 99–107. [DOI] [PubMed] [Google Scholar]
- Milbrath MO, Wenger Y, Chang CW, Emond C, Garabrant D, Gillespie BW, Jolliet O. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ Health Perspect 2009; 117(3):417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH (National Institute of Environmental Health Sciences). Dioxins; 2016. [Google Scholar]
- Patterson DG Jr, Turner WE, Caudill SP, Needham LL. Total TEQ reference range (PCDDs, PCDFs, cPCBs, mono-PCBs) for the US population 2001–2002. Chemosphere 2008; 73(1):S261–77. [DOI] [PubMed] [Google Scholar]
- Patterson DG Jr, Wong LY, Turner WE, Caudill SP, Dipietro ES, McClure PC, et al. Levels in the U.S. population of those persistent organic pollutants (2003–2004) included in the Stockholm Convention or in other Long-Range Transboundary Air Pollution Agreements. Environ Sci Technol 2009; 43(4):1211–8. [DOI] [PubMed] [Google Scholar]
- Pavuk M, Olson JR, Sjödin A, Wolff P, Turner WE, Shelton C, Dutton ND, Bartell S, Anniston Environmental Health Research Consortium. Serum concentrations of polychlorinated biphenyls (PCBs) in participants of the Anniston Community Health Survey. Sci Total Environ 2014a;473:286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuk M, Olson JR, Wattigney WA, Dutton ND, Sjödin A, Shelton C, Turner WE, Bartell SM, Anniston Environmental Health Research Consortium. Predictors of serum polychlorinated biphenyl concentrations in Anniston residents. Sci Total Environ 2014b;496:624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuk M, Dutton N, Turner WE, Sjödin A, Bartell S, Anniston Environmental Research Consortium. Dioxins, dibenzofurans, and non-ortho polychlorinated biphenyls in a subset of the Anniston Community Health Survey. Organohalogen Compd 2014c;76:1195–98. [Google Scholar]
- Pavuk M, Dutton ND, Sjödin A, Lewin M, Birnbaum LS. Temporal changes of serum concentrations of polychlorinated biphenyls and organochlorine pesticides in a residential cohort. Organohalogen Compd. 2015;77: 472–475. [Google Scholar]
- Rushneck DR, Beliveau A, Fowler B. Concentrations of dioxin-like PCB congeners in unweathered aroclors by HRGC/HRMS using EPA Method 1668A. Chemosphere 2004;54(1):79–87. [DOI] [PubMed] [Google Scholar]
- Schecter A, Gasiewicz T. Dioxins and health. Hoboken, NJ: John Wiley & Sons, Inc; 2003. P. 137–58 [Google Scholar]
- Silverstone AE, Rosenbaum PF, Weinstock RS, Bartell SM, Foushee HR, Shelton C, Pavuk M. Polychlorinated biphenyl (PCB) exposure and diabetes: results from the Anniston Community Health Survey. Environ Health Perspect 2012;120(5):727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Lapeza CR, Focant J- F, McGahee EE, Patterson D Jr. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem 2004;76:1921–7. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Caudill SP, Wong LY, Turner WE, Calafat AM. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the National Health and Nutrition Examination Survey: 2003–2008. Environ Sci Technol 2014;48(1):753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Startin JR. Dioxins in food In: Schecter A, editor. Dioxins and health. Hoboken, NJ: John Wiley & Sons, Inc; 2003. P. 89–136 [Google Scholar]
- Turner W, DiPietro E, Lapeza C, Green V, Gill J, Patterson DG. A fast universal automated cleanup system for the isotope-dilution HRMS analysis of PCDDs, PCDFs, coplanar PCBs, PCB congeners, and persistent pesticides from the same serum sample. Organohalogen Compd 1997;31(31):26–31. [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson R. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 2006;93(2):223–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Dioxin-like chemicals (pg/g wet weight) in ACHS II participants stratified by race.
Supplemental Table 2. Comparison of ACHS (2007–2007) and ACHS II (2014) PCDDs, PCDFs, non-ortho PCBs, mono-ortho PCBs, and TEQs (pg/g lipid) of individuals from the pilot study (n=35)
Supplemental Table 3. Arithmetic mean concentrations of NHANES dioxin-like chemicals (1999–2010) among participants at least 20 years old. PCDDs, PCDFs, and non-ortho PCBs are in pg/g lipid. Mono-ortho PCBs are in ng/g lipid.
Supplemental Table 4. Limit of detection values (pg/g lipid) for ACHS II and NHANES 2003/04.