Abstract
Background:
Maternal immune system regulation is critical for maintenance of a healthy pregnancy and fetal development. Exposure to phenols and parabens is widespread, and may be linked to systemic inflammation and alteration of circulating immunological biomarkers.
Objective:
We sought to characterize associations between repeated measures of individual urinary phenols, parabens and plasma inflammatory markers across pregnancy.
Methods:
In the LIFECODES prospective birth cohort, we conducted a nested preterm birth case-control study, including 130 cases and 352 controls. In urine samples collected from each participant at up to four study visits during pregnancy, we measured concentrations of six phenols and four parabens, as well as five plasma inflammatory markers. We used multivariable linear mixed models to analyze repeated measures of exposures on inflammatory markers. We created and applied inverse probability weights to account for the sampling approach.
Results:
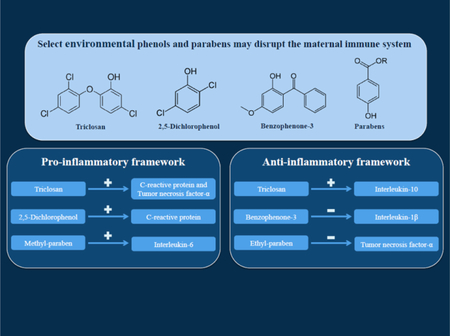
We observed bidirectional associations between select phenols and parabens and inflammatory markers. An interquartile range increase in triclosan (55.2 ng/mL) was associated with a 12.5% (95% CI: 3.67, 22.0) increase in C-reactive protein, a 7.95% (95% CI: 1.95, 14.3) increase in interleukin 10, and a 7.93% (95% CI: 3.82, 12,2) increase in tumor necrosis factor-α. Additionally, an interquartile range increase in 2,5-dichlorophenol (11.0 ng/mL) was associated with a 10% increase in C-reactive protein (95% CI: 1.92, 18.7). Conversely, an interquartile range increase in ethyl paraben (10.4 ng/mL) was associated with a 7.7% decrease in interleukin-1β (95% CI: −14.1, −0.86).
Conclusions:
Our findings can be organized into two thematic frameworks, one where concentrations of urinary phenols and parabens during pregnancy reflected a pro-inflammatory relationship with immunological biomarkers, and the other contrary theme – an anti-inflammatory relationship. These findings have implications for fetal development and reproductive outcomes, and emphasize the need for further research on immunological mechanisms of phenol and paraben action during pregnancy.
Keywords: Chlorinated phenols, cytokines, reproductive immunology
Graphical abstract
1. INTRODUCTION
Regulation of the maternal immune system during gestation is critical for the maintenance of a healthy pregnancy. Cells of the maternal immune system guide the innate and adaptive immune responses in order to protect the mother and fetus against viral and bacterial infections, and regulate cell-to-cell signaling to ensure successful implantation, placental development, and fetal growth leading to parturition (Chatterjee et al., 2014; Chau et al., 2016; Racicot et al., 2014). Further, maternal immune modulation by the fetal-placental immune system promotes tolerance of the foreign paternal antigens present in fetal tissues (Morelli et al., 2015). Importantly, these immune signaling pathways rely on complex networks of cytokines, which have historically been codified as pro-inflammatory and anti-inflammatory (Chatterjee et al., 2014; Romero et al., 2007; Wilczyński, 2005). Within the repertoire of maternal cytokines are the pro-inflammatory cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), and the anti-inflammatory cytokine interleukin-10 (IL-10) (Chatterjee et al., 2014; Chau et al., 2016; Chiesa et al., 2015). In addition to these cytokines, C-reactive protein (CRP) is a nonspecific inflammatory marker involved in the innate immune response (Wang et al., 2017).
Sterile inflammation – defined as an inflammatory response in the absence of detectable infection – during pregnancy, can adversely affect fetal growth, contribute to perinatal morbidity, and lead to reproductive complications. Although the specific mechanisms are not well understood, the elevation of reactive oxygen species, alteration of vascularization, and promotion of leukocytic activity at the maternal-fetal interface are hypothesized to play a role (Kim et al., 2015; Lissauer et al., 2017; Romero et al., 2007; 2016; Thompson et al., 2015). Immune perturbations due to environmental exposures can potentially disrupt immune signaling thereby tending toward an inflammatory response.
Human exposure to phenols and parabens is widespread, due to their use in several consumer food and personal care products, as well as their post-industrial fate in various ecosystems worldwide (Andra et al., 2015; Błędzka et al., 2014; Centers for Disease Control and Prevention, 2016). Phenols such as bisphenol-S (BPS) are increasingly used as chemically analogous alternatives to bisphenol-A (BPA), and found in industrial and commercial products that contain polycarbonate and plastic linings (e.g., canned and packaged food container linings and thermal receipts) (Andra et al. 2015; Rochester and Bolden, 2015). BPA and BPS are dissimilar in that BPA is a compound that binds two phenols with an ethyl group, whereas in BPS, a sulfonyl group binds those phenols. In addition, several anti-microbial, pharmaceutical, and personal care products contain phenols and parabens such as benzophenone-3 (BP3), triclosan (TCS), triclocarban (TCB), methyl-paraben (MPB), ethyl-paraben (EPB), propyl-paraben (PPB), and butyl-paraben (BPB) (Andra et al. 2015; Błędzka et al. 2014; Centers for Disease Control and Prevention, 2016; Goodman et al., 2017). Furthermore, exposure to herbicides and room deodorizers lead to measureable levels of phenol metabolites such as 2-4-diclorophenol (2-4-DCP) and 2-5-dichlorophenol (2-5-DCP) (Centers for Disease Control and Prevention, 2016; Ye et al., 2014). Exposure to phenols and parabens is ubiquitous and persistent, and it is critical to investigate the implications of these exposures on vulnerable populations, such as pregnant women and developing fetuses. Modulation of the maternal cytokine profile and inflammatory pathways may be a target of phenol and paraben action through interactions with maternal immune cells and peripheral tissues (Kiyama and Wada-Kiyama, 2015; Kovats, 2012; Rogers et al., 2013). Alterations in circulating peripheral maternal cytokines can affect important cellular processes such as leukocyte differentiation, proliferation, and migration (R. Druckmann and M.-A. Druckmann, 2005; Thompson et al., 2015).
Although several in vitro and animal studies have assessed the immunological mechanisms of action for these select phenols and parabens, very few human studies have been conducted during pregnancy. In the LIFECODES prospective birth cohort, we previously demonstrated that urinary levels of BPA were associated with circulating plasma inflammatory markers during pregnancy (Ferguson et al., 2016). In the present analysis, we analyze an expanded panel of phenols and parabens in the same cohort. The objective of this present study was to test for changes in circulating maternal plasma inflammatory markers in association with urinary concentrations of phenols and parabens. Additionally, we sought to determine potential windows of vulnerability during gestation. We hypothesized that exposure to select phenols and parabens would result in higher levels of maternal inflammatory markers as a result of toxicant interaction with maternal immune cells and peripheral tissues.
2. METHODS
2.1. Study Population
Between 2006 and 2008, 1,600 pregnant women were enrolled in the LIFECODES prospective birth cohort at the Brigham and Women’s Hospital in Boston, MA. Participants were eligible for recruitment if they were 18 years of age or older and their pregnancy was less than 15 weeks gestation at the initial study visit. From the LIFECODES cohort, 1,181 participants were followed to term, and delivered live, single infants. Among these participants, 130 women delivered preterm (< 37 weeks gestation). We constructed an unmatched, nested case-control study within the larger cohort, and randomly selected 352 women who delivered after 37 weeks gestation. The present study received institution review board approval from the Brigham and Women’s Hospital. Additional details regarding recruitment can be found elsewhere (Ferguson et al 2016, 2014b; McElrath et al., 2012).
Participants of this study attended up to four study visits throughout their pregnancy, and the range of each visit is as follows: visit 1 (4.71 – 19.1 weeks), visit 2 (14.9 – 32.1 weeks), visit 3 (22.9 – 36.3 weeks), and visit 4 (33.1 – 38.3 weeks). At the initial study visit, we administered questionnaires to collect demographic and health-related information. During each of the four study visits we administered physical examinations and collected both urine and plasma samples. Biological samples were stored at −80° Celsius, and subsequently analyzed for biomarkers of environmental toxicants and endogenous immunological compounds. Birth outcomes, including preterm birth status, were abstracted from medical records and assessed via questionnaire at a later study visit.
2.2. Measurement of Phenols and Parabens
From the 482 study participants, we collected a total of 1,628 urine samples, which were used to analyze a panel of phenols and parabens (2-4-DCP, 2-5-DCP, BP3, TCS, MPB, EPB, PPB, BPB, TCB, and BPS). Exposure biomarkers were quantified using isotope dilution-liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS) at NSF International (Ann Arbor, MI, USA). Analytical methods for ID-LC-MS/MS were designed and modified from a protocol developed by the Centers for Disease Control and Prevention (CDC) and detailed in depth in a previous study (Ferguson et al., 2016, 2017; Lewis et al., 2013). Briefly, urine samples underwent enzymatic deconjugation followed by solid phase extraction, and subsequently were analyzed with a triple quadrupole mass spectrometer.
When exposure biomarker values were below the limit of detection (LOD), we assigned a value of the LOD/√2, an imputation method that generally applies to exposure distributions that are not highly skewed and have at least 50 percent detection (Hornung and Reed, 1990). Due to variations in urinary dilution within the population, urinary biomarker (UB) concentration were corrected using specific gravity (SG), for descriptive and bivariate statistics, as follows:
| [1] |
UBSG is the specific gravity-adjusted urinary biomarker concentration (ng/mL), UB is the measured and uncorrected urinary biomarker concentration, the constant SGMedian is the specific gravity population median, and SG is the observed specific gravity of the individual urine sample (Meeker et al., 2009). In regression models, we regressed each inflammatory biomarker on phenols and parabens (not individually adjusted or corrected for SG) and adjusted for SG as a covariate.
2.3. Measurement of Inflammatory Biomarkers
We collected 1,585 plasma samples from the study subjects to measure endogenous biomarkers related to inflammation and immune signaling, including the pro-inflammatory markers CRP, IL-1β, IL-6, and TNF-α. We also measured an anti-inflammatory marker, IL-10. The selection of these inflammatory biomarkers was based the following factors: evidence of high detection in previous human studies; reported associations with adverse birth outcomes; and limited budget to scale up measurements in this large study sample (Boyle et al., 2017; Kumar et al., 2013; Taylor et al., 2016; Wei et al., 2010). All measurements were performed at the Cancer Center Immunology Core at the University of Michigan (Ann Arbor, MI, USA). CRP was measured using a DuoSet enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) with a minimum LOD of 10 pg/mL and a maximum LOD of 100 μg/mL. One sample with a CRP measurement below the lower LOD was imputed with LOD/√2, while another sample with a CRP measurement above the upper LOD was assigned with the value of 100 μg/mL. Each of the cytokines were simultaneously measured using the Milliplex MAP High Sensitivity Human Cytokine Magnetic Bead Panel and had an LOD of 0.128 ng/mL (EMD Millipore Corp., St. Charles, MO). Among the cytokine measurements, 32 samples reported as <0.128 pg/mL and were imputed with the LOD/√2. Additional details on detection rates and assay sensitivity were previously described (Ferguson et al. 2016, 2014a).
2.4. Statistical Analyses
Each of the analytical steps for this study was performed using R version 3.4.0. Due to the sampling approach of our nested case-control study, we designed weights related to the inverse probability of over-representation of preterm birth cases in order for our study to resemble the proportions of preterm birth in a general population (Richardson et al., 2007). We applied these weights for all statistical analyses (univariate, bivariate, and regression). Each of the highly detected exposure and endogenous biomarkers had distributions that were right-skewed; therefore we transformed each of the skewed variables using the natural log to comply with assumptions of normality for linear mixed models (LMMs). Two phenols – TCB and BPS – had very low detection rates (7.3% and 21% respectively), therefore we excluded them from LMMs. For any LMMs, we used the general additive mixed model (GAMM) function from the mgcv package (version 1.8) in R. We further assessed the appropriateness of our transformations by comparing residuals of LMMs with and without transformations.
For descriptive statistics of exposure analytes, we calculated geometric means (GM) and geometric standard deviations (GSD) by study visit and demographic covariates. We tested for bivariate differences using simple LMMs with random subject specific intercepts for single analytes regressed on each of these variables individually. We assessed differences in levels of categorical covariates by regressing each exposure analyte against individual demographic variables using LMMs with a random subject specific intercept. Spearman correlation coefficients were also calculated for each exposure analyte and inflammatory pair at each study visit.
In adjusted analyses, models for repeated measures analyses were built by assessing bivariate associations, and covariates were eligible for model selection if they were associated with at least one exposure analyte and one inflammatory marker using LMMs with random subject specific intercepts. The variables that fit these criteria included: baseline maternal age, gestational age at each study visit, specific gravity at each study visit, baseline maternal education level (High school, technical school, some college, or college graduate), baseline maternal race/ethnicity (White, African-American, or other), maternal alcohol use and smoking during pregnancy (determined by each study visit as yes/no), baseline health insurance provider – an indicator of socioeconomic status (Private/HMO/Self-pay vs. Medicaid/SSI/MassHealth), baseline maternal body mass index (BMI) at first study visit, and infant sex (male/female). From crude LMMs of inflammatory markers regressed against exposure analytes, we incorporated variables in a stepwise manner in an order that sequentially prioritized the variables with the most bivariate associations. In our stepwise approach, potential covariates were selected for the final model if they changed the beta coefficient of at least two exposure analytes by 10 percent or more. Covariates did not vary across models. We further evaluated model fit and the linearity of exposure analytes with respect to outcome inflammatory markers using penalized splines within the GAMM function. We assessed smoothing plots and compared the Aikaike information criterion (AIC) between models with and without penalized splines, and found that splines for exposures did not improve the model fit. We also assessed nonlinear relationships between outcome biomarkers and both maternal age and gestational age using penalized spline functions, and only a nonlinear term for gestational age improved model fit and was carried forward to the main analyses.
The final group of variables used in LMMs included individual continuous natural log-transformed inflammatory markers regressed on natural log-transformed exposure analytes, specific gravity, penalized spline term for gestational age, study visit, maternal age, health insurance provider, and BMI at initial visit. We further assessed the appropriateness of fitting random slopes and random intercepts. Based on AIC, we determined that all LMMs would be fit using solely random subject specific intercepts. To interpret regression results from models with continuous natural log-transformed predictors, we converted the beta coefficients and corresponding 95% confidence intervals (CI) to the percent change in individual inflammatory markers corresponding to an interquartile range (IQR) difference in exposure analyte. This conversion is calculated using the following equation:
| [2] |
To address the issues of false positive associations when conducting multiple statistical tests, we calculated q-values using the Benjamini and Hochberg (1995) method. Each immune biomarker was treated as a family of tests (8 total tests with exposure analytes for each outcome biomarker). We interpreted higher confidence associations with q-values at a minimum threshold of 0.1, and recognized that tests of association with higher q-values are at greater risk of being false positives.
2.5. Sensitivity Analyses
In addition to our primary statistical analyses, we also conducted sensitivity analyses. First, we fit a simpler model, where we only adjusted for specific gravity and a penalized spline term for gestational age. One of the phenols, 2-5-DCP, appeared to have a non-linear relationship with CRP. Therefore, as a sensitivity analysis, we categorized 2-5-DCP into quartiles and regressed this form of 2-5-DCP for models with all of the inflammatory markers to compare our results with the continuous form of 2-5-DCP. Additionally, given that EPB and BPB had moderately lower detections rates (59.5% and 68.4% respectively), we explored other forms of operationalizing these exposure variables into four categories (below LOD [reference group], and tertiles above LOD). Furthermore, we tested for statistical interaction between each exposure analyte and study visit to determine if our LMM results varied and changed across individual study visits. To evaluate potential differences in effect estimates by fetal sex, we tested statistical interaction terms between exposure analytes and infant sex to determine sex differences in effect estimates. We also conducted a sensitivity analysis to test for differences in effect estimates by case status, where we tested statistical interaction terms between exposure analytes and preterm birth status and further conducted stratified analyses by preterm birth status.
We previously investigated the relationship between phthalates and BPA in relation to inflammatory biomarkers in the LIFECODES cohort. In those studies we observed that two toxicants were associated with IL-6: mono-carboxypropyl phthalate (MCPP) and BPA (Ferguson et al. 2016; 2015). Based on these findings, we conducted sensitivity analyses where we further adjusted our individual LMMs for MCPP and BPA in separate models to evaluate potential confounding of these toxicants on the present panel of phenols and parabens.
3. RESULTS
In Table 1, we report univariate descriptive statistics of demographic variables, and corresponding bivariate analyses with exposure analytes by presenting the median and IQR for each exposure analyte in categories of demographic and health-related covariates. We also indicated differences in exposure analytes (p <0.05) across categories of covariates using LMMs of individual exposure analytes regressed on covariates, adjusted for specific gravity and including random subject specific intercepts, in Table 1.
Table 1.
LIFECODES cohort profile and select weighted percentiles of specific gravity corrected urinary analyte measurements by demographic characteristics in all samples measured (N = 482 participants, 1628 urine samples).
| Population characteristics | Count (percent)a | 2-4-DCP (ng/mL) |
2-5-DCP (ng/mL) |
BP3 (ng/mL) |
BPS (ng/mL) |
BPB (ng/mL) |
|---|---|---|---|---|---|---|
| Median (25th, 75th) | Median (25th, 75th) | Median (25th, 75th) | Median (25th, 75th) | Median (25th, 75th) | ||
| Age | ||||||
| 18–24 years old | 54 (11.2%) | 1.55 (0.67, 4.06) | 20.9 (6.06, 93.3) | 12.1 (5.76, 46.2) | 0.27 (0.19, 0.43) | 0.26 (0.13, 1.28) |
| 25–29 years old | 95 (19.7%) | 0.79 (0.46, 1.64)* | 3.97 (1.42, 16.2)* | 32.0 (8.08, 203)* | 0.35 (0.23, 0.70) | 0.98 (0.22, 5.78)* |
| 30–34 years old | 190 (39.3%) | 0.66 (0.4, 1.19)* | 2.65 (1.33, 7.15)* | 57.4 (14.3, 330)* | 0.41 (0.23, 0.70) | 1.17 (0.27, 7.50)* |
| 35+ years old | 143 (29.7%) | 0.53 (0.34, 1.12)* | 2.17 (1.15, 6.39)* | 60.3 (18.8, 299)* | 0.38 (0.23, 0.70) | 0.90 (0.24, 6.38)* |
| Race/ethnicity | ||||||
| White | 282 (58.4%) | 0.56 (0.35, 1.07) | 2.03 (1.09, 4.63) | 85.1 (24.0, 373) | 0.42 (0.25, 0.70) | 1.65 (0.31, 8.59) |
| African-American | 77 (16.0%) | 0.98 (0.55, 2.33)* | 10.8 (4.18, 40.1)* | 14.0 (5.52, 52.5)* | 0.30 (0.20, 0.60)* | 0.39 (0.16, 2.76)* |
| Other | 123 (25.6%) | 0.95 (0.5, 2.83)* | 8.03 (2.28, 44.2)* | 17.8 (6.66, 85.5)* | 0.30 (0.21, 0.53) | 0.42 (0.16, 2.66)* |
| Education | ||||||
| High school degree | 68 (14.5%) | 1.25 (0.62, 3.83) | 16.0 (4.95, 59.0) | 11.5 (4.35, 31.3) | 0.28 (0.19, 0.53) | 0.42 (0.14, 3.88) |
| Technical school | 77 (16.4%) | 0.79 (0.44, 2.1)* | 6.59 (2.11, 30.0)* | 20.0 (7.38, 152)* | 0.32 (0.22, 0.65) | 0.45 (0.16, 3.59) |
| Junior college or some college | 139 (29.4%) | 0.67 (0.39, 1.34)* | 3.03 (1.19, 9.15)* | 54.6 (14.3, 252)* | 0.42 (0.23, 0.70)* | 1.21 (0.30, 7.28)* |
| College graduate | 187 (39.8%) | 0.55 (0.36, 1.05)* | 2.07 (1.16, 4.54)* | 85.0 (23.2, 433)* | 0.39 (0.23, 0.70)* | 1.23 (0.29, 7.73)* |
| Health insurance provider | ||||||
| Private/HMO/self-pay | 385 (81.9%) | 0.62 (0.37, 1.17) | 2.44 (1.20, 7.10) | 61.1 (16.2, 340) | 0.38 (0.23, 0.70) | 1.12 (0.26, 7.02) |
| Medicaid/SSI/MassHealth | 85 (18.1%) | 1.33 (0.64, 3.66)* | 16.2 (4.95, 62.7)* | 12.1 (5.73, 31.1)* | 0.30 (0.21, 0.61)* | 0.30 (0.14, 1.98)* |
| BMI at initial visit | ||||||
| b25 kg/m2 | 250 (52.4%) | 0.61 (0.39, 1.20) | 2.17 (1.14, 5.79) | 61.2 (15.6, 371) | 0.38 (0.23, 0.70) | 1.37 (0.30, 8.28) |
| 25–29.9 kg/m2 | 126 (26.4%) | 0.70 (0.40, 1.50) | 4.18 (1.76, 14.2)* | 38.8 (10.5, 164)* | 0.38 (0.22, 0.60) | 0.55 (0.19, 5.15)* |
| ≥30 kg/m2 | 102 (21.2%) | 0.98 (0.49, 1.94)* | 9.45 (2.75, 35.2)* | 23.5 (7.58, 98.2)* | 0.32 (0.20, 0.63)* | 0.42 (0.16, 3.56)* |
| Tobacco use | ||||||
| No smoking during pregnancy | 445 (93.5%) | 0.69 (0.40, 1.46) | 3.07 (1.37, 11.4) | 43.4 (12.2, 260) | 0.38 (0.23, 0.70) | 0.86 (0.23, 6.0) |
| Smoked during pregnancy | 31 (6.52%) | 0.81 (0.43, 1.60) | 4.79 (2.02, 16.3) | 19.4 (7.95, 93.8)* | 0.30 (0.19, 0.60) | 0.67 (0.20, 5.33) |
| Alcohol use | ||||||
| No alcohol use during pregnancy | 452 (95.8%) | 0.68 (0.41, 1.44) | 3.12 (1.37, 11.2) | 42.3 (11.5, 249) | 0.38 (0.23, 0.70) | 0.86 (0.23, 6.07) |
| Alcohol use during pregnancy | 20 (4.25%) | 0.96 (0.51, 1.86) | 4.76 (1.75, 22.6) | 42.8 (20.3, 458) | 0.32 (0.21, 0.56) | 0.67 (0.21, 4.32) |
| Fetal sex | ||||||
| Male | 214 (44.3%) | 0.73 (0.42, 1.76) | 3.30 (1.43, 14.4) | 40.5 (10.3, 252) | 0.35 (0.23, 0.64) | 0.70 (0.19, 5.69) |
| Female | 268 (55.7%) | 0.66 (0.40, 1.24) | 3.14 (1.37, 9.83) | 42.7 (12.8, 252) | 0.38 (0.22, 0.70) | 1.05 (0.26, 6.45) |
| Population characteristics | EPB (ng/mL) |
MPB (ng/mL) |
PPB (ng/mL) |
TCB (ng/mL) |
TCS (ng/mL) |
|---|---|---|---|---|---|
| Median (25th, 75th) | Median (25th, 75th) | Median (25th, 75th) | Median (25th, 75th) | Median (25th, 75th) | |
| Age | |||||
| 18–24 years old | 1.43 (0.71, 8.22) | 284 (77.4, 723) | 59.9 (11.8, 165) | 1.18 (0.88, 1.76) | 10.1 (2.63, 75.7) |
| 25–29 years old | 1.78 (0.76, 12.3) | 195 (83.1, 523) | 47.0 (12.3, 130) | 1.49 (1.01, 2.64) | 10.4 (3.69, 48.8) |
| 30–34 years old | 2.66 (1.04, 17.5) | 186 (76.5, 417) | 50.2 (17.8, 130) | 1.76 (1.11, 3.53) | 11.4(4.23, 61.2) |
| 35+ years old | 2.52 (0.92, 15.5) | 155 (57.8, 340)* | 37.9 (10.0, 93.4) | 1.63 (1.11, 3.02) | 10.3 (3.41, 61.2) |
| Race/ethnicity | |||||
| White | 2.71 (1.16, 18.1) | 171 (64.3, 350) | 42.9 (12.8, 109) | 1.76 (1.11, 3.02) | 11.7 (4.02, 64.5) |
| African-American | 1.55 (0.71, 7.42) | 382 (140, 908)* | 78.3 (25.9, 282)* | 1.32 (0.96, 2.24)* | 9.66 (3.10, 38.8) |
| Other | 1.66 (0.71, 11.2) | 154 (56.8, 501) | 35.9 (8.52, 118) | 1.32 (0.96, 2.35) | 9.96 (3.14, 79.6) |
| Education | |||||
| High school degree | 1.53 (0.59, 7.24) | 252 (78.5, 688) | 60.0 (11.6, 198) | 1.24 (0.92, 2.12) | 8.25 (2.91, 70.5) |
| Technical school | 1.36 (0.70, 8.40) | 251 (92.9, 617) | 62.1 (15.3, 167) | 1.41 (0.96, 2.12) | 7.66 (3.02, 41.9) |
| Junior college or some college | 2.86 (1.05, 14.4) | 206 (80.3, 410) | 51.2 (15.8, 119) | 1.92 (1.11, 3.53) | 14.2 (4.34, 52.4) |
| College graduate | 2.66 (1.18, 19.0)* | 148 (58.5, 341) | 37.7 (11.6, 98.2) | 1.63 (1.11, 3.02) | 11.9 (3.55, 76.2) |
| Health insurance provider | |||||
| Private/HMO/self-pay | 2.66 (0.97, 17.5) | 178 (68.2, 400) | 44.7 (14.2, 121) | 1.63 (1.06, 3.02) | 11.5 (3.80, 64.5) |
| Medicaid/SSI/MassHealth | 1.33 (0.65, 5.15)* | 252 (79.2, 606)* | 50.4 (10.9, 154) | 1.24 (0.92, 1.92)* | 6.55 (3.02, 39.0) |
| BMI at initial visit | |||||
| <25 kg/m2 | 3.11 (1.07, 20.1) | 192 (79.0, 420) | 48.8 (15.7, 122) | 1.63 (1.11, 3.53) | 11.0 (3.54, 58.4) |
| 25–29.9 kg/m2 | 1.91 (0.89, 8.91) | 175 (61.8, 483) | 43.0 (11.4, 136) | 1.51 (1.11, 2.64) | 11.1 (3.54, 74.1) |
| ≥30 kg/m2 | 1.33 (0.56, 6.24)* | 155 (44.8, 413) | 39.6 (8.51, 129) | 1.24 (0.88, 2.35) | 10.3 (3.51, 45.1) |
| Tobacco use | |||||
| Smoked during pregnancy | 2.28 (0.89, 15.2) | 185 (68.3, 444) | 46.4 (13.6, 129) | 1.58 (1.06, 3.02) | 10.9 (3.54, 61.7) |
| No smoking during pregnancy | 2.13 (0.76, 12.7) | 210 (90.0, 603) | 40.9 (11.1, 119) | 1.21 (0.92, 1.92) | 10.2 (3.53, 64.9) |
| Alcohol use | |||||
| Alcohol use during pregnancy | 2.27 (0.89, 14.2) | 185 (68.8, 449) | 45.2 (13.2, 129) | 1.51 (1.06, 3.02) | 10.9 (3.53, 62.5) |
| No alcohol use during pregnancy | 2.13 (0.76, 24.9) | 237 (89.1, 458) | 58.8 (13.6, 122) | 1.51 (1.06, 2.64) | 11.2 (4.34, 67.9) |
| Fetal sex | |||||
| Male | 2.10 (0.76, 10.5) | 170 (63.0, 432) | 42.1 (11.5, 121) | 1.51 (1.06, 2.64) | 11.5 (3.53, 74.4) |
| Female | 2.66 (0.97, 19.0) | 196 (75.9, 449) | 47.7 (15.2, 129) | 1.63 (1.06, 3.02) | 9.96 (3.60, 50.5) |
Abbreviations: BMI, body mass index; HMO, health maintenance organization; SSI, supplemental security income; 2-4-DCP (2,4 diclorophenol), 2-5-DCP (2,5 diclorophenol), BP3 (ben-zophenone 3), BPS (bisphenol S), BPB (butyl paraben), EPB (ethyl paraben), MPB (methyl paraben), PPB (propyl paraben), TCB (triclocarban), TCS (triclosan), CRP (C-reactive protein), IL 1β (interleukin 1β), IL 6 (interleukin 6), IL 10 (interleukin 10), TNF α (tumor necrosis factor α).
Weighted by case-control sampling probabilities to represent the general sampling population.
Difference in urinary analytes (P < 0.05) in the category compared to reference (first category listed) using linear mixed models adjusted for specific gravity and with a random intercept for each subject.
Our study population was predominantly White and over the age of 30, and consisted mostly of participants that reported no alcohol or tobacco use during pregnancy. A majority of the study participants also had private health insurance (81.9%) and some form of higher education (85.6%). Participants under the age of 25 had higher levels of 2-4-DCP (0.76 – 1.02 ng/mL higher median concentration than older age groups; P-trend<0.001), and 2-5-DCP (16.9 – 18.7 ng/mL higher median concentration than older age groups; P-trend<0.001), and also lower levels of BPB (0.64 – 0.91 ng/mL lower median concentration than older age groups; P-trend=0.001). Urinary concentrations of exposure analytes also varied by race/ethnicity, such that white participants had higher levels of BP3 (67.3 – 71.1 ng/mL higher median concentration than non-White groups; P-trend <0.001) and BPB (1.23 – 1.26 ng/mL higher median concentration than non-White groups; P-trend <0.01). In contrast, compared to the White participants, African-American participants had higher levels 2-4-DCP (0.42 ng/mL higher median concentration; P-value<0.001), 2-5-DCP (8.77 ng/mL higher median concentration; P-value<0.001), MPB (211 ng/mL higher median concentration; P-value<0.001), and PPB (35.4 ng/mL higher median concentration; P-value<0.001). There were also differences in several exposure analytes by socioeconomic variables. Compared to participants with private health insurance, participants with public health insurance had higher levels of 2-4-DCP (0.71 ng/mL higher median concentration; P-value<0.001), 2-5-DCP (13.8 ng/mL higher median concentration, P-value<0.001), and MPB (74 ng/mL higher median concentration, P-value<0.05). In contrast, participants with private health insurance had higher levels of BP3 (49 ng/mL higher median concentration; P-value<0.001), BPB (0.82 ng/mL higher median concentration; P-value<0.001), and EPB (1.33 ng/mL higher median concentration; P-value<0.05), compared to participants with public health insurance.
Distributions of immunological biomarkers across study visits are reported in Table 2. We observed overall high detection (>95%) of CRP, IL-10, IL-6, and TNF- α. IL-1β had slightly lower detection (78%). CRP had the highest concentrations (overall geometric mean: 5.72 μg/mL). Among the cytokines, we observed the highest concentrations for IL-10 (overall geometric mean 13.6 pg/mL). The results in Supplemental Table 1 include the detection rates, GM, GSD, and distribution of individual exposure analytes. These estimates are reported for the overall study population and stratified by study visits. Among the exposure analytes, 2-4-DCP, 2-5-DCP, BP3, MPB, PPB, and TCS had overall detection rates above 75%, whereas the overall detection rates of EPB and BPB were 59.5% and 68.4% respectively. The remaining analytes, TCB and BPS, were detected in fewer than 21% of samples. Compared to the other analytes, MPB had the highest overall weighted and SG-corrected GM (161 ng/mL), followed by BP3 (57.8 ng/mL).
Table 2.
Weighted distributions of plasma inflammatory biomarkers by study visit of sample collection during pregnancy in the LIFECODES cohort (N = 482 subjects).
| Plasma inflammatory biomarker | Visit | N samples | Overall % > LOD | Geometric mean (geometric SD) | Select percentiles |
||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | 90 | 95 | |||||
| C-reactive protein (μg/mL) | Total | 1585 | 99.9% | 5.72 (2.82) | 2.82 | 5.26 | 11.0 | 24.0 | 34.4 |
| 1 | 417 | 4.83 (3.10) | 2.22 | 4.16 | 9.27 | 23.9 | 38.3 | ||
| 2 | 403 | 6.50 (2.74) | 3.13 | 5.93 | 13.9 | 27.4 | 37.4 | ||
| 3 | 387 | 6.22 (2.73) | 3.24 | 6.06 | 12.8 | 23.9 | 34.4 | ||
| 4 | 378 | 5.53 (2.63) | 2.99 | 5.36 | 9.41 | 21.3 | 29.3 | ||
| IL 1β (pg/mL) | Total | 1585 | 78.0% | 0.29 (3.43) | 0.14 | 0.26 | 0.49 | 1.12 | 2.80 |
| 1 | 417 | 0.32 (3.37) | 0.16 | 0.28 | 0.52 | 1.24 | 3.14 | ||
| 2 | 403 | 0.31 (3.31) | 0.15 | 0.27 | 0.47 | 1.18 | 2.68 | ||
| 3 | 387 | 0.27 (3.47) | 0.13 | 0.23 | 0.50 | 1.05 | 2.66 | ||
| 4 | 378 | 0.27 (3.54) | 0.12 | 0.24 | 0.49 | 1.05 | 2.10 | ||
| IL 6 (pg/mL) | Total | 1585 | 97.9% | 1.48 (3.19) | 0.81 | 1.33 | 2.35 | 5.16 | 11.8 |
| 1 | 417 | 1.55 (3.50) | 0.80 | 1.34 | 2.51 | 7.00 | 14.9 | ||
| 2 | 403 | 1.35 (3.29) | 0.74 | 1.21 | 2.16 | 4.86 | 10.9 | ||
| 3 | 387 | 1.34 (3.03) | 0.80 | 1.24 | 2.17 | 4.17 | 9.28 | ||
| 4 | 378 | 1.70 (2.90) | 0.96 | 1.54 | 2.53 | 5.80 | 11.9 | ||
| IL 10 (pg/mL) | Total | 1585 | 99.9% | 13.6 (2.56) | 8.98 | 13.2 | 19.4 | 31.5 | 56.0 |
| 1 | 417 | 13.5 (2.78) | 8.76 | 13.0 | 20.2 | 30.6 | 58.8 | ||
| 2 | 403 | 13.9 (2.51) | 9.16 | 13.3 | 19.4 | 33.0 | 52.6 | ||
| 3 | 387 | 13.4 (2.41) | 9.03 | 13.1 | 18.9 | 30.3 | 55.4 | ||
| 4 | 378 | 13.7 (2.54) | 8.92 | 13.5 | 18.9 | 30.6 | 59.7 | ||
| TNF α (pg/mL) | Total | 1585 | 99.9% | 3.02 (1.83) | 2.19 | 2.99 | 4.25 | 5.92 | 7.21 |
| 1 | 417 | 2.87 (1.84) | 2.07 | 2.91 | 4.07 | 5.63 | 6.92 | ||
| 2 | 403 | 3.06 (1.83) | 2.25 | 2.97 | 4.32 | 5.93 | 7.21 | ||
| 3 | 387 | 2.95 (1.78) | 2.19 | 2.98 | 4.07 | 5.78 | 6.69 | ||
| 4 | 378 | 3.23 (1.84) | 2.23 | 3.28 | 4.60 | 6.27 | 8.01 | ||
Abbreviations: Limit of detection (LOD); Interleukin 1β (IL 1β); Interleukin 6 (IL 6); Interleukin 10 (IL 10); Tumor necrosis factor α (TNF α).
Correlations between exposure analytes and inflammatory markers can be found in Supplemental Table 2. We observed the most positive correlations between CRP and the analytes 2-4-DCP (rs=0.21), 2-5-DCP (rs=0.22), and TCS (rs=0.11) at visit 1. In contrast, the relationship between BP3 and IL-1β (rs=0.17) was most positive at visit 4 compared to the first three visits.
The percent change in inflammatory markers in relation to exposure analytes in final adjusted models are presented in Table 3. Results from the simple models can be found in Supplemental Table 3. We observed that an IQR increase in 2-5-DCP (11.0 ng/mL) was associated with a 10% elevation of CRP (95% confidence interval [CI]: 1.92, 18.7). An IQR increase in TCS (55.2 ng/mL) was positively associated with multiple inflammatory markers: 12.5% increase in CRP (95% CI: 3.67, 22.0), 7.95% increase in IL-10 (95% CI: 1.95, 14.3), and 7.93% increase in TNF-α (95% CI: 3.82, 12.2). Additionally, an IQR increase in MPB (359 ng/mL) was positively associated with a 6.69% increase in IL-6 (95% CI: 0.02, 13.8). Two inverse associations were also observed. An IQR increase in BP3 (192 ng/mL) was associated with a 3.69% decrease in TNF-α (95% CI: −7.09, −0.17) in the adjusted models, but in the simple models, BP3 was associated with a 3.16% decrease in TNF-α (95% CI: −6.49, 0.30). Additionally, IQR increase in EPB (10.4 ng/mL) was associated with a 7.7% decrease in IL-1β (95% CI:−14.1, −0.86) in the adjusted models, and 6.9% decrease in the simple models (95% CI: −13.2, −0.14).
Table 3.
Repeated measures analyses in the LIFECODES cohort: Percent change (95% CI) in plasma inflammatory marker concentrations in relation to an interquartile range difference in urinary exposure biomarkers during pregnancy.
| Adjusted modelsa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Urinary analyte |
CRP |
IL-1β |
IL-6 |
|||||||
| IQR (ng/mL) | %∆ (95% CI) | p-value | q-value | %∆ (95% CI) | p-value | q-value | %∆ (95% CI) | p-value | q-value | |
| 2-4-DCP | 1.18 | 2.50 (−4.21, 9.68) | 0.48 | 0.54 | −0.92 (−6.91, 5.46) | 0.77 | 0.88 | −3.37 (−9.26, 2.90) | 0.29 | 0.55 |
| 2-5-DCP | 11.0 | 10.0 (1.92, 18.7) | 0.015* | 0.06b | −3.72 (−10.5, 3.53) | 0.31 | 0.67 | −0.60 (−7.59, 6.93) | 0.87 | 0.87 |
| BP3 | 192 | 0.79 (−6.44, 8.59) | 0.84 | 0.83 | 1.05 (−5.83, 8.43) | 0.77 | 0.88 | −1.60 (−8.32, 5.61) | 0.65 | 0.74 |
| BPB | 5.12 | 7.17 (−2.22, 17.5) | 0.14 | 0.22 | −6.28 (−13.9, 2.04) | 0.13 | 0.54 | −3.59 (−11.5, 5.03) | 0.40 | 0.55 |
| EPB | 10.4 | 3.36 (−4.31, 11.6) | 0.40 | 0.54 | −7.70 (−14.1, −0.86) | 0.03* | 0.22 | −4.20 (−10.9, 2.95) | 0.24 | 0.55 |
| MPB | 359 | 5.56 (−1.49, 13.1) | 0.13 | 0.25 | −0.15 (−6.37, 6.48) | 0.96 | 0.96 | 6.69 (0.02, 13.8) | 0.049* | 0.40 |
| PPB | 98.2 | 6.40 (−0.25, 13.5) | 0.06 | 0.16 | −2.36 (−8.01, 3.63) | 0.43 | 0.69 | 2.94 (−3.05, 9.30) | 0.34 | 0.55 |
| TCS | 55.2 | 12.5 (3.67, 22.0) | 0.005* | 0.04b | 3.85 (−3.81, 12.1) | 0.33 | 0.67 | 3.32 (−4.34, 11.6) | 0.41 | 0.55 |
| Adjusted modelsa | ||||||
|---|---|---|---|---|---|---|
| Urinary analyte | IL-10 |
TNF-α |
||||
| %∆ (95% CI) | p-value | q-value | %∆ (95% CI) | p-value | q-value | |
| 2-4-DCP | −1.05 (−5.57, 3.68) | 0.66 | >0.90 | −1.30 (−4.40, 1.89) | 0.42 | 0.67 |
| 2-5-DCP | 0.58 (−4.75, 6.21) | 0.83 | >0.90 | 0.27 (−3.38, 4.06) | 0.89 | >0.90 |
| BP3 | −0.34 (−5.47, 5.07) | 0.90 | 0.90 | −3.69 (−7.09, −0.17) | 0.04* | 0.16 |
| BPB | 0.80 (−5.42, 7.44) | 0.80 | >0.90 | −0.42 (−4.66, 4.00) | 0.85 | >0.90 |
| EPB | −3.33 (−8.37, 2.00) | 0.22 | 0.86 | −3.14 (−6.61, 0.46) | 0.09 | 0.23 |
| MPB | 0.34 (−4.38, 5.29) | 0.89 | >0.90 | 1.42 (−1.85, 4.80) | 0.40 | 0.80 |
| PPB | −1.53 (−5.82, 2.97) | 0.50 | >0.90 | −0.05 (−3.05, 3.03) | 0.97 | 0.97 |
| TCS | 7.95 (1.95, 14.3) | 0.009* | 0.07b | 7.93 (3.82, 12.2) | <0.001* | 0.001b |
Abbreviations: IQR (interquartile range), 2-4-DCP (2,4 diclorophenol), 2-5-DCP (2,5 diclorophenol), BP3 (benzophenone 3), BPB (butyl paraben), EPB (ethyl paraben), MPB (methyl paraben), PPB (propyl paraben), TCS (triclosan), CRP (C-reactive protein), IL 1β (interleukin 1β), IL 6 (interleukin 6), IL 10 (interleukin 10), TNF α (tumor necrosis factor α).
Linear mixed models adjusted for specific gravity at sample collection, smoothing term for gestational age, study visit, maternal age, health insurance provider, and BMI at first study visit, and includes random subject specific intercepts; n = 1426 samples for all analyses.
q < 0.1.
P < 0.05.
In sensitivity analyses, we categorized 2-5-DCP into quartiles, and EPB and BPB into four categories (below LOD, and tertiles above LOD). Trends across levels for 2-5-DCP, EPB and BPB remained consistent with the continuous findings in Table 3 (also see Supplemental Table 4). We tested interaction terms between exposure analytes and infant sex, and did not observe any association in the interaction terms (P-values > 0.05; data not shown). We did not observe associations for interaction terms between study visits and exposure analytes (P-values > 0.05; data not shown), except for EPB and IL-1β (P-interaction=0.05; data not shown).
The sensitivity analyses of interactions by preterm birth status are reported in Supplemental Table 5, and should be interpreted alongside the findings from Table 3. Notably, we observed increased positive associations between 2,5-DCP and CRP among cases compared to controls. An IQR difference in 2,5-DCP (7.2 ng/mL) among preterm birth cases was associated with a 15.4% increase in CRP (95% CI: −0.16, 33.3), and an IQR difference in 2,5-DCP (11.5 ng/mL) among controls was associated with a 3.28% increase in CRP (95% CI: −5.36, 12.7) (Supplemental Table 5). We also observed a greater inverse association between BPB and IL-1β among preterm birth cases compared to controls, and since we did not observe a similar association in the overall population, this finding might indicate a relationship that is unique to cases but not generalizable to the overall study population (Supplemental Table 5).
Finally, results from the additional adjustment of MCPP and BPA alongside individual phenols and parabens are reported in Supplemental Table 5. Overall these results revealed that most of the notable associations from Table 3 were consistently associated (p<0.05) and within 10% of the effect estimate magnitude (Supplemental Table 6). The exception was the association between MPB and IL-6 (p>0.05), after adjustment of MCPP and BPA (Supplemental Table 6).
4. DISCUSSION
To our knowledge, this is the largest epidemiological study yet conducted to characterize associations between repeated measurements of maternal inflammatory markers in relation to urinary phenols and parabens during pregnancy. The results of the mixed effects models can be organized into two thematic frameworks, one where concentrations of urinary phenols and parabens during pregnancy reflected a pro-inflammatory relationship with immunological biomarkers, and the other contrary theme – an anti-inflammatory relationship. First, in support of our preliminary hypothesis, our study indicated that urinary levels of TCS, 2-5-DCP and MPB were positively associated with pro-inflammatory markers across pregnancy. In the second group of results in the anti-inflammatory framework, we observed that increases in EPB and BP3 were associated with lower concentrations of pro-inflammatory markers. Additionally, we observed a positive association between TCS and the anti-inflammatory marker IL-10. Our sensitivity analyses suggest that these associations do not vary by study visit during pregnancy or fetal sex, with the exception of the associations between EPB and IL-1β. However, we did observe evidence of differences in associations between 2,5-DCP and CRP between preterm birth cases and controls. There were also suggestive findings that the associations between BPB and IL-1β may differ between cases and controls. Our findings inform several potential biomarker candidates of immune perturbations.
One other prospective birth cohort study conducted by investigators from our research team characterized preliminary associations between phenols, parabens, and inflammatory markers in pregnant women from Northern Puerto Rico (Watkins et al., 2015). Up to two repeated measurements of inflammatory markers and three repeated measurements of urinary phenol analytes from 54 participants were analyzed (Watkins et al., 2015). In the earlier Puerto Rico study, TCS was positively associated with the pro-inflammatory marker IL-6, consistent with our pro-inflammatory findings of TCS. Further, the initial study reported inverse associations between BP3, BPB, and the pro-inflammatory marker CRP, consistent with the anti-inflammatory finding of BP3 in the current study. Differences between the two studies that might influence the conflicting results could be due to several factors, such as sample size, geographical location, and socioeconomic dissimilarities in study populations. Additional human studies need to be conducted to validate and provide greater confidence in the associations that we observed.
Studies have explored the relationships between toxicants and inflammatory markers outside the context of women during pregnancy. One case crossover study of 14 adults assessed levels of inflammatory markers with usage of TCS-containing products, but did not report significant relationships with CRP, IL6, or TNF-α (Poole et al., 2016). Other human studies investigated select phenols and parabens in relation to additional immunological biomarkers and outcomes in adults and children, highlighting evidence that these toxicants may play a role in allergy and sensitization (Cashman and Warshaw, 2005; Clayton et al., 2010; Goodman et al., 2017; Savage et al., 2012; Spanier et al., 2014). Although these studies do not provide direct comparisons to ours, the immunological outcomes of allergy and sensitization parallel molecular signaling pathways related to inflammation and immunomodulation.
Animal studies have examined peripheral tissues to provide evidence of potential mechanisms by which phenols and parabens interact with the immune system. Dermal exposure to TCS in mice for example, indicated that TCS causes greater leukocyte infiltration to exposed tissue and acts as an adjuvant in immunomodulation of lymphocyte populations in local draining lymph nodes – in this case, changing the proportions and profiles of immune cells such as T-cells, B-cells, dendritic cells, and natural killer cells (Anderson et al., 2012; 2015; Marshall et al., 2017). In another study, oral exposure to rats with the paraben BPB resulted in offspring with elevated brain tissue concentrations of IL-1β, IL-6, and TNF-α, suggesting that exposure to parabens alters cytokines of peripheral tissues (Hegazy et al., 2015). Due to molecular similarity, parallels can also be drawn from animal studies of BPA. Studies of mice have shown that oral BPA exposure can also alter the proportions of immune cells in select tissues, and increase the concentration of several circulating cytokines and chemokines (O’Brien et al., 2014; O’Brien et al., 2013; Yan et al., 2008). In alignment with these animal studies, our previous study of BPA within the LIFECODES cohort indicated that higher levels of urinary BPA was associated with elevated IL-6 concentrations in plasma (Ferguson et al., 2016). Although these animal studies highlight potential immunological consequences of phenol and paraben exposure, the physiology of the immune system at the maternal-fetal interface is distinct from peripheral tissues (Hunt et al., 2006).
The associations we reported within the pro- and anti-inflammatory immunological frameworks of our results shed light on potential biomarkers of immune disruption associated with human exposure to phenols and parabens. Toxicants that elicited a pro-inflammatory association in our study include 2-5-DCP, TCS, and MPB. Previous in vitro and animal studies have highlighted that chlorinated phenolic compounds such as 2-5-DCP and TCS can interact with estrogen receptors in immune cells and peripheral tissue (Kiyama and Wada-Kiyama, 2015). Ligand activity of estrogen receptors can stimulate cytokine production in innate immune cells (Nair et al., 2017). For example, when stimulated with estrogen, M1 macrophages can induce a pro-inflammatory response by producing the cytokines IL-12, IL-6, and TNF-α (Brown et al., 2014; Zhang et al., 2017). Mechanistic studies should be conducted to explore estrogen receptor signaling as a plausible link between endocrine disrupting toxicants and immunological target sites.
Increasing concentrations of EPB, TCS, and BP3 were associated with an anti-inflammatory framework. Previous in vitro and animal studies have highlighted that parabens can also interact with estrogen receptors, in addition to progesterone receptors – which are nuclear receptors that help regulate immunomodulation and immune tolerance (Kiyama and Wada-Kiyama, 2015; Nair et al., 2017). Progesterone receptor signaling can modulate lymphocytes by shifting T-cell populations towards differentiated TH2 cell types, which predominantly produce anti-inflammatory cytokines (R. Druckmann and M.-A. Druckmann, 2005; Wilczyński, 2005). Another hallmark of progesterone receptor signaling is the production of anti-inflammatory cytokines such as IL-10 within M2 macrophages, dendritic cells, and uterine natural killer cells (R. Druckmann and M.-A. Druckmann, 2005; Nair et al., 2017). Interestingly, estrogen receptors are also present and active in uterine natural killer cells, however, unlike their pro-inflammatory role in M1 macrophages, estrogen receptors in uterine natural killer cells stimulate the expression of the anti-inflammatory cytokines IL-10 and TGF-β (Nair et al., 2017). The interpretation of the observed inverse association between BP3 and TNF-α is less clear. This association should be explored and validated in additional human studies. These findings provide opportunities to build hypotheses of phenol and paraben action on immune targets and should be further explored in mechanistic studies.
In the third anti-inflammatory finding, elevated EPB concentrations were associated with reduced IL-1β. Produced predominantly by macrophages, IL-1β is an integral product of inflammasomes, which are cytoplasmic protein complexes that elicit inflammatory responses, and of particular importance at the placental and fetal membranes (Romero et al., 2016; Scott et al., 2017). The inverse associations that we observed were drawn from linear mixed models of strictly main effect terms. The sensitivity analyses of interaction terms between individual exposure analytes and study visits indicated that the association between EPB and IL-1β differed across study visits, becoming positive by visit 4. Together, the effect of EPB on IL-1β changes across pregnancy, and we observed uncertainty with this pairwise association. Further investigations of windows of susceptibility are required to more clearly understand this complex relationship between EPB and IL-1β.
The stratified results by case status indicate that there may potentially be differences in associations between 2,5-DCP and CRP between participants that delivered preterm compared to those delivered term. This finding suggests that preterm birth cases may be more susceptible to the pro-inflammatory relationship between 2,5-DCP and CRP. These relationships need to be further explored in additional longitudinal case-control studies and mechanistic animal studies in order to better understand the biological basis of differences in effect estimates between cases of preterm birth and term birth.
Our study has limitations that should be highlighted to improve future studies. This study characterized single pollutant associations, whereas in most settings, humans are exposed to several toxicants and complex mixtures of these toxicants may be acting through our proposed immunological mechanisms. In this case, the advantage of conducting single pollutant analyses is that we can focus on characterizing associations for specific toxicants, which can have more direct policy implications. The study design that we implemented is also limited because we are unable to assess causality between phenols and inflammatory markers. However, randomized controlled trials would not be feasible due to ethical implications of exposure assignment. We measured circulating inflammatory markers, and as such were unable to assess local inflammation at the maternal-fetal interface. Furthermore, the four cytokines that we measured represent only a fraction of the cytokine repertoire within the maternal immune system. We have plans to expand measurements of cytokines and chemokines in our prospective birth cohort in Northern Puerto Rico. In addition, we conducted several comparisons between exposure analytes and immunological biomarkers, and recognize that some of the associations we detected may be due to chance. Although methods such as the Bonferroni correction can be deployed to adjust for multiple comparisons by setting a stricter p-value threshold, doing so may be too conservative (Perneger, 1998). We applied the Benjamini-Hochberg (1995) method to calculate false discovery rates and prioritize findings in the context of multiple comparisons. Several of our findings were robust to false discovery rate calculations with lower observed q-values. However, the associations between EPB and IL-1β, MPB and IL-6, and BP3 and TNF-α were of lower confidence as each had elevated q-values. Studies with larger sample sizes and independent samples can test for replication, determining whether these suggestive associations are consistent or by chance. Last, although we applied inverse probability weights, there are still constraints for the generalizability of our results to the U.S. population. Despite this limitation, the observed associations within our nested case-control population can still likely provide generalizable results for readers to consider when evaluating the potential effects of phenol exposure on disruption of the maternal immune system during pregnancy.
There are several strengths to our study as well. First, we obtained biological samples from a large sample size of pregnant women. We obtained up to four repeated measurements of biological samples across pregnancy. From these samples, we measured a diverse breadth of inflammatory biomarkers and urinary phenol analytes. Repeated measurements contribute to a robust and comprehensive exposure assessment across pregnancy. Furthermore, repeated measurements also afford greater statistical power to characterize associations, which provide more information than single spot urine measures. Another strength was that our study participants were recruited early in pregnancy, which allowed us to comprehensively test potential windows of vulnerability. Last, the plasma sample analysis in our study used high sensitivity assays, which contributed to high detection rates of inflammatory markers.
In our study, we characterized several associations between phenols, parabens, and inflammatory markers. These associations provide the reader with tools for developing hypotheses of immune mechanisms by which phenols and parabens affect reproductive health. Investigators should consider measuring immunological biomarkers when assessing these toxicants in prospective birth cohorts in order to better understand the relationship between phenols, parabens and the maternal immune system.
5. CONCLUSION
We propose that the weight of evidence for immune perturbations warrants further investigation of immunological biomarkers during pregnancy in relation to exposure levels of phenols and parabens. Due to the complexity of receptor signaling in immune cells, it is difficult to make conclusions about the magnitude that phenols and parabens contribute towards inflammatory processes during pregnancy. Modulation of the maternal immune system during pregnancy may be a critical precursor for adverse reproductive and developmental outcomes. Future human and animal immunological biomarker studies should be conducted to better understand the complex mechanisms linking phenol and parabens exposure to immune perturbations during pregnancy.
Supplementary Material
Highlights:
Maternal phenol exposure during pregnancy was associated with systemic plasma immune biomarkers
2,5-Dichlorophenol and triclosan were both positively associated with C-reactive protein
Triclosan was positively associated with the pro-inflammatory cytokine tumor necrosis factor-α and the anti-inflammatory cytokine interleukin-10
Acknowledgements:
Subject recruitment and sample collection was originally funded by Abbott Diagnostics.
Funding:
This work was also supported by National Institute of Environmental Health Sciences, National Institutes of Health (grants R01ES018872, P42ES017198, P01ES022844, P30ES017885, P50ES026049, U2CES026553).
Support for Max Aung was provided in part by a grant from the Robert Wood Johnson Foundation Health Policy Research Scholars program.
Support for Kelly Ferguson was provided by the Intramural Research Program of the National Institute of Environmental Health Sciences.
Abbreviations:
- 2,4-DCP
2,4-diclorophenol
- 2,5-DCP
2,5-dichlorophenol
- BP3
Benzophenone-3
- BPS
Bisphenol-S
- BPB
Butyl paraben
- EPB
Ethyl paraben
- MPB
Methyl paraben
- PPB
Propyl paraben
- TCB
Triclocarban
- TCS
Triclosan
- CRP
C-reactive protein
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- TNF-α
Tumor necrosis factor-α
- ID-LC-MS/MS
Isotopic dilution-liquid chromatography-tandem mass spectrometry
- LOD
Limit of detection
- SG
Specific gravity
- ELISA
enzyme-linked immunosorbent assay
- GAMM
general additive mixed model
- LMM
Linear mixed model
- GM
Geometric mean
- GSD
Geometric standard deviation
- AIC
Aikaike information criterion
- IQR
Interquartile range
- CI
Confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they have no financial or potential competing interests.
References
- Anderson SE, Franko J, Kashon ML, Anderson KL, Hubbs AF, Lukomska E, Meade BJ, 2012. Exposure to Triclosan Augments the Allergic Response to Ovalbumin in a Mouse Model of Asthma. Toxicological Sciences 132, 96–106. doi: 10.1093/toxsci/kfs328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Meade BJ, Long CM, Lukomska E, Marshall NB, 2015. Investigations of immunotoxicity and allergic potential induced by topical application of triclosan in mice. Journal of Immunotoxicology 13, 165–172. doi: 10.3109/1547691X.2015.1029146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andra SS, Charisiadis P, Arora M, van Vliet-Ostaptchouk JV, Makris KC, 2015. Biomonitoring of human exposures to chlorinated derivatives and structural analogs of bisphenol A. Environment International 85, 352–379. doi: 10.1016/j.envint.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300. [Google Scholar]
- Boyle AK, Rinaldi SF, Norman JE, Stock SJ, 2017. Preterm birth: Inflammation, fetal injury and treatment strategies. Journal of Reproductive Immunology 119, 62–66. doi: 10.1016/j.jri.2016.11.008 [DOI] [PubMed] [Google Scholar]
- Brown MB, Chamier von M, Allam AB, Reyes L, 2014. M1/M2 macrophage polarity in normal and complicated pregnancy. Front. Immunol 5, 209. doi: 10.3389/fimmu.2014.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Błędzka D, Gromadzińska J, Wąsowicz W, 2014. Parabens. From environmental studies to human health. Environment International 67, 27–42. doi: 10.1016/j.envint.2014.02.007 [DOI] [PubMed] [Google Scholar]
- Cashman AL, Warshaw EM, 2005. Parabens: A Review of Epidemiology, Structure, Allergenicity, and Hormonal Properties. Dermatitis (formerly American Journal of Contact Dermatitis) 16, 057. doi: 10.2310/6620.2005.05008 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2016. Chemical factsheets [WWW Document] URL https://www.cdc.gov/biomonitoring/chemical_factsheets.html (accessed 10.31.17).
- Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM, 2014. Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy. Front. Immunol 5, 58– 7. doi: 10.3389/fimmu.2014.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau A, Markley JC, Juang J, Tsen LC, 2016. Cytokines in the perinatal period - Part I. International Journal of Obstetric Anesthesia 26, 39–47. doi: 10.1016/j.ijoa.2015.12.005 [DOI] [PubMed] [Google Scholar]
- Chiesa C, Pacifico L, Natale F, Hofer N, Osborn JF, Resch B, 2015. Fetal and early neonatal interleukin-6 response. Cytokine 76, 1–12. doi: 10.1016/j.cyto.2015.03.015 [DOI] [PubMed] [Google Scholar]
- Clayton EMR, Todd M, Dowd JB, Aiello AE, 2010. The Impact of Bisphenol A and Triclosan on Immune Parameters in the U.S. Population, NHANES 2003–2006. Environ Health Perspect 119, 390–396. doi: 10.1289/ehp.1002883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann R, Druckmann M-A, 2005. Progesterone and the immunology of pregnancy. The Journal of Steroid Biochemistry and Molecular Biology 97, 389–396. doi: 10.1016/j.jsbmb.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Cantonwine DE, McElrath TF, Mukherjee B, Meeker JD, 2016. Repeated measures analysis of associations between urinary bisphenol-A concentrations and biomarkers of inflammation and oxidative stress in pregnancy. Reproductive Toxicology 66, 93–98. doi: 10.1016/j.reprotox.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Chen Y-H, Mukherjee B, Meeker JD, 2014a. Longitudinal Profiling of Inflammatory Cytokines and C-reactive Protein during Uncomplicated and Preterm Pregnancy. Am J Reprod Immunol 72, 326–336. doi: 10.1111/aji.12265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Ko Y-A, Mukherjee B, Meeker JD, 2014b. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environment International 70, 118–124. doi: 10.1016/j.envint.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Mukherjee B, Loch-Caruso R, Meeker JD, 2015. Associations between Maternal Biomarkers of Phthalate Exposure and Inflammation Using Repeated Measurements across Pregnancy. PLoS ONE 10, e0135601–12. doi: 10.1371/journal.pone.0135601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Meeker JD, Cantonwine DE, Mukherjee B, Pace GG, Weller D, McElrath TF, 2017. Environmental phenol associations with ultrasound and delivery measures of fetal growth. Environment International 112, 243–250. doi: 10.1016/j.envint.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, Naiman DQ, LaKind JS, 2017. Systematic review of the literature on triclosan and health outcomes in humans. Crit. Rev. Toxicol 122, 1–51. doi: 10.1080/10408444.2017.1350138 [DOI] [PubMed] [Google Scholar]
- Hegazy HG, Ali EHA, Elgoly AHM, 2015. Interplay between pro-inflammatory cytokines and brain oxidative stress biomarkers: Evidence of parallels between butyl paraben intoxication and the valproic acid brain physiopathology in autism rat model. Cytokine 71, 173–180. doi: 10.1016/j.cyto.2014.10.027 [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene 5, 46–51. doi: 10.1080/1047322x.1990.10389587 [DOI] [Google Scholar]
- Hunt JS, McIntire RH, Petroff MG, 2006. Immunobiology of human pregnancy, in: Knobil and Neill’s Physiology of Reproduction pp. 2759–2785. doi: 10.1016/B978-012515400-0/50056-7 [DOI] [Google Scholar]
- Kim CJ, Romero R, Chaemsaithong P, Kim J-S, 2015. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. American Journal of Obstetrics and Gynecology 213, S53–69. doi: 10.1016/j.ajog.2015.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama R, Wada-Kiyama Y, 2015. Estrogenic endocrine disruptors: Molecular mechanisms of action. Environment International 83, 11–40. doi: 10.1016/j.envint.2015.05.012 [DOI] [PubMed] [Google Scholar]
- Kovats S, 2012. Estrogen receptors regulate an inflammatory pathway of dendritic cell differentiation: Mechanisms and implications for immunity. Hormones and Behavior 62, 254–262. doi: 10.1016/j.yhbeh.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Begum N, Prasad S, Agarwal S, Sharma S, 2013. IL-10, TNF-α & IFN-γ: Potential early biomarkers for preeclampsia. Cellular Immunology 283, 70–74. doi: 10.1016/j.cellimm.2013.06.012 [DOI] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD, Peterson KE, Lee JM, Pace GG, Cantoral A, Téllez-Rojo MM, 2013. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere 93, 2390–2398. doi: 10.1016/j.chemosphere.2013.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissauer D, Kilby MD, Moss P, 2017. Maternal effector T cells within decidua: The adaptive immune response to pregnancy? Placenta 1–5. doi: 10.1016/j.placenta.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Marshall NB, Lukomska E, Nayak AP, Long CM, Hettick JM, Anderson SE, 2017. Topical application of the anti-microbial chemical triclosan induces immunomodulatory responses through the S100A8/A9-TLR4 pathway. Journal of Immunotoxicology 14, 50–59. doi: 10.1080/1547691X.2016.1258094 [DOI] [PubMed] [Google Scholar]
- McElrath TF, Lim KH, Pare E, Rich-Edwards J, Pucci D, Troisi R, Parry S, 2012. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. The American Journal of Obstetrics & Gynecology 207, 407.e1–407.e7. doi: 10.1016/j.ajog.2012.08.010 [DOI] [PubMed] [Google Scholar]
- Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, Téllez-Rojo MM, 2009. Urinary Phthalate Metabolites in Relation to Preterm Birth in Mexico City. Environ Health Perspect 117, 1587–1592. doi: 10.1289/ehp.0800522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli S, Mandal M, Goldsmith LT, Kashani BN, Ponzio NM, 2015. The maternal immune system during pregnancy and its influence on fetal development. RRB 171–20. doi: 10.2147/RRB.S80652 [DOI] [Google Scholar]
- Nair RR, Verma P, Singh K, 2017. Immune-endocrine crosstalk during pregnancy. General and Comparative Endocrinology 242, 18–23. doi: 10.1016/j.ygcen.2016.03.003 [DOI] [PubMed] [Google Scholar]
- O’Brien E, Bergin IL, Dolinoy DC, Zaslona Z, Little RJA, Tao Y, Peters-Golden M, Mancuso P, 2014. Perinatal bisphenol A exposure beginning before gestation enhances allergen sensitization, but not pulmonary inflammation, in adult mice. J Dev Orig Health Dis 5, 121–131. doi: 10.1017/S204017441400004X [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien E, Dolinoy DC, Mancuso P, 2013. Perinatal bisphenol A exposures increase production of pro-inflammatory mediators in bone marrow-derived mast cells of adult mice. Journal of Immunotoxicology 11, 205–212. doi: 10.3109/1547691X.2013.822036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneger TV, 1998. What’s wrong with Bonferroni adjustments. BMJ : British Medical Journal 316, 1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AC, Pischel L, Ley C, Suh G, Goodrich JK, Haggerty TD, Ley RE, Parsonnet J, 2016. Crossover Control Study of the Effect of Personal Care Products Containing Triclosan on the Microbiome. mSphere 1, e00056.–15–10. doi: 10.1128/mSphere.00056-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racicot K, Kwon J-Y, Aldo P, Silasi M, Mor G, 2014. Understanding the Complexity of the Immune System during Pregnancy. Am J Reprod Immunol 72, 107–116. doi: 10.1111/aji.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DB, Rzehak P, Klenk J, Weiland SK, 2007. Analyses of Case–Control Data for Additional Outcomes. Epidemiology 18, 441–445. doi: 10.1097/EDE.0b013e318060d25c [DOI] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL, 2015. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect 1–9. doi: 10.1289/ehp.1408989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JA, Metz L, Yong VW, 2013. Review: Endocrine disrupting chemicals and immune responses: A focus on bisphenol-A and its potential mechanisms. Molecular Immunology 53, 421– 430. doi: 10.1016/j.molimm.2012.09.013 [DOI] [PubMed] [Google Scholar]
- Romero R, Gotsch F, Pineles B, Kusanovic JP, 2007. Inflammation in Pregnancy: Its Roles in Reproductive Physiology, Obstetrical Complications, and Fetal Injury. Nut. Rev 65, 194–202. doi: 10.1301/nr.2007.dec.S194-S202 [DOI] [PubMed] [Google Scholar]
- Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, Than NG, Chiang PJ, Dong Z, Xu Z, Tarca AL, Abrahams VM, Hassan SS, Yeo L, Gomez-Lopez N, 2016. A Role for the Inflammasome in Spontaneous Labor at Term. Am J Reprod Immunol 11, n/a–n/a. doi: 10.1111/aji.12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JH, Matsui EC, Wood RA, Keet CA, 2012. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. Journal of Allergy and Clinical Immunology 130, 453–460.e7. doi: 10.1016/j.jaci.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LM, Bryant AH, Rees A, Down B, Jones RH, Thornton CA, 2017. Production and regulation of interleukin-1 family cytokines at the materno-fetal interface. Cytokine 99, 194–202. doi: 10.1016/j.cyto.2017.07.005 [DOI] [PubMed] [Google Scholar]
- Spanier AJ, Fausnight T, Camacho TF, Braun JM, 2014. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. Allergy Asthma Proc 35, 475– 481. doi: 10.2500/aap.2014.35.3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BD, Ness RB, Klebanoff MA, Zoh R, Bass D, Hougaard DM, Skogstrand K, Haggerty CL, 2016. First and second trimester immune biomarkers in preeclamptic and normotensive women. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health 6, 388–393. doi: 10.1016/j.preghy.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PA, Khatami M, Baglole CJ, Sun J, Harris SA, Moon E-Y, Al-Mulla F, Al-Temaimi R, Brown DG, Colacci AM, Mondello C, Raju J, Ryan EP, Woodrick J, Scovassi AI, Singh N, Vaccari M, Roy R, Forte S, Memeo L, Salem HK, Amedei A, Hamid RA, Lowe L, Guarnieri T, Bisson WH, 2015. Environmental immune disruptors, inflammation and cancer risk. CARCIN 36, S232–S253. doi: 10.1093/carcin/bgv038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tan G-J, Han L-N, Bai Y-Y, He M, Liu H-B, 2017. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol 14, 135–150. doi: 10.11909/j.issn.1671-5411.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Ferguson KK, Del Toro LVA, Alshawabkeh AN, Cordero JF, Meeker JD, 2015. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. International Journal of Hygiene and Environmental Health 218, 212–219. doi: 10.1016/j.ijheh.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S-Q, Fraser W, Luo Z-C, 2010. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol 116, 393–401. doi: 10.1097/AOG.0b013e3181e6dbc0 [DOI] [PubMed] [Google Scholar]
- Wilczyński JR, 2005. Th1/Th2 cytokines balance—yin and yang of reproductive immunology. European Journal of Obstetrics & Gynecology and Reproductive Biology 122, 136–143. doi: 10.1016/j.ejogrb.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Yan H, Takamoto M, Sugane K, 2008. Exposure to Bisphenol A Prenatally or in Adulthood Promotes Th2 Cytokine Production Associated with Reduction of CD4+CD25+ Regulatory T cells. Environ Health Perspect 1–6. doi: 10.1289/ehp.10829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wong L-Y, Zhou X, Calafat AM, 2014. Urinary Concentrations of 2,4-Dichlorophenol and 2,5-Dichlorophenol in the U.S. Population (National Health and Nutrition Examination Survey, 2003–2010): Trends and Predictors. Environ Health Perspect 1–5. doi: 10.1289/ehp.1306816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-H, He M, Wang Y, Liao A-H, 2017. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front. Immunol 8, 387–12. doi: 10.3389/fimmu.2017.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


