Abstract
Early recognition of neoantigen-expressing cells is complex, involving multiple immune cell types. In this study, in vivo, we examined how antigen-presenting cell subtypes coordinate and induce an immunological response against neoantigen-expressing cells, particularly in the absence of a pathogen-associated molecular pattern, which is normally required to license antigen-presenting cells to present foreign or self-antigens as immunogens. Using two reductionist models of neoantigen-expressing cells and two cancer models, we demonstrated that natural IgM is essential for the recognition and initiation of adaptive immunity against neoantigen-expressing cells. Natural IgM antibodies form a cellular immune complex with the neoantigen-expressing cells. This immune complex licenses surveying monocytes to present neoantigens as immunogens to CD4+ T cells. CD4+ T helper cells, in turn, use CD40L to license cross-presenting CD40+ Batf3+ dendritic cells to elicit a cytotoxic T cell response against neoantigen-expressing cells. Any break along this immunological chain reaction results in the escape of neoantigen-expressing cells. This study demonstrates the surprising, essential role of natural IgM as the initiator of a sequential signaling cascade involving multiple immune cell subtypes. This sequence is required to coordinate an adaptive immune response against neoantigen-expressing cells.
Keywords: Ly6C+ monocytes, XCR1+ Batf3+ dendritic cells, neoantigens, cancer, natural IgM
The immune system recognizes and attacks cancerous cells that express neoantigens, or cell surface markers associated with cellular pathology and aberrant growth (1, 2). Many forms of cancer are thus recognized and destroyed by the intact immune system. This process is also known to involve adaptive immune pathways that result in the differentiation of naive T cells into effector T cells. Such T cell differentiation nearly universally implicates pathogen-associated molecular patterns (PAMPs) or non-PAMP adjuvants (such as Alum)—but in the case of tumor cells, early neoantigen recognition and elimination occurs in the absence of PAMPs. Further understanding how the immune system recognizes neoantigen and orchestrates a T cell–mediated adaptive immune response against neoantigen-expressing cells in the absence of PAMPs could tell us how cancers are suppressed by the healthy immune system, and provide new key targets for diagnosis and intervention.
Studies focusing on extrinsic tumor suppressor mechanisms have paid much attention to the roles played by danger-associated molecular patterns, dendritic cells (DCs), natural killer (NK) cells, and T cells during the recognition and clearance of neoantigen-expressing cells (1, 2). However, less attention has been paid to the contribution and role of natural IgM in neoantigen recognition. We demonstrate that natural IgM recognizes cell surface neoantigens and license antigen-presenting cells (APCs) to initiate an adaptive immune response.
Natural IgM antibodies are found early in ontogeny and throughout life (3–7), even in the absence of deliberate antigenic stimulation and in germ-free animals (8, 9). It has been characterized to display specificities with weak affinity for a number of common microbial determinants, and provide an early and broad humoral defense against a wide variety of pathogens (10–18). Most notably, natural IgM specificities also recognize many self-antigens that have been shown to aid in the clearance of apoptotic cells (18, 19). Moreover, natural IgM is a key player in immune complex formation, and several studies ex vivo have observed natural IgM binding on tumors in immunohistochemistry sections and tumor lysates in Western blots (20–23). Whether natural IgM recognition of neoantigen-expressing cells occurs in vivo is unclear. We hypothesize here that a cellular immune complex formation initiated by natural IgM (i.e., antigen–antibody complex) is likely to play a critical part during the early recognition and elimination phase of precancerous cells (24–26).
Using two reductionist models of neoantigen-expressing cells (expressing normal levels of major histocompatibility complex class I [MHCI]) and two cancer models (urethane-induced spontaneously occurring cancer and the B16F10 melanoma cell line) (27, 28), we provide evidence that natural IgM antibodies is required for the elimination of neoantigen-expressing cells in mice lacking a diverse natural IgM repertoire. This mouse displays a relatively normal lymph node (LN) architecture (unlike B-cell deficient mice, μMT mice) where all APC subtypes and naive T cell subsets are present. Furthermore, we demonstrate that the elimination of neoantigen-expressing cells requires a sequential signaling interaction among five different immune cell types, which was conceptually supported with the experimental use of over 15 cellular immune and mechanistically deficient mice.
In mice, there are predominantly three LN-trafficking APCs: Ly6C+ monocytes and two overarching DC subtypes, which are named after the transcription factors that regulate their development, Batf3+ DCs and Irf4+ DCs (29–33). Although Ly6C+ monocytes can present exogenous antigens to transgenic CD4 and CD8 T cells in vitro and elicit any branch in the adaptive immune system (34), less is known about their major contributions in adaptive immunity in vivo and in the absence of pathogens. On the other hand, DC subtypes have been extensively studied and shown to have diverse functional roles. Batf3+ and Irf4+ DCs differ in their expression levels of transcription factors, phagocytic receptors, cytokine production, and pattern-recognition receptors, such as Toll-like receptors (TLRs) and C-type lectins. They also differ in T cell imprinting, antigen acquisition, processing, and presentation (35–39). These differences among DC subtypes imply that they play distinct functional roles in the clearance of neoantigen-expressing cells. Specifically, Batf3+ DCs mainly present exogenous antigen to CD8+ T cells, whereas Irf4+ DCs predominantly present exogenous antigen to CD4+ T cells (37, 40, 41). Moreover, the antigens that these APC subtypes acquire can be significantly different. We and others have demonstrated the selective ability of Batf3+ DCs and Ly6C+ monocytes, but not Irf4+ DCs, to take up dying cells (efferocytosis) (42), migrate to the draining LNs, and present exogenous cell-associated antigen peptides on MHCI (i.e., cross-presentation). These can then be recognized by cognate CD8+ T cells (37, 43–46), of which Batf3+ DCs display a preferential role in cross-presentation and cross-priming of neoantigen-expressing cells.
Based on our knowledge of APC antigen presentation, a key question that arose in this study was, in the absence of an identifiable PAMP, what initiates an immune response against neoantigen-expressing cells? This question arose because we and others have demonstrated that only a PAMP-activated, antigen-bearing APC can differentiate a naive T cell into an effector T cell (47–49). Here, we propose a role for an initial immune complex formation due to natural IgM antibody binding, followed by CD4+ T helper cell CD40L-CD40 ligation. CD4+ T cells license antigen-bearing Batf3+ DC subtypes to present neoantigens in an immunogenic fashion to cognate CD8 T cells, which then selectively target neoantigen-expressing cells.
Methods
Mice
C57BL/6 Ly5.1 (CD45.1) or Ly5.2 (CD45.2) wild-type (WT) mice (6–8 week old) were purchased from Charles River or Jackson Research Laboratory. 129SvEv, Batf3−/−, CCR2−/−, CD11ccre, Ifr4fl/fl, CCR7−/−, PMEL, TLR3−/−, TLR7−/−, CD11b−/−, IL12−/−, IL27−/−, CD4−/−, IAb−/−, CD40L−/−, CD40−/−, IFN-γ reporter, μMT, Act-mOVA, and IghelMD4 mice were purchased from Jackson Laboratory. AID−/−, FcRγ−/−, and STING−/− mice were kindly provided by Drs. Tasuko Honjo, Erwin Gelfand, and John Cambier. OT-I and OT-II transgenic mice purchased from Jackson Laboratory were crossed with C57BL/6 Ly5.1. Double knockouts, IL12−/−IL27−/− and TLR3−/−TLR7−/−, were created in house. All mice were genotyped upon arrival and before their use. Mice were housed in a specific pathogen-free environment at National Jewish Health, an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited institution, and used in accordance with protocols approved by the Institutional Animal Care and Use Committee, and which conform to NIH guidelines.
Male and 129 Neoantigen Rejection Model
Male neoantigen rejection model
C57BL/6 T cells from male CD45.1 OT-I mice were used to examine acceptance or rejection of male cells in female C57BL/6 mice (50). 129 Neoantigen Rejection Model: C57BL/6 CD45.1 OT-I mice were crossed with 129SvEv mice to create an F1 129/BL6 OT-I mouse. F1 129/BL6 OT-I cells express non-MHCII mismatches due to allelic variations outside of the H-Y locus (51, 52). Female 129/BL6 OT-I cells were used to introduce neoantigens into female C57BL/6 mice. Model set up: two million CD45.1 (male or 129/BL6 female) OT-I cells were transferred intravenously into congenic recipients. The next day, mice were anesthetized and given intranasal (IN) (0.22-μm filtered to remove aggregates) 2 μg ovalbumin (OVA), resulting in the expansion of adoptively transferred neoantigen-expressing T cells. Mice were then rechallenged with 100 μg OVA at Day 18 to recall adoptively transferred cells. At 2 days after rechallenge, the lung-draining LNs were examined for the presence (recall) or absence (rejection) of adoptively transferred neoantigen-expressing cells.
Cancer Models
Urethane model
Spontaneous lung tumors were induced with intraperitoneal injections of 1 mg/g urethane (ethyl carbamate; Sigma-Aldrich) weekly for 6 weeks. Mice were killed 20 weeks after the last intraperitoneal injection of urethane. An experienced reader was blinded to the sample for tumor counts under a dissecting microscope, where the entire lung was carefully dissected. Tumor numbers of each lung were averaged and statistically analyzed.
Melanoma model
Pulmonary metastatic melanoma was induced by intravenously injecting 2.0 × 105 B16F10 melanoma cell line (CRL-6475; ATCC). Mice were killed at Day 16. The lungs were first perfused with cold PBS and then inflated through the trachea with 1% agarose. Tumor counts were performed on the dorsal and ventral side of the lungs.
Antibodies used, flow analysis, Western blot, ex vivo immune complex (IC) experiment, tetramer+ staining, and bone marrow chimeras are described in the Methods in the data supplement.
Statistical Analysis
Statistical analysis was conducted using InStat and Prism software (GraphPad). All results are expressed as the mean (±SEM). Statistical tests were performed using two-tailed Student’s t test. A value of P less than 0.05 was considered statistically significant.
Results
Nonredundant Role of APC Subtypes in the Clearance of Neoantigen-Expressing Cells
Batf3+ DCs are required for the elimination of neoantigen-expressing cells and antitumor immunity (31, 47, 50). Because we previously demonstrated that Batf3+ DCs require direct activation to present neoantigens as an immunogen (47), we first asked, in the absence of PAMPs, what is the main endogenous cellular mechanism that licenses Batf3+ DCs to present neoantigens in an immunogenic fashion to CD8+ T cells? Based on previous findings (37), Batf3+ DCs do not readily present cell-associated antigen to CD4+ T cells, and it has been shown that CD4+ T cells are required for the elimination of neoantigen-expressing cells (27, 50). Therefore, if Batf3+ DCs are not directly activating CD4+ T cells, then we hypothesized that other immune cell types upstream of the Batf3+ DCs are playing a significant role in ultimately licensing the Batf3+ DCs to elicit a cytotoxic T cell response against neoantigen-expressing cells (Figure 1A). Our hypothesis states that, first, natural IgM antibodies are required for the initial recognition of neoantigens. The binding of natural IgM on a neoantigen-expressing cell results in the formation of a cell-bound immune complex, which is then, second, acquired by an LN-trafficking monocyte (34). Third, due to the acquisition of an immune complex, antigen-presenting monocytes become activated and licensed to present neoantigens as immunogens, resulting in the priming of cognate CD4+ T cells. Fourth, the activated CD4+ T cells use CD40L to license Batf3+ DCs via CD40 to cross-prime a CD8 cytotoxic T cell (CTL) response against the neoantigen-expressing cells (Figure 1A). Figure 1 sets out to examine this overarching hypothesis.
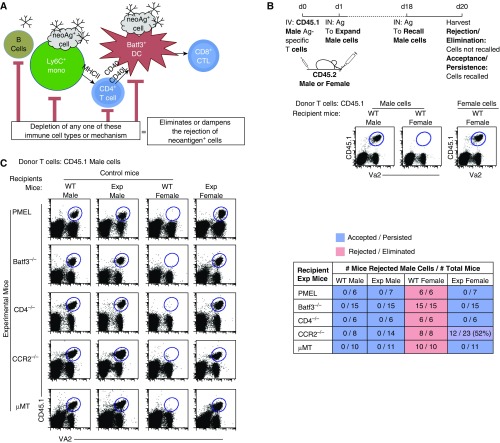
Figure 1.
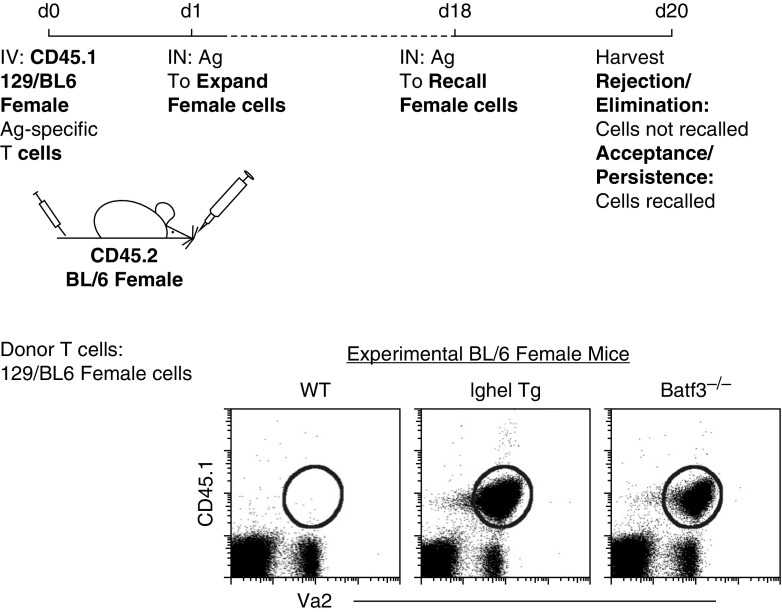
Key players involved in the coordinated, immunological cascade against neoantigen-expressing cells: B cells, T cells, lymph node (LN) monocytes, and dendritic cells (DCs). (A) Schematic diagram of our stated and examined hypothesis. The rejection and elimination of neoantigen-expressing cells requires a chain-link leukocyte reaction. (B) Top: the experimental scheme used to determine, if minor antigen–mismatched cells (neoantigen-expressing cells) are accepted or rejected in female mice. Bottom: flow plots show the recall of adoptively transferred CD45.1 male cells into wild-type (WT) male or female mice (left) compared with CD45.1 female cells into WT female mice (right). (C) Flow data illustrate three controls: two negative controls (acceptance, male cells into male WT and knockout [KO]/transgenic mice, referred to as experimental [Exp] mice) and one positive control (rejection, male cells into WT female mice). Experimental mice are PMEL (CD8+ T cell transgenic, hypo-CD8+ T cell repertoire), Batf3−/− (lack Batf3+ DCs), CD4−/− (lack CD4+ T cells), CCR2−/− (lack LN monocytes), and μMT (lack peripheral B cells) female mice. Data are representative of two to three individual experiments with n = 3–5 mice per group. Table displays the combined experiments with the number of mice that rejected male cells (numerator) over the number of total mice examined (denominator). Acceptance is shown in blue, rejection in red. Ag = antigen; CTL = cytotoxic T lymphocytes; IN = intranasal; IV = intravenous; MHCII = major histocompatibility complex class II; Va2 = Vα2.
Neoantigens are mutated, newly formed antigens not present in the normal genome. To address the immune cells and mechanisms involved in the neoantigen recognition and elimination, we developed a consistent, reproducible, reductionist model. Although not a cancer model per se, this neoantigen-expressing cell model does not require PAMPs for its recognition and elimination, as new non–MHC-associated proteins are introduced into female mice. It is well established that adoptively transferred male cells are completely rejected (i.e., eliminated) in syngeneic female mice (53–57). This is due to the development of a CTL response against the Y chromosome–associated antigens, which are absent in female mice (27, 50). To illustrate our model (Figure 1B), we first isolated CD45.1 male cells (from OT-I mice, OVA-specific T cells) and adoptively transferred them into syngeneic CD45.2 WT male and female mice. One day after adoptive transfer of CD45.1 male cells, recipient CD45.2 mice were immunized via the intranasal route with 2 μg of soluble OVA to induce proliferative expansion of adoptively transferred OT-I male cells in the host mouse. To assess the rejection or acceptance of the adoptively transferred male cells, at Day 18, mice were challenged with 100 μg of OVA (i.e., a recall response). Two days after challenge (i.e., Day 20), the draining LNs were examined for the presence or absence of adoptively transferred CD45.1 male cells (Figure 1B). As expected, there was no recall of the adoptively transferred CD45.1 male cells (i.e., the cells were rejected) in syngeneic WT female mice compared with syngeneic WT male mice (50). This rejection was not due to the presence of other neoantigens besides the H-Y antigens, as adoptively transferred CD45.1 female OT-I T cells were not rejected in syngeneic WT female mice (Figure 1B). Moreover, it is important to note that, in this model, the TCR specificity is irrelevant, and any male transgenic T cell could be used, as illustrated in Reference 50. This is because the recipient WT female mice are recognizing and eliminating adoptively transferred male cells expressing perceived neoantigens (i.e., H-Y antigens).
Next, we examined whether a break along the hypothesized immune cell interactions resulted in an inability to eliminate neoantigen-expressing cells. To ascertain the reliability of the experimental group, each experiment was accompanied by three control groups: two negative control groups comprised of WT and knockout/cell-deficient male mice, where acceptance (i.e., recall) of male cells was expected regardless of the presence or absence of an APC subtype or lymphocyte population, and a positive control group, comprised of WT female mice, where the complete rejection of transferred syngeneic male cells was anticipated (Figure 1C). Focusing on the experimental group, male cells were not rejected in female mice that lacked either: 1) a diverse CD8+ T cell repertoire (i.e., in PMEL mice, wherein greater than 90% of the CD8+ T cells are specific for an enzyme involved in pigment synthesis); 2) Batf3+ DC (Batf3−/− mice); 3) CD4+ T cells (CD4−/− mice); 4) surveying, tissue-trafficking monocytes (CCR2−/− mice); or 5) B cells (μMT mice). Deletion of CD4+ T cells with anti-CD4 antibody showed similar results as CD4−/− mice (see Figure E1 and scatter plot frequency in Figure E2 in the data supplement). Importantly, when male cells were transferred into individual genetic male mutant hosts, the transferred male cells were able to mount a recall response (Figure 1C, second column). This result further demonstrated that each mutation interrogated did not individually impede the recall response. We also examined other types of leukocytes or cellular mediators known to induce antigen-specific CTL response, and found that none of the examined mutants appeared to have a major role in the rejection of adoptively transferred male cells in female mice (i.e., depletion of NK cells using anti-NK1.1, FcR common γ chain FcRγ−/−, IL-12−/−/IL-27−/−, and TLR3−/−/TLR7−/− mice; Figure E1). Overall, these data support the concept that each APC subtype examined herein plays a critical and unique role in this immunological process.
A Diverse Polyclonal IgM Repertoire Is Required for the Rejection of Neoantigen-Expressing Cells
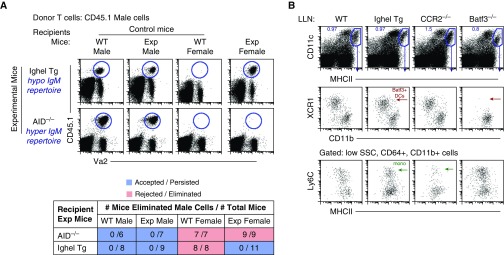
In the absence of B cells (i.e., μMT mice), we observed a recall of adoptively transferred male cells in μMT female mice compared with WT female mice (Figure 1C). As B cells can also function as APCs, it was important to discriminate between their requirements as APCs versus producers of serum antibodies. To test if natural IgM antibodies were required for rejection, we again used the H-Y antigen model with IghelMD4 female mice as recipients, where greater than 90% of IgM-secreting B cells are specific for hen egg lysozyme (58). Thus, in these mice, the specificity of serum IgM is severely restricted to hen egg lysozyme. When female IghelMD4 mice were used as recipients, no rejection of male cells was observed (Figure 2A). Furthermore, this was in striking contrast to activation-induced cytidine deaminase deficient (AID−/−) female recipients, the B cells of which are unable to undergo Ig class switch recombination, and have only serum IgM and lack all other Ig isotypes (59). Here, male cells were completely rejected after adoptive transfer (Figure 2A). Hence, even though all APC subtypes are present in IghelMD4 mice (Figure 2B and Figure E3), male cells escape recognition and elimination. These data demonstrate that a diverse polyclonal IgM, but not IgG, repertoire is required for the rejection of neoantigen-expressing cells. In addition, we further suggest that: 1) natural IgM includes specificities able to recognize neoantigen-expressing cells; and 2) IgM is required to initiate the immune response against neoantigen-expressing cells, as all other cell types, APC subtypes and CD4 and CD8 lymphocytes, are present in IghelMD4 mice.
Figure 2.
Natural IgM antibodies are essential for the initial recognition and elimination of neoantigen-expressing cells. (A) Flow data illustrate three controls: two negative controls (acceptance, male cells into male WT and KO/transgenic [Tg] mice) and one positive control (rejection, male cells into WT female mice). Exp mice are IghelMD4 (hypo-IgM repertoire) AID−/− (hyper-IgM repertoire) female mice. Data represent two independent experiments consisting of n = 3–5 animals per group. Table displays combined experiments with the number of mice that rejected male cells (numerator) over the number of total mice examined (denominator). Acceptance is shown in blue, rejection in red. (B) Lung-draining LN (LLN) flow plots. Top row: live cells plotted as CD11c versus MHCII to illustrate migratory DCs, gated blue. Middle row: migratory DCs are plotted as XCR1 versus CD11b to illustrate the two overarching DC subtypes: XCR1+, CD11blow Batf3+ DCs (red arrow) and CD11b+, Irf4+ DCs. Batf3−/− mice lack Batf3+ DCs, whereas IghelMD4 (Ighel) mice contain Batf3+ DCs. Bottom row: LN cells pregated on low side scatter (SSC), CD64+, CD11b+ cells and plotted as Ly6C versus MHCII to illustrate LN Ly6C+ monocytes. CCR2−/− mice display significantly diminished Ly6C+ monocytes (green arrow), whereas IghelMD4 mice display a normal quantity of LN monocytes.
To further support the concept that natural IgM antibodies are required for neoantigen recognition and elimination, we used a different experimental approach in vivo. This model takes advantage of the fact that OVA antigen alone, although foreign in mice, does not, by itself, elicit an immune response. If OVA-expressing female cells are transferred into syngeneic female mice in the absence of a PAMP or non-PAMP adjuvant, such as Alum, no immune response is elicited against the linked OVA antigen (37, 47). We hypothesized that, if male OVA-expressing cells, compared with female OVA-expressing cells, were transferred to WT female recipients, then the adoptively transferred male cells would form an immune complex, which would be acquired by relevant APCs, leading to the presentation of the linked-antigen, OVA, expressed by the male cell to be presented as an immunogen to endogenous OVA-specific T cells.
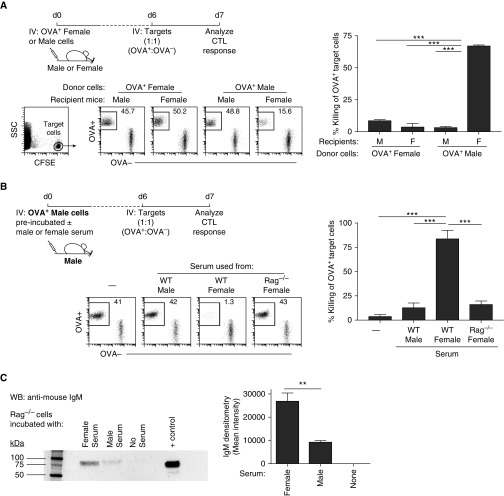
To test this hypothesis, we employed an in vivo CTL killing assay against the linked, nonimmunogenic antigen (i.e., OVA) expressed by the adoptively transferred cells (27, 60). In this experiment (outlined in Figure 3A), OVA-expressing female or male cells from Act-membrane-bound OVA (mOVA) mice were adoptively transferred into female or male mice. After 6 days, CFSE-labeled female target cells, OVA+ (CD45.2) and OVA− (CD45.1) cells, were transferred at a 1:1 ratio into the same hosts. One day after the transfer of CFSE-labeled target cells, an in vivo CTL response against the linked OVA antigen was assessed by measuring the killing of the OVA+ target cells compared with the OVA− target cells (Figure 3A). As anticipated, WT female mice that received OVA-expressing female cells displayed no killing of OVA+ target cells. Similarly, WT male mice that received OVA-expressing male cells displayed no killing of OVA+ target cells (Figure 3A). However, WT female mice that received OVA-expressing male cells subsequently displayed significant cytotoxic activity directed toward OVA+ target cells. This induced CTL activity was supported by the increased reporter expression of IFN-γ from endogenous OVA-specific CD8+ T cells (Figure E4). Furthermore, similar observations were made using GFP-expressing cells (Figure E5). Thus, these data strongly suggest that, in contrast to OVA-expressing female cells, OVA-expressing male cells are acquired in an immunogenic fashion in female mice, which subsequently leads to the presentation of the associated foreign antigen, OVA, as an immunogen instead of a tolerogen.
Figure 3.
Female serum immunoglobulins bind male cells, resulting in its immunogenic uptake and antigen presentation. (A) Top: experimental design: ovalbumin (OVA)-expressing female and male cells were adoptively transferred into female mice; 6 days later, CD45.2 OVA+ and CD45.1 OVA− female target cells were transferred to assess in vivo CTL response against the linked antigen, OVA. Bottom: target cells were plotted as CD45.2 OVA+ versus CD45.1 OVA− cells. Right bar graph displays the percent killing of OVA+ target cells. (B) Top: experimental design (schematic diagram in E6), OVA-expressing male cells were either untreated (−) or preincubated WT male, WT female, or Rag−/− female serum before adoptive transfer into male mice; 6 days later, CD45.2 OVA+ and CD45.1 OVA− female target cells were transferred to assess in vivo CTL response against the linked antigen, OVA. Bottom: target cells were plotted as CD45.2 OVA+ versus CD45.1 OVA− cells. Right bar graph displays the percent killing of OVA+ target cells. (C) Western blot and densitometry analysis of IgM binding on male cells preincubated with female, male, or no serum. Positive control is WT serum. Data are representative of three to five independent experiments. **P < 0.005, ***P < 0.001. CFSE = carboxyfluorescein succinimidyl ester; WB = Western blot.
Using this model, we again addressed the contribution of serum IgM in the rejection of male cells in female hosts by preincubating the OVA-expressing male cells with either WT female or male serum for 45 minutes on ice (Figure 3B). We hypothesized that immunoglobulins in the WT female serum, unlike the WT male serum, would recognize and bind neoantigens expressed on the surface of male cells to form an immune complex. Formation of this cell-associated immune complex would result in the immunogenic uptake of the adoptively transferred OVA-expressing male cells in male mice (see illustrated diagram in Figure E6). To examine the endogenous immune response against the linked antigen, 6 days after adoptive transfer, the same in vivo CTL assay was performed as in Figure 3A. The results from the CTL assay demonstrated that the adoptive transfer of OVA-expressing male cells preincubated with female serum, compared with male serum or no serum, resulted in an immune response against the linked antigen (Figure 3B). These data further strongly suggest that components present in WT female serum, and not in WT male serum, specifically bind to male cells promoting their immunogenicity.
To confirm that antibodies were indeed responsible for this immunogenic observation, the male cells were preincubated with Rag−/− female serum (Rag−/− mice lack mature T and B cells, and thus any serum immunoglobulins). Indeed, OVA-expressing male cells preincubated with Rag−/− female serum displayed no immune response against the linked antigen (Figure 3B), suggesting that the lack of immunoglobulins in the Rag−/− serum resulted in adoptively transferred OVA-expressing male cells to be cleared in a nonimmunogenic fashion. Finally, as direct evidence of natural IgM antibody binding to neoantigen-expressing cells, a Western blot analysis of Rag−/− male cells incubated with either WT male or female serum was assessed. Western blot data showed that there was a significantly higher concentration of IgM bound to the male cells when incubated with WT female serum compared with WT male serum (Figure 3C). In summary, these data, together with results shown in Figure 2A using IghelMD4 Tg and AID−/− mice as hosts, provide strong support that natural IgM antibodies present in WT female serum form a cellular immune complex with male cells, resulting in neoantigen immunogenicity.
Ly6Chi Monocytes Need to Express MHCII to Elicit Rejection of Neoantigen-Expressing Cells
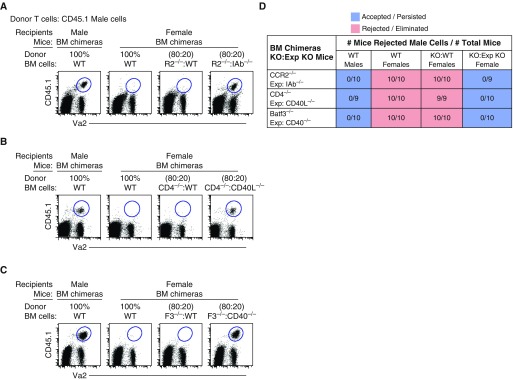
Although, we showed that surveying monocytes and Batf3+ DCs are both important for the elimination of male cells in female mice (Figure 1), we next explored more closely their dominant role in this process of rejection. LN monocytes have been shown to be highly efferocytic, and readily present exogenous antigen to CD4+ T cells (34, 42, 61–63). Therefore, we hypothesized that monocytes are APCs that substantially contribute to neoantigen presentation via MHCII to CD4+ T cells. Therefore, we created bone marrow (BM) chimeric female mice where 100% of tissue and LN monocytes lack MHCII expression, all other leukocytes were approximately 20% MHCII deficient. These mice were referred to as 80:20 BM cells, CCR2−/−:IAb−/− chimeric mice (64). As controls, the chimeric mice contained one negative and two positive controls. The negative control was recipient male mice that received 100% WT male BM cells. The positive controls were recipient female mice that received 100% WT female and 80:20, CCR2−/−:WT female BM cells, of which 100% of tissue and LN monocytes express MHCII. As expected, adoptively transferred male cells in male chimeric mice demonstrated a recall and acceptance response (Figure 4A). In contrast, adoptively transferred male cells in chimeric female mice reconstituted with either 100% WT female or 80:20 CCR2−/−:WT female BM cells resulted in its complete rejection (Figure 4A). However, female chimeric mice that contained 100% MHCII-deficient monocytes did not reject adoptively transferred male cells. Hence, these data suggest that, even when all other APCs express MHCII, such as B cells and DC subtypes, expression of MHCII on LN monocytes is required for and contributes to the development of neoantigen-specific CD4+ T cells.
Figure 4.
Surveying monocytes require MHCII expression to prime CD4+ T helper cells, which then licenses Batf3+ DCs via CD40 to cross-present antigen as an immunogen. Flow data illustrate three controls: one negative control (acceptance, male cells in 100% chimeric WT male mice) and two positive controls (rejection, male cells in 100% chimeric WT female mice and [A] 80:20 bone marrow [BM] cell replenishment of circulating WT monocytes [CCR2−/−:WT], [B] WT CD4+ T cells [CD4−/−:WT] or [C] WT Batf3+ DCs [Batf3−/−:WT]). Exp chimeric female mice consist of 80:20 BM replenishment of 100% circulating (A) MHCII-deficient monocytes (CCR2−/−:IAb−/−) (APC MHCII expression, E6), (B) CD40L-deficient CD4+ T cells (CD4−:CD40L−/−), or (C) CD40-deficient Batf3+ DCs (Batf3−/−:CD40−/−). Data represent two independent experiments consisting of n = 4–5 animals per group. (D) Table displays the combined experiments with the number of mice that rejected male cells (numerator) over the number of total mice examined (denominator). Acceptance is shown in blue, rejection in red.
CD40L–CD40 Cross-Talk between CD4+ T Cells and Batf3+ DCs Is Required for the Rejection of Neoantigen-Expressing Cells
Next, we hypothesized that CD4+ T cells license Batf3+ DCs via CD40 to elicit antigen-specific CTL response. Therefore, we examined the need for CD40L and CD40 interaction and expression on CD4+ T cells and Batf3+ DCs. We created two sets of BM chimeric mice where 100% of CD4+ T cells lacked CD40L and 100% of Batf3+ DCs lacked CD40 (80:20 BM cells, CD4−/−:CD40L−/− and Batf3−/−:CD40−/−); all other leukocytes were only 20% deficient for either CD40L or CD40 (Figures 4B and 4C). As hypothesized, chimeric female mice that contained either 100% CD40L-deficient CD4+ T cells or CD40-deficient Batf3+ DCs displayed no rejection against the adoptively transferred neoantigen-expressing cells (Figures 4B–4D). These data suggest that, even when other APC subtypes express CD40, such as monocytes, Irf4+ DCs, and B cells, Ag-specific CD4+ T cells require the expression of CD40L to activate and license Batf3+ DCs via CD40 to induce Ag-specific CTL response.
Using a Non–H-Y Antigen Model, a Diverse Polyclonal IgM Repertoire and Batf3+ DCs are Required for the Rejection and Elimination of Neoantigen-Expressing Cells
Finally, using a different neoantigen-expressing cell model, independent of H-Y antigen recognition, we examined whether natural IgM antibodies and Batf3+ DCs were required. We crossed 129SvEv mouse with C57BL/6 OT-I mouse and created an F1 129/BL6 OT-I mouse. Although 129SvEv and C57BL/6 mice are MHC matched, they are heavily mismatched outside of the MHC locus, due to multiple allelic variations (65). Hence, if 129SvEv cells are transferred into C57BL/6 mice, 129SvEv cells will be completely rejected, due to the perceived neoantigens (51, 52). Similar to the H-Y model, transferred female 129/BL6 cells into WT female mice resulted in complete rejection of the 129/BL6 cells (Figure 5). However, when female 129/BL6 cells were transferred into IghelMD4 and Batf3−/− female mice, elimination of the 129/BL6 neoantigen-expressing female cells did not occur (Figure 5). In summary, the data demonstrate that both natural IgM antibodies and Batf3+ DCs are required for recognition and elimination of neoantigen-expressing cells.
Figure 5.
129SvEv female cells were not rejected in C57BL/6 female mice lacking a diverse natural IgM antibody repertoire or Batf3+ DCs. Schematic diagram illustrates experimental design. At Day 20, CD45.1 female 129/BL6 cells were recalled in CD45.2 IghelMD4 (Ighel) and Batf3−/− female mice compared with WT female mice, where complete rejection of 129/BL6 cells occurred. Flow plot represents n = 8 mice per group.
A Diverse Polyclonal IgM Repertoire Is Required for the Clearance and Elimination of Urethane-induced Lung Tumors and Metastatic Melanoma Cells
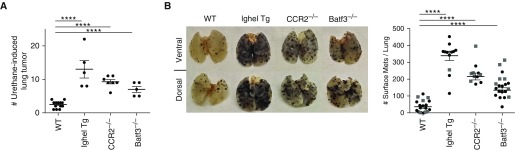
Finally, we set out to examine whether a diverse polyclonal IgM repertoire was also required for antitumor immunity. First, we used the urethane model, which results in a spontaneously occurring, chemically induced lung adenocarcinoma. As anticipated, at 20 weeks after urethane treatment, WT C57BL/6 mice developed roughly one to three tumors per mouse (Figure 6A) (66, 67). Mice known to be susceptible to cancer were also examined: Batf3−/− and CCR2−/− mice, which lack either Batf3+ DCs or Ly6C+ monocytes, respectively. Both strains displayed approximately a three- to fivefold increase in tumor development compared with WT mice (Figure 6A). However, the greatest tumor development was observed in the IghelMD4 mice (lacking a diverse polyclonal IgM repertoire) containing roughly 15 tumors per mouse (Figure 6A). The development of multiple tumors in the IghelMD4 mice was not due to the lack of splenic or LN APC subtypes known to be involved in tumor clearance (i.e., Batf3+ DCs and LN monocytes; Figure 2B). Furthermore, in a second tumor model, injection of B16F10 melanoma cells, which rapidly results in metastatic melanoma in the lungs of mice after 16 days, showed a similar effect as in the urethane model (Figure 6B). The greatest number of metastatic melanoma nodules was observed in IghelMD4 mice compared with mice lacking either Batf3+ DC or Ly6C+ monocytes, and WT controls (Figure 6B). Overall, these data suggest that, even when all APC subtypes are present, a diverse IgM repertoire is required for antitumor immunity through the initial recognition of neoantigen-expressing tumors and activation of APCs.
Figure 6.
A complete natural IgM antibody repertoire is required for antitumor immunity. (A) The scatter plot displays the number of urethane-induced tumors observed per mouse in WT, IghelMD4 Tg, CCR2−/−, and Batf3−/− mice after 6 months post–urethane treatment. (B) At 16 days after intravenous injection of B16F10, melanoma cell line in WT, IghelMD4 Tg, CCR2−/−, and Batf3−/− mice was examined for melanoma tumor development. Left: pictures illustrate metastatic melanoma on the surface of whole lungs. Right: scatter plot displays the number of surface metastatic tumors developed per mouse. Circles indicate pure KO mouse, and squares indicate WT recipient mice reconstituted with 100% BM from the indicated KO mouse. Data combine three to five independent experiments. ****P < 0.0001.
Discussion
The immune system has evolved to recognize invading pathogens, but understanding how it recognizes and mounts a coordinated immune response against naturally occurring alterations of self-antigens during mutagenesis is ongoing (68, 69). Most cells undergo transcriptional and translational mutations approximately 1,000–10,000 times per day (69), and even more if a mutation successfully causes the cells to escape intrinsic repair mechanisms, in which case an intact immune system can suppress this escape, known as extrinsic tumor suppressor mechanisms. Therefore, after an abnormal cell escapes intrinsic tumor suppressor mechanisms, it is imperative to have this second line of defense that rapidly recognizes these abnormal cells.
Our findings suggest that natural IgM is a key player in neoantigen recognition and part of the “second-line” immune surveillance. Specifically, we demonstrated that natural IgM antibodies recognize neoantigens expressed on the surface of the adoptively transferred neoantigen-expressing cells, leading to the formation of an immune complex, which initiates the immune response against neoantigen-expressing cells. In our reductionist models, using either mice deficient for an immune cell type or chimeric mice lacking a required molecule that mediates adaptive immunity (i.e., CD40L on CD4+ T cells, MHCII on Ly6C+ monocytes, or CD40 on Batf3+ DCs), we demonstrated that there was virtually no redundancy in the role individual APC subtypes play in coordinating the rejection of neoantigen-expressing cells. Thus, we provide a holistic view of the dependency of each APC subtype and how they contribute to an immune response against neoantigen-expressing cells.
Initially, we showed that male cells escape recognition and elimination in syngeneic female mice when these mice lack either immune cell subtypes: B cells, CD4 T cells, CD8 T cells, or APC subtypes. Moreover, we focused on mice that were either hypo- or hyper-IgM, and demonstrated that mice lacking a diverse IgM repertoire demonstrated the escape of neoantigen-expressing cells. We also performed ex vivo experiments demonstrating directly the binding of IgM on male cells with female serum. Finally, we extended our findings to two cancer models and tested the hypothesis outlined in Figure 1A. In the absence of any one critical part within the immune cascade we identified—a diverse IgM repertoire, LN monocytes, or Batf3+ DCs—precancerous cells escaped and proliferated, resulting in the development of tumors with greater numbers over WT mice containing an intact immune system. The worst tumor growth was observed in mice lacking a diverse natural IgM repertoire (Figure 6).
Since the most striking data observed in tumor development was with the IghelMD4 mice, this leads us to hypothesize, and examine in the future, the following concept during the elimination phase of extrinsic tumor suppressor mechanisms. Similar to what is observed with invading pathogens, there are two lines of defense during the early elimination phase of abnormal cells, which have recently escaped intrinsic tumor suppressor mechanisms. The first line of defense would be the tagging of neoantigen-expressing cells with natural IgM antibodies for clearance by innate immune cells already present within the tissue, including interstitial macrophages and surveying monocytes. If this clearance fails, then IgM helps initiate a second line of defense based on an adaptive immune response. Here, LN-trafficking monocytes and Batf3+ DCs play a major role. Consistent with this hypothesis, when mice lack natural IgM, both the innate and the adaptive immune response is eliminated. This is likely why we observe the most extensive tumor development in natural IgM repertoire deficient mice compared with mice in which only the adaptive arm is compromised, such as in the Batf3+ DC–deficient mice.
Although our findings provide a scaffold to work from, there are most likely a number of mechanisms in the early stages of recognition not examined here. For example, once natural IgM binds to neoantigen and forms an immune complex, the activation of the classical complement cascade might be expected. Hence, the role of cell types known to secrete the first components of the complement cascade (e.g., interstitial macrophages) (70–74) are also likely involved in this process, along with receptors known to mediate this recognition. Clearly, future studies highlighting the initial recognition of neoantigens are needed to enhance our understanding of the endogenous processes and additional mechanisms involved in the early immune detection and elimination stage of neoantigen-expressing cells.
Footnotes
This work was supported by National Institutes of Health grants R01 HL115334 and R01 HL135001 (C.V.J.), R01 HL114381 (P.M.H.), and R01 AI052157 (R.M.T.), and by Department of Veterans Affairs Career Development Award 1IK2BX002401-01A2 (E.F.R.).
Author Contributions: S.M.A., R.M.T., P.M.H., and C.V.J. prepared the manuscript; S.M.A., S.L.G., E.F.R., and F.A.C. executed the experiments; all authors provided intellectual input, critical feedback, discussed results, and designed experiments.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0159OC on June 28, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 3.Dighiero G, Lymberi P, Holmberg D, Lundquist I, Coutinho A, Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985;134:765–771. [PubMed] [Google Scholar]
- 4.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–818. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 5.Mouthon L, Nobrega A, Nicolas N, Kaveri SV, Barreau C, Coutinho A, et al. Invariance and restriction toward a limited set of self-antigens characterize neonatal IgM antibody repertoires and prevail in autoreactive repertoires of healthy adults. Proc Natl Acad Sci USA. 1995;92:3839–3843. doi: 10.1073/pnas.92.9.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merbl Y, Zucker-Toledano M, Quintana FJ, Cohen IR. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest. 2007;117:712–718. doi: 10.1172/JCI29943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 2008;4:491–498. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto K, Handa H, Umehara K, Sasaki S. Germfree mice reared on an “antigen-free” diet. Lab Anim Sci. 1978;28:38–45. [PubMed] [Google Scholar]
- 9.Haury M, Sundblad A, Grandien A, Barreau C, Coutinho A, Nobrega A. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur J Immunol. 1997;27:1557–1563. doi: 10.1002/eji.1830270635. [DOI] [PubMed] [Google Scholar]
- 10.Briles DE, Nahm M, Schroer K, Davie J, Baker P, Kearney J, et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 13.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci USA. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajan B, Ramalingam T, Rajan TV. Critical role for IgM in host protection in experimental filarial infection. J Immunol. 2005;175:1827–1833. doi: 10.4049/jimmunol.175.3.1827. [DOI] [PubMed] [Google Scholar]
- 15.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81:3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou ZH, Zhang Y, Hu YF, Wahl LM, Cisar JO, Notkins AL. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe. 2007;1:51–61. doi: 10.1016/j.chom.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramaniam KS, Datta K, Quintero E, Manix C, Marks MS, Pirofski LA. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J Immunol. 2010;184:5755–5767. doi: 10.4049/jimmunol.0901638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 19.Grönwall C, Silverman GJ. Natural IgM: beneficial autoantibodies for the control of inflammatory and autoimmune disease. J Clin Immunol. 2014;34:S12–S21. doi: 10.1007/s10875-014-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creaney J, Ma S, Sneddon SA, Tourigny MR, Dick IM, Leon JS, et al. Strong spontaneous tumor neoantigen responses induced by a natural human carcinogen. OncoImmunology. 2015;4:e1011492. doi: 10.1080/2162402X.2015.1011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Díaz-Zaragoza M, Hernández-Ávila R, Ostoa-Saloma P. Recognition of tumor antigens in 4T1 cells by natural IgM from three strains of mice with different susceptibilities to spontaneous breast cancer. Oncol Lett. 2017;13:271–274. doi: 10.3892/ol.2016.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Díaz-Zaragoza M, Hernández-Ávila R, Viedma-Rodríguez R, Arenas-Aranda D, Ostoa-Saloma P. Natural and adaptive IgM antibodies in the recognition of tumor-associated antigens of breast cancer (Review) Oncol Rep. 2015;34:1106–1114. doi: 10.3892/or.2015.4095. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohn J, Roggenbuck D, Settmacher U, Döcke W, Volk HD, Von Baehr R, et al. Binding of natural human IgM auto-antibodies to human tumor cell lines and stimulated normal T lymphocytes. Immunol Lett. 1994;39:187–194. doi: 10.1016/0165-2478(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 24.Kumar R, Tuen M, Liu J, Nàdas A, Pan R, Kong X, et al. Elicitation of broadly reactive antibodies against glycan-modulated neutralizing V3 epitopes of HIV-1 by immune complex vaccines. Vaccine. 2013;31:5413–5421. doi: 10.1016/j.vaccine.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osato K. Antigen–antibody complexes in the immune response. I. Analysis of the effectiveness of complexes on the primary antibody response. Immunology. 1972;23:545–557. [PMC free article] [PubMed] [Google Scholar]
- 26.Hamaoka T, Kitagawa M. Antibody production in mice. II. The mechanism of antigenic stimulation in the secondary immune response. Immunology. 1971;20:191–203. [PMC free article] [PubMed] [Google Scholar]
- 27.Gavin MA, Dere B, Grandea AG, III, Hogquist KA, Bevan MJ. Major histocompatibility complex class I allele–specific peptide libraries: identification of peptides that mimic an H-Y T cell epitope. Eur J Immunol. 1994;24:2124–2133. doi: 10.1002/eji.1830240929. [DOI] [PubMed] [Google Scholar]
- 28.Bevan MJ. Priming for a cytotoxic response to minor histocompatibility antigens: antigen specificity and failure to demonstrate a carrier effect. J Immunol. 1977;118:1370–1374. [PubMed] [Google Scholar]
- 29.Plantinga M, Hammad H, Lambrecht BN. Origin and functional specializations of DC subsets in the lung. Eur J Immunol. 2010;40:2112–2118. doi: 10.1002/eji.201040562. [DOI] [PubMed] [Google Scholar]
- 30.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O’Shea JJ, et al. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174:2573–2581. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 33.Quantz MA, Bennett LE, Meyer DM, Novick RJ. Does human leukocyte antigen matching influence the outcome of lung transplantation? An analysis of 3,549 lung transplantations. J Heart Lung Transplant. 2000;19:473–479. doi: 10.1016/s1053-2498(00)00081-4. [DOI] [PubMed] [Google Scholar]
- 34.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 36.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desch AN, Randolph GJ, Murphy K, Gautier EL, Kedl RM, Lahoud MH, et al. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med. 2011;208:1789–1797. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim TS, Gorski SA, Hahn S, Murphy KM, Braciale TJ. Distinct dendritic cell subsets dictate the fate decision between effector and memory CD8(+) T cell differentiation by a CD24-dependent mechanism. Immunity. 2014;40:400–413. doi: 10.1016/j.immuni.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iborra S, Martínez-López M, Khouili SC, Enamorado M, Cueto FJ, Conde-Garrosa R, et al. Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires crosspriming by DNGR-1+ dendritic cells. Immunity. 2016;45:847–860. doi: 10.1016/j.immuni.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Förster R. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol. 2007;178:6861–6866. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 41.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 42.Larson SR, Atif SM, Gibbings SL, Thomas SM, Prabagar MG, Danhorn T, et al. Ly6C(+) monocyte efferocytosis and cross-presentation of cell-associated antigens. Cell Death Differ. 2016;23:997–1003. doi: 10.1038/cdd.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Contreras V, Urien C, Guiton R, Alexandre Y, Vu Manh TP, Andrieu T, et al. Existence of CD8α-like dendritic cells with a conserved functional specialization and a common molecular signature in distant mammalian species. J Immunol. 2010;185:3313–3325. doi: 10.4049/jimmunol.1000824. [DOI] [PubMed] [Google Scholar]
- 44.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 46.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desch AN, Gibbings SL, Clambey ET, Janssen WJ, Slansky JE, Kedl RM, et al. Dendritic cell subsets require cis-activation for cytotoxic CD8 T-cell induction. Nat Commun. 2014;5:4674. doi: 10.1038/ncomms5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kratky W, Reis e Sousa C, Oxenius A, Spörri R. Direct activation of antigen-presenting cells is required for CD8+ T-cell priming and tumor vaccination. Proc Natl Acad Sci USA. 2011;108:17414–17419. doi: 10.1073/pnas.1108945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spörri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 50.Atif SM, Nelsen MK, Gibbings SL, Desch AN, Kedl RM, Gill RG, et al. Cutting edge: roles for Batf3-dependent APCs in the rejection of minor histocompatibility antigen-mismatched grafts. J Immunol. 2015;195:46–50. doi: 10.4049/jimmunol.1500669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malarkannan S, Horng T, Eden P, Gonzalez F, Shih P, Brouwenstijn N, et al. Differences that matter: major cytotoxic T cell–stimulating minor histocompatibility antigens. Immunity. 2000;13:333–344. doi: 10.1016/s1074-7613(00)00033-9. [DOI] [PubMed] [Google Scholar]
- 52.Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 53.Ashman RB. Primary immune responses to H-Y in BALB/c-H-2k mice. Immunogenetics. 1983;18:125–129. doi: 10.1007/BF00368540. [DOI] [PubMed] [Google Scholar]
- 54.Goldberg EH, Shen FW, Tokuda S. Detection of H-Y (male) antigen on mouse lymph node cells by the cell to cell cytotoxicity test. Transplantation. 1973;15:334–336. doi: 10.1097/00007890-197303000-00013. [DOI] [PubMed] [Google Scholar]
- 55.Gordon RD, Mathieson BJ, Samelson LE, Boyse EA, Simpson E. The effect of allogeneic presensitization on H-Y graft survival and in vitro cell–mediated responses to H-y antigen. J Exp Med. 1976;144:810–820. doi: 10.1084/jem.144.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon RD, Simpson E. Immune-response gene control of cytotoxic T-cell responses to H-Y. Transplant Proc. 1977;9:885–888. [PubMed] [Google Scholar]
- 57.Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen, H-Y: a history of transplantation, immune response genes, sex determination and expression cloning. Annu Rev Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- 58.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 59.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 60.Tyznik AJ, Bevan MJ. The surprising kinetics of the T cell response to live antigenic cells. J Immunol. 2007;179:4988–4995. doi: 10.4049/jimmunol.179.8.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell–mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 62.Kool M, Soullié T, van Nimwegen M, Willart MA, Muskens F, Jung S, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langlet C, Tamoutounour S, Henri S, Luche H, Ardouin L, Grégoire C, et al. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol. 2012;188:1751–1760. doi: 10.4049/jimmunol.1102744. [DOI] [PubMed] [Google Scholar]
- 64.Chakarov S, Fazilleau N. Monocyte-derived dendritic cells promote T follicular helper cell differentiation. EMBO Mol Med. 2014;6:590–603. doi: 10.1002/emmm.201403841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Threadgill DW, Yee D, Matin A, Nadeau JH, Magnuson T. Genealogy of the 129 inbred strains: 129/SvJ is a contaminated inbred strain. Mamm Genome. 1997;8:390–393. doi: 10.1007/s003359900453. [DOI] [PubMed] [Google Scholar]
- 66.Dwyer-Nield LD, McQuillan J, Hill-Baskin A, Radcliffe RA, You M, Nadeau JH, et al. Epistatic interactions govern chemically-induced lung tumor susceptibility and Kras mutation site in murine C57BL/6J-ChrA/J chromosome substitution strains. Int J Cancer. 2010;126:125–132. doi: 10.1002/ijc.24743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Redente EF, Orlicky DJ, Bouchard RJ, Malkinson AM. Tumor signaling to the bone marrow changes the phenotype of monocytes and pulmonary macrophages during urethane-induced primary lung tumorigenesis in A/J mice. Am J Pathol. 2007;170:693–708. doi: 10.2353/ajpath.2007.060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hambach L, Goulmy E. Immunotherapy of cancer through targeting of minor histocompatibility antigens. Curr Opin Immunol. 2005;17:202–210. doi: 10.1016/j.coi.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 69.Brégeon D, Doetsch PW. Transcriptional mutagenesis: causes and involvement in tumour development. Nat Rev Cancer. 2011;11:218–227. doi: 10.1038/nrc3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gibbings SL, Thomas SM, Atif SM, McCubbrey AL, Desch AN, Danhorn T, et al. Three unique interstitial macrophages in the murine lung at steady state. Am J Respir Cell Mol Biol. 2017;57:66–76. doi: 10.1165/rcmb.2016-0361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ensan S, Li A, Besla R, Degousee N, Cosme J, Roufaiel M, et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat Immunol. 2016;17:159–168. doi: 10.1038/ni.3343. [DOI] [PubMed] [Google Scholar]
- 72.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bain CC, Hawley CA, Garner H, Scott CL, Schridde A, Steers NJ, et al. Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat Commun. 2016;7:ncomms11852. doi: 10.1038/ncomms11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]