Abstract
IL-8–dependent inflammation is a hallmark of host lung innate immunity to bacterial pathogens, yet in many human lung diseases, including chronic obstructive pulmonary disease, bronchiectasis, and pulmonary fibrosis, there are progressive, irreversible, pathological changes associated with elevated levels of IL-8 in the lung. To better understand the duality of IL-8–dependent host immunity to bacterial infection and lung pathology, we expressed human IL-8 transgenically in murine bronchial epithelium, and investigated the impact of overexpression on lung bacterial clearance, host immunity, and lung pathology and function. Persistent IL-8 expression in bronchial epithelium resulted in neutrophilia, neutrophil maturation and activation, and chemotaxis. There was enhanced protection against challenge with Pseudomonas aeruginosa, and significant changes in baseline expression of innate and adaptive immunity transcripts for Ccl5, Tlr6, IL-2, and Tlr1. There was increased expression of Tbet and Foxp3 in response to the Pseudomonas antigen OprF, indicating a regulatory T-cell phenotype. However, this enhanced bacterial immunity came at a high price of progressive lung remodeling, with increased inflammation, mucus hypersecretion, and fibrosis. There was increased expression of Ccl3 and reduced expression of Claudin 18 and F11r, with damage to epithelial organization leading to leaky tight junctions, all of which resulted in impaired lung function with reduced compliance, increased resistance, and bronchial hyperreactivity as measured by whole-body plethysmography. These results show that IL-8 overexpression in the bronchial epithelium benefits lung immunity to bacterial infection, but specifically drives lung damage through persistent inflammation, lung remodeling, and damaged tight junctions, leading to impaired lung function.
Keywords: bacterial infection, host immunity, IL-8, lung remodeling, tight junction
Clinical Relevance
Long-standing lung expression of IL-8 contributes to a double-edged sword of the inflammatory response in several lung diseases, including chronic obstructive pulmonary disease, bronchiectasis, and cystic fibrosis. The IL-8–driven mechanisms underlying these pathologies are poorly understood. The impact of IL-8 on host immunity to bacterial infection and pathogenesis in the lung was modeled through lung-targeted transgenic expression of human IL-8. Lung-targeted IL-8 results in enhanced protection from bacterial infection in the lung and drives downstream changes, including inflammation, mucus hypersecretion, lung remodeling, and fibrosis, with damaged, leaky tight junctions and reduced lung function. Thus, IL-8–mediated enhanced microbial immunity comes at a high price of progressive lung damage and reduced lung integrity.
IL-8 (CXCL8) is a CXC chemokine neutrophil chemoattractant that plays a central role in innate immunity to bacterial infection (1, 2), as evidenced by increased susceptibility to infection in defective IL-8 receptor binding (3). Elevated IL-8 is found in the BAL, sputum, and lung tissue in various chronic lung diseases, including chronic obstructive pulmonary disease (COPD), bronchiectasis, cystic fibrosis (CF), idiopathic pulmonary fibrosis, sarcoidosis, acute respiratory distress syndrome, and asthma (4–8). Several of these lung diseases are associated with polymorphisms in the IL-8 gene or its receptors (9–11). Furthermore, increased bronchial IL-8 and neutrophilia contribute to the inflammatory response to exposure to diesel exhaust particles (12).
Generally, elevated levels of IL-8 are associated with neutrophilia, the presence of which acutely or chronically may contribute to significant and often irreversible lung tissue damage (13). However, in addition to persistent neutrophilic inflammation, excessive mucus production, fibrosis, and lung tissue remodeling are also common features of many of these diseases. Several key molecules that mediate lung fibrosis also regulate IL-8 expression (14).
IL-8’s biological activities are mediated through binding to the G protein–coupled receptors CXCR1 and CXCR2 (15), which are found on different types of immune cells, including neutrophils, monocytes, and natural killer cells, as well as on smooth-muscle and endothelial cells (16–18). IL-8 receptor ligation activates neutrophil effector mechanisms such as degranulation and respiratory burst (19), and neutrophil-independent actions such as airway smooth-muscle migration and function (16, 17, 20). In bleomycin-induced fibrosis, CXCR2 receptor blockade reduces fibrosis and collagen deposition through a neutrophil-independent mechanism (21). Furthermore, CXCR2 receptor antagonists have undergone trials in bacterial challenge models and in clinical settings of lung inflammation, including bronchiectasis (22, 23).
IL-8 can be produced by diverse cell types, including myeloid cells (19). To interrogate mechanisms underpinning the protective and pathogenic roles for persistent IL-8 in lung disease, we generated transgenic mice constitutively expressing human IL-8 (hIL-8) under control of a bronchial epithelial cell–specific promoter. Mice lack the gene for IL-8, but the CXCR2 receptor is expressed and can mediate neutrophil chemotaxis upon binding the mouse proteins keratinocyte chemoattractant (KC) and macrophage-inflammatory protein 2 (MIP-2), and murine CXCR2 can also bind hIL-8 (24, 25). Murine KC is not a direct homolog of hIL-8 (26). Here, we report the characterization of lung pathology in hIL-8 transgenic mice and show that IL-8 expression in the mouse lung mediates neutrophilia, with enhanced neutrophil maturation, activation, and chemotaxis; protection against Pseudomonas challenge; and changes in innate and adaptive immunity transcripts. At the same time, it causes lung remodeling, with inflammation, mucus hypersecretion, fibrosis, and leaky tight junctions, all of which result in impaired lung function. This offers a new model for the study of chronic lung disease.
Methods
The experimental protocols used in this work are described in detail in the data supplement.
Mice
Lung-targeted hIL-8 transgenics were generated using a construct carrying hIL-8, subcloned into a pBluescript II vector downstream of the CC10 promoter and upstream of the rabbit β-globin-poly(A) sequence (Figure E1A in the data supplement). The transgenic founder was backcrossed onto C57BL/6. Mouse experiments were performed in accordance with UK Home Office legislation under project license PPL 70/7708.
RT-PCR and Real-Time PCR analysis
RNA was extracted from tissue and cDNA was reverse transcribed using SuperScript III.
PCR array
cDNA samples were run on murine innate and adaptive immune response (PAMM-052Z), murine fibrosis (PAMM-120A), or murine tight junction (PAMM-143Z) RT2 Profiler PCR array plates (Qiagen UK) on a Stratagene Mx3000p RT-PCR machine. Data were analyzed using Partek Genomics Suite version 6.6 (Partek).
BAL and Lung Tissue Preparation
BAL fluid and lung tissue were harvested. Snap-frozen lung samples were prepared for ELISA by homogenization. The lung tissue was disaggregated. BAL and lung cells were stained by Wright-Giemsa for differential cell counting.
ELISA
Paired antibodies were used for cytokine ELISAs. Albumin concentrations in BAL fluid were determined by ELISA.
Immunohistochemistry
Immunohistochemical staining was performed on wax-embedded lung sections using primary antibodies in combination with the appropriate biotinylated secondary antibodies. Measurements of the smooth-muscle diameter around the bronchioles and luminal area on smooth muscle actin (SMA)-stained lung sections were performed. Immunofluorescence staining of Claudin 18 and hIL-8 proteins was done with the use of rabbit anti-mouse Claudin 18 and goat anti–hIL-8, in combination with donkey anti-rabbit Alexa Fluor 546 and donkey anti-goat Alexa Fluor 680. Epithelial/tight-junction damage was scored.
Histological Scoring of Lung Inflammation and Fibrosis
Sections were stained with hematoxylin and eosin, periodic acid–Schiff, or Masson’s Trichrome.
Neutrophil Chemotaxis
Lung tissue was disaggregated and cells were resuspended for chemotaxis assays. Plates were incubated for 2.5 hours and the number of migrated cells was quantitated.
Flow Cytometry
Neutrophil oxidative burst assays with dihydrorhodamine 123 were performed using peripheral blood mononuclear cells and lung neutrophils.
Pseudomonas aeruginosa Infection
Mice were infected intranasally with 2 × 106 cfu P. aeruginosa (Xen41) and culled for analysis at predefined experimental endpoints. The relative quantity of P. aeruginosa in lung tissue was determined using primers specific for the ecfX gene (27).
T-Cell Assay
Mice were immunized with 25 μg of outer membrane porin F (OprF) in TiterMax Gold adjuvant. On Day 10, draining lymph node cells were harvested for ELISpot or short-term culture with OprF antigen. T-cell antigen responses were quantified by IFN-γ ELISpot.
Measurement of Airway Resistance and Compliance
Mice were anesthetized and the trachea was cannulated. Resistance and compliance measurements were taken in an artificial ventilator in response to PBS and increasing doses of methacholine.
Measurement of Bronchial Hyperreactivity
Bronchial hyperreactivity was measured by recording respiratory pressure curves via whole-body plethysmography.
Isolation of Airway Smooth-Muscle Cells and Ca2+ Flux Assays
Smooth-muscle cells isolated from lung tissue were incubated with a Fluo-4 dye before they were stimulated by the rapid addition of calcium ionophore. Baseline measurements were taken and data were acquired for at least 5 minutes after stimulation by continuous measurement using a FACSCalibur (BD Biosciences).
Results
hIL-8 Expression in the Lung Promotes Neutrophilia in Transgenic Mice
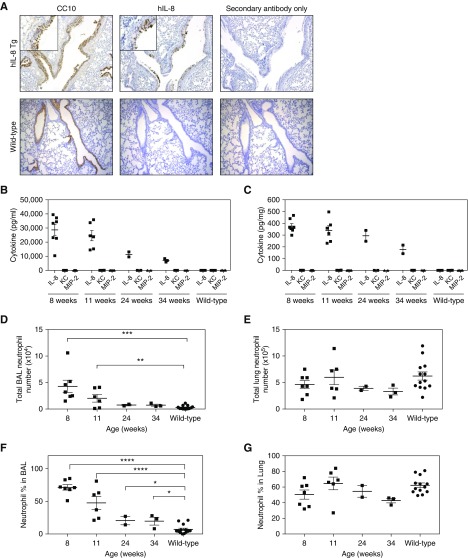
We generated transgenic mice expressing hIL-8 under control of the bronchial epithelial cell–specific promoter CC10 (Figure E1A). The mice showed hIL-8 transcription limited to the lung, with minor transcription in the brain (Figure E1B). Immunocytochemistry of lung tissue with antibodies specific for CC10 and hIL-8 showed positive staining limited to bronchial epithelial cells, with no hIL-8 expression in alveolar or vascular structures of the lung (Figure 1A). hIL-8 protein was detectable in BAL (Figure 1B), lung homogenate (Figure 1C), and serum (Figure E2A) of hIL-8 transgenic mice. There was no significant production of the murine orthologs KC and MIP-2. The amount of IL-8 protein produced decreased with increasing age, as did hIL-8 transcription in the lung (Figures E2B and E3A–E3C). In keeping with the function of hIL-8 as a neutrophil chemoattractant (1, 2), transgenic mice had increased numbers (Figure 1D) and percentages (Figure 1F) of neutrophils in BAL. This was not seen in the lung parenchyma (Figures 1E and 1G), presumably because neutrophils recruited to lung tissue move into BAL in response to hIL-8 secretion by the bronchial epithelium.
Figure 1.
Expression of human IL-8 (hIL-8) in transgenic (Tg) mice is localized to the bronchial epithelium and is associated with recruitment of neutrophils into the airways. (A) Localization of hIL-8 protein in the bronchial epithelium of the lung was established by immunohistochemical staining for hIL-8 and Clara cell 10-kD protein (CC10) in consecutive paraffin-embedded lung sections (×20 magnification). (B and C) Levels of hIL-8 protein and the murine orthologs keratinocyte cytokine (KC) and macrophage‐inflammatory protein‐2 (MIP‐2) were determined by ELISA in (B) BAL fluid and (C) homogenized lung samples of hIL-8 Tg mice (8 weeks [n = 7], 11 weeks [n = 6], 24 weeks [n = 2], and 34 weeks [n = 3] old) and in a cohort of wild-type mice (11–34 weeks old, n = 13). (D–G) The total numbers of neutrophils and the neutrophil percentage in BAL (D and F) and lung (E and G) were determined by differential cell counting of Wright-Giemsa–stained cytospins. Error bars represent mean ± SEM. Statistical significance was determined using an unpaired t test (*P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.0001).
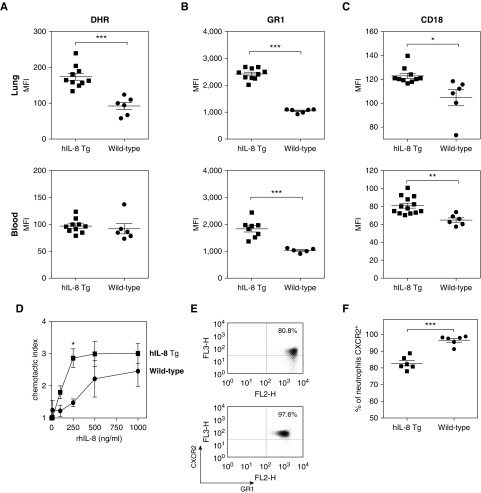
Having established that lung-targeted expression of hIL-8 results in neutrophilia, we next investigated the activation status and maturation of neutrophils in the lungs and periphery. Upon neutrophil activation, the cell adhesion molecule CD18 is upregulated (28), and degranulation and respiratory burst processes are initiated. H2O2 is a major product of the neutrophil respiratory burst (29). Neutrophils from the lung tissue of hIL-8 transgenics (but not from peripheral blood) show significantly enhanced production of H2O2 (Figure 2A). Expression of the maturation marker Gr-1 and activation marker CD18 was increased on lung and peripheral blood neutrophils from transgenic mice (Figures 2B and 2C). The chemotactic response of lung neutrophils from hIL-8 transgenics to a gradient of recombinant hIL-8 in vitro was significantly increased compared with that of neutrophils from wild-type animals (Figure 2D). hIL-8 binds and signals through the CXCR2 receptor on neutrophils, resulting in internalization of the receptor (30). Lower expression levels of the CXCR2 receptor were seen on neutrophils from hIL-8 transgenics (Figures 2E and 2F).
Figure 2.
Neutrophils in the lungs of hIL-8 Tg mice are more mature, more activated, and more chemotactic to rhIL-8. (A) Nicotinamide adenine dinucleotide phosphate oxidase activity in neutrophils from the lung and peripheral blood of hIL-8 Tg (n = 10, squares) and wild-type (n = 6, circles) mice, 15–18 weeks old, was measured using a dihydrorhodamine (DHR) assay. (B and C) Expression of the neutrophil maturation marker lymphocyte antigen 6 complex G/C (Gr-1) (B) and the neutrophil activation marker CD18 (C) was measured by flow cytometry. (D) Lung neutrophils from hIL-8 Tg mice (n = 5, squares) showed an enhanced chemotactic response to rhIL-8 in vitro compared with cells from wild-type animals (n = 4, circles) (mice 26–37 weeks old). (E and F) Flow cytometry showed cell-surface expression of the hIL-8 receptor CXCR2 on lung neutrophils from hIL-8 Tg mice (n = 6, squares) compared with wild-type mice (n = 6, circles) (mice 9–15 weeks old). Error bars represent mean ± SEM. Statistical significance was determined using an unpaired t test (*P < 0.05, **P < 0.005, ***P < 0.0005). FL2 = fluorescence detector 2; MFI = mean fluorescence intensity; rhIL-8 = recombinant human IL-8.
hIL-8 Transgenics Have Improved Survival after Lung Infection with P. aeruginosa
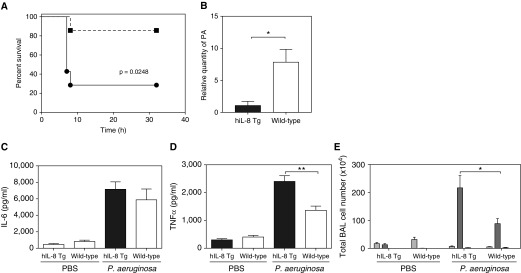
A preactivated respiratory niche of mature, activated neutrophils might be considered advantageous in the host defense against microbial pathogens. This was investigated in a model of acute lung bacterial infection by P. aeruginosa (Figure 3). hIL-8 transgenics showed significantly improved protection against acute challenge with 2 × 106 cfu P. aeruginosa (Figure 3A). Seventy percent of the wild-type mice reached the humane endpoint within 7 hours of infection, compared with just 15% of the hIL-8 transgenic group (P = 0.0248). P. aeruginosa in lung tissue was quantified 7 hours after infection in a separate cohort of mice, and bacterial load was found to be significantly reduced in the hIL-8 transgenics at this time point (Figure 3B). No difference in IL-6 was seen between the two groups (Figure 3C), but protection was associated with an enhanced TNF-α response and neutrophil infiltration (Figures 3D and 3E).
Figure 3.
Lung expression of hIL-8 in Tg mice confers protection from infection with Pseudomonas aeruginosa (PA). (A) hIL-8 Tg (squares) and wild-type control (circles) mice (8–10 weeks old, n = 7 per group) were infected intranasally with 2 × 106 cfu of PA and disease severity was monitored over a 32-hour period. (B) A separate cohort of 13- to 14-week-old mice (n = 5 per group) were killed at 6 hours after infection and the relative quantity of PA present in the lung tissue was determined by real-time PCR. (C and D) Eight-week-old hIL-8 Tg (black bars) and wild-type (white bars) mice were given 2 × 106 cfu of PA (n = 6 per group) or PBS (n = 5 per group) intranasally and culled at 6 hours after infection. (C) TNF-α and (D) IL-6 concentrations in BAL fluid were determined by ELISA. (E) Total BAL inflammatory cell counts were determined using Wright-Giemsa–stained cytospins. The numbers of macrophages (light gray bars), neutrophils (gray bars), and lymphocytes (dark gray bars) are shown. Error bars represent mean ± SEM. Statistical significance was determined using the log-rank (Mantel-Cox) test for disease severity curves, and an unpaired t test for all other data (*P < 0.05, **P < 0.005).
Chronic hIL-8 Expression Impacts Innate and Adaptive Arms of the Immune System
Thus far, hIL-8 expression in transgenic mice had been shown to have a pronounced effect on innate, neutrophilic immune pathways at the site of hIL-8 expression and to some extent in the periphery. However, the innate and adaptive arms of the immune system are rarely activated in isolation. A PCR array of relevant genes was analyzed in lung tissue from (uninfected) hIL-8 transgenic and wild-type mice (Figure 4A and Table E1). The transgenic mice showed enhanced transcription of genes involved in chemoattraction and recognition of bacterial lipoproteins such as Ccl5 and Tlr6. In addition, genes associated with adaptive immunity, particularly T-helper cell type 1 (Th1) responses, such as Il2, Tbx21, and Ifng, were also upregulated. Tlr1 was downregulated.
Figure 4.
Expression of hIL-8 in the lung is associated with transcriptional changes in adaptive and innate immunity–related genes. (A) Lung RNA was isolated from hIL-8 Tg (n = 5) and wild-type (n = 5) mice, 20 weeks of age. An SA Biosciences innate and adaptive immune system PCR array was run for all samples. A heat map shows gene expression that was statistically different (P < 0.05) between the two groups with a fold change of ±1.2 and above. A color scale is used to represent a fold increase (red) and a fold decrease (blue) in gene expression. Statistical significance was determined using a one-way ANOVA. Data shown are all genes that were statistically different with a fold change greater than 1.2 and P < 0.05. To interrogate differences in adaptive immune responses between Tg and wild-type mice, 8- to 9-week-old hIL-8 Tg (n = 12, squares) and wild-type (n = 5, circles) mice were primed subcutaneously in the footpad with P. aeruginosa antigen outer membrane porin F (OprF) in combination with TiterMax adjuvant. Ten days after immunization, the draining popliteal lymph node (DLN) and spleen were removed. (B) hIL-8 Tg (squares) and wild-type (circles) CD4+ T cell responses to OprF from both DLN (i) and spleen (ii) were assayed by IFN-γ ELISpot. (C–J) DLN and spleen cells from hIL-8 Tg (solid bars) and wild-type (open bars) mice were additionally cultured with 25 μg/ml of OprF for 3 days before cell lysis for real-time PCR analysis of Tbet (C), Foxp3 (D), Gata3 (E), RORγt (F), Ifng (G), IL-10 (H), Tgfb1 (I), and IL-17A (J) transcripts. Error bars represent mean ± SEM. Statistical significance was determined using an unpaired t test (*P < 0.05, **P < 0.005). SFC = spot-forming cells.
To investigate T-cell responses to bacterial antigen in hIL-8 transgenics, mice were immunized with the P. aeruginosa protein OprF. In recall responses to antigen, the number of IFN-γ–producing cells in the draining lymph node was equivalent between transgenics and wild-types (Figures 4B and 4G). Tbet expression was greatly enhanced (Figure 4C). This may be explained by the concomitant upregulation of other genes associated with a more regulatory phenotype in Tbet+ regulatory T cells (Foxp3, Il10, and Tgfb1) (Figures 4D, 4H, and 4I) (31, 32). There was no evidence of differences in transcription factors or cytokines associated with Th2 or Th17 T-cell responses (Figures 4E, 4F, and 4J).
Lung Inflammation, Mucus Hypersecretion, and Fibrosis
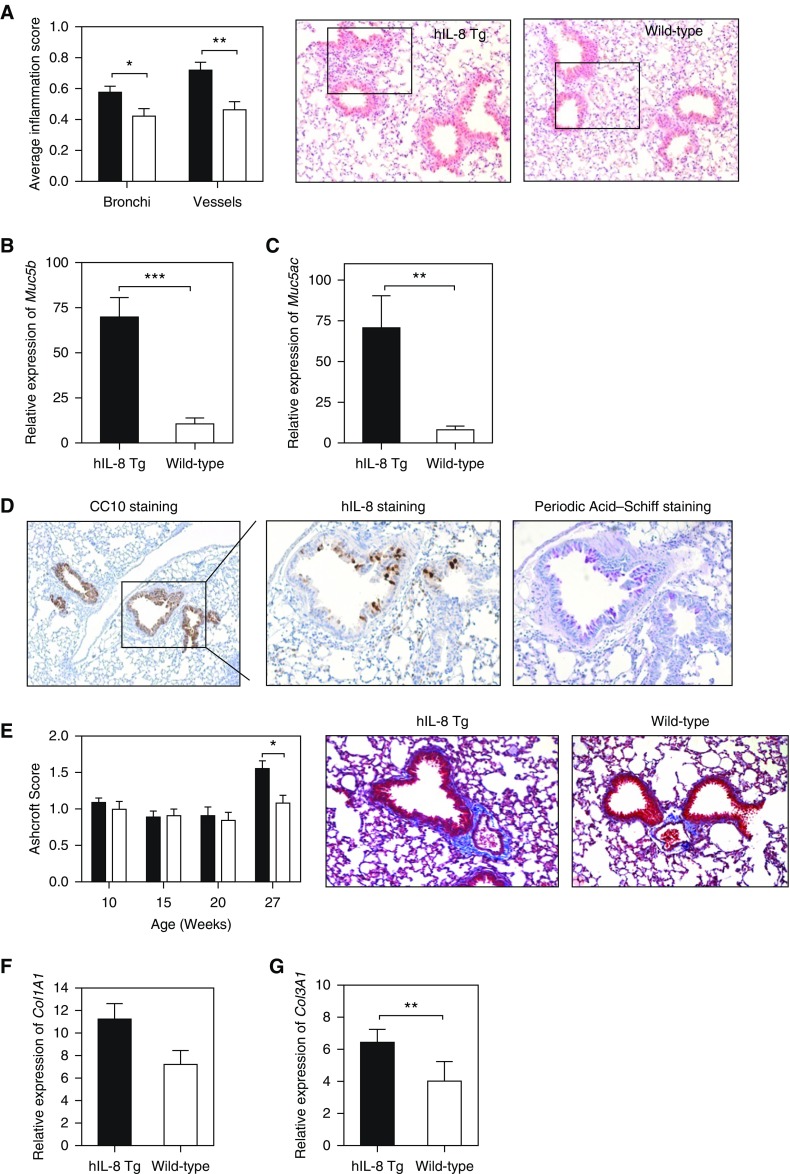
From the infection studies, it was clear that the hIL-8 transgenics displayed an enhanced lung host defense. Investigation turned to whether long-standing exposure to hIL-8 would result in pathological changes in the lung that are classically observed in human chronic lung diseases. A histological comparison of (uninfected) mice showed inflammation around bronchi and blood vessels (Figure 5A) accompanied by increased transcription of Muc5b and Muc5ac, the major mucin protein components of airway mucus (33). These were increased sixfold and 10-fold, respectively (Figures 5B and 5C). Excessive mucus production is a pathological feature of many chronic lung diseases, including asthma, COPD, bronchiectasis, and CF (34). Mucus hypersecretion, as evidenced by periodic acid–Schiff staining, was frequently observed in transgenic lung tissue and colocalized to areas of the bronchial epithelium with the greatest production of hIL-8 protein (Figure 5D). This was not seen in wild-type mouse lung.
Figure 5.
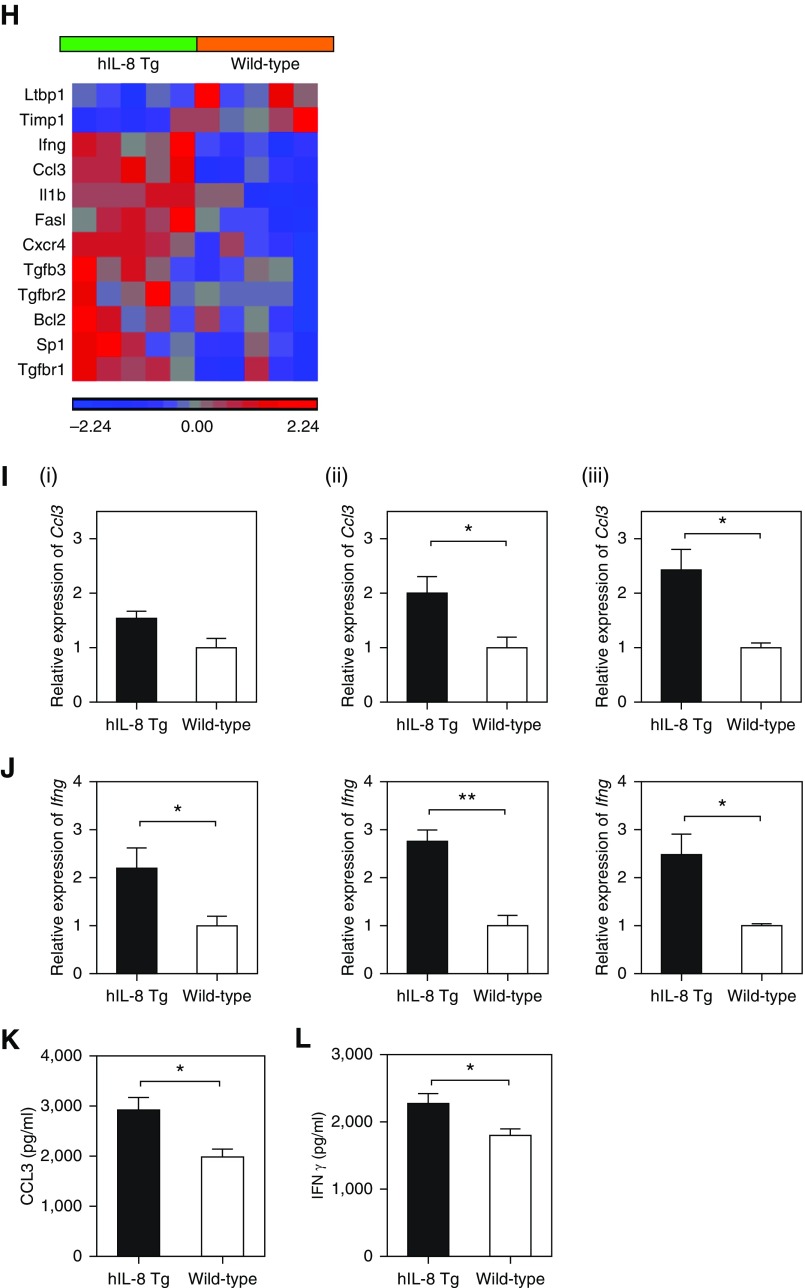
Expression of hIL-8 in lung associated with inflammation, mucus hypersecretion, and fibrosis. (A) A validated scoring system for bronchi and blood vessels was used to assess levels of inflammation in hematoxylin and eosin–stained paraffin lung sections from hIL-8 Tg (n = 40, solid bars, 10–29 week old) and wild-type (n = 22, open bars, 10–20 weeks old) mice. A representative image from each group is shown (×20 magnification). (B and C) The relative expression levels of mucin genes (B) Muc5b and (C) Muc5ac in lung tissue from hIL-8 Tg (n = 27, 10–30 weeks old) and wild-type (n = 13, 10–30 weeks old) mice were determined by real-time PCR. (D) Periodic acid–Schiff staining for mucus was colocalized with immunohistochemical staining for hIL-8 using consecutive paraffin-embedded lung sections (×40 magnification). (E) The Ashcroft scoring system was used to measure the degree of fibrosis in Masson’s Trichrome–stained lung tissue from hIL-8 Tg and wild-type mice (10–27 weeks old, n = 9–18 per group). Representative images are shown (×20 magnification). (F and G) The relative expression levels of the fibrotic markers (F) Col1A1 and (G) Col3A1 were measured by real-time PCR (n = 17 hIL-8 Tg and n = 13 wild-type mice, 11–30 weeks old). (H) A PCR array of genes implicated in fibrotic pathways was performed on lung RNA isolated from 20-week-old hIL-8 (n = 5) and wild-type (n = 5) animals. The heat map shows genes with a fold change of ±1.2 and above, and where P < 0.05. Statistical significance was determined using a one-way ANOVA. (I) Ccl3 and (J) Ifng transcripts were compared in lung tissue from hIL-8 Tg and wild-type mice at (i) 10 weeks, (ii) 20 weeks, and (iii) 27 weeks of age (n = 5 per group). (K) CCL3 and (L) IFN-γ protein levels were also measured in homogenized lung tissue from 20-week-old mice. Error bars represent mean ± SEM. Statistical significance was determined using an unpaired t test (*P < 0.05, **P < 0.005, ***P < 0.0005).
We next investigated another key pathological feature, lung fibrosis, using Masson’s Trichrome staining of lung tissue (Figure 5E). Fibrotic changes, as measured by Ashcroft scoring, were more apparent in lung tissue from hIL-8 transgenic mice compared with controls in older mice (>27 wk). A transcriptional analysis showed increased expression of transcripts for the extracellular matrix proteins collagen I and collagen III (Figures 5F and 5G), and a significant difference between transgenics and controls was seen for expression of collagen III (P = 0.0039). Further evidence that fibrotic processes occurred in the lungs of older hIL-8 transgenics came from a transcriptional array of fibrotic genes in lung tissue of 20-week-old mice (Figure 5H and Table E2). Key genes in fibrotic pathways, such as Ifng, Ccl3, Il1b, Sp1, Tgfbr1, and Tgfbr2, were upregulated in hIL-8 transgenics, as was Cxcr4, which is chemotactic for lymphocytes. Timp1, a metalloproteinase inhibitor, was downregulated. Increased transcription of Ccl3 was apparent in hIL-8 transgenic lungs from 20 weeks of age, whereas differences in Ifng transcription were present from as early as 10 weeks (Figures 5I and 5J). Increased levels of CCL3 and IFN-γ protein were present in homogenized lung tissue (Figures 5K and 5L).
Lung Remodeling Leads to Reduced Lung Function
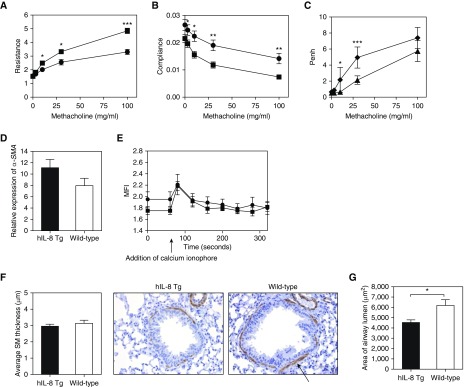
A major physiological consequence of irreversible structural damage to the airways is reduced lung function. We hypothesized that the lung inflammation, mucus hypersecretion, and fibrosis observed in the lungs of hIL-8 transgenic mice would negatively impact lung-function parameters such as airway resistance and compliance. We observed increased airway resistance and reduced lung compliance in hIL-8 transgenics in response to methacholine challenge (Figures 6A and 6B). There was evidence of increased bronchial hyperreactivity in older mice (<18 compared with >42 weeks of age), as measured by enhanced pause (Penh) (Figure 6C).
Figure 6.
Chronic IL-8 exposure results in impaired lung function with increased airway resistance and reduced compliance. (A and B) The lung-function parameters of airway (A) resistance and (B) compliance were measured in 12- to 15-week-old hIL-8 Tg (n = 8, squares) and wild-type (n = 6, circles) animals. (C) Enhanced pause (Penh) was measured in hIL-8 Tg mice of different ages (one group 9–12 weeks old, n = 8, triangles; and one group 41–44 weeks old, n = 4, diamonds). (D) α-Smooth muscle actin (α-SMA) transcripts in lung tissue from hIL-8 Tg (n = 27, solid bars) and wild-type (n = 13, open bars) mice (11–30 weeks old) were compared by real-time PCR. (E) Flow cytometry was used to determine the kinetics and magnitude of calcium flux in response to stimulant in airway smooth muscle cells derived from 8-week-old hIL-8 Tg (n = 5, squares) and wild-type (n = 4, circles) lung tissue. (F) Immunohistochemical staining of SMA in paraffin-embedded lung sections of 12- to 15-week-old hIL-8 Tg (n = 9) and wild-type (n = 6) mice was used to quantify the average thickness of SMA around bronchi. The arrow indicates positive staining for smooth muscle actin. Representative images are shown (×20 magnification). (G) The average luminal area of the bronchi was calculated. (H–K) The relative expression levels of transcripts for the epithelial-to-mesenchymal transition–related proteins E-cadherin (H), occludin (I), vimentin (J), and fibronectin (K) were compared in lung tissue from hIL-8 Tg and wild-type mice at (i) 10 weeks, (ii) 20 weeks, and (iii) 27 weeks of age (n = 5 per group). Error bars represent mean ± SEM. Statistical significance was determined using an unpaired t test (*P < 0.05, **P < 0.005, ***P < 0.0005).
The changes in lung function did not result from increased smooth-muscle mass, a feature of airway remodeling that is postulated to contribute to abnormal lung resistance in diseases such as COPD and asthma (35). Expression of α-SMA was not significantly increased in the lung tissue of hIL-8 transgenics (Figure 6D). Furthermore, lung-function changes did not result from major differences in smooth-muscle function, as primary smooth-muscle cells from transgenics and wild-type control mice showed similar magnitudes and kinetics of calcium flux in response to stimulus (Figure 6E). Immunocytochemistry of lung sections from hIL-8 transgenic and wild-type animals showed no difference in the thickness of α-SMA surrounding the bronchi (Figure 6F). The luminal area of airways was smaller in transgenic mice than in control mice (Figure 6G).
α-SMA, along with collagen I and collagen III, is a marker for epithelial-to-mesenchymal transition (EMT) (36), a profibrotic process that is believed to play a role in the pathogenesis of several lung diseases, including COPD (37). Hallmark EMT comprises downregulation of epithelial transcripts such as E-cadherin and occludin, and upregulation of mesenchymal transcripts such as vimentin and fibronectin. There was no significant E-cadherin downregulation in the lungs of hIL-8 transgenics, although occludin transcription was reduced twofold in older mice (Figures 6H and 6I). Neither vimentin nor fibronectin was upregulated, and fibronectin transcripts were significantly reduced (Figures 6J and 6K). Taken together, the data suggest that long-standing hIL-8 exposure results in a reduction in lung function that is not related to changes in smooth muscle nor a consequence of a classic EMT program.
Longstanding hIL-8 Exposure Results in Disruption of Tight-Junction Integrity
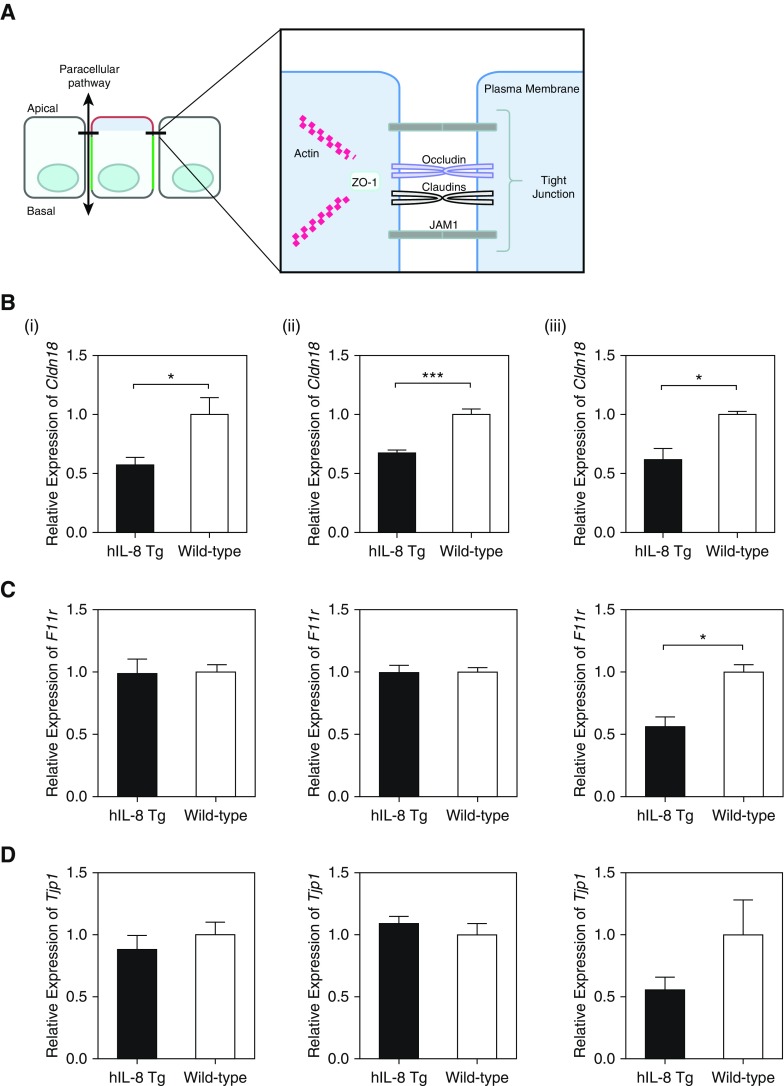
Although the data thus far did not support a role for EMT in lung remodeling, significant downregulation of transcripts for occludin and fibronectin was observed. Occludin is a key protein of epithelial tight junctions (Figure 7A), which play a vital role in maintaining the barrier function and integrity of the epithelium. Disruption of epithelial integrity has been suggested to be compromised in lung diseases such as CF (38). Fibronectin is a component of the extracellular matrix, which is important in promoting the formation of these barrier complexes (39). Transcripts for other tight-junction proteins (Claudin 18, Jam1, and ZO-1) were downregulated in hIL-8 transgenic mice compared with controls (Figures 7B–7D), particularly in older mice (>27 wk old). A gene array identified reduced transcription of additional tight-junction proteins such as Magi3, Jam2, and Cgn (Table E3), and upregulated transcription of Cldn4, which has been shown to be protective against acute lung injury (40). The data suggest that the integrity of lung epithelial tight junctions might be compromised. The possibility of tight-junction disruption was investigated by immunocytochemistry of lung epithelium from older mice, and the results showed considerable damage to epithelial cell organization, junctions, and polarity, with a significantly increased epithelial damage score (Figures 7E and 7F). This effect was not seen in epithelium from other tissues that did not express the IL-8 transgene. Disrupted organization of epithelial tight junctions was closely correlated with IL-8 expression (Figure 7E). Lung epithelial damage correlated with functional impairment of tight-junction permeability, as the BAL albumin concentration was increased fivefold in hIL-8 transgenics (Figure 7G). Collectively, these data suggest that long-standing exposure of lung tissue to hIL-8 activates inflammatory and regulatory processes, which over time result in damage to the bronchial epithelium, including loss of tight-junction integrity, and cause long-term and irreversible lung remodeling with a concomitant decline in lung function.
Figure 7.
Chronic exposure to hIL-8 in the lung results in altered expression of tight junction–associated proteins, damage to the bronchial epithelium, and functionally leaky tight junctions. (A) A schematic diagram of key tight junction–associated proteins is shown. (B–D) The relative expression levels of transcripts for the tight junction proteins Claudin 18 (B), JAM1 (C), and ZO-1 (D) were compared in lung tissue from hIL-8 Tg (black bars) and wild-type (white bars) mice at (i) 10 weeks, (ii) 20 weeks, and (iii) 27 weeks of age (n = 5 per group). (E) Representative immunofluorescence staining for DAPI (blue), Claudin 18 (green), and hIL-8 (red) in frozen lung and small-intestine tissue sections from 26- to 27-week-old hIL-8 Tg (n = 6) and wild-type (n = 6) mice is shown. (F) Images were scored by four blinded individuals using a predefined scale to assess epithelial damage. Data are shown as (i) average scores for hIL-8 Tg and wild-type groups, and (ii) the percentage of images per group assigned a score of 0–1 (light gray bars) or 2–3 (dark gray bars). (G) The concentration of albumin in the BAL fluid from hIL-8 Tg (squares, n = 14) and wild-type (circles, n = 6) mice at 10–15 weeks of age was determined by ELISA. Error bars represent mean ± SEM. Statistical significance was determined using an unpaired t test (*P < 0.05, **P < 0.005, ***P < 0.0005).
Discussion
We have characterized the impact of long-standing expression of high levels of hIL-8 protein in the murine lung. We demonstrated neutrophilia and protection from bacterial challenge, but also showed that enhanced immunity comes at the cost of chronic inflammation, with evidence of damage to the integrity of the bronchial epithelium, lung remodeling processes, and a decline in lung function.
It is a given that the IL-8/CXCR2 axis for neutrophil recruitment is a first-line innate defense for respiratory host immunity to bacterial and fungal pathogens. For example, an increased acute-phase IL-8 response is observed in pneumococcal pneumonia (41). However, potent respiratory IL-8–mediated immunity is a double-edged sword, as illustrated by the number of chronic inflammatory lung diseases that feature elevated local IL-8 levels, including CF, COPD, idiopathic pulmonary fibrosis, bronchiectasis, sarcoidosis, acute respiratory distress syndrome, and asthma (4–8). Previous studies have attempted to explore specific functions of IL-8 or its murine homolog, KC, through targeted expression in transgenic strains or through administration of recombinant cytokines (26, 42, 43). Tsai and colleagues showed increased lung bacterial clearance and improved survival after infection with Klebsiella pneumoniae in a transgenic expressing lung-targeted KC (42). This was associated with polymorphonuclear leukocyte trafficking to the lung. hIL-8 gene expression in our model is confined to the bronchial epithelium, an important physiological source of this cytokine in the human lung (44). The levels of hIL-8 protein reported here (range 5,891–39,466 pg/ml) in mouse BAL fluid are significant. High hIL-8 levels have been reported in the literature for patients with COPD (250–750 pg/ml), CF (3,800 pg/ml), and asthma (30 pg/ml) (4, 45, 46). However, hIL-8 is a less potent chemoattractant for murine neutrophils than for human cells (25), and it is difficult to make direct comparisons between BAL hIL-8 levels found in transgenic mice and those observed in patients with COPD.
We have shown that recruitment of mature and activated neutrophils into the airways of hIL-8 transgenic mice confers protection from acute P. aeruginosa infection. Persistent P. aeruginosa infection is a major problem in bronchiectasis and CF, and the key role for neutrophils in the innate immune response to this pathogen is well described (47, 48). Neutrophils are an important source of proinflammatory IL-1β during acute P. aeruginosa pneumonia (49). Here, we report upregulation of IL-1β transcripts in the lungs of older hIL-8 transgenic mice. The documented actions of IL-1β in driving lung fibrotic processes (50) serve as a possible link between expression of hIL-8 and the lung remodeling and fibrosis seen in our mice. Other profibrotic proteins that are upregulated in the hIL-8 transgenic lung and can be secreted by activated neutrophils include IFN-γ and CCL3 (51, 52). Although, the exact cell type from which the transcripts were derived is not known as experiments looking at the relative transcription of fibrotic genes used RNA made from whole lung. CCL3 is only elevated in the lung tissue of older mice, whereas IFN-γ transcripts are increased in mice as young as 10 weeks. Both probably contribute to the inflammatory response, fibrosis, and remodeling observed in these mice. Studies showing that IFN-γ has a negative effect on tight-junction integrity (38) have demonstrated the interrelatedness of these pathologies.
Although it is possible that the source of increased IFN-γ in our model is activated neutrophils, the observation that the archetypal Th1-cell transcription factor Tbet was also upregulated suggests that hIL-8 expression also triggers adaptive immune pathways. Neutrophils lie within the innate arm of the immune system, but other studies have highlighted their potential as antigen-presenting cells for T-cell activation (53). It is also possible that production of reactive oxygen species by activated neutrophils results in collateral damage to the lung mucosa, or that loss of tight-junction integrity exposes antigens that are capable of triggering an adaptive T-cell response. Our data regarding T-cell immunity to the P. aeruginosa antigen OprF show that regulatory mechanisms of the adaptive immune system were also engaged in the hIL-8 transgenics, presumably as a consequence of long-term exposure to proinflammatory signals such as IFN-γ. Induction of Foxp3 and the antiinflammatory cytokines Il10 and Tgfb1 means that overactivation of Th1 immunity may be partially counterbalanced. In addition to Tgfb1 upregulation in T cells, lung tissue from hIL-8 transgenic mice was shown to have increased levels of the TGF-β receptors Tgfbr1 and Tgfbr2. It is not surprising that regulatory mechanisms, including those elicited through TGF-β signaling, should be upregulated in a situation where the inflammatory trigger (namely, hIL-8) is continuously present. This will also be the case in patients with chronic lung diseases such as COPD, bronchiectasis, and CF, where underlying factors predispose to repeated bacterial infection. The caveat to controlling inflammation through antiinflammatory mechanisms such as TGF-β signaling is that these mechanisms are also potent inducers of tissue fibrosis. Disruption of epithelial tight junctions in hIL-8 transgenics was profound, and apparent in significant leakage of albumin into the BAL.
The data presented here provide insight into the consequences of long-term exposure to hIL-8 in the lung. IL-8 is an integral component of innate immunity to infection, and as such, overexpression is beneficial in combating bacterial infection. However, if levels of IL-8 are elevated over a long timescale, the resultant inflammatory cascades that are initiated and consequent antiinflammatory and regulatory mechanisms that are deployed will culminate in long-term structural damage to the tissue, with inflammation, mucus hypersecretion, remodeling, damaged and leaky epithelial tight junctions, and fibrosis all leading to reduced lung function. For patients suffering from chronic lung disease associated with elevated levels of IL-8, therapeutic interventions to temper excessive proinflammatory effects may be beneficial.
Acknowledgments
Acknowledgment
The authors thank Professor Clare Lloyd and the MRC & Asthma UK Centre in Allergic Mechanisms of Asthma for support with lung-function studies. They also acknowledge the support of the NIHR Imperial BRC Multiparameter Flow Cytometry and Confocal Imaging Facility (Hammersmith Campus, Imperial College London) and the Transgenics and Embryonic Stem Cell Facility (MRC London Institute of Medical Sciences) and Central Biomedical Services at the South Kensington and Hammersmith Campus, Imperial College London. The authors thank Dr. Michael Poidinger for his help and advice with the bioinformatic analysis, Michael Spencer for help with T-cell studies, Professor Jeffery Whitsett for providing the CC10 promoter, Dr. Helen Bodmer for supplying the rabbit β-globin poly(A) cassette, and Amarjit Naura and Hamid Boulares for sharing their protocols relating to the isolation and culture of primary smooth-muscle cells from mouse lung.
Footnotes
Supported by Medical Research Council grant G108-495 (R.J.B.), the Welton Foundation grant P14475 (R.J.B.), the National Institutes of Health–National Institute of Allergy and Infectious Diseases Large Scale T Cell Epitope Discovery Program grant HHSN27220090046C (R.J.B. and D.M.A.), and the National Institute for Health Research Biomedical Research Centre funding scheme grant P46708 (R.J.B.). K.Q. was supported by an MRC and Asthma UK Centre Ph.D. studentship. X.C. was supported by an Asthma UK project grant 05/045 (R.J.B.). M.S. was supported by a 4-year Wellcome Trust–National Institutes of Health Ph.D. studentship (WT095472MA) and J.S. was supported by a Wellcome Trust Clinician Scientist fellowship (100046/Z/12/Z).
Author Contributions: C.J.R., K.Q., X.C., A.S., S.T., F.A.-J., K.N., K.C., S.A.W., S.A.M., M.S., J.S., J.M., and T.P. performed experiments. C.J.R. and K.Q. designed experiments, analyzed the data, and helped prepare the manuscript. D.M.A. interpreted data and helped prepare the manuscript. R.J.B. conceived the research, acquired funding, supervised the research, designed experiments, analyzed data, interpreted data, and wrote the manuscript. All authors read, commented on, and agreed with the content of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0007OC on June 12, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, et al. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schröder JM, Mrowietz U, Morita E, Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987;139:3474–3483. [PubMed] [Google Scholar]
- 3.Auer PL, Teumer A, Schick U, O’Shaughnessy A, Lo KS, Chami N, et al. Rare and low-frequency coding variants in CXCR2 and other genes are associated with hematological traits. Nat Genet. 2014;46:629–634. doi: 10.1038/ng.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGarvey LP, Dunbar K, Martin SL, Brown V, Macmahon J, Ennis M, et al. Cytokine concentrations and neutrophil elastase activity in bronchoalveolar lavage and induced sputum from patients with cystic fibrosis, mild asthma and healthy volunteers. J Cyst Fibros. 2002;1:269–275. doi: 10.1016/s1569-1993(02)00098-x. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto C, Yoneda T, Yoshikawa M, Fu A, Tokuyama T, Tsukaguchi K, et al. Airway inflammation in COPD assessed by sputum levels of interleukin-8. Chest. 1997;112:505–510. doi: 10.1378/chest.112.2.505. [DOI] [PubMed] [Google Scholar]
- 6.Car BD, Meloni F, Luisetti M, Semenzato G, Gialdroni-Grassi G, Walz A. Elevated IL-8 and MCP-1 in the bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Crit Care Med. 1994;149:655–659. doi: 10.1164/ajrccm.149.3.8118632. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal A, Baker CS, Evans TW, Haslam PL. G-CSF and IL-8 but not GM-CSF correlate with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur Respir J. 2000;15:895–901. doi: 10.1034/j.1399-3003.2000.15e14.x. [DOI] [PubMed] [Google Scholar]
- 8.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma : evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119:1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 9.Ahn MH, Park BL, Lee SH, Park SW, Park JS, Kim DJ, et al. A promoter SNP rs4073T>A in the common allele of the interleukin 8 gene is associated with the development of idiopathic pulmonary fibrosis via the IL-8 protein enhancing mode. Respir Res. 2011;12:73. doi: 10.1186/1465-9921-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillian AD, Londono D, Dunn JM, Goddard KA, Pace RG, Knowles MR, et al. CF Gene Modifier Study Group. Modulation of cystic fibrosis lung disease by variants in interleukin-8. Genes Immun. 2008;9:501–508. doi: 10.1038/gene.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matheson MC, Ellis JA, Raven J, Walters EH, Abramson MJ. Association of IL8, CXCR2 and TNF-α polymorphisms and airway disease. J Hum Genet. 2006;51:196–203. doi: 10.1007/s10038-005-0344-7. [DOI] [PubMed] [Google Scholar]
- 12.Behndig AF, Mudway IS, Brown JL, Stenfors N, Helleday R, Duggan ST, et al. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J. 2006;27:359–365. doi: 10.1183/09031936.06.00136904. [DOI] [PubMed] [Google Scholar]
- 13.Simpson JL, Phipps S, Gibson PG. Inflammatory mechanisms and treatment of obstructive airway diseases with neutrophilic bronchitis. Pharmacol Ther. 2009;124:86–95. doi: 10.1016/j.pharmthera.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, et al. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167:1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Horuk R, Rice GC, Bennett GL, Camerato T, Wood WI. Characterization of two high affinity human interleukin-8 receptors. J Biol Chem. 1992;267:16283–16287. [PubMed] [Google Scholar]
- 16.Morohashi H, Miyawaki T, Nomura H, Kuno K, Murakami S, Matsushima K, et al. Expression of both types of human interleukin-8 receptors on mature neutrophils, monocytes, and natural killer cells. J Leukoc Biol. 1995;57:180–187. doi: 10.1002/jlb.57.1.180. [DOI] [PubMed] [Google Scholar]
- 17.Govindaraju V, Michoud MC, Al-Chalabi M, Ferraro P, Powell WS, Martin JG. Interleukin-8: novel roles in human airway smooth muscle cell contraction and migration. Am J Physiol Cell Physiol. 2006;291:C957–C965. doi: 10.1152/ajpcell.00451.2005. [DOI] [PubMed] [Google Scholar]
- 18.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 19.Walz A, Peveri P, Aschauer H, Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987;149:755–761. doi: 10.1016/0006-291x(87)90432-3. [DOI] [PubMed] [Google Scholar]
- 20.Takeda N, Sumi Y, Préfontaine D, Al Abri J, Al Heialy N, Al-Ramli W, et al. Epithelium-derived chemokines induce airway smooth muscle cell migration. Clin Exp Allergy. 2009;39:1018–1026. doi: 10.1111/j.1365-2222.2009.03238.x. [DOI] [PubMed] [Google Scholar]
- 21.Russo RC, Guabiraba R, Garcia CC, Barcelos LS, Roffê E, Souza AL, et al. Role of the chemokine receptor CXCR2 in bleomycin-induced pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol. 2009;40:410–421. doi: 10.1165/rcmb.2007-0364OC. [DOI] [PubMed] [Google Scholar]
- 22.Herbold W, Maus R, Hahn I, Ding N, Srivastava M, Christman JW, et al. Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect Immun. 2010;78:2620–2630. doi: 10.1128/IAI.01169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Soyza A, Pavord I, Elborn JS, Smith D, Wray H, Puu M, et al. A randomised, placebo-controlled study of the CXCR2 antagonist AZD5069 in bronchiectasis. Eur Respir J. 2015;46:1021–1032. doi: 10.1183/13993003.00148-2015. [DOI] [PubMed] [Google Scholar]
- 24.Bozic CR, Gerard NP, von Uexkull-Guldenband C, Kolakowski LF, Jr, Conklyn MJ, Breslow R, et al. The murine interleukin 8 type B receptor homologue and its ligands. Expression and biological characterization. J Biol Chem. 1994;269:29355–29358. [PubMed] [Google Scholar]
- 25.Rot A. Chemotactic potency of recombinant human neutrophil attractant/activation protein-1 (interleukin-8) for polymorphonuclear leukocytes of different species. Cytokine. 1991;3:21–27. doi: 10.1016/1043-4666(91)90006-y. [DOI] [PubMed] [Google Scholar]
- 26.Singer M, Sansonetti PJ. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J Immunol. 2004;173:4197–4206. doi: 10.4049/jimmunol.173.6.4197. [DOI] [PubMed] [Google Scholar]
- 27.Clifford RJ, Milillo M, Prestwood J, Quintero R, Zurawski DV, Kwak YI, et al. Detection of bacterial 16S rRNA and identification of four clinically important bacteria by real-time PCR. PLoS One. 2012;7:e48558. doi: 10.1371/journal.pone.0048558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 29.Weiss SJ, Young J, LoBuglio AF, Slivka A, Nimeh NF. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981;68:714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feniger-Barish R, Ran M, Zaslaver A, Ben-Baruch A. Differential modes of regulation of cxc chemokine-induced internalization and recycling of human CXCR1 and CXCR2. Cytokine. 1999;11:996–1009. doi: 10.1006/cyto.1999.0510. [DOI] [PubMed] [Google Scholar]
- 31.Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine AG, Mendoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature. 2017;546:421–425. doi: 10.1038/nature22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J. 2002;361:537–546. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol. 2006;34:661–665. doi: 10.1165/rcmb.2006-0035SF. [DOI] [PubMed] [Google Scholar]
- 35.Lambert RK, Wiggs BR, Kuwano K, Hogg JC, Paré PD. Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol (1985) 1993;74:2771–2781. doi: 10.1152/jappl.1993.74.6.2771. [DOI] [PubMed] [Google Scholar]
- 36.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milara J, Peiró T, Serrano A, Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68:410–420. doi: 10.1136/thoraxjnl-2012-201761. [DOI] [PubMed] [Google Scholar]
- 38.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218–3234. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koval M, Ward C, Findley MK, Roser-Page S, Helms MN, Roman J. Extracellular matrix influences alveolar epithelial claudin expression and barrier function. Am J Respir Cell Mol Biol. 2010;42:172–180. doi: 10.1165/rcmb.2008-0270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L219–L227. doi: 10.1152/ajplung.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Endeman H, Meijvis SC, Rijkers GT, van Velzen-Blad H, van Moorsel CH, Grutters JC, et al. Systemic cytokine response in patients with community-acquired pneumonia. Eur Respir J. 2011;37:1431–1438. doi: 10.1183/09031936.00074410. [DOI] [PubMed] [Google Scholar]
- 42.Tsai WC, Strieter RM, Wilkowski JM, Bucknell KA, Burdick MD, Lira SA, et al. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J Immunol. 1998;161:2435–2440. [PubMed] [Google Scholar]
- 43.Asfaha S, Dubeykovskiy AN, Tomita H, Yang X, Stokes S, Shibata W, et al. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology. 2013;144:155–166. doi: 10.1053/j.gastro.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cromwell O, Hamid Q, Corrigan CJ, Barkans J, Meng Q, Collins PD, et al. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alpha. Immunology. 1992;77:330–337. [PMC free article] [PubMed] [Google Scholar]
- 45.Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14:1015–1022. doi: 10.1183/09031936.99.14510159. [DOI] [PubMed] [Google Scholar]
- 46.Hollander C, Sitkauskiene B, Sakalauskas R, Westin U, Janciauskiene SM. Serum and bronchial lavage fluid concentrations of IL-8, SLPI, sCD14 and sICAM-1 in patients with COPD and asthma. Respir Med. 2007;101:1947–1953. doi: 10.1016/j.rmed.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Koh AY, Priebe GP, Ray C, Van Rooijen N, Pier GB. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect Immun. 2009;77:5300–5310. doi: 10.1128/IAI.00501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lovewell RR, Patankar YR, Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2014;306:L591–L603. doi: 10.1152/ajplung.00335.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patankar YR, Mabaera R, Berwin B. Differential ASC requirements reveal a key role for neutrophils and a noncanonical IL-1β response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2015;309:L902–L913. doi: 10.1152/ajplung.00228.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borthwick LA. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin Immunopathol. 2016;38:517–534. doi: 10.1007/s00281-016-0559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ethuin F, Gérard B, Benna JE, Boutten A, Gougereot-Pocidalo MA, Jacob L, et al. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest. 2004;84:1363–1371. doi: 10.1038/labinvest.3700148. [DOI] [PubMed] [Google Scholar]
- 52.Charmoy M, Brunner-Agten S, Aebischer D, Auderset F, Launois P, Milon G, et al. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog. 2010;6:e1000755. doi: 10.1371/journal.ppat.1000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandilands GP, Ahmed Z, Perry N, Davison M, Lupton A, Young B. Cross-linking of neutrophil CD11b results in rapid cell surface expression of molecules required for antigen presentation and T-cell activation. Immunology. 2005;114:354–368. doi: 10.1111/j.1365-2567.2004.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]