Abstract
Reversible phosphorylation of proteins on tyrosine residues is an essential signaling mechanism by which diverse cellular processes are closely regulated. The tight temporal and spatial control of the tyrosine phosphorylation status of proteins by protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs) is critical to cellular homeostasis as well as to adaptations to the external environment. Via regulation of cellular signaling cascades involving other protein kinases and phosphatases, receptors, adaptor proteins, and transcription factors, PTKs and PTPs closely control diverse cellular processes such as proliferation, differentiation, migration, inflammation, and maintenance of cellular barrier function. Given these key regulatory roles, it is not surprising that dysfunction of PTKs and PTPs is important in the pathogenesis of human disease, including many pulmonary diseases. The roles of various PTKs and PTPs in acute lung injury and repair, pulmonary fibrosis, pulmonary vascular disease, and inflammatory airway disease are discussed in this review. It is important to note that although there is overlap among many of these proteins in various disease states, the mechanisms by which they influence the pathogenesis of these conditions differ, suggesting wide-ranging roles for these enzymes and their potential as therapeutic targets.
Keywords: phosphorylation, kinase, phosphatase
Phosphorylation is the most common type of post-translational protein modification, and its impact on control of diverse cellular processes is ubiquitous. Protein kinases represent a family of enzymes that transfer a phosphate group from ATP to specific amino acids, most commonly on serine (S), threonine (T), or tyrosine (Y) residues (1). In contrast, protein phosphatases remove a phosphate group from these residues. An estimated 30% of all proteins can be phosphorylated on at least one residue, and 2–3% of the eukaryotic genome encodes a kinase or phosphatase (1). Of the 518 human protein kinases, 90 encode an enzyme that is relatively specific for tyrosine residues and thus are classified as protein tyrosine kinases (PTKs). Compared with kinases, there are comparatively fewer protein phosphatases (only ∼200), and of these, 108 are selective for tyrosine residues and thus are classified as protein tyrosine phosphatases (PTPs) (2, 3). A smaller number of kinases or phosphatases can phosphorylate or dephosphorylate both serine/threonine and tyrosine residues and are therefore termed dual-specificity kinases or phosphatases, respectively (4, 5).
Tight control of cellular tyrosine phosphorylation via PTKs and PTPs is critical to cellular homeostasis and impacts diverse cellular functions, ranging from proliferation and differentiation to migration, metabolism, immunity, and cell death (1). Phosphorylation and dephosphorylation of proteins are intimately tied to the activity of signaling molecules and are essential for the regulation of protein–protein interactions (6). PTKs and PTPs play fundamental roles in diverse essential physiological cellular processes, including maintenance of cellular barriers, inflammation, and regulation of cellular signaling pathways (Figure 1). However, if these key molecules become dysregulated, they have the potential to contribute to the pathogenesis of diverse disease processes. In general, much more is known about the function of PTKs than PTPs, although recent studies have begun to elucidate the roles of PTPs in physiological and pathophysiological processes. This review focuses on the roles of PTKs and PTPs in the pathogenesis of various forms of human lung disease.
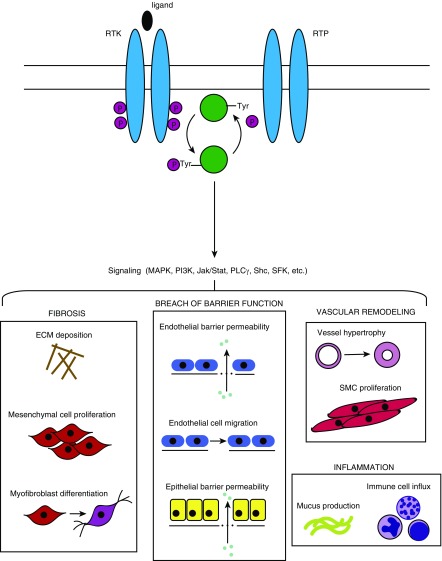
Figure 1.
Basic protein tyrosine kinase and protein tyrosine phosphatase activity and consequences for pulmonary pathology. RTKs and PTKs, located on the cell surface, bind ligands on their extracellular domain, which induces dimerization and phosphorylation of the intracellular catalytic domain. The active enzyme either phosphorylates or dephosphorylates the substrate (in the case of kinases or phosphatases, respectively). Subsequent downstream signaling can involve multiple signaling cascades and pathways, resulting in diverse physiologic consequences that are relevant to the pathogenesis of various pulmonary disease states. ECM = extracellular matrix; Jak/Stat = Janus kinase/signal transducers and activators of transcription; MAPK = mitogen-activated protein kinase; P = phosphate; PLCγ = phospholipase Cγ; RTK = receptor tyrosine kinase; RTP = receptor tyrosine phosphatase; Tyr = tyrosine; SFK = Src family kinase; Shc = Src homology 2 domain-containing transforming protein 2; SMC = smooth muscle cell.
Classification and Mechanisms of Activation of PTKs and PTPs
PTKs and PTPs are categorized into receptor type and nonreceptor type (2, 3). Receptor-type tyrosine kinases (RTKs) and receptor-type tyrosine phosphatases (RTPs) are located on cell membranes and typically transduce signals to intracellular signaling pathways via ligand binding to the extracellular domain. Oligomerization (often dimerization) and subsequent autophosphorylation or dephosphorylation of the intracellular catalytic (kinase or phosphatase) or regulatory domains generally ensues. This is followed by recruitment and activation of downstream signaling molecules and binding of cytoplasmic adaptors and regulators, ultimately resulting in modulation of cellular responses depending on the cell type and specific signal transduction pathways that are activated (3, 7–9). In response to ligand binding, activation of most growth factor–type receptors is transient, with rapid activation followed by rapid inactivation, giving tight temporal control over signaling pathways. Others, such as the discoidin domain receptors, are RTKs that bind to soluble collagen and demonstrate a slow and sustained phosphorylation. Importantly, these receptors have been implicated in the pathogenesis of human interstitial lung diseases (ILDs) (10–12).
Alternatively, RTKs and RTPs can be activated by G protein–coupled receptors (GPCRs) in a ligand-independent manner. GPCRs and RTKs often act together to control physiological processes. For example, GPCRs have been shown to regulate processes in the lung such as surfactant production (13), smooth muscle contraction (14), inflammatory cytokine production, and alterations in vascular endothelial permeability (15). The actions of GPCRs and RTKs may be synergistic or antagonistic. When GPCRs stimulate RTK activity, this mechanism is termed transactivation (16). For example, epidermal growth factor receptor (EGFR) induction by GPCR agonists is comparable in duration and effect to activation of EGFR by low concentrations of its ligand, epidermal growth factor (EGF) (3, 16). In contrast to RTKs and RTPs, nonreceptor PTKs and PTPs do not contain an extracellular or transmembrane domain, cannot bind ligands, and typically are restricted to the regulation of signaling pathways within the cytoplasm (3, 17).
Another key mechanism controlling the activation and inactivation of PTKs and PTPs is oxidation. Oxidative stress is a feature of many physiological processes, such as aging, as well as of pathophysiological processes, including diverse acute and chronic lung diseases (18). Reactive oxygen species (ROS), the by-products of cellular oxidative metabolism, are generated during oxidative stress and can be derived from a variety of oxidant-generating systems such as the mitochondrial electron transport chain and oxidases such as the NADPH oxidases (19, 20). Stimulation of cells with growth factors including EGF, PDGF, and transforming growth factor (TGF)-β results in ROS production, and there is evidence that ROS participate in signal transduction pathways involved in cellular responses to growth factor stimulation, such as growth, motility, and apoptosis. Importantly, both PTKs and PTPs are targets of ROS, and oxidative modification to specific amino acids can regulate their catalytic and adaptor functions (21, 22). PTPs are particularly susceptible to oxidant modification by ROS, in part because of critical cysteine residues in their highly conserved catalytic domains that are readily oxidized (23). PTPs known to be regulated by this mechanism include PTP1B, PTP-α, CD45, and SHP-1 (Src homology region 2 domain-containing phosphatase 1) (22, 24–26). These oxidative modifications can result in conformational alterations to the protein that result in changes in responsiveness to ligands, inhibitors, and activators that persist until the PTP is reduced or regenerated (22). The downstream signaling consequences of these oxidative modifications of PTPs are often enhancement of the response of counterpart PTKs (21, 22). Furthermore, emerging evidence suggests that PTKs, including Src, vascular endothelial growth factor receptor (VEGFR), EGFR, fibroblast growth factor receptor (FGFR), and c-abl, are also subject to direct redox regulation, suggesting that oxidative modifications are pivotal in control of signal transduction pathways directly relevant to fibrogenesis (18, 22, 27).
Among the key signaling pathways that are controlled by PTPs and PTKs are the mitogen-activated protein kinase (MAPK), PI3K, and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathways. These pathways have important implications for many human disease states. An example illustrating the importance of RTKs and RTPs in control of cellular signaling pathways involves EGFR. Binding of the ligand EGF to its receptor, EGFR, induces activation of the receptor’s intrinsic tyrosine kinase activity, leading to autophosphorylation and activation of downstream signaling molecules and adaptor proteins, including phospholipase Cγ, PI3K, Shc (Src homology 2 domain-containing transforming protein 2), GRB2 (growth factor receptor-bound protein 2), MAPK, Src (abbreviation for sarcoma), JAK, and FAK (focal adhesion kinase) (8, 28–30). EGFR signaling is also downregulated by PTPs, including LAR (leukocyte common antigen-related protein), PTP1B, and SHP-1, that dephosphorylate the receptor and its substrates, resulting in signal attenuation (31). The importance of RTKs as oncogenes in the pathogenesis of cancer, including certain types of lung cancer, underscores the importance of these signaling proteins in human disease (8).
Many PTKs and PTPs have been implicated in important pulmonary diseases, including idiopathic pulmonary fibrosis (IPF), acute respiratory distress syndrome (ARDS), pulmonary vascular disease, and inflammatory airway diseases. Several of these proteins are involved in multiple disease processes and contribute to pathophysiological processes by distinct mechanisms (see Figure 1). Furthermore, inhibitors of tyrosine kinases in particular have been evaluated extensively in vitro, in animal models and in human clinical trials, at times with great success, though often with unintended and unexpected consequences. Several of the specific proteins implicated in pulmonary disease, as well as the mechanisms by which they contribute to these disease states, in addition to the potential benefits and risks of specific inhibitors, are discussed in depth in the following sections.
IPF
IPF is the most common of the idiopathic interstitial pneumonias and carries a strikingly poor prognosis, with median survival time from diagnosis of only 2–3 years (32–35). IPF is characterized by diffuse, progressive fibrosis leading to destruction of lung tissue and respiratory compromise (33, 34). IPF is a heterogeneous disorder with a complex pathophysiology. Although still incompletely understood, the risk factors; genetic predispositions; clinical, radiological, and histopathological phenotypes; and cellular and molecular basis of fibrogenesis have been extensively characterized (36). IPF is a disease characterized by recurrent and/or nonresolving injury to the distal lung epithelium, resulting in production of cytokines and growth factors that promote myofibroblast differentiation and deposition of excess extracellular matrix (ECM) components (33, 37, 38). TGF-β is an important cytokine that is intimately involved in fibrosis of the lung and other organs (39). In IPF, TGF-β contributes to fibrogenesis in many ways, including promotion of fibroblast proliferation, activation of myofibroblasts, and induction of expression of numerous proinflammatory and fibrogenic cytokines (40). Several PTKs that control key steps in the TGF-β signaling pathway have been implicated in the pathogenesis of pulmonary fibrosis, as discussed below. The effects of PTKs on TGF-β signaling can be both positive and negative. For example, TGF-β can be phosphorylated in the cytoplasmic tail by Src, which promotes downstream fibrogenic TGF-β signaling cascades (41). In contrast, FGF2 downregulates TGF-β receptor type 1 expression and reduces cellular responses to TGF-β ligand (42–44). Other TGF-β–independent effects of tyrosine kinases and phosphatases also drive profibrotic responses. Although the role of PTKs is well defined in IPF, the contribution of PTPs is currently less well understood. Recent studies highlight the roles of PTPs in the process of fibrogenesis in the lung and other organs, and these are discussed below.
Role of PTKs in IPF
Platelet-derived growth factor receptors
Platelet-derived growth factor receptor (PDGFR)-α and PDGFR-β are RTKs whose ligands are members of the PDGF family of growth factors that include PDGF-A, -B, -C, and -D. As a fibroblast chemoattractant and stimulator of collagen synthesis, PDGF signaling plays important roles in response to tissue injury and in both wound healing and scar formation (3, 45, 46). Intratracheal administration of PDGF-BB in mice is sufficient to induce mesenchymal cell proliferation and collagen deposition (47). Animal models of pulmonary fibrosis also demonstrate elevated concentrations of PDGF ligand and receptor after treatment with bleomycin or other experimental fibrogenic stimuli (48–50). Conversely, inhibition of the PDGFR attenuates fibrosis in a rat model (51). In humans with IPF, concentrations of PDGF are elevated in the BAL fluid (46). Lung fibroblasts isolated from patients with IPF exhibit higher expression of PDGFRs than those of nonfibrotic control individuals (3, 52–54).
FGFRs
FGFRs represent a family of RTKs that function in wound healing, promoting fibroblast proliferation and ECM deposition (3, 55). In animal models of bleomycin-induced pulmonary fibrosis, FGF-2 inhibition attenuated the development of pulmonary fibrosis in part by inhibiting the effects of TGF-β (56). In vitro FGF-2 stimulates ECM synthesis by lung fibroblasts isolated from patients with IPF (57). In patients, higher FGFR2-β expression has been observed in lung fibroblasts isolated from patients with IPF (54), and concentrations of FGF-2 were increased in BAL fluid from patients with IPF compared with healthy control individuals and correlated with poorer physiological function (58). In contrast to FGF-2, other FGFs, including FGF-7 and FGF-10, have been shown to have protective (antifibrotic) effects in both patients and animal models (3, 52, 53).
EGFRs
EGFRs have multiple ligands, including EGF (Erb/Neu), TGF-α, and ErbB (59). TGF-α, via activation of EGFR, has been shown to promote pulmonary fibrosis, and in rodent models of bleomycin-induced fibrosis, EGFR and TGF-α expression are increased (3, 60, 61). Analogous findings are seen in human IPF lung tissue (62). Inhibition of EGFR and its Erb ligands protected against fibrosis in murine models (51, 63).
Src
Src family kinases (SFKs) comprise a large family of protooncogenic non-RTKs. In the pathogenesis of experimental pulmonary fibrosis, Src kinases are key in mediating the activity of TGF-β signaling by activating TGF-β receptor type 2 and other downstream targets via tyrosine phosphorylation (41). In addition, Src promotes fibroblast migration and invasion (64). In vitro Src is activated by TGF-β, and inhibition of Src reduces myofibroblast differentiation of fibroblasts (64). In vivo inhibition of Src protects against bleomycin-induced fibrosis in mice (64). Other tyrosine kinases, both receptor and nonreceptor, including VEGFR, other members of the SFKs (64), JAK, c-kit, and c-abl (3, 45, 65), have also been implicated in the pathogenesis of pulmonary fibrosis, but a discussion of these kinases is beyond the scope of this review.
Role of PTPs in IPF
SHP-2
The PTP SHP-2 is a nonreceptor PTP that has a wide range of physiological functions and plays critical roles in the regulation of developmental signaling pathways, as evidenced by the fact that SHP-2–knockout mice die early during embryogenesis (6). SHP-2 has been shown to exert antifibrotic effects in the lung. In epithelial cell–specific SHP-2–knockout mice, expression of pulmonary surfactant proteins was reduced, and mice developed spontaneous pulmonary fibrosis (66). In addition, in myeloid-specific SHP-2–knockout mice, bleomycin-induced fibrosis was accelerated (67). Conversely, mice with SHP-2 gain-of-function mutations were protected in the bleomycin model of pulmonary fibrosis. In vitro overexpression of SHP-2 in human and mouse lung fibroblasts reduced responsiveness of cells to profibrotic stimuli, as assessed by attenuated myofibroblast differentiation, whereas reduction of SHP-2 concentrations was sufficient to induce myofibroblast differentiation. Finally, human IPF lungs showed downregulation of SHP-2 with absence of this phosphatase within fibroblastic foci (68). Taken together, these observations suggest an important antifibrotic function of SHP-2.
PTP-α
PTP-α is a widely expressed receptor-type PTP that has recently been implicated in the pathogenesis of fibrosis in the lung, periodontal tissue, and joints (69–72). Global PTP-α–knockout mice are protected from experimental models of pulmonary fibrosis, and in vitro, fibroblasts lacking PTP-α exhibited blunted profibrotic responses to TGF-β stimulation (70). PTP-α serves as a checkpoint for TGF-β profibrotic signaling, and as a well-known activator of Src, its effects on the TGF-β pathway may be mediated by Src activity, thus linking both tyrosine phosphorylation and dephosphorylation in the pathogenesis of IPF.
ARDS
ARDS, a frequent complication in critically ill patients, is characterized by noncardiogenic pulmonary edema, hypoxemia, bilateral radiographic infiltrates, decreased pulmonary compliance, and respiratory failure (73–75). The definition of ARDS has recently been updated to reflect gradations in the severity of disease, with mild, moderate, and severe disease defined by the degree of hypoxemia (76). The histopathological hallmarks of the disease include interstitial and alveolar edema, inflammatory and hemorrhagic alveolar infiltrates, destruction of the alveolar epithelium, and hyaline membrane formation (77). Few therapeutic options have been shown to be of benefit in patients with ARDS, and currently, most therapy is directed at avoiding injurious mechanical ventilation using low-Vt ventilation strategies. The pathogenesis of ARDS is complex and involves multiple inflammatory mediators and disruption of endothelial and epithelial barrier function (73–75, 78). Barrier breakdown can occur with disruption of endothelial intercellular junctions (adherens junctions and tight junctions) and changes in intercellular contractile forces. Phosphorylation of intercellular junctional proteins can affect cell–ECM and cell–cell interactions (79), and enhanced tyrosine phosphorylation of junctional proteins (via inhibition of PTPs) is associated with changes in vascular permeability through formation and dissociation of adherens junctions and regulation of stress fiber formation, leading to increased permeability of the endothelial monolayer (79, 80). Several PTKs and PTPs have been implicated in the pathogenesis of endothelial injury and barrier dysfunction via mechanisms that include neutrophil chemoattraction, activation, and production of ROS, leading to increased vascular endothelial cell permeability (81).
Role of PTKs in ARDS
VEGFR
VEGF and its receptors are crucial for vascular development, and VEGF is a potent mediator of increased vascular permeability via induction of fenestrations in endothelial cells (82, 83). Most effects of VEGF on endothelial cells, including those related to cell proliferation, angiogenesis, and vascular permeability, are mediated by VEGFR-2, which is increased under conditions of hypoxia (84). Ligand binding to VEGFR-2 results in activation of multiple downstream kinases, including p38 MAPK, FAK, and SFKs (82, 83, 85). Downstream effects include endothelial cell migration and VEGF-induced endothelial permeability (85, 86). In animal models of acute lung injury (ALI), including LPS or acid instillation and injurious mechanical ventilation, VEGF and VEGFR-2 concentrations are increased (87–89). In patients with ARDS, plasma VEGF concentrations are significantly elevated compared with those in normal control individuals (86). However, intrapulmonary concentrations of VEGF are lower in patients with ARDS and normalize during recovery, suggesting a more complex role for VEGF in the genesis of and recovery from ALI (86).
EGFR
In addition to its roles in pulmonary fibrosis, the EGFR family of RTKs is implicated in the pathogenesis of ARDS through regulation of airway and alveolar epithelial barrier function (81). EFGR signaling can be either protective or injurious, depending on the experimental model and activation status of the receptor (59). In the setting of acute injury (as in models of mechanical stretch or scratch wounds), EGFR ligand is shed and EGFR is activated, resulting in proliferation, spreading, and motility of epithelial cells (90–93). Cell spreading in response to activation can enhance epithelial repair (94). However, EGFR can also promote lung injury by disrupting cell–cell adhesions, resulting in epithelial barrier dysfunction mediated by rearrangement of apical junctional complexes or expression of matrix metalloproteinases (59, 95–98). In animal models, inhibition of HER2 (human epidermal growth factor receptor 2), a member of the EGFR family, attenuated lung injury (99).
Src and SFKs
SFKs play key roles in regulating inflammatory responses, including in the milieu of ALI and ARDS (100). In ventilator-, oxidant-, and LPS-induced animal models of lung injury, Src and other SFK activity is increased (101, 102), and, conversely, Src inhibitors reduce lung injury, neutrophil influx, endothelial permeability, and chemokine/cytokine concentrations (103, 104). The molecular mechanisms that underlie SFK actions in ALI include regulation of vascular permeability as well as recruitment and activation of inflammatory cells (100). SFKs mediate phosphorylation of myosin light chains via myosin light-chain kinase activity, thereby regulating structural changes that can affect endothelial permeability (100). Src may also regulate endothelial barrier function by phosphorylation of the junctional proteins VE-cadherin and β-catenin; dissociation of these proteins from their cytoskeletal anchors can disrupt the endothelial barrier (100). The use of Src inhibitors in vivo reduces lung permeability (101). In addition to their role in barrier function, SFKs also act to enhance immune cell responses by influencing neutrophil adhesion and degranulation (105, 106).
Role of PTPs in ARDS
SHP-2
SHP-2 is important in the maintenance of the endothelial barrier, particularly via its interactions with adherens junction proteins (79, 80). SHP-2 associates with VE-cadherin via β-catenin and regulates adherens junction integrity by maintaining dephosphorylation of β-catenin (107). In vitro inhibition of SHP-2 results in increased tyrosine phosphorylation of VE-cadherin and β-catenin and subsequent disruption of endothelial monolayers. The in vivo consequence of this inhibition is the development of pulmonary edema in rat models (79). In cell culture, stimulation with LPS or thrombin reduces SHP-2 concentrations and correlates with decreased FAK phosphorylation (a substrate of SHP-2) as well as reduced SHP-2–FAK protein interactions, which are key to maintaining endothelial barrier functions (80). Finally, overexpression of SHP-2 increases resistance of the endothelial monolayer and blocks stimulation-induced permeability in vitro (80), suggesting that activation of SHP-2 preserves barrier function and is protective against edema formation. SHP-2 may therefore hold promise as a future therapeutic option to restore vascular barrier function in ALI and ARDS (80).
PTP1B
PTP1B is a nonreceptor PTP that plays several critical roles in cellular homeostasis and metabolism. Among these functions is the dephosphorylation of several RTKs, including EGFR and PDGFR, and transmembrane cadherin proteins (N-, E-, and VE-cadherins) (79). Through its actions (dephosphorylation) on these cadherin proteins, PTP1B strengthens intercellular adherens junctions by reducing tyrosine phosphorylation of associated β-catenin (108). In vitro inhibition of PTP1B has been shown to increase pulmonary endothelial cell permeability. In rodent models, increased pulmonary edema was observed after inhibition of PTP1B (79).
Vascular endothelial protein tyrosine phosphatase
Vascular endothelial protein tyrosine phosphatase (VE-PTP) is a transmembrane PTP essential for the development and maintenance of the integrity of adherens junctions. VE-PTP dephosphorylates VE-cadherin, resulting in reduction in VE-cadherin endocytosis (109). This augments adherens junction integrity and preserves endothelial barrier function (110). VE-PTP expression is regulated by hypoxia-inducible factors (HIFs), specifically HIF-2α, which induces expression of VE-PTP (111). HIFs, including HIF-2α, are essential mediators of adaptive responses to hypoxia and tissue ischemia and regulate the barrier function of endothelial monolayers, in part through induction of expression of VE-PTP (110).
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) is a progressive disease of the small pulmonary arteries characterized by vascular remodeling, medial hypertrophy, intimal thickening, and the formation of plexiform lesions, the histological hallmark lesion of PAH. Multiple cellular processes contribute to this vascular remodeling, including proliferation of endothelial and smooth muscle cells, accumulation of inflammatory cells, and deposition of ECM components. Multiple cytokines, including TGF-β and bone morphogenetic proteins, have been implicated in the disease process, and mutations in bone morphogenetic protein receptor type II are noted in familial and idiopathic cases of PAH (112). Several PTKs have been implicated in the pathogenesis of PAH.
Role of PTKs in Pulmonary Hypertension
PDGFR
Binding of the PDGFR by its PDGF ligands results in autophosphorylation of the receptor and the formation of docking sites for signaling molecules, including those of the MAPK and SFK pathways and activation of STAT transcription factors (113, 114). PDGFR promotes smooth muscle cell proliferation and pulmonary vascular remodeling (115). In animal models, including large-animal models, inhibition of PDGF reduces right ventricular hypertrophy and remodeling of the pulmonary arteries (116). PDGFR overexpression is seen in animal models of PAH and in humans with the disease (114, 117).
VEGFR
VEGFR is fundamental to angiogenesis and to the physiology of the vascular endothelium. Thus, its role in the pathogenesis of PAH is highly plausible. Interestingly, somewhat conflicting data exist regarding the potentially protective and injurious roles of VEGF signaling (114). VEGF expression is decreased in some experimental models of PAH, and its overexpression is protective against the development of PAH (118, 119). Other rodent studies show vascular remodeling after treatment with a VEGF inhibitor (120). However, in humans, plexiform lesions have higher expression of VEGFR (121). Thus, there may be a dual role for VEGF and its receptor in the pathogenesis of PH, characterized by protection in the early phases with later pathogenic increases in expression resulting in vascular remodeling in the lungs (122). The dichotomy that emerges from the opposing pro- and antiangiogenic roles of various VEGF isoforms may make therapeutic targeting of this receptor problematic.
c-kit
c-kit is a membrane-bound tyrosine kinase that acts as the receptor for stem cell factor and is expressed in bone marrow–derived cells. It is responsible, in part, for mobilization of bone marrow–derived progenitor cells to the lungs in settings of hypoxia and injury (114, 123). In humans, c-kit–positive cells are found in remodeled pulmonary arteries and plexiform lesions of patients with PAH. In addition, circulating c-kit concentrations are elevated in patients with PAH (124). Inhibition of c-kit by tyrosine kinase inhibitors (TKIs), including imatinib, reduces c-kit–positive cells and associated pulmonary vascular remodeling and right ventricular hypertrophy in murine models of pulmonary hypertension (114, 125).
Other kinases, including FGFR and SFKs, are increased in endothelial cells or smooth muscle cells of patients with PAH (114). EGFR also likely plays a role in PAH pathogenesis via induction of smooth muscle cell proliferation (126). It is noteworthy that PAH can be induced by TKIs, which is discussed further in the section entitled The Promise of Specific Inhibitors of Tyrosine Kinases or Phosphatases in the Treatment of Pulmonary Disease.
Role of PTPs in Pulmonary Hypertension
Compared with tyrosine kinases, far less is known about the role of PTPs in the development of PAH. Few studies have examined the associations between PTPs and PAH. Interestingly, it has been shown that in hypoxia-driven models of PAH, expression of numerous PTPs, including T-cell PTP, PTP1B, SHP-2, and others, is reduced, and there is an overall reduction in PTP activity. These PTPs may play important roles as negative regulators of the phosphorylation and activity of PTKs, such as PDGFR, which are important drivers of PAH (115). More studies that delve into the role of PTPs are likely to be forthcoming and will be necessary to fully understand the pathogenesis of this complex disease.
Inflammatory Airway Diseases
Chronic obstructive pulmonary disease (COPD) and asthma are inflammatory airway diseases that are characterized by increased mucus production, airway inflammation, and airway obstruction (127–129). Although the pathogenesis, demographics, and etiologies of these conditions vary, they share common features pathologically and clinically. Both are diseases of chronic inflammation of the airways, although the types of infiltrating leukocytes are different in patients with asthma, in whom they are more likely to demonstrate eosinophils, mast cells, and CD4 lymphocytes, whereas in patients with COPD, neutrophils, macrophages, and CD8 lymphocytes predominate. Cough and breathlessness are shared clinical features, and physiologically, both diseases manifest as reduced FEV1/FVC on pulmonary function testing. Much of the therapeutic arsenal is shared between these diseases. Furthermore, subgroups of patients with either disease are resistant to standard treatments and are refractory to steroids. PTK and PTP signaling have been implicated in the pathogenesis of airway disease, particularly in patients with severe or steroid-resistant phenotypes (130–132).
Role of Tyrosine Kinases in Inflammatory Airway Disease
EGFR
EGFR contributes to the pathogenesis of asthma and COPD in several ways. Upregulated expression of EGFR in the airway epithelium has been documented in patients with severe chronic asthma (90, 133–135). Increased expression of EGFR can result in the transformation of mesenchymal cells to myofibroblasts with subsequent ECM deposition that contributes to pathological airway remodeling. EGFR signaling is also involved in the recruitment of inflammatory cells such as eosinophils (136) and contributes to goblet cell metaplasia and overproduction of mucus (137). EGFR is increased in the airway epithelial cells of smokers as compared with nonsmokers (138, 139). EGFR activation may also contribute to the risk for lung cancer in smokers with COPD. Several TKIs have been used therapeutically in animal models of asthma, and the results suggest beneficial effects on airway remodeling and mucus production (135).
PDGFR
PDGFR signaling is increased after experimental asthma induced by allergen exposure, with resultant smooth muscle proliferation and airway remodeling (140). More severe asthma phenotypes have been associated with higher expression of PDGFR (141, 142).
SFKs
Several SFK members are implicated in the pathogenesis of inflammatory airway disease. For example, Syk kinase plays a role in signaling in immune cells, including regulation of T- and B-lymphocyte development and activation, as well as in eosinophil survival (135, 143). Syk also induces mast cell degranulation and histamine release (144). Mice with Syk deletions or treated with Syk inhibitors are protected from ovalbumin-induced asthma (145–147). Src itself has also been implicated in COPD pathogenesis (135, 148). Src activity is increased in vitro by cigarette smoke, and in vivo Src inhibitors are protective against the inflammatory cell infiltrates and airspace enlargement induced by smoke exposure (149).
JAK
JAK, which activates STAT cytoplasmic factors that control immune gene expression, is also involved in hypersecretory airway disease (150). JAK/STAT signaling pathways regulate neutrophilic inflammation in severe asthma (151) and are also involved in Th2 (T-helper cell type 2) responses through cytokine signaling (135, 152).
Role of PTPs in Inflammatory Airway Disease
PTEN
The phosphatase and tensin homolog (PTEN) is reduced in patients with asthma after allergen challenge, and, conversely, PTEN overexpression prevented the development of asthma (153, 154). In patients with COPD, single-nucleotide polymorphisms in PTEN are highly associated with the disease (155). PTEN expression is reduced in the lungs of patients with COPD and correlates with worse pulmonary physiology (FEV1). The mechanism by which PTEN reduction contributes to COPD development is hypothesized to be related to increased PI3K signaling leading to enhanced inflammation (156).
SHP-1
SHP-1 is a tyrosine phosphatase that controls innate and adaptive immune responses (157). Reductions in SHP-1 activity worsened airway inflammation and obstruction in murine models of asthma (158) and COPD, and SHP-1–deficient mice exhibit enhanced mucus production (159), suggesting a protective role of this phosphatase in airway pathophysiology. At baseline, SHP-1–deficient mice display airway and lung parenchymal cellular infiltrates, including eosinophils and macrophages, increased BAL cell counts, and mucus metaplasia due to increased expression of mucin gene MUC5AC, as compared with wild-type mice. These responses are likely due to upregulation of Th2 cytokines (160, 161). It is also noteworthy that tyrosine kinases and phosphatases have been strongly implicated and targeted in the pathogenesis and treatment of lung cancer, but this complex topic is beyond the scope of this review.
The Promise of Specific Inhibitors of Tyrosine Kinases or Phosphatases in the Treatment of Pulmonary Disease
The development of targeted TKIs, the first of which was imatinib in 2001, has fundamentally changed the treatment options for diverse diseases ranging from cancer to inflammatory and autoimmune diseases (162, 163). Within the realm of in vivo animal models of pulmonary disease, TKIs have been shown to mitigate disease development and severity. Unfortunately, few have translated well into human studies (see Table 1), although there are notable exceptions.
Table 1.
Recent and Ongoing Trials of Tyrosine Kinase Inhibitors in Lung Disease
| Drug | Disease | Trials* | Primary Pathway(s) Targeted |
|---|---|---|---|
| Nintedanib | IPF | Tomorrow INPULSIS 1, 2 | VEGF, FGF, PDGF |
| Nintedanib | LAM | Nintedanib for LAM (phase II) | PDGF, FGF, VEGF |
| Imatinib | PAH | IMPRES | PDGF, c-KIT |
| Imatinib | LAM | LAMP-1 | VEGF |
| Imatinib | IPF | Gleevec IPF Study | PDGF |
| Imatinib | Asthma | KIA | c-KIT |
| Dasatinib | IPF | Targeting proinflammatory cells in IPF | Src |
| Sorafenib | PAH | Dosing in patients with PAH (phase I) | VEGF, Raf-1 kinase |
| Sorafenib | Hepatopulmonary syndrome | SHPS (phase II) | VEGF |
| Saracatinib | LAM | SLAM-1 | Src |
Definition of abbreviations: FGF = fibroblast growth factor; IMPRES = Imatinib (QTI571) in Pulmonary Arterial Hypertension study; IPF = idiopathic pulmonary fibrosis; KIA = Effects of c-Kit Inhibition by Imatinib in Patients with Severe Refractory Asthma study; LAM = lymphangioleiomyomatosis; LAMP-1 = LAM Pilot Study with Imatinib Mesylate 1; PAH = pulmonary arterial hypertension; PDGF = platelet-derived growth factor; SHPS = Sorafenib for Hepatopulmonary Syndrome; SLAM-1 = Tolerability of Saracatinib in Subjects with Lymphangioleiomyomatosis; VEGF = vascular endothelial growth factor.
Cancer trials excluded (see www.clinicaltrials.gov).
One such success in the use of TKIs for pulmonary disease intervention is the approval of the multiple-TKI nintedanib (OFEV; Boehringer Ingelheim) in 2014 for the treatment of IPF. Together with pirfenidone (Esbriet; Genentech), nintedanib is one of two drugs to receive U.S. Food and Drug Administration approval for this condition, with the primary benefit being reduced decline of FVC (164, 165). Nintedanib is a small-molecule inhibitor of several tyrosine kinases, including PDGFR, FGFR, VEGFR, and several others, that acts by competitively binding the ATP site of the receptor kinase, thereby blocking downstream intracellular signaling. Mechanisms by which nintedanib functions as an antifibrotic agent include antagonism of fibroblast proliferation and migration and myofibroblast differentiation (166).
Other TKIs have been studied in the treatment of human lung disease. The Src kinase inhibitor saracatinib has been shown experimentally to mitigate the lymphangioleiomyomatosis (LAM) phenotype in vitro and in vivo (167). Saracatinib is currently in phase II trials in patients with LAM (www.clinicaltrials.gov NCT02737202). Similarly, imatinib is under investigation in a phase I trial for LAM (www.clinicaltrials.gov NCT03131999). Although imatinib appeared promising as a therapeutic for IPF, with in vivo and in vitro studies showing reduced myofibroblast differentiation and ECM deposition (65) as well as attenuated fibrosis in bleomycin-induced animal models of pulmonary fibrosis (168, 169), trials of imatinib in humans have been disappointing, with no effect on survival or decline in lung function (170).
Most TKIs inhibit multiple kinases simultaneously (171). This is in part because the majority of TKIs target the ATP-binding site of tyrosine kinases, a region that is highly conserved among these enzymes (172, 173). Nintedanib, for example, inhibits at least seven receptor and nonreceptor tyrosine kinases (PDGFR, FGFR, VEGFR, Flt-3, Src, Lck, and Lyn) with half-maximal inhibitory concentration values ranging from 13 to 610 nmol·/L− (VEFGR-3 and FGFR-4, respectively) (174). To date, selectivity among these agents is primarily based on minor differences in kinase domain structures and conformational states (172, 173). Enhanced selectivity with fewer off-target effects could be achieved by the development of substrate-competitive inhibitors that will selectively block the kinase(s) implicated in aberrant signaling, rather than focusing on ATP-competitive inhibitors that are inherently nonspecific (175).
With issues of target selectivity in mind, it is important to note the potential risk of pulmonary complications arising from the use of TKIs. Despite the experimental benefits of TKIs in diseases such as pulmonary hypertension and pulmonary fibrosis, these drugs have paradoxically been reported to induce both ILD and pulmonary hypertension. TKI-induced ILD has been documented with use of 16 (57%) of the approved agents, including gefitinib, erlotinib, and sorafenib (176) (see Table 2). Frequency of disease, severity, and time from drug administration to disease onset vary among susceptible patients (176, 177). Thus, when choosing treatment with TKIs, caution must be used and careful monitoring observed, particularly in cases of patients with preexisting ILD.
Table 2.
Interstitial Lung Disease Injury Patterns Associated with Common Tyrosine Kinase Inhibitors
| Drug |
Injury Pattern |
| Gefitinib | DAD, HP, IP, alveolar hemorrhage |
| Erlotinib | BO, HP |
| Sorafenib | BO, COP, IP |
| Imatinib | IP |
Definition of abbreviations: BO = bronchiolitis obliterans; COP = cryptogenic organizing pneumonia; DAD = diffuse alveolar damage; HP = hypersensitivity pneumonitis; IP = interstitial pneumonia.
An additional treatment paradox exists in the case of pulmonary hypertension. Multiple TKIs have shown benefit in mitigating experimental pulmonary hypertension (114). Imatinib has been tested as a potential therapeutic agent in patients with PAH because of its inhibition of PDGF and c-kit signaling, although the results did not demonstrate improvement in key clinical outcomes (178, 179). However, there are also multiple reported cases of pulmonary hypertension induced by TKIs, including dasatinib, ponatinib, bosutinib, and lapatinib (178). Interestingly, no cases of imatinib-induced pulmonary hypertension have been reported (178). The mechanisms by which TKIs induce pulmonary hypertension are incompletely understood but may be related specifically to Src inhibition in the case of dasatinib, which results in Src inhibition–mediated vasoconstriction that is frequently improved or reversed after discontinuation of the drug (178, 180). Other Src-independent mechanisms include generation of ROS that induce pulmonary endothelial cell dysfunction and apoptosis (178). Overall, pulmonary hypertension is a rare but serious complication of TKI use. Fortunately, many cases appear to be reversible, and mortality caused by TKI-induced pulmonary hypertension is rare (178). Dasatinib has also been shown to cause pleural effusions in a dose-dependent manner related to endothelial cell injury and increased permeability (178, 181).
Given the potential pitfalls and adverse effects of these agents, improved targeting of TKI pathways is needed to prevent unwanted adverse effects of these promising agents. Phosphatase inhibitors have been used less commonly in the treatment of human diseases, and to date, we know of no phosphatase inhibitors that have been trialed in human lung disease, though, as noted above, there are several potential targets of great interest (182, 183). Vanadate, a potent phosphatase inhibitor, has been used as an insulin mimetic in human diabetes (184). There are many challenges and barriers to the generation of specific phosphatase inhibitors targeting the highly conserved catalytic domain, as noted above (185). Like TKIs, an alternative strategy to achieve selectivity would be to target specific substrate binding or regulatory domains of PTP. For receptor-type PTPs, it might also be possible to target the extracellular domain with antibodies or peptides. Given the promise of drugs targeting PTK and PTP with respect to the pathobiology of many respiratory diseases, we hope to see innovative therapeutic approaches that target these molecules and pathways in experimental models with translation to human disease in the coming years.
Conclusions
The diverse roles of PTKs and PTPs in pulmonary disease underscore the importance of the process of tyrosine phosphorylation in human physiology. Multiple members of these families of enzymes are key in the pathogenesis of human disease states, and they function in varied ways to maintain cellular homeostasis, including maintaining integrity of cellular barriers and regulating diverse signaling cascades involved in physiological and pathological processes such as inflammation, repair, and fibrosis. TKIs have considerable promise in the treatment of human disease, including many lung diseases. Tyrosine phosphatase inhibitors, though less available as treatment modalities, also hold great promise as therapeutic agents. Future studies that focus on the roles of PTPs and PTKs in human lung disease and their potential for treatment are warranted.
Footnotes
Supported by National Institutes of Health (NIH) grants ES023932 and HL132950 (G.P.D.) and NIH/National Center for Advancing Translational Sciences (CTSA) Colorado CTSA grant KL2 TR001080 and a Parker B. Francis Fellowship from the Francis Family Foundation (Y.A.)
Author Contributions: Y.A. and G.P.D. both contributed to the drafting and editing of this manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0049TR on May 29, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ubersax JA, Ferrell JE., Jr Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 2.Sacco F, Perfetto L, Castagnoli L, Cesareni G. The human phosphatase interactome: an intricate family portrait. FEBS Lett. 2012;586:2732–2739. doi: 10.1016/j.febslet.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimminger F, Günther A, Vancheri C. The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1426–1433. doi: 10.1183/09031936.00149614. [DOI] [PubMed] [Google Scholar]
- 4.Dhanasekaran N, Premkumar Reddy E. Signaling by dual specificity kinases. Oncogene. 1998;17:1447–1455. doi: 10.1038/sj.onc.1202251. [DOI] [PubMed] [Google Scholar]
- 5.Patterson KI, Brummer T, O’Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- 6.Qu CK. The SHP-2 tyrosine phosphatase: signaling mechanisms and biological functions. Cell Res. 2000;10:279–288. doi: 10.1038/sj.cr.7290055. [DOI] [PubMed] [Google Scholar]
- 7.Pawson T, Warner N. Oncogenic re-wiring of cellular signaling pathways. Oncogene. 2007;26:1268–1275. doi: 10.1038/sj.onc.1210255. [DOI] [PubMed] [Google Scholar]
- 8.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 10.Bian H, Nie X, Bu X, Tian F, Yao L, Chen J, et al. The pronounced high expression of discoidin domain receptor 2 in human interstitial lung diseases. ERJ Open Res. 2018;4:00138–2016. doi: 10.1183/23120541.00138-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrera-Herrera ML, Quezada-Calvillo R. DDR2 plays a role in fibroblast migration independent of adhesion ligand and collagen activated DDR2 tyrosine kinase. Biochem Biophys Res Commun. 2012;429:39–44. doi: 10.1016/j.bbrc.2012.10.103. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Bian H, Bu X, Zhang S, Zhang P, Yu J, et al. Targeting of discoidin domain receptor 2 (DDR2) prevents myofibroblast activation and neovessel formation during pulmonary fibrosis. Mol Ther. 2016;24:1734–1744. doi: 10.1038/mt.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang MY, Hilton MB, Seaman S, Haines DC, Nagashima K, Burks CM, et al. Essential regulation of lung surfactant homeostasis by the orphan G protein-coupled receptor GPR116. Cell Rep. 2013;3:1457–1464. doi: 10.1016/j.celrep.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penn RB, Bond RA, Walker JK. GPCRs and arrestins in airways: implications for asthma. Handb Exp Pharmacol. 2014;219:387–403. doi: 10.1007/978-3-642-41199-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun L, Ye RD. Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin. 2012;33:342–350. doi: 10.1038/aps.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cattaneo F, Guerra G, Parisi M, De Marinis M, Tafuri D, Cinelli M, et al. Cell-surface receptors transactivation mediated by G protein-coupled receptors. Int J Mol Sci. 2014;15:19700–19728. doi: 10.3390/ijms151119700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller WT. Determinants of substrate recognition in nonreceptor tyrosine kinases. Acc Chem Res. 2003;36:393–400. doi: 10.1021/ar020116v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karki R, Zhang Y, Igwe OJ. Activation of c-Src: a hub for exogenous pro-oxidant-mediated activation of Toll-like receptor 4 signaling. Free Radic Biol Med. 2014;71:256–269. doi: 10.1016/j.freeradbiomed.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sies H, Berndt C, Jones DP. Oxidative sstress. Annu Rev Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 20.Robinson JC, Graham BB, Rouault TC, Tuder RM. The crossroads of iron with hypoxia and cellular metabolism: implications in the pathobiology of pulmonary hypertension. Am J Respir Cell Mol Biol. 2014;51:721–729. doi: 10.1165/rcmb.2014-0021TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28:509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 22.Corcoran A, Cotter TG. Redox regulation of protein kinases. FEBS J. 2013;280:1944–1965. doi: 10.1111/febs.12224. [DOI] [PubMed] [Google Scholar]
- 23.Schwertassek U, Haque A, Krishnan N, Greiner R, Weingarten L, Dick TP, et al. Reactivation of oxidized PTP1B and PTEN by thioredoxin 1. FEBS J. 2014;281:3545–3558. doi: 10.1111/febs.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fialkow L, Chan CK, Downey GP. Inhibition of CD45 during neutrophil activation. J Immunol. 1997;158:5409–5417. [PubMed] [Google Scholar]
- 25.Juarez JC, Manuia M, Burnett ME, Betancourt O, Boivin B, Shaw DE, et al. Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc Natl Acad Sci USA. 2008;105:7147–7152. doi: 10.1073/pnas.0709451105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weibrecht I, Böhmer SA, Dagnell M, Kappert K, Ostman A, Böhmer FD. Oxidation sensitivity of the catalytic cysteine of the protein-tyrosine phosphatases SHP-1 and SHP-2. Free Radic Biol Med. 2007;43:100–110. doi: 10.1016/j.freeradbiomed.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Heppner DE, van der Vliet A. Redox-dependent regulation of epidermal growth factor receptor signaling. Redox Biol. 2016;8:24–27. doi: 10.1016/j.redox.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. 2005;1:2005.0010. doi: 10.1038/msb4100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 2017;9:E52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 2014;24:26–34. doi: 10.1016/j.tcb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiganis T. Protein tyrosine phosphatases: dephosphorylating the epidermal growth factor receptor. IUBMB Life. 2002;53:3–14. doi: 10.1080/15216540210811. [DOI] [PubMed] [Google Scholar]
- 32.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 33.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 34.Raghu G. Idiopathic pulmonary fibrosis: increased survival with “gastroesophageal reflux therapy”: fact or fallacy? Am J Respir Crit Care Med. 2011;184:1330–1332. doi: 10.1164/rccm.201110-1842ED. [DOI] [PubMed] [Google Scholar]
- 35.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 36.Wolters PJ, Blackwell TS, Eickelberg O, Loyd JE, Kaminski N, Jenkins G, et al. Time for a change: is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet Respir Med. 2018;6:154–160. doi: 10.1016/S2213-2600(18)30007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aschner Y, Downey GP. Transforming growth factor-β: master regulator of the respiratory system in health and disease. Am J Respir Cell Mol Biol. 2016;54:647–655. doi: 10.1165/rcmb.2015-0391TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9:111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 41.Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-β type II receptor and regulates TGF-β stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- 42.Fafeur V, Terman BI, Blum J, Böhlen P. Basic FGF treatment of endothelial cells down-regulates the 85-KDa TGF β receptor subtype and decreases the growth inhibitory response to TGF-β1. Growth Factors. 1990;3:237–245. doi: 10.3109/08977199009043908. [DOI] [PubMed] [Google Scholar]
- 43.Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, et al. FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2012;2:1684–1696. doi: 10.1016/j.celrep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen PY, Qin L, Li G, Tellides G, Simons M. Fibroblast growth factor (FGF) signaling regulates transforming growth factor beta (TGFβ)-dependent smooth muscle cell phenotype modulation. Sci Rep. 2016;6:33407. doi: 10.1038/srep33407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beyer C, Distler JH. Tyrosine kinase signaling in fibrotic disorders: translation of basic research to human disease. Biochim Biophys Acta. 2013;1832:897–904. doi: 10.1016/j.bbadis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Antoniades HN, Bravo MA, Avila RE, Galanopoulos T, Neville-Golden J, Maxwell M, et al. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990;86:1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi ES, Lee H, Yin S, Piguet P, Sarosi I, Kaufmann S, et al. Platelet-derived growth factor causes pulmonary cell proliferation and collagen deposition in vivo. Am J Pathol. 1996;149:539–548. [PMC free article] [PubMed] [Google Scholar]
- 48.Bonner JC, Lindroos PM, Rice AB, Moomaw CR, Morgan DL. Induction of PDGF receptor-α in rat myofibroblasts during pulmonary fibrogenesis in vivo. Am J Physiol. 1998;274:L72–L80. doi: 10.1152/ajplung.1998.274.1.L72. [DOI] [PubMed] [Google Scholar]
- 49.Gurujeyalakshmi G, Hollinger MA, Giri SN. Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. Am J Physiol. 1999;276:L311–L318. doi: 10.1152/ajplung.1999.276.2.L311. [DOI] [PubMed] [Google Scholar]
- 50.Zhuo Y, Zhang J, Laboy M, Lasky JA. Modulation of PDGF-C and PDGF-D expression during bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2004;286:L182–L188. doi: 10.1152/ajplung.00083.2003. [DOI] [PubMed] [Google Scholar]
- 51.Rice AB, Moomaw CR, Morgan DL, Bonner JC. Specific inhibitors of platelet-derived growth factor or epidermal growth factor receptor tyrosine kinase reduce pulmonary fibrosis in rats. Am J Pathol. 1999;155:213–221. doi: 10.1016/S0002-9440(10)65115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupte VV, Ramasamy SK, Reddy R, Lee J, Weinreb PH, Violette SM, et al. Overexpression of fibroblast growth factor-10 during both inflammatory and fibrotic phases attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 2009;180:424–436. doi: 10.1164/rccm.200811-1794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci USA. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hostettler KE, Zhong J, Papakonstantinou E, Karakiulakis G, Tamm M, Seidel P, et al. Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Respir Res. 2014;15:157. doi: 10.1186/s12931-014-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dosanjh A. The fibroblast growth factor pathway and its role in the pathogenesis of lung disease. J Interferon Cytokine Res. 2012;32:111–114. doi: 10.1089/jir.2011.0079. [DOI] [PubMed] [Google Scholar]
- 56.Yu ZH, Wang DD, Zhou ZY, He SL, Chen AA, Wang J. Mutant soluble ectodomain of fibroblast growth factor receptor-2 IIIc attenuates bleomycin-induced pulmonary fibrosis in mice. Biol Pharm Bull. 2012;35:731–736. doi: 10.1248/bpb.35.731. [DOI] [PubMed] [Google Scholar]
- 57.Hetzel M, Bachem M, Anders D, Trischler G, Faehling M. Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung. 2005;183:225–237. doi: 10.1007/s00408-004-2534-z. [DOI] [PubMed] [Google Scholar]
- 58.Inoue Y, King TE, Jr, Tinkle SS, Dockstader K, Newman LS. Human mast cell basic fibroblast growth factor in pulmonary fibrotic disorders. Am J Pathol. 1996;149:2037–2054. [PMC free article] [PubMed] [Google Scholar]
- 59.Finigan JH, Downey GP, Kern JA. Human epidermal growth factor receptor signaling in acute lung injury. Am J Respir Cell Mol Biol. 2012;47:395–404. doi: 10.1165/rcmb.2012-0100TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vallath S, Hynds RE, Succony L, Janes SM, Giangreco A. Targeting EGFR signalling in chronic lung disease: therapeutic challenges and opportunities. Eur Respir J. 2014;44:513–522. doi: 10.1183/09031936.00146413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Madtes DK, Busby HK, Strandjord TP, Clark JG. Expression of transforming growth factor-α and epidermal growth factor receptor is increased following bleomycin-induced lung injury in rats. Am J Respir Cell Mol Biol. 1994;11:540–551. doi: 10.1165/ajrcmb.11.5.7524566. [DOI] [PubMed] [Google Scholar]
- 62.Baughman RP, Lower EE, Miller MA, Bejarano PA, Heffelfinger SC. Overexpression of transforming growth factor-α and epidermal growth factor-receptor in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:57–61. [PubMed] [Google Scholar]
- 63.Nethery DE, Moore BB, Minowada G, Carroll J, Faress JA, Kern JA. Expression of mutant human epidermal receptor 3 attenuates lung fibrosis and improves survival in mice. J Appl Physiol (1985) 2005;99:298–307. doi: 10.1152/japplphysiol.01360.2004. [DOI] [PubMed] [Google Scholar]
- 64.Hu M, Che P, Han X, Cai GQ, Liu G, Antony V, et al. Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J Pharmacol Exp Ther. 2014;351:87–95. doi: 10.1124/jpet.114.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-β and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X, Zhang Y, Tao B, Teng L, Li Y, Cao R, et al. Loss of Shp2 in alveoli epithelia induces deregulated surfactant homeostasis, resulting in spontaneous pulmonary fibrosis. FASEB J. 2012;26:2338–2350. doi: 10.1096/fj.11-200139. [DOI] [PubMed] [Google Scholar]
- 67.Tao B, Jin W, Xu J, Liang Z, Yao J, Zhang Y, et al. Myeloid-specific disruption of tyrosine phosphatase Shp2 promotes alternative activation of macrophages and predisposes mice to pulmonary fibrosis. J Immunol. 2014;193:2801–2811. doi: 10.4049/jimmunol.1303463. [DOI] [PubMed] [Google Scholar]
- 68.Tzouvelekis A, Yu G, Lino Cardenas CL, Herazo-Maya JD, Wang R, Woolard T, et al. SH2 domain-containing phosphatase-2 is a novel antifibrotic regulator in pulmonary fibrosis. Am J Respir Crit Care Med. 2017;195:500–514. doi: 10.1164/rccm.201602-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sap J, D’Eustachio P, Givol D, Schlessinger J. Cloning and expression of a widely expressed receptor tyrosine phosphatase. Proc Natl Acad Sci USA. 1990;87:6112–6116. doi: 10.1073/pnas.87.16.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aschner Y, Khalifah AP, Briones N, Yamashita C, Dolgonos L, Young SK, et al. Protein tyrosine phosphatase α mediates profibrotic signaling in lung fibroblasts through TGF-β responsiveness. Am J Pathol. 2014;184:1489–1502. doi: 10.1016/j.ajpath.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stanford SM, Svensson MN, Sacchetti C, Pilo CA, Wu DJ, Kiosses WB, et al. Receptor protein tyrosine phosphatase α-mediated enhancement of rheumatoid synovial fibroblast signaling and promotion of arthritis in mice. Arthritis Rheumatol. 2016;68:359–369. doi: 10.1002/art.39442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rajshankar D, Sima C, Wang Q, Goldberg SR, Kazembe M, Wang Y, et al. Role of PTPα in the destruction of periodontal connective tissues. PLoS One. 2013;8:e70659. doi: 10.1371/journal.pone.0070659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 74.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 77.Burnham EL, Janssen WJ, Riches DW, Moss M, Downey GP. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J. 2014;43:276–285. doi: 10.1183/09031936.00196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol. 2009;40:519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grinnell KL, Chichger H, Braza J, Duong H, Harrington EO. Protection against LPS-induced pulmonary edema through the attenuation of protein tyrosine phosphatase-1B oxidation. Am J Respir Cell Mol Biol. 2012;46:623–632. doi: 10.1165/rcmb.2011-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chichger H, Braza J, Duong H, Harrington EO. SH2 domain-containing protein tyrosine phosphatase 2 and focal adhesion kinase protein interactions regulate pulmonary endothelium barrier function. Am J Respir Cell Mol Biol. 2015;52:695–707. doi: 10.1165/rcmb.2013-0489OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aschner Y, Zemans RL, Yamashita CM, Downey GP. Matrix metalloproteinases and protein tyrosine kinases: potential novel targets in acute lung injury and ARDS. Chest. 2014;146:1081–1091. doi: 10.1378/chest.14-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 83.Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997;57:765–772. [PubMed] [Google Scholar]
- 84.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–L221. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 85.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 86.Thickett DR, Armstrong L, Millar AB. A role for vascular endothelial growth factor in acute and resolving lung injury. Am J Respir Crit Care Med. 2002;166:1332–1337. doi: 10.1164/rccm.2105057. [DOI] [PubMed] [Google Scholar]
- 87.Medford AR, Millar AB. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax. 2006;61:621–626. doi: 10.1136/thx.2005.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karmpaliotis D, Kosmidou I, Ingenito EP, Hong K, Malhotra A, Sunday ME, et al. Angiogenic growth factors in the pathophysiology of a murine model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2002;283:L585–L595. doi: 10.1152/ajplung.00048.2002. [DOI] [PubMed] [Google Scholar]
- 89.Gurkan OU, O’Donnell C, Brower R, Ruckdeschel E, Becker PM. Differential effects of mechanical ventilatory strategy on lung injury and systemic organ inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2003;285:L710–L718. doi: 10.1152/ajplung.00044.2003. [DOI] [PubMed] [Google Scholar]
- 90.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, et al. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–1374. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 91.Savla U, Waters CM. Mechanical strain inhibits repair of airway epithelium in vitro. Am J Physiol. 1998;274:L883–L892. doi: 10.1152/ajplung.1998.274.6.L883. [DOI] [PubMed] [Google Scholar]
- 92.Ryan RM, Mineo-Kuhn MM, Kramer CM, Finkelstein JN. Growth factors alter neonatal type II alveolar epithelial cell proliferation. Am J Physiol. 1994;266:L17–L22. doi: 10.1152/ajplung.1994.266.1.L17. [DOI] [PubMed] [Google Scholar]
- 93.Leslie CC, McCormick-Shannon K, Shannon JM, Garrick B, Damm D, Abraham JA, et al. Heparin-binding EGF-like growth factor is a mitogen for rat alveolar type II cells. Am J Respir Cell Mol Biol. 1997;16:379–387. doi: 10.1165/ajrcmb.16.4.9115748. [DOI] [PubMed] [Google Scholar]
- 94.Geiser T, Jarreau PH, Atabai K, Matthay MA. Interleukin-1β augments in vitro alveolar epithelial repair. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1184–L1190. doi: 10.1152/ajplung.2000.279.6.L1184. [DOI] [PubMed] [Google Scholar]
- 95.Bierman A, Yerrapureddy A, Reddy NM, Hassoun PM, Reddy SP. Epidermal growth factor receptor (EGFR) regulates mechanical ventilation-induced lung injury in mice. Transl Res. 2008;152:265–272. doi: 10.1016/j.trsl.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Correa-Meyer E, Pesce L, Guerrero C, Sznajder JI. Cyclic stretch activates ERK1/2 via G proteins and EGFR in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L883–L891. doi: 10.1152/ajplung.00203.2001. [DOI] [PubMed] [Google Scholar]
- 97.Terakado M, Gon Y, Sekiyama A, Takeshita I, Kozu Y, Matsumoto K, et al. The Rac1/JNK pathway is critical for EGFR-dependent barrier formation in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L56–L63. doi: 10.1152/ajplung.00159.2010. [DOI] [PubMed] [Google Scholar]
- 98.Chen SP, Zhou B, Willis BC, Sandoval AJ, Liebler JM, Kim KJ, et al. Effects of transdifferentiation and EGF on claudin isoform expression in alveolar epithelial cells. J Appl Physiol (1985) 2005;98:322–328. doi: 10.1152/japplphysiol.00681.2004. [DOI] [PubMed] [Google Scholar]
- 99.Faress JA, Nethery DE, Kern EF, Eisenberg R, Jacono FJ, Allen CL, et al. Bleomycin-induced pulmonary fibrosis is attenuated by a monoclonal antibody targeting HER2. J Appl Physiol (1985) 2007;103:2077–2083. doi: 10.1152/japplphysiol.00239.2007. [DOI] [PubMed] [Google Scholar]
- 100.Okutani D, Lodyga M, Han B, Liu M. Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol. 2006;291:L129–L141. doi: 10.1152/ajplung.00261.2005. [DOI] [PubMed] [Google Scholar]
- 101.Khadaroo RG, He R, Parodo J, Powers KA, Marshall JC, Kapus A, et al. The role of the Src family of tyrosine kinases after oxidant-induced lung injury in vivo. Surgery. 2004;136:483–488. doi: 10.1016/j.surg.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 102.Severgnini M, Takahashi S, Rozo LM, Homer RJ, Kuhn C, Jhung JW, et al. Activation of the STAT pathway in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1282–L1292. doi: 10.1152/ajplung.00349.2003. [DOI] [PubMed] [Google Scholar]
- 103.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 104.Severgnini M, Takahashi S, Tu P, Perides G, Homer RJ, Jhung JW, et al. Inhibition of the Src and Jak kinases protects against lipopolysaccharide-induced acute lung injury. Am J Respir Crit Care Med. 2005;171:858–867. doi: 10.1164/rccm.200407-981OC. [DOI] [PubMed] [Google Scholar]
- 105.Yan SR, Berton G. Regulation of Src family tyrosine kinase activities in adherent human neutrophils: evidence that reactive oxygen intermediates produced by adherent neutrophils increase the activity of the p58c-fgr and p53/56lyn tyrosine kinases. J Biol Chem. 1996;271:23464–23471. doi: 10.1074/jbc.271.38.23464. [DOI] [PubMed] [Google Scholar]
- 106.Mócsai A, Jakus Z, Vántus T, Berton G, Lowell CA, Ligeti E. Kinase pathways in chemoattractant-induced degranulation of neutrophils: the role of p38 mitogen-activated protein kinase activated by Src family kinases. J Immunol. 2000;164:4321–4331. doi: 10.4049/jimmunol.164.8.4321. [DOI] [PubMed] [Google Scholar]
- 107.Timmerman I, Hoogenboezem M, Bennett AM, Geerts D, Hordijk PL, van Buul JD. The tyrosine phosphatase SHP2 regulates recovery of endothelial adherens junctions through control of β-catenin phosphorylation. Mol Biol Cell. 2012;23:4212–4225. doi: 10.1091/mbc.E12-01-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Balsamo J, Arregui C, Leung T, Lilien J. The nonreceptor protein tyrosine phosphatase PTP1B binds to the cytoplasmic domain of N-cadherin and regulates the cadherin-actin linkage. J Cell Biol. 1998;143:523–532. doi: 10.1083/jcb.143.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gong H, Gao X, Feng S, Siddiqui MR, Garcia A, Bonini MG, et al. Evidence of a common mechanism of disassembly of adherens junctions through Gα13 targeting of VE-cadherin. J Exp Med. 2014;211:579–591. doi: 10.1084/jem.20131190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gong H, Rehman J, Tang H, Wary K, Mittal M, Chaturvedi P, et al. HIF2α signaling inhibits adherens junctional disruption in acute lung injury. J Clin Invest. 2015;125:652–664. doi: 10.1172/JCI77701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Le Bras A, Lionneton F, Mattot V, Lelièvre E, Caetano B, Spruyt N, et al. HIF-2α specifically activates the VE-cadherin promoter independently of hypoxia and in synergy with Ets-1 through two essential ETS-binding sites. Oncogene. 2007;26:7480–7489. doi: 10.1038/sj.onc.1210566. [DOI] [PubMed] [Google Scholar]
- 112.Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation. 2005;111:534–538. doi: 10.1161/01.CIR.0000156326.48823.55. [DOI] [PubMed] [Google Scholar]
- 113.Claesson-Welsh L. Platelet-derived growth factor receptor signals. J Biol Chem. 1994;269:32023–32026. [PubMed] [Google Scholar]
- 114.Godinas L, Guignabert C, Seferian A, Perros F, Bergot E, Sibille Y, et al. Tyrosine kinase inhibitors in pulmonary arterial hypertension: a double-edge sword? Semin Respir Crit Care Med. 2013;34:714–724. doi: 10.1055/s-0033-1356494. [DOI] [PubMed] [Google Scholar]
- 115.ten Freyhaus H, Dagnell M, Leuchs M, Vantler M, Berghausen EM, Caglayan E, et al. Hypoxia enhances platelet-derived growth factor signaling in the pulmonary vasculature by down-regulation of protein tyrosine phosphatases. Am J Respir Crit Care Med. 2011;183:1092–1102. doi: 10.1164/rccm.200911-1663OC. [DOI] [PubMed] [Google Scholar]
- 116.Jankov RP, Kantores C, Belcastro R, Yi S, Ridsdale RA, Post M, et al. A role for platelet-derived growth factor β-receptor in a newborn rat model of endothelin-mediated pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1162–L1170. doi: 10.1152/ajplung.00180.2004. [DOI] [PubMed] [Google Scholar]
- 117.Humbert M, Monti G, Fartoukh M, Magnan A, Brenot F, Rain B, et al. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J. 1998;11:554–559. [PubMed] [Google Scholar]
- 118.Partovian C, Adnot S, Eddahibi S, Teiger E, Levame M, Dreyfus P, et al. Heart and lung VEGF mRNA expression in rats with monocrotaline- or hypoxia-induced pulmonary hypertension. Am J Physiol. 1998;275:H1948–H1956. doi: 10.1152/ajpheart.1998.275.6.H1948. [DOI] [PubMed] [Google Scholar]
- 119.Campbell AI, Zhao Y, Sandhu R, Stewart DJ. Cell-based gene transfer of vascular endothelial growth factor attenuates monocrotaline-induced pulmonary hypertension. Circulation. 2001;104:2242–2248. doi: 10.1161/hc4201.097838. [DOI] [PubMed] [Google Scholar]
- 120.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, McMahon G, Waltenberger J, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 121.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 122.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Cool C, Wood K, Tuder RM, et al. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;291:L668–L676. doi: 10.1152/ajplung.00491.2005. [DOI] [PubMed] [Google Scholar]
- 123.Schwarz J. Emerging role of c-kit+ progenitor cells in pulmonary hypertension. Am J Respir Crit Care Med. 2011;184:5–7. doi: 10.1164/rccm.201104-0664ED. [DOI] [PubMed] [Google Scholar]
- 124.Montani D, Perros F, Gambaryan N, Girerd B, Dorfmuller P, Price LC, et al. c-kit-Positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2011;184:116–123. doi: 10.1164/rccm.201006-0905OC. [DOI] [PubMed] [Google Scholar]
- 125.Gambaryan N, Perros F, Montani D, Cohen-Kaminsky S, Mazmanian M, Renaud JF, et al. Targeting of c-kit+ haematopoietic progenitor cells prevents hypoxic pulmonary hypertension. Eur Respir J. 2011;37:1392–1399. doi: 10.1183/09031936.00045710. [DOI] [PubMed] [Google Scholar]
- 126.Dahal BK, Cornitescu T, Tretyn A, Pullamsetti SS, Kosanovic D, Dumitrascu R, et al. Role of epidermal growth factor inhibition in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2010;181:158–167. doi: 10.1164/rccm.200811-1682OC. [DOI] [PubMed] [Google Scholar]
- 127.Angelis N, Porpodis K, Zarogoulidis P, Spyratos D, Kioumis I, Papaiwannou A, et al. Airway inflammation in chronic obstructive pulmonary disease. J Thorac Dis. 2014;6(Suppl 1):S167–S172. doi: 10.3978/j.issn.2072-1439.2014.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kudo M, Ishigatsubo Y, Aoki I. Pathology of asthma. Front Microbiol. 2013;4:263. doi: 10.3389/fmicb.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cukic V, Lovre V, Dragisic D, Ustamujic A. Asthma and chronic obstructive pulmonary disease (COPD) – differences and similarities. Mater Sociomed. 2012;24:100–105. doi: 10.5455/msm.2012.24.100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007 J Allergy Clin Immunol 20071205SupplS94–S138.[Published erratum appears in J Allergy Clin Immunol 2008;121:1330.] [DOI] [PubMed] [Google Scholar]
- 131.Wenzel SE, Busse WW National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Severe asthma: lessons from the Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:14–21, quiz 22–23. doi: 10.1016/j.jaci.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 132.Barnes PJ. Kinases as novel therapeutic targets in asthma and chronic obstructive pulmonary disease. Pharmacol Rev. 2016;68:788–815. doi: 10.1124/pr.116.012518. [DOI] [PubMed] [Google Scholar]
- 133.Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, et al. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med. 1998;157:1907–1912. doi: 10.1164/ajrccm.157.6.9609040. [DOI] [PubMed] [Google Scholar]
- 134.Polosa R, Puddicombe SM, Krishna MT, Tuck AB, Howarth PH, Holgate ST, et al. Expression of c-erbB receptors and ligands in the bronchial epithelium of asthmatic subjects. J Allergy Clin Immunol. 2002;109:75–81. doi: 10.1067/mai.2002.120274. [DOI] [PubMed] [Google Scholar]
- 135.Guntur VP, Reinero CR. The potential use of tyrosine kinase inhibitors in severe asthma. Curr Opin Allergy Clin Immunol. 2012;12:68–75. doi: 10.1097/ACI.0b013e32834ecb4f. [DOI] [PubMed] [Google Scholar]
- 136.Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, et al. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am J Physiol Lung Cell Mol Physiol. 2011;300:L414–L421. doi: 10.1152/ajplung.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Casalino-Matsuda SM, Monzón ME, Forteza RM. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol. 2006;34:581–591. doi: 10.1165/rcmb.2005-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Takeyama K, Jung B, Shim JJ, Burgel PR, Dao-Pick T, Ueki IF, et al. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2001;280:L165–L172. doi: 10.1152/ajplung.2001.280.1.L165. [DOI] [PubMed] [Google Scholar]
- 139.de Boer WI, Hau CM, van Schadewijk A, Stolk J, van Krieken JH, Hiemstra PS. Expression of epidermal growth factors and their receptors in the bronchial epithelium of subjects with chronic obstructive pulmonary disease. Am J Clin Pathol. 2006;125:184–192. doi: 10.1309/W1AX-KGT7-UA37-X257. [DOI] [PubMed] [Google Scholar]
- 140.Hirota JA, Ask K, Farkas L, Smith JA, Ellis R, Rodriguez-Lecompte JC, et al. In vivo role of platelet-derived growth factor-BB in airway smooth muscle proliferation in mouse lung. Am J Respir Cell Mol Biol. 2011;45:566–572. doi: 10.1165/rcmb.2010-0277OC. [DOI] [PubMed] [Google Scholar]
- 141.Wu LS, Tan CY, Wang LM, Lin CG, Wang JY. Variant in promoter region of platelet-derived growth factor receptor-α (PDGFRα) gene is associated with the severity and allergic status of childhood asthma. Int Arch Allergy Immunol. 2006;141:37–46. doi: 10.1159/000094180. [DOI] [PubMed] [Google Scholar]
- 142.Leung TF, Wong GW, Ko FW, Li CY, Yung E, Lam CW, et al. Analysis of growth factors and inflammatory cytokines in exhaled breath condensate from asthmatic children. Int Arch Allergy Immunol. 2005;137:66–72. doi: 10.1159/000085106. [DOI] [PubMed] [Google Scholar]
- 143.Yanagi S, Inatome R, Takano T, Yamamura H. Syk expression and novel function in a wide variety of tissues. Biochem Biophys Res Commun. 2001;288:495–498. doi: 10.1006/bbrc.2001.5788. [DOI] [PubMed] [Google Scholar]
- 144.MacGlashan D, Jr, Lavens-Phillips S. Characteristics of the free cytosolic calcium timelag following IgE-mediated stimulation of human basophils: significance for the nonreleasing basophil phenotype. J Leukoc Biol. 2001;69:224–232. [PubMed] [Google Scholar]
- 145.Wex E, Bouyssou T, Duechs MJ, Erb KJ, Gantner F, Sanderson MP, et al. Induced Syk deletion leads to suppressed allergic responses but has no effect on neutrophil or monocyte migration in vivo. Eur J Immunol. 2011;41:3208–3218. doi: 10.1002/eji.201141502. [DOI] [PubMed] [Google Scholar]
- 146.Yamamoto N, Hasegawa H, Seki H, Ziegelbauer K, Yasuda T. Development of a high-throughput fluoroimmunoassay for Syk kinase and Syk kinase inhibitors. Anal Biochem. 2003;315:256–261. doi: 10.1016/s0003-2697(03)00026-5. [DOI] [PubMed] [Google Scholar]
- 147.Matsubara S, Koya T, Takeda K, Joetham A, Miyahara N, Pine P, et al. Syk activation in dendritic cells is essential for airway hyperresponsiveness and inflammation. Am J Respir Cell Mol Biol. 2006;34:426–433. doi: 10.1165/rcmb.2005-0298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.El-Hashim AZ, Khajah MA, Renno WM, Babyson RS, Uddin M, Benter IF, et al. Src-dependent EGFR transactivation regulates lung inflammation via downstream signaling involving ERK1/2, PI3Kδ/Akt and NFκB induction in a murine asthma model. Sci Rep. 2017;7:9919. doi: 10.1038/s41598-017-09349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Geraghty P, Hardigan A, Foronjy RF. Cigarette smoke activates the proto-oncogene c-Src to promote airway inflammation and lung tissue destruction. Am J Respir Cell Mol Biol. 2014;50:559–570. doi: 10.1165/rcmb.2013-0258OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pernis AB, Rothman PB. JAK-STAT signaling in asthma. J Clin Invest. 2002;109:1279–1283. doi: 10.1172/JCI15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nakajima H, Hirose K. Role of IL-23 and Th17 cells in airway inflammation in asthma. Immune Netw. 2010;10:1–4. doi: 10.4110/in.2010.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kudlacz E, Conklyn M, Andresen C, Whitney-Pickett C, Changelian P. The JAK-3 inhibitor CP-690550 is a potent anti-inflammatory agent in a murine model of pulmonary eosinophilia. Eur J Pharmacol. 2008;582:154–161. doi: 10.1016/j.ejphar.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 153.Kwak YG, Song CH, Yi HK, Hwang PH, Kim JS, Lee KS, et al. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest. 2003;111:1083–1092. doi: 10.1172/JCI16440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pouliot P, Bergeron S, Marette A, Olivier M. The role of protein tyrosine phosphatases in the regulation of allergic asthma: implication of TC-PTP and PTP-1B in the modulation of disease development. Immunology. 2009;128:534–542. doi: 10.1111/j.1365-2567.2009.03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hosgood HD, III, Menashe I, He X, Chanock S, Lan Q. PTEN identified as important risk factor of chronic obstructive pulmonary disease. Respir Med. 2009;103:1866–1870. doi: 10.1016/j.rmed.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yanagisawa S, Baker JR, Vuppusetty C, Fenwick P, Donnelly LE, Ito K, et al. Decreased phosphatase PTEN amplifies PI3K signaling and enhances proinflammatory cytokine release in COPD. Am J Physiol Lung Cell Mol Physiol. 2017;313:L230–L239. doi: 10.1152/ajplung.00382.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol. 2000;12:361–378. doi: 10.1006/smim.2000.0223. [DOI] [PubMed] [Google Scholar]
- 158.Kamata T, Yamashita M, Kimura M, Murata K, Inami M, Shimizu C, et al. src homology 2 domain-containing tyrosine phosphatase SHP-1 controls the development of allergic airway inflammation. J Clin Invest. 2003;111:109–119. doi: 10.1172/JCI15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jang MK, Kim SH, Lee KY, Kim TB, Moon KA, Park CS, et al. The tyrosine phosphatase, SHP-1, is involved in bronchial mucin production during oxidative stress. Biochem Biophys Res Commun. 2010;393:137–143. doi: 10.1016/j.bbrc.2010.01.102. [DOI] [PubMed] [Google Scholar]
- 160.Zhang L, Oh SY, Wu X, Oh MH, Wu F, Schroeder JT, et al. SHP-1 deficient mast cells are hyperresponsive to stimulation and critical in initiating allergic inflammation in the lung. J Immunol. 2010;184:1180–1190. doi: 10.4049/jimmunol.0901972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Oh SY, Zheng T, Kim YK, Cohn L, Homer RJ, McKenzie AN, et al. A critical role of SHP-1 in regulation of type 2 inflammation in the lung. Am J Respir Cell Mol Biol. 2009;40:568–574. doi: 10.1165/rcmb.2008-0225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Patterson H, Nibbs R, McInnes I, Siebert S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol. 2014;176:1–10. doi: 10.1111/cei.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thomas A, Rajan A, Giaccone G. Tyrosine kinase inhibitors in lung cancer. Hematol Oncol Clin North Am. 2012;26:589–605. doi: 10.1016/j.hoc.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 165.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 166.Fala L. OFEV (nintedanib): first tyrosine kinase inhibitor approved for the treatment of patients with idiopathic pulmonary fibrosis. Am Health Drug Benefits. 2015;8:101–104. [PMC free article] [PubMed] [Google Scholar]
- 167.Tyryshkin A, Bhattacharya A, Eissa NT. SRC kinase is a novel therapeutic target in lymphangioleiomyomatosis. Cancer Res. 2014;74:1996–2005. doi: 10.1158/0008-5472.CAN-13-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Aono Y, Nishioka Y, Inayama M, Ugai M, Kishi J, Uehara H, et al. Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 2005;171:1279–1285. doi: 10.1164/rccm.200404-531OC. [DOI] [PubMed] [Google Scholar]
- 169.Azuma M, Nishioka Y, Aono Y, Inayama M, Makino H, Kishi J, et al. Role of α1-acid glycoprotein in therapeutic antifibrotic effects of imatinib with macrolides in mice. Am J Respir Crit Care Med. 2007;176:1243–1250. doi: 10.1164/rccm.200702-178OC. [DOI] [PubMed] [Google Scholar]
- 170.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR Imatinib-IPF Study Investigators. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 171.Kitagawa D, Yokota K, Gouda M, Narumi Y, Ohmoto H, Nishiwaki E, et al. Activity-based kinase profiling of approved tyrosine kinase inhibitors. Genes Cells. 2013;18:110–122. doi: 10.1111/gtc.12022. [DOI] [PubMed] [Google Scholar]
- 172.Broekman F, Giovannetti E, Peters GJ. Tyrosine kinase inhibitors: Multi-targeted or single-targeted? World J Clin Oncol. 2011;2:80–93. doi: 10.5306/wjco.v2.i2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Levitzki A, Mishani E. Tyrphostins and other tyrosine kinase inhibitors. Annu Rev Biochem. 2006;75:93–109. doi: 10.1146/annurev.biochem.75.103004.142657. [DOI] [PubMed] [Google Scholar]
- 174.Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1434–1445. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Levitzki A. Tyrosine kinase inhibitors: views of selectivity, sensitivity, and clinical performance. Annu Rev Pharmacol Toxicol. 2013;53:161–185. doi: 10.1146/annurev-pharmtox-011112-140341. [DOI] [PubMed] [Google Scholar]
- 176.Min JH, Lee HY, Lim H, Ahn MJ, Park K, Chung MP, et al. Drug-induced interstitial lung disease in tyrosine kinase inhibitor therapy for non-small cell lung cancer: a review on current insight. Cancer Chemother Pharmacol. 2011;68:1099–1109. doi: 10.1007/s00280-011-1737-2. [DOI] [PubMed] [Google Scholar]
- 177.Shah RR. Tyrosine kinase inhibitor-induced interstitial lung disease: clinical features, diagnostic challenges, and therapeutic dilemmas. Drug Saf. 2016;39:1073–1091. doi: 10.1007/s40264-016-0450-9. [DOI] [PubMed] [Google Scholar]
- 178.Weatherald J, Chaumais MC, Montani D. Pulmonary arterial hypertension induced by tyrosine kinase inhibitors. Curr Opin Pulm Med. 2017;23:392–397. doi: 10.1097/MCP.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 179.Hoeper MM, Barst RJ, Bourge RC, Feldman J, Frost AE, Galié N, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation. 2013;127:1128–1138. doi: 10.1161/CIRCULATIONAHA.112.000765. [DOI] [PubMed] [Google Scholar]
- 180.Nagaraj C, Tang B, Bálint Z, Wygrecka M, Hrzenjak A, Kwapiszewska G, et al. Src tyrosine kinase is crucial for potassium channel function in human pulmonary arteries. Eur Respir J. 2013;41:85–95. doi: 10.1183/09031936.00211811. [DOI] [PubMed] [Google Scholar]
- 181.Wang X, Roy A, Hochhaus A, Kantarjian HM, Chen TT, Shah NP. Differential effects of dosing regimen on the safety and efficacy of dasatinib: retrospective exposure-response analysis of a phase III study. Clin Pharmacol. 2013;5:85–97. doi: 10.2147/CPAA.S42796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Scott LM, Lawrence HR, Sebti SM, Lawrence NJ, Wu J. Targeting protein tyrosine phosphatases for anticancer drug discovery. Curr Pharm Des. 2010;16:1843–1862. doi: 10.2174/138161210791209027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McConnell JL, Wadzinski BE. Targeting protein serine/threonine phosphatases for drug development. Mol Pharmacol. 2009;75:1249–1261. doi: 10.1124/mol.108.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Goldfine AB, Simonson DC, Folli F, Patti ME, Kahn CR. In vivo and in vitro studies of vanadate in human and rodent diabetes mellitus. Mol Cell Biochem. 1995;153:217–231. doi: 10.1007/BF01075941. [DOI] [PubMed] [Google Scholar]
- 185.He RJ, Yu ZH, Zhang RY, Zhang ZY. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol Sin. 2014;35:1227–1246. doi: 10.1038/aps.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]