Abstract
Mechanical ventilation with O2-rich gas (MV-O2) inhibits alveologenesis and lung growth. We previously showed that MV-O2 increased elastase activity and apoptosis in lungs of newborn mice, whereas elastase inhibition by elafin suppressed apoptosis and enabled lung growth. Pilot studies suggested that MV-O2 reduces lung expression of prosurvival factors phosphorylated epidermal growth factor receptor (pEGFR) and Krüppel-like factor 4 (Klf4). Here, we sought to determine whether apoptosis and lung growth arrest evoked by MV-O2 reflect disrupted pEGFR-Klf4 signaling, which elafin treatment preserves, and to assess potential biomarkers of bronchopulmonary dysplasia (BPD). Five-day-old mice underwent MV with air or 40% O2 for 8–24 hours with or without elafin treatment. Unventilated pups served as controls. Immunoblots were used to assess lung pEGFR and Klf4 proteins. Cultured MLE-12 cells were exposed to AG1478 (EGFR inhibitor), Klf4 siRNA, or vehicle to assess effects on proliferation, apoptosis, and EGFR regulation of Klf4. Plasma elastase and elafin levels were measured in extremely premature infants. In newborn mice, MV with air or 40% O2 inhibited EGFR phosphorylation and suppressed Klf4 protein content in lungs (vs. unventilated controls), yielding increased apoptosis. Elafin treatment inhibited elastase, preserved lung pEGFR and Klf4, and attenuated the apoptosis observed in lungs of vehicle-treated mice. In MLE-12 studies, pharmacological inhibition of EGFR and siRNA suppression of Klf4 increased apoptosis and reduced proliferation, and EGFR inhibition decreased Klf4. Plasma elastase levels were more than twofold higher, without a compensating increase of plasma elafin, in infants with BPD, compared to infants without BPD. These findings indicate that pEGFR-Klf4 is a novel prosurvival signaling pathway in lung epithelium that MV disrupts. Elafin preserves pEGFR-Klf4 signaling and inhibits apoptosis, thereby enabling lung growth during MV. Together, our animal and human data raise the question: would elastase inhibition prevent BPD in high-risk infants exposed to MV-O2?
Keywords: bronchopulmonary dysplasia, EGFR and Klf4 proteins, elastase inhibition, lung growth arrest, neonatal chronic lung disease
Clinical Relevance
This paper presents novel and important findings related to the molecular basis (EGFR-Klf4 signaling) and potential treatment (by elafin, which inhibits elastase activity and activates pEGFR-Klf4 signaling) of neonatal lung injury caused by lengthy mechanical ventilation. The paper also presents new and important clinical data demonstrating that elevated plasma levels of neutrophil elastase and increased urinary excretion of desmosine, an elastin degradation product, may serve as biomarkers of evolving bronchopulmonary dysplasia (neonatal chronic lung disease).
Respiratory failure often complicates the postnatal course of extremely preterm infants whose lungs are incompletely developed. Treatment of such infants with mechanical ventilation (MV) and supplemental O2 can be lifesaving, but if prolonged, it can elicit a chronic form of lung injury called bronchopulmonary dysplasia (BPD) (1). The clinical and pathological features of this condition have changed considerably in recent years, likely reflecting major advances in perinatal care, including routine antenatal glucocorticoid treatment, postnatal surfactant replacement, and better respiratory and nutritional support. The salient pathological feature of this BPD variant, here defined as neonatal chronic lung disease (nCLD), is impaired formation of alveoli and pulmonary microvessels (2–4), often attributed to lengthy exposure of developing lungs to high concentrations of inspired O2 (5) and associated lung cell apoptosis (6, 7). It is noteworthy, however, that nCLD rarely (if ever) develops without a history of MV, and sometimes occurs after only a brief exposure to extra O2 (8).
The incidence of nCLD remains high, especially among extremely preterm (<28 weeks gestation) infants (9), who remain at risk for long-term respiratory dysfunction and disease, including bronchial asthma during childhood, and pulmonary emphysema in adults (10). Effective treatment or prevention of ventilator-induced lung injury (VILI) evolving into nCLD likely will derive from elucidating molecular mechanisms that cause the initial injury, which is a central aim of this study.
Authentic animal models of nCLD, featuring lengthy MV-O2 of surfactant-treated, premature neonatal baboons and lambs, showed lung growth arrest linked to failed formation of alveoli and pulmonary microvessels, with epithelial and endothelial cell apoptosis, and matrix remodeling in response to increased lung elastase activity (11–15). Studies with these animal models have identified dysregulated signaling of various growth factors (e.g., vascular endothelial growth factor [VEGF], platelet-derived growth factor [PDGF], and transforming growth factor β [TGF-β]) in the pathogenesis of nCLD. Studies of mechanically ventilated newborn mice likewise showed impaired alveolarization and angiogenesis linked to aberrant VEGF, PDGF, and TGF-β signaling, with degradation and dispersion of elastin and increased apoptosis (16–18). These adverse effects of MV-O2 were suppressed by treatment with the elastase inhibitor elafin, which enabled alveolar formation and lung growth (19).
Because epidermal growth factor receptor (EGFR) signaling plays a critical role in lung development (20–22) and can promote epithelial cell survival and proliferation (23), we queried whether prolonged MV-O2 of newborn mice might inhibit EGFR signaling, thereby contributing to lung growth arrest, as seen in nCLD. Preliminary studies showed that MV-O2 reduced EGFR activity and also suppressed lung expression of Krüppel-like factor 4 (Klf4), a zinc-finger transcription factor that plays a major role in regulating the postnatal proliferation, migration, and differentiation of intestinal epithelial cells (24, 25). Therefore, we performed studies to test the hypothesis that apoptosis and lung growth arrest evoked by MV-O2 reflect disrupted phosphorylated EGFR (pEGFR)-Klf4 signaling, and to determine whether treatment with elafin, which enabled lung growth in prior studies of mechanically ventilated newborn mice (19, 26), would preserve pEGFR-Klf4 signaling and protect against apoptosis during lengthy MV-O2.
In addition to studies with neonatal mice and cultured MLE cells, we conducted human studies in which premature infants born at <32 weeks of gestation were followed in the neonatal ICU at Lucile Packard Children’s Hospital to determine whether infants destined to acquire nCLD had higher levels of elastase and lower levels of elafin in plasma, and greater urinary excretion of desmosine, an elastin breakdown product, than infants without nCLD.
Methods
Experimental Design
This investigation comprised five sets of experiments: 1) 8-hour pilot studies to assess the short-term effects of MV-O2 on lung expression of pEGFR, Klf4, and Akt (cell survival); 2) 24-hour studies to determine the long-term effects of MV-Air and MV-O2 on the lung protein content of pEGFR and Klf4, and cell survival; 3) 16- to 24-hour studies to assess the impact of elafin on pEGFR-Klf4 signaling and apoptosis during MV-O2; 4) in vitro studies to assess EGFR-Klf4 linkage and its impact on survival of cultured MLE-12 cells (ATCC); and 5) clinical studies of plasma elastase and elafin levels in preterm infants with evolving nCLD.
In vivo studies
Three-day-old (8-h pilot studies) and 5-day-old (subsequent 8-h and 16- to 24-h studies) full-term Balb/c mice were randomly assigned to either MV or unventilated controls (see data supplement for respiratory data; Table E1). In a subset of 16- to 24-hour studies, recombinant human elafin (Proteo-Biotech-AG) was given intratracheally (1 μg/g body weight [bw]) immediately before MV began. In the 16-hour studies, an additional elafin dose (2 μg/g bw) was given subcutaneously after 8 hours of MV. Ventilation data, anesthesia, routine care, and physiological monitoring (16, 19) are described in the data supplement. Surgical procedures and experimental protocols were approved by Stanford University’s institutional animal care and use committee. For further details, see the data supplement.
Tissue Assays
Elastase activity assay
Lung tissue was stored at −80°C for measurement of serine elastase activity using the DQ-elastin substrate assay (15). For further details, see the data supplement.
Protein extraction and immunoblots
Lungs were frozen in liquid N2 and stored at −80°C for protein extraction and immunoblot protein measurements as previously described (16, 17). For further details, see the data supplement.
RNA extraction and mRNA measurement
RNA extraction and qRT-PCR were performed as previously described (16) to measure mRNA expression relative to 18S ribosomal RNA. For further details, see the data supplement.
Immunohistochemistry to assess Klf4 protein
Standard immunohistochemistry techniques, as previously described (15, 19), were applied for semiquantitative assessments of nuclear Klf4 protein in the lungs of newborn mice that received MV-O2 for 24 hours after treatment with either Ringer’s lactate (R/L) or elafin compared with controls. For further details, see the data supplement.
Cell Culture Studies
pEGFR inhibition
MLE-12 cells were exposed to AG1478, an EGFR inhibitor, or vehicle (DMSO). For further details, see the data supplement.
siRNA transfection
Anti-Klf4 siRNA or control siRNA was transfected into subcultured MLE-12 cells. For further details, see the data supplement.
Cell survival
Apoptosis was assessed using the Caspase-Glo 3/7 assay. Proliferation was assessed by MTT assay. Total luminescence and absorbance were measured in a plate reader. For further details, see the data supplement.
Clinical Biomarker Studies
Fifty-five infants born at <32 weeks of gestation were enrolled into a longitudinal cohort of patients at Lucile Packard Children’s Hospital. Stanford University’s institutional review board approved the study. After informed parental consent was obtained, the infants were followed until 36 weeks after conception to determine whether they had acquired nCLD. Plasma samples were assayed by ELISA for human neutrophil elastase (HNE) and elafin, and urine desmosine was measured by mass spectrometry. For further details, see the data supplement.
Statistics
Continuous outcomes are expressed as mean ± SD. We used the two-sample t test or ANOVA followed by a Bonferroni, Kruskal-Wallis, or Dunn’s multiple-comparison test to compare outcomes between groups, and linear regression to assess associations (27). Prism-6 software (GraphPad) was used for statistical analyses, with P < 0.05 denoting a significant difference. For further details, see the data supplement.
Results
MV Inhibits EGFR Activation and Klf4 Expression, and Reduces Cell Survival in Lungs of Newborn Mice
Our first goal was to determine whether the lung cell apoptosis and impaired alveolarization observed in newborn mice after lengthy MV with either air or 40% O2, as previously reported (18), might be linked to defective EGFR signaling, which plays a critical role in postnatal lung growth and development (20–22), and if so, whether this might lead to downstream suppression of Klf4, a gene known for its role in generating induced pluripotent stem cells (28) and contributing to epithelial cell survival and proliferation (23–25).
Pilot studies showed that MV-O2 for 8 hours reduced the lung abundance of pEGFR protein by ∼50%, indicating reduced receptor activation (Figure E1A). Concurrently, the lung abundance of phosphorylation of Akt (pAkt) protein decreased by >50%, consistent with diminished cell survival (Figure E1B). Neither EGFR nor its ligands (amphiregulin, TGF-α, and epiregulin) showed a decrease in mRNA expression in response to MV-O2 for 8 hours (Figure E2), whereas lung mRNA expression of both EGFR and amphiregulin increased after 8 hours of MV-O2. The lung abundance of EGFR and amphiregulin proteins was unchanged compared with control values after MV-O2 for 8 hours (Figures E1A and E3A), demonstrating that reduced pEGFR was not attributable to diminished lung expression of EGFR or its ligand amphiregulin. Amphiregulin protein, however, did increase after MV-O2 for 24 hours (Figure E3B). The finding that amphiregulin was increased but pEGFR was decreased after 24 hours of MV-O2 suggests that the delayed increased abundance of ligand likely developed in response to decreased activation of pEGFR, without a corresponding increase of EGFR phosphorylation, perhaps related to proteolytic activity linked to lengthy MV-O2. MV with either air or 40% O2 (MV-Air, MV-O2) for 8 hours suppressed lung mRNA expression of Klf4 (Figure E4), evoking speculation that disrupted pEGFR-Klf4 signaling might account for the lung growth arrest observed in newborn mice after prolonged MV-O2.
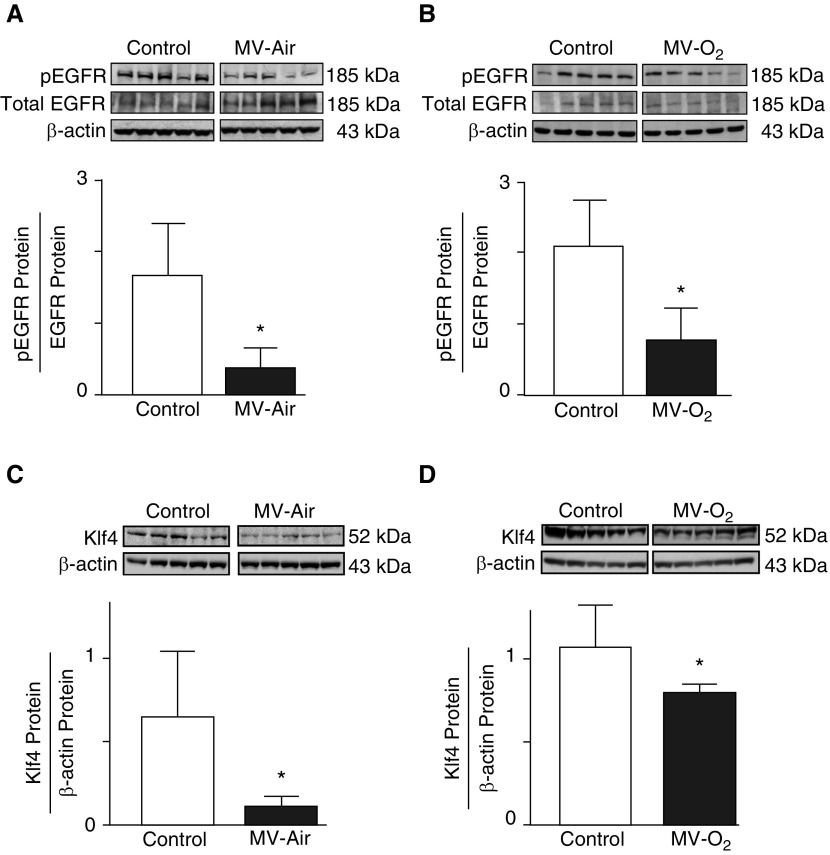
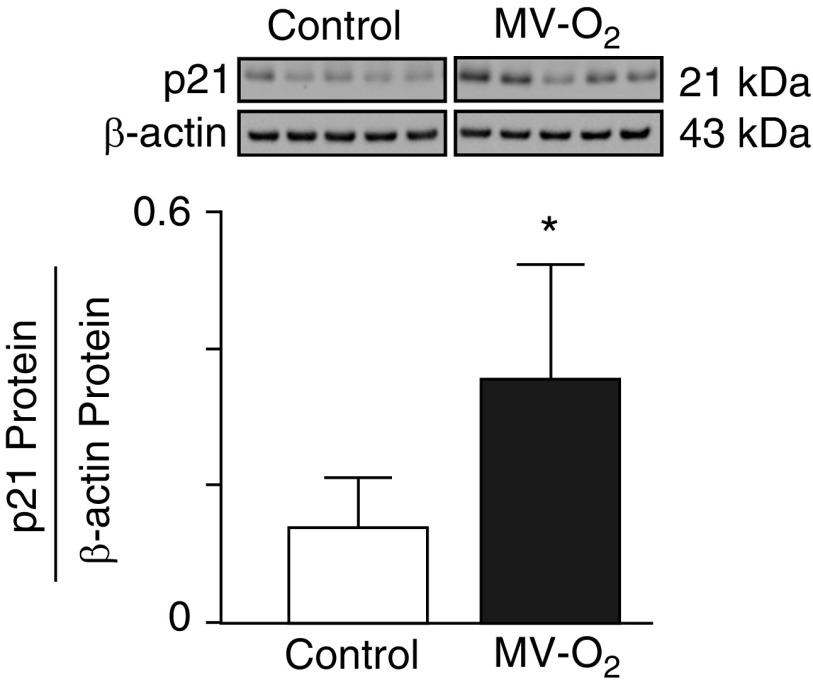
These preliminary results led us to assess the impact of longer MV (24 h) with either air or 40% O2 on the lung abundance of pEGFR, Klf4, and p21 (cell survival index) proteins. MV-Air and MV-O2 for 24 hours decreased the lung content of pEGFR (Figures 1A and 1B) and Klf4 (Figures 1C and 1D) proteins. Reduced pEGFR and Klf4 protein abundance, elicited by MV, yielded a more than twofold increase of p21 protein (Figure 2), reflecting diminished lung cell survival.
Figure 1.
(A and B) Mechanical ventilation (MV) with either air (MV-Air) or 40% O2 (MV-O2) for 24 hours inhibits phosphorylation of epidermal growth factor receptor (pEGFR) protein in lungs of 5-day-old mice. pEGFR, total EGFR, and β-actin proteins were measured by immunoblot of lung homogenates from mice after 24 hours of exposure to MV-Air (A) or MV-O2 (B). Control pups breathed air or 40% O2 without MV for 24 hours. The graphs display pEGFR relative to total EGFR protein, with β-actin as a loading control (n = 5/group; mean and SD; *P < 0.05). (C and D) Exposure to MV-Air or MV-O2 for 24 hours reduced the Krüppel-like factor 4 (Klf4) protein content in lungs of 5-day-old mice. Klf4 and β-actin proteins were measured by immunoblot of total lung homogenates from mice after 24 hours of exposure to MV-Air (C) or MV-O2 (D). Control pups breathed air or 40% O2 without MV for 24 hours. The graphs display Klf4 protein relative to β-actin (n = 5/group; mean and SD; *P < 0.05).
Figure 2.
MV-O2 for 24 hours increased p21 (cyclin-dependent kinase inhibitor-1) in lungs of 5-day-old mice compared with control pups that breathed 40% O2 without MV for 24 hours. p21 protein was measured by immunoblot of total lung homogenates and is expressed relative to β-actin (n = 5/group; mean and SD; *P < 0.05).
Inhibiting EGFR Phosphorylation or Suppressing Klf4 Decreases the Survival of Cultured Lung Epithelial Cells
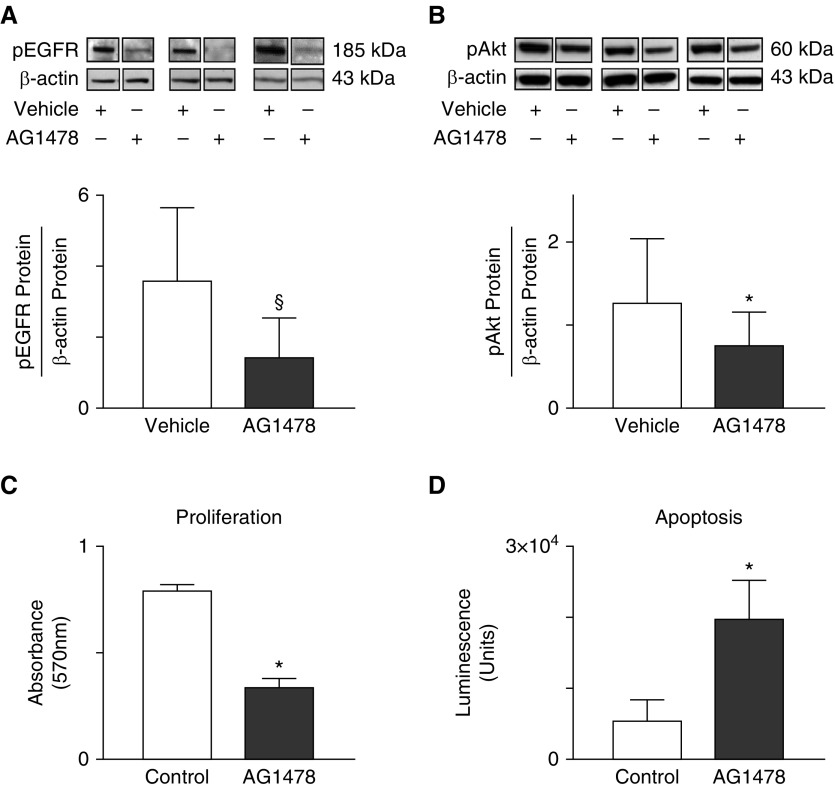
These findings prompted us to consider a possible link between impaired pEGFR-Klf4 signaling and lung cell survival in studies using cultured MLE-12 cells. Exposure of MLE-12 cells to tyrphostin (AG1478), an EGFR tyrosine kinase inhibitor, reduced pEGFR (Figure 3A) in three independent experiments, whereas the cell content of total EGFR protein was unchanged (data not shown). pEGFR inhibition decreased pAkt protein by 40% (Figure 3B), indicating reduced cell survival. Consistent with these findings, cell proliferation decreased (Figure 3C) and apoptosis increased (Figure 3D) in response to AG1478.
Figure 3.
(A and B) EGFR regulates Akt signaling and survival of cultured MLE cells. MLE-12 cells were grown to 80% confluency, starved with serum-reduced medium overnight, and then exposed to AG1478 (10 μM) for 60 minutes in serum-rich medium. (A) Tyrphostin, also known as AG1478, attenuates pEGFR, as indicated by consistently reduced pEGFR relative to β-actin protein (n = 3; mean and SD; §P = 0.07 by paired t test). (B) Inhibiting activation of EGFR by exposing cells to AG1478 blocks phosphorylation of Akt (pAkt), expressed relative to β-actin. Three representative immunoblots from a total of six studies are shown (n = 6/group; mean and SD; *P < 0.05 by Wilcoxon signed-rank test). (C and D) Inhibition of pEGFR by exposure to AG1478 reduces proliferation and increases apoptosis of cultured MLE-12 cells compared with cells exposed to vehicle (DMSO). (C) To assess proliferation, cells were grown in 96-well plates until they were nearly confluent, exposed to serum-reduced medium overnight, and then exposed to AG1478 for 24 hours. Cell proliferation was measured with the MTT assay (n = 6; mean and SD; *P < 0.05). (D) To assess apoptosis, cells were grown in 96-well plates until they were nearly confluent, exposed to serum-reduced medium overnight, and then exposed to AG1478 for 9 hours in serum-free medium. Apoptosis was measured with the Caspase-Glo 3/7 assay (n = 6; mean and SD; *P < 0.05).
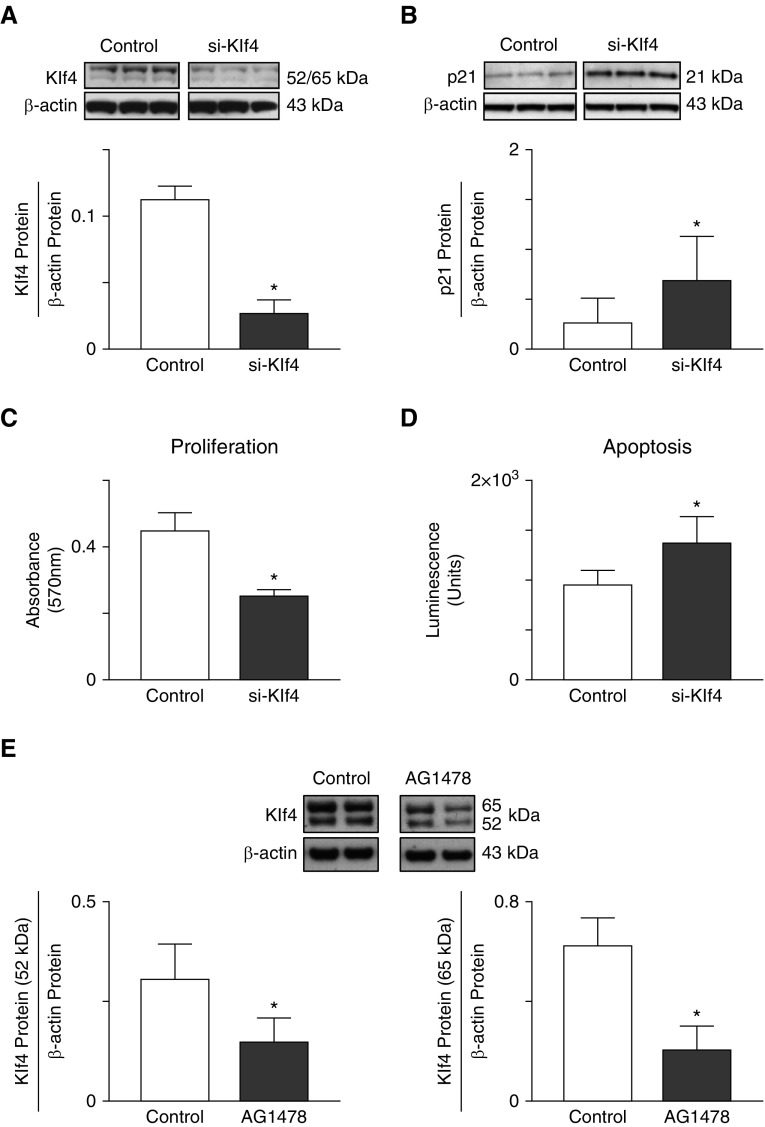
Based on our in vivo data supporting a possible association between Klf4 expression and epithelial cell survival, we tested the effect of siRNA suppression of Klf4 protein abundance in cultured MLE-12 cells (Figure 4A), and found that it increased p21 protein, a downregulator of cell-cycle progression, by more than twofold (Figure 4B). Likewise, Klf4 suppression by siRNA transfection yielded reduced proliferation (Figure 4C) and increased apoptosis (Figure 4D) of MLE-12 cells, confirming the importance of Klf4 in maintaining epithelial cell viability.
Figure 4.
(A and B) Suppression of Klf4 reduces survival of cultured MLE-12 cells. (A) Transfection with siRNA against Klf4 using lipofectamine reduces Klf4 protein abundance in MLE-12 cells 72 hours after transfection compared with cells transfected with noncoding siRNA (control). Klf4 protein was assessed by immunoblot and related to β-actin (mean and SD; n = 3; *P < 0.05). (B) The cell content of p21 protein, a downregulator of cell-cycle progression, is increased in Klf4-depleted cells compared with Klf4-replete cells after 72 hours in serum-rich culture medium (three blots per group, shown from a total of n = 6 experiments; mean and SD; *P < 0.05). (C and D) Klf4 suppression by siRNA transfection results in decreased proliferation and increased apoptosis of MLE-12 cells cultured for 24 hours in serum-reduced medium compared with Klf4-expressing (control) cells. Cell proliferation was measured by the MTT assay (n = 5; mean and SD; *P < 0.05). MLE-12 cell apoptosis, measured by the Caspase-Glo 3/7 assay, is increased in Klf4-depleted cells compared with Klf4-replete cells after 72 hours in culture with serum-rich medium (n = 6; mean and SD; *P < 0.05). (E) Inhibition of EGFR phosphorylation reduces Klf4 protein content in cultured mouse lung epithelial cells. Cells were grown to confluence and exposed to Tyrphostin (AG1478, 10 μM) for 24 hours in serum-rich medium. Klf4 protein was assessed by immunoblot, and quantitative densitometry was performed for two bands, one at 52 kD (left panel) and another at 65 kD (right panel), relative to β-actin. Two representative immunoblots/group, and the corresponding densitometric measurements for each band obtained from five studies are displayed (n = 5; mean and SD; *P < 0.05).
EGFR Signaling Regulates Klf4 Protein Expression in Lung Epithelial Cells
To test for linkage between EGFR activation and Klf4 content in lung epithelial cells, MLE-12 cells were grown to confluence and treated with AG1478 for 24 hours in FBS-rich medium. In five such studies, the cell content of Klf4 protein decreased by ≥50% in response to inhibition of pEGFR (Figures 4E and 4F), suggesting that EGFR activation has an important role in regulating Klf4 abundance in MLE cells.
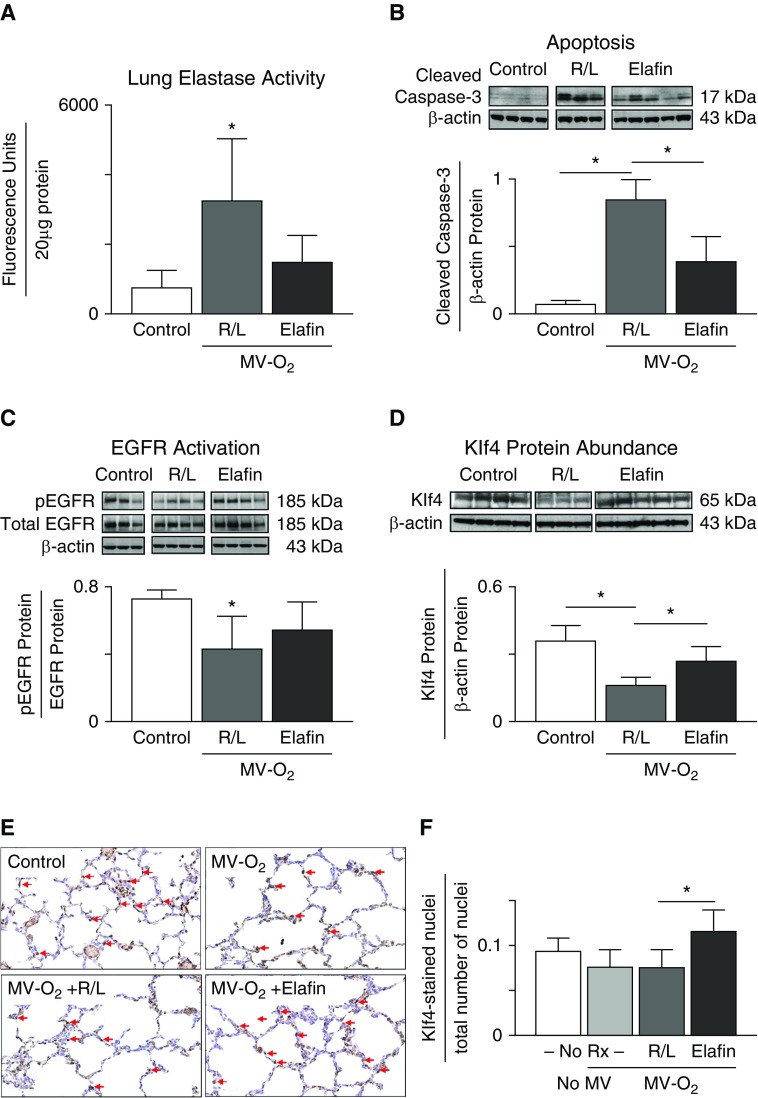
Elafin Treatment during MV-O2 Suppresses Lung Elastase Activity and Apoptosis, Preventing the Reduced pEGFR and Klf4 Observed in the Lungs of Control Mice
Prior studies from our lab showed that inhibiting protease activity, specifically elastase and matrix metalloproteinase-9 (MMP-9) activity, in mechanically ventilated newborn mice by treatment with elafin prevented the degradation of lung elastin that occurred in control pups exposed to lengthy MV-O2, and thereby suppressed apoptosis, which allowed for alveolar septation and lung growth (19). We questioned whether the protective effect of elafin on lung growth during MV-O2 might relate to preserved pEGFR-Klf4 signaling. We discovered that elafin treatment of mechanically ventilated 5-day-old mice, besides suppressing lung elastase activity (Figure 5A) and apoptosis (Figure 5B), mitigated the decrease of pEGFR (Figure 5C) and Klf4 (Figure 5D) observed in the lungs of vehicle-treated control pups during MV-O2. Immunohistochemistry for Klf4 in histologic sections of lung taken from newborn pups after MV-O2 for 24 hours showed that elafin treatment increased the lung abundance of nuclear Klf4 protein compared with that observed in R/L-treated controls (Figures 5E and 5F).
Figure 5.
(A and B) Treatment with elafin suppresses elastase activity and protects the lungs of 5-day-old mice from apoptosis during lengthy MV with 40% O2 (MV-O2). (A) For this set of studies, pups received MV-O2 for 16 hours after treatment with elafin (1 μg/g body weight [bw]) or vehicle (Ringer’s lactate [R/L]) via tracheotomy, followed by subcutaneous injection of either elafin (2 μg/g bw) or R/L at the midpoint (8 h) of each study. Unventilated control pups breathed 40% O2 without MV for 16 hours. Elastase activity was measured in whole-lung homogenates by the DQ-elastin assay (n = 3–4/group; mean and SD; *P < 0.05). (B) For this set of studies, pups were treated via tracheotomy with either elafin (1 μg/g bw) or R/L just before MV-O2 for 24 hours. Unventilated control pups breathed 40% O2 without MV for 24 hours. Apoptosis was measured in lung protein extracts by immunoblot, using cleaved caspase-3 as an indicator of apoptosis; β-actin served as the loading control (n = 3–5/group; mean and SD; *P < 0.05). (C and D) Elafin treatment mitigates the decrease of pEGFR and Klf4 protein abundance in the lungs of mechanically ventilated 5-day-old mice. (C) For this set of studies, pups received MV-O2 for 16 hours after treatment with either elafin (1 μg/g bw) or vehicle (R/L) delivered via tracheotomy; a second dose of elafin (2 μg/g bw) or R/L was given by subcutaneous injection at the midpoint of each study. Control pups breathed 40% O2 without MV. pEGFR, expressed relative to total EGFR protein, was reduced in the lungs of R/L-treated pups after MV-O2 for 16 hours, and this change was attenuated by elafin treatment (n = 3–4/group; mean and SD; *P < 0.05). (D) For this set of studies, pups received MV-O2 for 24 hours after treatment with either elafin (1 μg/g bw) or R/L given via tracheotomy. The lung content of Klf4 protein, expressed relative to β-actin, was reduced in R/L-treated pups, an effect that was attenuated by elafin (n = 3–5/group; mean and SD; *P < 0.05). (E and F) Elafin treatment increased nuclear Klf4 protein in lungs of 5-day-old mice after MV-O2 for 24 hours. (E) Representative images showing Klf4-stained lung sections obtained from unventilated pups (control) that breathed 40% O2 for 24 hours, and pups exposed to MV-O2 for 24 hours after receiving either no treatment (No Rx, No MV-O2) or intratracheal treatment with vehicle (R/L, MV-O2 + R/L) or with elafin (1 μg/g bw; MV-O2 + elafin); red arrows denote Klf4 stained nuclei. (F) Respective quantitative data for Klf4-stained nuclei relative to the total number of nuclei for the four groups of mice (n = 3–4/group; mean and SD; *significant difference between groups, P < 0.05).
High Plasma Levels of HNE Serve as a Biomarker of nCLD in Premature Infants
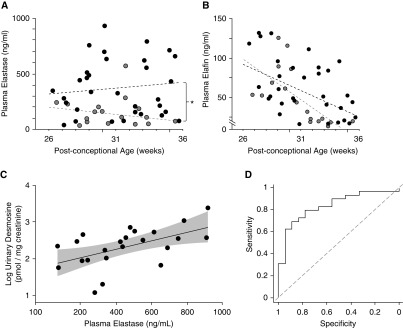
Previous reports postulated that HNE might play a critical role in the pathogenesis of nCLD (29–31). Those studies showed elevated elastase in airway secretions of preterm infants with evolving nCLD. Based on these reports, and observations of increased lung elastase activity in preterm lambs with evolving nCLD (15) and newborn mice exposed to lengthy MV-O2 (17), we conducted a prospective study to determine whether plasma levels of HNE might enable early postnatal detection of nCLD. Of 55 infants born at <32 weeks of gestation (29 ± 3 weeks, birth weight 1.4 ± 0.6 kg) enrolled in the study, 15 acquired nCLD, as assessed by the need for supplemental O2 at 36 weeks after conception. All infants with nCLD were born at ≤29 weeks of gestation and received respiratory support (MV or continuous positive airway pressure) for varying periods, yielding a 55% incidence (15/27) of nCLD in this group of extremely premature infants. Additional details are provided in the data supplement, which includes two tables listing demographic and clinical data for infants with and without nCLD.
Plasma HNE levels were significantly higher in infants with evolving nCLD than in infants without nCLD (401 ± 143 vs. 177 ± 179 ng/ml, respectively; P < 0.01) (Figure 6A). Plasma elafin levels were similar in infants with and without evolving nCLD (63 ± 23 vs. 55 ± 27 ng/ml, respectively) (Figure 6B), suggesting that preterm infants with evolving nCLD were unable to generate a sufficient compensatory response to counterbalance heightened HNE levels. Urine desmosine excretion did not differ significantly between infants who acquired nCLD and those who did not. There was, however, a significant correlation between plasma HNE and urine desmosine levels among infants with nCLD (Figure 6C). The value of plasma HNE for differentiating between infants with and without nCLD was assessed by means of a receiver operating characteristic (ROC) analysis, which yielded an area under the ROC curve of 0.835 (95% confidence interval, 0.73–0.94), confirming a robust discriminating power for HNE measurements to predict evolving nCLD (Figure 6D). Adjustments for gestational age did not alter this strong relationship between HNE and outcome.
Figure 6.
(A and B) Human neutrophil elastase (HNE) protein (ng/ml) and elafin protein (ng/ml) levels in plasma of very premature infants (gestational age <32 weeks at birth), showing significantly greater elastase levels over time (A), without a corresponding increase of elafin levels over time (B), in infants with evolving neonatal chronic lung disease (nCLD; black circles; n = 15) compared with control infants who did not acquire nCLD (gray circles; n = 8). Dashed black regression lines represent plasma levels over time for very premature infants with evolving nCLD; dashed gray regression lines represent plasma levels over time for control very premature infants who did not acquire nCLD. For plasma elastase levels (A), the difference between groups (nCLD compared with non-nCLD) was significant, *P < 0.01. For plasma elafin levels (B), there was no significant difference between nCLD and non-nCLD, though there was a trend (P > 0.05) for elafin levels to be higher in the infants with nCLD compared with those without nCLD in the interval between 32 and 36 weeks gestation. (C) A log plot of urine desmosine concentration, expressed relative to urine creatinine concentration, correlates with elastase concentrations measured in plasma samples obtained from 15 preterm infants with evolving nCLD. The oblique line represents the regression, and the shaded area is the 95% confidence interval (CI) for the regression line (r = 0.54, P < 0.01). The 23 plotted points include measurements made on repeated samples obtained from some of the infants; the regression is adjusted for repeated measures. (D) Receiver operating characteristic (ROC) curve for the relationship between HNE measured in plasma and the development of nCLD among premature infants. The value of plasma HNE for differentiating between nCLD and no nCLD was assessed by the area under the ROC curve, which at 0.835 (95% CI, 0.730–0.938) provided evidence of a robust discriminating power for HNE measured in plasma as a predictor of evolving nCLD. Adjustments for gestational age did not alter this strong relationship between HNE and outcome.
Discussion
MV-O2 in Newborn Mice: Model of Failed Alveolarization Leading to Lung Growth Arrest
nCLD typically develops in extremely preterm infants whose incompletely developed lungs are exposed to lengthy MV-O2. Impaired alveolarization, associated with lung cell apoptosis and increased deposition of disordered matrix elastin, are prominent features of nCLD. These features are recapitulated in newborn mice, wherein prolonged MV-O2 increases lung elastase activity, causing dysregulated elastin synthesis and assembly, apoptosis, and defective alveologenesis (16–18). Treatment with elafin, a serine elastase inhibitor that is expressed in humans but not in rodents, mitigates the adverse pulmonary effects of MV-O2 in newborn mice (19, 26).
Elafin Suppresses Lung Elastase Activity, Reduces Apoptosis, and Preserves the pEGFR-Klf4 Axis
The molecular mechanisms responsible for the lung growth arrest seen in nCLD remain obscure, but likely reflect aberrant signaling between various growth and transcription factors within the developing lung. This study identifies a novel prosurvival EGFR-Klf4 link between EGFR and Klf4 that is inhibited during lengthy MV of newborn mice. Elafin treatment reduces apoptosis in ventilated newborn lungs, as it blocks elastase activity and preserves pEGFR-Klf4 signaling. This could account for elafin’s benefit in promoting alveologenesis and lung growth in ventilated newborn mice (19, 26). These experimental findings, coupled with our observation of increased plasma elastase in preterm infants with evolving nCLD, support speculation that elafin treatment might prevent or lessen the severity of neonatal lung injury evoked by MV-O2. This notion merits further study in authentic large animal models of lengthy MV-O2 that mimic the clinical and pathological features of nCLD.
EGFR in Lung Growth and Injury
EGFR signaling regulates lung development (20–22) and cell homeostasis (32, 33). Prior studies showed that EGF ligand stimulates the branching morphogenesis and growth of embryonic mouse lungs in culture (20), and that EGFR mediates stretch-induced transdifferentiation of cultured fetal MLE cells (34), indicating that EGFR signaling plays an important role in stimulating epithelial cell proliferation and lung growth. Conversely, the lungs of mice in which EGFR is deficient exhibit impaired alveologenesis and greatly diminished surface area for gas exchange (21, 22). A recent study showed that EGFR signaling selectively stimulates the proliferation of alveolar type II cells (23). The same report presented compelling evidence that EGFR plays an important role in self-renewal of the stem cell program, promoting expansion of epithelial progenitor cells and thereby contributing to lung growth and regeneration after injury. Our studies of ventilated newborn mice show that mechanical stretch of the lungs during early postnatal life inhibits EGFR phosphorylation and causes reduced lung cell survival and impaired alveologenesis. The in vitro studies with MLE-12 cells confirm the importance of EGFR activation in preserving lung epithelial cell survival and proliferation.
Our observation that lengthy MV-O2 of newborn mice not only suppresses EGFR activation but also increases lung mRNA and protein expression of the EGFR ligand amphiregulin evokes speculation that MV-induced inhibition of pEGFR can trigger epithelial release of its ligands. A study of preterm lambs also noted a striking increase in gene expression of EGFR ligands, including amphiregulin, in response to MV (35). Likewise, in a study of isolated, perfused adult mouse lungs, high-pressure MV yielded a more than fivefold increase of amphiregulin gene expression (36). A study that examined the genomic response of cultured primary rat alveolar epithelial cells exposed to cyclic stretch for 6 hours showed a fourfold increase of amphiregulin mRNA (37), confirming that lung epithelial cells produce EGFR ligand in response to mechanical stretch.
In another study, Bierman and colleagues explored a possible role of EGFR signaling in the lung injury that occurs in adult mice exposed to MV (38). In contrast to our study, in which MV with modest tidal volumes (7–10 μl/g bw) for 24 hours suppressed pEGFR in the lungs of newborn mice, pEGFR increased in the lungs of adult mice subjected to MV for 2 hours with very large tidal volumes (30 ml/kg bw). This MV strategy, which was applied specifically to cause VILI in mature mice, yielded significant neutrophil migration and protein leak into BAL fluid, which was attenuated by treatment with the EGFR inhibitor AG1478 (38). The authors concluded that EGFR-activated signaling could contribute to the lung injury observed in adult mice exposed to high-volume MV. Several major differences in study design might account for the apparently conflicting results between the two studies: 1) the mouse strains differed (Balb/c newborns vs. CD1 adults), 2) the ventilation strategies differed (much smaller tidal volumes and longer MV in newborns vs. adults), and 3) the stages of alveolar formation differed greatly, which presumably accounts for the contrasting pathologies observed in newborn (lung growth arrest with impaired alveologenesis) versus adult (prominent inflammation and edema) lungs exposed to MV. However, we cannot exclude the possibility that the response of EGFR signaling to MV or MV-O2 is developmentally regulated in mice. The findings of our study indicate that EGFR signaling likely plays an important role in alveolar formation, in contradistinction to the adult lung, wherein MV-activated EGFR signaling might contribute to the development of VILI.
Klf4 Increases in the Lungs at Birth and Is Suppressed by MV
Klf4, which belongs to a family of zinc finger–containing transcription factors, regulates cell survival through its antiapoptotic activity and interaction with cell-cycle–regulating molecules, such as cyclin-dependent kinase inhibitor p21 and the tumor suppressor protein p53 (39–42). Klf4 is one of four transcription factors that, when applied together with Oct3/4, Sox2, and c-Myc, can reprogram embryonic stem cells or adult fibroblasts to generate induced pluripotent stem cells (28).
The role of Klf4 in lung development is unclear. Klf4-null mice die soon after birth with epithelial barrier dysfunction and rapid leak of body fluids through the skin, without documented lung pathology (43). Klf4 was shown to play a critical role in maintaining genetic stability in mouse embryonic fibroblasts by protecting against oxidative stress and DNA damage, and modulating associated repair processes (44, 45). Klf4 gene expression is abruptly upregulated in the lungs at birth in response to air breathing and increased oxygenation. Klf4 protein is expressed in neonatal lung fibroblasts and epithelial cells, wherein it is postulated to regulate proliferation, apoptosis, and myofibroblast differentiation (46). Here, we show for the first time that Klf4 mRNA expression and protein abundance are reduced in the lungs of newborn mice exposed to MV. As Klf4 is known to inhibit apoptosis, the finding of Klf4 suppression likely accounts, at least partially, for the increased p21 protein abundance and apoptosis detected in the lungs of newborn mice after lengthy MV-O2. Our observation that Klf4 suppression in cultured lung epithelial cells increased apoptosis and reduced proliferation supports this notion. Klf4 protein in lung epithelial cells decreased in response to EGFR inhibition, showing a regulatory link between EGFR and Klf4, which when disrupted can lead to apoptosis and defective alveologenesis, as seen in nCLD.
Elafin Preserves EGFR-Klf4 Signaling in Lungs of Newborn Mice during MV-O2
Elafin is a low-molecular-weight (∼6 kD) inhibitor of HNE and proteinase-3. It is expressed in several human tissues, notably skin and lung (47), and is secreted by airway epithelial cells (48, 49). Elafin is not constitutively expressed in mice (50), but in a previous study we showed that neonatal mice genetically modified to express elafin in their pulmonary vascular endothelium were protected against the adverse effects of MV on lung growth (26). Whereas wild-type pups, in response to MV-O2 for up to 36 hours, exhibited elastin degradation and defective alveologenesis linked to increased lung elastase activity, inflammation (associated with increased activation of NF-κB and TGF-β), and apoptosis, these changes were prevented or greatly attenuated in elafin-expressing pups. In a related study, intratracheal treatment of mechanically ventilated newborn mice with recombinant human elafin yielded similar results: elafin treatment blocked elastase and MMP-9 activity, as well as NF-κB and TGF-β activation, thereby preventing the elastin degradation, increased apoptosis, and defective alveolar formation noted in lungs of vehicle-treated mice after MV-O2 for 24 hours (19).
These earlier studies led us to explore the possible impact of elafin treatment on the altered EGFR-Klf4 signaling and lung cell apoptosis that occurred in response to lengthy MV-O2. The mechanism by which elafin helps preserve pEGFR and Klf4 protein abundance in lungs of pups on MV-O2 is unclear. Although elafin’s benefit in preserving pEGFR-Klf4 signaling may relate to elastase and MMP-9 inhibition, other actions of elafin merit consideration. These include the aforementioned inhibitory effects of elafin on NF-κB and TGF-β activation, as previously reported in studies using the same mechanically ventilated neonatal mouse model (19, 26). Similarly, a study done with adult mice confirmed that treatment with an inhibitor of neutrophil elastase (Sivelestat) or NF-κB (SN50) reduced the severity of lung injury associated with short-term, high-tidal-volume MV (51). Thus, besides inhibiting elastase and MMP-9 activity, elafin may help to prevent or attenuate neonatal lung injury by suppressing the inflammatory response to mechanical stretch via inhibition of NF-κB or TGF-β, or both. In addition, our group recently showed that elafin treatment effectively reversed experimental pulmonary hypertension in rats, and promoted angiogenesis by increasing bone morphogenetic protein receptor 2 (BMPR2) signaling, an effect linked mechanistically to enhanced interaction of BMPR2 with caveolin-1 via elafin-mediated stabilization of endothelial surface caveolin-1 (52). A related study showed that stretch-induced activation of EGFR and Akt in rat glomerular mesangial cells requires phosphorylation of caveolin-1 (53). Based on the finding that elafin can promote endothelial cell proliferation and angiogenesis via caveolin-1–dependent amplification of BMPR2 signaling, we speculate that elafin similarly may stabilize caveolin-1 on the surface of lung epithelial cells, thereby enabling EGFR phosphorylation and Klf4 expression, contributing to enhanced cell survival.
The molecular pathway through which EGFR-Klf4 signaling occurs remains unknown. Two previous reports, however, have provided compelling evidence that Erk5, sometimes referred to as Mek5, may be a key pathway linking pEGFR and Klf4. The first of these studies was reported 20 years ago, showing that the EGF is a potent activator of Erk5, which is part of a distinct MAP-kinase signaling pathway required for EGF-induced cell proliferation and progression through the cell cycle (54). A subsequent study identified Klf4 as an important downstream target of Erk5, and provided strong evidence that Erk5 activation in human primary endothelial cells elicits an overall protective phenotype, notably featuring increased resistance to apoptosis, and decreased inflammatory potential—a protective effect that Klf4 expression reproduced in the endothelial cells (55). These interesting studies, which were conducted exclusively with endothelial cells, raise the question of whether the same pathway (EGF-pEGFR-Erk5-Klf4) plays a critical role in homeostasis/survival and proliferation of lung epithelial cells exposed to cyclic stretch and/or hyperoxia, and whether elafin treatment would favorably impact this pathway with respect to Erk5 activation and Klf4 expression.
HNE in Plasma as a Biomarker of Evolving nCLD
Abundant, disorganized lung elastin is a prominent feature of evolving nCLD. Previous reports on lung pathology of infants who died with BPD described a striking accumulation of thickened, tortuous, and irregularly distributed elastic fibers in distal lung parenchyma, which was associated with decreased septation and fewer alveoli compared with control lungs (56, 57). Urine desmosine, a biomarker of elastin breakdown, increased during the first week of MV in infants with evolving BPD (58). Degradation of lung elastin was attributed to inflammation associated with infection and O2 toxicity (59). In a study of very premature infants who were especially susceptible to lung injury and BPD, treatment with an α1-proteinase inhibitor during the first 2 weeks after birth significantly decreased the incidence of pulmonary hemorrhage, with a nearly significant decline (P = 0.06) in the incidence of BPD (60).
These clinical reports, coupled with increased lung elastase activity measured in preterm infants with evolving BPD (29–31) and relevant animal models (15, 17, 19, 26), provided a strong rationale to determine whether measuring plasma HNE in very premature infants might serve as a biomarker of lung injury, and perhaps help to define the risk for BPD. Our finding that plasma HNE levels were more than twofold higher among extremely premature infants with evolving nCLD compared with nonafflicted infants confirms the potential value of early postnatal measurements of HNE as an indicator of lung injury and a prelude to the development of nCLD. The failure of plasma elafin levels to increase in response to the postnatal increase of HNE observed in premature infants at risk for nCLD raises the question of whether early treatment with an elastase inhibitor (elafin) might help to protect against development of nCLD—a question worthy of further study in authentic animal models of this disease.
Working Model
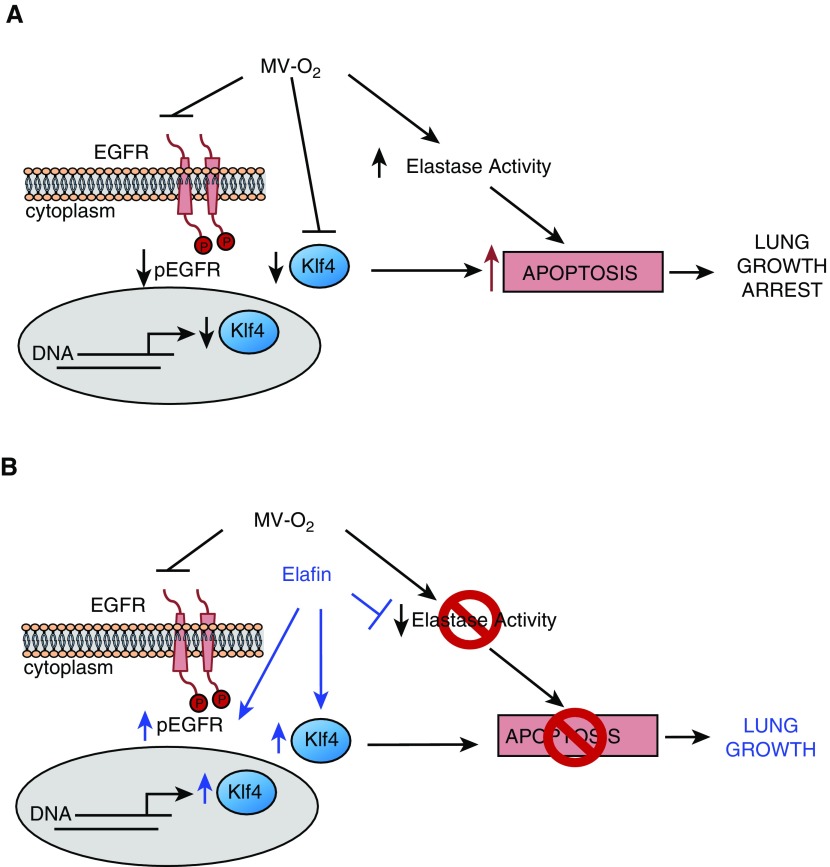
Figure 7 shows our working model, depicting how MV-O2 can lead to apoptosis and lung growth arrest in newborn mice, and how intratracheal treatment with recombinant human elafin can help preserve lung growth during lengthy MV-O2. As shown in Figure 7A, prolonged cyclic stretch with O2-rich gas increases lung elastase activity and inhibits pEGFR, which downregulates expression of the nuclear transcription factor Klf4, thereby increasing apoptosis and disrupting alveologenesis. As shown in Figure 7B, elafin treatment inhibits elastase activity and enables EGFR phosphorylation. Increased pEGFR upregulates Klf4 protein abundance in lung epithelial cells, and together these effects suppress apoptosis and promote lung growth during lengthy MV-O2. The mechanism by which elafin activates EGFR-Klf4 signaling is unclear, but several known actions of elafin might be operative in sustaining the EGFR-Klf4 axis. By inhibiting elastase activity, elafin might block proteolytic cleavage of EGFR or its ligands from the extracellular membrane, thereby promoting EGFR activation and enabling EGFR-Klf4 signaling. Elafin also inhibits inflammation associated with activation of NF-κB and TGF-β, which in turn may help preserve the prosurvival link between EGFR and Klf4. As elafin was shown to promote endothelial cell proliferation and angiogenesis via caveolin-1 amplification of BMPR signaling, it is possible that elafin has a similar effect of augmenting the interaction of caveolin-1 with EGFR on the surface of lung epithelial cells exposed to MV-O2, thereby promoting EGFR activation and downstream Klf4 signaling.
Figure 7.
Our working model, depicting how MV-O2 causes apoptosis and lung growth arrest in newborn mice, and how treatment with recombinant human elafin helps promote lung growth during MV-O2. (A) MV-O2 inhibits pEGFR and suppresses Klf4 protein abundance in epithelial cells while also evoking inflammation and increased elastase activity in the developing lung. The resulting epithelial cell apoptosis blunts alveolar formation and lung growth. (B) Elafin treatment inhibits lung elastase activity and also enables pEGFR, thus preserving Klf4 expression in lung epithelial cells. In so doing, elafin protects against apoptosis and enables lung growth during MV-O2.
The increased elastase activity measured in lungs of newborn mice exposed to lengthy MV-O2 supports our clinical observations that elastase levels in plasma and urinary desmosine excretion are significantly increased in infants with evolving nCLD. These findings suggest that HNE measurement in plasma may serve as an early postnatal biomarker of evolving BPD.
Acknowledgments
Acknowledgment
The authors thank Anne Coates and Jackie Zirbes for their assistance with the clinical component of this work, enrolling patients, collecting specimens, and gathering data. The authors also thank Michelle Fox for administrative help, and Prof. Dr. Margarete Odenthal and Dr. Iris Macheleidt of the Institute of Pathology, University Hospital Cologne, for assistance with the Klf4 immunostaining. Elafin was a kind gift from Prof. Oliver Wiedow of the University of Kiel and Proteo Biotech, Kiel German, where Birge Bargmann and Barbara Kahike of Proteo facilitated the transfer.
Footnotes
Supported by National Institutes of Health grants HL-086631 (R.D.B.) and P01HL108797 (M.R. and R.D.B.), and Deutsche Forschungsgemeinschaft grants DFG-AL1636/1-1, 2-1 (M.A.A.A.) and DFG-PR14251-1 (S.P.)
Author Contributions: Conception and design of the work: M.A.A.A., M.K., R.E., S.P., C.M., M.R., and R.D.B. Acquisition, analysis, and interpretation of data: M.A.A.A., M.K., R.E., S.P., C.M., S.M., J. Masumi, S.K., L.M.M., L.T., J. Mohr, D.V.H., M.R., and R.D.B. Drafting the manuscript: M.A.A.A., R.E., C.M., and S.M. Critically reviewing the draft, and preparing and extensively revising the final manuscript: M.R. and R.D.B.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0332OC on June 12, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 2.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 4.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 5.Warner BB, Stuart LA, Papes RA, Wispé JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275:L110–L117. doi: 10.1152/ajplung.1998.275.1.L110. [DOI] [PubMed] [Google Scholar]
- 6.Hargitai B, Szabó V, Hajdú J, Harmath A, Pataki M, Farid P, et al. Apoptosis in various organs of preterm infants: histopathologic study of lung, kidney, liver, and brain of ventilated infants. Pediatr Res. 2001;50:110–114. doi: 10.1203/00006450-200107000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Das KC, Ravi D, Holland W. Increased apoptosis and expression of p21 and p53 in premature infant baboon model of bronchopulmonary dysplasia. Antioxid Redox Signal. 2004;6:109–116. doi: 10.1089/152308604771978417. [DOI] [PubMed] [Google Scholar]
- 8.Rojas MA, Gonzalez A, Bancalari E, Claure N, Poole C, Silva-Neto G. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr. 1995;126:605–610. doi: 10.1016/s0022-3476(95)70362-4. [DOI] [PubMed] [Google Scholar]
- 9.Van Marter LJ. Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med. 2009;14:358–366. doi: 10.1016/j.siny.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192:134–156. doi: 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999;160:1333–1346. doi: 10.1164/ajrccm.160.4.9810071. [DOI] [PubMed] [Google Scholar]
- 12.Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol. 2002;282:L811–L823. doi: 10.1152/ajplung.00325.2001. [DOI] [PubMed] [Google Scholar]
- 13.Pierce RA, Albertine KH, Starcher BC, Bohnsack JF, Carlton DP, Bland RD. Chronic lung injury in preterm lambs: disordered pulmonary elastin deposition. Am J Physiol. 1997;272:L452–L460. doi: 10.1152/ajplung.1997.272.3.L452. [DOI] [PubMed] [Google Scholar]
- 14.Albertine KH, Jones GP, Starcher BC, Bohnsack JF, Davis PL, Cho SC, et al. Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am J Respir Crit Care Med. 1999;159:945–958. doi: 10.1164/ajrccm.159.3.9804027. [DOI] [PubMed] [Google Scholar]
- 15.Bland RD, Xu L, Ertsey R, Rabinovitch M, Albertine KH, Wynn KA, et al. Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1370–L1384. doi: 10.1152/ajplung.00367.2006. [DOI] [PubMed] [Google Scholar]
- 16.Bland RD, Mokres LM, Ertsey R, Jacobson BE, Jiang S, Rabinovitch M, et al. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1099–L1110. doi: 10.1152/ajplung.00217.2007. [DOI] [PubMed] [Google Scholar]
- 17.Bland RD, Ertsey R, Mokres LM, Xu L, Jacobson BE, Jiang S, et al. Mechanical ventilation uncouples synthesis and assembly of elastin and increases apoptosis in lungs of newborn mice. Prelude to defective alveolar septation during lung development? Am J Physiol Lung Cell Mol Physiol. 2008;294:L3–L14. doi: 10.1152/ajplung.00362.2007. [DOI] [PubMed] [Google Scholar]
- 18.Mokres LM, Parai K, Hilgendorff A, Ertsey R, Alvira CM, Rabinovitch M, et al. Prolonged mechanical ventilation with air induces apoptosis and causes failure of alveolar septation and angiogenesis in lungs of newborn mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L23–L35. doi: 10.1152/ajplung.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilgendorff A, Parai K, Ertsey R, Jain N, Navarro EF, Peterson JL, et al. Inhibiting lung elastase activity enables lung growth in mechanically ventilated newborn mice. Am J Respir Crit Care Med. 2011;184:537–546. doi: 10.1164/rccm.201012-2010OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warburton D, Seth R, Shum L, Horcher PG, Hall FL, Werb Z, et al. Epigenetic role of epidermal growth factor expression and signalling in embryonic mouse lung morphogenesis. Dev Biol. 1992;149:123–133. doi: 10.1016/0012-1606(92)90269-m. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 22.Miettinen PJ, Warburton D, Bu D, Zhao JS, Berger JE, Minoo P, et al. Impaired lung branching morphogenesis in the absence of functional EGF receptor. Dev Biol. 1997;186:224–236. doi: 10.1006/dbio.1997.8593. [DOI] [PubMed] [Google Scholar]
- 23.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghaleb AM, McConnell BB, Kaestner KH, Yang VW. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Krüppel-like factor 4 gene. Dev Biol. 2011;349:310–320. doi: 10.1016/j.ydbio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McConnell BB, Yang VW. Mammalian Krüppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilgendorff A, Parai K, Ertsey R, Juliana Rey-Parra G, Thébaud B, Tamosiuniene R, et al. Neonatal mice genetically modified to express the elastase inhibitor elafin are protected against the adverse effects of mechanical ventilation on lung growth. Am J Physiol Lung Cell Mol Physiol. 2012;303:L215–L227. doi: 10.1152/ajplung.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zar J. Biostatistical analysis. Upper Saddle River, NJ: Prentice Hall; 1998. [Google Scholar]
- 28.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, et al. Elastase and alpha 1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. Role of inflammation in the pathogenesis of bronchopulmonary dysplasia. J Clin Invest. 1983;72:656–666. doi: 10.1172/JCI111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogden BE, Murphy SA, Saunders GC, Pathak D, Johnson JD. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis. 1984;130:817–821. doi: 10.1164/arrd.1984.130.5.817. [DOI] [PubMed] [Google Scholar]
- 31.Walti H, Tordet C, Gerbaut L, Saugier P, Moriette G, Relier JP. Persistent elastase/proteinase inhibitor imbalance during prolonged ventilation of infants with bronchopulmonary dysplasia: evidence for the role of nosocomial infections. Pediatr Res. 1989;26:351–355. doi: 10.1203/00006450-198910000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Plopper CG, St George JA, Read LC, Nishio SJ, Weir AJ, Edwards L, et al. Acceleration of alveolar type II cell differentiation in fetal rhesus monkey lung by administration of EGF. Am J Physiol. 1992;262:L313–L321. doi: 10.1152/ajplung.1992.262.3.L313. [DOI] [PubMed] [Google Scholar]
- 33.Merklinger SL, Jones PL, Martinez EC, Rabinovitch M. Epidermal growth factor receptor blockade mediates smooth muscle cell apoptosis and improves survival in rats with pulmonary hypertension. Circulation. 2005;112:423–431. doi: 10.1161/CIRCULATIONAHA.105.540542. [DOI] [PubMed] [Google Scholar]
- 34.Huang Z, Wang Y, Nayak PS, Dammann CE, Sanchez-Esteban J. Stretch-induced fetal type II cell differentiation is mediated via ErbB1-ErbB4 interactions. J Biol Chem. 2012;287:18091–18102. doi: 10.1074/jbc.M111.313163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillman NH, Gisslen T, Polglase GR, Kallapur SG, Jobe AH. Ventilation-induced increases in EGFR ligand mRNA are not altered by intra-amniotic LPS or ureaplasma in preterm lambs. PLoS One. 2014;9:e96087. doi: 10.1371/journal.pone.0096087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolinay T, Kaminski N, Felgendreher M, Kim HP, Reynolds P, Watkins SC, et al. Gene expression profiling of target genes in ventilator-induced lung injury. Physiol Genomics. 2006;26:68–75. doi: 10.1152/physiolgenomics.00110.2005. [DOI] [PubMed] [Google Scholar]
- 37.Yerrapureddy A, Tobias J, Margulies SS. Cyclic stretch magnitude and duration affect rat alveolar epithelial gene expression. Cell Physiol Biochem. 2010;25:113–122. doi: 10.1159/000272056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bierman A, Yerrapureddy A, Reddy NM, Hassoun PM, Reddy SP. Epidermal growth factor receptor (EGFR) regulates mechanical ventilation-induced lung injury in mice. Transl Res. 2008;152:265–272. doi: 10.1016/j.trsl.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Whitney EM, Gao SY, Yang VW. Transcriptional profiling of Krüppel-like factor 4 reveals a function in cell cycle regulation and epithelial differentiation. J Mol Biol. 2003;326:665–677. doi: 10.1016/S0022-2836(02)01449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW. Krüppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15:92–96. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghaleb AM, Katz JP, Kaestner KH, Du JX, Yang VW. Krüppel-like factor 4 exhibits antiapoptotic activity following gamma-radiation-induced DNA damage. Oncogene. 2007;26:2365–2373. doi: 10.1038/sj.onc.1210022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Geiman DE, Shields JM, Dang DT, Mahatan CS, Kaestner KH, et al. The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J Biol Chem. 2000;275:18391–18398. doi: 10.1074/jbc.C000062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 44.El-Karim EA, Hagos EG, Ghaleb AM, Yu B, Yang VW. Krüppel-like factor 4 regulates genetic stability in mouse embryonic fibroblasts. Mol Cancer. 2013;12:89–101. doi: 10.1186/1476-4598-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu C, La Rosa S, Hagos EG. Oxidative DNA damage causes premature senescence in mouse embryonic fibroblasts deficient for Krüppel-like factor 4. Mol Carcinog. 2015;54:889–899. doi: 10.1002/mc.22161. [DOI] [PubMed] [Google Scholar]
- 46.Jean J-C, George E, Kaestner KH, Brown LAS, Spira A, Joyce-Brady M. Transcription factor Klf4, induced in the lung by oxygen at birth, regulates perinatal fibroblast and myofibroblast differentiation. PLoS One. 2013;8:e54806. doi: 10.1371/journal.pone.0054806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nara K, Ito S, Ito T, Suzuki Y, Ghoneim MA, Tachibana S, et al. Elastase inhibitor elafin is a new type of proteinase inhibitor which has a transglutaminase-mediated anchoring sequence termed “cementoin”. J Biochem. 1994;115:441–448. doi: 10.1093/oxfordjournals.jbchem.a124357. [DOI] [PubMed] [Google Scholar]
- 48.Sallenave JM, Silva A, Marsden ME, Ryle AP. Secretion of mucus proteinase inhibitor and elafin by Clara cell and type II pneumocyte cell lines. Am J Respir Cell Mol Biol. 1993;8:126–133. doi: 10.1165/ajrcmb/8.2.126. [DOI] [PubMed] [Google Scholar]
- 49.Sallenave JM, Silva A. Characterization and gene sequence of the precursor of elafin, an elastase-specific inhibitor in bronchial secretions. Am J Respir Cell Mol Biol. 1993;8:439–445. doi: 10.1165/ajrcmb/8.4.439. [DOI] [PubMed] [Google Scholar]
- 50.Sallenave JM, Cunningham GA, James RM, McLachlan G, Haslett C. Regulation of pulmonary and systemic bacterial lipopolysaccharide responses in transgenic mice expressing human elafin. Infect Immun. 2003;71:3766–3774. doi: 10.1128/IAI.71.7.3766-3774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li LF, Lai YT, Chang CH, Lin MC, Liu YY, Kao KC, et al. Neutrophil elastase inhibitor reduces ventilation-induced lung injury via nuclear factor-κB and NF-κB repressing factor in mice. Exp Biol Med (Maywood) 2014;239:1045–1057. doi: 10.1177/1535370214529393. [DOI] [PubMed] [Google Scholar]
- 52.Nickel NP, Spiekerkoetter E, Gu M, Li CG, Li H, Kaschwich M, et al. Elafin reverses pulmonary hypertension via Caveolin-1-dependent bone morphogenetic protein signaling. Am J Respir Crit Care Med. 2015;191:1273–1286. doi: 10.1164/rccm.201412-2291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang B, Peng F, Wu D, Ingram AJ, Gao B, Krepinsky JC. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell Signal. 2007;19:1690–1700. doi: 10.1016/j.cellsig.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee J-D. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–716. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- 55.Ohnesorge N, Viemann D, Schmidt N, Czymai T, Spiering D, Schmolke M, et al. Erk5 activation elicits a vasoprotective endothelial phenotype via induction of Kruppel-like factor 4 (KLF4) J Biol Chem. 2010;285:26199–26210. doi: 10.1074/jbc.M110.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Margraf LR, Tomashefski JF, Jr, Bruce MC, Dahms BB. Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis. 1991;143:391–400. doi: 10.1164/ajrccm/143.2.391. [DOI] [PubMed] [Google Scholar]
- 57.Thibeault DW, Mabry SM, Ekekezie II, Truog WE. Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics. 2000;106:1452–1459. doi: 10.1542/peds.106.6.1452. [DOI] [PubMed] [Google Scholar]
- 58.Bruce MC, Wedig KE, Jentoft N, Martin RJ, Cheng PW, Boat TF, et al. Altered urinary excretion of elastin cross-links in premature infants who develop bronchopulmonary dysplasia. Am Rev Respir Dis. 1985;131:568–572. doi: 10.1164/arrd.1985.131.4.568. [DOI] [PubMed] [Google Scholar]
- 59.Bruce MC, Schuyler M, Martin RJ, Starcher BC, Tomashefski JF, Jr, Wedig KE. Risk factors for the degradation of lung elastic fibers in the ventilated neonate. Implications for impaired lung development in bronchopulmonary dysplasia. Am Rev Respir Dis. 1992;146:204–212. doi: 10.1164/ajrccm/146.1.204. [DOI] [PubMed] [Google Scholar]
- 60.Stiskal JA, Dunn MS, Shennan AT, O’Brien KK, Kelly EN, Koppel RI, et al. α1-Proteinase inhibitor therapy for the prevention of chronic lung disease of prematurity: a randomized, controlled trial. Pediatrics. 1998;101:89–94. doi: 10.1542/peds.101.1.89. [DOI] [PubMed] [Google Scholar]