Abstract
Objectives. To examine 23-year trends in both physically and cognitively healthy life expectancy from age 65 years in the Netherlands.
Methods. We used 8 waves between 1993 and 2016 from the nationally representative Longitudinal Aging Study Amsterdam (12 948 observations). We calculated physically and cognitively healthy life expectancies by using the Sullivan life table method and tested prevalence trends over time by using generalized estimating equations.
Results. Total life expectancy at age 65 years rose from 14.7 to 18.7 years (men) and from 19.2 to 21.4 years (women). Life expectancy in poor physical health increased nonlinearly from 1.8 to 2.9 years for men; for women it fluctuated around 5.7 years. Meanwhile, life expectancy in good cognitive health increased linearly from 11.0 to 15.7 years (men) and from 13.4 to 18.0 years (women). The proportion of people with poor physical and poor cognitive health combined did not increase, averaging 5.9% (men) and 8.7% (women).
Conclusions. This multiwave study shows that a negative trend in physically healthy life expectancy is accompanied by a positive trend in cognitively healthy life expectancy.
In view of population aging and the need to contain health care costs, many Western countries are reforming their long-term care system.1 One such reform is to cut back on long-term care services. This policy assumes that in the near future, fewer older people will need these services, because they will stay healthy to older ages. Accumulated evidence on trends in healthy and unhealthy life years from age 65 years onward, however, suggests that the rise in life expectancy is not accompanied by a similar rise in healthy life years.2,3 In particular, years with mild physical disability have been shown to increase while years with severe physical disability remain more or less constant.4 If these trends continue, increasing numbers of older people will have to pay for care themselves, or go without care.
The rise in the number of very old people and the relatively high costs of dementia care call for monitoring trends in cognitive health.5,6 Trend studies on cognitive impairment or dementia generally show decreases in prevalence and incidence.7–9 Moreover, increases have been reported in years with good cognitive health that come close to or even exceed the increase in life expectancy at age 65 years.4,10 These studies suggest a delay of years with poor cognitive health, but evidence for this is not unequivocal.11 So far, it seems that the prospects for life expectancy in good cognitive health are more favorable than for life expectancy in good physical health.
Evidence on trends in cognitively healthy life expectancy is recent and limited to a few countries. Trend studies on physically healthy life expectancy have accumulated over several decades and have shown differences in trends across countries and across time periods within 1 country.12 This body of research has taught us to be cautious about generalizing across periods and geographic regions.13 So far, no evidence exists on trends in cognitively healthy life expectancy for the Netherlands over more than a decade. The current study contributes evidence from a nationwide, representative study in the Netherlands, across a period of 23 years. Life expectancy from age 65 years is distinguished into life expectancy in good, fair, and poor physical health; life expectancy in good and fair-to-poor cognitive health; and their combination. A joint study of physically and cognitively healthy life years is rare, but provides the necessary evidence to properly assess future health and long-term care costs.
METHODS
We used data from the nationally representative Longitudinal Aging Study Amsterdam (LASA). Participants were drawn from the population registries of 11 municipalities in 3 socio-culturally distinct geographic areas in the Netherlands (west, northeast, and south) and are followed at 3-year intervals with exactly the same face-to-face interviews and tests. The first cohort, recruited in 1992 to 1993, included 3107 participants aged 55 to 85 years. After 10 and 20 years, a second and a third cohort aged 55 to 64 years were added, including 1002 and 1023 participants, respectively. The samples were weighted so that together they represented the distribution of population density of the Netherlands. Participants who refuse a face-to-face interview are offered a 15-minute telephone interview, in which either the participant or a proxy can participate. Details on the sampling and data collection have been published elsewhere.14
For the current study, we selected subsamples aged 65 years or older from participants at each wave between 1993 and 2016. (Although each wave takes place in 2 successive calendar years, in the following, for brevity, the waves are denoted by the second year, as the majority of the participants were interviewed in that year.) This study design defined a partly different cohort at each wave, because after each time interval of 3 years, new individuals aged 65 to 67 years are included. Up to 2002, these newly included participants were members of the original cohort. From 2006 to 2012, they were members of the cohort added in 2002, and in 2016, they were members of the cohort added in 2012. The number of participants at each wave varied from 1415 to 2003 for physical health and from 1240 to 2129 for cognitive health.
Measures
Physical health.
The definition of physical health took into account both multimorbidity and disability. First, multimorbidity, defined as the presence of at least 2 chronic diseases, is a complex condition that may have serious consequences for functioning and quality of life.15 Second, disability is associated with health and long-term care use and quality of life.16 Thus, multimorbidity and disability combined may be considered to reflect the physical health states that are relevant to older people’s use of care.
We measured multimorbidity by self-reports of major chronic diseases: chronic obstructive lung diseases (including emphysema, bronchitis, and asthma), coronary heart diseases, peripheral arterial disease, stroke, diabetes, cancer, and arthritis (including osteoarthritis and rheumatoid arthritis). In addition, participants could indicate a maximum of 2 other chronic diseases. The comparability of these self-reports with general practitioner records was satisfactory and remained stable across the period 1992 to 2009.17
We measured disability by self-reports of difficulty or need of help with 6 activities: climbing stairs, dressing, getting up from a chair, cutting one’s toenails, walking 400 meters, and using own or public transportation. We defined mild disability as difficulty with at least 1 activity and severe disability as needing help with at least 1 activity.18
Chronic diseases and disability were included in both the face-to-face and the telephone interview. We derived 3 physical health states:
Good physical health: no multimorbidity and no mild disability;
Fair physical health: (1) multimorbidity with at most mild disability, (2) mild disability with or without multimorbidity, or (3) severe disability without multimorbidity; and
Poor physical health: both multimorbidity and severe disability.
Cognitive health.
In keeping with most previous studies, we measured cognitive functioning with the Mini-Mental State Examination (MMSE), which includes 23 items with a maximum score of 30 when all responses are correct.19 This test was included only in the face-to-face interview. We distinguished 2 cognitive health states, because the available numbers did not allow examination of a third state. The cut-off between good and fair or poor followed Jagger et al.4:
Good cognitive health: MMSE score of 26 or higher, and
Fair or poor cognitive health: MMSE score of 25 or lower.
Covariates.
We obtained age and gender from the municipal registries. We defined level of education as number of years of schooling, ranging from 5 to 18 years.
Data Analysis
We calculated healthy and unhealthy life expectancies by using the Sullivan life table method for 8 calendar years corresponding to the LASA waves for 1993, 1996, 1999, 2002, 2006, 2009, 2012, and 2016.20 We used generalized estimating equations (GEEs) to statistically test trends in the prevalence of good and poor health. Each step is explained next.
We obtained life tables for the national population for each calendar year stated from Statistics Netherlands. We derived annual survival probabilities for men and women from age 65 years onward. At age 65 years, the survival probability was set to 1. At age 66 years, the survival probability was 1 minus the proportion of deaths at age 65 years; at age 67 years, the survival probability was the survival probability at age 66 years minus the proportion of deaths at age 66 years, and so on. If a person died, this person was assumed to have lived 0.5 year. The resulting probabilities added up to the total life expectancy from age 65 years.
To obtain age-specific prevalences of good and poor health, we used LASA data from each wave. In the higher age groups the number of cases was relatively small; thus, we refrained from using age-specific prevalence rates directly derived from the data because these would be too unstable. Instead, for each calendar year and gender, we fitted a polynomial of the association of health with age to derive age-specific prevalence rates.21
Following the Sullivan method, for each age year from 65 years onward, we multiplied the national life table–derived survival probability by the LASA-derived prevalence of good or poor health in this age year. We summed these age-specific probabilities to the life expectancy in good or poor health. As during the study period total life expectancy increased, we calculated not only the number of years but also the proportion of remaining life with and without health problems.
A systematic statistical test for trends in healthy life expectancy across multiple waves is not available. Therefore, we tested trends in the prevalence of good and poor health in the LASA data by using GEEs after pooling the data across the 8 waves, with year as the main determinant and adjustment for age. Because there is an overlap in participants across the waves, we accounted for the interdependence of the data by including a 7-dependent correlation matrix.22 For this trend analysis, we selected same-age participants (ages 65–85 years) at each wave. To test for nonlinear trends, we added a quadratic term and reported it if significant. As the MMSE is known for its sensitivity to education, which might lead to misclassification, and as the level of education has increased in subsequent cohorts,23 we examined all trends in additional GEE models adjusted for educational level.
We performed a series of sensitivity analyses to address (1) a potential practice effect in the MMSE in continuing participants, (2) the use of proxy information for physical health, (3) nonmortality attrition and item nonresponse for cognitive health, and (4) other cutpoints for fair physical health problems and fair or poor cognitive health (Appendix, available as a supplement to the online version of this article at http://www.ajph.org).
RESULTS
During the period 1993 to 2016, total life expectancy at age 65 years rose from 14.7 to 18.7 years (27.2%) for men and from 19.2 to 21.4 years (11.5%) for women (Table 1).
TABLE 1—
Descriptive Characteristics, Total Life Expectancy, and Proportion of Life Expectancy in Good and Poor Physical Health and in Good Cognitive Health at Age 65 Years, by Gender: Longitudinal Aging Study Amsterdam, The Netherlands, 1993–2016
| 1993 | 1996 | 1999 | 2002 | 2006 | 2009 | 2012 | 2016 | Difference 2016–1993, No. (%) or Percentage Points | |
| Men | |||||||||
| No. for physical health | 972 | 881 | 750 | 665 | 644 | 613 | 603 | 650 | |
| No. for cognitive health | 1031 | 809 | 697 | 611 | 572 | 564 | 540 | 590 | |
| Age, y, mean (range) | 75.7 (65.0–85.6) | 76.2 (65.1–88.7) | 76.3 (65.0–91.8) | 76.0 (65.0–94.0) | 75.5 (65.0–97.9) | 75.3 (65.0–100.6) | 75.4 (65.0–104.2) | 74.7 (65.0–102.8) | |
| Years of education, mean | 9.2 | 9.5 | 9.8 | 10.0 | 10.3 | 10.8 | 10.9 | 11.4 | |
| Total life expectancy, y | 14.7 | 15.1 | 15.5 | 16.0 | 17.1 | 17.8 | 18.2 | 18.7 | +4.0 (27.2) |
| Proportion life expectancy in good physical health | 46.6 | 37.6 | 33.6 | 32.4 | 32.7 | 30.5 | 22.8 | 25.0 | −21.6 |
| Proportion life expectancy in poor physical health | 12.3 | 13.8 | 15.2 | 14.6 | 19.1 | 17.9 | 17.0 | 15.7 | +3.4 |
| Proportion life expectancy in good cognitive health | 74.8 | 78.0 | 81.5 | 83.8 | 81.1 | 82.8 | 85.5 | 83.7 | +8.9 |
| Women | |||||||||
| No. for physical health | 1031 | 1010 | 950 | 900 | 863 | 809 | 812 | 795 | |
| No. for cognitive health | 1098 | 920 | 884 | 797 | 739 | 721 | 700 | 695 | |
| Age, y, mean (range) | 75.3 (65.0–85.6) | 76.1 (65.0–88.8) | 76.6 (65.0–91.7) | 77.1 (65.0–94.3) | 76.7 (65.0–98.6) | 76.9 (65.1–100.1) | 76.7 (65.0–102.3) | 76.0 (65.0–102.0) | |
| Years of education, mean | 7.8 | 8.0 | 8.1 | 8.3 | 8.7 | 9.0 | 9.3 | 10.0 | |
| Total life expectancy, y | 19.2 | 19.4 | 19.5 | 19.7 | 20.5 | 21.1 | 21.2 | 21.4 | +2.2 (11.5) |
| Proportion life expectancy in good physical health | 28.7 | 26.0 | 24.5 | 22.7 | 21.2 | 20.2 | 14.9 | 18.9 | −9.8 |
| Proportion life expectancy in poor physical health | 28.3 | 27.8 | 25.2 | 26.0 | 28.8 | 28.7 | 32.9 | 28.9 | +0.6 |
| Proportion life expectancy in good cognitive health | 69.9 | 73.7 | 76.1 | 78.2 | 78.9 | 82.4 | 82.3 | 84.4 | +14.5 |
Physical Health
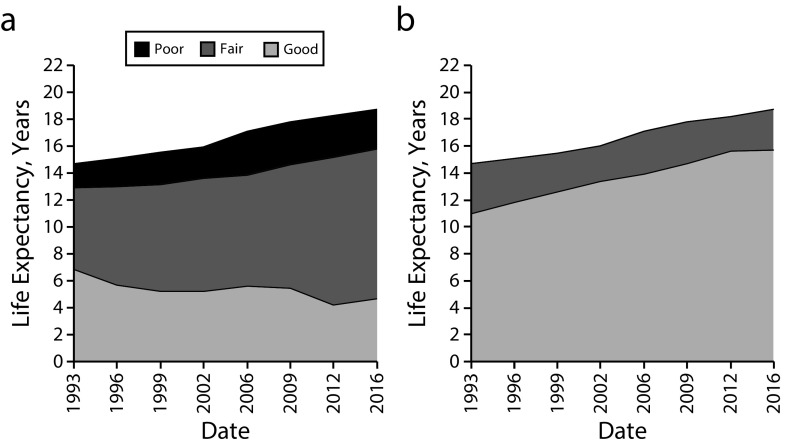
Life expectancy in good physical health decreased from 6.9 to 4.7 years for men (Figure 1). A steady decrease occurred in the 1990s, after which the pattern was more irregular, with a low of 4.2 years in 2012. For women, physically healthy life expectancy decreased as well, from 5.5 to 4.0 years (Figure 2). This decrease continued throughout the study period, with a low of 3.2 years in 2012. Life expectancy in poor physical health increased for men from 1.8 to 2.9 years, with a steady increase occurring up to 2006, after which an improvement set in. For women, life expectancy in poor physical health showed an increase from 5.4 to 6.2 years. This increase occurred only in the late 2000s, with a high of 7.0 years in 2012.
FIGURE 1—
Life Expectancy for Men Aged 65 Years in (a) Good, Fair, and Poor Physical Health and in (b) Good and Fair or Poor Cognitive Health: Longitudinal Aging Study Amsterdam, The Netherlands, 1993–2016
Note. The years in good, fair, and poor health are stacked vertically so that for each calendar year they sum to the total life expectancy. Good physical health = no multimorbidity and no mild disability; fair physical health = multimorbidity, disability, or both; poor physical health = multimorbidity and severe disability; good cognitive health = Mini-Mental State Examination (MMSE) ≥ 26; fair or poor cognitive health = MMSE ≤ 25.
FIGURE 2—
Life Expectancy for Women Aged 65 Years in (a) Good, Fair, and Poor Physical Health and in (b) Good and Fair or Poor Cognitive Health: Longitudinal Aging Study Amsterdam, The Netherlands, 1993–2016
Note. The years in good, fair, and poor health are stacked vertically so that for each calendar year they sum to the total life expectancy. Good physical health = no multimorbidity and no mild disability; fair physical health = multimorbidity, disability, or both; poor physical health = multimorbidity and severe disability; good cognitive health = Mini-Mental State Examination (MMSE) ≥ 26; fair or poor cognitive health = MMSE ≤ 25.
The proportional decrease in life expectancy in good physical health continued throughout the study period, with, for both men and women, a small rise after 2012 (Table 1). By contrast, we saw a proportional increase in life expectancy in poor health for men up to 2009 with a decrease after that, whereas for women this proportion fluctuated, with a low in 1999 and a high in 2012.
Taking all 8 LASA waves into account, we tested trends in the prevalence of good and poor health (Table 2). The prevalence of good physical health showed a decrease for men that flattened off, as demonstrated by significant odds ratios (ORs) for both the linear and the quadratic terms for year (ORyear = 0.912; 95% confidence interval [CI] = 0.887, 0.938, and ORyear2 = 1.002; 95% CI = 1.001, 1.003). For women, we found a linear decrease (ORyear = 0.971; 95% CI = 0.962, 0.980). The prevalence of poor physical health for men showed an initial increase that also flattened off (ORyear = 1.069; 95% CI = 1.035, 1.104, and ORyear2 = 0.998; 95% CI = 0.996, 0.999). For women, it showed stability (ORyear = 1.008; 95% CI = 0.999, 1.017).
TABLE 2—
Tests of Trends Over Time in Good and Poor Physical Health, and in Fair or Poor Cognitive Health: Longitudinal Aging Study Amsterdam, The Netherlands, 1993–2016
| OR (95% CI) | |
| Physical health, men | |
| Good physical healtha | |
| Linear term | 0.912 (0.887, 0.938) |
| Quadratic term | 1.002 (1.001, 1.003) |
| Poor physical healthb | |
| Linear term | 1.069 (1.035, 1.104) |
| Quadratic term | 0.998 (0.996, 0.999) |
| Physical health, women | |
| Good physical healtha | 0.971 (0.962, 0.980) |
| Poor physical healthb | 1.008 (0.999, 1.017) |
| Cognitive health, men | |
| Fair or poor cognitive healthc | 0.973 (0.962, 0.983) |
| Cognitive health, women | |
| Fair or poor cognitive healthc | 0.963 (0.953, 0.973) |
| Physical and cognitive health combined, men | |
| Good overall healthd | |
| Linear term | 0.922 (0.895, 0.950) |
| Quadratic term | 1.002 (1.001, 1.003) |
| Poor overall healthe | 0.989 (0.971, 1.007) |
| Physical and cognitive health combined, women | |
| Good overall healthd | 0.978 (0.968, 0.988) |
| Poor overall healthe | 0.971 (0.957, 0.986) |
Note. CI = confidence interval; OR = odds ratio. Using generalized estimating equations adjusted for age and gender, with year as the independent variable. If the quadratic term of year was significant (P < .05), the OR (95% CI) for this term is reported along with the OR (95% CI) for the linear term; if the quadratic term was not significant, by default only the OR (95% CI) for the linear term is reported.
At most 1 chronic disease and no difficulty with any activity.
Multimorbidity and needing help with at least 1 activity.
Mini-Mental State Examination (MMSE) ≤ 25.
At most 1 chronic disease, no difficulty with any activity, and MMSE ≥ 26.
Multimorbidity, needing help with at least 1 activity, and MMSE ≤ 25.
Adding years of education to the GEE models did not change the trend for men. For women, the negative trend in good health became more negative and the nonsignificant positive trend in poor health became statistically significant (ORyear = 1.016; 95% CI = 1.007, 1.025).
Cognitive Health
Life expectancy from age 65 years in good cognitive health showed increases from 11.0 to 15.7 years for men and from 13.4 to 18.0 years for women; both increases continued at more or less the same pace throughout the study period (Figures 1 and 2).
A proportional increase was concentrated for men in the 1990s, a period with relatively little change in total life expectancy from age 65 years (Table 1). Women showed a greater and more steady increase than men, which can be attributed partly to the smaller rise in their life expectancy, and partly to their greater absolute increase in cognitively healthy life expectancy. These increases exceed those in total life expectancy.
The GEE models showed linear decreases in the prevalence of fair or poor cognitive health for both men (ORyear = 0.973; 95% CI = 0.962, 0.983) and women (ORyear = 0.963; 95% CI = 0.953, 0.973) over time. Adding years of education to these models halved the decreases both for men (ORyear = 0.986; 95% CI = 0.975, 0.997) and for women (ORyear = 0.980; 95% CI = 0.969, 0.991), but the decreases remained significant.
Physical and Cognitive Health Combined
We assessed trends in 2 extreme health categories: good in both physical and cognitive health (good overall health), and poor in physical and fair or poor in cognitive health (poor overall health). The number of participants with poor overall health per wave was too small to calculate unhealthy life years.
Averaged across waves, 5.9% of men and 8.7% of women were in poor overall health. The great majority (86.4%) of those in good physical health also had good cognitive health, whereas 34.4% of those in poor physical health also had fair or poor cognitive health.
The decline in the trend in good overall health was only slightly less negative compared with that in good physical health (ORyear = 0.922; 95% CI = 0.895, 0.950, and ORyear2 = 1.002; 95% CI = 1.001, 1.003, for men and ORyear = 0.978; 95% CI = 0.968, 0.988, for women). Regarding poor overall health, the trend for men was linear and stable, but for women it showed a significant linear decrease (ORyear = 0.989; 95% CI = 0.971, 1.007, and OR = 0.971; 95% CI = 0.957, 0.986, respectively). Thus, for this group in poor overall health, the decrease in fair or poor cognitive health compensated for the increase (men) or stability (women) in poor physical health.
DISCUSSION
In this study, we examined national trends in physically and cognitively healthy life expectancy over the relatively long period of 23 years. This study provides, to our knowledge, the first national estimates of long-term trends in cognitively healthy life expectancy for the Netherlands. The findings show a substantial decline in physically healthy life expectancy and a smaller increase in life expectancy in poor physical health. There were fluctuations across the study period, with, for men, the worsening trend leveling off after the mid-2000s. For women, an improvement was apparent only after 2012, but as this concerns only 1 time point, conclusions about leveling off cannot be drawn. In contrast, life expectancy in good cognitive health showed increases that continued throughout the study period.
Strengths and Limitations
Our study had several strengths. First, it was based on a nationally representative data set, the LASA. Second, exactly the same measurement instruments were used at each LASA wave. Third, we performed our estimates of healthy and unhealthy life expectancy for 8 points in time, covering more than 2 decades. With only 2 time points, which most earlier studies have used,4 it cannot be derived whether trends accelerate or decelerate in specific periods; also, measurement error may over- or underestimate trends. Finally, the addition to LASA of new cohorts in 2002 and 2012 allows the examination of trends over a long period in the relevant age group of 65 years and older. Had new cohorts not been added, the minimum age in 2016 would have been 78, allowing the study of trends only in participants aged 78 years and older.
This study also had limitations. First, we derived our criteria to define less-than-good physical and cognitive health from previous studies,4,17 but in relation to health care use, stricter criteria may be needed. People with difficulty in only 1 activity may not be at substantially higher risk of needing care than their peers with no difficulty in any activity; also, people with scores of 24 to 25 on the MMSE may have functioned at this level during their lifetime, which means that they do not have age-related cognitive impairment.24 Our sensitivity analyses, however, revealed virtually the same trends over time for narrower definitions of fair physical health and fair or poor cognitive health (Table A, available as a supplement to the online version of this article at http://www.ajph.org).
Second, during the 23 years covered by our study, the reporting behavior of successive cohorts might have changed because of higher expectations of medical care, less tolerance for mild health problems, greater awareness of chronic disease in general, and more frequent attribution of similar complaints to a disease instead of to old age.17,25 These developments may result in more recent cohorts reporting fair rather than good health more easily.
Third, the use of proxy information on physical health for respondents who were unable to self-report might have affected the trend over time if the association between physical health and use of proxies had changed over time. However, a sensitivity analysis showed that this association had not changed over time (see “Proxy information,” in the Appendix, available as a supplement to the online version of this article at http://www.ajph.org).
Fourth, although the MMSE is designed as a screening instrument for cognitive impairment and dementia, additional information is needed to distinguish age-related cognitive impairment from dementia. Cognitive impairment per se may not be associated with health care use to the same extent as dementia.26,27
Fifth, the use of longitudinal data entails that a substantial number of participants were tested repeatedly, raising the possibility of a practice effect in the MMSE. However, participants in more recent cohorts who were tested the same number of times showed consistent improvements in cognitive health compared with earlier cohorts (see “Practice effect,” in the Appendix). A further issue concerning longitudinal data is nonmortality attrition, which proved to be associated with cognitive impairment at the previous wave. Additional analyses showed that this association was of similar size across waves and that nonmortality attrition did not affect the trend in cognitive health (see “Attrition,” in the Appendix). The proportion of participants with nonresponse on the MMSE did increase among participants in fair or poor cognitive health at the previous wave, and did not increase among participants in good cognitive health at the previous wave. However, the trend in cognitive health was not affected when this proportion was included in the model. These various sensitivity analyses suggest that practice effect and nonresponse attrition had no substantial effects on the positive trends observed in cognitive health.
Relation to Other Studies
Our findings fit in with those from other western countries, in particular regarding the increase in cognitively healthy life expectancy.4,9 Regarding physically healthy life expectancy, the findings are a little more pessimistic, in that we note a small increase in life years in poor health, whereas other studies show stability or a small decrease. However, in most studies, a single measure of physical disability is used.3,4 Indeed, when we examined the trends in mild and severe disability alone, we found that the increase in mild disability came to a halt in the 2000s and that severe disability showed a small decline across the study period (data available upon request). Therefore, any increase in fair physical health after 2002 is likely to be driven by an increase in multimorbidity. Such increase has been found in other countries.28 Meanwhile, we maintain that our health measure, which combines multimorbidity and disability, better reflects the issues that older people are confronted with than disability alone. Our reasons for this are that the prevalence of multimorbidity is expected to increase because of further advances in medical treatment and care,28 that multimorbidity represents a complex condition with serious consequences for functioning and well-being,15 and that multimorbidity did not become less disabling during the period covered by our study.29
The positive trend observed in cognitive health could be attributed more than 50% to the rise in the level of education, but remained significant after we accounted for education. Various authors have attributed the improvement in cognitive functioning not only to increased access to education but also more meaningful and active learning,30 and to an increase in occupations with high complexity.31 More distal explanatory factors include better nutrition, smaller and more affluent families in which parents pay more attention to their children, and greater information density in society.32 Particularly relevant to later life is the recent improvement in the control of cardiovascular risk factors such as hypertension and cholesterol, which may help slow cognitive decline.10,33
Thus, various developments are likely drivers of the observed improvement in cognitive health. In contrast, the negative physical health trend for women would have been more pronounced had women’s level of education not increased over time. Further rises in the level of education of the older population may be expected, so that increases in life expectancy in poor physical health may continue to be counteracted by increases in the level of education.34
Public Health Implications
In light of current policy to contain the costs of long-term care, this study’s findings are pertinent to the question whether trends in physically and cognitively healthy life expectancy warrant reductions in the supply of elder care. Because of the increase in less-than-good physical health, future health care needs may change toward more social and rehabilitative care as well as toward more medical care geared to multimorbidity.25 The small increase in life expectancy in poor physical health was accompanied by a clear decrease in life expectancy in fair or poor cognitive health. The trend in poor physical and fair or poor cognitive health combined showed no increase for men and even a decrease for women. Although the proportion of people with poor overall health is small, it accounts for a relatively large share of health care costs.25,35 Thus, one might argue that an increase in demand for long-term care might be limited, were it not for the expected absolute increase in the number of oldest-old people. Interventions to improve both physical and cognitive health in the older population remain important.
ACKNOWLEDGMENTS
We greatly acknowledge funding for the Longitudinal Aging Study Amsterdam by grants from the Netherlands Ministry of Health Welfare and Sports, Directorate of Long-Term Care, the Netherlands Organization for Scientific Research (file number 480-10-014), and the Network for Studies on Pensions Aging and Retirement (LMVP2014.01).
Note. The funders have no role in the study design; the collection, analysis, and interpretation of data; in the writing of this report; and in the decision to submit it for publication.
HUMAN PARTICIPANT PROTECTION
The VU University Medical Centre’s Medical Ethics Evaluation Committee approved the study (archive numbers 92/138 and 2002/141). Informed consent was obtained from all individual participants included in the study.
Footnotes
See also Crimmins, p. 1582.
REFERENCES
- 1.Da Roit B. The Netherlands: the struggle between universalism and cost containment. Health Soc Care Community. 2012;20(3):228–237. doi: 10.1111/j.1365-2524.2011.01050.x. [DOI] [PubMed] [Google Scholar]
- 2.European Health and Life Expectancy Information System (EHLEIS) Health expectancy in the Netherlands. EHLEIS Country Reports, Issue 8, 2015. Available at: http://www.eurohex.eu. Accessed May 25, 2018.
- 3.Freedman VA, Wolf DA, Spillman BC. Disability-free life expectancy over 30 years: a growing female disadvantage in the US population. Am J Public Health. 2016;106(6):1079–1085. doi: 10.2105/AJPH.2016.303089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jagger C, Matthews FE, Wohland P et al. Medical Research Council. Cognitive Function and Ageing Collaboration. A comparison of health expectancies over two decades in England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2016;387(10020):779–786. doi: 10.1016/S0140-6736(15)00947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thatcher AR, Cheung SL, Horiuchi S, Robine J-M. The compression of deaths above the mode. Demogr Res. 2010;22:505–538. doi: 10.4054/DemRes.2010.22.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews FE, Arthur A, Barnes LE et al. Medical Research Council. Cognitive Function and Ageing Collaboration. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382(9902):1405–1412. doi: 10.1016/S0140-6736(13)61570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham heart study. N Engl J Med. 2016;374(6):523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu YT, Fratiglioni L, Matthews FE et al. Dementia in Western Europe: epidemiological evidence and implications for policy making. Lancet Neurol. 2016;15(1):116–124. doi: 10.1016/S1474-4422(15)00092-7. [DOI] [PubMed] [Google Scholar]
- 10.Crimmins EM, Saito Y, Kim JK. Change in cognitively healthy and cognitively impaired life expectancy in the United States: 2000–2010. SSM Popul Health. 2016;2:793–797. doi: 10.1016/j.ssmph.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perera G, Pedersen L, Ansel D et al. Dementia prevalence and incidence in a federation of European electronic health record databases: the European Medical Informatics Framework resource. Alzheimers Dement. 2018;14(2):130–139. doi: 10.1016/j.jalz.2017.06.2270. [DOI] [PubMed] [Google Scholar]
- 12.Robine J-M, Michel J-P. Looking forward to a general theory on population aging. J Gerontol A Biol Sci Med Sci. 2004;59(6):M590–M597. doi: 10.1093/gerona/59.6.m590. [DOI] [PubMed] [Google Scholar]
- 13.Deeg DJH. Robine and Michel’s “Looking forward to a general theory on population aging”: population aging: the benefit of global versus local theory. J Gerontol A Biol Sci Med Sci. 2004;59(6):M600. doi: 10.1093/gerona/59.6.m600. [DOI] [PubMed] [Google Scholar]
- 14.Hoogendijk EO, Deeg DJ, Poppelaars J et al. The Longitudinal Aging Study Amsterdam: cohort update 2016 and major findings. Eur J Epidemiol. 2016;31(9):927–945. doi: 10.1007/s10654-016-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marengoni A, Angleman S, Melis R et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Geerts J, Van den Bosch K. Transitions in formal and informal care utilization amongst older Europeans: the impact of national contexts. Eur J Ageing. 2011;9(1):27–37. doi: 10.1007/s10433-011-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galenkamp H, Huisman M, Braam AW, Schellevis FG, Deeg DJ. Disease prevalence based on older people’s self-reports increased, but patient–general practitioner agreement remained stable, 1992–2009. J Clin Epidemiol. 2014;67(7):773–780. doi: 10.1016/j.jclinepi.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Galenkamp H, Braam AW, Huisman M, Deeg DJ. Seventeen-year time trend in poor self-rated health in older adults: changing contributions of chronic diseases and disability. Eur J Public Health. 2013;23(3):511–517. doi: 10.1093/eurpub/cks031. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Mathers CD, Robine J-M. How good is Sullivan’s method for monitoring changes in population health expectancies? J Epidemiol Community Health. 1997;51(1):80–86. doi: 10.1136/jech.51.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero-Ortuno R, Kenny RA. The frailty index in Europeans: association with age and mortality. Age Ageing. 2012;41(5):684–689. doi: 10.1093/ageing/afs051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. New York, NY: Cambridge University Press; 2003. [Google Scholar]
- 23.Flynn JR. Massive IQ gains in 14 nations: what IQ tests really measure. Psychol Bull. 1987;101(2):171–191. [Google Scholar]
- 24.Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 25.Parker MG, Thorslund M. Health trends in the elderly population: getting better and getting worse. Gerontologist. 2007;47(2):150–158. doi: 10.1093/geront/47.2.150. [DOI] [PubMed] [Google Scholar]
- 26.Andrews JS, Desai U, Kirson NY et al. Functional limitations and health care resource utilization for individuals with cognitive impairment without dementia: findings from a United States population-based survey. Alzheimers Dement (Amst) 2016;6:65–74. doi: 10.1016/j.dadm.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comijs HC, Dik MG, Deeg DJH, Jonker C. The impact of change in cognitive functioning and cognitive decline on disability, well-being, and the use of health care services in older persons: results of the Longitudinal Aging Study Amsterdam. Dement Geriatr Cogn Disord. 2005;19(5-6):316–323. doi: 10.1159/000084557. [DOI] [PubMed] [Google Scholar]
- 28.Crimmins EM, Beltrán-Sánchez H. Mortality and morbidity trends: is there compression of morbidity? J Gerontol B Psychol Sci Soc Sci. 2011;66(1):75–86. doi: 10.1093/geronb/gbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoeymans N, Wong A, van Gool CH et al. The disabling effect of diseases: a study on trends in diseases, activity limitations, and their interrelationships. Am J Public Health. 2012;102(1):163–170. doi: 10.2105/AJPH.2011.300296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blair C, Gamson DA, Thome S, Baker DP. Rising mean IQ: cognitive demand of mathematics education for young children, population exposure to formal schooling, and the neurobiology of the prefrontal cortex. Intelligence. 2005;33(1):93–106. [Google Scholar]
- 31.Marioni RE, Valenzuela MJ, Van den Hout A, Brayne C, Matthews FE. Active cognitive lifestyle is associated with positive cognitive health transitions and compression of morbidity from age sixty-five. PLoS One. 2012;7(12):e50940. doi: 10.1371/journal.pone.0050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bordone V, Scherbov S, Steiber N. Smarter every day: the deceleration of population ageing in terms of cognition. Intelligence. 2015;52:90–96. [Google Scholar]
- 33.Dodge HH, Zhu J, Hughes TF et al. Cohort effects in verbal memory function and practice effects: a population-based study. Int Psychogeriatr. 2017;29(1):137–148. doi: 10.1017/S1041610216001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joung IMA, Kunst AE, van Imhoff E, Mackenbach JP. Education, aging, and health: to what extent can the rise in educational level relieve the future health (care) burden associated with population aging in the Netherlands? J Clin Epidemiol. 2000;53(9):955–963. doi: 10.1016/s0895-4356(99)00232-2. [DOI] [PubMed] [Google Scholar]
- 35.Wouterse B, Huisman M, Meijboom BR, Deeg DJ, Polder JJ. Modeling the relationship between health and health care expenditures using a latent Markov model. J Health Econ. 2013;32(2):423–439. doi: 10.1016/j.jhealeco.2012.11.005. [DOI] [PubMed] [Google Scholar]