Abstract
Frequently reported poor dietary habits of young adults increase their risk of metabolic syndrome (MetS). Excess adiposity is the most established predictor of MetS, and numerous anthropometric measures have been proposed as proxy indicators of adiposity. We aimed to assess prevalence of MetS in young adult population and to make comparison between weight- and shape-oriented measures of adiposity to identify the best index in association with measured body fat and as a risk predictor for MetS. Healthy males and females aged 18–25 years from the Northwest of England were recruited using convenience sampling (n=550). As part of the assessment of the overall health of young adults, the biochemical variables and adiposity measures BMI, waist circumference (WC), waist-to-height ratio (WHtR), waist-to-hip ratio (WHR), new BMI, Body Adiposity Index (BAI), Clinica Universidad de Navarra-Body Adiposity Estimator (CUN-BAE), and A Body Shape Index (ABSI) were assessed. Linear regression analysis was used to investigate the association between the proxy indices of adiposity and measured percentage body fat. The odds ratio with 95% confidence interval was used to investigate the relationship between cardiometabolic (CM) risk factors and proxy measures of adiposity. The discriminatory power of these measures for diagnosis of MetS was investigated using area under the receiver operating characteristic curve. Body weight-related indicators of adiposity, particularly CUN-BAE, had stronger association with measured body fat compared with body shape-related indices. In relation with MetS, body shape-related indices, particularly elevated WC and WHtR, had stronger associations with CM risk compared with body weight-related measures. Amongst all indices, the best predictor for CM risk was WHtR, while ABSI had the weakest correlation with body fat, MetS, and CM risk. Indices directly associated with WC and specifically WHtR had greater diagnostic power in detection of CM risk in young adults.
1. Introduction
Emerging adulthood has been characterized with poor dietary habits [1, 2]. These poor dietary habits have been associated with the transition to independence, stress, academic and peer pressure, and taking responsibility for food choice when starting to study at university [3–5]. Several studies have reported that university students fail to meet the dietary guidelines [6–8] and gain weight in the university years [9–12], which can have adverse health consequences leading to an increased risk of obesity, type 2 diabetes, and cardiovascular diseases in later life [13, 14]. The existence of metabolic syndrome (MetS) in young adults can be a predictor of these chronic conditions in older adults [15, 16].
MetS is defined as a cluster of metabolic conditions associated with abdominal obesity including elevated blood pressure, impaired glucose tolerance, insulin resistance, elevated triglycerides, and low level of high-density lipoprotein cholesterol concentrations [17]. Similarly, the term “cardiometabolic risk” (CM) is characterized by the existence of the elements of MetS, namely, central obesity, impaired glucose metabolism, hypertension, and dyslipidaemia [18, 19]. Within the UK, several studies have investigated the prevalence and correlates of MetS in ethnic minority groups [20–23] and/or in patients with particular clinical conditions [24–28]; however, such research in emerging adulthood in the UK is scarce [29].
Amongst metabolic conditions of MetS, abdominal adiposity is of particular importance as it independently predicts the risk of other comorbidities and metabolic conditions [30]. Several anthropometric measures have been used as proxy indicators of central or whole-body adiposity.
Body mass index (BMI) developed by Adolphe Quetelet in 1832 [31] has been extensively used as a traditional proxy measure of adiposity [32]. BMI is a weight-for-height measure and by nature unable to distinguish between fat mass and muscle mass and unable to establish regional fat distribution [33]. These two substantial limitations question the discriminatory power of BMI in practice as it can potentially produce false diagnosis of adiposity, overestimate fat accumulation in tall people, and underestimate it in short people [32, 34, 35]. Furthermore, the limitation in estimating central obesity matters, as abdominal fat, is a more specific CM risk predictor compared with overall body fatness [33].
Waist circumference (WC) has been recommended with the advantage of assessing central adiposity [36, 37]; however, its application in practice has been questioned due to different cutoff points for men and women and emerging evidence showing a variation in diagnostic thresholds between ethnic groups [33, 37–40]. Similarly, the proposed ratio of waist circumference to hip circumference (WHR) as a measure of relative fat distribution requires specific gender and ethnic group cutoff points [41, 42]. Further, throughout weight loss with reduction of circumferences of both waist and hip, the ratio of waist to hip circumferences may not change substantially and therefore limits the practical utility of the measure for the CM risk management [43].
To eliminate the confounding impact of height on the association between anthropometry and CM risk [44, 45], waist-to-height ratio (WHtR) was proposed as a simple, noninvasive, and effective screening tool [46–55] benefiting from the extensive literature to support its use in relation with CM risk [56–62] and cross validation with a widely used universal cutoff point measure for identification of the abdominal obesity in different ethnic groups [63–70]. Despite this, not only has the superiority of WHtR to other anthropometric measures, as a better predictor of central adiposity and chronic diseases been questioned [71–74], but also the use of its universal yardstick for establishing central obesity in different ethnic groups has been challenged [54, 75–81].
In recent years, several body weight- and shape-associated measures of adiposity were proposed to address the limitations of the aforementioned established measures.
A correction to the equation of BMI was offered to produce a better predictor of the postoperative complications amongst colorectal cancer patients [82], but its validity and discriminatory power in relation with CM risk has yet to be tested in large samples.
Bergman et al. proposed another measure, Body Adiposity Index (BAI), calculated from hip circumference and height (Table 1) as a predictor of percentage body fat, which was validated against dual-energy X-ray absorptiometry (DXA) measurements in a large sample of Mexican adults [83]. Several studies confirmed validity and practical use of BAI [84–96]; nonetheless, an extensive body of knowledge from studies in the range of ethnic groups and patient populations questioned its validity in comparison with reference methods and/or in association with the CM risk [97–128].
Table 1.
Anthropometric indicators of adiposity used in this study, reference, and equation for calculation.
| Measure | Author (year) | Equation |
|---|---|---|
| Anthropometric indicators of adiposity related to body weight | ||
| Body mass index (BMI) | Gysel (1974) [31] | BMI = body weight (kg)/height (m)2 |
| New body mass index (New BMI) | Van Vugt et al. (2015) [82] | New BMI = 1.3 × (weight (kg)/height (m)2) |
| Clinica Universidad de Navarra-Body Adiposity Estimator (CUN-BAE) | Gomez-Ambrosi et al. (2012) [129] | BF% = −44.988 + (0.503 × age (years)) + (10.689 × sex) + (3.172 × BMI (kg/m2))−(0.026 × BMI2 (kg/m2)) + (0.181 × BMI (kg/m2) × sex) − (0.02 × BMI (kg/m2) × age) − (0.005 × BMI2 (kg/m2) × sex) + (0.00021 × BMI2 (kg/m2) × age), where male = 0 and female = 1 |
|
| ||
| Anthropometric indicators of adiposity related to body shape | ||
| Waist circumference (WC) | WHO (2008) [37] | Circumference of the waist measured in standardized position as advised by the WHO (cm) |
| Waist-to-hip ratio (WHR) | WHO (2008) [37] | WHR = waist circumference (cm)/hip circumference (cm) |
| Waist-to-height ratio (WHtR) | Ashwell (1995) [46] | WHtR = waist circumference (cm)/height (cm) |
| Body Adiposity Index (BAI) | Bergman et al. (2011) [83] | BAI (percentage body fat, BF%) = (hip circumference (cm)/height (m)1.5) − 18. |
| A Body Shape Index (ABSI) | Krakauer and Krakauer (2012) [134] | ABSI = waist circumference (cm)/(BMI (kg/m2)0.66 × height (m)0.5) |
The Clinica Universidad de Navarra-Body Adiposity Estimator (CUN-BAE) has been proposed to estimate percentage body fat from BMI, gender, and age [129] (Table 1); however, preliminary promising findings [130] and clinical usefulness were debated in some other studies [131–133]. A Body Shape Index (ABSI) was developed taking into consideration WC as a proxy measure of abdominal obesity but adjusting for weight and height [134]. Several studies confirmed the practical validity of ABSI [135–142]; however, others questioned its clinical use because of the limited association with measures of body fat [143], mortality [144, 145], and CM risk [146–152].
The inconsistencies, limitations, and discrepancies on reported validity of anthropometric indicators of adiposity demonstrate a gap in knowledge and a need to conduct further studies in the field. We recognise that the above anthropometric indices have been proposed as proxy indicators for total adiposity or central adiposity. Since the former invariably includes body weight and the latter typically includes waist circumference, within the current study, we categorised them as anthropometric proxy indicators related to body weight or body shape. Table 1 shows the body weight and body shape associated proxy indicators of adiposity with reference to the predictive equation model used to calculate each index.
Studies assessing the practical use of the anthropometric proxy indicators of adiposity either investigate their validity against a notional reference method or study their associations with chronic disease. The rationale for this approach is that in principle, a valid anthropometric proxy measure of adiposity must generally have a strong correlation with measured percentage body fat [153] and/or strong association with comorbidities and indeed a discriminatory power to predict their risk [154].
In the current study, to assess the predictive discriminatory power of different anthropometric measures of adiposity, we preliminarily compared them against an objective index measured, and then compared their associations with CM risk markers taking into consideration known confounding factors such as the level of physical activity. Therefore, the aim of this study was to assess prevalence of MetS in our young adult population and investigate which proxy measure of anthropometric adiposity has the strongest association (a) with measured percentage body fat and (b) with CM risk indices in healthy young adults in Northwest of England.
2. Materials and Methods
2.1. Study Design and Participants
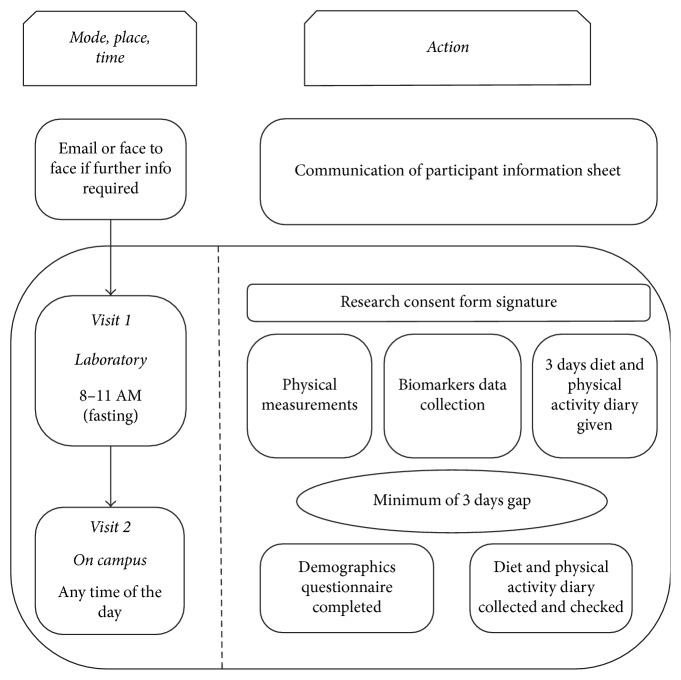
Five hundred and fifty (236 male and 314 female) participants aged 18–25 years were recruited in a cross-sectional study. The study was conducted within the framework of the Collaborative Investigation on Nutritional Status of Young Adults (CINSYA) in the city of Liverpool, UK. Participants were recruited by convenience sampling from universities across the Northwest of England between 2014 and 2016 and attended two clinical visits (Figure 1). All participants gave their written informed consent for inclusion in the study, prior to participation. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Ethics Committee.
Figure 1.
Schematic demonstrating the design of study.
Demographic data were collected by the questionnaire using questions extracted from the validated questionnaires of the UK National Diet and Nutrition Survey (NDNS) [155].
2.2. Physical Measurements
Body composition, fat and fat-free mass, total body water, and the overall percentage body fat were assessed by Tanita MC-180MA, which is a multifrequency bioelectrical impedance body composition analyser (Tanita Ltd, Tokyo, Japan). For this assessment, participants were in light clothing (i.e., commonly, 0.5 kg estimated weight of the light clothing automatically deducted by the equipment), while they removed their shoes and socks before stepping on the equipment. Tanita MC-180MA also measured body mass to the nearest 0.1 kg, which was used for calculation of BMI and other anthropometric indices.
Height was determined in Frankfort plane position using a SECA 201 stadiometer (SECA GMBH & Co., Hamburg, Germany). The systolic and diastolic blood pressure (SBP and DBP, respectively) was measured in a seated position by Omron 907 professional blood pressure monitor (Omron Corporation, Kyoto, Japan) twice; in the start and toward the end of the first visit, the SBP and DBP were recorded as the mean of the two measurements. The circumferences of waist and hip were measured using nonstretchable tape measure over light clothing as advised within the literature [40].
Traditional BMI calculated as body weight divided by squared height (kg/m2) was classified into two categories as normal (18.5–24.9 kg/m2) and overweight or obese (≥25 kg/m2). The new BMI, which was calculated as 1.3 ∗ weight/height2 (kg/m2), was also classified into two categories using the same cutoff points: normal (18.5–24.9 kg/m2) and overweight or obese (≥25 kg/m2) [82]. ABSI [134], BAI [83], and CUN-BAE [129] were calculated based on the earlier-suggested formulae (Table 1). Since there are no population specific defined cutoff points for these three measures and also for WHR, sex-specified medians for each one was used to categorise participants into two groups (equal or more than median or lower than median). Abdominal obesity was assessed using WC and WHtR. Based on WC, participants were divided into abdominal obese, where the WC was ≥102 cm in male and ≥88 cm in female, or nonabdominally obese, where WC was <102 cm in male and <88 cm in female [156]. WHtR was calculated by dividing WC by height, which was classified as abdominal obese or nonabdominally obese using cutoff point 0.50 [50]. With regard to the cutoff points for body fat measured by the bioelectrical impedance body fat analyser, as per the manufacturer's guidelines, ≥20% of total body fat in males and ≥33% in females were considered as excessive body fat, whilst lower amounts were defined as normal values.
2.3. Diet and Physical Activity
A three-day integrated diet and physical activity diary were used to assess energy and nutrient intake and to estimate energy expenditure. The diet diary was extracted from the validated questionnaires of the UK's National Diet and Nutrition Survey (NDNS) [155] with minimal adjustments. To improve compliance and enhance accuracy, standardised guidelines used in NDNS, a completed example, and food portion pictures were supplied and prompts on time, place, and portion sizes were shown in the diet diary. The diaries were analysed for energy, macronutrients, and micronutrients using microdiet dietary analysis software (Microdiet v3, Downlee systems Ltd, Salford, UK). A validated 3-day physical activity diary produced by Bouchard et al. was used to assess physical activity. The analysed output of the diary produced total energy expenditure as kcal/kg/day and min/day spent in light/moderate/vigorous activity [157].
2.4. Biomarkers
The full procedure of capillary whole blood lipid and glucose analysis was detailed previously [158]. In brief, participants fasted overnight for least 8 hours before capillary puncture of whole blood sample was obtained. After cleaning the site with alcohol and drying it, a capillary sample of 35 µl was collected using a lancet and capillary tube/plunger with heparin anticoagulant. The sample was injected into the equipment cassette, which was inserted to the analyser. The Alere LDX (Alere, San Diego, CA) was used as a point of care capillary whole blood glucose and lipid analyser to assess total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG). These variables were then used by the analyser to calculate low-density lipoprotein cholesterol (LDL-C) using the Friedewald equation [158] and the ratio of TC/HDL. The fasting blood glucose concentration was also measured by the analyser.
2.5. Statistical Analysis
Data analysis was conducted using SPSS version 23 for Windows (IBM SPSS, Inc., Armonk, NY, USA). All data are expressed as mean ± standard error (SE) of mean. The required sample size (n=543) was estimated with 95% confidence interval and prediction of the prevalence of MetS to be around 8% (based on the midpoint of the values reported in comparable age groups [146, 159]) and with setting a margin of error and a potential for dropout. Because of the relatively large sample size of the study (n > 500), we did not perform the Kolmogorov–Smirnov or Shapiro–Wilk test to assess the normal distribution of the data; however, the data were scanned to remove SPSS-identified outliers based on 95% confidence interval for the mean as well as participants with improbable energy intake (i.e., reported average calorie intake <800 kcal or >4200 kcal in line with the previous literature [160]) and/or participants who did not have their anthropometric data completed. Out of 565 potential participants who contributed to study, fifteen participants were excluded as obvious outliers of normal distributions or missing key anthropometric information. Data from 550 participants (236 males and 314 females) with productive profile were used in the final analytical dataset.
Descriptive statistics were performed to establish the demographic profile of the study population. Inferential statistics were used to address the main research questions. To investigate the strengths of the association between the proxy measures of anthropometric adiposity and measured body fat, regression analysis was used.
To investigate the relationship between CM risk factors and BMI, new BMI, ABSI, CUN-BAE, BAI, WC, WHR, and WHtR, the odds ratio was calculated with 95% confidence interval from linear and logistic models in crude and adjusted models, respectively, controlling for the effect of smoking, age, and physical activity in the adjusted model. To investigate the discriminatory ability of each proxy measure of anthropometric adiposity over the possible values to detect CM risk, area under the receiver operating characteristic (ROC) curve (AUC) was quantified and tested. Further details of the statistical analysis procedure have been described previously [146].
3. Results
3.1. Participants' Characteristics
A total of 550 young adults participated in the study, and the participants' mean age and BMI were 21.2 years and 24.2 kg/m2, respectively. Of these participants, 57.1% were female and 96.2% were British. Most of the participants were single (80.1%), and 14.0% of them were current smoker. Means of all serum lipids, fasting blood sugar, and diastolic blood pressure were in the normal range. Mean systolic blood pressure of participants was 123.1 mmHg (Table 2).
Table 2.
Characteristics of the study population.
| Index | Mean ± SE or % |
|---|---|
| Age (years) | 21.19 ± 0.10 |
| BMI (kg/m2) | 24.18 ± 0.18 |
| Body fat (%) | 24.60 ± 0.39 |
| WC (cm) | 80.51 ± 0.50 |
| CUN-BAE | 26.03 ± 0.36 |
| BAI | 45.08 ± 0.22 |
| ABSI | 0.00030 ± 0.0000035 |
| New BMI (kg/m2) | 24.11 ± 0.18 |
| WHR | 0.80 ± 0.003 |
| WHtR | 0.47 ± 0.003 |
| Total cholesterol (mg/dL) | 158.70 ± 1.49 |
| LDL-C (mg/dL) | 104.83 ± 16.56 |
| HDL-C (mg/dL) | 59.30 ± 5.59 |
| Triglyceride (mg/dL) | 115.44 ± 12.07 |
| Fasting blood sugar (mg/dL) | 89.82 ± 0.50 |
| Systolic blood pressure (mmHg) | 123.14 ± 0.61 |
| Diastolic blood pressure (mmHg) | 75.59 ± 0.45 |
| Female (%) | 57.1 |
| British (home students) (%) | 96.2 |
| Single (%) | 80.1 |
| Smoker (%) | 14.0 |
| Population at risk based on BMI∗ (%) | 32.5 |
| Population at risk based on new BMI∗ (%) | 31.8 |
| Population at risk based on WC∗ (%) | 12.2 |
| Population at risk based on WHtR∗ (%) | 28.5 |
| Population at risk based on excessive measured body fat∗ (%) | 32.7 |
∗Percentage of the population classified at risk is calculated using accepted boundary values for the anthropometric indices: BMI/new BMI ≥ 25 kg/m2, abdominal obese: WC ≥ 102 cm in males and ≥88 cm in females, elevated WHtR: ≥0.5, and excess body fat: body fat ≥20% in male and ≥33% in female.
3.2. Prevalence of CM Risk Factors
The prevalence of CM risk factors is shown in Figure 2. Overall, 6.8% of participants were affected by MetS, 57.6% of them had at least one risk factor, and 18.1% at least two risk factors for cardiometabolic diseases. The most prevalent CM risk factor among biochemical markers was low-serum HDL-C levels (30.7%), whilst the lowest prevalent one was elevated LDL-C levels (8.1%) (Figure 2).
Figure 2.
Prevalence of cardiometabolic risk in the study population.
The prevalence of risk factors related to the biochemical test is based on total cholesterol ≥200 mg/dL, LDL ≥130 mg/dL, HDL-C <40 mg/dL in male and <50 mg/dL in female, triglyceride ≥150 mg/dL, fasting blood sugar ≥100 mg/dL, hypertension SBP ≥130.0, and/or DBP ≥85.0 mmHg. Metabolic syndrome was defined as the presence of three or more of the following components: (1) abdominal adiposity (elevated waist circumference); (2) low-serum HDL-C (<50 mg/dL); (3) high-serum triacylglycerol levels (≥150 mg/dL); (4) elevated blood pressure (≥130/85 mmHg); (5) abnormal glucose homeostasis (fasting plasma glucose level ≥110 mg/dL).
3.3. Association with Percentage Body Fat
The Pearson correlation coefficient from linear regression tests showed statistically significant association between all proxy anthropometric indicators of adiposity in comparison with measured percentage body fat (P < 0.0001). The strength of the correlation with measured body fat (%) was BMI: r = 0.546, new BMI: r = 0.589, CUN-BAE: r = 0.828, WC: r = 0.307, WHtR: r = 0.479, BAI: r = 0.681, WHR: r = −0.012, and ABSI: r = −0.426.
3.4. Association with Cardiometabolic Risk
The correlation between different anthropometric measurements and serum lipids and fasting blood sugar are presented in Table 3. Fasting blood sugar was positively correlated to traditional BMI, WC, WHR, and new BMI. Total cholesterol was also directly correlated with body fat percent, traditional BMI, WHtR, new BMI, CUN-BAE, and BAI. Serum TG was weakly correlated to WHR. Other biochemical risk factors were not significantly correlated with proxy anthropometric measures of adiposity.
Table 3.
Linear regression of proxy anthropometric measures of adiposity with cardiometabolic risk.1
| Blood sugar | Total cholesterol | LDL-C | HDL-C | TG | |
|---|---|---|---|---|---|
| Body fat percent | 0.027 | 0.173§ | −0.004 | −0.016 | 0.026 |
| BMI | 0.131§ | 0.103§ | −0.006 | −0.049 | 0.052 |
| New BMI | 0.106§ | 0.129§ | −0.004 | −0.033 | 0.054 |
| CUN-BAE | 0.001 | 0.177§ | 0.024 | −0.052 | 0.037 |
| WC | 0.236§ | 0.069 | 0.025 | −0.052 | 0.055 |
| WHR | 0.208§ | 0.032 | 0.013 | 0.022 | 0.087§ |
| WHtR | 0.195 | 0.126§ | 0.040 | −0.031 | 0.061 |
| BAI | 0.028 | 0.160§ | 0.050 | −0.036 | 0.005 |
| ABSI | −0.070 | −0.078 | 0.028 | 0.048 | −0.040 |
1Using linear regression;§P < 0.05.
Crude and multivariable-adjusted odds ratio (OR) and 95% CI for the presence of at least one risk factor, at least two risk factors for CM diseases, and also MetS (i.e., at least three risk factors for CM diseases) are shown in Table 4. All body weight-related or body shape-related anthropometric indicators of adiposity were associated with increased risk of having MetS, at least one risk factor or at least two risk factors of CM diseases except for ABSI. We observed that higher ABSI decreased the risk of MetS by 75% in the crude model; however, controlling for various potential confounders disappeared this association. ABSI was also not related to the risk of at least one risk factor or at least two risk factors of CM diseases. Overall, the direct link between body shape-related indicators of adiposity and CM risks was stronger than body weight-related measures. Amongst the body shape-related anthropometric indicators of adiposity, abdominal adiposity, particularly elevated WC, was the best predictor of CM risks. Amongst the body weight-related anthropometric indicators of adiposity, the best predictor for MetS was CUN-BAE, whereas the best predictor for at least one risk factor or at least two risk factors of CM diseases was the new BMI.
Table 4.
Multivariate-adjusted odds ratio (and 95% confidence intervals) for cardiometabolic risk associated with proxy indicators of anthropometric adiposity.1
| MetS | P value2 | At least two risk factors | P value2 | At least one risk factor | P value2 | |
|---|---|---|---|---|---|---|
| Crude model | ||||||
| Body fat percent | 7.47 (3.44, 16.22) | <0.0001 | 2.25 (1.42, 3.54) | <0.0001 | 1.96 (1.33, 2.89) | 0.001 |
| BMI | 10.50 (4.51, 24.43) | <0.0001 | 2.62 (1.66, 4.14) | <0.0001 | 2.36 (1.59, 3.51) | <0.0001 |
| New BMI | 9.23 (4.12, 20.66) | <0.0001 | 2.87 (1.82, 4.54) | <0.0001 | 2.71 (1.81, 4.07) | <0.0001 |
| CUN-BAE | 12.81 (3.88, 42.24) | <0.0001 | 1.82 (1.15, 2.87) | 0.010 | 1.92 (1.34, 2.74) | <0.0001 |
| WC | 32.40 (14.62, 71.80) | <0.0001 | 2.85 (1.60, 5.08) | <0.0001 | 3.21 (1.69, 6.07) | <0.0001 |
| WHR | 16.49 (3.92, 69.31) | <0.0001 | 2.05 (1.28, 3.30) | 0.003 | 1.43 (1.00, 2.04) | 0.048 |
| WHtR | 26.32 (9.14, 75.78) | <0.0001 | 2.98 (1.87, 4.73) | <0.0001 | 2.92 (1.90, 4.49) | <0.0001 |
| BAI | 9.32 (3.26, 26.71) | <0.0001 | 1.80 (1.14, 2.85) | 0.011 | 1.79 (1.26, 2.56) | 0.001 |
| ABSI | 0.25 (0.11, 0.56) | 0.001 | 0.71 (0.45, 1.11) | 0.137 | 0.72 (0.50, 1.02) | 0.066 |
|
| ||||||
| Adjusted model | ||||||
| Body fat percent | 5.33 (2.36, 12.07) | <0.0001 | 1.90 (1.16, 3.14) | 0.011 | 1.89 (1.24, 2.89) | 0.003 |
| BMI | 7.99 (3.15, 20.32) | <0.0001 | 2.19 (1.27, 3.78) | 0.005 | 2.54 (1.58, 4.07) | <0.0001 |
| New BMI | 6.60 (2.73, 15.95) | <0.0001 | 2.37 (1.39, 4.05) | 0.002 | 2.96 (1.84, 4.75) | <0.0001 |
| CUN-BAE | 9.02 (2.57, 31.73) | 0.001 | 1.37 (0.79, 2.35) | 0.258 | 1.88 (1.22, 2.90) | 0.004 |
| WC | 58.04 (18.30, 184.10) | <0.0001 | 2.65 (1.38, 5.09) | 0.003 | 2.96 (1.50, 5.86) | 0.002 |
| WHR | 16.26 (3.77, 70.12) | <0.0001 | 1.83 (1.09, 3.06) | 0.021 | 1.45 (0.98, 2.15) | 0.061 |
| WHtR | 20.88 (7.00, 62.29) | <0.0001 | 2.55 (1.53, 4.24) | <0.0001 | 3.07 (1.91, 4.91) | <0.0001 |
| BAI | 6.91 (2.37, 20.16) | <0.0001 | 1.62 (1.00, 2.63) | 0.050 | 1.77 (1.22, 2.58) | 0.003 |
| ABSI | 0.41 (0.17, 1.01) | 0.052 | 0.99 (0.58, 1.67) | 0.961 | 0.83 (0.55, 1.25) | 0.374 |
1Using logistic regression; 2derived from a Mantel–Haenszel extension chi-square test.
The ROC curve analysis examining the AUCs (and 95% CIs) of anthropometric measures in the prediction of MetS and cardiometabolic risks is shown in Table 5. The lowest AUC for all three MetS, at least one risk factor, or at least two risk factors of CM risk belonged to ABSI. Consistent with results of logistic regression, the highest AUC for MetS was related to WC and WHtR. The greatest AUC for at least two risk factors of CM was related to WHtR, which was not statistically different from body fat, BMI, WC, and new BMI. Although the highest AUC for at least one risk factor of CM also belonged to WHtR, this was not statistically different from all other indices (except for ABSI).
Table 5.
Area under curve analysis for cardiometabolic risk associated with body weight and body shape-related indicators of adiposity.
| MetS | At least two risk factors | At least one risk factor | |
|---|---|---|---|
| Body fat percent | 0.771 (0.694, 0.847) | 0.605 (0.544, 0.665) | 0.595 (0.545, 0.644) |
| BMI | 0.827 (0.747, 0.906) | 0.614 (0.548, 0.680) | 0.615 (0.566, 0.663) |
| New BMI | 0.826 (0.746, 0.907) | 0.637 (0.572, 0.702) | 0.630 (0.582, 0.678) |
| CUN-BAE | 0.768 (0.680, 0.856) | 0.596 (0.530, 0.661) | 0.600 (0.551, 0.650) |
| WC | 0.889 (0.831, 0.947) | 0.640 (0.575, 0.705) | 0.612 (0.563, 0.660) |
| WHR | 0.782 (0.723, 0.841) | 0.638 (0.575, 0.701) | 0.585 (0.536, 0.634) |
| WHtR | 0.892 (0.831, 0.953) | 0.663 (0.600, 0.727) | 0.631 (0.583, 0.680) |
| BAI | 0.776 (0.692, 0.860) | 0.581 (0.517, 0.646) | 0.599 (0.549, 0.648) |
| ABSI | 0.233 (0.140, 0.327) | 0.427 (0.358, 0.495) | 0.415 (0.365, 0.464) |
4. Discussions
To the best of our knowledge, this is the first study reporting the prevalence of CM risk and MetS in young adults in Northwest of the UK and the first that compared the association between variety of proxy indicators of adiposity with measured body fat and CM risk in this population. The study addressed its question about the clinical usefulness of different anthropometric indices of adiposity and contributes to our understanding of broad picture of nutritional status of young adults in the UK:
The current study demonstrated a significant and relatively strong correlation between most of indicators of adiposity and measured body fat and is also a strong association between these indicators and CM risk. Apart from ABSI, all other indices of adiposity were associated with CM risk when tested using multivariate-adjusted OR, while showing clear advantage for simple anthropometric indices based on waist circumference. The ROC examination also confirmed the usefulness of WC and particularly the superiority of WHtR based on the greatest AUC and therefore its diagnostic power in detection of MetS.
The strength of the correlation between CUN-BAE and measured body fat, which was in line with the previous literature [129], suggests CUN-BAE to be a potentially useful proxy measure of adiposity for our population; however, this strength was not replicated to the same extent when CUN-BAE was associated with CM risk in testing through multivariate OR and in particular, when the effect of potential confounding factors were taken into consideration in our multivariate-adjusted OR analysis. Furthermore, the prediction equation formula of CUN-BAE is rather complicated, and this limits the clinical usefulness of this measure in practice.
In addition to CUN-BAE, other indicators of anthropometric adiposity also showed statistically significant association with measured body fat, with the strength of the association declining gradually from BAI, new BMI, BMI, WHtR, WC, ABSI, and WHR, respectively; broadly showing strong correlations between body weight-related indicators of adiposity when compared with measured body fat. On the other hand, as seen with CUN-BAE, the body weight-related measures of adiposity did not produce superior association with CM risk factors limiting their clinical usefulness for the current population. Nonetheless, the strong association of the body weight-related indicators of adiposity with measured body fat was not surprising because these measures are understandably expected to have a better association with whole-body adiposity (rather than abdominal adiposity), often generated or validated based on the linear regression prediction equations against measured adiposity in cross-sectional studies [86, 94, 103, 113, 115, 125–129, 131, 132, 143], and they typically require further validation to establish their association with chronic noncommunicable diseases.
Amongst all body weight and body shape indicators of adiposity investigated, ABSI produced the weakest association with CM risk and negative association with percentage body fat. The current finding proposes that ABSI has substantial limitations for using in this population as the measure had no statistical association with CM risk factors and consequently insignificant association in multivariate-adjusted OR and the smallest AUC in the ROC curves amongst all body weight and body shape-related measures of anthropometric adiposity. This finding was in contrast with some previous studies [135, 137, 138, 142], whereas confirming some other studies [146–150]. This is difficult to explain these contrasting findings particularly in view of the different endpoint outcome variables used in different studies. For instance, the study by Krakauer and Krakauer [134] proposed ABSI as a predictor of premature mortality, whereas our study investigated the discriminatory power of this measure in distinguishing CM risk. Despite this, we thought that a potential explanation for the findings may be based on the nature and relationship between the variables used in ABSI's calculation. Conceptually, while ABSI was proposed to associate body shape with health outcomes independently of the variables defining body size (i.e., height, WC, and BMI) [134, 161], we argue that in the principle, the interrelationship between these defining variables may restrict its clinical usefulness. In particular, we support the previously proposed hypotheses that ABSI's dependency to body height may confound its capacity to distinguish CM risk in study populations [47, 48, 146, 161].
The ROC analysis demonstrates that the highest AUC belongs to WHtR confirming the discriminatory power of this measure for our target population; however, WC and new BMI also showed promising AUC for detection of MetS as potential alternatives. Similarly, the largest AUC in relation with one or two CM risk factors belonged to WHtR overall confirming the previously reported superiority of WHtR compared with other proxy measures of adiposity [52].
While the association between WHtR and measured body fat was significant, the strong association with CM risk factors in logistic regression and the excellent findings from test of the ROC curve give a collective evidence for the superiority of this measure in comparison with other anthropometric indicators of adiposity investigated. This is also important to consider the ease of use, simplicity, and clarity of the public health messages based on the WHtR (i.e., keep your waist size less than half of your height) [49], which makes it an excellent tool to be used in different settings including our population. The matter becomes more important when we consider the complexity of the prediction equation formulae of some of the investigated proxy indicators of adiposity and the necessity for establishing gender and ethnicity specific cutoff points in order to interpret their findings.
The pathophysiological mechanism to explain the association between WHtR and CM risk is yet to be fully elucidated. Height can reflect early life exposures. A prospective cohort study among Chilean adults suggests that individuals who had adverse environmental exposures during childhood were more likely to have short stature and abdominal adiposity, insulin resistance, and other CM risk factors in their adulthood [162]. In addition, due to inverse associations between height and mortality and CM morbidity, inclusion of the parameter of stature beside central adiposity measures such as WC might be the reason for the superiority of WHtR over BMI and WC [163, 164]. Alternatively, height may not only affect CM risk via independent mechanisms but also it might change CM risk via affecting other mediators. Schneider et al. indicated greater CM risk in short subjects compared with tall subjects when they were grouped by WC, but not WHtR, which suggests that these differences cannot be explained by height alone [165]. Moreover, it should be taken into account that height remains relatively unchanged during adulthood, and therefore, WHtR will change only by the changes in the waist measurement, whereas indices like waist-to-hip ratio are more likely to be changed over time since body size is changing, and consequently, hip and waist circumference would increase or decrease proportionately [52].
The current study has some limitations and strengths to be considered in the interpretation of the results and in the design and conduct of the future similar investigations:
The present study examined participants recruited through nonrandomized convenient sampling who might not be a fully representative sample of young adults residing in the NW of England. This may limit the generalizability of our findings, and the results should be interpreted carefully and in view of the above limitation. It is also important to consider that within this study, we only examined a group of anthropometric indicators of adiposity, and our analysis by no means has included all possible proxy indicators and data analysis approaches. For instance, a measure such as Body Roundness Index (BRI) [166] has recently produced promising indications of clinical use; however, the investigation of BRI did not fully fit the overall conceptual framework of the current study as the prediction equation includes body weight and body shape-related parameters within the same measure. Future studies should not only investigate the use of BRI but also consider other proxy indicators such Anthropometric Risk Index (ARI) [167] and Conicity Index (CI) [168], while implementing Z-score to adjust for age and gender variation in larger and more diverse populations.
Although we did not stratify our analysis by gender, its confounding effect was controlled. In addition, due to differences in fat and lean mass tissue between men and women, we categorized participants based on gender-specific cutoff points for anthropometric measures, which do not have predefined threshold that minimize its confounding effect.
In the current study and in consideration of our sample size, use of a lab-based objective reference method of assessment of body composition such as magnetic resonance imaging (MRI), dual-energy X-ray absorptiometry (DXA), and/or hydrodensitometry [169] was not plausible, and hence, we used a multifrequency bioelectrical impedance body composition analyzer with an advanced functionality to measure percentage body fat, for which the validity and clinical usefulness was demonstrated in previous studies [170, 171].
The current investigation measured basic anthropometric indices, and this was an advantage compared with some previous investigations using self-reported anthropometry. The assessors of anthropometry in this study were graduate nutritionists, who were trained by a qualified registered nutritionist with experience of assessment of anthropometry. The body weight and body shape-related measures used in the analysis were computed electronically, and this restricted the potential impact of the human error.
Although we considered various potential confounding factors in our analysis, the aetiology of the MetS and occurrence of the CM risk in the population is heterogonous and yet to be fully understood. Future studies should therefore control for a wider range of potential confounders including dietary, socioeconomic, and biochemical measures to elucidate the associations between anthropometric proxy measures and CM risk with elimination of potential mediating and moderating factors.
5. Conclusions
Overall, most of the body weight and body shape-related indicators of anthropometric adiposity showed statistically significant association with measured body fat and with CM risk factors. Body weight-related measures such as CUN-BAE and new BMI demonstrated strong association with measured adiposity but did not show adequate discriminatory power in identifying CM risk. On the other hand, body shape-related indicators of anthropometric adiposity such as BAI, WC, and WHtR showed mediocre association with measured percentage body fat and superior discriminatory power in identifying CM risk.
Overall, the acceptable statistically significant association of the WHtR against measured body fat and the strong association with CM risk in logistic regression and the AUC when testing the ROC curve of the WHtR, together with simplicity and clarity of the public health message in communication of the use of the measure, are amongst the reasons to propose WHtR as a clinically superior measure of anthropometric adiposity for our population. We therefore recommend the use of WHtR as a simple, effective, and clear measure for monitoring the CM risk within young adults.
Acknowledgments
The authors would like to acknowledge the assistance of several graduate nutrition students involved in recruiting participants and data collection and the work completed by Dr. Claire Macdonald–Clarke in proof reading of the introduction and methods of this manuscript. The authors would also like to thank young adults who participated in this study. The study was conducted as part of a larger study called the Collaborative Investigation on Nutritional Status of Young Adults (CINSYA) supported by the School of Health Sciences, Liverpool Hope University.
Data Availability
We will obviously be more than happy to submit our data to the Journal reviewers if necessary; however, the data will not be available to public as further manuscripts are expected to be submitted from the analysis of the data.
Disclosure
Partial results of this study were presented as an oral presentation at the scientific conference of the Nutrition Society, 11–14 July 2016, Dublin, Ireland [172].
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Nour M. M., McGeechan K., Wong A. T., et al. Diet quality of young adults enrolling in TXT2BFiT, a mobile phone-based healthy lifestyle intervention. JMIR Research Protocols. 2015;4(2):p. e60. doi: 10.2196/resprot.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorpe M. G., Kestin M., Riddell L. J., Keast R. S., McNaughton S. A. Diet quality in young adults and its association with food-related behaviours. Public Health Nutrition. 2014;17(8):1767–1775. doi: 10.1017/s1368980013001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papadaki A., Hondros G., Scott J. A., Kapsokefalou M. Eating habits of university students living at, or away from home in Greece. Appetite. 2007;49(1):169–176. doi: 10.1016/j.appet.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Barker M. E., Blain R. J., Russell J. M. The influence of academic examinations on energy and nutrient intake in male university students. Nutrition Journal. 2015;14(1):p. 98. doi: 10.1186/s12937-015-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Ansari W., Berg-Beckhoff G. Nutritional correlates of perceived stress among university students in Egypt. International Journal of Environmental Research and Public Health. 2015;12(11):14164–14176. doi: 10.3390/ijerph121114164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster H., Alaunyte I., Amirabdollahian F. An investigation in the quality of diet and adequacy of energy and macronutrient intake amongst male and female university students. Proceedings of the Nutrition Society. 2015;74(OCE5):p. E320. doi: 10.1017/s0029665115003675. [DOI] [Google Scholar]
- 7.Silliman K., Rodas-Fortier K., Neyman M. A Survey of dietary and exercise habits and perceived barriers to following a healthy lifestyle in a college population. Californian Journal of Health Promotion. 2004;2(2):10–19. [Google Scholar]
- 8.Deliens T., Van Crombruggen R., Verbruggen S., De Bourdeaudhuij I., Deforche B., Clarys P. Dietary interventions among university students: a systematic review. Appetite. 2016;105:14–26. doi: 10.1016/j.appet.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Crombie A. P., Ilich J. Z., Dutton G. R., Panton L. B., Abood D. A. The freshman weight gain phenomenon revisited. Nutrition Reviews. 2009;67(2):83–94. doi: 10.1111/j.1753-4887.2008.00143.x. [DOI] [PubMed] [Google Scholar]
- 10.Deforche B., Van Dyck D., Deliens T., De Bourdeaudhuij I. Changes in weight, physical activity, sedentary behaviour and dietary intake during the transition to higher education: a prospective study. International Journal of Behavioral Nutrition and Physical Activity. 2015;12(1):p. 16. doi: 10.1186/s12966-015-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deliens T., Clarys P., Van Hecke L., De Bourdeaudhuij I., Deforche B. Changes in weight and body composition during the first semester at university: a prospective explanatory study. Appetite. 2013;65:111–116. doi: 10.1016/j.appet.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Deliens T., Deforche B., De Bourdeaudhuij I., Clarys P. Changes in weight, body composition and physical fitness after 1.5 years at university. European Journal of Clinical Nutrition. 2015;69(12):1318–1322. doi: 10.1038/ejcn.2015.79. [DOI] [PubMed] [Google Scholar]
- 13.Roger V. L., Go A. S., Lloyd-Jones D. M., et al. Executive summary: heart disease and stroke statistics-2012 update a report from the american heart association. Circulation. 2012;125(1):188–197. doi: 10.1161/cir.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 14.Willett W. C., Koplan J. P., Nugent R., Dusenbury C., Puska P., Gaziano T. A. Prevention of chronic disease by means of diet and lifestyle changes. In: Jamison D. T., Breman J. G., Measham A. R., et al., editors. Disease Control Priorities in Developing Countries. 2nd. Washington, DC, USA: World Bank; 2006. [Google Scholar]
- 15.Mattsson N., Ronnemaa T., Juonala M., Viikari J. S., Raitakari O. T. The prevalence of the metabolic syndrome in young adults. The cardiovascular risk in young finns study. Journal of Internal Medicine. 2007;261(2):159–169. doi: 10.1111/j.1365-2796.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- 16.Usha S. M. R., Chandrika N., Shetty H. V., Reena R. A study of the components of metabolic syndrome in young adults. Biomedical Research. 2014;25(1):45–50. [Google Scholar]
- 17.Papakonstantinou E., Lambadiari V., Dimitriadis G., Zampelas A. Metabolic syndrome and cardiometabolic risk factors. Current Vascular Pharmacology. 2013;11(6):858–879. doi: 10.2174/15701611113116660176. [DOI] [PubMed] [Google Scholar]
- 18.van Vliet M., Heymans M. W., von Rosenstiel I. A., Brandjes D. P., Beijnen J. H., Diamant M. Cardiometabolic risk variables in overweight and obese children: a worldwide comparison. Cardiovascular Diabetology. 2011;10(1):p. 106. doi: 10.1186/1475-2840-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Vliet M., Von Rosenstiel I. A., Schindhelm R. K., Brandjes D. P., Beijnen J. H., Diamant M. Ethnic differences in cardiometabolic risk profile in an overweight/obese paediatric cohort in the Netherlands: a cross-sectional study. Cardiovascular Diabetology. 2009;8(1):p. 2. doi: 10.1186/1475-2840-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brady L. M., Williams C. M., Lovegrove J. A. Dietary PUFA and the metabolic syndrome in Indian Asians living in the UK. Proceedings of the Nutrition Society. 2004;63(1):115–125. doi: 10.1079/pns2003318. [DOI] [PubMed] [Google Scholar]
- 21.Goff L. M., Griffin B. A., Lovegrove J. A., et al. Ethnic differences in beta-cell function, dietary intake and expression of the metabolic syndrome among UK adults of South Asian, black African-Caribbean and white-European origin at high risk of metabolic syndrome. Diabetes and Vascular Disease Research. 2013;10(4):315–323. doi: 10.1177/1479164112467545. [DOI] [PubMed] [Google Scholar]
- 22.Tillin T., Forouhi N., Johnston D. G., McKeigue P. M., Chaturvedi N., Godsland I. F. Metabolic syndrome and coronary heart disease in South Asians, African-Caribbeans and white Europeans: a UK population-based cross-sectional study. Diabetologia. 2005;48(4):649–656. doi: 10.1007/s00125-005-1689-3. [DOI] [PubMed] [Google Scholar]
- 23.Tillin T., Forouhi N., Godsland I. F., McKeigue P. M., Chaturvedi N. Coronary heart disease and stroke mortality and metabolic syndrome in UK African Caribbeans and Europeans: a population based prospective cohort study. Diabetologia. 2005;48:p. A121. doi: 10.1007/s00125-005-1689-3. [DOI] [PubMed] [Google Scholar]
- 24.DiBello J. R., Ioannou C., Rees J., et al. Prevalence of metabolic syndrome and its components among men with and without clinical benign prostatic hyperplasia: a large, cross-sectional, UK epidemiological study. BJU International. 2016;117(5):801–808. doi: 10.1111/bju.13334. [DOI] [PubMed] [Google Scholar]
- 25.Elgalib A., Aboud M., Kulasegaram R., et al. The assessment of metabolic syndrome in UK patients with HIV using two different definitions: CREATE 2 study. Current Medical Research and Opinion. 2011;27(1):63–69. doi: 10.1185/03007995.2010.537212. [DOI] [PubMed] [Google Scholar]
- 26.Somani B., Khan S., Donat R. Screening for metabolic syndrome and testosterone deficiency in patients with erectile dysfunction: results from the first UK prospective study. BJU International. 2010;106(5):688–690. doi: 10.1111/j.1464-410x.2009.09145.x. [DOI] [PubMed] [Google Scholar]
- 27.Wei C., Ford A., Hunt L., Crowne E. C., Shield J. P. Abnormal liver function in children with metabolic syndrome from a UK-based obesity clinic. Archives of Disease in Childhood. 2011;96(11):1003–1007. doi: 10.1136/adc.2010.190975. [DOI] [PubMed] [Google Scholar]
- 28.Millar H. L. Prevalence of metabolic syndrome in a UK psychiatric population. European Psychiatry. 2010;25:p. 806. doi: 10.1016/s0924-9338(10)70797-5. [DOI] [Google Scholar]
- 29.Van Vliet-Ostaptchouk J. V., Nuotio M. L., Slagter S. N., et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocrine Disorders. 2014;14(1):p. 9. doi: 10.1186/1472-6823-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wander P. L., Boyko E. J., Leonetti D. L., McNeely M. J., Kahn S. E., Fujimoto W. Y. Change in visceral adiposity independently predicts a greater risk of developing type 2 diabetes over 10 years in Japanese Americans. Diabetes Care. 2013;36(2):289–293. doi: 10.2337/dc12-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gysel C. Adolphe Quetelet (1796–1874). The statistics and biometry of growth. L’ Orthodontie Française. 1974;45(1):643–677. [PubMed] [Google Scholar]
- 32.Shah N. R., Braverman E. R. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0033308.e33308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millar S. R., Perry I. J., Phillips C. M. Assessing cardiometabolic risk in middle-aged adults using body mass index and waist-height ratio: are two indices better than one? A cross-sectional study. Diabetology & Metabolic Syndrome. 2015;7(1):p. 73. doi: 10.1186/s13098-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heo M., Faith M. S., Pietrobelli A., Heymsfield S. B. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999–2004. American Journal of Clinical Nutrition. 2012;95(3):594–602. doi: 10.3945/ajcn.111.025171. [DOI] [PubMed] [Google Scholar]
- 35.Burton R. F. Why is the body mass index calculated as mass/height2, not as mass/height3? Annals of Human Biology. 2007;34(6):656–663. doi: 10.1080/03014460701732962. [DOI] [PubMed] [Google Scholar]
- 36.O’Donnell C. M., Tarnay C. M., Rapkin A. J. Waist circumference, not BMI, is a positive predictive factor in women who suffer from chronic abdominal and pelvic. Reproductive Sciences. 2008;15(2):p. 293a. [Google Scholar]
- 37.World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 38.Carroll J. F., Chiapa A. L., Rodriquez M., et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity. 2008;16(3):600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 39.Katzmarzyk P. T., Bray G. A., Greenway F. L., et al. Ethnic-specific BMI and waist circumference thresholds. Obesity. 2011;19(6):1272–1278. doi: 10.1038/oby.2010.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lear S. A., Humphries K. H., Kohli S., Birmingham C. L. The use of BMI and waist circumference as surrogates of body fat differs by ethnicity. Obesity. 2007;15(11):2817–2824. doi: 10.1038/oby.2007.334. [DOI] [PubMed] [Google Scholar]
- 41.Croft J. B., Keenan N. L., Sheridan D. P., Wheeler F. C., Speers M. A. Waist-to-hip ratio in a biracial population: measurement, implications, and cautions for using guidelines to define high risk for cardiovascular disease. Journal of the American Dietetic Association. 1995;95(1):60–64. doi: 10.1016/s0002-8223(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 42.Lear S. A., James P. T., Ko G. T., Kumanyika S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. European Journal of Clinical Nutrition. 2010;64(1):42–61. doi: 10.1038/ejcn.2009.70. [DOI] [PubMed] [Google Scholar]
- 43.Ashwell M., Gibson S. Waist to height ratio is a simple and effective obesity screening tool for cardiovascular risk factors: analysis of data from the British national diet and nutrition survey of adults aged 19–64 years. Obesity Facts. 2009;2(2):97–103. doi: 10.1159/000203363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burton R. F. Relations between body mass, height, fat mass, and waist circumference in American and Korean men and women. American Journal of Clinical Nutrition. 2015;101(3):685–686. doi: 10.3945/ajcn.114.102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burton R. F. The height dependence of fat-free mass, fat mass, and bone mineral content: insights into the body mass index. American Journal of Clinical Nutrition. 2011;94(2):612–613. doi: 10.3945/ajcn.111.018820. [DOI] [PubMed] [Google Scholar]
- 46.Ashwell M. A new shape chart for assessing the risks of obesity. Proceedings of the Nutrition Society. 1995;54:p. 86A. [Google Scholar]
- 47.Ashwell M., Lejeune S., Mcpherson K. Ratio of waist circumference to height may be a better indicator of need for weight management. British Medical Journal. 1996;312(7027):p. 377. doi: 10.1136/bmj.312.7027.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashwell M., Cole T., Dixon A. Ratio of waist circumference to height is strong predictor of intra-abdominal fat. British Medical Journal. 1996;313(7056):559–560. doi: 10.1136/bmj.313.7056.559d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashwell M. Plea for simplicity: use of waist-to-height ratio as a primary screening tool to assess cardiometabolic risk. Clinical Obesity. 2012;2(1-2):3–5. doi: 10.1111/j.1758-8111.2012.00037.x. [DOI] [PubMed] [Google Scholar]
- 50.Ashwell M., Gibson S. A proposal for a primary screening tool: “keep your waist circumference to less than half your height”. BMC Medicine. 2014;12(1):p. 207. doi: 10.1186/s12916-014-0207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashwell M., Gibson S. Waist-to-height ratio as an indicator of “early health risk”: simpler and more predictive than using a “matrix” based on BMI and waist circumference. BMJ Open. 2016;6(3) doi: 10.1136/bmjopen-2015-010159.e010159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashwell M., Gunn P., Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obesity Reviews. 2012;13(3):275–286. doi: 10.1111/j.1467-789x.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 53.Ashwell M., Mayhew L., Richardson J., Rickayzen B. Waist-to-height ratio is more predictive of years of life lost than body mass index. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0103483.e103483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uday S., Gorman S., Feltbower R. G., Mathai M. Ethnic variation in the correlation between waist to height ratio and total daily insulin requirement in children with type 1 diabetes: a cross-sectional study. Pediatric Diabetes. 2017;18(2):128–135. doi: 10.1111/pedi.12363. [DOI] [PubMed] [Google Scholar]
- 55.Hsieh S. D., Yoshinaga H. Waist/height ratio as a simple and useful predictor of coronary heart disease risk factors in women. Internal Medicine. 1995;34(12):1147–1152. doi: 10.2169/internalmedicine.34.1147. [DOI] [PubMed] [Google Scholar]
- 56.Cai L., Liu A., Zhang Y., Wang P. Waist-to-height ratio and cardiovascular risk factors among Chinese adults in Beijing. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069298.e69298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jayawardana R., Ranasinghe P., Sheriff M. H., Matthews D. R., Katulanda P. Waist to height ratio: a better anthropometric marker of diabetes and cardio-metabolic risks in South Asian adults. Diabetes Research and Clinical Practice. 2013;99(3):292–299. doi: 10.1016/j.diabres.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 58.Park Y. S., Kim J. S. Association between waist-to-height ratio and metabolic risk factors in Korean adults with normal body mass index and waist circumference. Tohoku Journal of Experimental Medicine. 2012;228(1):1–8. doi: 10.1620/tjem.228.1. [DOI] [PubMed] [Google Scholar]
- 59.Savva S. C., Lamnisos D., Kafatos A. G. Predicting cardiometabolic risk: waist-to-height ratio or BMI. A meta-analysis. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2013;6:403–419. doi: 10.2147/dmso.s34220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ware L. J., Rennie K. L., Kruger H. S., et al. Evaluation of waist-to-height ratio to predict 5 year cardiometabolic risk in sub-Saharan African adults. Nutrition, Metabolism and Cardiovascular Diseases. 2014;24(8):900–907. doi: 10.1016/j.numecd.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Zhou D., Yang M., Yuan Z. P., et al. Waist-to-Height Ratio: a simple, effective and practical screening tool for childhood obesity and metabolic syndrome. Preventive Medicine. 2014;67:35–40. doi: 10.1016/j.ypmed.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 62.Zhu Q., Shen F., Ye T., Zhou Q., Deng H., Gu X. Waist-to-height ratio is an appropriate index for identifying cardiometabolic risk in Chinese individuals with normal body mass index and waist circumference. Journal of Diabetes. 2014;6(6):527–534. doi: 10.1111/1753-0407.12157. [DOI] [PubMed] [Google Scholar]
- 63.Caminha T. C., Ferreira H. S., Costa N. S., et al. Waist-to-height ratio is the best anthropometric predictor of hypertension: a population-based study with women from a state of northeast of Brazil. Medicine. 2017;96(2) doi: 10.1097/md.0000000000005874.e5874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W. C., Chen I. C., Chang Y. C., Loke S. S., Wang S. H., Hsiao K. Y. Waist-to-height ratio, waist circumference, and body mass index as indices of cardiometabolic risk among 36,642 Taiwanese adults. European Journal of Nutrition. 2013;52(1):57–65. doi: 10.1007/s00394-011-0286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu L., Ping Z., Li L., Yang Y., Li C., Zhang M. Power and the cutoff value of waist-to-height ratio predicting metabolism syndrome. Wei Sheng Yan Jiu. 2012;41(6):992–996. [PubMed] [Google Scholar]
- 66.Meseri R., Ucku R., Unal B. Waist:height ratio: a superior index in estimating cardiovascular risks in Turkish adults. Public Health Nutrition. 2014;17(10):2246–2252. doi: 10.1017/s136898001300267x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajput R., Rajput M., Bairwa M., Singh J., Saini O., Shankar V. Waist height ratio: a universal screening tool for prediction of metabolic syndrome in urban and rural population of Haryana. Indian Journal of Endocrinology and Metabolism. 2014;18(3):394–399. doi: 10.4103/2230-8210.131201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sabah K. M., Chowdhury A. W., Khan H. I., et al. Body mass index and waist/height ratio for prediction of severity of coronary artery disease. BMC Research Notes. 2014;7(1):p. 246. doi: 10.1186/1756-0500-7-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tatsumi Y., Watanabe M., Kokubo Y., et al. Effect of age on the association between waist-to-height ratio and incidence of cardiovascular disease: the Suita study. Journal of Epidemiology. 2013;23(5):351–359. doi: 10.2188/jea.je20130004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu Z., Qi X., Dahl A. K., Xu W. Waist-to-height ratio is the best indicator for undiagnosed type 2 diabetes. Diabetic Medicine. 2013;30(6):e201–e207. doi: 10.1111/dme.12168. [DOI] [PubMed] [Google Scholar]
- 71.Agredo-Zuniga R. A., Aguilar-De Plata C., Suarez-Ortegon M. F. Waist:height ratio, waist circumference and metabolic syndrome abnormalities in Colombian schooled adolescents: a multivariate analysis considering located adiposity. British Journal of Nutrition. 2015;114(5):700–705. doi: 10.1017/s0007114515002275. [DOI] [PubMed] [Google Scholar]
- 72.Hori A., Nanri A., Sakamoto N., et al. Comparison of body mass index, waist circumference, and waist-to-height ratio for predicting the clustering of cardiometabolic risk factors by age in Japanese workers—Japan Epidemiology collaboration on occupational health study. Circulation Journal. 2014;78(5):1160–1168. doi: 10.1253/circj.cj-13-1067. [DOI] [PubMed] [Google Scholar]
- 73.Kodama S., Horikawa C., Fujihara K., et al. Comparisons of the strength of associations with future type 2 diabetes risk among anthropometric obesity indicators, including waist-to-height ratio: a meta-analysis. American Journal of Epidemiology. 2012;176(11):959–969. doi: 10.1093/aje/kws172. [DOI] [PubMed] [Google Scholar]
- 74.Sabo R. T., Ren C., Sun S. S. Comparing height-adjusted waist circumference indices: the fels longitudinal study. Open Journal of Endocrine and Metabolic Diseases. 2012;2(3):40–48. doi: 10.4236/ojemd.2012.23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Esmaillzadeh A., Mirmiran P., Azizi F. Comparative evaluation of anthropometric measures to predict cardiovascular risk factors in Tehranian adult women. Public Health Nutrition. 2006;9(1):61–69. doi: 10.1079/phn2005833. [DOI] [PubMed] [Google Scholar]
- 76.Rerksuppaphol S., Rerksuppaphol L. Optimal cut-off points of weight for height, waist circumference and waist-to-height ratio for defining overweight and obesity in Thai school-aged children. Journal of Research in Health Sciences. 2013;13(1):13–18. [PubMed] [Google Scholar]
- 77.Peng Y. G., Li Y., Guo M., et al. The optimal cut-off value of waist-to-height ratio for detecting severe central obesity and low body weight adult Chinese population. Zhonghua Xin Xue Guan Bing Za Zhi. 2013;41(7):607–610. [PubMed] [Google Scholar]
- 78.Peng Y., Li W., Wang Y., Bo J., Chen H. The cut-off point and boundary values of waist-to-height ratio as an indicator for cardiovascular risk factors in Chinese adults from the PURE study. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0144539.e0144539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marrodan M. D., Martinez-Alvarez J. R., Gonzalez-Montero De Espinosa M., Lopez-Ejeda N., Cabanas M. D., Prado C. Diagnostic accuracy of waist to height ratio in screening of overweight and infant obesity. Medicina Clínica. 2013;140(7):296–301. doi: 10.1016/j.medcli.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 80.El Mabchour A., Delisle H., Vilgrain C., Larco P., Sodjinou R., Batal M. Specific cut-off points for waist circumference and waist-to-height ratio as predictors of cardiometabolic risk in black subjects: a cross-sectional study in Benin and Haiti. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2015;8:513–523. doi: 10.2147/dmso.s88893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bohr A. D., Laurson K., McQueen M. B. A contemporary cutoff for the waist-to-height ratio predicting metabolic syndrome in young American adults. BMC Public Health. 2016;16(1):p. 295. doi: 10.1186/s12889-016-2964-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Vugt J. L., Cakir H., Kornmann V. N., et al. The new body mass index as a predictor of postoperative complications in elective colorectal cancer surgery. Clinical Nutrition. 2015;34(4):700–704. doi: 10.1016/j.clnu.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 83.Bergman R. N., Stefanovski D., Buchanan T. A., et al. A better index of body adiposity. Obesity. 2011;19(5):1083–1089. doi: 10.1038/oby.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Godoy-Matos A. F., Moreira R. O., Valerio C. M., Mory P. B., Moises R. S. A new method for body fat evaluation, body adiposity index, is useful in women with familial partial lipodystrophy. Obesity. 2012;20(2):440–443. doi: 10.1038/oby.2011.343. [DOI] [PubMed] [Google Scholar]
- 85.Melmer A., Lamina C., Tschoner A., et al. Body adiposity index and other indexes of body composition in the SAPHIR study: association with cardiovascular risk factors. Obesity. 2013;21(4):775–781. doi: 10.1002/oby.20289. [DOI] [PubMed] [Google Scholar]
- 86.Silva M. I., Vale B. S., Lemos C. C., Torres M. R., Bregman R. Body adiposity index assess body fat with high accuracy in nondialyzed chronic kidney disease patients. Obesity. 2013;21(3):546–552. doi: 10.1002/oby.20261. [DOI] [PubMed] [Google Scholar]
- 87.Zwierzchowska A., Grabara M., Palica D., Zajac A. BMI and BAI as markers of obesity in a Caucasian population. Obesity Facts. 2013;6(6):507–511. doi: 10.1159/000356402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gupta S., Kapoor S. Body adiposity index: its relevance and validity in assessing body fatness of adults. ISRN Obesity. 2014;2014:5. doi: 10.1155/2014/243294.243294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuhn P. C., Vieira Filho J. P., Franco L., Dal Fabbro A., Franco L. J., Moises R. S. Evaluation of body adiposity index (BAI) to estimate percent body fat in an indigenous population. Clinical Nutrition. 2014;33(2):287–290. doi: 10.1016/j.clnu.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 90.Djibo D. A., Araneta M. R., Kritz-Silverstein D., Barrett-Connor E., Wooten W. Body adiposity index as a risk factor for the metabolic syndrome in postmenopausal Caucasian, African American, and Filipina women. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2015;9(2):108–113. doi: 10.1016/j.dsx.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garcia A. I., Nino-Silva L. A., Gonzalez-Ruiz K., Ramirez-Velez R. Body adiposity index as marker of obesity and cardiovascular risk in adults from Bogota, Colombia. Endocrinología y Nutrición. 2015;62(3):130–137. doi: 10.1016/j.endonu.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 92.Gonzalez-Ruiz K., Correa-Bautista J. E., Ramirez-Velez R. Body adiposity and its relationship of metabolic syndrome components in Colombian adults. Nutricion Hospitalaria. 2015;32(4):1468–1475. doi: 10.3305/nh.2015.32.4.9164. [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez-Ruiz K., Correa-Bautista J. E., Ramirez-Velez R. Evaluation of the body adiposity index in predicting percentage body fat among Colombian adults. Nutricion Hospitalaria. 2015;32(1):55–60. doi: 10.3305/nh.2015.32.1.9087. [DOI] [PubMed] [Google Scholar]
- 94.Thivel D., O’Malley G., Pereira B., Duche P., Aucouturier J. Comparison of total body and abdominal adiposity indexes to dual x-ray absorptiometry scan in obese adolescents. American Journal of Human Biology. 2015;27(3):334–338. doi: 10.1002/ajhb.22643. [DOI] [PubMed] [Google Scholar]
- 95.Zaki M. E., Kamal S., Reyad H., et al. The validity of body adiposity indices in predicting metabolic syndrome and its components among egyptian women. Open Access Macedonian Journal of Medical Sciences. 2016;4(1):25–30. doi: 10.3889/oamjms.2016.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bi X., Tey S. L., Leong C., Quek R., Loo Y. T., Henry C. J. Correlation of adiposity indices with cardiovascular disease risk factors in healthy adults of Singapore: a cross-sectional study. BMC Obesity. 2016;3(1):p. 33. doi: 10.1186/s40608-016-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Appelhans B. M., Kazlauskaite R., Karavolos K., et al. How well does the body adiposity index capture adiposity change in midlife women?: the SWAN fat patterning study. American Journal of Human Biology. 2012;24(6):866–869. doi: 10.1002/ajhb.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gibson C. D., Atalayer D., Flancbaum L., Geliebter A. Body adiposity index (BAI) correlates with BMI and body fat pre- and post-bariatric surgery but is not an adequate substitute for BMI in severely obese women. International Journal of Body Composition Research. 2012;10(1):9–14. [PMC free article] [PubMed] [Google Scholar]
- 99.Lemacks J. L., Liu P. Y., Shin H., Ralston P. A., Ilich J. Z. Validation of body adiposity index as a measure of obesity in overweight and obese postmenopausal white women and its comparison with body mass index. Menopause: The Journal of The North American Menopause Society. 2012;19(11):1277–1279. doi: 10.1097/gme.0b013e31825408e5. [DOI] [PubMed] [Google Scholar]
- 100.Lopez A. A., Cespedes M. L., Vicente T., et al. Body adiposity index utilization in a Spanish Mediterranean population: comparison with the body mass index. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035281.e35281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schulze M. B., Thorand B., Fritsche A., et al. Body adiposity index, body fat content and incidence of type 2 diabetes. Diabetologia. 2012;55(6):1660–1667. doi: 10.1007/s00125-012-2499-z. [DOI] [PubMed] [Google Scholar]
- 102.Snijder M. B., Nicolaou M., Van Valkengoed I. G., Brewster L. M., Stronks K. Newly proposed body adiposity index (BAI) by Bergman et al. is not strongly related to cardiovascular health risk. Obesity. 2012;20(6):1138–1139. doi: 10.1038/oby.2011.338. [DOI] [PubMed] [Google Scholar]
- 103.Suchanek P., Kralova Lesna I., Mengerova O., Mrazkova J., Lanska V., Stavek P. Which index best correlates with body fat mass: BAI, BMI, waist or WHR? Neuro Enocrinology Letters. 2012;33(2):78–82. [PubMed] [Google Scholar]
- 104.Bennasar-Veny M., Lopez-Gonzalez A. A., Tauler P., et al. Body adiposity index and cardiovascular health risk factors in Caucasians: a comparison with the body mass index and others. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063999.e63999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cerqueira M., Amorim P., Magalhaes F., et al. Validity of body adiposity index in predicting body fat in a sample of Brazilian women. Obesity. 2013;21(12):E696–E699. doi: 10.1002/oby.20543. [DOI] [PubMed] [Google Scholar]
- 106.Edston E. A correlation between the weight of visceral adipose tissue and selected anthropometric indices: an autopsy study. Clinical Obesity. 2013;3(3-4):84–89. doi: 10.1111/cob.12021. [DOI] [PubMed] [Google Scholar]
- 107.Elisha B., Rabasa-Lhoret R., Messier V., Abdulnour J., Karelis A. D. Relationship between the body adiposity index and cardiometabolic risk factors in obese postmenopausal women. European Journal of Nutrition. 2013;52(1):145–151. doi: 10.1007/s00394-011-0296-y. [DOI] [PubMed] [Google Scholar]
- 108.Esco M. R. The accuracy of the body adiposity index for predicting body fat percentage in collegiate female athletes. Journal of Strength and Conditioning Research. 2013;27(6):1679–1683. doi: 10.1519/jsc.0b013e3182712714. [DOI] [PubMed] [Google Scholar]
- 109.Freedman D. S., Ogden C. L., Goodman A. B., Blanck H. M. Skinfolds and coronary heart disease risk factors are more strongly associated with BMI than with the body adiposity index. Obesity. 2013;21(1):E64–E70. doi: 10.1002/oby.20054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lam B. C., Lim S. C., Wong M. T., et al. A method comparison study to validate a contemporary parameter of obesity, the body adiposity index, in Chinese subjects. Obesity. 2013;21(12):E634–E639. doi: 10.1002/oby.20504. [DOI] [PubMed] [Google Scholar]
- 111.Moliner-Urdiales D., Artero E. G., Lee D. C., Espana-Romero V., Sui X., Blair S. N. Body adiposity index and all-cause and cardiovascular disease mortality in men. Obesity. 2013;21(9):1870–1876. doi: 10.1002/oby.20399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vinknes K. J., Elshorbagy A. K., Drevon C. A., et al. Evaluation of the body adiposity index in a Caucasian population: the Hordaland health study. American Journal of Epidemiology. 2013;177(6):586–592. doi: 10.1093/aje/kws271. [DOI] [PubMed] [Google Scholar]
- 113.Lutoslawska G., Malara M., Tomaszewski P., et al. Relationship between the percentage of body fat and surrogate indices of fatness in male and female Polish active and sedentary students. Journal of Physiological Anthropology. 2014;33(1):p. 10. doi: 10.1186/1880-6805-33-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sung Y. A., Oh J. Y., Lee H. Comparison of the body adiposity index to body mass index in Korean women. Yonsei Medical Journal. 2014;55(4):1028–1035. doi: 10.3349/ymj.2014.55.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Z. Q., Liu Y. H., Xu Y., et al. The validity of the body adiposity index in predicting percentage body fat and cardiovascular risk factors among Chinese. Clinical Endocrinology. 2014;81(3):356–362. doi: 10.1111/cen.12351. [DOI] [PubMed] [Google Scholar]
- 116.Al-Daghri N. M., Al-Attas O. S., Wani K., et al. Sensitivity of various adiposity indices in identifying cardiometabolic diseases in Arab adults. Cardiovascular Diabetology. 2015;14(1):p. 101. doi: 10.1186/s12933-015-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Belarmino G., Horie L. M., Sala P. C., Torrinhas R. S., Heymsfield S. B., Waitzberg D. L. Body adiposity index performance in estimating body fat in a sample of severely obese Brazilian patients. Nutrition Journal. 2015;14(1):p. 130. doi: 10.1186/s12937-015-0119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Datta Banik S., Das S. Body mass index and body adiposity index in relation to percent body fat: a study in adult men of three endogamous groups of South Bengal. HOMO. 2015;66(1):90–99. doi: 10.1016/j.jchb.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 119.Yu Y., Wang L., Liu H., et al. Body mass index and waist circumference rather than body adiposity index are better surrogates for body adiposity in a Chinese population. Nutrition in Clinical Practice. 2015;30(2):274–282. doi: 10.1177/0884533614564468. [DOI] [PubMed] [Google Scholar]
- 120.Zhao D., Zhang Y. Body mass index (BMI) predicts percent body fat better than body adiposity index (BAI) in school children. Anthropologischer Anzeiger. 2015;72(3):257–262. doi: 10.1127/anthranz/2015/0499. [DOI] [PubMed] [Google Scholar]
- 121.Carpio-Rivera E., Hernandez-Elizondo J., Salicetti-Fonseca A., Solera-Herrera A., Moncada-Jimenez J. Predictive validity of the body adiposity index in costa rican students. American Journal of Human Biology. 2016;28(3):394–397. doi: 10.1002/ajhb.22800. [DOI] [PubMed] [Google Scholar]
- 122.Chen X., He C., Ma Y., et al. Association of metabolic syndrome with various anthropometric and atherogenic parameters in the Kazakh population in China. Lipids in Health and Disease. 2016;15(1):p. 166. doi: 10.1186/s12944-016-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu P. J., Ma F., Lou H. P., Zhu Y. N. Body roundness index and body adiposity index: two new anthropometric indices to identify metabolic syndrome among Chinese postmenopausal women. Climacteric. 2016;19(5):433–439. doi: 10.1080/13697137.2016.1202229. [DOI] [PubMed] [Google Scholar]
- 124.Motamed N., Rabiee B., Keyvani H., et al. The best obesity indices to discriminate type 2 diabetes mellitus. Metabolic Syndrome and Related Disorders. 2016;14(5):249–253. doi: 10.1089/met.2015.0133. [DOI] [PubMed] [Google Scholar]
- 125.Ramirez-Velez R., Correa-Bautista J. E., Gonzalez-Ruiz K., Vivas A., Garcia-Hermoso A., Triana-Reina H. R. Predictive validity of the body adiposity index in overweight and obese adults using dual-energy X-ray absorptiometry. Nutrients. 2016;8(12):p. 737. doi: 10.3390/nu8120737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Verma M., Rajput M., Sahoo S. S., Kaur N., Rohilla R. Correlation between the percentage of body fat and surrogate indices of obesity among adult population in rural block of Haryana. Journal of Family Medicine and Primary Care. 2016;5(1):154–159. doi: 10.4103/2249-4863.184642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ramirez-Velez R., Correa-Bautista J. E., Gonzalez-Ruiz K., et al. Body adiposity index performance in estimating body fat percentage in Colombian college students: findings from the FUPRECOL-adults study. Nutrients. 2017;9(1):p. 40. doi: 10.3390/nu9010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Segheto W., Coelho F. A., Cristina Guimaraes Da Silva D., et al. Validity of body adiposity index in predicting body fat in Brazilians adults. American Journal of Human Biology. 2017;29(1) doi: 10.1002/ajhb.22901. [DOI] [PubMed] [Google Scholar]
- 129.Gomez-Ambrosi J., Silva C., Catalan V., et al. Clinical usefulness of a new equation for estimating body fat. Diabetes Care. 2012;35(2):383–388. doi: 10.2337/dc11-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zubiaga Toro L., Ruiz-Tovar Polo J., Diez-Tabernilla M., Giner Bernal L., Arroyo Sebastian A., Calpena Rico R. CUN-BAE formula and biochemical factors as predictive markers of obesity and cardiovascular disease in patients before and after sleeve gastrectomy. Nutricion Hospitalaria. 2014;30(2):281–286. doi: 10.3305/nh.2014.30.2.7581. [DOI] [PubMed] [Google Scholar]
- 131.Lara J., Siervo M., Bertoli S., et al. Accuracy of three contemporary predictive methods for measurements of fat mass in healthy older subjects. Aging Clinical and Experimental Research. 2014;26(3):319–325. doi: 10.1007/s40520-013-0169-8. [DOI] [PubMed] [Google Scholar]
- 132.Fuster-Parra P., Bennasar-Veny M., Tauler P., Yanez A., Lopez-Gonzalez A. A., Aguilo A. A comparison between multiple regression models and CUN-BAE equation to predict body fat in adults. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0122291.e0122291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martin V., Davila-Batista V., Castilla J., et al. Comparison of body mass index (BMI) with the CUN-BAE body adiposity estimator in the prediction of hypertension and type 2 diabetes. BMC Public Health. 2016;16(1):p. 82. doi: 10.1186/s12889-016-2728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Krakauer N. Y., Krakauer J. C. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0039504.e39504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sardarinia M., Ansari R., Azizi F., Hadaegh F., Bozorgmanesh M. Mortality prediction of a body shape index versus traditional anthropometric measures in an Iranian population: Tehran lipid and glucose study. Nutrition. 2017;33:105–112. doi: 10.1016/j.nut.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 136.Biolo G., Di Girolamo F. G., Breglia A., et al. Inverse relationship between “a body shape index” (ABSI) and fat-free mass in women and men: insights into mechanisms of sarcopenic obesity. Clinical Nutrition. 2015;34(2):323–327. doi: 10.1016/j.clnu.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 137.Bozorgmanesh M., Sardarinia M., Hajsheikholeslami F., Azizi F., Hadaegh F. CVD-predictive performances of “a body shape index” versus simple anthropometric measures: Tehran lipid and glucose study. European Journal of Nutrition. 2016;55(1):147–157. doi: 10.1007/s00394-015-0833-1. [DOI] [PubMed] [Google Scholar]
- 138.Dhana K., Ikram M. A., Hofman A., Franco O. H., Kavousi M. Anthropometric measures in cardiovascular disease prediction: comparison of laboratory-based versus non-laboratory-based model. Heart. 2015;101(5):377–383. doi: 10.1136/heartjnl-2014-306704. [DOI] [PubMed] [Google Scholar]
- 139.Dhana K., Kavousi M., Ikram M. A., Tiemeier H. W., Hofman A., Franco O. H. Body shape index in comparison with other anthropometric measures in prediction of total and cause-specific mortality. Journal of Epidemiology and Community Health. 2016;70(1):90–96. doi: 10.1136/jech-2014-205257. [DOI] [PubMed] [Google Scholar]
- 140.Duncan M. J., Mota J., Vale S., Santos M. P., Ribeiro J. C. Associations between body mass index, waist circumference and body shape index with resting blood pressure in Portuguese adolescents. Annals of Human Biology. 2013;40(2):163–167. doi: 10.3109/03014460.2012.752861. [DOI] [PubMed] [Google Scholar]
- 141.Fatema K., Rahman B., Zwar N. A., Milton A. H., Ali L. Short-term predictive ability of selected cardiovascular risk prediction models in a rural Bangladeshi population: a case-cohort study. BMC Cardiovascular Disorders. 2016;16(1):p. 105. doi: 10.1186/s12872-016-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Malara M., Keska A., Tkaczyk J., Lutoslawska G. Body shape index versus body mass index as correlates of health risk in young healthy sedentary men. Journal of Translational Medicine. 2015;13(1):p. 75. doi: 10.1186/s12967-015-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Santos D. A., Silva A. M., Matias C. N., et al. Utility of contemporary body indices in predicting fat mass in elite athletes. Nutrition. 2015;31(7-8):948–954. doi: 10.1016/j.nut.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 144.He S., Zheng Y., Wang H., Chen X. Assessing the relationship between a body shape index and mortality in a group of middle-aged men. Clinical Nutrition. 2016;37(3):p. 1088. doi: 10.1016/j.clnu.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 145.Dhana K., Koolhas C., Schoufour J., et al. Association of anthropometric measures with fat and fat-free mass in the elderly: the Rotterdam study. Maturitas. 2016;88:96–100. doi: 10.1016/j.maturitas.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 146.Haghighatdoost F., Sarrafzadegan N., Mohammadifard N., Asgary S., Boshtam M., Azadbakht L. Assessing body shape index as a risk predictor for cardiovascular diseases and metabolic syndrome among Iranian adults. Nutrition. 2014;30(6):636–644. doi: 10.1016/j.nut.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 147.Liu P. J., Ma F., Lou H. P., Zhu Y. N. Comparison of the ability to identify cardiometabolic risk factors between two new body indices and waist-to-height ratio among Chinese adults with normal BMI and waist circumference. Public Health Nutrition. 2016;20(6):984–991. doi: 10.1017/s1368980016003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Motamed N., Rabiee B., Hemasi G. R., et al. Body roundness index and waist-to-height ratio are strongly associated with non-alcoholic fatty liver disease: a population-based study. Hepatitis Monthly. 2016;16(9) doi: 10.5812/hepatmon.39575.e39575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hardy D. S., Stallings D. T., Garvin J. T., Xu H., Racette S. B. Best anthropometric discriminators of incident type 2 diabetes among white and black adults: a longitudinal ARIC study. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0168282.e0168282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Janghorbani M., Aminorroaya A., Amini M. Comparison of different obesity indices for predicting incident hypertension. High Blood Pressure & Cardiovascular Prevention. 2017;24(2):157–166. doi: 10.1007/s40292-017-0186-3. [DOI] [PubMed] [Google Scholar]
- 151.Zaid M., Ameer F., Munir R., et al. Anthropometric and metabolic indices in assessment of type and severity of dyslipidemia. Journal of Physiological Anthropology. 2017;36(1):p. 19. doi: 10.1186/s40101-017-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang N., Chang Y., Guo X., Chen Y., Ye N., Sun Y. A Body Shape Index and Body Roundness Index: two new body indices for detecting association between obesity and hyperuricemia in rural area of China. European Journal of Internal Medicine. 2016;29:32–36. doi: 10.1016/j.ejim.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 153.Gibney M. J. Introduction to Human Nutrition. 2nd. Hoboken, NJ, USA: Wiley-Blackwell; 2009. [Google Scholar]
- 154.Larsson B., Svardsudd K., Welin L., Wilhelmsen L., Bjorntorp P., Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. BMJ. 1984;288(6428):1401–1404. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. National Diet and Nutrition Survey: results from years 1, 2, 3 and 4 (combined) of the rolling programme (2008/2009-2011/2012)2014.
- 156.Hsieh S. D., Yoshinaga H., Muto T. Waist-to-height ratio, a simple and practical index for assessing central fat distribution and metabolic risk in Japanese men and women. International Journal of Obesity. 2003;27(5):610–616. doi: 10.1038/sj.ijo.0802259. [DOI] [PubMed] [Google Scholar]
- 157.Bouchard C., Tremblay A., Leblanc C., Lortie G., Savard R., Theriault G. A method to assess energy expenditure in children and adults. American Journal of Clinical Nutrition. 1983;37(3):461–467. doi: 10.1093/ajcn/37.3.461. [DOI] [PubMed] [Google Scholar]
- 158.Donato L. J., Deobald G. R., Wockenfus A. M., Hornseth J. M., Saenger A. K., Karon B. S. Comparison of two point of care devices for capillary lipid screening in fasting and postprandial adults. Clinical Biochemistry. 2015;48(3):174–176. doi: 10.1016/j.clinbiochem.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 159.Vergetaki A., Linardakis M., Papadaki A., Kafatos A. Presence of metabolic syndrome and cardiovascular risk factors in adolescents and university students in Crete (Greece), according to different levels of snack consumption. Appetite. 2011;57(1):278–285. doi: 10.1016/j.appet.2011.05.309. [DOI] [PubMed] [Google Scholar]
- 160.Willett W. Nutritional Epidemiology. 2nd. Oxford, UK: Oxford University Press; 1998. [Google Scholar]
- 161.Maessen M. F., Eijsvogels T. M., Verheggen R. J., Hopman M. T., Verbeek A. L., de Vegt F. Entering a new era of body indices: the feasibility of a body shape index and body roundness index to identify cardiovascular health status. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0107212.e107212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Koch E., Romero T., Romero C. X., et al. Early life and adult socioeconomic influences on mortality risk: preliminary report of a “pauper rich” paradox in a Chilean adult cohort. Annals of Epidemiology. 2010;20(6):487–492. doi: 10.1016/j.annepidem.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 163.Langenberg C., Shipley M. J., Batty G. D., Marmot M. G. Adult socioeconomic position and the association between height and coronary heart disease mortality: findings from 33 years of follow-up in the Whitehall Study. American Journal of Public Health. 2005;95(4):628–632. doi: 10.2105/2004.046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barker D. J., Osmond C., Golding J. Height and mortality in the counties of England and wales. Annals of Human Biology. 1990;17(1):1–6. doi: 10.1080/03014469000000732. [DOI] [PubMed] [Google Scholar]
- 165.Schneider H. J., Klotsche J., Silber S., Stalla G. K., Wittchen H. U. Measuring abdominal obesity: effects of height on distribution of cardiometabolic risk factors risk using waist circumference and waist-to-height ratio. Diabetes Care. 2011;34(1):p. e7. doi: 10.2337/dc10-1794. [DOI] [PubMed] [Google Scholar]
- 166.Thomas D. M., Bredlau C., Bosy-Westphal A., et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. 2013;21(11):2264–2271. doi: 10.1002/oby.20408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Krakauer N. Y., Krakauer J. C. An anthropometric risk index based on combining height, weight, waist, and hip measurements. Journal of Obesity. 2016;2016:9. doi: 10.1155/2016/8094275.8094275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Valdez R. A simple model-based index of abdominal adiposity. Journal of Clinical Epidemiology. 1991;44(9):955–959. doi: 10.1016/0895-4356(91)90059-i. [DOI] [PubMed] [Google Scholar]
- 169.Heyward V. H., Wagner D. R. Applied body Composition Assessment. 2nd. Champaign, IL, USA: Human Kinetics; 2004. [Google Scholar]
- 170.Nigam P., Misra A., Colles S. L. Comparison of DEXA-derived body fat measurement to two race-specific bioelectrical impedance equations in healthy Indians. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2013;7(2):72–77. doi: 10.1016/j.dsx.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 171.Wan C. S., Ward L. C., Halim J., et al. Bioelectrical impedance analysis to estimate body composition, and change in adiposity, in overweight and obese adolescents: comparison with dual-energy x-ray absorptiometry. BMC Pediatrics. 2014;14(1):p. 249. doi: 10.1186/1471-2431-14-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Amirabdollahian F., Macdonald-Clarke C. J., Lees E. K., Harrison T., Davies I. G. Traditional and novel correlates of adiposity and cardiometabolic risk among young healthy adults in the North West of England. Proceedings of the Nutrition Society. 2016;75(OCE3) doi: 10.1017/s0029665116002457. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We will obviously be more than happy to submit our data to the Journal reviewers if necessary; however, the data will not be available to public as further manuscripts are expected to be submitted from the analysis of the data.