Abstract
Background and Aim:
Nalbuphine as an adjuvant intrathecally can produce significant analgesia with minimal side effects. However, no research has been done with isobaric ropivacaine. We, therefore, in this prospective, randomised double-blind study tried to find the optimal dose of intrathecal nalbuphine with isobaric 0.75% ropivacaine for elective lower limb surgeries.
Materials and Methods:
One hundred American Society of Anaesthesiologists I and II patients undergoing elective lower limb surgery were divided into four groups randomly: groups A, B, C and D, who received 0.5 mL normal saline or 0.4, 0.8 and 1.6 mg nalbuphine made up to 0.5 mL normal saline added to 22.5 mg (total volume 3.5 mL) isobaric 0.75% ropivacaine, respectively. The onset of sensory and motor block, two-segment regression time, duration of sensory and motor block, Visual Analogue Scale (VAS) and the incidence of adverse effects were compared between the groups.
Results:
The onset of both sensory and motor blockade was faster with addition of 0.4, 0.8 and 1.6 mg of nalbuphine when compared with ropivacaine alone; however, it was not statistically significant (P > 0.05). Two-segment regression time and duration of analgesia and motor blockade were highest with 1.6 mg of nalbuphine followed by 0.8, 0.4 and plain 0.75% ropivacaine (P < 0.05). The duration of sensory blockade in all four groups was slightly more than the duration of motor blockade. VAS readings were comparable in all nalbuphine groups when compared with ropivacaine group. Haemodynamic variability among the four groups was comparable. Incidence of adverse effects was highest in the 1.6-mg group when compared with others, although it was statistically insignificant (P > 0.05).
Conclusion:
Nalbuphine can be a good alternative to other opioids as an adjuvant intrathecally to prolong postoperative analgesia with a minimal side effect profile. Addition of nalbuphine to isobaric 0.75% ropivacaine gives the added advantage of significant analgesia with early motor recovery. We infer from our study that when compared with 1.6 mg of nalbuphine, both 0.4 and 0.8 mg nalbuphine are equally good as adjuvants to isobaric 0.75% ropivacaine in elective lower limb surgeries with prolonged analgesia, a reliable block with equal efficacy but with lesser side effects.
Key words: Intrathecal nalbuphine, postoperative analgesia, ropivacaine
INTRODUCTION
Spinal anaesthesia is the most popular and effective regional anaesthetic technique used for lower limb surgeries. Various local anaesthetics commonly used for spinal anaesthesia are lignocaine, bupivacaine, levobupivacaine and ropivacaine.[1],[2] Nowadays, ropivacaine is gaining increasing popularity because of reduced risk of central nervous system and cardiac toxicity, early ambulation and discharge with good quality of postoperative analgesia.[3] Various adjuvants have since been added to local anaesthetics to increase the quality and duration of spinal blockade as well as prolongation of postoperative analgesia. Intrathecal administration of morphine was first described by Wang in a group of eight patients with genitourinary malignancies in 1979, which was later followed up by various other studies. Since then, the use of intrathecal opioids has become a widely accepted technique for providing effective postoperative pain relief.[4] Intrathecal opioids reduce the release of gamma amino butyric acid and glycine by a calcium-independent process from dorsal horn neurons.[5] Nalbuphine is a mixed opioid agonist–antagonist which can prove to be particularly advantageous because of the potential to maintain or even enhance opioid-based analgesia while simultaneously eliminating the common μ-opioid side effects (nausea, emesis, pruritis, constipation, undesirable sedation, respiratory depression and the development of tolerance/dependence).[6],[7],[8]
Mukherjee et al. compared three different doses of nalbuphine (0.2, 0.4 and 0.8 mg) intrathecally as an adjunct to subarachnoid block with 0.5% bupivacaine and found 0.4 mg nalbuphine to be the most optimal dose.[9] Culebras et al. compared intrathecal morphine (0.2 mg) added to hyperbaric bupivacaine with different doses of intrathecal nalbuphine (0.2, 0.8 and 1.6 mg) added to hyperbaric bupivacaine in caesarean section and concluded 0.8 mg to be the most optimal dose.[10] In this prospective, randomised double-blind study, we tried to compare three different doses of intrathecal nalbuphine (0.4, 0.8 and 1.6mg) as adjuvants to isobaric 0.75% ropivacaine and tried to find the optimal dose of nalbuphine required to produce prolonged postoperative analgesia with minimal side effects in patients undergoing elective lower limb surgeries.
MATERIALS AND METHODS
After obtaining approval from the institutional ethics committee and written informed consent from patients, 100 adult American Society of Anaesthesiologists (ASA) physical status I and II patients age between 18 and 60 years scheduled for elective lower limb surgery were included in this prospective study. Patients with a history of adverse response to ropivacaine or nalbuphine, pregnant patients, patients with comorbid diseases such as diabetes and hypertension, patients receiving phenothiazine, hypnotics or other central nervous system depressants or suffering from peripheral or central neurological, cardiac, respiratory, hepatic, renal disease or with body weight more than 100 kg or less than 40 kg and patients having contraindication to SAB (Sub arachnoid block) were excluded from study.
Patients were randomly allocated to one of the four groups by computer-generated randomization: Group A: (n = 25) – received normal saline (NS) 0.5 mL + isobaric ropivacaine 0.75% (3 mL).
Group B: (n = 25) – received nalbuphine 0.4 mg, made up to 0.5 mL volume with NS, mixed with 22.5 mg of isobaric ropivacaine 0.75% (total volume 3.5 mL).
Group C: (n = 25) – received nalbuphine 0.8 mg, made up to 0.5 mL volume with NS, mixed with 22.5 mg of isobaric ropivacaine 0.75% (total volume 3.5 mL).
Group D: (n = 25) – received nalbuphine 1.6 mg, made up to 0.5 mL volume with NS, mixed with 22.5 mg of isobaric ropivacaine 0.75% (total volume 3.5 mL).
The study drug was injected by an independent anaesthesiologist in a double-blind fashion who did not participate in observation or collection of data. Both the patient and the anaesthesiologist were blinded to the patient's group assignment and all recordings were performed by an anaesthesiologist, who was blinded to the randomisation schedule.
All the patients were asked to fast for at least 8 h before the procedure. Baseline vital parameters were recorded. After securing intravenous (18 G) access in dorsum of the left hand and attaching routine monitors, preloading with Ringer's lactate solution 10 mL/kg over 10 min was done. Under all aseptic and antiseptic precautions, SAB was performed with 3.5 mL of the study drug injected in L3/4 or L4/5 intervertebral space, using a 25-G Quincke spinal needle, in the sitting position. Then, the patients were placed in the supine or lateral position for surgery. Advanced equipments and drugs for resuscitation, airway management and ventilation were kept ready, in anticipation of any untoward event.
The segmental level of sensory block to pinprick was evaluated bilaterally along the mid-axillary line using a short beveled 27-G needle. The onset of sensory blockade (time taken from the end of injection to loss of pinprick sensation at T10 dermatome) and complete motor blockade (time taken from the end of injection to development of grade 3 motor block), modified Bromage's criteria, highest level of sensory blockade, duration of sensory blockade (two-segment regression time from highest level of sensory blockade), duration of motor blockade (time required for motor blockade return to Bromage's grade I from the time of onset of motor blockade) and duration of effective analgesia [time from the intrathecal injection to the first analgesic requirement, Visual Analogue Scale (VAS) score 3.5 or more] were recorded.
Both sensory and motor block were assessed at 2, 4 and 5 min, and then at 5-min intervals for first 30 min. Assessment was continued at 30-min intervals following the completion of surgery till the patient complained of pain. The motor block of both legs was assessed using the modified Bromage scale (0 = full movement, 1 = unable to raise extended leg, 2 = unable to flex knee, 3 = no movement) and assessment was continued till normal motor function returned. The changes in pulse rate, systolic and diastolic blood pressure, oxygen saturation (SpO2) and respiratory rate were recorded at 0, 2, 5, 10, 15 and 30 min and then at 15-min intervals up to 300 min after SAB, or up to the end point of study. Any adverse effects were recorded.
The intensity of pain was assessed by VAS at 0, 10, 15, 30 and 60 min and then at 30-min intervals till 300 min after injection or until the patient received a rescue analgesic. Patients reporting a VAS score 3.5 or more received rescue analgesics in the form of injection Inj. diclofenac 75 mg i.m. and the study ended. Incidence of nausea, vomiting and pruritus was noted. Nausea and vomiting were treated with Inj. ondansetron 4 mg i.v. and pruritus with anti-histaminics.
Statistical analysis
Data were analyzed using Student's t-test (paired and unpaired), one-way analysis of variance and Fisher's test with the help of GraphPad InStat (GraphPad Prism Software, Inc, La Jolla, CA, USA). The results are expressed as mean, standard deviation and range values. A P value less than 0.05 was considered statistically significant.
Assuming an increase in duration of sensory block of about 20% with addition of nalbuphine to 0.75% ropivacaine using standard programs and with the power of 80% and Type 1 error of 5%, the sample size required was calculated as 25 patients in each group.
RESULTS
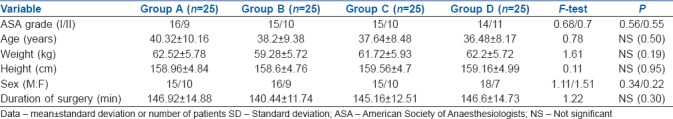
One hundred patients were enrolled in this study by dividing them into four groups of 25 each. The four groups of patients A, B, C and D did not differ significantly with respect to ASA grade, age, sex, weight, height and duration of surgery as shown in Table 1.
Table 1.
Demographic data in groups A, B, C and D (mean±SD)
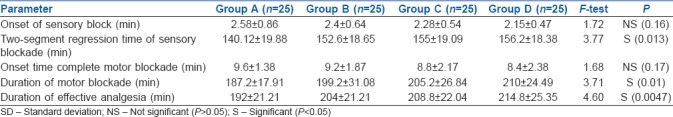
The onset of sensory and motor block was found to be statistically insignificant (P > 0.05) in all four groups. Two-segment regression time, duration of motor blockade and duration of effective analgesia were significantly prolonged (P < 0.05) progressively in groups B, C and D with addition of incremental doses of nalbuphine when compared with group A which had ropivacaine alone [Table 2].
Table 2.
Sensory block, motor block and analgesia in groups A, B, C and D (mean±SD)
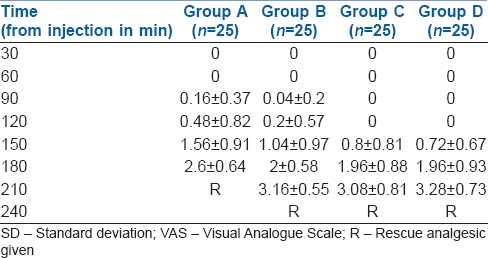
Groups B, C and D recorded comparable VAS of 3.16, 3.08 and 3.28 at 210 min, whereas group A recorded VAS of 2.6 at 180 min. Rescue analgesics were given when the VAS was 3.5 or more and the study terminated [Table 3].
Table 3.
Comparison of VAS scores among groups (mean±SD)
All the four groups did not vary significantly in terms of intraoperative mean heart rate, systolic and diastolic blood pressure, mean arterial pressure [Figure 1], respiratory rate and SPO2. There was no statistically significant difference in the incidence of adverse effects among the four groups although group D had more incidence of hypotension, bradycardia and nausea/vomiting than others [Figure 2].
Figure 1.
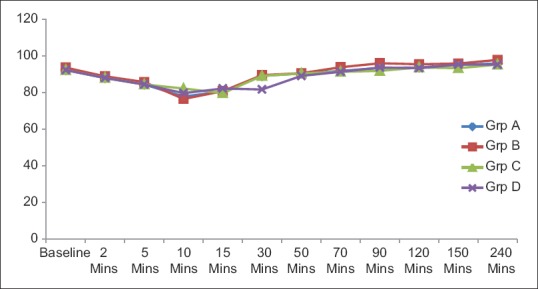
Haemodynamic comparison of all the groups (P > 0.05)
Figure 2.
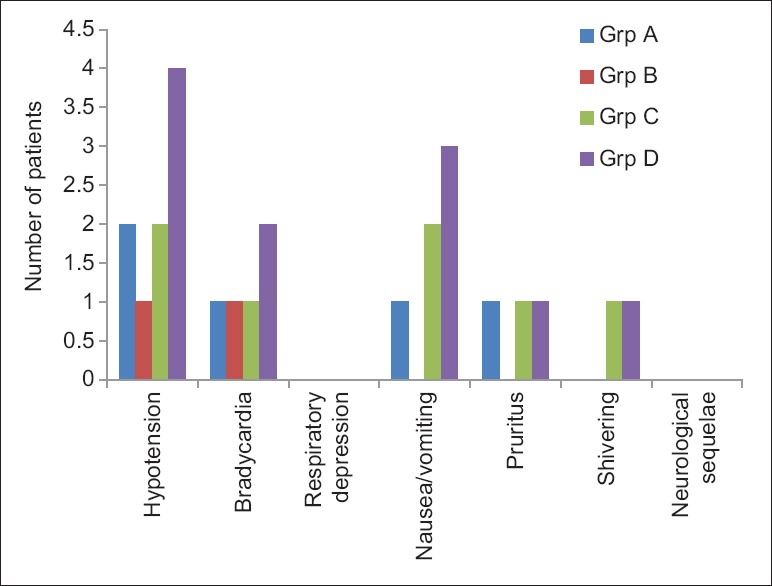
Frequency of adverse effects in each group (P > 0.05)
DISCUSSION
Spinal opioids can provide profound analgesia with fewer central and systemic adverse effects than with opioids administered systematically.[11] The technique of intrathecal opioid administration along with local anaesthetics has been studied extensively and found to provide superior quality of analgesia in a variety of surgical procedures.[12],[13] The rationale for the combination of opioids and local anaesthetics is that these two types of drugs eliminate pain by acting at two different sites. Local anaesthetics act at the nerve axon, whereas opioids act at the receptor site in the spinal cord.[14] Intrathecal opioids used as adjuncts also allow early ambulation of patients because of their sympathetic and motor nerve sparing activities.[15]
The most commonly used intrathecal opioids are mu agonist drugs that provide excellent analgesia and also carry along with them various mu-mediated side effects. Nalbuphine is a mixed agonist–antagonist opioid which has agonistic activity at the kappa receptors providing good intraoperative and postoperative analgesia and has antagonistic activity at the mu receptors, thereby exhibiting less mu-mediated side effects.[16]
We used isobaric 0.75% ropivacaine with three different doses of nalbuphine in our study as it blocks sensory nerve fibers more readily than motor fibers thus helping early ambulation and is now gaining popularity due to its reduced cardiac toxicity with overdose.[17] In our study, we tried to find the most optimal dose of nalbuphine as an adjuvant to ropivacaine which can provide adequate analgesia with minimal side effects in elective lower limb surgeries.
The onset time of both sensory and motor blocks reduced incrementally in groups A, B, C and D (2.58, 2.4, 2.28 and 2.15 min, respectively) with the 1.6- and 0.8-mg group having the fastest onset time; however, they were not statistically significant (P > 0.05). Two-segment regression time, duration of analgesia and duration of motor blockade were significantly prolonged with incremental doses of nalbuphine (P < 0.05). All groups with nalbuphine (0.4, 0.8 and 1.6 mg) had comparable VAS readings when compared with plain ropivacaine group. Rescue analgesics were thus required earlier in the plain ropivacaine group (180 min). All the groups were statistically comparable in terms of adverse effects although the 1.6-mg group had slightly higher incidence of adverse effect than the other groups.
The duration of sensory blockade in all four groups was slightly more than the duration of motor blockade. These results of our study are consistent with other studies. For example, Whiteside et al. compared 15 mg of either 0.5% ropivacine or 0.5% bupivacaine in 8% glucose and reported that ropivacaine provided reliable spinal anaesthesia of shorter duration and with less hypotension than bupivacaine.[18] Similarly, McNamee et al. reported that intrathecal administration of 17.5 mg plain ropivacaine 0.5% or plain bupivacaine 0.5% resulted in a similar effective spinal anaesthesia for total hip arthroplasty.[19]
Very few studies have been done comparing different doses of intrathecal nalbuphine as an adjuvant and none as far as we know with isobaric ropivacaine. Most studies have found intrathecal nalbuphine to produce a significant analgesia accompanied by minimal pruritus and respiratory depression.[20]
Our findings correlate with Culebras et al. who compared intrathecal morphine (0.2 mg) added to hyperbaric bupivacaine with different doses of intrathecal nalbuphine (0.2, 0.8 and 1.6 mg) added to hyperbaric bupivacaine in caesarean section and their study concluded that intrathecal nalbuphine 0.8 mg provided good intraoperative and early postoperative analgesia without side effects.[10]
Mukherjee et al. used different doses of nalbuphine intrathecally (0.2, 0.4 and 0.8 mg) added to 0.5% hyperbaric bupivacaine intrathecally and concluded that the duration of sensory block and the duration of effective analgesia were prolonged with the doses 0.4 and 0.8 mg, but the side effects were higher with the dose 0.8 mg. Our study produced similar results in terms of onset of sensory and motor blocks and duration of effective analgesia and motor blockade but lesser side effects were seen with the 0.8-mg group, probably because of use of isobaric ropivacaine in place of hyperbaric bupivacaine.[9]
Yoon et al. studied 60 obstetric patients scheduled for caesarean section under SAB to receive morphine 0.1 mg or nalbuphine 1 mg or morphine 0.1 mg with nalbuphine 1 mg in addition to 0.5% bupivacaine 10 mg and concluded that effective analgesia was prolonged in the morphine group and morphine with nalbuphine group, but the incidence of pruritus was significantly lower in the nalbuphine group, while the incidence of nausea and vomiting did not differ in different groups.[21] In our study, we found the incidence of adverse effects including nausea and vomiting to be statistically insignificant in all groups with nalbuphine.
Nalbuphine is being used intrathecally for more than 10 years now without any adverse neurotoxic effects.[9] Various animal studies have also been conducted to confirm that nalbuphine is not neurotoxic. Rawal et al. in a sheep model showed that even large doses of 15–24 mg of napbuphine were not associated with hypertensive changes in spinal cord or any behavioural or systematic histopathologic abnormalities.[22]
CONCLUSION
Intrathecal nalbuphine can be a good adjuvant to subarachnoid block as it can prolong both sensory and motor blockade with minimal side effects. From our study, we can infer that when compared with 1.6 mg nalbuphine, both 0.4 and 0.8 mg nalbuphine can be used safely intrathecally with isobaric 0.75% ropivacaine in elective lower limb surgery as they both provide prolonged analgesia and a reliable motor block with equal efficacy but with lesser side effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lim Y, Ocampo CE, Sia AT. A comparison of duration of analgesia of intrathecal 2.5 mg of bupivacaine, ropivacaine, and levobupivacaine in combined spinal epidural analgesia for patients in labor. Anesth Analg. 2004;98:235–9. doi: 10.1213/01.ANE.0000094338.80430.C5. [DOI] [PubMed] [Google Scholar]
- 2.Casati A, Moizo E, Marchetti C, Vinciguerra F. A prospective, randomized, double-blind comparison of unilateral spinal anesthesia with hyperbaric bupivacaine, ropivacaine, or levobupivacaine for inguinal herniorrhaphy. Anesth Analg. 2004;99:1387–92. doi: 10.1213/01.ANE.0000132972.61498.F1. [DOI] [PubMed] [Google Scholar]
- 3.Sultan MA, Ali Shams TM, Mageed NA, El-ebidy MG. Intrathecal hyperbaric ropivacaine versus hyperbaric bupivacaine in geriatric hypertensive patients. Benha M J. 2005;22:479. [Google Scholar]
- 4.Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149–51. doi: 10.1097/00000542-197902000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Kerchief GAB, Zhou M. Presynaptic suppression of dorsal horn inhibitory transmission by m-opioid receptors. J Neurophysiol. 2002;88:520–2. doi: 10.1152/jn.2002.88.1.520. [DOI] [PubMed] [Google Scholar]
- 6.American Society of Anaesthesiologists Task Force on Neuraxial Opioids. Horlocker TT, Burton AW, Connis RT, Hughes SC, Nickinovich DG, Palmer CM, et al. Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration. Anesthesiology. 2009;110:218–30. doi: 10.1097/ALN.0b013e31818ec946. [DOI] [PubMed] [Google Scholar]
- 7.Eisenach JC, Carpenter R, Curry R. Analgesia from a peripherally active kappa-opioid receptor agonist in patients with chronic pancreatitis. Pain. 2003;101:89–95. doi: 10.1016/s0304-3959(02)00259-2. [DOI] [PubMed] [Google Scholar]
- 8.Charuluxananan S, Kyokong O, Somboonviboon W, Lertmaharit S, Ngamprasertwong P, Nimcharoendee K. Nalbuphine versus propofol for treatment of intrathecal morphine-induced pruritus after cesarean delivery. Anesth Analg. 2001;93:162–5. doi: 10.1097/00000539-200107000-00032. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee A, Pal A, Agrawal J, Mehrotra A, Dawar N. Intrathecal nalbuphine as an adjuvant to subarachnoid block: What is the most effective dose? Anesth Essays Res. 2011;5:171–5. doi: 10.4103/0259-1162.94759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culebras X, Gaggero G, Zatloukal J, Kern C, Marti R. Advantages of intrathecal nalbuphine, compared with intrathecal morphine, after cesarean delivery: An evaluation of postoperative analgesia and adverse effects. Anesth Analg. 2000;91:601–5. doi: 10.1097/00000539-200009000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Morgan M. The rational use of intrathecal and extradural opiods. Br J Anaesth. 1989;63:165–88. doi: 10.1093/bja/63.2.165. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya R, Dutta B. Postoperative analgesia with local anesthetic and opioid combination using double space CSE technique. Indian J Anaesth. 2007;51:409–14. [Google Scholar]
- 13.Chakraborty S, Chakrabarti J, Bhattacharya D. Intrathecal tramadol added to bupivacaine as spinal anesthetic increases analgesic effect of the spinal blockade after major gynecological surgeries. Indian J Pharmacol. 2008;40:4180–2. doi: 10.4103/0253-7613.43166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357–8. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- 15.Terajima K, Onodera H, Kobayashi M, Yamanaka H, Ohno T, Konuma S, et al. Efficacy of intratecal morphine for analgesia following elective ceaserean section: Comparison with previous delivery. J Nippon Med Sch. 2003;70:327–33. doi: 10.1272/jnms.70.327. [DOI] [PubMed] [Google Scholar]
- 16.Gunion MW, Marchionne AM, Anderson CT. Use of the mixed agonist-antagonist nalbuphine in opioid based analgesia. Acute Pain. 2004;6:29–39. [Google Scholar]
- 17.Malinovsky JM, Charles F, Kick O, Lepage JY, Malinge M, Cozian A, et al. Intrathecal anesthesia: Ropivacaine versus bupivacaine. Anesth Anal. 2000;91:1457–60. doi: 10.1097/00000539-200012000-00030. [DOI] [PubMed] [Google Scholar]
- 18.Whiteside JB, Burke, Wildsmith JA. Comparison of ropivacine 0.5% (in glucose) with bupivacine 0.5% (in glucose 8%) for spinal anaesthesia for elective surgery. Br J Anaesth. 2003;18:521–5. doi: 10.1093/bja/aeg077. [DOI] [PubMed] [Google Scholar]
- 19.McNamee DA, McClelland AM, Scott S, Milligan KR, Westman L, Gustafsson U. Spinal anaesthetic: Comparison of plain ropivacaine 5mg/ml with bupivacaine 5mg/ml for major orthopedic surgery. Br J Anaesth. 2002;89:702–6. [PubMed] [Google Scholar]
- 20.Fournier R, Gamulin Z, Macksay M, Van Gessel E. Intrathecal morphine versus nalbuphine for post operative pain relief after total hip replacement. Anesthesiology. 1998;89:867. doi: 10.1034/j.1399-6576.2000.440808.x. [DOI] [PubMed] [Google Scholar]
- 21.Yoon JY, Jee YS, Hong JY. A Comparison of Analgesic Effects and side effects of intrathecal morphine, nalbuphine and morphine-nalbuphine mixture for pain relief during a caesarean section. Korean J Anaesthesiol. 2002;42:627–33. [Google Scholar]
- 22.Rawal N, Nuutinen L, Lovering SL, Gobuty AH, Hargardine J, Lehmkuhl L, et al. Behavioral and histopathologic effects following intrathecal administration of butorphanol, sufentanil, and nalbuphine in sheep. Anesthesiology. 1991;75:1025–34. doi: 10.1097/00000542-199112000-00015. [DOI] [PubMed] [Google Scholar]


