Abstract
Fluoroscopy is a method used to provide real time x‐ray imaging of the body during medical procedures to assist with medical diagnosis and treatment. Recent technological advances have seen an increase in the number of fluoroscopic examinations being performed. Nurses are an integral part of the team conducting fluoroscopic investigations and are often located close to the patient resulting in an occupational exposure to radiation. The purpose of this review was to examine recent literature which investigates occupational exposure received by nursing staff during cardiovascular fluoroscopic procedures. Articles published between 2011 and 2017 have been searched and comprehensively reviewed on the referenced medical search engines. Twenty‐four relevant studies were identified among which seventeen investigated nursing dose comparative to operator dose. Seven researched the effectiveness of interventions in reducing occupational exposure to nursing staff. While doctors remain at the highest risk of exposure during procedures, evidence suggests that nursing staff may be at risk of exceeding recommended dose limits in some circumstances. There is also evidence of inconsistent use of personal protection such as lead glasses and skull caps by nursing staff to minimize radiation exposure. Conclusions: The review has highlighted a lack of published literature focussing on dose to nurses. There is a need for future research in this area to inform nursing staff of factors which may contribute to high occupational doses and of methods for minimizing the risk of exposure, particularly regarding the importance of utilizing radiation protective equipment.
Keywords: fluoroscopy, nursing, occupational exposure, radiation exposure, systematic review
Abbreviations
- ALARA
as low as reasonably achievable
- CV
cardiovascular
- DAP
Dose Area Product
- DSA
digital subtraction angiography
- EP
electrophysiology
- EVAR
endovascular aneurysm repair
- Hp(0.07)
calibration of a dose meter to detect the personal dose equivalent at 0.07 mm depth in tissue
- Hp(3)
calibration of a dose meter to detect the personal dose equivalent at 3 mm depth in tissue
- Hp(10)
calibration of a dose meter to detect the personal dose equivalent at 10 mm depth in tissue
- IC
interventional cardiology
- ICRP
International Commission on Radiological Protection
- INR
interventional neuro‐radiology
- IR
interventional radiology
- KAP
kerma area product
- mSv
milliSievert
- NR
neuroradiology
- PDM
personal dose meter
1. INTRODUCTION
Fluoroscopy is a method used to provide real time imaging of the body during medical procedures. It utilizes x‐rays which pass through the patient to visualize internal structures. Historically x‐ray fluoroscopy was primarily used for diagnosis, but recent advances in both imaging and procedural equipment have led to considerable growth in the range of fluoroscopically guided procedures, particularly in the field of interventional cardiology, (IC) and vascular intervention.1, 2, 3 Interventional cardiovascular (CV) cases are often less costly than surgery and allow medical intervention to be conducted in a minimally invasive way, reducing the risk to the patient.4
Although very useful for imaging, ionizing radiation may result in several detrimental effects to those exposed, including cellular damage, malignancies, and cataracts.5, 6, 7, 8 The greatest risk of occupational exposure occurs when the primary x‐ray beam strikes the patient's skin and scatters, a portion of the x‐ray photons are absorbed and scatter in the patient's body.9 Scattered radiation levels near the patient can be relatively high, even under routine working conditions, and staff are subsequently exposed while conducting CV procedures.1, 10
There has been justifiable concern over the dose received by the physicians operating in this environment, but data detailing exposure to supporting staff during fluoroscopic procedures are scarce.1, 11, 12 The fundamental premise is to keep exposure to ionizing radiation as low as reasonably achievable (ALARA)6, 13 and organizations such as the International Commission on Radiological Protection (ICRP) recommend dose limits to those that are occupationally exposed.14 Staff radiation monitoring is performed as locally legislated to ensure that departments are complying with regulatory occupational dose limits, but problems with effective monitoring have been highlighted partly due to the attitude and radiation safety culture of staff.15 Poor adherence to the ICRP recommendation to conduct measurements using two dosimeters, one worn above and the other underneath the lead apron, as well as irregular use of personal dosimeters and has been emphasized,16 and it has been reported that appropriate dosimetry is essential to provide reasonable estimations of dose to the lens of the eye.17, 18, 19
There has been increasing concern over recent epidemiological evidence suggesting that radiation‐induced cataracts can occur at much lower doses than previously assumed.20, 21, 22 Staff involved in fluoroscopic CV procedures have demonstrated an elevated incidence of radiation‐associated lens changes.16, 21, 23, 24, 25, 26 In response, in 2011 the ICRP recommended reducing the occupational dose limit for the eye from 150 mSv (millisievert) to 20 mSv per year.27 This has resulted in numerous studies investigating the lens dose received by fluoroscopic operators, but there is very little research evaluating the risk of occupational eye exposure for nursing and allied health staff.1, 11, 19
Nurses are an integral part of the team conducting CV procedures, and many cases require staff to stand adjacent to the patient resulting in inadvertent exposure to radiation. To minimize the risk of exposure, it is vital that occupational dose to individuals is monitored and quantified. To date, the occupational exposure to nurses within the CV setting is widely unexplored.
1.A. Review objective
The purpose of this review is to provide a current account of research specifically examining occupational dose to nursing staff during x‐ray guided CV procedures. It will compare results of publications within procedural contexts, critically review the findings, and assess areas in which further research would be beneficial.
2. MATERIALS AND METHODS
A search for relevant literature published between 2011 and 2017 was undertaken between November 2016 and June 2017 to retrieve articles related to occupational radiation dose to nursing staff present during fluoroscopically guided CV procedures. A combination of keywords was used correlated to occupational radiation dose to nurses, i.e.: “nurse occupational dose”, “nursing fluoroscopy”, “staff fluoroscopy dose”, and “occupational fluoroscopy dose”. Search terms were purposefully general to ensure that articles which did not explicitly articulate ‘cardiovascular’ terminology were included in the initial screening for suitability for inclusion in the review. Due to the relatively small number of identified studies, reference lists of located manuscripts were also used to detect additional articles. Due to the rapid advancements in both imaging and procedural equipment in the last decade, searches were limited to those published after 2010 to ensure relevance to current operating practices.
A total of thirty potentially relevant articles were identified and of these six articles were excluded from the review as the investigated radiation doses to nurses were not directly related to the imaging of the CV system as illustrated in Fig. 1. The literature was subsequently reviewed, analyzed, and compared. A summary of selected articles is provided in Table 1.
Figure 1.
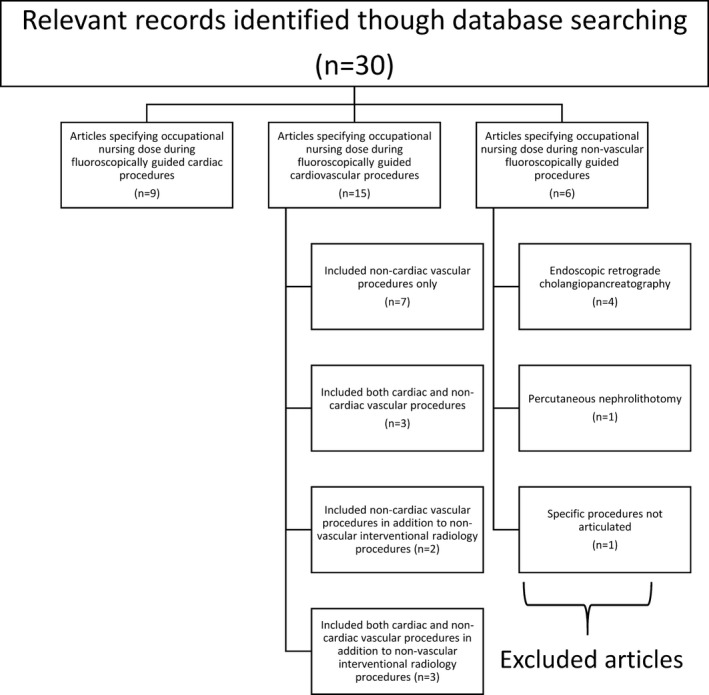
Flow diagram of study selection and exclusion process.
Table 1.
Summary of reviewed literature
| First author; year; location | Studied population | Cases | Collection period | Phantom measurements | Clinical | Intervention |
|---|---|---|---|---|---|---|
| Domienik, J. (2012) Poland1 | Cardiologist* Nurse* |
Vas IC (D + I) (n = 79) RFA (n = 11) PPM/ICD (n = 20) CRT/CRT‐D (n = 11) |
* | y‐ for calibration of dosimeters Hp(0.07) | y | n |
| Chohan, M. (2015) United States of America11 |
Patient (n = 24) Radiologist (n = 1) Scout nurse* |
Vas NR D (n = 18) I (n = 6) |
July 2011 to Dec. 2011 | n | y | n |
| Chida, K. (2013) Japan12 |
IR physician (n = 18) nurse (n = 7) Radiographer (n = 8) |
Vas IC D (n = 5280) I (n = 1326) |
During 2009 | n | y | n |
| Antic, V (2012) Serbia19 |
Primary operator (n = 13) Secondary operator (n = 8) Scrub nurse (n = 18) Radiographer (n = 12) |
Vas IC (D + I) (n = 106) |
* | n | y | n |
| Sailer, A. ( 2015) *25 |
Primary operator* Second operator* Scrub nurse* Scout nurse* Radiographer* Anaesthesiologist* |
EVAR (n = 22) TEVAR (n = 11) FEVAR (n = 11) |
Sept. 2013–Jan. 2014 | n | y | n |
| Nuraeni, N. (2016) Indonesia 29 |
Radiologist (n = 1) Scrub nurse (n = 1) Scout nurse (n = 1) Radiographer (n = 1) |
Vas NR (D + I) (n = 8) |
* | n | y | n |
| Mohapatra, A. (2013) * 31 |
Primary operator Secondary operator Total (n = 101) Scrub nurse * Radiographer * |
FEVAR (n = 39) | Oct. 2011–Feb. 2012 | n | y | n |
| Korir, G. (2012) Kenya 32 |
Physician* Nurse* Radiographer* Neurologists* Clinical staff* Total (n = 216) |
Vas INR Vas IC (D + I) (n = 54) |
Nov. 2007–end time * | n | y | n |
| Omar, A. (2017) Sweden 34 |
IR and IC physician (n varied per room) Scrub nurse Scout nurse Anaesthetist Anaesthetic nurse |
Vas IR, IC and INR NVas IR R1 (n = 200) R2 (n = 55) R3 (n = 80) R4 (n = 10) |
R1 (hybrid IR OR)—11 months R2 (IR)—2 months R3 (IC)—3 months R4 (INR)—3 months |
n | y | n |
| Racadio, J (2014) 35 |
IR physician (n = 4) IR fellow (n = 4) Nurse ^ (n = 3) Radiographer (n = 7) Anaesthetist * |
Vas IR (n = 38) NonVas IR (n = 207) CP (n = 97) OP (n = 148) |
CP–12 weeks OP–17 weeks |
n | y |
CP–blinded OP–unblinded |
| Baumann, F. (2015) * 36 |
IR physician and fellows* Scout nurse ^ * Radiographer * Anaethetist * |
Vas and NonVas IR (D + I) CP (n = 88) LP (n = 50) OP (n = 114) |
CP—6 weeks LP—6 weeks OP—10 weeks year * |
n | y |
CP—blinded LP—unblinded, not evaluated OP—unblinded and evaluated |
| Sandblom, V. (2013) Sweden 37 |
Cardiologist (n = 3) Nurse (n = 10) |
Vas IC (D + I) CP (n = 80) OP (n = 81) |
CP—1 month OP—1 month |
n | y |
CP—blinded OP—unblinded |
| James, R. (2015) United States of America 38 |
Radiologist (n = 2) Scrub nurse* Scout nurse* Total (n = 25) |
Vas NR (D) CP (n = 60) OP (n = 60) |
Apr. 2012–Aug. 2013 | n | y |
CP—blinded OP—unblinded |
| Butcher, R. (2015) Australia 39 |
Scrub nurse* Scout nurse* Total (n = 10) |
Vas IR (D + I) CP (n = 28) OP (n = 28) |
* | n | y |
CP—blinded OP—unblinded |
| Haga, Y. (2017) Japan 44 |
Cardiologist (n = 12) Nurse (n = 11) |
Vas IC (D) (n = 1707) Vas IC (I) (n = 902) |
Sept. 2015–Feb. 2016 | n | y | n |
| Gilligan, P. (2015) * 45 |
Cardiologist (n = 14) Nurse ^ * Cardiac Technicians * Radiographer * |
IC (total n*) | 3 times within 7 months | n | y |
P1—standard shield P2—larger shield with lamellae and femoral cutout + additional flexible shield |
| McLean, D. (2016) * 46 |
Cardiologist * IC nurse ^ * IR operator (n = 6) IR nurse ^ (n = 9) IR radiographer (n = 2) ERCP operator * ERCP nurse ^ * |
Vas IR (n = 93) IC (n = 192) ERCP (n = 34) |
1 month per location | n | y | n |
| Efstathopoloulos, E. (2011) Greece 47 |
Cardiologist (n = 5) Radiologist (n = 5) Nurse (n = 3) |
IC (D) (n = 6) PPM (n = 1) Vas IR (D + I) (n = 11) |
Oct. 2008—Jan. 2009 | n | y | n |
| Omar, A. (2015) Sweden 48 |
Cardiologist (n = 1) Nurse (n = 3) |
IC * | 1 month | y | y | n |
| Rigatelli, G. (2016) Italy 49 |
Physician (n = 4) Nurse (n = 9) Radiographer (n = 7) |
IC (D + I) (n = 2130) Vas peripheral (D + I) (n = 440) INR (n = 60) |
12 months (2014) | y | y | n |
| Principi, S. (2015) Spain 52 |
P1—cardiologist (n = 9) P1—nurse ^ (n = 6) P2—cardiologist (n = 3) P2—nurse ^ (n = 1) |
Vas IC (D + I) * |
P1—2 weeks P2—7 weeks |
n | y | n |
| Urboniene, A. (2015) Lithuania 53 |
IC physician (n = 114) IC nurse (n = 137) |
Vas IC (n*) Non Vas IC (n*) |
2012‐2013 1 month for the eyes |
n | y | n |
| Komemushi, A. (2014 * 63 |
IR physician (n = 3) Nurse (n = 5) ED physician (n = 1) |
Vas IR Non Vas IR CG (n = 50) NCG (n = 43) |
Mar.—May 2012 | n | y |
CG—nurse alerted operator before approaching patient NCG—no alert |
| Mori, H. (2015) Japan 64 |
IR nurse (n = 27) IC nurse (n = 42) |
Vas IR (n*) Vas IC (n*) |
* | n | y |
P1—change dosimeters P2—staff education P3—additional portable lead shields P4—reducing radiation parameters |
Summary of review literature. RFA: radiofrequency ablation; PPM: permanent pacemaker; ICD: implantable cardioverter defibrillator; CRT: cardiac resynchronization therapy; EVAR: endovascular aortic repair; TEVAR: thoracic aortic repair; FEVAR: fenestrated aortic repair; INR: interventional neuroradiology; NR: neuroradiology; IC: interventional cardiology; Vas: vascular; D: diagnostic; I: interventional; CP: closed phase; OP: open phase; LP: learning phase; R: room; OR: operating room; P1: Phase 1; P2: Phase 2; P3: Phase 3; P4: Phase 4; ERCP: endoscopic retrograde cholangio‐pancreatography; CG: call group; NCG: no call group; ^: role not articulated; *: not articulated.
2.A. Radiation dose monitoring
It has been demonstrated that the dose to nursing staff during fluoroscopic procedures can be similar or higher than that received by the physician28, 29, 30 with evidence of an increasing trend toward higher dose levels to nurses working in this environment.28 It is therefore important to quantify the radiation exposure to individuals working within fluoroscopic departments.31, 32, 33
Typically, the devices used to evaluate the individual cumulative radiation exposure are personal dosimeters, which are usually badges worn by occupationally exposed staff during procedures. The ICRP recommends the proper use of personal monitoring badges in interventional fluoroscopic laboratories to monitor and audit occupational radiation dose.14 There was a variety of styles, anatomical positioning, and calibration of dosimeters utilized in the reviewed literature (Table 2). Active dosimetry systems, such as DoseAware (Philips Medical Systems, Amsterdam, The Netherlands) provide real time visualization of radiation dose rate. It consists of a personal dosimeter worn by staff [Fig. 2(a)], a wireless base station which displays live radiation exposure information transmitted from individual dosimeters [Fig. 2(b)], a download cradle [Fig. 2(c)], and computer software which downloads badge data for analysis [Fig. 2(d)]. Several studies evaluated the effectiveness of immediate exposure information on staff behavior by monitoring dose received by DoseAware31, 34, 35, 36, 37, 38 or other real time systems.39 The blinded, or closed phase measurements were downloaded from badges worn when staff were not able to view the base station display. During the unblinded, or open phase staff could visualize the real time dose rate information on the base station and modify behavior.
Table 2.
Location, calibration, and dose values of dosimeters
| Reference: First author (year) | Cases | Monitored staff‐1 | Badge location | Calibration | Dosimeter type | Monitored staff‐2 | Badge location | Calibration | Dosimeter type | Monitored staff‐3 | Badge location | Calibration | Dosimeter type | Monitored staff dose‐1 | Monitored staff dose‐2 | Monitored staff dose‐3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domienik, J. (2012) 1 |
Vas IC Non Vas IC |
Nurse (n*) |
1—FH (E) 2—RF L 3—RF R 4—W L 5—W R 6—K L 7—K R 8—An L 9—An R |
Hp(0.07) | TLD | Cardiologist * |
1—FH (E) 2—RF L 3—RF R 4—W L 5—W R 6—K L 7—K R 8—An L 9—An R |
Hp(0.07) | TLD |
MD/case# 1—15.7μSv 2—26 μSv 3—24.3 μSv 4—24.6 μSv 5—23.7 μSv 6—14.5 μSv 7—13 μSv 8 & 9—33.3 μSv |
MD/case# 1—67.6 μSv 2—203 μSv 3—205 μSv 4—133 μSv 5—115 μSv 6—72.8 μSv 7—43.1 μSv 8 & 9 –108 μSv |
|||||
| Chohan, M. (2015) 11 | Vas INR | Scout nurse (n*) | 1—eye R (E) | * | ED |
Radiologist (n = 1) |
1—eye L (E) 2—eye L (U) |
* | ED | pt | 1‐head (E) | * | TLD |
1‐MD/case 30 + /‐ 60 μSv |
1—MD/case 80 + /‐ 190 μSv 2 –MD/case 5 + /‐ 16 μSv |
cranial entrance MD/case 220270 + /‐221170 μSv |
| Chida, K. (2013) 12 | Vas IC | Nurse ^ (n = 7) |
1—Ch (U) 2—Co (E) |
Hp(10) | PGD |
Physician (n = 18) |
1—Ch (U) 2—Co (E) |
Hp(10) | PGD | rad (n = 8) |
1—Ch (U) 2—Co (E) |
Hp(10) | PGD |
annual MD equiv./year 4730 + /‐720 μSv |
annual MD equiv./year 19840+/‐12450 μSv |
annual MD equiv./year 1300 + /‐ 1000 μSv |
| Antic, V (2012) 19 | Vas IC |
Scrub nurse (n = 18) second physician (n = 8) |
1—eye L (E) | Hp(3) | APD |
Cardiologist (n = 8) |
1—eye L (E) | Hp(3) | APD | rad (n = 12) | 1—eye L (E) | Hp(3) | APD |
MD/case 1—33 μSv |
MD/case 1—121 μSv |
MD /case 1—12μSv |
| Sailer, A. ( 2015) 25 | EVARS (angio) | Scrub nurse (n*) | 1‐Ch (E) | Hp(10) | APD |
Radiologist (n*) Cardiologist (n*) Gastroenterologist (n*) |
1—Co (E) | Hp(10) | APD | Scout nurse (n*) | 1—Co (E) | Hp(10) | APD |
MD/case 17 μSv |
MD/case 170 μSv |
MD/case 4 μSv |
| Nuraeni, N. (2016) 29 | Vas INR |
Nurse (n = 2) A & B |
1—eye side * (E)‐A only 2—Ch (U) 3—Co (E) 4—Co (U) 5—G (E) 6—G (U) 7—F (E)‐A only |
1‐ Hp(3) 2 to 6‐Hp(10) 7‐ Hp(0.07) |
TLD |
Radiologist (n = 1) |
1—eye side * 2—Ch (U) 3—Co (E) 4—Co (U) 5—G (E) 6—G (U) 7—F (E) |
1—Hp(3) 2‐6 Hp(10) 7—Hp(0.07) |
TLD | rad (n = 1) |
1—eye side * 2—Ch (U) 3—Co (E) 4—Co (U) 5—G (E) 6—G (U) 7—F (E) |
1‐ Hp(3) 2 to 6‐Hp(10) 7—Hp(0.07) |
TLD |
Highest dose 2—2 μSv (A) |
Highest dose 2—1 μSv |
Highest dose 2—1 μSv |
| Mohapatra, A. (2013) 31 | EVARS | Scrub nurse (n*) | 1‐Co (E) | Hp(10) | APD |
Primary & Assistant surgeons (n = 101) |
1—Co (E) | Hp(10) | APD |
Dosimeter on equipment |
1‐on anaethetic equipment |
Hp(10) | APD |
MD/case 26μSv |
MD/case 125μSv |
MD/case 268μSv |
| Korir, G. (2012) 32 |
Vas IC Vas IR |
Nurse ^ (n*) | 1—Co (E) | * | TLD |
Cardiologist (n*) Radiologist (n*) |
1—Co (E) | * | TLD | rad (n*) | 1—Co (E) | * | TLD | MD/case‐ 270 μSv | MD/case‐ 340 μSv | MD/case—220 μSv |
| Omar, A. (2017) 34 |
Vas IC Non Vas IC R1 & 2 Vas IC R3 Vas INR R4 |
Scrub nurse (n*) | 1—Ch (E) | Hp(10) | APD |
Radiologist (n > 14) Cardiologist (n = 6) |
1—Ch (E) | Hp(10) | APD | Scout nurse (n*) | 1—Ch (E) | Hp(10) | APD |
equiv. eye dose/case calculated from T APD# R1‐13 μSv R2‐51 μSv R3—5.7 μSv R4‐11 μSv |
equiv. eye dose/case calculated from T APD# R1—60 μSv R2—190 μSv R3—66 μSv R4—8.6 μSv |
equiv. eye dose/case calculated from T APD# R1—2.7 μSv R2—8.9 μSv R3—5.7 μSv R4—3.0 μSv |
| Racadio, J (2014) 35 |
Vas IR Non Vas IR |
Nurse ^ (n = 3) | 1—Ch (E) | Hp(10) | APD |
Radiologist (n = 4) N or F from pt |
1—Ch (E) | Hp(10) | APD | IR fellow (n = 4) | 1—Ch (E) | Hp(10) | APD | * |
CP median~ N—0.15μSv/min F—4.14μSv/min OP median~ N—0.02μSv/min F‐4.12μSv/min |
CP median~—0.0μSv/min OP median~—0.0μSv/min |
| Baumann, F. (2015) 36 |
Vas IR Non Vas IR |
Nurse ^ (n*) | 1—Co (E) | Hp(10) | APD | Radiologist (n*) | 1—Co (E) | Hp(10) | APD | Anaethetist (n*) | 1—Co (E) | Hp(10) | APD |
Avg. of all staff (drs, nurse, primary physician, fellow, rad)~ CP—42.79 μSv/min OP—19.81 μSv/min |
anaethetist*~ CP—16.9 μSv/min OP—8.9 μSv/min |
|
| Sandblom, V. (2013) 37 | Vas IC |
Scrub nurse (n = 10) CP‐69 cases OP–73 cases |
1—Ch (E) | Hp(10) | APD |
Cardiologist (n = 3) |
1—Ch (E) | Hp(10) | APD |
median/case CP—4.3 μSv OP—2.5 μSv |
median/case CP—9.9 μSv OP—8.5 μSv |
|||||
| James, R. (2015) 38 | Vas INR |
scrub nurse (n=<26) CP—60 cases OP—60 cases |
1—Ch (E) 2—Ch (E) |
1—* 2‐Hp(10) |
1—TLD 2—APD |
Radiologist (n = 2) A & B OP—30 cases each CP—30 cases each |
1—Ch (E) 2—Ch (E) |
1—* 2‐Hp(10) |
1—TLD 2—APD |
Scrub nurse (n=<26) CP—60 cases OP—60 cases |
1—TLD 2—APD |
1—* 2—Hp(10) |
1—TLD 2—APD |
CP MD‐0.045 μSv/Gy‐cm2 OP MD‐0.02 μSv/Gy‐cm2 dose divided by DAP |
A‐CP MD ‐0.028 μSv/Gy‐cm2 A‐OP MD‐0.051 μSv/Gy‐cm2 B‐CP MD ‐0.243 μSv/Gy‐cm2 B‐OP MD‐0.069 μSv/Gy‐cm2 dose divided by DAP |
B‐CP MD‐0.033 μSv/Gy‐cm2 B‐OP MD‐0.015 μSv/Gy‐cm2 dose divided by DAP |
| Butcher, R. (2015) 39 | Vas IR |
Scrub nurse (n = 10) CP—14 cases OP—14 cases |
1—Ch (U) | * | SM |
Scout nurse (n = 10) CP—12 cases OP—12 cases |
Ch (U) | * | SM |
MD/case CP—2.18 μSv OP—0.674 μSv |
MD/case CP—3.25 μSv OP—0.009 μSv |
|||||
| Haga, Y. (2017) 44 | Vas IC | Nurse ^ (n = 11) |
1—eye L (E) 2—Co (E) |
1—Hp(3) 2—Hp(0.07) |
1—TLD 2—PGD |
Cardiologist A—with LG (n = 9) B—without LG (n = 3) |
1—eye L (E) 2—eye L (U) A only 3—Co (E) |
1—Hp(3) 2—Hp(3) 3—Hp(0.07) |
1—TLD 2—TLD 3—PGD |
est. annual dose 1—3300 + /‐2000 μSv 2—4000 + /‐2400 μSv |
est. annual dose A 1 –15800 + /‐ 6600 μSv 2—6200 + /‐ 2600 μSv 3—22800 + /‐ 12800 μSv est. annual dose B 1—12600 + /‐ 10200 μSv 3—10000 + /‐5200 μSv |
|||||
| Gilligan, P. (2015) 45 |
Vas IC Non Vas IC |
Scrub nurse (n*) P1—Standard shield P2—larger shield + pt drape |
1—Co (E) | Hp(10) | EPD |
Cardiologist (n = 14) P1—standard shield P2—larger shield + pt drape |
1‐Co (E) | Hp(10) | EPD |
rad (n*) P1—standard shield P2—larger shield + pt drape |
1‐ Co (E) | Hp(10) | EPD |
median P1—1 μSv P2—0.1 μSv |
median P1—15.4 μSv P2—7.3 μSv |
median P1—4.2 μSv P2—2.5 μSv |
| McLean, D. (2016) 46 |
Vas IR IC ERCP |
Nurse A—Cardiology (n*) B—Angiography (n = 9) C—ERCP cases (n*) |
1—eye L (E) | Hp(3) | TLD |
A—cardiologist (n*) B—radiologist (n = 6) C—gastroenterologist (n*) |
1—eye L (E) | Hp(3) | TLD |
median eye dose! A‐130 μSv B‐100 μSv C‐360 μSv |
median eye dose! A‐340 μSv B‐930 μSv C‐1510 μSv |
|||||
| Efstathopoloulos, E. (2011) 47 |
IC PPM Vas IR |
Nurse ^ (n = 3) |
1—eye L (E) 2—central FH (E) 3—MF L (E) 4—W L (E) 5—MF R (E) 6—W R (E) 7—leg L (E) 8—leg R (E) |
Hp(0.07) | TLD |
Radiologist (n = 5) Cardiologist (n = 5) |
1—eye L (E) 2—central FH (E) 3—MF L hand (E) 4—L W (E) 5—MF R hand (E) 6—R W (E) 7—L leg (E) 8—R leg (E) |
Hp(0.07) | TLD |
MD/case 1—1μSv 2—4μSv 3—4μSv 4—26μSv 5—2μSv 6—26μSv 7—15μSv 8‐18μSv |
MD/case 1—37μSv 2—64μSv 3—324μSv 4—485μSv 5—88μSv 6—108μSv 7—124μSv 8‐103μSv |
|||||
| Omar, A. (2015) 48 | Vas IC | Nurse ^ (n = 3) |
1—eye R & L (E) 2—Ch (E) 3—central FH (E) |
Hp(10) |
1—TLD 2—APD 3‐APD |
Cardiologist (n = 1) |
1—eye R & L (E) 2—Ch (E) 3—central FH (E) |
Hp(10) |
1—TLD 2—APD 3‐APD |
quantitative measurements * | ||||||
| Rigatelli, G. (2016) 49 |
IC Vas IR INR |
A‐nurse <165 cm tall (n = 6) B‐nurse >165 cm tall (n = 3) |
1‐Ch (E) | * | TLD |
A‐physician <165 cm tall (n = 2) B‐ physician >165 cm tall (n = 2) |
1—Ch (E) | * | TLD |
A‐rad <165 cm tall (n = 4) B‐rad >165 cm tall (n = 3) |
1—Ch (E) | * | TLD |
Annual mean for all staff A—4550 + /‐ 4000 μSv B—1950 + /‐ 1000 μSv |
||
| Principi, S. (2015) 52 | Vas IC |
Nurse ^ P1 (n = 6) P2 (n = 1) |
1—eye L (E) 2—Ch (E) |
1—Hp(3) 2‐Hp(10)/Hp(0.07) |
TLD |
Cardiologist P1 (n = 9) P2 A (n = 2) B (n = 1) |
1—eye L (E) 2—Ch (E) P2B—eye L (E & U) |
1—Hp(3) 2‐Hp(10)/Hp(0.07) |
TLD |
nurse MD/case# P1‐17 μSv P2—13 + /‐5 μSv |
dr MD/case# P1‐ 114 μSv P2—97 μSv P2B—mean U/E—3.5 |
|||||
| Urboniene, A. (2015) 53 |
Vas IC Non Vas IC |
Nurse ^ 1 & 2 (n = 137) 3 (n = 8) |
1—T (U) 2—Co (E) 3—eye (E) |
1—Hp(10) 2—Hp(10) 3—Hp(3) |
TLD |
Physician 1 & 2 (n = 114) 3 (n = 42) |
1—T (U) 2—Co (E) 3—eye (E) |
1—Hp(10) 2—Hp(10) 3—Hp(3) |
TLD |
Avg. annual dose# 2 –1490 μSv (Avg. over 9 hospitals) est. eye dose/year# 3 ‐1 600 μSv/yr |
Avg. annual dose# 2—14500 μSv (Avg. over 9 hospitals) est. eye dose/ year# 3‐ 2300 μSv/yr (Avg. over 18 physicians) |
|||||
| Komemushi, A. (20a14)63 |
Vas IR Non Vas IR |
Scout nurse (n = 5) A—No CG B—CG |
1—Ch (E) 2—T (U) |
Hp(10) | PDM |
Radiologist (n = 4) A—No CG B—CG |
1—Ch (E) 2—T (U) |
Hp(10) | PDM |
MD/case 1A—0.51 + /‐ 1.17 μSv (A) 1B ‐0.16 + /‐ 0.41 μSv (B) 2A & B—below detectable limit (A & B) |
MD/case 1A—8.70 + /‐ 12.70 μSv 1B ‐8.88 + /‐ 13.38 μSv 2A—0.65 + /‐ 1.45 μSv 2B‐0.48 + /‐ 1.03 μSv |
|||||
| Mori, H. (2015)64 |
Vas IC Vas IR |
Vascular IR nurse (n = 69) |
1—T (U) 2—Co (E) |
Hp(10) | PGD | a reduction of annual effective dose to approx 1/3 or baseline dose after education, and a reduction to 2/5 of baseline after reduction in pulse rates | ||||||||||
Vas: vascular; Non Vas – non vascular radiology procedures; IC: interventional cardiology; IR: interventional radiology; INR: interventional neuro‐radiology; EVAR: endovascular aortic repair; Ch: chest; Co: collar; T: trunk; W: wrist; K: knee; F: finger; RF: ring finger; MF: middle finger; G: gonad; FH: forehead; An: ankle; L: left; R: right; P: phase; (E): external to protective equipment; (U): under protective equipment; APD: active personal dosimeter; ED: electronic dosimeter; EPD: electronic personal dosimeter; PDM: personal dosimeter; PGD: phosphate glass dose meter; SM: survey meter; TLD: thermoluminescent dosimeter; CP: closed phase; OP: open phase; LP: learning phase; LG: lead glasses; CG: call group; NCG: no call group; rad: radiographer; pt: patient; dr; doctor; Avg.: average; MD: mean dose; DAP : dose area product; μSv:microsievert; Gy.cm2 : gray‐centimetres squared; N: near; F: far; *: not articulated; ^: role not articulated; #: average calculated from data; ~: normalized with fluoroscopy time; !: dose normalized by cumulative KAP; equiv.: equivalent; est.: estimated.
Figure 2.
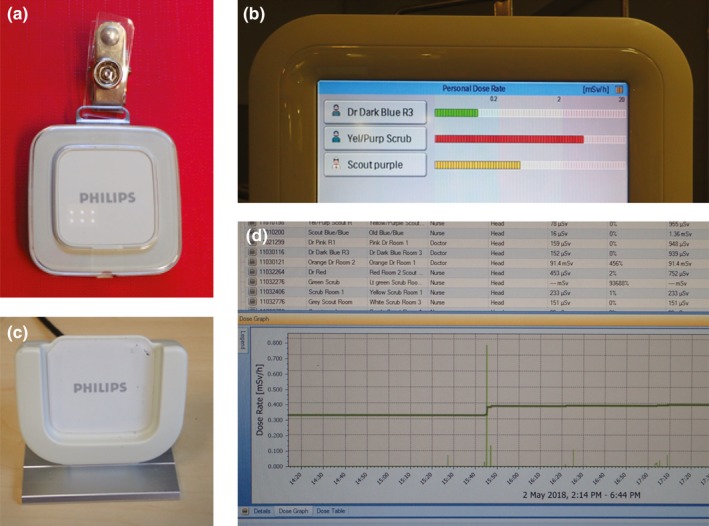
Components of a real time feedback monitoring system. (a) personal dosimeter. (b) base station. (c) download cradle. (d) dose manager software.
Baumann et al. report the overall mean staff dose per fluoroscopic minute was 42.79 vs 19.81 μSv/min (P < 0.05) comparing the closed and open phases,36 and Racadio et al. also demonstrate that the dose to staff was higher in the closed phase with a median of 3.01 μSv/min than in the open phase 0.56 μSv/min.35 Similarly, Butcher et al. reports a mean personal percentage dose reduction for scrub nurses from 0.065% (SD, 0.12) in the closed phase to 0.03% (SD, 0.034) in the open phase, while scout nurses decreased from 0.06% (SD, 0.11) measured during the closed phase, to 0.009% (SD, 0.01).39 None of these reductions were reported as statistically significant with one cited explanation the possibility that the nurses had a restricted view of the readout monitor during cases, but it is acknowledged that real time dose feedback can be effective in dose reduction.35, 36, 37, 38, 39
2.B. The effect of equipment and staff location
Radiation scatter is the primary mechanism of operator and staff exposure, and understanding the factors that can affect its magnitude and distribution is essential.40 As X‐ray scatter from the patient is the primary source of radiation dose to in‐room personnel,41 staff location within the fluoroscopy room influences the level of occupational exposure.1, 19, 42 In x‐ray guided CV procedures, the area of greatest scatter alters as the geometry of the x‐ray tube changes (Fig. 3).43 Nursing staff may undertake several roles within fluoroscopic suites, and the in‐room location of the nurse may vary during procedures. In many of the reviewed articles, the role of the nurse was not well‐defined and it was unclear whether staff were performing the scrub or scout role12, 32, 35, 44, 45, 46 and consequently reported data may represent an average of the dose of both duties.
Figure 3.
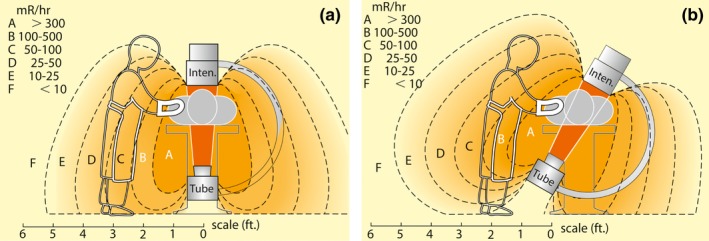
Exposure levels during fluoroscopy. (a): straight under table tube orientation. (b): central ray 30° from vertical. Reprinted with permission from Personnel exposure during fluoroscopy procedures, Postgraduate Radiology 8:162–173, 1988. 1 millirem (mR) is equivalent to 0.01 millisievert (mSv).
Mohapatra et al. investigated several staff roles and found that there was surprising variation in doses to different personnel present during the same procedure.31 The authors also identified that personal behavior within the fluoroscopic suite alters dose considerably. Depending on their responsibilities during the procedure nurses may have greater opportunity of deliberately increasing their distance from the patient resulting in a decrease in dose.1, 25, 29, 39
Some authors investigated dose in relation to proximity to the x‐ray tube.25, 34, 38, 47, 48, 49 Explanatory diagrammatic representation of the position of staff was provided in several articles25, 38, 47, 48, 49 which allows comparison by dosimetric location rather than assigned role. Specific articulation of staff distances from the x‐ray tube or table31, 47, 49 was constructive when comparing occupational doses.
2.C. Lead shielding
Lead shielding refers to the use of lead, or lead equivalent products to shield staff from radiation. Variations in accessibility and utilization of lead shielding devices by staff in fluoroscopic suites have been well documented50, 51 and this has been reflected in reported use of personal protection in the reviewed studies (Table 3). Thyroid shields were either not worn12, 44 or inconsistently worn by staff at some centers.52 Only one reviewed article specifically articulated the use of a lead skull cap during fluoroscopic procedures and was utilized by the operator only.11 Lead glasses also had varying degrees of use with several studies reporting that while doctors routinely used lead eye protection, nursing staff did not.11, 19, 44, 47, 53
Table 3.
Protective equipment utilized by staff
| Reference: First author (year) | Lead coat | Thyroid shield | Lead glasses | Table mounted lead drape | Ceiling mounted lead shield | Lead cap | Lead gloves | Additional shielding | X‐ray tube orientation |
|---|---|---|---|---|---|---|---|---|---|
| Domienik, J. (2012) 1 | y | * |
*—nurses y—drs (use varied) |
R1—y R2—y R3—use varied R4—use varied R5—n R6—y |
R1—y R2—use varied R3—use varied R4—n R5—n R6—n |
n | * | * |
OT (R5) UT (R1‐4;6) |
| Chohan, M. (2015) 11 | y | y | y | y | y |
n‐ nurses y—drs |
n | Additional lead shield on anaesthetic side | Biplane |
| Chida, K. (2013) 12 | y | n | * | y | y | n | * | * | UT |
| Antic, V (2012) 19 | y | y |
0%—nurses 46%—doctors |
y | y | n | n | nil | UT (x2) |
| Sailer, A. ( 2015) 25 | y | y | n | n | n | n | n | nil | UT |
| Nuraeni, N. (2016) 29 | y | y | * | y | y | * | * | * | biplane |
| Mohapatra, A. (2013) 31 | y | y | y—use varied | y | y (2) | n | * | Floor shield for anaethetic team (infrequently used) | UT |
| Korir, G. (2012) 32 | * | * | * | * | * | n | * | * | * |
| Omar, A. (2017) 34 | y | * | * |
R1—y (x2) R2—y R3—y R4—y |
R1 (hybrid IR OR)—y (x2) R2 (IR)—y (x2) R3 (IC)—y (large) R4 (IR)—y |
* | * | Mobile full body radiation protection shield available in R1, 3 and 4 |
UT (R1‐3) biplane (R4) |
| Racadio, J (2014) 35 | y | * | * | y—use varied | y—use varied | * | * | * | UT |
| Baumann, F. (2015) 36 | y | y | n | * | * | n | n | * | * |
| Sandblom, V. (2013) 37 | y | * | * | * | y | * | * | * | * |
| James, R. (2015) 38 | * | * | * | * | y | * | * | Standing stationary full body length leaded acrylic barrier | Biplane |
| Butcher, R. (2015) 39 | y | * | * | * | * | * | * | * | UT |
| Haga, Y. (2017) 44 | y | n |
0%—nurses 75%—drs |
* | n | n | n | Nil | UT |
| Gilligan, P. (2015) 45 | * | * |
n—nurses y—drs (use varied) |
* | y | n | * | * | UT |
| McLean, D. (2016) 46 | * | * |
50%—IC staff 30%—Vas IR staff 0%—ERCP staff |
* |
y—IC y—IR Angiography n—ERCP |
n | n | * | UT |
| Efstathopoloulos, E. (2011) 47 | y | y |
0%—nurses 71%—radiologists 83%—cardiologists |
y | y | n | Available but not used |
Mobile floor screen 78%—IR 14%—IC |
UT |
| Omar, A. (2015) 48 | y | * | y! | * | y | y | * | * | UT |
| Rigatelli, G. (2016) 49 | y | * | * | * | Phantom measurements taken with and without CML | * | * | * | UT |
| Principi, S. (2015) 52 | y |
17%—nurses 100%—drs |
0%—nurses 11%—drs |
y | y—78% | n | n | nil | UT |
| Urboniene, A. (2015) 53 | y | y | 50%—IR staff | * |
y—use varied ~ 76% of workers were protected with lead screens or glasses |
n | * | * | * |
| Komemushi, A. (2014)63 | y | * | * | y | y | n | * | * | UT |
| Mori, H. (2015) 64 | y | * | * | y | * | * | * | Portable radiation shielding screens | UT |
Vas: vascular; R: room; CML: Ceiling mounted lead; IC: interventional cardiology; IR: interventional radiology; ERCP: endoscopic retrograde cholangiopancreatography; drs: doctors: UT: undertable; OT: overtable; OR: operating room; !: ?protective glasses’ unclear whether this is lead or plastic; * : not articulated; y: yes; n: no.
Consideration should also be given to the location of lead protection. This may include items such as ceiling mounted lead glass, table mounted, or stand‐alone lead shields (Fig. 4). This equipment provides a barrier between the scattered radiation from the patient and the staff member, but correct positioning is vital for effective dose minimization.54
Figure 4.
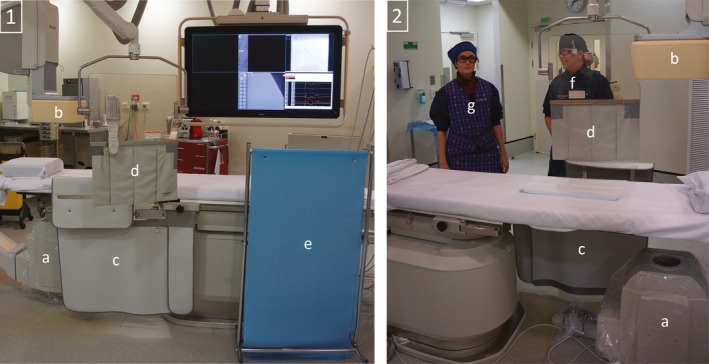
Lead protection and staff position: 1:View from operating side; 2: View from non‐operating side; (a) x‐ray tube; (b) x‐ray detector; (c) Table mounted lead drapes with extension panel; (d) Movable ceiling mounted lead glass shield with lead drapes; (e) Moveable stand‐alone shield; (f) Common location for flurosocopic operator; (g) Common location for scrub nurse.
The importance of careful positioning of the movable ceiling mounted lead shield has been previously reported55 especially when using biplane equipment,56 and this was echoed in the reviewed literature.1, 11, 19, 25, 31, 32, 34, 35, 46, 48, 52, 53 Several authors declared the absence of ceiling25, 44, 46 and table‐mounted lead shields25 when no other additional lead protection such as lead glasses or skull caps were worn by staff.25, 44 It has been highlighted previously that some fluoroscopic staff have access to a ceiling mounted lead shield but choose not to utilize it50 and this was also found to be the case in a number of reviewed manuscripts.1, 34, 35, 52, 53
2.D. Eye dose
While many dosimeters are worn underneath protective lead aprons, it is important to monitor dose for the unprotected areas of the body exposed to radiation.19 Ideally a dedicated dosimeter should be worn adjacent to the eye closest to the x‐ray tube and monitor lens dose using the operational quantity personal dose equivalent Hp(3)18, 56, 57 which means it is designed to detect dose to the lens at a depth of 3 mm. Dosimeters are also available in Hp(10) and Hp(0.07) which estimate values for dose of deep organs and skin dose, respectively. Several of the reviewed manuscripts recorded eye dose at the level of the eye1, 11, 19, 29, 44, 46, 47, 48, 53, 58 and some utilized multiple dosimeters around the face or head (Table 2).1, 11, 47, 48, 52
Several studies positioned dosimeters external to protective lenses19, 44, 46, 47, 48 which gives an approximation of the unprotected dose to the eyes, but not the actual dose incident on the lens of the monitored staff member.19, 46, 48 To assess the benefit of protective lead glasses Haga et al. measured doses both inside and outside the lead eye ware and found the shielding effect was approximately 60% reduction in measured radiation dose in a clinical IC setting.44
Several of the reviewed research investigated whether eye dose to personnel would exceed the recommended ICRP dose limits. A number of authors found that staff eye doses were within acceptable limits, but it is observed that some of these findings relate to the pre‐2012 ICRP recommended limit of 150 mSv per year, not the revised limit of 20 mSv per year. With the new eye limits applied, nurses in Korir et al. study, with a mean dose of 270 μSv per case, and physicians in Domienik et al. and Efstathopoulos et al., with procedural eye doses of 67.6 and 64 μSv, respectively, may be at risk of exceeding the current recommendations. Domienik et al. goes on to report an annual estimated eye dose for one operator of 247 mSv, which not only exceeds the new limit of 20 mSv, but definitively exceeded the old limit of 150 mSv. Mulitple reviewed studies highlighted the fact that this new eye dose limit could be exceeded by the operator when bad practices are followed, radiation protection tools are not used appropriately,34, 47 or when protective eyeglasses are not worn.11, 19, 34, 44, 46, 52, 53, 59, 60
With a recommended equivalent dose limit of 500 mSv in a year for the hands and feet, even the highest recorded average extremity dose of 485 μSv at the left wrist of a physician47 would require participation in over 1000 fluoroscopic cases within a year to be at risk of exceeding the recommended limit.
Chohan et al. demonstrated that scout nurses would receive 39 mSv of cumulative exposure per year and were at risk of exceeding the recommended ICRP eye limit11 and Antic et al. noted that a scrub nurse could exceed the limit if over 600 procedures per year were performed in this role.19 McLean et al.46 identified that the nursing staff received three of the highest six doses in the angiographic suite and noted that, while not routinely the closest to the patient, nurses were present during a large number of procedures. Chida et al. established that individual nurses were present for over double the number of coronary cases as interventionalists (average 754 ± 352 times vs 293 ± 145 times, respectively).12 Nuraeni et al. reported that a single monitored nurse, due to her proximity to the x‐ray tube and her habit of bowing her head during procedures, resulted in a similar eye exposure as the operator.29 If findings of nursing dose measured of 0.27 mSv per case at the collar in Korir et al.32 study were extrapolated, nurses would exceed the eye dose after only 75 cases.
2.E. Imaging parameters
Mohapatra et al. found that digital subtraction angiography (DSA) acquisition runs, as opposed to fluoroscopy accounted for “a large fraction of individuals’ doses”31 (p. 702) which has been highlighted by other researchers.61, 62 James et al. reported changes in behavior regarding the use of DSA in cerebral angiography as a result of real time feedback from the scrub nurse's dosimeter which monitored a difference in the mean dose of 0.045 μSv/Gy‐cm2 during the closed phase, to 0.02 μSv/Gy‐cm2 during the open phase.38
It was demonstrated that reducing staff proximity to the x‐ray tube during fluoroscopic activation can be achieved by better communication between the operator and the nurse,38, 63 limiting DSA acquisitions31 and increasing staff distance during acquisitions especially when using large tube angles.31, 38 Adequate staff training and education were also seen as essential, and this was successfully supplemented by using real time feedback monitors.34, 37
2.F. Staff education
Mori investigated nursing doses before and after staff were provided with practical education.64 This resulted in a decrease in annual effective dose from 1.33 to 0.47 mSv, which corresponds to similar studies.65, 66 Several authors articulated the need for appropriate training to heighten staff awareness to ideally result in the active participation of staff in optimizing occupational exposure.32, 34, 35, 48, 52, 67
3. DISCUSSION
While lead aprons were universally worn, it was concerning to note the irregular use of other radiation protection (Table 3). The use of lead glasses is especially important in the absence of a ceiling mounted lead shield and provides protection from the formation of radiation‐induced subcapsular cataracts.33 Although the reviewed literature was unconvincing in demonstrating a staff commitment to utilizing eye protection, a vast number of authors acknowledged the advantage of lead glasses,1, 11, 19, 32, 34, 35, 44, 46, 48, 53 and hopefully, this signals a trend toward greater compliance. Haga et al. report the mean ± the standard deviation for dosimeter measurements external to, and inside of protective lead glasses as being 7.9 ± 3.3 mSv and 3.1 ± 1.3 mSv/6 months, respectively, concluding the shielding effect was approximately 60%.44 The reviewed publications almost universally recommend the diligent use of appropriately positioned lead shielding and protective eyewear during fluoroscopic procedures.
Due to cardiac motion, DSA is infrequently used in cardiology procedures which may result in lower occupational doses as demonstrated by McLean et al.46 in reported lower extrapolated annual eye dose to nurses involved in fluoroscopic cardiac procedures (1.32 mGy) compared to vascular interventions (6.06 mGy). Authors investigating endovascular aortic repairs which, in theory, should expose staff to increased levels of radiation due to the proximity of staff to the irradiated area, the thickness of the imaged body part, and the use of DSA report mean nursing doses of 17 μSv (measured at the chest)25 and 26 μSv (measured at collar level).31 Omar et al. (2017) report a higher equivalent eye dose received by nurses assisting during interventional neuroradiology procedures compared with the physician (11 vs 8.6 μSv).34
Ideally DSA runs should be limited where possible,5, 31, 35, 36, 68 magnification should be increased,31 and the pressure injector should be utilized to allow staff to stand further away from the patient during acquisitions.31, 38 James et al.38 reported modification of staff behavior during cerebral DSA due to real time monitoring. One physician substituted fluoro‐save where possible for visualization of the femoral artery, which has been shown to reduce dose by 95%.62 The pressure injector was more consistently used, as opposed to injecting by hand, thus allowing personnel to step back during DSA acquisitions which may have contributed to the significant decrease in mean dose for physician B from 0.243 μSv/Gy‐cm2 during the closed phase, to 0.069 μSv/Gy‐cm2 during the open phase. It was also reported that during the open phase the scrub nurses utilized the operating physician as a personal shield by stepping behind them to reduce exposure.38 Physicians should also let other in‐room staff know of an impending DSA acquisition so that the staff know to not approach the patient and stay behind shielding if possible.38, 63
Research indicates a considerable number of parameters which can cause a significant variation in resultant dose levels during fluoroscopic cases, even within the same type of procedures.1 The Optimization of RAdiation protection for MEDical (ORAMED) staff study also revealing a large variability of practices between cases and workplaces.56 Given the variation in procedure type, operator, tube geometry, and staff position, correlation of dose conditions within differing procedures proved difficult. This was exacerbated by the different reporting values used by the authors.
The ICRP notes that radiation training may be lacking which may result in a radiation safety issue for staff as well as patients69 and recommends that departments implement an effective optimization program through training and raising consciousness of radiology protection in individuals.70 The effectiveness in dose reduction to staff following radiation education has been highlighted65, 66, 71 as has the need for radiation training of occupationally exposed nursing staff.72
Several authors noted that nursing staff are at risk of exceeding recommended dose levels if radiation protection tools are not properly used. Given the variables that exist for nursing staff during fluoroscopic procedures, dose minimization is not as simple as increasing distance from the source of the scattered radiation. Given the invisible nature of radiation, staff should be provided with appropriate information and training to highlight factors which influence dose allowing them to become conscious contributors to personal dose minimization.
3.A. Limitations of current evidence
Several limitations have been identified in the current literature. Many of the articles reviewed had relatively small sample sizes either due to the number of staff or procedures, or a relatively short data collection period. Evaluation of occupational nursing dose during fluoroscopic procedures is vital, and it is recommended that monitoring of nurse doses should be implemented as part of a robust quality assurance program. This review has highlighted the need for additional research to evaluate radiation exposure to nurses during fluoroscopic procedures. It would be constructive for future investigations to specifically articulate the location of the nurse during procedures and divide the monitoring per position as well as monitoring the dose to the individual. Having multiple dosimeters evaluating eye and extremity dose would also be beneficial.
3.B. Strengths and limitations of the review
To the author's knowledge, this is the first review to examine literature reporting dose to nursing staff during fluoroscopic CV procedures. One limitation of the review is the difficulty in making direct comparisons of nursing dose in the reviewed studies due to the variability of staff role and position, the wide variety of procedures, the type, calibration, and location of the dosimeters and the differing parameters in the reporting of dose.
4. CONCLUSION
This literature review was undertaken to highlight research specifically investigating the occupational dose received by nursing staff within fluoroscopic examinations and to critically review the findings. Nursing staff should be aware of the effect that x‐ray tube angle, orientation, and acquisition type has on potential exposure and use this knowledge to position themselves and lead shielding correctly to minimize risk. Appropriate education and training should be provided to inform nursing staff working within CV fluoroscopic suites of dose reduction techniques and the importance of utilizing protective equipment. Departments should also provide adequate shielding options for personnel to ensure that occupational radiation dose is kept as low as reasonably achievable.
Of all the reviewed literature, only three authors looked purely at dose to nurses during fluoroscopic procedures39, 63, 64 indicating that more studies are needed focussing on the occupational dose to nursing staff during x‐ray guided CV procedures.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1. Domienik J, Brodecki M, Rusicka D. A study of the dose distribution in the region of the eye lens and extremities for staff working in interventional cardiology. Radiat Meas. 2012;47:130–138. [Google Scholar]
- 2. Perisinakis K, Solomou G, Stratakis J, Damilakis J. Data and methods to assess occupational exposure to personnel involved in cardiac catheterization procedures. Phys Med. 2016;32:386–392. [DOI] [PubMed] [Google Scholar]
- 3. Heidbuchel H, Wittkampf FHM, Vano E, et al. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. EP Europace. 2014;16:946–964. [DOI] [PubMed] [Google Scholar]
- 4. Mahesh M. Radiation Dose Management for Fluoroscopically Guided Interventional Medical Procedures, Vol 39. Alexandria, VA: American Association of Physicists in Medicine; 2012:5789–5790. [Google Scholar]
- 5. Colangelo JE, Johnston J, Killion JB, Wright DL. Radiation biology and protection. Radiol Technol. 2009;80:421–441. [PubMed] [Google Scholar]
- 6. Hall EJ, Giaccia AJ. Radiobiology for the Radiologist, 7th ed Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 7. Rühm W, Woloschak GE, Shore RE, et al. Dose and dose‐rate effects of ionizing radiation: a discussion in the light of radiological protection. Radiat Environ Biophys. 2015;54:379–401. [DOI] [PubMed] [Google Scholar]
- 8. Rühm W, Azizova TV, Bouffler SD, et al. Dose‐rate effects in radiation biology and radiation protection. Ann ICRP. 2016;45:262–279. [DOI] [PubMed] [Google Scholar]
- 9. Dave JK. Why is the X‐Ray tube usually located underneath the patient instead of above the patient for interventional fluoroscopic procedures? AJR Am J Roentgenol. 2016;207:W24. [DOI] [PubMed] [Google Scholar]
- 10. Toossi MTB, Mehrpouyan M, Nademi H, Fardid R. Preliminary results of an attempt to predict over apron occupational exposure of cardiologists from cardiac fluoroscopy procedures based on DAP (dose area product) values. Austr Physi Eng Sci Med. 2015;38:83–91. [DOI] [PubMed] [Google Scholar]
- 11. Chohan MO, Sandoval D, Buchan A, Murray‐Krezan C, Taylor CL. Cranial radiation exposure during cerebral catheter angiography. J Neurointerv Surg. 2014;6:633–636. [DOI] [PubMed] [Google Scholar]
- 12. Chida K, Kaga Y, Haga Y, et al. Occupational dose in interventional radiology procedures. Am J Roentgenol. 2013;200:138–141. [DOI] [PubMed] [Google Scholar]
- 13. Lanzer P. Catheter‐Based Cardiovascular Interventions: A Knowledge‐Based Approach, vol. 1. Berlin, Heidelberg: Springer; 2014. [Google Scholar]
- 14. Vano E. Basis for standards: ICRP activities. Radiat Prot Dosimetry. 2015;165:30–33. [DOI] [PubMed] [Google Scholar]
- 15. Simeonov G, Mundigl S, Janssens A. Radiation protection of medical staff in the latest draft of the revised Euratom Basic Safety Standards directive. Radiat Meas. 2011;46:1197–1199. [Google Scholar]
- 16. Vano E, Kleiman NJ, Duran A, Romano‐Miller M, Rehani MM. Radiation‐associated lens opacities in catheterization personnel: results of a survey and direct assessments. J Vasc Interv Radiol. 2013;24:197–204. [DOI] [PubMed] [Google Scholar]
- 17. Pradhan A, Lee J, Kim J. On the scenario of passive dosimeters in personnel monitoring: relevance to diagnostic radiology and fluoroscopy‐based interventional cardiology. J Med Phys. 2016;41:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Implications for Occupational Radiation Protection of the New Dose Limit for the Lens of the Eye. In: Agency IAE, ed. IAEA_TECDOC. Vol 1731. Vienna; 2014.
- 19. Antic V, Ciraj‐Bjelac O, Rehani M, Aleksandric S, Arandjic D, Ostojic M. Eye lens dosimetry in interventional cardiology: results of staff dose measurements and link to patient dose levels. Radiat Protect Dosimetry. 2013;154:276–284. [DOI] [PubMed] [Google Scholar]
- 20. Worgul BV, Kundiev Y, Likhtarev I, Sergienko N, Wegener A, Medvedovsky CP. Use of subjective and nonsubjective methodologies to evaluate lens radiation damage in exposed populations ‐ an overview. Radiat Environ Biophys. 1996;35:137–144. [DOI] [PubMed] [Google Scholar]
- 21. Jacob S, Boveda S, Bar O, et al. Interventional cardiologists and risk of radiation‐induced cataract: results of a French multicenter observational study. Int J Cardiol. 2013;167:1843–1847. [DOI] [PubMed] [Google Scholar]
- 22. Haskal ZJ. Get protected: the eyes have it. J Vasc Interv Radiol. 2013;24:205–206. [DOI] [PubMed] [Google Scholar]
- 23. Vano E, Kleiman NJ, Duran A, Rehani MM, Echeverri D, Cabrera M. Radiation cataract risk in interventional cardiology personnel. Radiat Res. 2010;174:490–495. [DOI] [PubMed] [Google Scholar]
- 24. Koukorava C, Farah J, Struelens L, et al. Efficiency of radiation protection equipment in interventional radiology: a systematic Monte Carlo study of eye lens and whole body doses. J Radiol Prot. 2014;34:509–528. [DOI] [PubMed] [Google Scholar]
- 25. Sailer AM, Schurink GWH, Bol ME, et al. Occupational radiation exposure during endovascular aortic repair. Cardiovasc Interv Radiol. 2015;38:827–832. [DOI] [PubMed] [Google Scholar]
- 26. Ciraj‐Bjelac O, Rehani M, Minamoto A, Sim KH, Liew HB, Vano E. Radiation‐induced eye lens changes and risk for cataract in interventional cardiology. Cardiology. 2012;123:168–171. [DOI] [PubMed] [Google Scholar]
- 27. Boal TJ, Pinak M. Dose limits to the lens of the eye: International Basic Safety Standards and related guidance. Ann ICRP. 2015;44:112–117. [DOI] [PubMed] [Google Scholar]
- 28. Almasri HY, Kakinohana Y, Yogi T. Occupational radiation monitoring at a large medical center in Japan. Radiol Phys Technol. 2014;7:271–276. [DOI] [PubMed] [Google Scholar]
- 29. Nuraeni N, Hiswara E, Kartikasari D, Waris A, Haryanto F. Occupational radiation doses during interventional procedures. J Phys Conf Ser. 2016;694:012032. [Google Scholar]
- 30. Dumonceau JM, Garcia‐Fernandez F, Verdun F, et al. Radiation protection in digestive endoscopy: European society of digestive endoscopy (ESGE) guideline. Endoscopy. 2012;44:408–424. [DOI] [PubMed] [Google Scholar]
- 31. Mohapatra A, Greenberg RK, Mastracci TM, Eagleton MJ, Thornsberry B. Radiation exposure to operating room personnel and patients during endovascular procedures. J Vasc Surg. 2013;58:702–709. [DOI] [PubMed] [Google Scholar]
- 32. Korir GK, Ochieng BO, Wambani JS, Korir IK, Jowi CY. Radiation exposure in interventional procedures. Radiat Prot Dosimetry. 2012;152:339–344. [DOI] [PubMed] [Google Scholar]
- 33. Miller DL, Balter S, Cardella JF, et al. Occupational radiation protection in interventional radiology: A Joint Guideline of the Cardiovascular and Interventional Radiology Society of Europe and the Society of Interventional Radiology. Cardiovasc Interv Radiol. 2010;33:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Omar A, Kadesjo N, Palmgren C, Marteinsdottir M, Segerdahl T, Fransson A. Assessment of the occupational eye lens dose for clinical staff in interventional radiology, cardiology and neuroradiology. J Radiol Prot. 2017;37:145–159. [DOI] [PubMed] [Google Scholar]
- 35. Racadio J, Nachabe R, Carelsen B, et al. Effect of real‐time radiation dose feedback on pediatric interventional radiology staff radiation exposure. J Vasc Interv Radiol. 2014;25:119–126. [DOI] [PubMed] [Google Scholar]
- 36. Baumann F, Katzen BT, Carelsen B, Diehm N, Benenati JF, Peña CS. The effect of realtime monitoring on dose exposure to staff within an interventional radiology setting. Cardiovasc Interv Radiol. 2015;38:1105–1111. [DOI] [PubMed] [Google Scholar]
- 37. Sandblom V, Mai T, Almén A, et al. Evaluation of the impact of a system for real‐time visualisation of occupational radiation dose rate during fluoroscopically guided procedures. J Radiol Prot. 2013;33:693–702. [DOI] [PubMed] [Google Scholar]
- 38. James RF, Wainwright KJ, Kanaan HA, et al. Analysis of occupational radiation exposure during cerebral angiography utilizing a new real time radiation dose monitoring system. J Neurointerv Surg. 2015;7:503–508. [DOI] [PubMed] [Google Scholar]
- 39. Butcher RL, Gaggini R, Thoirs K. Does wearing a real‐time visual dosimeter reduce the personal radiation dose for interventional radiology nurses? An observational comparative study. J Radiol Nurs. 2015;34:137–142. [Google Scholar]
- 40. Christopoulos G, Makke L, Christakopoulos G, et al. Optimizing radiation safety in the cardiac catheterization laboratory: a practical approach. Catheter Cardiovasc Interv. 2016;87:291–301. [DOI] [PubMed] [Google Scholar]
- 41. Topol EJ, Teirstein PS. Textbook of Interventional Cardiology E‐Book, 7th ed Philadelphia: Elsevier; 2015. [Google Scholar]
- 42. Bushberg JT. The Essential Physics of Medical Imaging, 3 ed Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 43. Haqqani OP, Agarwal PK, Halin NM, Iafrati MD. Defining the radiation “scatter cloud” in the interventional suite. J Vasc Surg. 2013;58:1339–1345. [DOI] [PubMed] [Google Scholar]
- 44. Haga Y, Chida K, Kaga Y, Sota M, Meguro T, Zuguchi M. Occupational eye dose in interventional cardiology procedures. Scient Rep (Nature Publisher Group). 2017;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gilligan P, Lynch J, Eder H, et al. Assessment of clinical occupational dose reduction effect of a new interventional cardiology shield for radial access combined with a scatter reducing drape: shield/drape combination reduces staff doses. Catheter Cardiovasc Interv. 2015;86:935–940. [DOI] [PubMed] [Google Scholar]
- 46. McLean D, Hadaya D, Tse J. Eye dose to staff involved in interventional and procedural fluoroscopy. J Phys Conf Ser. 2016;694:012054. [Google Scholar]
- 47. Efstathopoulos EP, Pantos I, Andreou M, et al. Occupational radiation doses to the extremities and the eyes in interventional radiology and cardiology procedures. Br J Radiol. 2011;84:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Omar A, Marteinsdottir M, Kadesjo N, Fransson A. On the feasibility of utilizing active personal dosimeters worn on the chest to estimate occupational eye lens dose in x‐ray angiography. J Radiol Prot. 2015;35:271–284. [DOI] [PubMed] [Google Scholar]
- 49. Rigatelli G, Panin S, Fiorrevanti R, et al. Impact of operators’ height on individual radiation exposure measurements during catheter‐based cardiovascular interventions. J Interv Cardiol. 2016;29:83–88. [DOI] [PubMed] [Google Scholar]
- 50. Wilson‐Stewart K. A survey of radiation protection utilization and accessibility within Australian cardiac angiography laboratories. J Radiol Nurs. 2017;36:112–116. [Google Scholar]
- 51. Lynskey GE III, Powell DK, Dixon RG, Silberzweig JE. Radiation protection in interventional radiology: survey results of attitudes and use. J Vasc Interv Radiol. 2013;24:1547–1551. [DOI] [PubMed] [Google Scholar]
- 52. Principi S, Soler CD, Ginjaume M, Vilagrasa MB, Escutia JJR, Duch MA. Eye lens dose in interventional cardiology. Radiat Prot Dosimetry. 2015;165:289–293. [DOI] [PubMed] [Google Scholar]
- 53. Urboniene A, Sadzeviciene E, Ziliukas J. Assessment of eye lens doses for workers during interventional radiology procedures. Radiat Prot Dosimetry. 2015;165:299–303. [DOI] [PubMed] [Google Scholar]
- 54. Fetterly KA, Magnuson DJ, Tannahill GM, Hindal MD, Mathew V. Effective use of radiation shields to minimize operator dose during invasive cardiology procedures. JACC: Cardiovasc Interv. 2011;4:1133–1139. [DOI] [PubMed] [Google Scholar]
- 55. Kuon E, Schmitt M, Dahm JB. Significant reduction of radiation exposure to operator and staff during cardiac interventions by analysis of radiation leakage and improved lead shielding. Am J Cardiol. 2002;89:44–49. [DOI] [PubMed] [Google Scholar]
- 56. Carinou E, Brodecki M, Domienik J, et al. Recommendations to reduce extremity and eye lens doses in interventional radiology and cardiology. Radiat Meas. 2011;46:1324. [DOI] [PubMed] [Google Scholar]
- 57. Bordy JM, Gualdrini G, Daures J, Mariotti F. Principles for the design and calibration of radiation protection dosemeters for operational and protection quantities for eye lens dosimetry. Radiat Prot Dosimetry. 2011;144:257–261. [DOI] [PubMed] [Google Scholar]
- 58. Principi S, Farah J, Ferrari P, Carinou E, Clairand I, Ginjaume M. The influence of operator position, height and body orientation on eye lens dose in interventional radiology and cardiology: Monte Carlo simulations versus realistic clinical measurements. Phys Med. 2016;32:1111–1117. [DOI] [PubMed] [Google Scholar]
- 59. Zagorska A, Romanova K, Hristova‐Popova J, Vassileva J, Katzarov K. Eye lens exposure to medical staff during endoscopic retrograde cholangiopancreatography. Phys Med‐Eur J Med Phys. 2015;31:781–784. [DOI] [PubMed] [Google Scholar]
- 60. O'Connor U, Gallagher A, Malone L, O'Reilly G. Occupational radiation dose to eyes from endoscopic retrograde cholangiopancreatography procedures in light of the revised eye lens dose limit from the International Commission on Radiological Protection. Br J Radiol. 2013;56:20120289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sanchez R, Vano E, Fernandez JM, Gallego JJ. Staff radiation doses in a real‐time display inside the angiography room. Cardiovasc Interv Radiol. 2010;33:1210–1214. [DOI] [PubMed] [Google Scholar]
- 62. Pearl MS, Torok C, Wang JX, Wyse E, Mahesh M, Gailloud P. Practical techniques for reducing radiation exposure during cerebral angiography procedures. J Neurointerv Surg. 2015;7:141–145. [DOI] [PubMed] [Google Scholar]
- 63. Komemushi A, Suzuki S, Sano A, et al. Radiation dose of nurses during IR procedures: a controlled trial evaluating operator alerts before nursing tasks. J Vasc Interv Radiol. 2014;25:1195–1199. [DOI] [PubMed] [Google Scholar]
- 64. Mori H. Action research regarding the optimisation of radiological protection for nurses during vascular interventional radiology. J Radiol Prot. 2015;35:457–466. [DOI] [PubMed] [Google Scholar]
- 65. Abatzoglou I, Koukourakis M, Konstantinides S. Reduction of the radiation dose received by interventional cardiologists following training in radiation protection. Radiat Prot Dosimetry. 2013;155:119–121. [DOI] [PubMed] [Google Scholar]
- 66. Sheyn DD, Racadio JM, Ying J, Patel MN, Racadio JM, Johnson ND. Efficacy of a radiation safety education initiative in reducing radiation exposure in the pediatric IR suite. Pediat Radiol. 2008;38:669–674. [DOI] [PubMed] [Google Scholar]
- 67. Mori H, Koshida K, Ichikawa K. Estimation of personal dose based on the dependent calibration of personal dosimeters in interventional radiology. Nihon Hoshasen Gijutsu Gakkai Zasshi. 2007;63:852–861. [DOI] [PubMed] [Google Scholar]
- 68. Vano E. Radiation exposure to cardiologists: how it could be reduced. Heart. 2003;89:1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Agency IAE . Fluoroscopy in Operating Theatres; 2013. https://rpop.iaea.org/RPOP/RPoP/Content/InformationFor/HealthProfessionals/4_InterventionalRadiology/fluoroscopy-operating-theatres/index.htm. Accessed 03 July, 2017, 2017.
- 70. ICRP . The optimisation of radiological protection: broadening the process. Ann ICRP. 2006;36:69–87. [DOI] [PubMed] [Google Scholar]
- 71. Mavrikou I, Kottou S, Tsapaki V, Neofotistou V. High patient doses in interventional cardiology due to physicians’ negligence: how can they be prevented? Radiat Prot Dosimetry. 2008;129:67–70. [DOI] [PubMed] [Google Scholar]
- 72. Ohno K, Kaori T. Effective education in radiation safety for nurses. Radiat Prot Dosimetry. 2011;147:343–345. [DOI] [PubMed] [Google Scholar]


