Abstract
Background
Glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) are incretin hormones. By lowering blood glucose in a glucose-dependent manner, incretin-based therapies represent a novel and promising intervention to treat hyperglycaemia in hospital settings. We performed a systematic review of the literature for all current applications of incretin-based therapies in the peri-operative and critical care settings.
Methods
We searched MEDLINE, the Cochrane Library, and Embase databases for all randomised controlled trials using exogenous GLP-1, GLP-1 receptor agonists, exogenous GIP and dipeptidyl peptidase IV inhibitors in the setting of adult peri-operative care or intensive care. We defined no comparator treatment. Outcomes of interest included blood glucose, frequency of hypoglycaemia and insulin administration.
Results
Of the 1190 articles identified during the initial literature search, 38 fulfilled criteria for full-text review, and 19 single-centre studies were subsequently included in the qualitative review. Of the 18 studies reporting glycaemic control, improvement was reported in 15, defined as lower glucose concentrations in 12 and as reduced insulin administration (with similar glucose concentrations) in 3. Owing to heterogeneity, meta-analysis was possible only for the outcome of hypoglycaemia. This revealed an incidence of 7.4% in those receiving incretin-based therapies and 6.8% in comparator groups (P = 0.94).
Conclusions
In small, single-centre studies, incretin-based therapies lowered blood glucose and reduced insulin administration without increasing the incidence of hypoglycaemia.
Trial registration
PROSPERO, CRD42017071926.
Electronic supplementary material
The online version of this article (10.1186/s13054-018-2197-4) contains supplementary material, which is available to authorized users.
Keywords: DPP-IV inhibitors, GIP, GLP-1, Glucose control, Hyperglycaemia, Hypoglycaemia, Intensive care, Peri-operative care
Background
Hyperglycaemia occurs frequently in the peri-operative period and during critical illness, even in patients without a history of diabetes mellitus [1–3]. Usual management of hyperglycaemia in these settings primarily involves intravenous infusions of insulin, with the dose titrated according to intermittent measurement of blood glucose [4]. This strategy is somewhat complicated and labour–intensive, and it increases the risk of hypoglycaemia and glycaemic variability, which are both associated with adverse outcome [3, 5–10].
The incretin effect is the physiological phenomenon observed following the ingestion of glucose, which results in endogenous insulin secretion almost two-fold greater than after a comparable intravenous glucose load [11]. This process is attributed to the enterohormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) that have insulinotropic and glucagonostatic properties [12]. The insulinotropic response is glucose-dependent, meaning that even when GLP-1 and GIP are administered in pharmacological doses, there is negligible risk of hypoglycaemia [12].
GLP-1 and GIP are rapidly metabolised by the enzyme dipeptidyl peptidase IV (DPP-IV) [12]. Accordingly, incretin-based therapies necessitate a continuous infusion of either exogenous GLP-1 or GIP, administration of a DPP-IV-resistant receptor agonist (GLP-1 receptor agonists, first-in-class drug exenatide), or a DPP-IV antagonist that increases endogenous GLP-1 and GIP concentrations (first-in-class drug sitagliptin) [12]. All currently available and applicable drugs are named in Additional file 1.
GLP-1 receptor agonists and DPP-IV inhibitors are now established therapies for the management of patients with type 2 diabetes mellitus (T2DM) [13]. The efficacy and safety profiles of incretin-based therapies have fostered enthusiasm for use of these agents as adjuncts or alternatives to insulin for glycaemic control in the operating room and intensive care unit (ICU). The purpose of this systematic review was to evaluate the safety and efficacy of incretin therapies for glucose control in the operating room and ICU.
Methods
This systematic review was prospectively registered in the PROSPERO database (PROSPERO identifier CRD42017071926) and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14].
Eligibility criteria
Studies eligible for inclusion were prospective randomised controlled trials using an incretin-based therapy in the operating room and/or the ICU. Studies published in any language and without publication date restriction were considered. Paediatric, animal and observational studies were excluded.
Search strategy
We performed an unrestricted electronic database search of the MEDLINE, Cochrane Library and Embase databases from their inception to 13 February 2018. Our search included terms to specify the intervention (incretin therapy), setting (peri-operative and ICU care) and study type (prospective randomised controlled trials). Searches included synonyms and combinations of the following terms: ‘operating room’, ‘OR’, ‘peri-operative period’, ‘ICU’, ‘critical care’, ‘incretin therapy’, ‘GLP-1’, ‘GIP’ and ‘DPP-IV inhibitor’, as well as generic names of the currently marketed forms of these medications. Our complete search terms and methodology are available as additional material (see Additional file 1) and accessible via PROSPERO. Reference lists of retrieved papers were also reviewed for potentially eligible studies not captured in the primary search. We defined no specific comparator for any intervention.
Study selection
After deletion of duplicate studies, two investigators (AHH, MPP) screened all titles and abstracts using Rayyan [15]. Relevant studies were then evaluated in full text for eligibility, with any conflicts resolved by a third investigator (JH). The authors of conference abstracts and published protocols without subsequent full texts were contacted to request the data and/or manuscript.
Risk-of-bias assessment
Two authors independently assessed the quality of the research methodology of all randomised controlled trials using the Cochrane Collaboration’s Risk of Bias Tool [16].
Data extraction
We extracted data including study characteristics (author, publication year, country, design, funding source and sample size), setting (operating room, ICU, post-cardiac surgery), patient characteristics (demographics) and intervention and comparator parameters (incretin therapy, route, dose and duration, as well as additional treatments). We did not predefine primary outcomes in this scoping exploratory systematic review; all reported outcomes were recorded and summarised if reported across multiple studies. Owing to the expected heterogeneity of interventions, comparators, settings and outcomes, we did not plan a meta-analysis of outcomes. Owing to the frequency with which hypoglycaemia was reported across studies, we decided to retrospectively perform a meta-analysis of this outcome. This was not feasible for all other outcomes.
Statistical analysis
For data extraction and meta-analysis, we used Review Manager version 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). We used a random effects model because of expected clinical heterogeneity between trials. Results of the meta-analysis were expressed as Mantel-Haenszel odds ratios with 95% CIs because of the dichotomous outcome. As markers for inter-trial heterogeneity, we used τ2, χ2 and I2 statistics.
Results
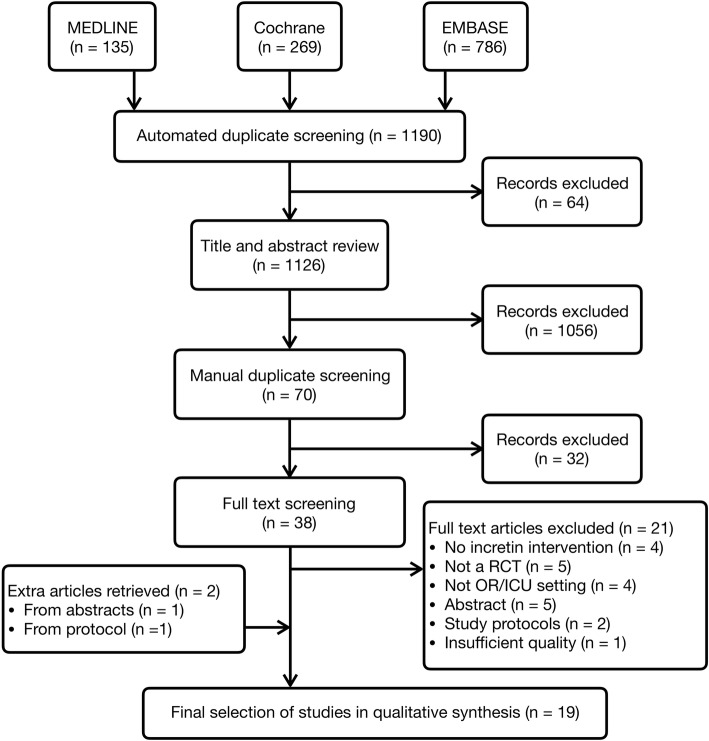
Our search yielded 1126 citations, and after elimination of duplicates, abstracts and full texts, 19 studies were included in this systematic review (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. ICU Intensive care unit, OR Operating room, RCT Randomised controlled trial
Study characteristics
Characteristics of the included studies are summarised in Table 1, including setting of care, duration, type and dose of intervention, and reported outcomes [17–34]. In total, 1410 patients participated in these studies, of whom 988 were known to have T2DM. All studies recruited patients in a single centre. Comparator groups included placebo or combinations of intravenous or subcutaneous insulin.
Table 1.
Study characteristics
| Author, year | Participants, setting, n | DM, n (%) | Intervention duration | Intervention, dose, n | Comparator, n | Standard glycaemic therapy | Outcome parameters |
|---|---|---|---|---|---|---|---|
| Besch, 2017 [19] | CABG, OR + ICU n = 104 |
22 (21%) | 48 h | Exenatide IV 25 ng/min n = 53 |
Standard glycaemic therapy n = 51 |
Continuous insulin IV + bolus regimen | Glycaemia Insulin administration, complications, LoS |
| Brackbill, 2012 [20] | CABG, ward, n = 62 |
62 (100%) | 4 d | Sitagliptin PO 100 mg q.d. n = 30 |
Placebo n = 32 |
Basal bolus insulin SC regimen | Glycaemia LoS |
| Deane, 2009 [21] | Mechanically ventilated, ICU n = 7 |
0 (0%) | 240 min | GLP-1 IV 1.2 pmol/kg/min n = 7 |
Placebo n = 7 |
None | Glycaemia Insulinaemia, Glucagon, GLP-1 |
| Deane, 2010 [22] | Mechanically ventilated, ICU n = 25 |
0 (0%) | 360 min | GLP-1 IV 1.2 pmol/kg/min n = 25 |
Placebo n = 25 |
None | Glycaemia Gastric emptying, glucose absorption, Insulinaemia, Glucagon |
| Deane, 2011 [23] | Mechanically ventilated, ICU n = 11 |
11 (100%) | 240 min | GLP-1 IV 1.2 pmol/kg/min n = 11 |
Placebo n = 11 |
None | Glycaemia Insulinaemia, C-peptide, glucagon, FFA |
| Galiatsatos, 2014 [24] | Surgical/burn, ICU n = 18 |
9 (50%) | 72 h | GLP-1 IV 1.5 pmol/kg/min n = 9 |
Saline n = 9 |
Intensive insulin treatment protocol | Glycaemia Insulin administration, glucagon, C-peptide, CV medication |
| Garg, 2017 [35] | In hospital, ward (74% surgical) n = 66 |
66 (100%) | 5 d | Saxagliptin PO 5 mg q.d. n = 33 |
Basal bolus insulin SC regimen n = 33 |
Corrective insulin bolus regimen | Glycaemia Insulin administration, Treatment failure, LoS |
| Holmberg, 2014 [25] | CABG, OR n = 62 |
12 (19%) | 390 min | Exenatide IV 43 ng/min n = 21 |
RIPC n = 20 / Placebo n = 21 |
Unknown | Cardiac enzymes Complications, LoS |
| Kar, 2015 [26] | Mechanically ventilated, ICU n = 20 |
0 (0%) | 300 min | GIP IV 4 pmol/kg/min n = 20 |
Placebo n = 20 |
None | Glycaemia Gastric emptying, glucose absorption, insulinaemia |
| Kohl, 2014 [27] | CABG, OR n = 77 |
11 (14%) | 72 h | GLP-1 IV 1.5 pmol/kg/min n = 37 |
Placebo n = 40 |
Continuous insulin IV + bolus regimen | Glycaemia Insulinaemia, glucagon, GLP-1, cortisol, FFA. |
| Lee, 2013 [28] | Mechanically ventilated, ICU n = 20 |
0 (0%) | 300 min | GIP IV 4 pmol/kg/min n = 20 |
Standard glycaemic therapy n = 20 |
GLP-1 IV 1.2 pmol/kg/min (300 min) |
Glycaemia Insulinaemia, glucagon, GLP-1, GIP, |
| Lipš, 2017 [17] | CABG, OR n = 38 |
26 (68%) | 72 h | Exenatide IV 20 ng/min n = 19 |
Placebo n = 19 |
Intensive insulin treatment protocol | Glycaemia Echocardiography, CV medications, complications |
| Meier, 2004 [29] | Major surgery, ward n = 8 |
100 (100%) | 8 h | GLP-1 IV 1.2 pmol/kg/min n = 8 |
Placebo n = 8 |
None | Glycaemia Insulinaemia, C-peptide, glucagon, GLP-1 |
| Miller, 2017 [30] | Mechanically ventilated, ICU n = 12 |
0 (0%) | 270 min | GLP-1 IV 1.2 pmol/kg/min n = 12 |
Placebo n = 12 |
None | Glycaemia Glucose absorption |
| Müssig, 2008 [31] | CABG, ICU n = 20 |
100 (100%) | 12 h | GLP-1 IV 3.6 pmol/kg/min n = 10 |
Continuous insulin IV n = 10 |
Corrective insulin bolus regimen | Glycaemia Insulin administration, haemodynamics |
| Pasquel, 2017 [32] | In hospital, ward (16% surgical) n = 277 |
100 (100%) | 10 d | Sitagliptin PO 100 mg q.d. n = 138 |
Bolus insulin regimen n = 139 |
Basal (glargine) insulin regimen | Glycaemia Insulin administration, complications, treatment failure |
| Polderman, 2018 [18] | Surgical, OR n = 150 |
100 (100%) | 2 d | Liraglutide SC 0.6 mg + 1.2 mg n = 44 |
GIK infusion n = 53/Bolus insulin algorithm n = 53 |
Bolus insulin treatment algorithm | Glycaemia Insulin administration, Potassium, nausea, complications |
| Sokos, 2007 [34] | CABG, OR n = 20 |
5 (25%) | 60 h | GLP-1 IV 1.5 pmol/kg/min n = 10 |
Standard insulin therapy n = 10 |
Standard insulin therapy | Glycaemia LVEF, haemodynamics |
| Umpierrez, 2014 [33] | In hospital, ward (45% surgical) n = 90 |
100 (100%) | 10 d | Sitagliptin PO 100 mg q.d. n = 27 / Sitagliptin + basal insulin n = 29 |
Basal bolus insulin regimen n = 26 |
Correction bolus insulin regimen | Glycaemia Insulin administration, complications, treatment failure |
Abbreviations: b.i.d. Twice per day, CABG Coronary artery bypass grafting, CV Cardiovascular, d Days, DM Diabetes mellitus, FFA Free fatty acids, GIK Glucose-insulin-potassium infusion, GIP Gastric inhibitory polypeptide, GLP-1 Glucagon-like peptide-1, h Hours, ICU Intensive care unit, IV Intravenously, LoS Length of stay, min Minutes, LVEF Left ventricular ejection fraction, OR Operating room, PO By mouth, q.d Once per day, RIPC Remote ischaemic preconditioning, SC Subcutaneous
All secondary outcomes are in italics
Risk of bias
A summary of the risk of bias in the included studies is presented in Figs. 2 and 3. Randomisation sequence generation was often briefly described and therefore assessed as unclear. Allocation concealment carried a low risk of bias in most studies and was scored as unclear only if it remained unmentioned in the manuscript. Most trials were blinded and adequately described as such. In some trials the intervention was not blinded; however, if the primary outcome was a measurable physiological variable (e.g., glucose), a low risk of bias was ascribed. Only one trial was deemed to have a high risk of bias owing to both open-label administration of study drug and an outcome measure (insulin administration) that has the capacity to be influenced by the knowledge of treatment allocation [19]. With limited numbers of patients per study and short follow-up periods for the main outcome parameters, attrition bias was deemed low in all studies. Because most studies reported similar outcomes (Table 1), the risk of selective reporting between studies was considered low. The majority of studies had registered protocols demonstrating consistent reporting of outcomes, and in only one case was there a discrepancy between reported and registered outcomes [24]. Other potential sources of bias identified were an early termination due to slow enrolment [18], deviation from baseline reporting for some outcomes [22] and one study published as a letter to the editor with consequent brief reporting and unclear identification of sources of bias [31].
Fig. 2.
Review authors’ judgements about each risk-of-bias item presented as percentages across all included studies. Green = low risk of bias; yellow = unclear risk of bias; red = high risk of bias
Fig. 3.

Review authors’ judgements about each risk-of-bias item for each included study. Green = low risk of bias; yellow = unclear risk of bias; red = high risk of bias
Efficacy of intervention
A measurement of glycaemic control was reported as the primary outcome in 17 of 19 included studies. We summarise all primary outcomes in Table 2.
Table 2.
Summary of main outcomes of all included studies
| Author, year | Main outcome | Result |
|---|---|---|
| Meier, 2004 [29] | GLP-1 IV lowered mean glucose levels | + |
| Sokos, 2007 [34] | GLP-1 IV reduced peri-operative glucose levels | + |
| Müssig, 2008 [31] | GLP-1 IV reduced insulin administration with comparable glycaemic control | + |
| Deane, 2009 [21] | GLP-1 IV lowered mean post-prandial glucose levels | + |
| Deane, 2010 [22] | GLP-1 IV lowered mean post-prandial glucose levels | + |
| Deane, 2011 [23] | GLP-1 IV lowered mean post-prandial glucose levels | + |
| Galiatsatos, 2014 [24] | GLP-1 IV did not lower mean glucose levels | – |
| Kohl, 2014 [27] | GLP-1 IV lowered mean glucose levels | + |
| Miller, 2017 [30] | GLP-1 IV reduced intestinal glucose absorption | + |
| Kar, 2015 [26] | GIP IV did not lower mean glucose levels | – |
| Lee, 2013 [28] | GIP IV did not lower mean glucose levels | – |
| Polderman, 2018 [18] | Liraglutide SC reduced post-operative glucose levels | + |
| Holmberg, 2014 [25] | Exenatide IV did not lower post-operative cardiac enzymes | – |
| Besch, 2017 [19] | Exenatide IV did not increase number of patient that spend > 50% in target range | – |
| Lipš, 2017 [17] | Exenatide IV did not improve left ventricular ejection fraction | – |
| Garg, 2017 [35] | Saxagliptin PO resulted in similar glucose levels compared with basal bolus insulin | + |
| Pasquel, 2017 [32] | Sitagliptin PO as adjunct to basal insulin resulted in similar glucose levels compared with bolus insulin | + |
| Umpierrez, 2014 [33] | Sitagliptin PO resulted in similar glucose levels compared with basal bolus insulin | – |
| Brackbill, 2012 [20] | Sitagliptin PO did not lower the mean postoperative glucose levels | – |
Abbreviations: GIP Gastric inhibitory polypeptide, GLP-1 Glucagon-like peptide-1, IV Intravenously, PO By mouth, SC Subcutaneous
+ = study positive for primary outcome, − = study negative for primary outcome
Intra-operative glucose lowering
A number of studies assessed the effect of GLP-1 receptor stimulation as an adjunct to standard insulin therapy during cardiac surgery. The first of these randomised 20 patients to a continuous intravenous infusion of GLP-1 (1.5 pmol kg− 1 min− 1) or placebo, commencing 12 h pre-operatively and continuing for 48 h post-operatively. GLP-1 resulted in lower mean glucose in the pre- and peri-operative periods, with nearly half the insulin administered to achieve comparable glycaemic control in the post-operative periods [34]. In 77 patients undergoing elective cardiac surgery, using the same dose of intravenous GLP-1 infused intra-operatively, Kohl and colleagues reported that mean blood glucose values were 0.68 mmol L− 1 lower for subjects receiving GLP-1 compared with those receiving placebo (95% CI, 0.13–1.22 mmol L− 1; P = 0.015) [27]. Lipš and colleagues randomised 38 patients with decreased left ventricular function undergoing coronary artery bypass grafting (CABG) to a 72-h infusion of intravenous exenatide (20 ng min− 1) or placebo as an adjuvant to standard insulin therapy [17]. Patients receiving exenatide demonstrated lower peri-operative mean blood glucose (6.4 ± 0.5 vs. 7.3 ± 0.8 mmol/L; P < 0.001) and a greater percentage of time in the target range of 4.5–6.5 mmol/L (54.8% ± 14.5% vs. 38.6% ± 14.4%; P = 0.001). In a similar study of 104 patients undergoing elective CABG, Besch and colleagues did not observe a statistical difference in the glycaemic outcome of interest (time in target range) between intravenous exenatide (25 ng min− 1) and placebo; however, exenatide was insulin-sparing with a longer time to commencement of insulin and significantly less insulin administered [19]. Polderman and colleagues compared pre- and intra-operative subcutaneous liraglutide (0.6 mg + 1.2 mg) (a GLP-1 receptor agonist) with an intravenous glucose-insulin-potassium infusion and an insulin bolus regimen [18]. Median plasma glucose 1 h post-operatively was lower in the liraglutide group (6.6 mmol L− 1) than in both the continuous insulin infusion (7.5 mmol L− 1) and insulin bolus (7.6 mmol L− 1) groups (P = 0.015). In this study, liraglutide showed an insulin-sparing effect, with fewer episodes of insulin administration and reduced total insulin administration.
Post-operative glucose lowering
In their vanguard study, Meier and colleagues randomised eight patients with T2DM who had undergone major surgery within the preceding week to 8-h infusions of intravenous GLP-1 (1.2 pmol kg− 1 min− 1) and placebo in a cross-over fashion [29]. GLP-1 ‘normalised’ blood glucose (fasting < 7 mmol/L) in the cohort within 150 min, whereas patients remained hyperglycaemic (> 8 mmol/L) in the control arm [29]. In a further study of post-operative glycaemic control in T2DM, Müssig and colleagues randomised patients to GLP-1 (3.6 pmol kg− 1 min− 1) or standard intravenous insulin in the 12 h following CABG [31]. Glycaemic control was comparable between groups; however, the GLP-1 cohort had significantly less insulin administered during the first 6 h following surgery [31].
Studies assessing the efficacy of the oral DPP-IV inhibitor sitagliptin for post-operative glycaemic control in patients with T2DM have reported varied results. In the study by Brackbill and colleagues the post-CABG addition of sitagliptin (100 mg once daily) to standard subcutaneous basal insulin and regular oral hypoglycaemic agents did not result in any difference in glycaemia or insulin administration [20]. Two related studies on the ward, one [33] a pilot preceding a larger trial [32], which included both medical and surgical patients (Table 1), assessed sitagliptin (100 mg once daily) as an adjunct to a basal insulin when compared with a standard basal bolus insulin regimen. The primary outcome of the larger trial was non-inferiority of mean blood glucose. Sitagliptin group was non-inferior to standard care and was associated with less total daily insulin requirement (24 ± 16 U/d vs. 34 ± 20 U/d; P < 0.001) [32]. Garg and colleagues compared the oral DPP-IV inhibitor saxagliptin (5 mg once daily) with basal bolus insulin in a non-critically ill population of hospitalised patients with T2DM, predominantly in the post-operative period [35]. Saxagliptin was non-inferior to basal bolus insulin for glycaemic control as determined by the daily mean blood glucose (primary outcome), with saxagliptin treatment causing less glycaemic variability [35].
Intensive care unit
Deane and colleagues have assessed continuous intravenous infusions of GLP-1 in a series of cross-over trials in heterogeneous cohorts of mechanically ventilated patients [21–23, 30]. At a dose of 1.2 pmol kg− 1 min− 1 infused over 270 to 330 min, GLP-1 reduced the glycaemic response to small intestinal nutrient delivery in patients with T2DM [23] and to intra-gastric and small intestinal nutrient delivery in patients not known to have T2DM [21, 22, 30]. Enteral nutrient-stimulated hyperglycaemia was attenuated but not suppressed completely at this dose, with the glucose-lowering effect more prominent in those patients without a history of diabetes. This group also evaluated the glycaemic effect of intravenous infusions of GIP during intra-gastric and small intestinal nutrient administration in mechanically ventilated patients, and, in contrast to the profound glucose-lowering effect of GIP in health, they reported no glucose-lowering effect when GIP was given as stand-alone therapy or added to GLP-1 [26, 28]. Galiatsatos and colleagues compared an extended intravenous GLP-1 infusion (1.5 pmol kg− 1 min− 1 for 72 h) with placebo as an adjunct to intensive insulin therapy in critically ill surgical patients. They reported no difference in mean blood glucose or insulin use between groups, but substantially less glycaemic variability (given by the co-efficient of variation of mean glucose) was observed in the GLP-1 cohort [24].
Hypoglycaemia
Data regarding hypoglycaemia are summarised in Table 3. The threshold to diagnose moderate hypoglycaemia ranged from < 2.8 to < 4.0 mmol/L. The incidence of moderate hypoglycaemia in the incretin arm varied from zero to 17%, except for one outlier with a reported incidence of 36% (8 of 23 patients) [25]. In the latter trial intravenous exenatide was infused at double the dose of subsequent trials, and it is unclear whether insulin was concurrently administered [25]. Meta-analysis revealed no difference in incidence of hypoglycaemia (incretin-based therapy 36 of 484 [7.4%] vs. comparator 36 of 540 [6.7%], P = 0.96). Of note, incretin-based therapies were administered with insulin in 10 of the 14 studies reporting hypoglycaemia (Table 1).
Table 3.
Analysis of hypoglycaemia in reported studies
| Author, year | Threshold to define hypoglycaemia | Incretin | Comparator | Weight | Odds ratio M-H, random, 95% CI |
P value | Odds ratio M-H, random, 95% CI |
||
|---|---|---|---|---|---|---|---|---|---|
| n | group | n | group | ||||||
| Besch, 2017 [19] | 3.3 mmol L− 1 | 2 | 53 | 1 | 51 | 8.1% | 1.96 [0.17, 22.32] | 0.58 |

|
| Brackbill, 2012 [20] | 3.3 mmol L− 1 | 5 | 30 | 2 | 32 | 12.9% | 3.00 [0.54, 16.81] | 0.06 | |
| Deane, 2010 [22] | 3.0 mmol L− 1 | 0 | 25 | 0 | 25 | Not estimable | 1 | ||
| Galiatsatos, 2014 [24] | 2.8 mmol L− 1 | 1 | 9 | 3 | 9 | 7.8% | 0.25 [0.02, 3.04] | 0.58 | |
| Garg, 2017 [35] | 3.9 mmol L− 1 | 1 | 33 | 1 | 33 | 5.7% | 1.00 [0.06, 16.69] | 1 | |
| Holmberg, 2014 [25] | 4.0 mmol L− 1 | 8 | 21 | 0 | 41 | 6.1% | 52.26 [2.83, 966.6] | 0.003 | |
| Kar, 2015 [26] | Not stated | 0 | 24 | 0 | 24 | Not estimable | 1 | ||
| Kohl, 2014 [27] | 3.8 mmol L− 1 | 0 | 37 | 0 | 40 | Not estimable | 1 | ||
| Lipš, 2017 [17] | 3.3 mmol L− 1 | 2 | 19 | 4 | 19 | 11.9% | 0.44 [0.07, 2.76] | 0.12 | |
| Meier, 2004 [29] | 4.0 mmol L−1 | 0 | 8 | 0 | 8 | Not estimable | 1 | ||
| Müssig, 2008 [31] | Not stated | 0 | 10 | 0 | 10 | Not estimable | 1 | ||
| Pasquel, 2017 [33] | 3.9 mmol L−1 | 13 | 138 | 17 | 139 | 24.7% | 0.75 [0.35, 1.60] | 0.45 | |
| Polderman, 2018 [18] | 4.0 mmol L−1 | 1 | 44 | 5 | 106 | 9.5% | 0.47 [0.05, 4.14] | 0.26 | |
| Sokos, 2007 [34] | 3.3 mmol L−1 | 1 | 10 | 2 | 10 | 7.4% | 0.44 [0.03, 5.88] | 0.39 | |
| Umpierrez, 2014 [33] | 3.9 mmol L−1 | 3 | 56 | 2 | 26 | 11.8% | 0.68 [0.11, 4.33] | 0.86 | |
| Total (95% CI) | 484 | 540 | 100% | 0.97 [0.47, 2.02] | 0.94 | ||||
| Total events | 37 | 37 | |||||||
M-H Mantel-Haenszel
Heterogeneity: Tau2 = 0.39, Chi2 = 12.94, df = 9 (P = 0.17); I2 = 30%
Test for overall effect: Z = 0.08 (P = 0.94)
Non-glycaemic effects
Owing to the heterogeneity of definitions and infrequency of reporting of non-glycaemic end-points, quantitative analysis of these data was not possible. Plasma insulin and glucagon concentrations were reported in eight studies [21–24, 26–28]. GLP-1 was reported to increase plasma insulin levels [23, 29] or insulin/glucose ratios [21, 22] in enterally fed critically ill and post-operative patients. However, this insulinotropic effect was not observed in studies that sampled blood intra-operatively in fasted patients [27, 34]. The effect of GLP-1 on glucagon concentration was similarly heterogeneous, with several studies reporting a glucagonostatic effect [24, 29, 34] and others reporting no difference [21, 22, 27]. The addition of GIP to a GLP-1 regimen in critically ill patients did not have an additional insulinotropic effect [28], and GIP as a sole agent was not shown to have an effect on plasma insulin or glucagon concentrations in critically ill patients [26].
In the critically ill, GLP-1 slows gastric emptying when emptying is relatively normal, but it appears to have minimal effect when emptying is already delayed [22], whereas GIP appears to have no effect on gastric motility [26]. Similarly, GLP-1 delayed enteral glucose absorption, even when nutrient was delivered directly into the small intestine [23, 30], whereas GIP had no effect [26].
Five studies compared the cardiovascular effects of GLP-1 or a GLP-1 receptor agonist with placebo [17, 24, 25, 31, 34]. In these studies there were no differences in cardiac enzymes [17, 25], echocardiographic measurements of left ventricular function [17, 34], haemodynamic parameters (heart rate, mean arterial pressure, pulmonary artery diastolic pressure) [31, 34] or vasoactive medication requirement [17, 24, 25, 31].
There was no difference in the incidence of post-operative nausea and vomiting in studies comparing placebo with intravenous exenatide [19], oral sitagliptin [32] and subcutaneous liraglutide [18]. However, pre-operative nausea was more common when subcutaneous liraglutide was administered the night before surgery (13% vs. 0%, P = 0.007, n = 150) [18]. Incretin-based therapies have not been reported to increase post-operative complications or serious adverse events [17–19, 25, 32].
Diabetes mellitus
Eight studies were performed exclusively in patients with T2DM [18, 20, 23, 29, 31–33, 35], five studies in patients without T2DM [21, 22, 26, 28, 30] and a further six studies in mixed cohorts of patients with and without T2DM (Table 1) [17, 19, 24, 25, 27, 34]. None of the studies recruiting mixed populations reported subgroup analyses according to diabetic status. Owing to the heterogeneity of interventions and outcomes, it was not possible to draw meaningful conclusions on the effects of incretins in patients with T2DM compared with those without.
Discussion
We systematically reviewed all randomised controlled trials of incretin-based interventions performed in the operating room and/or ICU setting and identified 19 studies which included 1410 patients in aggregate. Most studies reported a reduction in blood glucose or glycaemic variability when incretin-based therapies were used as a sole agent and/or a decrease in insulin administration when used as adjuvant therapy. Incretin-based therapies did not significantly reduce the incidence of hypoglycaemia. Incretin-based therapies did appear to attenuate glycaemic variability, although the latter was infrequently reported.
A number of studies attempted to delineate mechanisms underlying glucose-lowering in this cohort. The recognised insulinotropic effect of GLP-1 was consistently demonstrated in enterally fed patients, whereas glucagonostasis was less reliably reported. In small, single-centre studies, exogenous GLP-1 slowed gastric emptying in the setting of normal gastric motility and delayed intestinal glucose absorption, both of which likely contribute to attenuating nutrient stimulated hyperglycaemia [22, 30].
Although compliance with GLP-1 receptor agonists is relatively good in ambulant patients with T2DM, the primary reason for discontinuation of therapy is gastrointestinal discomfort, particularly nausea and vomiting [36, 37]. Critically ill and post-operative patients are at increased risk of nausea and vomiting, and it is therefore somewhat surprising that only three of the studies reported this side effect. Notwithstanding the relatively small number of patients studied, it is reassuring that incretin therapy did not appear to further increase the risk of post-operative nausea and vomiting.
Large trials in ambulant patients with T2DM have reported beneficial cardiovascular effects with GLP-1 receptor agonists [38–40]. This signal is supported by preliminary animal and observational human data identifying potential cardioprotective properties of incretin-based therapies [41, 42]. This provides a persuasive rationale for the use of GLP-1 in the setting of cardiac surgery. In murine models, GLP-1 decreases ischaemia-induced myocardial damage [41], and in patients with heart failure, exogenous GLP-1 has been associated with improvements in left ventricular ejection fraction, myocardial oxygen uptake and 6-min walk distance [42]. However, the most recent trial in patients with diabetes and heart failure observed no difference in time to death or rehospitalisation for heart failure [43]. None of the studies included in this review reported any differences in acute indices of cardiac performance between incretin-based therapies and control.
Strengths and limitations
Strengths of this systematic review include the structured search, complete retrieval of the identified research and validated methods in accordance with the PRISMA statement. However, there are some limitations. We found marked clinical heterogeneity between the studies, including the dose and type of incretin therapy and duration of intervention, ranging from 4 h to 10 days. In addition, there were substantial differences in the glycaemic control strategies of the control arms, ranging from blinded placebo to open-label intravenous insulin. The broad scope of this review revealed a marked heterogeneity in the populations studied, which included patients undergoing elective cardiac surgery, ward surgical patients and mechanically ventilated critically ill patients. Furthermore, there were trials performed exclusively in patients with pre-existing diabetes, whereas in other trials patients with pre-existing diabetes were excluded, and still others included both groups of patients. Inferences should therefore be circumspect because it is increasingly recognised that hyperglycaemia does not represent the same insult to all patients and may be modified by patients’ pre-morbid glycaemic control [44]. It should be noted, however, that the majority of included patients were diagnosed with DM. Although all of the studies assessed ‘glycaemic control’, there was substantial variation in the outcomes reported, such that meta-analysis was possible only on the variable of hypoglycaemia. Finally, most studies were small, single-centre trials and thus underpowered to detect differences in clinical and patient-centred outcomes and safety end-points.
Future directions
Taken together, these data signal the potential for incretin-based therapies, particularly GLP-1-based regimens, as effective glucose-lowering agents with a relatively low incidence of hypoglycaemia. However, owing to the limitations of the original studies, it is not possible to draw definitive conclusions regarding the role of incretin therapies in the operating room and ICU. Future studies are required to determine (1) the population most likely to benefit; (2) optimal dosing regimens, including the role for combination therapy with insulin; and (3) clinical efficacy and safety outcomes.
Conclusions
Incretin-based therapies represent a promising, novel approach to glucose control in the peri-operative period and during critical illness, with a low risk of hypoglycaemia. Further studies with larger sample sizes [45] are required to determine the optimal agent and dosing regimen and effects on patient-centred outcomes.
Additional file
Methodology of systematic review on incretins in peri-operative and intensive care (PDF 88 kb)
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on request.
Abbreviations
- CABG
Coronary artery bypass grafting
- CV
Cardiovascular
- DM
Diabetes mellitus
- DPP-IV
Dipeptidyl peptidase IV
- FFA
Free fatty acids
- GIK
Glucose-insulin-potassium infusion
- GIP
Glucose-dependent insulinotropic peptide
- GLP-1
Glucagon-like peptide 1
- ICU
Intensive care unit
- IV
Intravenously
- LoS
Length of stay
- LVEF
Left ventricular ejection fraction
- PO
By mouth
- RIPC
Remote ischaemic preconditioning
- SC
Subcutaneous
- T2DM
Type 2 diabetes mellitus
Authors’ contributions
AHH, MPP and JH were responsible for the data collection, data input, study design, analysis and drafting of the manuscript. MWH, JHD, BP, AMD and JH were responsible for critical review, revisions and editorial assistance. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
AHH, BP and JH receive ongoing research support from Novo Nordisk.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abraham H Hulst, Email: a.h.hulst@amc.uva.nl.
Mark P Plummer, Email: mark.philip.plummer@gmail.com.
Markus W Hollmann, Email: m.w.hollmann@amc.uva.nl.
J Hans DeVries, Email: j.h.devries@amc.uva.nl.
Benedikt Preckel, Phone: +31 20 5669111, Email: b.preckel@amc.uva.nl.
Adam M Deane, Email: adam.m.deane@gmail.com.
Jeroen Hermanides, Email: j.hermanides@amc.uva.nl.
References
- 1.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 2.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35:2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 3.Krinsley JS, Egi M, Kiss A, Devendra AN, Schuetz P, Maurer PM, et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care. 2013;17:R37. doi: 10.1186/cc12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Diabetes care in the hospital. Diabetes Care. 2016;39(Suppl 1):S99–104. doi: 10.2337/dc16-S016. [DOI] [PubMed] [Google Scholar]
- 5.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85:217–224. doi: 10.4065/mcp.2009.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krinsley JS, Schultz MJ, Spronk PE, Harmsen RE, van Braam Houckgeest F, van der Sluijs JP, et al. Mild hypoglycemia is independently associated with increased mortality in the critically ill. Crit Care. 2011;15:R173. doi: 10.1186/cc10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali NA, O’Brien JM, Dungan K, Phillips G, Marsh CB, Lemeshow S, et al. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36:2316–2321. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plummer MP, Finnis ME, Horsfall M, Ly M, Kar P, Abdelhamid YA, et al. Prior exposure to hyperglycaemia attenuates the relationship between glycaemic variability during critical illness and mortality. Crit Care Resusc. 2016;18:189–197. [PubMed] [Google Scholar]
- 9.Hermanides J, Vriesendorp TM, Bosman RJ, Zandstra DF, Hoekstra JB, Devries JH. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38:838–842. doi: 10.1097/CCM.0b013e3181cc4be9. [DOI] [PubMed] [Google Scholar]
- 10.Polderman JAW, Hollmann MW, DeVries JH, Preckel B, Hermanides J. Perioperative hyperglycemia and glucose variability in gynecologic laparotomies. J Diabetes Sci Technol. 2016;10:145–150. doi: 10.1177/1932296815595985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plummer MP, Chapman MJ, Horowitz M, Deane AM. Incretins and the intensivist: what are they and what does an intensivist need to know about them? Crit Care. 2014;18:205. doi: 10.1186/cc13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deane AM, Jeppesen PB. Understanding incretins. Intensive Care Med. 2014;40:1751–1754. doi: 10.1007/s00134-014-3435-0. [DOI] [PubMed] [Google Scholar]
- 13.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429–442. doi: 10.1007/s00125-014-3460-0. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J. P. T., Altman D. G., Gotzsche P. C., Juni P., Moher D., Oxman A. D., Savovic J., Schulz K. F., Weeks L., Sterne J. A. C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928–d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipš M, Mráz M, Kloučková J, Kopecký P, Dobiáš M, Křížová J, et al. The effect of continuous exenatide infusion on cardiac function and perioperative glucose control in cardiac surgery patients: a single-blind, randomized, controlled trial. Diabetes Obes Metab. 2017;19:1818–1822. doi: 10.1111/dom.13029. [DOI] [PubMed] [Google Scholar]
- 18.Polderman JAW, Van Steen SCJ, Thiel B, Godfried MB, Houweling PL, Hollmann MW, et al. Peri-operative management of patients with type-2 diabetes mellitus undergoing non-cardiac surgery using liraglutide, glucose–insulin–potassium infusion or intravenous insulin bolus regimens: a randomised controlled trial. Anaesthesia. 2018;73:332–339. doi: 10.1111/anae.14180. [DOI] [PubMed] [Google Scholar]
- 19.Besch G, Perrotti A, Mauny F, Puyraveau M, Baltres M, Flicoteaux G, et al. Clinical effectiveness of intravenous exenatide infusion in perioperative glycemic control after coronary artery bypass graft surgery. Anesthesiology. 2017;127:775–787. doi: 10.1097/ALN.0000000000001838. [DOI] [PubMed] [Google Scholar]
- 20.Brackbill ML, Rahman A, Sandy JS, Stam MD, Harralson AF. Adjunctive sitagliptin therapy in postoperative cardiac surgery patients: a pilot study. Int J Endocrinol. 2012;2012:1–6. doi: 10.1155/2012/810926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deane AM, Chapman MJ, Fraser RJL, Burgstad CM, Besanko LK, Horowitz M. The effect of exogenous glucagon-like peptide-1 on the glycaemic response to small intestinal nutrient in the critically ill: a randomised double-blind placebo-controlled cross over study. Crit Care. 2009;13:R67. doi: 10.1186/cc7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deane AM, Chapman MJ, Fraser RJL, Summers MJ, Zaknic AV, Storey JP, et al. Effects of exogenous glucagon-like peptide-1 on gastric emptying and glucose absorption in the critically ill: relationship to glycemia. Crit Care Med. 2010;38:1261–1269. doi: 10.1097/CCM.0b013e3181d9d87a. [DOI] [PubMed] [Google Scholar]
- 23.Deane AM, Summers MJ, Zaknic A, Chapman MJ, Fraser RJL, Di Bartolomeo AE, et al. Exogenous glucagon-like peptide-1 attenuates the glycaemic response to postpyloric nutrient infusion in critically ill patients with type-2 diabetes. Crit Care. 2011;15:R35. doi: 10.1186/cc9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galiatsatos P, Gibson BR, Rabiee A, Carlson O, Egan JM, Shannon RP, et al. The glucoregulatory benefits of glucagon-like peptide-1 (7-36) amide infusion during intensive insulin therapy in critically ill surgical patients: a pilot study. Crit Care Med. 2014;42:638–645. doi: 10.1097/CCM.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmberg FEO, Ottas KA, Andreasen C, Perko MJ, Møller CH, Engstrøm T, et al. Conditioning techniques and ischemic reperfusion injury in relation to on-pump cardiac surgery. Scand Cardiovasc J. 2014;48:241–248. doi: 10.3109/14017431.2014.923930. [DOI] [PubMed] [Google Scholar]
- 26.Kar P, Cousins CE, Annink CE, Jones KL, Chapman MJ, Meier JJ, et al. Effects of glucose-dependent insulinotropic polypeptide on gastric emptying, glycaemia and insulinaemia during critical illness: a prospective, double blind, randomised, crossover study. Crit Care. 2015;19:20. doi: 10.1186/s13054-014-0718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohl BA, Hammond MS, Cucchiara AJ, Ochroch EA. Intravenous GLP-1 (7-36) amide for prevention of hyperglycemia during cardiac surgery: a randomized, double-blind, placebo-controlled study. J Cardiothorac Vasc Anesth. 2014;28:618–625. doi: 10.1053/j.jvca.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Lee MY, Fraser JD, Chapman MJ, Sundararajan K, Umapathysivam MM, Summer MJ, et al. The effect of exogenous glucose- dependent insulinotropic polypeptide in combination with glucagon-like peptide-1 on glycemia in the critically ill. Diabetes Care. 2013;36:3333–3336. doi: 10.2337/dc13-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meier JJ, Weyhe D, Michaely M, Senkal M, Zumtobel V, M a N, et al. Intravenous glucagon-like peptide 1 normalizes blood glucose after major surgery in patients with type 2 diabetes. Crit Care Med. 2004;32:848–851. doi: 10.1097/01.CCM.0000114811.60629.B5. [DOI] [PubMed] [Google Scholar]
- 30.Miller A, Deane AM, Plummer MP, Cousins CE, Chapple LAS, Horowitz M, et al. Exogenous glucagon-like peptide-1 attenuates glucose absorption and reduces blood glucose concentration after small intestinal glucose delivery in critical illness. Crit Care Resusc. 2017;19:37–42. [PubMed] [Google Scholar]
- 31.Müssig K, Oncü A, Lindauer P, Heininger A, Aebert H, Unertl K, et al. Effects of intravenous glucagon-like peptide-1 on glucose control and hemodynamics after coronary artery bypass surgery in patients with type 2 diabetes. Am J Cardiol. 2008;102:646–647. doi: 10.1016/j.amjcard.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Pasquel FJ, Gianchandani R, Rubin DJ, Dungan KM, Anzola I, Gomez PC, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial. Lancet Diabetes Endocrinol. 2017;5:125–133. doi: 10.1016/S2213-8587(16)30402-8. [DOI] [PubMed] [Google Scholar]
- 33.Umpierrez GE, Gianchandani R, Smiley D, Jacobs S, Wesorick D, Newton C, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes. J Clin Endocrinol Metab. 2014;99:3430–3435. doi: 10.2337/dc13-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sokos GG, Bolukoglu H, German J, Hentosz T, Magovern GJ, Maher TD, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 35.Garg R, Schuman B, Hurwitz S, Metzger C, Bhandari S. Safety and efficacy of saxagliptin for glycemic control in non-critically ill hospitalized patients. BMJ Open Diabetes Res Care. 2017;5:e000394. doi: 10.1136/bmjdrc-2017-000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vilsboll T., Christensen M., Junker A. E., Knop F. K., Gluud L. L. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344(jan10 2):d7771–d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sikirica M, Martin A, Wood R, Leith A, Piercy J, Higgins V. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes Targets Ther. 2017;10:403–412. doi: 10.2147/DMSO.S141235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 40.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 42.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 43.Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction. JAMA. 2016;316:500–508. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plummer MP, Bellomo R, Cousins CE, Annink CE, Sundararajan K, Reddi BAJ, et al. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med. 2014;40:973–980. doi: 10.1007/s00134-014-3287-7. [DOI] [PubMed] [Google Scholar]
- 45.Hulst AH, Visscher MJ, Godfried MB, Thiel B, Gerritse BM, Scohy TV, et al. Study protocol of the randomised placebo-controlled GLOBE trial: GLP-1 for bridging of hyperglycaemia during cardiac surgery. BMJ Open. 2018;8:e022189. doi: 10.1136/bmjopen-2018-022189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methodology of systematic review on incretins in peri-operative and intensive care (PDF 88 kb)