Abstract
Many questions about the long-term effects of combination antiretroviral therapy (cART) on clinical outcomes in people living with HIV (PLWH) and their impact on health systems remain unanswered. The Collaboration of Observational HIV Epidemiological Research Europe (COHERE) was formed in 2005 to pool and harmonize existing longitudinal data on people living with HIV in Europe, to answer key research questions that could not be addressed adequately by individual cohorts. Key research questions include long-term prognosis, rare outcomes, and variations across patient groups, settings and health systems. COHERE uses the HIV Cohorts Data Exchange Protocol, a standardized and validated method of data structure and transfer, to compile data from over 40 cohorts of PLWH residing in Europe, representing 331 481 individuals, including 2808 children (<13), representing 2 135 896 person-years of follow-up. COHERE compiles data on clinical characteristics, antiretroviral therapy and other medications, HIV seroconversion, opportunistic infections, laboratory results and socio demographic data. External collaborators interested in conducting a project in COHERE should submit a project proposal to the Regional Coordinating Centres in Bordeaux and Copenhagen for review by COHERE’s governing bodies (see www.cohere.org for further information).
Why was COHERE set up?
Widespread access to effective combination antiretroviral therapy (cART), beginning in 1996, dramatically reduced the number of AIDS-related events and deaths in people living with HIV (PLWH) in high-income settings.1 The study of prognosis and specific clinical outcomes therefore requires larger populations. The Collaboration of Observational HIV Epidemiological Research Europe (COHERE) was founded in 2005 to continue to advance epidemiological research on the prognosis of PLWH in Europe. COHERE has expanded and strengthened collaborative efforts in Europe and facilitated those with other regions by ensuring that longitudinal data, the product of early investments in clinic-based databases and observational studies, were compiled and harmonized. In 2011, COHERE joined three other European HIV collaborations, PENTA, EuroSIDA, and CASCADE to form “EuroCoord”, a Network of Excellence funded by the European Commission Seventh Framework Programme.2 COHERE also collaborates with the ART Cohort Collaboration (ART-CC) and the International Epidemiologic Databases to Evaluate AIDS (IeDEA) global network.3, 4
How does COHERE operate?
COHERE operates according to the principles set out in Box 1. Projects in COHERE provide added value by only addressing scientific questions that cannot be answered by participating cohorts.
Box 1. COHERE’s Principles.
COHERE should neither threaten nor compete with the scientific agendas of participating cohorts/cohort collaborations.
The individual contributing cohorts must express their interest in participating in COHERE.
The scientific questions addressed by COHERE are determined by consensus according to both their scientific relevance and originality (not being addressed elsewhere).
Individual cohorts may veto the use of their data in any new project.
Two Regional Coordinating Centres (RCCs), based at the University of Bordeaux’s Institut de Santé Publique, d’Épidémiologie et de Développement (ISPED) in Bordeaux, France and the Center for Health and Infectious Diseases Research (CHIP), Department of Infectious Diseases and Rheumatology, Rigshospitalet, in Copenhagen, Denmark, maintain COHERE’s infrastructure. The COHERE Steering Committee (SC) -- composed of representatives from the participating cohorts -- oversees the COHERE Collaboration, ensuring compliance with its principles; it also elects the Chair and the “Regional Representatives” to the COHERE Executive Committee (EC). The EC -- composed of three representatives from each of the two regions, and the two RCC Heads -- acts as the functional link between the RCCs and the SC.
COHERE projects are organized by “themes” (Prognosis and the effect of antiretroviral therapy (ART), Hepatitis, Opportunistic Infections, Malignancies, Late Presentation, and Socio-economic Inequalities) to encourage collaboration and streamline the project proposal process. Theme Leads stimulate scientific enquiry within their theme and develop projects. A detailed account of how COHERE operates is described in the Manual of Operations (www.cohere.org).
Who participates in COHERE?
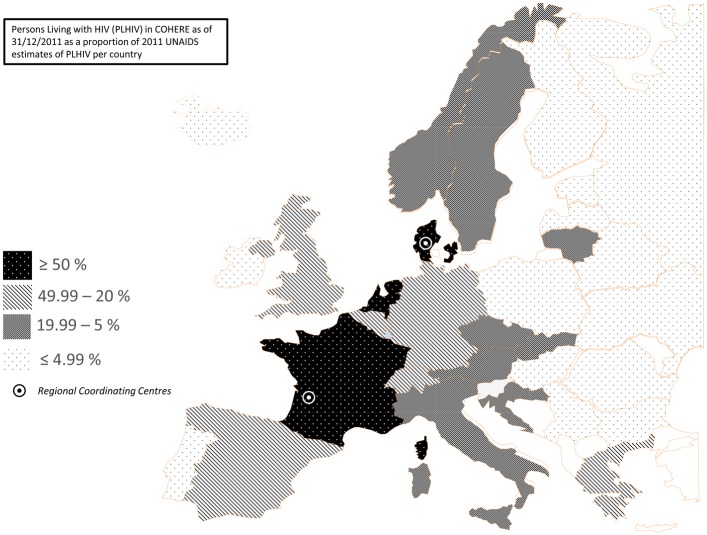
COHERE has grown from 33 cohorts in 2005 to 40 in 2015. COHERE initially approached cohorts because of their proven ability to address scientific questions and collect good quality data at clinical sites. As COHERE is a project-based collaboration, the data pooled in annual mergers depend on the projects included. Western European countries with longstanding national cohorts contribute a large proportion of person-years of follow-up, but there is an increasing number of individuals in care in Eastern Europe, primarily via the EuroSIDA network.5 Figure 1 presents the number of people living with HIV (excluding deaths) included in COHERE as of 31/12/2011 as a percentage of UNAIDS 2011 estimates of people living with HIV by country.
Figure 1.
COHERE includes both clinic/hospital-based cohorts of HIV-infected individuals, where data are extracted primarily from medical records in the context of routine care, and interval cohorts of specific populations of HIV-infected people, where data are collected at regular intervals that are unrelated to participants ongoing health care. Since people with HIV are seen regularly over a long period of time at a clinic/hospital, and are not just attending at times when they are symptomatic, the group of people seen at a given hospital naturally forms a cohort. Table 1a and 1b present a complete list of the cohorts, their characteristics and funding sources. All COHERE cohorts follow local ethical standards.
Table 1a.
Description of the cohort type, eligibility criteria, period of enrolment, and location of data collection of the adult or mixed (adult/paediatric) cohorts participating in COHERE (circa 2015)
| Cohort | Cohort Type | Eligibility criteria | Beginning of Enrollment | End of Enrollment | Data Collection | N Sites/ Countries | Location of sites |
|---|---|---|---|---|---|---|---|
| AHIVCOS | Hospital-based, Surveillance System | All HIV+ persons in care at 6/7 national centres | 1/1/1996 | Prospectively | 6 | Austria (National) | |
|
| |||||||
| AMACS | Clinic-based, Hospital-based | All HIV-1+ persons in at affiliated sites for at least 1 year, alive on 1/1/1996 | 1/1/1996 | Both prospectively and retrospectively | 13 | Greece (Regions : Attiki, Patras, Alexandroupolis) | |
|
| |||||||
| ANRS CO2 SEROCO | Interval cohort | HIV diagnosis <1 year before enrollment or a known date of infection identified by incomplete evokative Western-blot or an interval of less than 2 years between a negative and a positive ELISA | 1/1/1988 | 12/31/2009 | Prospectively | 25 | France (Paris area, Marseille, Nice) |
|
| |||||||
| ANRS CO3 AQUITAINE | Hospital-based | HIV-1+ >= age 13, seen at least once at a site & signed informed consent | 1/1/1987 | Prospectively | 13 | France (Region : Aquitaine) | |
|
| |||||||
| ANRS CO4 FHDH | Hospital-based | HIV-1 or HIV-2+ individuals who have provided written informed consent | 1/1/1989 | 12/31/2065 | Prospectively | 70 | France (National, except Aquitaine) |
|
| |||||||
| ANRS CO6 PRIMO | Interval cohort | HIV 1+ patients presenting during primary infection in sites | 1/1/1996 | Prospectively | 80 | France (National) | |
|
| |||||||
| ANRS CO8 APROCO-COPILOTE | Hospital-based | HIV-infected patients >= 18 years old, naive for protease inhibitors | 1/4/1997 | 6/6/1999 | Prospectively | 49 | France (National) |
|
| |||||||
| ANRS CO13 HEPAVIH | Hospital-based | Phase I : [12/2005, 12/2008]: HIV-1/HCV chronically infected >= age 18, Phase II : [9/2011, 3/2016]: HIV-1/HCV chronically infected >= age 18, beginning anti- HCV treatment comprising Telaprevir or Boceprevir, or having cleared HCV spontaneously in the absence of anti-HCV treatment. Phase III : [Q1/2014, Q1/2016] : Individuals who have received, are receiving, or will receive within the next 6 months combination therapy with new anti-HCV drugs, with or without peginterferon and/or ribavirin (temporary authorization, full marketing authorization, or within clinical trials) & individuals previously included in ANRS clinical trials evaluating new anti-HCV drugs |
11/10/2005 | Prospectively generally, and Prospective & Retrospective for Phase III | 27 | France (National) | |
|
| |||||||
| ATHENA | Clinic-based | Any HIV+ person entering care in one of the 27 adult (28 including Curacao) or 4 pediatric HIV treatment centers in the Netherlands and who does not object to standardized collection of data obtained as part of routine care | 1/1/1998 | Both prospectively and retrospectively | 31 | The Netherlands, Curacao (National) | |
|
| |||||||
| Bonn-Cologne Cohort | Clinic-based, Hospital-based | HIV+ | 1/5/1988 | Both prospectively and retrospectively | 2 | Germany (Bonn, Cologne) | |
|
| |||||||
| CASCADE | Clinic-based, Hospital-based | HIV+ with well estimated dates of HIV seroconversion | 1/1/1979 | Both prospectively and retrospectively | - | 11 countries* | |
|
| |||||||
| Clinserv | Clinic-based, Hospital-based | All patients HIV+ presenting at the clinical sites after 0/0/1999 | 1/1/1999 | Both prospectively and retrospectively | 18 | Germany | |
|
| |||||||
| CoRIS | Clinic-based | Confirmed HIV+, cART-naive, attending participating sitesl aged >16 & signed informed consent | 1/1/2004 | Prospectively | 37 | Spain (Multi-region) | |
| CoRIS-MD | Clinic-based | HIV+ | 1/1/1997 | 12/31/2003 | Retrospectively | 10 | Spain (Multi-region) |
|
| |||||||
| DHK | Hospital-based | HIV+ & in care in HIV treatment centres | 1/1/1995 | Prospectively | 8 | Denmark (National) | |
|
| |||||||
| ECS | Clinic-based, Hospital-based | Pregnant HIV+ women, diagnosed before or during pregnancy or as a result of HIV testing intrapartum, delivering liveborn infant. | 7/15/1985 | Prospectively | 10 countries | 10 countries^ | |
|
| |||||||
| EuroSIDA | Clinic-based, Hospital-based, Interval cohort | Aged > 16 & prebooked hospital appointment | 1/6/1994 | Both prospectively and retrospectively | 109 sites, 34 countries | 34 countries^^ | |
|
| |||||||
| Frankfurt | Clinic-based | HIV+ > age 16 in care at affiliated sites | 1/1/1987 | Prospectively | 5 | Germany (Frankfurt) | |
|
| |||||||
| Gemes Haemo | Clinic-based, Surveillance System | HIV+ haemophilics infected in early 1980s | 5/26/1999 | 4/26/1999 | Both prospectively and retrospectively | 2 | Spain (Madrid, Barcelona) |
|
| |||||||
| Georgian National HIV Cohort | Clinic-based, Hospital-based, Surveillance System | HIV+ adults | 1/1/2007 | Both prospectively and retrospectively | 1 | Georgia | |
|
| |||||||
| ICC (INMI Clinical Cohort) | Clinic-based, Hospital-based | HIV+ individuals in care at site | 1/1/1995 | Prospectively | 1 | Italy (Rome) | |
|
| |||||||
| ICONA | Hospital-based | HIV+, ART-naive, >= age 18 & signed informed consent | 1/1/1997 | Prospectively | 42 | Italy | |
|
| |||||||
| Infectious Disease Database (IDD) San Raffaele | Hospital-based | All HIV+ patients in care at site | 1/1/1991 | Prospectively | 1 | Italy (Milan) | |
|
| |||||||
| Italian MASTER Cohort | Clinic-based | HIV-1 or HIV-2+ (antibody test or positive HIV RNA) in care in participating sites | 1/1/1997 | Both prospectively and retrospectively | 8 | Italy (multi-city) | |
|
| |||||||
| Modena | Clinic-based | All new HIV diagnosis in adult patients (>= 18 year) since 1985, inhabitant in Province of Modena and reffered to Regional Surveillance System. Data were retrospectively collected from 1992. | 1/1/1992 | 12/31/2014 | Retrospectively | 1 | Italy (Modena) |
|
| |||||||
| PISCIS | Clinic-based, Hospital-based | HIV+ >= age 16 in care at one of the sites after January 1st 1998, irrespective of the stage of disease or degree of immunosuppression. | 1/1/1998 | Both prospectively and retrospectively | 11 | Spain (Region : Catalonia) | |
|
| |||||||
| SHCS | Clinic-based, Hospital-based | Any HIV+ persons >= age 18 | 1/1/1988 | Prospectively | 7 | Switzerland (National) | |
|
| |||||||
| St Pierre | Clinic-based, Hospital-based | HIV+ & at least one visit at affiliated sites | 1/25/1980 | Prospectively | 1 | Belgium (Brussels) | |
|
| |||||||
| Swedish InfCare HIV cohort | Clinic-based, Hospital-based, Surveillance System | All HIV+ persons in care in Sweden, opt out system | 1/1/1983 | Both prospectively and retrospectively | 29 | Sweden (National) | |
|
| |||||||
| UK Collaborative HIV Cohort Study (UK CHIC) | Clinic-based | HIV+ persons > age 16 & <= 1 visit at affilitated site after 1/1/1995 | 1/1/2001 | Both prospectively and retrospectively | 19 | United Kingdom (National) | |
|
| |||||||
| VACH | Hospital-based | HIV+ persons > age 16 & 1st visit at affiliated site. | 1/1/1997 | Prospectively | 23 | Spain | |
Austria, France, Germany, Greece, Italy, Netherlands, Norway, Spain (Badalona, Barcelona, Madrid Valencia), Sweden, Switzerland, United Kingdom
Belgium, Denmark, Germany, Netherlands, Poland, Italy, Spain, Sweden, Ukraine, United Kingdom
Argentina, Austria, Belarus, Belgium, Bosnia-Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Georgia, Greece, Hungary, Iceland, Ireland, Israel, Italy, Latvia, Lithuania, Luxembourg,Netherlands, Norway, Poland, Portugal, Romania, Russia, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Ukraine, United Kingdom)
Table 1b.
Description of the cohort type, eligibility criteria, period of enrolment, and location of data collection of paediatric or adolescent cohorts participating in COHERE (circa 2015)
| Name of Cohort | Cohort Type | Eligibility Criteria | Enrolment period | Data Collection | N Sites/Countries | Location of sites |
|---|---|---|---|---|---|---|
| AALPHI | Clinic & community-based, Interval cohort | HIV+ age 13–21 and in paediatric care in UK, HIV-uninfected age 13–23, sibling of HIV-infected or has HIV+ parent | 06/01/2012–12/31/2014 | Prospectively | - | United Kingdom (Region: England) |
| ANRS CO10 EPF | Interval cohort | HIV+ children included at birth (born to HIV-pregnant women enrolled in the CO1-EPF cohort), or, since 2005, at time of first HIV care management in the clinical participating sites | 09/07/1985 - | Prospectively | 24 | France |
| CHIPS | Clinic-based, Hospital-based, Surveillance System | All HIV+ children living in the UK/Ireland | 04/01/2000- | Prospectively | - | United Kingdom (National), Ireland (National) |
| CoRISPE-cat | Hospital-based | HIV+ <18 years at diagnosis | 01/01/2008 - | Both prospectively and retrospectively | 13 | Spain (regions: Catalonia, Balearic Islands) |
| CoRISpeS-Madrid | Hospital-based | HIV+, infected before age 18 | 01/01/2002 - | Both prospectively and retrospectively | 59 | Spain (national) |
| KOMPNET Children Cohort | Clinic-based | HIV+, < 18 age | 06/01/2005 - | Both prospectively and retrospectively | 7 | Germany (National) |
| Madrid PMTCT Cohort | Hospital-based | HIV-1+ women during pregnnacy and their HIV-exposed infants | 1/2/0200 | Prospectively | 7 | Spain (Madrid) |
| NENEXP | Hospital-based | HIV+ pregnant women & HIV-exposed children, HAART-exposed children until 18 month-old age. | 01/01/2000 - | Prospectively | 12 | Spain (region: Catalonia) |
| NSHPC | Surveillance System | All pregnancies in HIV+ women living in the UK or Ireland, their exposed infants, and all children with HIV infection | 01/01/1990 - | Prospectively | 230 | United Kingdom & Ireland (National) |
| St Pierre Paediatric | Hospital-based | All HIV+ children/adolescents in care at site | 01/01/2000 - | Both prospectively and retrospectively | 1 | Beligum (Brussels) |
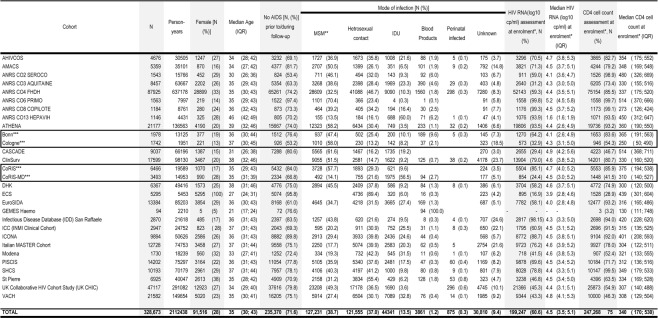
The 2014 merger included data from 331 481, including 2,808 children (<13), representing 2 135 896 person-years of follow-up. Table 2a highlights the demographic characteristics and prognostic markers of HIV in those aged 13 and older enrolled in adult cohorts. Approximately a quarter of the COHERE sample is female (27%). The median age at inclusion is 35 [IQR: 30, 43]. The primary mode of HIV transmission is sexual contact [homosexual/bisexual contact (38.7%), heterosexual contact (37%)], followed by injection drug use (IDU) (13.5%). Overall, 71.6% of the sample has never had a clinical AIDS diagnosis before or during enrolment. The median CD4 cell count at enrolment, defined as the period six months prior to and one month after enrolment date, was 340 cells/mm3 [IQR: 170, 530] in adults (Table 2a). Of those with an available CD4 cell counts at enrolment, 29% had <200 cells/mm3, 22% had between 200 and 350 cells/mm3, and 49% had >350 cells/mm3. Of those younger than age 13 (N=2808, representing 23 458), 93.7% were infected via vertical transmission and 73.4% had never had an AIDS diagnosis (Table 2b).
Table 2a.
Number of individuals, person-years of follow-up, patient demographics, prior AIDS status, mode of infection, median CD4 cell count and logarithm viral load in individuals older than 13 years old, (COHERE, 2014 merger)
Enrolment : measurements taken in the period six months prior to and one month after the variable “enrolment date”
Includes a small proportion of MSM+IDU
Cohorts who have merged administratively but which represent 'one cohort' insofar as COHERE governance
Table 2b.
Number of individuals, person-years of follow-up, patient demographics, prior AIDS status, mode of infection, median CD4 cell count and logarithm viral load in individuals enrolled in paediatric cohorts or enrolled in mixed cohorts and younger than 13 years old, (COHERE, 2014 merger
| Cohort | N | Person- Years | Female [N (%)] | Median Age (IQR) | No AIDS [N (%)] | HIV RNA (log10 cp/ml) assessment at enrolment*, N (%) | Median HIV RNA values (log10 cp/ml) at enrolment* (IQR) | CD4 cell count assessment at enrolment*, N (%) | Median CD4 values (cells/μl) at enrolment* (IQR) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||
| Sexual Contact | Blood products | Perinatal infected | Unknown | |||||||||||||||||||||
| ANRS CO10 EPF | 193 | 1583 | 112 | (58.0) | 0 | (0; 3) | 176 | (91.2) | 1 | (0.5) | 188 | (97.4) | 4 | (2.1) | 0 | - | - | - | 130 | (67) | 1535 | (708;2349) | ||
| ATHENA** | 254 | 1605 | 126 | (49.6) | 5 | (3; 8) | 204 | (80.3) | 5 | (2.0) | 235 | (92.5) | 14 | (5.5) | 241 | (95) | 2.5 | (1.7; 4.8) | 241 | (95) | 1000 | (620; 1550) | ||
| CHIPS | 1765 | 14793 | 914 | (51.8) | 4 | (0.7; 8) | 1281 | (72.6) | 33 | (1.9) | 1662 | (94.2) | 70 | (4.0) | 546 | (30) | 4.7 | (4.0; 5.4) | 618 | (35) | 553 | (254; 1000) | ||
| CORISPE-cat | 219 | 2834 | 129 | (58.9) | 1 | (0.3; 3) | 121 | (55.3) | 3 | (1.4) | 200 | (91.3) | 8 | (3.7) | 70 | (32) | 5.4 | (4.9; 5.9) | 79 | (36) | 1206 | (464; 2185) | ||
| CoRISpeS- Madrid | 318 | 2514 | 159 | (50.0) | 2 | (0.3; 5) | 237 | (74.5) | 3 | 0.9 | 5 | (1.6) | 294 | (92.5) | 16 | (5.0) | 235 | (73) | 5 | (4.3; 5.6) | 100 | (31) | 927 | (358; 1977) |
| KOMPNET Children Cohort | 59 | 129 | 28 | (47.5) | 8 | (4;11) | 49 | (83.1) | 53 | (89.8) | 6 | (10.2) | 44 | (75) | 1.7 | (1.7; 2.5) | 0 | - | - | - | ||||
|
| ||||||||||||||||||||||||
| TOTAL | 2808 | 23,458 | 1468 | (52.3) | 3 | (0.5; 8) | 1953 | (73.4) | 11 | 0.4 | 47 | (1.7) | 2632 | (93.7) | 118 | (4.2) | 1136 | (40) | 4.6 | (3.3; 5.4) | 1168 | (42) | 762 | (366;1425) |
Enrolment : measurements taken in the period six months prior to and one month after the variable “enrolment date”
Mixed (adult/paediatric) cohorts
How often have they been followed up?
As a consortium of cohorts comprising clinic and interval cohorts, patient follow-up varies. For clinic or hospital-based cohorts, average patient follow-up reflects current standards of care in those countries.
A derived measure of lost to follow-up (LTFU) was constructed by estimating the median last clinical encounter (defined as either visit and/or the date of last laboratory test) per active cohort. Those individuals who had not had a clinical encounter in the 18-months prior to this date were considered to be LTFU. Those who died during the same period were excluded. On average, 25% of the COHERE 2014 sample met this definition of LTFU, with variation between cohorts. LTFU in paediatric cohorts was estimated among those under age 17 as many cohorts discontinue follow-up at age 18. LTFU among paediatric patients aged <17 (N = 1,960) was 20% overall and ranged from 1.8–22% across cohorts.
What data are collected and how?
COHERE has benefited from dynamic data management processes, which have evolved to accommodate new projects and scientific questions. COHERE’s Data Managers (DMs) work with Project Leads and Statisticians to conduct preliminary surveys, feasibility studies and, occasionally, collect additional data. COHERE collects data on basic clinical information including: date of first HIV positive test, estimated date of seroconversion, cART and other medications, opportunistic infections, and laboratory results (CD4, CD8, plasma viral load values, hepatitis B and C serological tests, and HIV drug resistance tests), as well as socio-demographic data (see www.hicdep.org for more information about the definition of different variables). COHERE, via EuroCoord, conducts an inventory of data items and biological samples collected by participating cohorts. The submission of data to COHERE is facilitated by the use of the HIV Cohorts Data Exchange Protocol (HICDEP), a flexible data structure, developed in 2004, to guide the mapping of individual cohort data into a standard format to facilitate data merging.6
For approved projects, COHERE DMs organize data collection by developing a standardized operating procedure (SOP) for individual cohort DMs. Data are submitted in two stages via the HIV-Distributed Data Management (HIV-DDM) Tool.7 This implies that data submissions must therefore pass all format and edit checks, defined in HICDEP, before the submission can be completed. Additional inconsistencies are identified centrally. Cohorts are given an 8-week window to address said data inconsistencies before completing the second and final submission. Once data are merged, likely duplicate patient records between and within cohorts are identified using probability linkage. Data items used are gender, year of birth, treatment history, viral load measurements and CD4 cell counts. Duplicate records are reconciled based on prior agreements between participating cohorts. DMs identified and resolved 20,953 duplicate records in 2014. Cohorts resolve issues identified over time, ultimately improving data quality with each merger. To ensure transparency, the content of each merger together with cohort’s QA check feedback is summarized in a report. DMs extract data for projects based on specified and agreed eligibility criteria. After signing a data protection agreement, project leads are sent data extractions in a secure format.
What has been found?
Projects within the COHERE collaboration have led to the publication of 28 articles in peer-reviewed journals as of April, 2016, contributing high-quality evidence that has informed clinical and public health decision-making.
Prognosis and the effect of ART
The “Prognosis and the effect of ART” group focuses on clinical outcomes in patients treated with cART. The effect of age on the response to cART was studied in around 50,000 antiretroviral-naive individuals. Older individuals were characterized by low pre-ART CD4 cell counts, and experienced poorer immunological responses but better virologic responses, indicating those who are diagnosed or treated late are at increased risk of clinical events.8
Non-IDU HIV-infected individuals who achieved high CD4 cell counts after starting cART were found to have mortality patterns similar to those in the general population. Mortality was found to be persistently higher in individuals with a prior AIDS diagnosis 9, while the incidence of AIDS events continued to decline until CD4 cell counts were greater than 750 cells/mm3.10 In patients with viral suppression, the risk of a new AIDS events or death followed a CD4 cell count gradient, even benefiting those with a CD4 cell count ≥500 cells/mm3.11 Individuals who were virally suppressed on cART for more than three years but had incomplete CD4 cell recovery experienced substantially higher rates of mortality from both AIDS and non-AIDS causes, suggesting that these individuals should be monitored for diseases not conventionally considered HIV-related, especially non-AIDS defining cancers and liver diseases.12 Future research will focus on new markers of the risk of morbidity and cause-specific mortality, outcomes in individuals treated for many years, and outcomes in people aging with HIV, particularly in the context of multi-morbidity and polypharmacy.
The COHERE’s Pursuing Later Treatment Options (PLATO) II project looked at the rate of development of virologic failure in adults, adolescents and children. When virologic failure has occurred with at least two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), a non-nucleoside reverse transcriptase inhibitor (NNRTI) and a ritonavir-boosted protease inhibitor (PI), patients are said to have experienced triple class virologic failure (TCVF). Fewer than 9% of adult patients had experienced TCVF at year nine after starting cART.13 The risk of TCVF was somewhat higher in children and particularly higher in adolescents.14 Virologic suppression after TCVF was found to have increased from 20% in 2000 to 58% in 2009. Rates of AIDS and death also declined over time in people with TCVF.15 The incidence of TCVF in people on cART declined after 2008, and prevalence stabilized at around 2.5%.16 An approximately linear inverse relationship between log10 viral load and CD4 cell count in people with TCVF points to likely immunologic benefits of reducing viral load, even by modest amounts, without necessarily resulting in an undetectable viral load.17
Late Presentation
Late presentation is defined as an HIV-diagnosis with a CD4 cell count <350/mm3 or an AIDS diagnosis, regardless of CD4 cell count, within six months of HIV-diagnosis. This definition was applied to 84,524 PLWH presenting for care between January 1st, 2000 and June 30Th 2011 in Europe. Late presentation was present in over half (53.8%) of the sample. It decreased over time in both Central and Northern Europe among homosexual men and heterosexuals, but, in contrast, increased over time in Southern Europe among female heterosexuals and male IDUs and in Eastern Europe among IDUs. Late presentation was associated with increased mortality, especially in the first year after diagnosis, with significant variation across Europe.18 Further analyses study changes in late presentation within different regions and demographic groups since 2010.19 These findings have provided comprehensive evidence of patterns in late presentation in Europe and have informed discussions around earlier and more widespread testing for HIV and linkage to HIV care.20
Opportunistic Infections
Thirty per cent of HIV infected people either present late with an OI or are at significant risk of an OI.18 The COHERE OI group has described both the spectrum and incidence of OIs in patients on cART with high CD4 cell counts.10, 11 By including viral suppression as a cofactor, it was found that Pneumocystis jirovecii prophylaxis could safely be stopped in an additional 40% of patients when compared with guidelines based exclusively on CD4 cell counts,21 findings which informed both the American and European treatment guidelines 22, 23. The group is conducting similar analyses for toxoplasmosis and other OIs and intends to reassess the guidelines on the timing for discontinuing secondary prophylaxes against specific OIs.
While the early start of cART in the course of cryptococcal meningitis has been shown to be harmful in some clinical trials performed in resource-limited settings24, 25, in Western settings with advanced clinical monitoring this may not be the case.26 Current COHERE projects are examining the effect on mortality of the time of cART initiation after a diagnosis of cryptococcal meningitis or Toxoplasma gondii encephalitis. Preliminary results from a COHERE, NA-ACCORD and CNICS collaboration have shown that early cART did not increase mortality in AIDS patients with cryptococcal meningitis in high-income countries and overall mortality was lower than that reported by the clinical trials conducted in Africa.27 Current analyses explore how specific OIs influence long-term immune reconstitution, morbidity and mortality in the most recent cART era.
Malignancy
The COHERE malignancy group has focused on defining the incidence, risk factors and prognosis of HIV-associated cancers in the cART era, with a focus on systemic non-Hodgkin lymphoma (NHL) and primary brain lymphoma (PBL), Hodgkin’s lymphoma and, more recently, Kaposi’s sarcoma.28–30 The incidence of non-Hodgkin’s lymphoma, primary brain lymphoma and Kaposi’s sarcoma were substantially reduced in patients on cART, and timely initiation of therapy at high CD4 cell counts is important for preventing these malignancies.28, 30 In contrast, the incidence of Hodgkin’s lymphoma was not reduced by cART. Patients whose CD4 cell counts declined despite suppression of HIV-1 replication on cART were at increased risk of Hodgkin’s lymphoma.29 Comparative analyses are planned in collaboration with the African regions of IeDEA.4
Hepatitis
The immunological changes over the course of HCV treatment and their effect on mortality were estimated in 6,433 HIV-HCV-co-infected adults (≥16), 12% of whom had initiated HCV treatment (n=692 interferon and ribavirin; n=88 interferon alone). 31 CD4 cell counts decreased over the first 12 weeks but stabilized from week 24 onwards with no negative impact on mortality. The group is poised to monitor the effect of the introduction of direct-acting antiviral agents in co-infected patients.
Socio-economic inequalities
The Socio-economic inequalities group studies differences in key outcomes by sex, race/ethnicity, migrant status and educational level as a proxy for socioeconomic position. Even in European countries with universal health care systems, it has been documented that individuals with lower educational level do not benefit equally from timely cART initiation and have a poorer response to cART.32
Mortality in migrants has been found to be lower compared to native populations, which has been attributed to the “healthy migrant effect”. COHERE’s larger sample size has allowed this group to study mortality in men and women from multiple geographical origins separately, highlighting heterogeneity among migrant groups and revealing how certain groups are at an increased risk of mortality 33, work which was featured in the first issue of UNAIDS Science Now. The group plans to examine differences in cause-specific mortality by country of origin.
What are the main strengths and weaknesses?
COHERE in EuroCoord’s infrastructure is a unique research platform which has prompted collaborations both within and beyond Europe. EuroCoord’s cross-network work packages on data capture, HIV tuberculosis, migrant health and modelling and its interdisciplinary working groups (clinicians, virologists, epidemiologists, biostatisticians) have formalized this cross network collaboration and fostered intra-European capacity building. COHERE’s further investment in data harmonization (HICDEP) has benefitted other regional collaborations. Efforts to streamline data submission with the CASCADE and ART-CC cohort collaborations improved efficiency and reduced the workload of DMs. Finally, COHERE has provided excellent training opportunities for junior researchers (PhDs and Fellowships).
COHERE’s greatest strength is its size, enabling stratification of subgroups of interest 8 (e.g. across 10 age groups and sex-stratification) and the study of uncommon outcomes.28 The robustness of COHERE’s findings transcends Europe, benefiting the global HIV-patient community. COHERE data are highly representative of those in care in countries with large regional and national cohorts. Such cohorts enable COHERE to monitor trends across countries. However, adequate representation of marginalized groups such as migrant populations has become a challenge as a consequence of informed consent requirements in some countries as well as barriers to accessing care.34
Although COHERE uses HICDEP, the heterogeneity in data quality remains an ongoing challenge. In collaboration with ART-CC 3, COHERE has been an ideal platform for harmonizing the collection and validation of causes of deaths in HIV-1 infected individuals.
With a growing proportion of deaths now caused by non-AIDS events, accurately monitoring causes of death is critical to identify trends and evaluate risk factors. The progressive implementation of the “Coding Causes of Death in HIV” Protocol (CoDe), a uniform classification system for collecting and validating (via a centralized review process) data on causes of death and contributing factors in HIV-1 infected patients, developed by the D:A:D collaboration, has become a priority.35
Despite COHERE’s demonstrated ability to evolve and adapt to respond to new research questions, it is now facing the challenge of expanding cohort and clinic-based databases to include clinical outcomes that were not initially of interest. As the cohort of PLWH in Europe ages, comorbidities, such as cardiovascular, metabolic, neurocognitive, and bone diseases, have become increasingly relevant to the study of prognosis in the era of cART. If COHERE and its contributing cohorts were formed today, more emphasis would be placed on linking HIV databases with other health databases (e.g. cancer registries, hospital or other administrative databases, etc.).
COHERE from a patient’s perspective
COHERE has maintained close ties with the patient community via its patient representative on the COHERE SC. Data from COHERE helped to demonstrate the value of high quality treatment strategies and enabled people living with HIV understand the evolving nature of the epidemic as well as face the ongoing challenges of growing older with HIV. Findings from COHERE have informed and guided the patient community’s discussions with governments and authorities about treatment guidelines and standards of care.
Can I get hold of the data? Where can I find out more?
COHERE welcomes applications from Principal Investigators (PIs) of European cohorts interested in joining the COHERE collaboration. Interested cohorts must be willing to transform their data to fit HICDEP codes and adhere to the data submission timeline laid out in the COHERE Data Management SOP. COHERE DMs hold several explanatory webinars covering the data submission process to facilitate data transfer.
Those interested in using COHERE data to conduct a project can download a COHERE Project Proposal Form and a Data Specification Form from our website (www.cohere.org). Proposals from external investigators will undergo the same rigorous scrutiny as those from investigators within the study group (details outlined in the COHERE Manual of Operations, available at www.cohere.org).
Conclusion
COHERE is now a mature collaboration, which is unique in its size and coverage. It continues to produce new evidence on clinical outcomes, particularly AIDS-defining complications, late presentation and socio-economic inequalities, which inform clinical guidelines and public health policy recommendations. As PLWH live longer with a chronic infection, comparisons between them and cohorts of uninfected individuals are needed to disentangle the role of HIV infection from its long-term treatment and comorbidities, especially those linked to ageing.
Key Messages.
COHERE adds value by focusing on questions that cannot be addressed by single cohorts because of sample size requirements, ensuring the sustainability of individual cohorts.
Recent and exemplary reports by COHERE suggest that earlier and more widespread testing for HIV with linkage to care was required to reduce the incidence of late presentation.
Non-injection drug users living with HIV with CD4 cell counts above 500/mm3 after starting cART had mortality patterns similar to those in the general population.
COHERE has informed models of HIV progression and the effect of therapy which have been used to characterize HIV-infected populations, inform public health policy and serve as a basis for cost-effectiveness analysis.
Profile in a nutshell.
The Collaboration of Observational HIV Epidemiological Research Europe (COHERE) is a project-driven cohort consortium that was set-up to address scientific questions that could not be addressed by single cohorts because of sample size requirements.
COHERE has grown from 33 cohorts in 2005 to 40 in 2015. The 2014 merger, representing 14 projects, compiled data from 331 481 individuals from 34 European countries, including 2808 children (<13), representing 2 135 896 person-years of follow-up.
As a consortium of cohorts comprising clinic/hospital-based and interval cohorts, the frequency of patient follow-up varies. For clinic or hospital-based cohorts, average patient follow-up reflects current standards of care in those countries.
COHERE compiles data on clinical characteristics, antiretroviral therapy and other medications, estimated date of HIV seroconversion, opportunistic infections, laboratory results and socio demographic data according to the requirements of projects.
External collaborators interested in conducting a project in COHERE should submit a project proposal to the Regional Coordinating Centres in Bordeaux and Copenhagen for review by COHERE’s governing bodies (see www.cohere.org for further information).
Acknowledgments
Writing Committee: Geneviève Chêne*, Andrew Phillips, Dominique Costagliola, Jonathan Sterne, Hansjakob Furrer, Julia del Amo, Amanda Mocroft, Antonella d’Arminio Monforte, François Dabis, José M. Miro, Diana Barger, Monique Termote, Christine Schwimmer, Rikke Salbøl Brandt, Nina Friis-Moller, Dorthe Raben, David Haerry, Matthias Egger, Ian Weller, Stéphane De Wit
Steering Committee - Contributing Cohorts: Ali Judd (AALPHI), Robert Zangerle (AHIVCOS), Giota Touloumi (AMACS), Josiane Warszawski (ANRS CO1 EPF/ANRS CO11 OBSERVATOIRE EPF), Laurence Meyer (ANRS CO2 SEROCO), François Dabis (ANRS CO3 AQUITAINE), Murielle Mary Krause (ANRS CO4 FHDH), Jade Ghosn (ANRS CO6 PRIMO), Catherine Leport (ANRS CO8 COPILOTE), Linda Wittkop (ANRS CO13 HEPAVIH), Peter Reiss (ATHENA), Ferdinand Wit (ATHENA), Maria Prins (CASCADE), Heiner Bucher (CASCADE), Caroline Sabin (CHIC), Diana Gibb (CHIPS), Gerd Fätkenheuer (Cologne-Bonn), Julia Del Amo (CoRIS), Niels Obel (Danish HIV Cohort), Claire Thorne (ECS), Amanda Mocroft (EuroSIDA), Ole Kirk (EuroSIDA), Christoph Stephan (Frankfurt), Santiago Pérez-Hoyos (GEMES-Haemo), Osamah Hamouda (German ClinSurv), Barbara Bartmeyer (German ClinSurv), Nikoloz Chkhartishvili (Georgian National HIV/AIDS), Antoni Noguera-Julian (CORISPE-cat), Andrea Antinori (ICC), Antonella d’Arminio Monforte (ICONA), Norbert Brockmeyer (KOMPNET), Luis Prieto (Madrid PMTCT Cohort), Pablo Rojo Conejo (CORISPES-Madrid), Antoni Soriano-Arandes (NENEXP), Manuel Battegay (SHCS), Roger Kouyos (SHCS), Cristina Mussini (Modena Cohort), Pat Tookey (NSHPC), Jordi Casabona (PISCIS), Jose M. Miró (PISCIS), Antonella Castagna (San Raffaele), Deborah_Konopnick (St. Pierre Cohort), Tessa Goetghebuer (St Pierre Paediatric Cohort), Anders Sönnerborg (Swedish InfCare), Carlo Torti (Italian Master Cohort), Ramon Teira (VACH), Myriam Garrido (VACH). David Haerry (European AIDS Treatment Group)
Executive Committee: Stéphane de Wit (Chair, St. Pierre University Hospital), Jose M. Miró (PISCIS), Dominique Costagliola (FHDH), Antonella d’Arminio-Monforte (ICONA), Antonella Castagna (San Raffaele), Julia del Amo (CoRIS), Amanda Mocroft (EuroSida), Dorthe Raben (Head, Copenhagen Regional Coordinating Centre), Geneviève Chêne (Head, Bordeaux Regional Coordinating Centre). Paediatric Cohort Representatives: Ali Judd, Pablo Rojo Conejo.
Regional Coordinating Centres: Bordeaux RCC: Diana Barger, Christine Schwimmer, Monique Termote, Linda Wittkop; Copenhagen RCC: Maria Campbell, Nina Friis-Møller, Jesper Kjaer, Dorthe Raben, Rikke Salbøl Brandt.
Project Leads and Statisticians: Juan Berenguer, Julia Bohlius, Vincent Bouteloup, Heiner Bucher, Alessandro Cozzi-Lepri, François Dabis, Antonella d’Arminio Monforte, Mary-Anne Davies, Julia del Amo, Maria Dorrucci, David Dunn, Matthias Egger, Hansjakob Furrer, Marguerite Guiguet, Sophie Grabar, Ali Judd, Ole Kirk, Olivier Lambotte, Valériane Leroy, Sara Lodi, Sophie Matheron, Laurence Meyer, Jose M. Miró, Amanda Mocroft, Susana Monge, Fumiyo Nakagawa, Roger Paredes, Andrew Phillips, Massimo Puoti, Michael Schomaker, Colette Smit, Jonathan Sterne, Rodolphe Thiebaut, Claire Thorne, Carlo Torti, Marc van der Valk, Linda Wittkop, Natasha Wyss.
Funding: The COHERE study group has received unrestricted funding from: Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS), France; HIV Monitoring Foundation, The Netherlands; and the Augustinus Foundation, Denmark. The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under EuroCoord grant agreement n° 260694. A list of the funders of the participating cohorts can be found at www.COHERE.org.
Footnotes
Conflicts of Interest
DC was a member of the French Gilead HIV board up until 2015 and gave lectures for Janssen-Cilag, Merck-Sharp & Dohme-Chibret, ViiV and received travel/accommodations/meeting expenses from Gilead, ViiV, Janssen-Cilag. DC also conducted post-marketing studies for Janssen-Cilag, Merck-Sharp & Dohme-Chibret and ViiV. DC is currently a consultant at Innavirvax. HF has received grants from the Swiss National Science Foundation. His institution has received grants from ViiV, Abbvie, MSD, Janssen, Roche, BMS, and Gilead. The other co-authors have no conflicts of interest to declare.
References
- 1.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. The Lancet. 2003;362:22–9. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 2.EuroCoord: Enhancing clinical and epidemiological HIV research in Europe through cohort collaborations. 2014. [cited 2014 5 February]; Available from: http://www.eurocoord.net/
- 3.May MT, Ingle SM, Costagliola D, et al. Cohort profile: Antiretroviral Therapy Cohort Collaboration (ART-CC) Int J Epidemiol. 2013 doi: 10.1093/ije/dyt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egger M, Ekouevi DK, Williams C, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012;41:1256–64. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EuroSida. 2015. [cited 6 July, 2015]; Available from: http://www.cphiv.dk/Ongoing-Studies/EuroSIDA/About.
- 6.Kjaer J, Ledergerber B. HIV cohort collaborations: proposal for harmonization of data exchange. Antivir Ther. 2004;9:631–3. [PubMed] [Google Scholar]
- 7.HIV-Distributed Data Management. 2016. [cited 2016 8 April]; Available from: http://www.hiv-ddm.net/
- 8.Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS (London, England) 2008;22:1463–73. doi: 10.1097/QAD.0b013e3282f88d02. [DOI] [PubMed] [Google Scholar]
- 9.Lewden C, Bouteloup V, De Wit S, et al. All-cause mortality in treated HIV-infected adults with CD4 >/=500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol. 2012;41:433–45. doi: 10.1093/ije/dyr164. [DOI] [PubMed] [Google Scholar]
- 10.Mocroft A, Furrer HJ, Miro JM, et al. The incidence of AIDS-defining illnesses at a current CD4 count >/= 200 cells/muL in the post-combination antiretroviral therapy era. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57:1038–47. doi: 10.1093/cid/cit423. [DOI] [PubMed] [Google Scholar]
- 11.The Opportunistic Infections Project Team of the Collaboration of Observational HIVERiEiE. CD4 Cell Count and the Risk of AIDS or Death in HIV-Infected Adults on Combination Antiretroviral Therapy with a Suppressed Viral Load: A Longitudinal Cohort Study from COHERE. PLoS Med. 2012;9:e1001194. doi: 10.1371/journal.pmed.1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engsig FN, Zangerle R, Katsarou O, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;58:1312–21. doi: 10.1093/cid/ciu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodwick R, Costagliola D, Reiss P, et al. Triple-class virologic failure in HIV-infected patients undergoing antiretroviral therapy for up to 10 years. Arch Intern Med. 2010;170:410–9. doi: 10.1001/archinternmed.2009.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro H, Judd A, Gibb DM, et al. Risk of triple-class virological failure in children with HIV: a retrospective cohort study. Lancet. 2011;377:1580–7. doi: 10.1016/S0140-6736(11)60208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castagliola D, Ledergerber B, Torti C, et al. Trends in virological and clinical outcomes in individuals with HIV-1 infection and virological failure of drugs from three antiretroviral drug classes: a cohort study. Lancet Infect Dis. 2012;12:119–27. doi: 10.1016/S1473-3099(11)70248-1. [DOI] [PubMed] [Google Scholar]
- 16.Nakagawa F, Lodwick R, Costagliola D, et al. Calendar time trends in the incidence and prevalence of triple-class virologic failure in antiretroviral drug-experienced people with HIV in Europe. J Acquir Immune Defic Syndr. 2012;59:294–9. doi: 10.1097/QAI.0b013e31823fe66b. [DOI] [PubMed] [Google Scholar]
- 17.PLATO II COHERE in EuroCoord. Predictors of CD4+ T-Cell Counts of HIV Type 1–Infected Persons After Virologic Failure of All 3 Original Antiretroviral Drug Classes. Journal of Infectious Diseases. 2013;207:759–67. doi: 10.1093/infdis/jis752. [DOI] [PubMed] [Google Scholar]
- 18.Mocroft A, Lundgren JD, Sabin ML, et al. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE) PLoS Med. 2013;10:e1001510. doi: 10.1371/journal.pmed.1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mocroft ALJ, Kirk O for the Late Presentation Working Group of COHERE. HepHIV. Barcelona: 2014. Continued late presentation for HIV care across Europe. [Google Scholar]
- 20.Pharris A, Spiteri G, Noori T, Amato-Gauci AJ. Ten years after Dublin: principal trends in HIV surveillance in the EU/EEA, 2004 to 2013. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014;19:20968. doi: 10.2807/1560-7917.es2014.19.47.20968. [DOI] [PubMed] [Google Scholar]
- 21.Mocroft A, Reiss P, Kirk O, et al. Is it safe to discontinue primary Pneumocystis jiroveci pneumonia prophylaxis in patients with virologically suppressed HIV infection and a CD4 cell count <200 cells/microL? Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;51:611–9. doi: 10.1086/655761. [DOI] [PubMed] [Google Scholar]
- 22.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. 2015:19. [Google Scholar]
- 23.European AIDS Clinical Society (EACS) Guidelines Version 8.0. 2015. [Google Scholar]
- 24.Bisson GP, Molefi M, Bellamy S, et al. Early versus delayed antiretroviral therapy and cerebrospinal fluid fungal clearance in adults with HIV and cryptococcal meningitis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;56:1165–73. doi: 10.1093/cid/cit019. [DOI] [PubMed] [Google Scholar]
- 25.Boulware DR, MD, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, Taseera K, Nabeta HW, Schutz C, Williams DA, Rajasingham R, Rhein J, Thienemann F, Lo MW, Nielsen K, Bergemann KL, Kambugu A, Manabe YC, Janoff EN, Bohjanen PR, Meintjes G COAT Team. Timing of antiretroviral therapy after cryptococcal meningitis. N Engl J Med. 2014 [Google Scholar]
- 26.Lortholary O, Nicolas M, Soreda S, et al. Fluconazole, with or without dexamethasone for experimental cryptococcosis: impact of treatment timing. The Journal of antimicrobial chemotherapy. 1999;43:817–24. doi: 10.1093/jac/43.6.817. [DOI] [PubMed] [Google Scholar]
- 27.Ingle S, Miro J, Furrer H, et al. CROI. Seattle: 2015. Impact of ART on Mortality in Cryptococcal Meningitis Patients: High-Income Settings. [Google Scholar]
- 28.Bohlius J, Schmidlin K, Costagliola D, et al. Prognosis of HIV-associated non-Hodgkin lymphoma in patients starting combination antiretroviral therapy. AIDS (London, England) 2009;23:2029–37. doi: 10.1097/QAD.0b013e32832e531c. [DOI] [PubMed] [Google Scholar]
- 29.Bohlius J, Schmidlin K, Boue F, et al. HIV-1-related Hodgkin lymphoma in the era of combination antiretroviral therapy: incidence and evolution of CD4(+) T-cell lymphocytes. Blood. 2011;117:6100–8. doi: 10.1182/blood-2010-08-301531. [DOI] [PubMed] [Google Scholar]
- 30.Wyss NEM, Bohlius J. Kaposi Sarcoma in the Era of Combination Antiretroviral Therapy. Conference on Retroviruses and Opportunistic Infections (CROI); 2014 March 3–6, 2014; Boston. 2014. [Google Scholar]
- 31.Effect of hepatitis C treatment on CD4+ T-cell counts and the risk of death in HIV-HCV-coinfected patients: the COHERE collaboration. Antivir Ther. 2012;17:1541–50. doi: 10.3851/IMP2263. [DOI] [PubMed] [Google Scholar]
- 32.Lodi S, Dray-Spira R, Touloumi G, et al. Delayed HIV diagnosis and initiation of antiretroviral therapy: inequalities by educational level, COHERE in EuroCoord. AIDS (London, England) 2014;28:2297–306. doi: 10.1097/QAD.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 33.Mortality in migrants living with HIV in western Europe (1997–2013): a collaborative cohort study. The lancet HIV. 2015;2:e540–9. doi: 10.1016/S2352-3018(15)00203-9. [DOI] [PubMed] [Google Scholar]
- 34.ECDC. Improving HIV data comparability in migrant populations and ethnic minorities in EU/EEA/EFTA countries: findings from a literature review and expert panel. European Centre for Disease Control; 2011. [Google Scholar]
- 35.Kowalska JD, Friis-Moller N, Kirk O, et al. The Coding Causes of Death in HIV (CoDe) Project: initial results and evaluation of methodology. Epidemiology. 2011;22:516–23. doi: 10.1097/EDE.0b013e31821b5332. [DOI] [PubMed] [Google Scholar]