Abstract
Background
Rigid internal fixation (RIF) technology is a recently developed fracture fixation technique in which use of specific antibiotics before and after the operation and timely treatment of local infections is necessary.
Material/Methods
The bacteriocins were isolated from Lactobacillus rhamnosus L34. Twenty-four New Zealand White female rabbits were divided into 2 groups: bacteriocins and control group. After mandible fracture fixation, the rabbits were infected with S. aureus and subsequently injected with either bacteriocins or saline. The biofilm samples were harvested from rabbits euthanized on the 1st, 3rd, and 5th days and observed using a fluorescence microscope. Blood samples were collected at 1 h, 12 h, 1 day, 3 days, and 5 days after the injection of either bacteriocin or saline to test the level of C-reactive protein and TNF-α.
Results
Significant differences in the biofilm formation were evident between the bacteriocins and saline treatment group on days 1, 3, and 5. Moreover, the serum levels of TNF-α and CRP after treatment with bacteriocins were significantly lower than in controls.
Conclusions
Use of bacteriocins isolated from Lactobacillus rhamnosus L34 may be a promising way to control infections of mandible fracture after internal fixation in vivo.
MeSH Keywords: Bacteriocins, Biofilms, Mandible, Staphylococcus Aureus
Background
With the increasing incidence of open fractures due to high-energy injury, the number of resulting infections in hospitalized patients is also increasing [1]. Infection of fold open bone is difficult to prevent and to treat, so there is a critical need to address this problem in the clinical setting [2]. The incidences of mandible fracture are the first to be accounted in the accidents [3] and it is reported that mandible fractures account for 44.2% of all facial fractures [4]. S. aureus is one of the main pathogenic bacteria involved in bone fracture infections, and with the increasing trend of drug resistance in S. aureus, finding an effective antibiotic against S. aureus is urgent in treating the infection after fracture.
Rigid internal fixation (RIF) technology, a recently developed fracture fixation technique, has many advantages, such as less influence on the patient’s diet and nutrition intake, and employs the use of titanium, which has good biological compatibility, shows no obvious adverse reactions and, can be implanted in the body for a long time [5]. Although RIF is a successful treatment strategy, application of specific antibiotics before and after the operation and timely local treatment are necessary. The sustained release of antibiotics to the local site of infection is considered as an effective method for the prevention and control of infection of open fractures [6].
Lactic acid bacteria are probiotics which are widely used in the food fermentation industry [7]. They can inhibit or kill some pathogenic bacteria and food spoilage organisms, and are conducive to health [8]. In recent years, studies have shown that some lactic acid bacteria can produce an antimicrobial substances called bacteriocin, which has bacteriostatic activity of precursor peptides or polypeptides, and because of it has high efficacy, is non-toxic, leaves no residue, and has no resistance characteristics, is seen as an effective alternative of antibiotics [9]. A variety of studies have reported that bacteriocin isolated from lactic acid bacteria have strong antibacterial effects on common clinical pathogens. Our previous study showed that bacteriocin isolated from Lactobacillus plantarum can control infection after mandible fracture [10].
In the present study, we isolated a broad range of bacteriocins from Lactobacillus rhamnosus L34. We analyzed its antibacterial effect on infection after mandible fracture fixation and measured the serum concentrations of C-reactive protein and TNF-α in rabbits with bacterial infections. The aim of the present study was to verify the effects of bacteriocins in treatment of infection after mandible fracture fixation in vivo.
Material and Methods
Strains and culture conditions
Lactobacillus rhamnosus L34 was obtained from the Department of Microbiology, Zhejiang University. It was maintained on MRS agar plates (MRSA, Difco, Detroit, MI, USA) and grown overnight in Rogossa broth (MRS, Difco, USA) at 20°C with gentle agitation [11]. S. aureus ATCC 29213 was used to screen for antibacterial activity. Cultures of S. aureus were grown overnight in brain-heart infusion broth (BHI, Difco, USA) at 37°C, centrifuged at 2000 rpm for 5 min. The final concentrations of the cultures were adjusted to 0.5×105 CFU/ml using Densicheck (BioMérieux, Lyons, France).
Isolation of bacteriocins
L. rhamnosus L34 cultures were grown in the MRS broth at 37°C for 24 h. The cultures were then centrifuged at 7000 g for 10 min and cell-free supernatants were isolated. The pH of the supernatants was adjusted to 6.5 and treated with catalase (5 mg/ml) to eliminate the effect of hydrogen peroxide, then filtered through 0.22-μm pore size filters (Millipore, USA) to obtain a crude bacteriocin preparation [12].
Crude bacteriocin preparations were saturated with 70% ammonium sulfate and stored at 4°C to precipitate the protein. After 24 h, the precipitates were recovered by centrifugation (10 000 g at 4°C for 30 min), vacuum-dried, and dissolved in 1 ml PBS. Suspensions were then separated in Sephadex G-100 columns (1.6×36 cm) and the fractions (1 ml each) containing bacteriocins were collected and stored at −70°C [13].
Animals
All animals were handled in strict accordance with good animal practice and the animal work was approved by the College of Medicine, Zhejiang University Chancellor’s Animal Research Committee. Twenty-four New Zealand White female rabbits weighing 2.5–3.0 kg and aged 70–100 days were used in the study. Animals were housed in individual cages in a temperature-controlled room (23°C) with a 12-h light/dark cycle. Twelve rabbits were used for validation of the infection model and another 12 were assessed as the control group.
Model validation
Rabbits were initially anesthetized via inhalation of isoflurane (2%). The surgical procedure was then carried out as described in detail elsewhere [14]. The operation was performed on animals under general anesthesia induced by intramuscular injection of ketamine (25 mg/kg of body weight) followed by continuous inhalation of isoflurane (1%). A longitudinal gum incision was made and the periosteum was stripped to expose the mandible. The fracture of the mandible was made by means of a chisel or osteotome driven into the lines of the fracture. After fracture reduction, it was fixed with a mini-titanium board. The gums were finally closed to complete the procedure.
Immediately after the surgical wound was closed, rabbits were infected by injection of S. aureus (3×105 cfu/ml in a total of 0.5 mL inoculum) into the mandible fracture line. Subsequently, animals were divided into 2 groups: 12 rabbits were injected with 1 ml bacteriocins (0.5 mg/ml) and the other 12 rabbits were injected with 1 ml sterile saline solution as controls.
Detection of biofilm formation on the fixation
To evaluate the biofilm formation in our rabbit model, the biofilm samples were harvested from mini-titanium boards in rabbits euthanized on at days 1, 3, and 5 after infection. The samples were transferred to coverslips and fixed with 2.5% glutaraldehyde at 4°C. Tissues were then stained with 0.01% acridine orange solution (Sigma, USA) and observed at an excitation wavelength of 488 nm under a Nikon 80i microscope equipped with an argon laser [15]. All the images were analyzed and quantified using Image-Pro Plus 6.0 software (Media Cybernetics, USA). The fluorescence intensities of the biofilms are expressed as integrated optical density values (IOD) [16].
Determination of serum inflammatory cytokines
Blood samples from the 24 rabbits were collected at various time points (1 h, 12 h, 1 d, 3 d, and 5 d) after the injection of bacteriocins or saline. The blood samples were centrifuged at 3000 rpm for 5 min at 4°C and stored at −70°C. The protein levels of C-reactive protein (CRP) and TNF-α were measured using ELISA kits (Invitrogen, USA) [17]. All measurements were done in triplicate. The levels of CRP and TNF-α are expressed as mg/ml and pg/ml protein, respectively.
Statistical analysis
All tests were repeated 3 times, with consistent results. Data analysis was done using SPSS 14.0 software for Windows and the results were compared statistically using the t test. P values were considered statistically significant at p<0.05.
Results
The variation of biofilm formation on mini-titanium
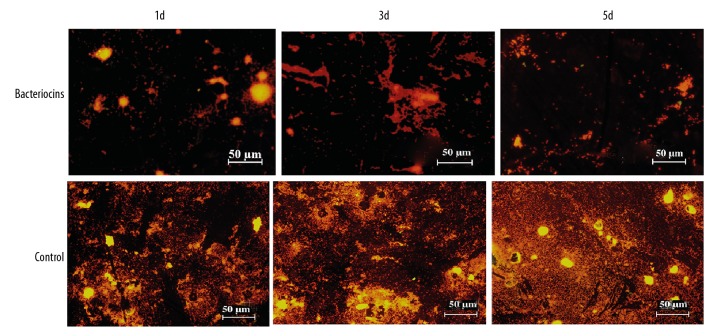
The progress of the surgery of mandible fracture fixation is shown in Figure 1. S. aureus were imaged for biofilm formation using a fluorescence microscope. As can be seen in Figure 2, significant variability in biofilm formation appeared at day 1 after the injection of either bacteriocins or saline (p<0.05, Figure 2). Notably, significant differences were evident between the group of bacteriocins and saline groups at days 3 and 5 (p<0.05, Figure 2). The variation in the IOD is shown in Figure 2 and the fluorescence image of the biofilm is shown in Figure 3.
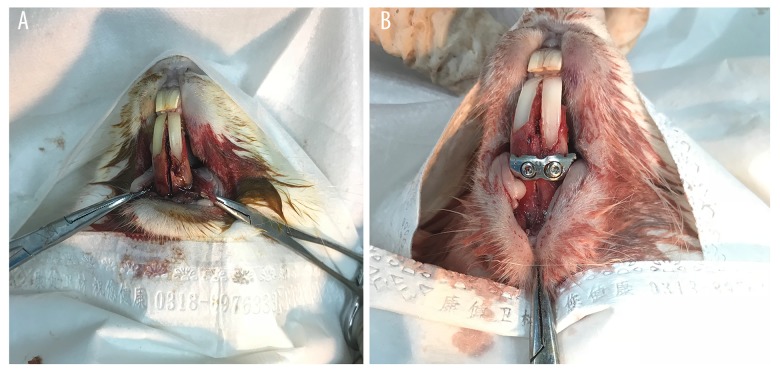
Figure 1.
Mandible fracture fixation surgery in the rabbit model (A: exposure of the mandible and artificial mandible fractures; B: mandible fracture fixation).
Figure 2.
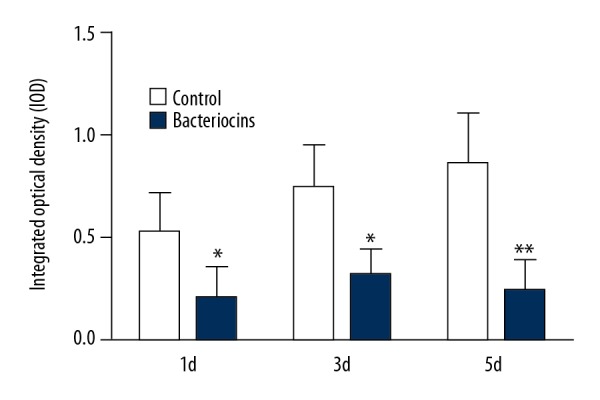
The integrated optical density (IOD) of biofilms in bacteriocins-injected rabbits and control group rabbits after 1 h, 12 h, 1 d, 3 d, and 5 d (mean ±SD, *, P<0.05)
Figure 3.
Fluorescent images of the biofilm of S. aureus in bacteriocins-injected rabbits and control group rabbits after 1 d, 3 d, and 5 d (stained with acridine orange).
Variations in the levels of serum inflammatory cytokines
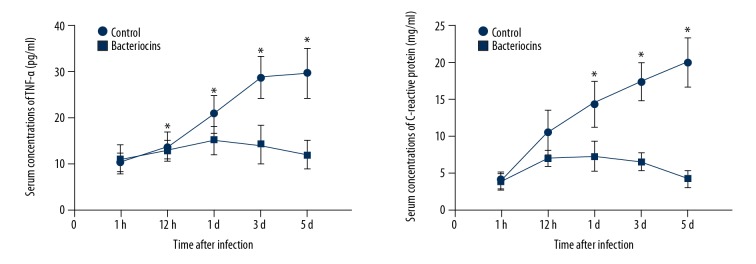
After 1 h and 12 h of the bacteriocins or saline injections, the serum levels of TNF-α and CRP in the bacteriocins treatment group and control group all increased; however, the serum level of TNF-α and CRP did not show significant differences between the bacteriocins treatment group and the control group at 1 h and 12 h (Figure 4). However, at days 1 and 3 following bacteriocins treatment, the serum levels of TNF-α and CRP were significantly lower than in controls (p<0.05, Figure 4). Importantly, at day 5 after infection, the serum levels of TNF-α and CRP were reduced to normal level (as observed after 1 h) and were significantly lower than in controls (p<0.05, Figure 4).
Figure 4.
Serum concentrations of C-reactive protein (CRP) and TNF-α in bacteriocins-injected rabbits and control group at 1 h, 12 h, 1 d, 3 d, and 5 d following infection (mean ±SD, * P<0.05).
Discussion
Clinically, the existence of S. aureus in infection is very common, and is the main pathogenic bacteria in nosocomial infections [18]. Owing to the extensive use of antibiotics, drug-resistant strains have developed [19]. Therefore, it becomes essential to identify safer and effective antibiotics or drug candidates.
Postoperative infection of internal fixation in orthopedic surgery is a catastrophic event, which not only increases the treatment cost, but also slows fracture healing, and in some cases can even led to deformity and dysfunction [20]. It is reported that the incidence of infection after orthopedics internal fixation implantation is 0.5–5% [21]; therefore, finding ways to prevent postoperative infection in orthopedics internal fixation implantation is a major problem. Lactic acid bacteria can produce a variety of antibacterial substances, such as organic acids, double b acyl, butanone, hydrogen peroxide, antifungal peptides, and bacteriocin [22]. Bacteriocins are bioactive proteins secreted by some bacteria; they can kill or inhibit other microbes in the same or similar living environment [23]. Compared to antibiotics, bacteriocins have added advantages as they can inhibit the closely related bacterial strains, and more importantly, they are non-toxic, with no adverse effect, no residue, and no drug resistance, and do not produce toxins when used for the treatment of infection [24]. In the present study, we found that bacteriocins exert a strong antibacterial effect on S. aureus.
The mandible is part of the lower region of the face, which is easily injured. There are several anatomical weak areas, which, when exposed to direct or indirect violence, become susceptible to breaks and fractures [25]. Mandible fracture fixation surgery is very common in China. Despite the routine use of antibiotics, postoperative infection occasionally occurs [26]. TNF-α and CRP are common inflammatory markers that can be used for monitoring infection [27]. We found that the levels of TNF-α and CRP were significantly lower after the injection of bacteriocins, which is in agreement with previous experimental results [28,29].
Although S. aureus is a standard strain used in testing, the bacterial species involved in mandible fracture infections after internal fixation are complicated and the pathogenic bacteria may be different from the standard strains. Therefore, our results may not reflect the true situation of infection in humans. In future, we will consider all aspects to conduct experiments in vivo.
Conclusions
In conclusion, bacteriocins isolated from Lactobacillus rhamnosus L34 significantly reduced the formation of biofilms and inflammation of mandible fractures after internal fixation. Thus, bacteriocins isolated from L. rhamnosus L34 may be promising in control of mandible fracture infection after internal fixation and could have potential clinical application in infection control in the future.
Footnotes
Conflicts of interest
None.
Source of support: This research was financially supported by the National Nature Science Fund of China (No. 30901686) and Zhejiang Provincial Natural Science Foundation of China (LY15H140005)
References
- 1.Forster AJ, Daneman N, van Walraven C. Influence of antibiotics and case exposure on hospital-acquired Clostridium difficile infection independent of illness severity. J Hosp Infect. 2017;95(4):400–9. doi: 10.1016/j.jhin.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Deirmengian C, Kardos K, Kilmartin P, et al. Diagnosing periprosthetic joint infection: Has the era of the biomarker arrived? Clin Ortho Relat Res. 2014;472(11):3254–62. doi: 10.1007/s11999-014-3543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oginni FO, Oladejo T, Alake DP, et al. Facial bone fractures in Ile-Ife, Nigeria: An update on pattern of presentation and care. J Maxil Oral Surg. 2016;15(2):184–90. doi: 10.1007/s12663-015-0826-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brasileiro BF, Passeri LA. Epidemiological analysis of maxillofacial fractures in Brazil: A 5-year prospective study. Oral Surg Oral Med. 2006;102(1):28–34. doi: 10.1016/j.tripleo.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy BC, D’Amico RS, Youngerman BE, et al. Long-term growth and alignment after occipitocervical and atlantoaxial fusion with rigid internal fixation in young children. J Neurosurg. 2016;17(1):94–102. doi: 10.3171/2015.5.PEDS14728. [DOI] [PubMed] [Google Scholar]
- 6.Uskoković V, Desai TA. In vitro analysis of nanoparticulate hydroxyapatite/chitosan composites as potential drug delivery platforms for the sustained release of antibiotics in the treatment of osteomyelitis. J Pharm Sci. 2014;103(2):567–79. doi: 10.1002/jps.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saez-Lara MJ, Gomez-Llorente C, Plaza-Diaz J, Gil A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. Biomed Res Int. 2015;2015 doi: 10.1155/2015/505878. 505878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stellato G, De Filippis F, La Storia A, Ercolini D. Coexistence of lactic acid bacteria and potential spoilage microbiota in a dairy processing environment. Appl Environ Microbiol. 2015;81(22):7893–904. doi: 10.1128/AEM.02294-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez RH, Zendo T, Sonomoto K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb Cell Fact. 2014;13(S1):1–13. doi: 10.1186/1475-2859-13-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu T, Liu YM. Antibacterial effect of bacteriocin isolated from Lactobacillus plantarum ATCC 8014 on postoperative infection of mandibular fracture in vivo. J Craniofac Surg. 2017;28(3):679–82. doi: 10.1097/SCS.0000000000003469. [DOI] [PubMed] [Google Scholar]
- 11.Rahman SA, Kahar AA, Mansor AB, Long K. Identification of potential indigenous microbe from local fermented vegetables with antimicrobial activity. Science Heritage Journal. 2017;1(1):1–3. [Google Scholar]
- 12.Ananou S, Maqueda M, Martínez-Bueno M, et al. Control of Staphylococcus aureus in sausages by enterocin AS-48. Meat Sci. 2005;71(3):549–56. doi: 10.1016/j.meatsci.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan R, Kumawat DK, Kumar S, Saxena AK. Purification and characterization of a bacteriocin from Lactobacillus rhamnosus L34. Ann Microbiol. 2013;63(1):387–92. [Google Scholar]
- 14.Perren SM. Evolution of the internal fixation of long bone fractures: the scientific basis of biological internal fixation: Choosing a new balance between stability and biology. Bone Joint J. 2002;84(8):1093–110. doi: 10.1302/0301-620x.84b8.13752. [DOI] [PubMed] [Google Scholar]
- 15.Zacheus OM, Iivanainen EK, Nissinen TK, et al. Bacterial biofilm formation on polyvinyl chloride, polyethylene and stainless steel exposed to ozonated water. Water Res. 2000;34(1):63–70. [Google Scholar]
- 16.Lin X, Chen X, Chen Y, et al. The effect of five probiotic lactobacilli strains on the growth and biofilm formation of Streptococcus mutans. Oral Dis. 2015;21(1):e128–34. doi: 10.1111/odi.12257. [DOI] [PubMed] [Google Scholar]
- 17.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res Clin Pract. 2005;69(1):29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Perl TM, Golub JE. New approaches to reduce Staphylococcus aureus nosocomial infection rates: treating S. aureus nasal carriage. Ann Pharmacother. 1998;32(1):7–16. doi: 10.1177/106002809803200104. [DOI] [PubMed] [Google Scholar]
- 19.Durante-Mangoni E, Del Franco M, Andini R, et al. Emergence of colistin resistance without loss of fitness and virulence after prolonged colistin administration in a patient with extensively drug-resistant Acinetobacter baumannii. Diagn Microbiol Infect Dis. 2015;82(3):222–26. doi: 10.1016/j.diagmicrobio.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Lin S, Mauffrey C, Hammerberg EM, et al. Surgical site infection after open reduction and internal fixation of tibial plateau fractures. Europ J Ortho Surg Traumatol. 2014;24(5):797–803. doi: 10.1007/s00590-013-1252-8. [DOI] [PubMed] [Google Scholar]
- 21.Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: A review. Biomater. 2000;21(24):2615–21. doi: 10.1016/s0142-9612(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 22.Klaenhammer TR. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Revi. 1993;12(1–3):39–85. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 23.Hanlin MB, Kalchayanand N, Ray P, Ray B. Bacteriocins of lactic acid bacteria in combination have greater antibacterial activity. J Food Prot. 1993;56(3):252–55. doi: 10.4315/0362-028X-56.3.252. [DOI] [PubMed] [Google Scholar]
- 24.Schwinghamer EA, Brockwell J. Competitive advantage of bacteriocin and phage-producing strains of Rhizobium trifolii in mixed culture. Soil Biol Biochem. 1978;10(5):383–87. [Google Scholar]
- 25.Schubert W, Kobienia BJ, Pollock RA. Cross-sectional area of the mandible. J Oral Maxillofac Surg. 1997;55(7):689–92. doi: 10.1016/s0278-2391(97)90577-2. [DOI] [PubMed] [Google Scholar]
- 26.Monaco G, Tavernese L, Agostini R, Marchetti C. Evaluation of antibiotic prophylaxis in reducing postoperative infection after mandibular third molar extraction in young patients. J Oral Maxillofac Surg. 2009;67(7):1467–72. doi: 10.1016/j.joms.2008.12.066. [DOI] [PubMed] [Google Scholar]
- 27.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-α) and essential hypertension. J Hum Hypertens. 2005;19(2):149–54. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 28.Abhari K, Shekarforoush SS, Hosseinzadeh S, et al. The effects of orally administered Bacillus coagulans and inulin on prevention and progression of rheumatoid arthritis in rats. Food Nutrit Res. 2016;60(1):1–8. doi: 10.3402/fnr.v60.30876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Espinosa-Martos I, Jiménez E, de Andrés J, et al. Milk and blood biomarkers associated to the clinical efficacy of a probiotic for the treatment of infectious mastitis. Benef Microbes. 2016;7(3):305–18. doi: 10.3920/BM2015.0134. [DOI] [PubMed] [Google Scholar]