Abstract
Background
Gemcitabine plus cisplatin (GP) is a novel regimen of induction chemotherapy (IC) for treating locoregional advanced nasopharyngeal cancer (NPC). This retrospective study aimed to compare the efficacy of GP and TP (paclitaxel plus cisplatin) regimens in tumor volume reduction after IC.
Material/Methods
Between January 2014 and July 2017, 44 patients with III–IVB stage NPC received GP IC followed by concurrent chemoradiotherapy. These patients were matched with 44 patients receiving TP IC according to clinical characteristics. The gross tumor volume of the primary site and positive lymph nodes were delineated by magnetic resonance imaging before and after IC, as well as the nasopharyngeal air cavities. The changes in tumor volume and nasopharyngeal air cavity after IC were calculated and compared between the 2 groups. Treatment toxicities and early survival outcomes were also reported.
Results
There were no differences in the initial tumor volume and nasopharyngeal cavity between the 2 groups. The volume changes after IC for the primary site, lymph nodes, and nasopharyngeal cavity were 31.4 (range, −0.97–75.8), 4.68 (range, −7.08–22.06), and 2.62 (range, 0.1–7.63) mL for GP and 23.36 (range, −59.14–83.58), 4.7 (range, −11.21–48.61), and 1.47 (range, −2.47–6.17) mL for TP, respectively. All comparisons favored the GP regimen. The toxicities of the 2 regimens were comparable and no survival differences were observed at follow-up (median, 18.7 months).
Conclusions
Changes in the tumor volume and nasopharyngeal air cavity showed that the GP regimen was significantly more effective than the TP regimen in tumor burden reduction. However, whether the advantages of GP can translate into survival benefits requires further investigation.
MeSH Keywords: Induction Chemotherapy; Nasopharyngeal Neoplasms; Toxicity Tests, Acute; Tumor Burden
Background
Nasopharyngeal carcinoma (NPC) is a poorly differentiated carcinoma arising from the mucosa of the nasopharynx, with high invasive and metastatic potential. Advances in radiotherapy techniques and an increasing wealth of knowledge on tumor behavior have changed this life-threatening malignancy to a potentially curable disease even at locoregionally advanced stage. The overall 5-year survival of NPC was >80% during the intensity-modulated radiotherapy (IMRT) era and the quality of life of patients is well maintained. However, distant metastasis will eventually develop in approximately 18% of patients, most of whom were at an advanced stage when first diagnosed [1–6]. Furthermore, the local control of advanced disease is not satisfactory with current chemoradiotherapy regimens. The 5-year local control rate of T4 disease is approximately 82%, which is lower than that of T1-2 disease (95%) [1,2]. Thus, the challenge of NPC treatment is to explore new strategies to reduce the distant metastasis rate and further improve disease control for locoregionally advanced diseases.
Concurrent chemoradiotherapy (CCRT) with or without adjuvant chemotherapy (AC) is the standard care for advanced stage NPC [7–9]. The role of induction chemotherapy (IC) has not been well established yet. However, compared to CCRT with/without AC, IC followed by CCRT has the advantage of immediate care after diagnosis and a high completion rate of chemotherapy. It also provides comparable disease control and may offer superior distant metastasis control among all forms of chemoradiation [10].
The commonly used regimens of IC are 5-fluorouracil plus cisplatin (PF); 5-fluorouracil, cisplatin, and docetaxel (TPF); and paclitaxel plus cisplatin (TP). These treatment regimens are used for both head and neck squamous cell carcinoma and NPC. TPF has been suggested to be superior to PF for head and neck cancer, but such comparisons have not been performed in NPC trials or for TP vs. TPF [11,12]. In fact, there is no consensus on how to choose a regimen for a given patient.
Gemcitabine plus cisplatin (GP) is another candidate for IC, although it is not widely used. In an early study comparing GP and PF, although a favorable trend in locoregional control was observed for the GP group, there were no significant differences in any of the endpoints [13]. However, in a recently published retrospective study, Zhao et al. reviewed the survival data of patients receiving GP, TP, or PF IC and found that, in some subgroups of patients, the GP regimen improved the overall survival (OS) more significantly than the TP or PF regimens [14]. Furthermore, the robust effect of GP on NPC treatment was validated by a phase 3 trial comparing GP and PF in treating recurrent and metastatic NPC. GP was proved to be more efficient than PF in terms of progression-free survival (PFS) and OS [15].
Induction chemotherapy has been generally used at the Sichuan Cancer Center for NPC with stage III–IVB. Since 2014, GP has been applied as an IC regimen at our center, particularly for locally advanced diseases. Herein, we report the early survival outcomes of patients receiving GP IC and compared them with those of patients receiving TP IC, which is another commonly used regimen in our center. As the follow-up for these patients is still short, the current study is mainly focused on tumor shrinkage after IC and early toxicity.
Material and Methods
Patient selection
This study retrospectively analyzed consecutive patients with newly diagnosed, histologically-proven NPC treated at the Sichuan Cancer Hospital between January 2014 and July 2017. The main inclusion criteria were: (1) stage III–IVb NPC; (2) treatment with IC (either TP or GP), followed by CCRT; (3) a Karnofsky performance score ≥70; and (4) age ≥18 years. To minimize heterogeneity between the TP and GP groups, patients receiving TP IC were matched with patients receiving GP IC based on the T category, N category, and clinical stage. This study was approved by the Ethics Review Board of Sichuan Cancer Hospital. Written informed consent was obtained from all patients prior to enrollment in the study.
Pretreatment evaluation
Prior to treatment, all patients underwent a medical history review and physical examination. Laboratory tests included hematological studies, biochemical profiles, and Epstein–Barr virus examination. Imaging examinations included fiber optic nasopharyngoscopy, magnetic resonance imaging (MRI) of the nasopharynx and neck, chest radiography, abdominal ultrasound, and bone scintigraphy. Some patients also underwent integrated positron emission tomography/computed tomography (PET/CT). All patients were staged according to the 7th edition of the International Union Against Cancer/American Joint Committee on Cancer (UICC/AJCC).
Chemotherapy
All patients underwent 2 cycles of IC prior to initiation of CCRT. Radiation was initiated at around 2 weeks after the second course of IC. The GP regimen was administered with gemcitabine 1000 mg/m2 on days 1 and 8 and cisplatin 80 mg/m2 on days 1–3, repeated every 3 weeks. The TP regimen was administered with paclitaxel 135 mg/m2 or docetaxel 75 mg/m2 on day 1 and cisplatin 80 mg/m2 on days 1–3, repeating every 3 weeks. CCRT consisted of tri-weekly cisplatin 80 mg/m2. Some patients received 1 cycle of AC using the same regimen as IC. Acute toxicities during IC and CCRT were graded according to the Common Terminology Criteria for Adverse Events (version 3.0).
Radiotherapy
All patients underwent IMRT. Patients were immobilized in a supine position by a thermoplastic mask from the head to shoulders. A planning CT scan of the region from the skull vertex to 3 cm below the sternoclavicular joint (slice thickness, 3 mm) was performed. The definition and delineation of the targets were in accordance with previous research [16]. In brief, the prescribed dose for the planning target volume was 66–76 Gy for the primary gross tumor volume (GTVnx); 60–70 Gy for the involved lymph nodes (GTVnd); 60–66 Gy for the high-risk clinic target volume (CTV1); 50–54 Gy for the low-risk clinic target volume (CTV2), which covered CTV1; and 50–54 Gy for the bilaterally lymphatic drainage region (CTVln). Each target was divided into 30–33 fractions. Radiotherapy planning was designed and optimized by use of the Eclipse treatment planning system (Varian, USA).
Tumor response assessment
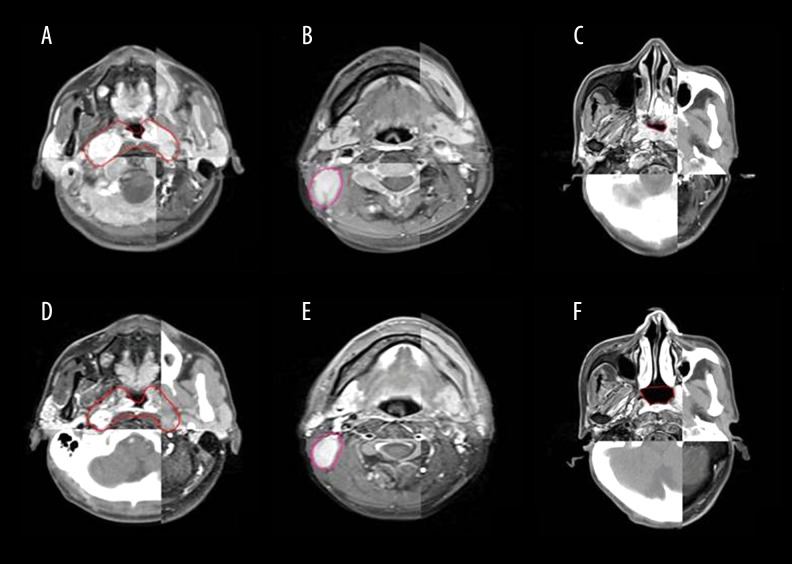
Response Evaluation Criteria in Solid Tumors were not used in this study due to irregular shapes of primary tumors in the nasopharynx. The tumor volume was delineated and measured using MIM software (MIM Software, Inc., USA). An experienced physician (S. Zhang) who was blinded to the chemotherapy regimen manually outlined the GTVnx, GTVnd, and nasopharyngeal cavity (NC) according to CT and MRI fusion images before and after IC (Figure 1). Because the border between tumors and soft tissue is often unclear, an additional approach was applied to measure tumor shrinkage. First, the NC was outlined according to the nasopharyngeal walls and tumor edges. The anterior border of the NC was defined as the lower turbinate posterior margin. The enlargement of the NC can represent tumor shrinkage. Because the NC has well-defined borders, it should be more objective than tumor volume evaluation. The criteria for positive lymph nodes were based on the MRI criteria [17]. For patients with involvement of more than 1 lymph node, a maximum of 3 lymph nodes were selected for delineating and calculating the mean value to represent whole lymph node volume. The volume of GTVnx before IC was determined as F-GTV (formal GTV); correspondingly, the volume of GTVnx after IC was L-GTVnx (latter GTV). The volume of the lymph node and NC before and after IC was also added using the same prefix as GTVnx (F-GTVnd/L-GTVnd, F-NC/L-NC). The degree of regression in volume (DRV%) was calculated by the difference in value of F-GTVnx (F-GTVnd) minus L-GTVnx (L-GTVnd) then divided by F-GTVnx (F-GTVnd).
Figure 1.
Representative images of contouring tumor volume and nasopharyngeal air cavity according to computed tomography and magnetic resonance imaging fusion images: (A) GTVnx before induction chemotherapy (IC), (B) GTVnd before IC, (C) NC before IC, (D) GTVnx after IC, (E) GTVnd after IC, and (F) NC after IC. Note that all outlines are manually drawn. GTV – gross tumor volume; GTVnd – gross tumor volume of lymph node involved; NC – nasopharyngeal cavity.
Statistical analysis
Statistical analysis was performed using the SPSS 19.0 statistical software package (IBM, Armonk, NY, USA). The t test was used to evaluate numerical variables, and the chi-square test was used to assess categorical variables between groups. Kaplan-Meier survival curves were drawn, and the log-rank test was performed to analyze survival rates. Data are reported as mean + standard error of mean. Two-tailed p<0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 1396 patients with non-metastatic NPC were treated between January 2014 and July 2017 at the Sichuan Cancer Hospital. Of these, 44 patients received GP IC and 86 patients received TP IC. This study included all 44 patients in the GP group and 44 patients in the TP group. Both groups were well-matched in terms of sex, T category, N category, and clinical stage. However, the median age of patients in the GP group was slightly higher than that of patients in the TP group (Table 1).
Table 1.
Baseline characteristics of patients and tumors.
| Characteristics | TP group (n=44) | GP group (n=44) |
|---|---|---|
| No. (%) | No. (%) | |
| Age (median, years) | 48 | 50 |
| Gender | ||
| Male | 32 (73) | 33 (75) |
| Female | 12 (27) | 11 (25) |
| T category | ||
| T2 | 2 (5) | 2 (5) |
| T3 | 4 (9) | 4 (9) |
| T4 | 38 (86) | 38 (86) |
| N category | ||
| N0 | 4 (9) | 4 (9) |
| N1 | 7 (16) | 7 (16) |
| N2 | 24 (54) | 24 (54) |
| N3 | 9 (21) | 9 (21) |
| Clinical stage | ||
| III | 3 (7) | 3 (7) |
| IVa | 32 (73) | 32 (73) |
| IVb | 9 (20) | 9 (20) |
GP – gemcitabine plus cisplatin; TP – paclitaxel plus cisplatin.
Volumes of GTVnx and GTVnd
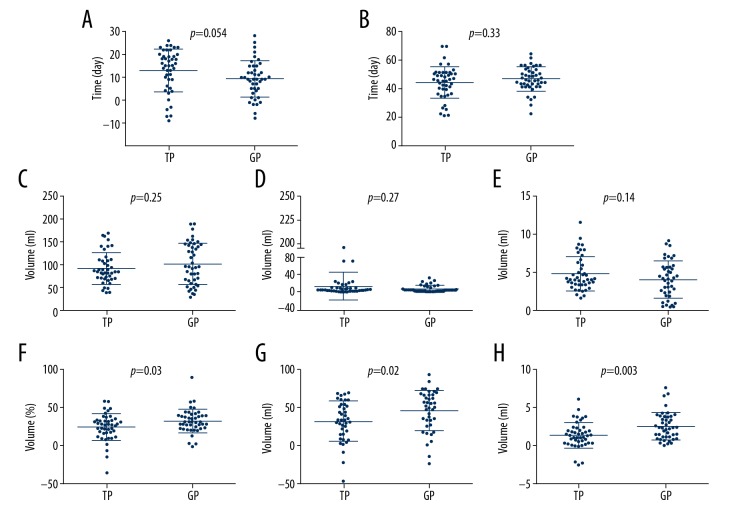
The median time between completion of the second course of chemotherapy and post-chemotherapy MRI and between pre-chemotherapy and post-chemotherapy MRI were 9 and 46 days for the GP group and 15 and 45 days for TP group, respectively (Figure 2A, 2B). There were no statistically significant differences in the length of time between the 2 groups, excluding the effect of time-related bias on change in tumor volume.
Figure 2.
Comparison of tumor volume and nasopharyngeal air cavity changes before and after induction chemotherapy. (A) Time interval between completion of 2nd induction chemotherapy and post-chemotherapy magnetic resonance imaging (MRI). (B) Time interval between pre-chemotherapy and post-chemotherapy MRI. (C) Primary tumor volume before induction chemotherapy. (D) Nodal volume before induction chemotherapy. (E) Volume of nasopharyngeal air cavity. (F) Degree of regression (DRV) in primary tumor volume. (G) DRV in nodal volume. (H) Volume change in nasopharyngeal air cavity.
The mean volumes of the F-GTVnx in the TP and GP groups before IC were 94.59 mL (range, 38.70–170.16) and 102.20 mL (range, 28.55–190.86), respectively. The mean volumes of F-GTVnd in the TP and GP groups before IC were 13.63 mL (range, 0.79–196.84) and 7.96 mL (range, 0.66–32.24), respectively. The mean volumes of the NC in the TP and GP groups before IC were 4.70 mL (range, 1.51–11.40) and 3.96 mL (range, 0–8.98), respectively. Unpaired t tests revealed no significant differences in F-GTVnx, F-GTVnd, and F-NC (Figure 2C–2E) between the 2 groups.
After IC, the volume changes in the primary site, lymph nodes, and nasopharyngeal cavity were 23.36 (range, −59.14–83.58), 4.7 (range, −11.21–48.61), and 1.47 (range, −2.47–6.17) mL for the TP group, and 31.4 (range, −0.97–75.8), 4.68 (range, −7.08–22.06), and 2.62 (range, 0.1–7.63) mL for the GP group, respectively. The mean DRV of GTVnx was 23.07% (range, −35.81–58.45%) and 31.89% (range, −1.96–89.92%), respectively, for the TP and GP groups. The mean DRV of GTVnd was 33.11% (range, −47.26–68.87%) and 46.07% (range, −23.15–93.28%), respectively, for the TP and GP groups. The DRV for both GTVnx and GTVnd were significantly higher in the GP group than in the TP group (Figure 2F, 2G). The increase in the NC after IC was also greater in the GP group than in the TP group (Figure 2H).
Survival analysis
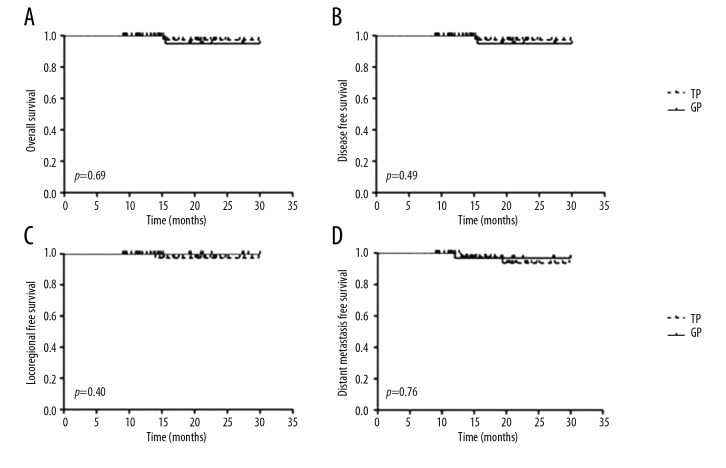
The median follow-up for the cohort was 18.7 (range, 9.03–30) months. In the TP group, 1 patient presented local recurrence, 2 patients presented distant metastasis, and 1 patient died. In the GP group, 1 patient presented distant metastasis and 1 patient died. For the entire cohort, the 1-year OS, disease-free survival (DFS), locoregionally-free survival (LRFS), and distant metastasis-free survival (DMFS) rates were 96.5%, 95.7%, 98.5%, and 97.2%, respectively. The 1-year OS (TP vs. GP, 97.3% vs. 95%), DFS (94.9% vs. 96.9%), LRFS (97.4% vs. 100%), and DMFS (97.5% vs. 96.9%) were not significantly different between the 2 groups (Figure 3).
Figure 3.
Kaplan-Meier survival curves. (A) Overall survival, (B) Disease-free survival, (C) Locoregional-free survival, and (D) Distant metastasis-free survival of patients with nasopharyngeal cancer in different groups.
Acute toxicity
The major acute toxicities during IC are listed in Table 2. Of the grade I–II hematologic toxicities (HT), leucopenia was the most common and occurred in 33 (75%) and 31 (70%) patients, followed by neutropenia [27 (61%) vs. 25 (57%)] and thrombocytopenia [10 (23%) vs. 13 (30%)] in the TP and GP groups, respectively. The most common grade III–IV HT was also leucopenia [8 (18%) vs. 6 (14%)], followed by neutropenia [6 (14%) vs. 4 (9%)] and thrombocytopenia [3 (7%) vs. 7(16%)] in the TP and GP groups, respectively. No statistically significant differences were observed between the 2 groups with respect to grade I–II or III–IV HT. Of non-HT toxicities, liver dysfunction was the most common adverse effect. During IC, no renal damage or grade III–IV liver dysfunction were observed. However, 6 (14%) and 11 (25%) patients showed grade I–II liver dysfunction in the TP and GP groups, respectively. Although there were more patients with liver dysfunction in the GP group than in the TP group, the difference was not statistically significant.
Table 2.
Acute toxicities during induction chemotherapy.
| Toxicity | GP group (n=44) | TP group (n=44) | p Value | |||
|---|---|---|---|---|---|---|
| Grade I–II (%) | Grade III–IV(%) | Grade I–II (%) | Grade III–IV (%) | Grade I–II | Grade III–IV | |
| Hematological | ||||||
| Leukemia | 31 (70) | 6 (14) | 33 (75) | 8 (18) | 0.63 | 0.58 |
| Neutropenia | 25 (57) | 4 (9) | 27 (61) | 6 (14) | 0.66 | 0.50 |
| Thrombocytopenia | 13 (30) | 7 (16) | 10 (23) | 3 (7) | 0.47 | 0.18 |
| Non-hematological | ||||||
| Liver dysfunction | 11 (25) | 0 | 6 (14) | 0 | 0.18 | – |
| Kidney dysfunction | 0 | 0 | 0 | 0 | – | – |
GP – gemcitabine plus cisplatin; TP – paclitaxel plus cisplatin.
The main acute toxicities during CCRT are summarized in Table 3. All patients completed the radiotherapy course on schedule. Dermatitis, the most common radiation toxicity, occurred in 25 (57%) vs. 28 (64%) and 7 (16%) vs. 8 (18%) patients at grade I–II and grade III–IV, respectively, in the TP and GP groups. Toxicity of grade III–IV was acceptable in both groups. There was no significant difference in the incidence of adverse effects during CCRT between the 2 groups, whether grade I–II or III–IV.
Table 3.
Acute toxicities during concurrent chemotherapy.
| Toxicity | GP group (n=44) | TP group (n=44) | p Value | |||
|---|---|---|---|---|---|---|
| Grade I–II (%) | Grade III–IV(%) | Grade I–II (%) | Grade III–IV (%) | Grade I–II | Grade III–IV | |
| Oral mucositis | 20 (46) | 12 (27) | 22 (50) | 10 (23) | 0.67 | 0.62 |
| Dermatitis | 25 (57) | 7 (16) | 28 (64) | 8 (18) | 0.51 | 0.78 |
| Xerostomia | 18 (41) | 9 (20) | 21 (48) | 8 (18) | 0.52 | 0.79 |
GP – gemcitabine plus cisplatin; TP – paclitaxel plus cisplatin.
Discussion
Gemcitabine is a novel pyrimidine analogue that inhibits DNA synthesis [18]. The role of a gemcitabine-containing regimen in NPC treatment is still being developed. For treating recurrent and metastatic NPC in a randomized phase 3 trial, a gemcitabine-cisplatin regimen was demonstrated to be superior to a 5-Fu-cisplatin regimen in terms of PFS (median, 7.0 vs. 5.6 months, p<0.001) and OS (median, 29.1 vs. 20.9 months, p=0.0025), making it the new standard of care for metastatic NPC [15]. For IC, retrospective studies have demonstrated the efficacy of GP IC for locoregionally advanced diseases. Wang et al. [19] reported 4-year local recurrence-free survival, regional recurrence-free survival, DMFS, and OS rates of 86.9%, 90.6%, 79.8%, and 81.9%, respectively. Wu [20] reported the corresponding survival rates of 93.2%, 92.3%, 89.0%, and 82.1%, respectively, at 5-year follow-up, indicating excellent disease control.
Although the efficacy of GP has been proven by these studies, the results are inconsistent when compared with those of other IC regimens in randomized trials. In an early study that compared GP and PF, although a favorable trend in locoregional control was observed for the GP group, there were no significant differences in any of the endpoints [13]. A phase 2/3 prospective trial that compared a gemcitabine, cisplatin, and paclitaxel regimen with CCRT alone also found no difference in the OS or DFS [21]. However, in a retrospective study, it was reported that GP and TP regimens led to significantly higher DFS and OS compared with those of PF regimens. In some subgroups of patients, including male patients and patients with bilateral neck metastasis, the GP regimen led to a significantly better OS than the TP or PF regimens [14].
In the present study, we reported early survival outcomes of using a GP regimen as IC at our center, with a main focus on tumor response after IC. Data were collected from a total of 44 patients, of which 86.4% (38 of 44) had T4 stage disease. The tumor volume reduction was measured after IC and was compared between patients receiving GP IC and those receiving TP IC. To maintain a balanced baseline, patients in the TP group were carefully matched with patients in the GP group with respect to the clinical stage and tumor volume. The volume change was analyzed by 2 methods. The results of both methods show that, at the dosage currently used, GP was more effective than TP in tumor volume reduction.
Tumor volume has been well established as an independent prognostic factor [16,22]. More cell-killing by upfront IC is associated with better control following CCRT. In fact, recent studies have proven that tumor response to IC might be a useful predictor of prognosis. Peng et al. [23] analyzed 399 patients who received IC and found that complete response was a favorable prognostic factor for failure-free survival (FFS) and partial response for FFS and OS when compared with stable disease. Furthermore, these authors performed a secondary analysis of a phase 3 prospective trial comparing IC with radiotherapy alone and reached similar conclusions [24]. In line with these findings, the results of the present study suggest that GP might deliver better tumor control than TP as an induction regimen.
However, because the median follow-up of our patients was only 18.7 months, the TP group had only 1 local failure and 2 distant metastases, whereas the GP group had only 1 distant metastasis and no locoregional failures. Both groups showed excellent disease control and there were no significant differences in any of the endpoints between the 2 regimens. A longer follow-up is required to prove whether GP has the advantage of disease control over TP in IC.
The acute toxicity rates during IC in both the TP and GP groups were acceptable. There were no statistically significant differences in hematological or non-hematological adverse effects between the 2 groups. In the present study, the incidence of grade I–II leucopenia and thrombocytopenia in patients in the GP group was similar to that reported by He et al. [25]. However, the incidence of grade III–IV thrombocytopenia was slightly higher in the GP group than in the TP group (16% vs. 7%). The incidence of grade III–IV neutropenia was approximately 9%, which was much lower than that reported by Yau et al. (52%) [13]. The high dose of gemcitabine used in Yau’s study (1250 mg/m2 rather than 1000 mg/m2, administered on days 1 and 8) might account for this difference. No kidney dysfunction was observed in the TP and GP groups. The incidence of grade I–II liver toxicity was higher in the GP group than in the TP group; however, this difference was not statistically significant. In another study using gemcitabine and nedaplatin as IC, the incidences of grade I–II and grade III-IV liver dysfunction reached 53% and 9.3%, respectively, suggesting that liver function should be closely monitored during gemcitabine-based chemotherapy [26]. During CCRT, the common toxicities, including mucositis, dermatitis, and xerostomia, did not differ between the 2 groups. All patents completed the entire course of irradiation and it was well tolerated. Only 1 patient in the GP group experienced grade IV mucositis and required tube feeding.
The purpose of the present study was limited to providing evidence that GP might be more efficient than TP for tumor volume reduction. Because the sample size was only 44 patients for each group, our conclusion remains to be validated in large-scale studies. In addition, the present study only assessed the 1-year OS, which was not significantly different between the 2 regimens. Assessing whether GP is superior to TP for tumor control requires further long-term follow-ups with the patients enrolled in this study, and well-designed prospective trials are also warranted.
Conclusions
For treating locoregional NPC via IC, the GP regimen had more powerful effects than the TP regimen on the reduction of the primary tumor and involved lymph node volume. Both regimens had comparable acute toxicities. Whether tumor regression after GP induction could transform into survival benefits must be confirmed by outcomes of long-term follow-up studies.
Footnotes
Conflicts of interest
None.
Source of support: This study was supported by the National Natural Science Foundation of China (Grant number 81771921)
References
- 1.Ou X, Zhou X, Shi Q, et al. Treatment outcomes and late toxicities of 869 patients with nasopharyngeal carcinoma treated with definitive intensity modulated radiation therapy: New insight into the value of total dose of cisplatin and radiation boost. Oncotarget. 2015;6:38381–97. doi: 10.18632/oncotarget.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zong J, Lin S, Lin J, et al. Impact of intensity-modulated radiotherapy on nasopharyngeal carcinoma: Validation of the 7th edition AJCC staging system. Oral Oncol. 2015;51:254–59. doi: 10.1016/j.oraloncology.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Mao YP, Tang LL, Chen L, et al. Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin J Cancer. 2016;35:103. doi: 10.1186/s40880-016-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Setton J, Han J, Kannarunimit D, et al. Long-term patterns of relapse and survival following definitive intensity-modulated radiotherapy for non-endemic nasopharyngeal carcinoma. Oral Oncol. 2016;53:67–73. doi: 10.1016/j.oraloncology.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su SF, Han F, Zhao C, et al. Treatment outcomes for different subgroups of nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Chin J Cancer. 2011;30:565–73. doi: 10.5732/cjc.010.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng H, Chen L, Zhang J, et al. Induction chemotherapy improved long-term outcomes of patients with locoregionally advanced nasopharyngeal carcinoma: A propensity matched analysis of 5-year survival outcomes in the era of intensity-modulated radiotherapy. J Cancer. 2017;8:371–77. doi: 10.7150/jca.16732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ying J, Xiu YC, Yan XS, et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2012;10:1717–25. doi: 10.1007/s00432-012-1219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–17. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 9.Lee AWM, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005;23:6966–75. doi: 10.1200/JCO.2004.00.7542. [DOI] [PubMed] [Google Scholar]
- 10.Ribassin-Majed L, Marguet S, Lee A, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol. 2017;35:498–505. doi: 10.1200/JCO.2016.67.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng H, Chen L, Li WF, et al. Tumor response to neoadjuvant chemotherapy predicts long-term survival outcomes in patients with locoregionally advanced nasopharyngeal carcinoma: A secondary analysis of a randomized phase 3 clinical trial. Cancer. 2017;123:1643–52. doi: 10.1002/cncr.30520. [DOI] [PubMed] [Google Scholar]
- 12.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 13.Yau TK, Lee AW, Wong DH, et al. Induction chemotherapy with cisplatin and gemcitabine followed by accelerated radiotherapy and concurrent cisplatin in patients with stage IV(A–B) nasopharyngeal carcinoma. Head Neck. 2006;28:880–87. doi: 10.1002/hed.20421. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Xu M, Jiang W, et al. Induction chemotherapy for the treatment of non-endemic locally advanced nasopharyngeal carcinoma. Oncotarget. 2017;8:6763–74. doi: 10.18632/oncotarget.14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: A multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388:1883–92. doi: 10.1016/S0140-6736(16)31388-5. [DOI] [PubMed] [Google Scholar]
- 16.Feng M, Wang W, Fan Z, et al. Tumor volume is an independent prognostic indicator of local control in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Radiat Oncol. 2013;8:208. doi: 10.1186/1748-717X-8-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King AD, Ahuja AT, Leung SF, et al. Neck node metastases from nasopharyngeal carcinoma: MR imaging of patterns of disease. Head Neck. 2000;22:275–81. doi: 10.1002/(sici)1097-0347(200005)22:3<275::aid-hed10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Barton-Burke M. Gemcitabine: A pharmacologic and clinical overview. Cancer Nurs. 1999;22:176–83. doi: 10.1097/00002820-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Wang FZ, Sun QQ, Jiang C, et al. Gemcitabine/cisplatin induction chemotherapy before concurrent chemotherapy and intensity-modulated radiotherapy improves outcomes for locoregionally advanced nasopharyngeal carcinoma. Oncotarget. 2017;8:96798–808. doi: 10.18632/oncotarget.18245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M, Ou D, He X, Hu C. Corrigendum to “Long-term results of a phase II study of gemcitabine and cisplatin chemotherapy combined with intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma”. Oral Oncol. 2017;73:118–23. doi: 10.1016/j.oraloncology.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Tan T, Lim W, Fong K, et al. Concurrent chemo-radiation with or without induction gemcitabine, carboplatin, and paclitaxel: A randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2015;91:952–60. doi: 10.1016/j.ijrobp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Guo R, Sun Y, Yu XL, et al. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother Oncol. 2012;104:294–99. doi: 10.1016/j.radonc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Peng H, Chen L, Zhang Y, et al. The tumour response to induction chemotherapy has prognostic value for long-term survival outcomes after intensity-modulated radiation therapy in nasopharyngeal carcinoma. Sci Rep. 2016;6:24835. doi: 10.1038/srep24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509–20. doi: 10.1016/S1470-2045(16)30410-7. [DOI] [PubMed] [Google Scholar]
- 25.He X, Ou D, Ying H, et al. Experience with combination of cisplatin plus gemcitabine chemotherapy and intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2012;269:1027–33. doi: 10.1007/s00405-011-1669-9. [DOI] [PubMed] [Google Scholar]
- 26.Jin T, Chen X, Liu J. Evaluation of the efficacy and safety of a neoadjuvant gemcitabine and nedaplatin regimen followed by radiotherapy or concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma. Oncol Lett. 2015;10:1123–30. doi: 10.3892/ol.2015.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]