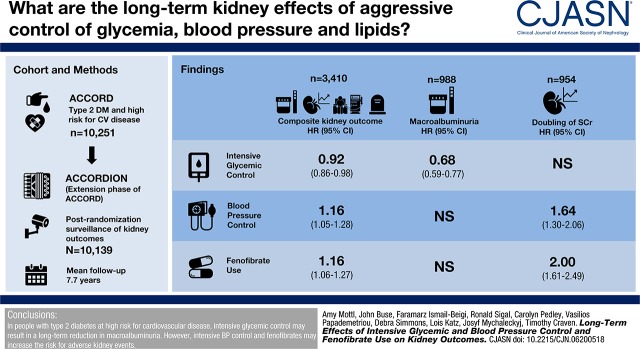
Abstract
Background and objectives
In people with type 2 diabetes, aggressive control of glycemia, BP, and lipids have resulted in conflicting short-term (<5 years) kidney outcomes. We aimed to determine the long-term kidney effects of these interventions.
Design, setting, participants, & measurements
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) was a multifactorial intervention study in people with type 2 diabetes at high risk for cardiovascular disease (n=10,251), to examine the effects of intensive glycemic control (hemoglobin A1c <6.0% versus 7%–7.9%), BP control (systolic BP <120 mm Hg versus <140 mm Hg) or fenofibrate versus placebo added to simvastatin on cardiovascular events and death. The glycemia trial lasted 3.7 years and participants were followed for another 6.5 years in ACCORDION, the ACCORD Follow-On Study. The post hoc primary composite kidney outcome was defined as incident macroalbuminuria, creatinine doubling, need for dialysis, or death by any cause. Cox proportional hazards regression estimated the effect of each intervention on the composite outcome and individual components. In secondary outcome analyses, competing risk regression was used to account for the risk of death in incident kidney outcomes. Analyses were adjusted for sociodemographics, randomization groups, and clinical factors.
Results
There were 988 cases of incident macroalbuminuria, 954 with doubling of creatinine, 351 requiring dialysis, and 1905 deaths. Hazard ratios (HRs) for the composite outcome with intensive glycemic, BP control, and fenofibrate use compared with standard therapy were 0.92 (95% confidence interval [95% CI], 0.86 to 0.98), 1.16 (95% CI, 1.05 to 1.28), and 1.16 (95% CI, 1.06 to 1.27). Multivariable, secondary outcome analyses showed that in the glycemia trial, only macroalbuminuria was significantly decreased (HR, 0.68; 95% CI, 0.59 to 0.77). In the BP and lipid trials, only creatinine doubling was affected (HR, 1.64; 95% CI, 1.30 to 2.06 and HR, 2.00; 95% CI, 1.61 to 2.49, respectively).
Conclusions
In people with type 2 diabetes at high risk for cardiovascular disease, intensive glycemic control may result in a long-term reduction in macroalbuminuria; however, intensive BP control and fenofibrates may increase the risk for adverse kidney events.
Keywords: diabetic nephropathy; clinical trial; fibrates; glycemic control; blood pressure control; Fenofibrate; blood pressure; Diabetes Mellitus, Type 2; creatinine; Simvastatin; Glycated Hemoglobin A; lipids; Random Allocation; renal dialysis; Blood Pressure Determination; Blood Glucose; Cardiovascular Diseases; kidney
Visual Abstract
Introduction
Diabetic kidney disease (DKD) is the leading cause of ESKD, resulting in an annual fiscal burden of billions to society and a marked decrease in quality of life for the individual (1,2). The vast majority of patients with DKD do not develop ESKD because of the very high incidence of death in this population (3,4). The risks for ESKD and cardiovascular events and death increase with rising albuminuria and declining eGFR (5,6). The reverse is also applicable, as the presence of cardiovascular disease increases the incidence of creatinine doubling and ESKD (7,8). Although patients with diabetes and cardiovascular disease are at very high risk for progressive kidney disease and death, the optimal management of these patients is still unclear.
For decades, clinical trials have sought the ideal clinical targets for glycemic and BP control and lipid therapies to provide reduction in kidney risk. Historic trials led to the commonly accepted notion that reduction in hemoglobin A1c (HbA1c) <7% and BP <140/90 mm Hg decrease the risk for progression of DKD (9,10). More recent trials have examined the kidney effects of further reductions in HbA1c to <6%–6.5% and systolic BP to <120 mm Hg. Although these trials have consistently found reductions in albuminuria, results are conflicting with regard to creatinine doubling and ESKD (11–15). The effect of lipid therapies on DKD have been more consistent: statin medications do not appear to affect DKD progression but are recommended for cardiovascular protection in this high-risk population (16); fibrates have been found to cause a rise in creatinine that is reversible with drug withdrawal and so are felt to not increase the risk for long-term kidney events (17–19).
The majority of these studies were conducted over a relatively short time period. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial continued to monitor consenting participants in the ACCORD follow-on trial (ACCORDION) for several years after trial completion, for a total mean follow-up of 7.7 years. In this post hoc follow-up study, we aimed to determine effects of intensive glycemic and BP control and fenofibrate use on long-term kidney outcomes.
Materials and Methods
Study Population
The full protocol for the main ACCORD trial has been previously published (20,21). Inclusion criteria included (1) type 2 diabetes and HbA1c≥7.5%; (2) age 40–79 years and known cardiovascular disease; or (3) age 55–79 years with anatomic evidence of significant atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two risk factors for cardiovascular disease (dyslipidemia, hypertension, obesity, or current smoker status). Exclusion criteria included body mass index >45 kg/m2 and serum creatinine >132.6 µmol/L (1.5 mg/dl). Potential participants also had to be eligible for the BP and/or lipid trials, each of which had additional criteria as previously published (22,23). There were 77 clinical centers in the United States and Canada; ethics committees at each center approved the protocol, which adhered to the Declaration of Helsinki. All participants provided written, informed consent. The parent ACCORD study is registered with Clinicaltrials.gov under identifier NCT00000620. Individual deidentified participant data including the protocols for ACCORD and ACCORDION, the data dictionary, manual of procedures, and forms are currently available at https://biolincc.nhlbi.nih.gov/studies/accord/.
Study Design, Measurements, and Outcomes
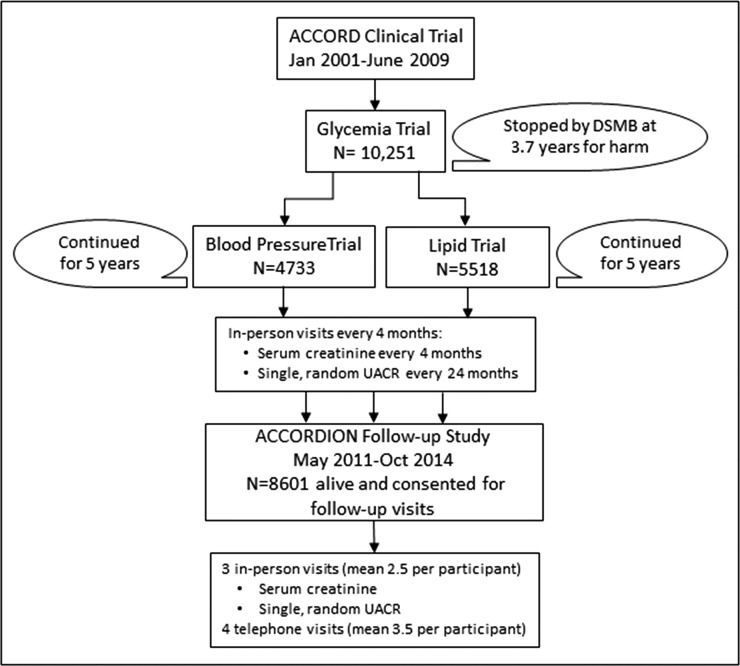
The ACCORD trial was a double two-by-two factorial, parallel treatment trial (see Figure 1) in which patients were randomized to one of two glycemic treatment arms, with the intensive arm targeting HbA1c<6.0% and the standard arm targeting HbA1c 7.0%–7.9%. On the basis of eligibility criteria, half of the participants were allotted to a lipid intervention consisting of randomization to fenofibrate versus placebo, both in addition to statin therapy. The other half were allotted to one of two randomly assigned BP treatment arms, with the intensive arm targeting systolic BP <120 mm Hg and the standard arm targeting systolic BP <140 mm Hg. The ACCORD glycemia trial lasted for a mean of 3.7 years; it was stopped early because of the Data Safety Monitoring Board finding of excess mortality in the intensive glucose-lowering group (24). Participants were subsequently managed according to the standard glucose protocol and monitored for an additional 17 months while the BP and lipid trials were completed.
Figure 1.
Schematic of the ACCORD and ACCORDION study designs and surveillance for kidney outcomes. DSMB, Data Safety Monitoring Board; ACCORD, Action to Control Cardiovascular Risk in Diabetes; ACCORDION, ACCORD Follow-On Study.
All surviving participants who could be contacted were invited to participate in the follow-up study (ACCORDION) for a mean period of 7.7 years (median 5.6 years) from the time of initial randomization (see Figure 1) (25). During the ACCORDION trial follow-up period, participants were seen or contacted for surveillance of cardiovascular outcomes, death, and dialysis. The chronicity and indications for dialysis were not obtained. Surveillance for deaths in the United States was supplemented for participants withdrawn or lost to follow-up using the National Death Index. Serum creatinine and urine albumin-to-creatinine (UACR) were measured centrally on two occasions, separated by approximately 30 months. eGFR was calculated using the CKD Epidemiology Collaboration Creatinine Equation (26).
All participants from the ACCORD trial with any surveillance postrandomization for kidney outcomes were included in analyses. Participants without surveillance for kidney outcomes (n=112) either died, withdrew, or were lost to follow-up. We defined the primary composite kidney outcome as incident macroalbuminuria (UACR>300 μg/mg), creatinine doubling, self-reported need for dialysis, or death from any cause. Secondary outcomes included individual end points comprising the primary outcome. Participants with overt albuminuria were excluded from analyses of incident macroalbuminuria but were maintained in analyses of the composite outcome if they sustained one of the other outcomes (incident doubling of creatinine or ESKD).
Statistical Analyses
All analyses used an intention-to-treat approach. The probability of reaching the composite kidney outcome was calculated using the cumulative incidence function. Cox proportional hazards regression estimated the effect of each intervention on the primary composite kidney outcome. In secondary outcome analyses of the individual (nonfatal) composite outcome components, a competing risk regression was used to estimate the subdistribution hazard ratio (SHR), which accounts for the competing risk of death (27). Multivariable regression analyses included baseline values for age, sex, ethnicity, study site, randomization to tight BP group, randomization to fenofibrate group, previous cardiovascular event, eGFR, UACR, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, HbA1c, and systolic BP as covariates. Estimates of treatment assignment in each subtrial (intensive versus standard control in BP or fibrate versus placebo in lipid) were performed using contrast statements in proportional hazards regression models.
Differential effects of treatment arm assignment during active treatment versus posttrial follow-up were examined for the primary kidney outcome using two-way interactions between a time-varying indicator event occurrence before or after the end of the active intervention period and treatment arm assignment. Interaction terms were also explored for sex, ethnicity, and baseline CKD status (defined as macroalbuminuria or eGFR<60 ml/min per 1.73 m2). If the interaction term was significant to P<0.1, we performed stratified analyses. For all other analyses, we utilized a nominal P value of <0.05, without correction for multiple testing. All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC).
Results
Baseline characteristics of participants, stratified by randomization in the glycemia trial, are displayed in Table 1. The presence of any CKD was common, with 37% of participants having eGFR<60 ml/min per 1.73 m2 and/or UACR≥30 μg/mg. In those with CKD, the severity was mild in the vast majority, with 10% having an eGFR<60 ml/min per 1.73 m2 and 5% with macroalbuminuria.
Table 1.
Characteristics of 10,139 participants of the ACCORD study at baseline according to randomization to intensive versus standard glycemic control
| Baseline Characteristicsa | Intensive Therapy | Standard Therapy | ||
|---|---|---|---|---|
| N | Mean±SD or N (%) | N | Mean±SD or N (%) | |
| Age, yr | 5074 | 62±7 | 5065 | 62±7 |
| Women, % | 5074 | 1959 (39%) | 5065 | 1940 (38%) |
| Non-Hispanic white, % | 5074 | 3266 (64%) | 5065 | 3305 (65%) |
| Glycated hemoglobin, % | 5066 | 8.3±1.1 | 5053 | 8.3±1.1 |
| BP, mm Hg | 5050 | 5046 | ||
| Systolic | 136±17 | 137±17 | ||
| Diastolic | 75±11 | 75±11 | ||
| Cholesterol, g/dl | 5048 | 5035 | ||
| Total | 183±42 | 183±41.6 | ||
| LDL | 105±34 | 105±34 | ||
| HDL | 42±12 | 42±12 | ||
| Triglycerides | 191±148 | 190±149 | ||
| Body mass index, kg/m | 5071 | 32±6 | 5062 | 32±6 |
| Waist circumference, cm | 5019 | 107±14 | 5021 | 107±14 |
| Previous cardiovascular event, % | 5074 | 1804 (36%) | 5065 | 1755 (35%) |
| Baseline CKD status, %b | 5042 | 5022 | ||
| No CKD | 3173 (63%) | 3163 (63%) | ||
| Stage 1 | 626 (12%) | 641 (13%) | ||
| Stage 2 | 714 (14%) | 713 (14%) | ||
| Stage 3a | 425 (8%) | 419 (8%) | ||
| Stage 3b | 98 (2%) | 92 (2%) | ||
| Stage 4 | 6 (0.1%) | 0 | ||
| Baseline UACR, % | 5042 | 5022 | ||
| ≥30 μg/mg | 1247 (25%) | 1225 (24%) | ||
| ≥300 μg/mg | 226 (5%) | 241 (5%) | ||
| ≥1000 μg/mg | 97 (2%) | 99 (2%) | ||
| eGFR, ml/min per 1.73 m2b | 5074 | 83±17 | 5065 | 84±17 |
| UACR, μg/mgc | 5042 | 13.5 [6.8, 43.8] | 5022 | 13.6 [6.8, 44.4] |
| ACEI/ARB use, % | 5074 | 3428 (68%) | 5065 | 3445 (68.0%) |
| Thiazolidinedione use, % | 5074 | 986 (19%) | 5065 | 976 (19%) |
ACCORD, Action to Control Cardiovascular Risk in Diabetes; UACR, urine albumin-to-creatinine ratio; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Includes all ACCORD trial participants with at least some surveillance for primary outcome. Description of ACCORDION study population is available (43).
eGFR was calculated using the CKD Epidemiology Collaboration Creatinine Equation. Stage 1 CKD: eGFR≥90 ml/min per 1.73 m2 and UACR≥30 μg/mg; stage 2 CKD: eGFR 60–89 ml/min per 1.73 m2 and UACR≥30 μg/mg; stage 3a CKD: eGFR 45–59 ml/min per 1.73 m2; stage 3b CKD: eGFR 30–44 ml/min per 1.73 m2; stage 4 CKD: eGFR 15–29 ml/min per 1.73 m2.
Data are presented as median [IQR].
Mean follow-up for kidney-related outcomes was 7.7 (median 5.7 years) for the glycemic intervention. Among 10,139 participants with at least some follow-up surveillance for kidney-related outcomes, 7076 (69%) either experienced the primary kidney outcome during the ACCORD trial or had assessment for kidney outcomes during the ACCORDION post-trial follow-up. One third (n=3410) of participants reached the primary composite kidney outcome. There were 988 cases of incident macroalbuminuria, 954 with doubling of creatinine, 351 participants requiring dialysis, and 1905 deaths. Of the 3410 participants with the primary composite kidney outcome, two thirds (n=2249) had two or more components of the composite outcome. Because the need for dialysis was not adjudicated, some may have been due to AKI requiring transient dialysis. The final serum creatinine was <2.0 mg/dl in 257 (73%) out of 351 participants self-reporting an occurrence of dialysis.
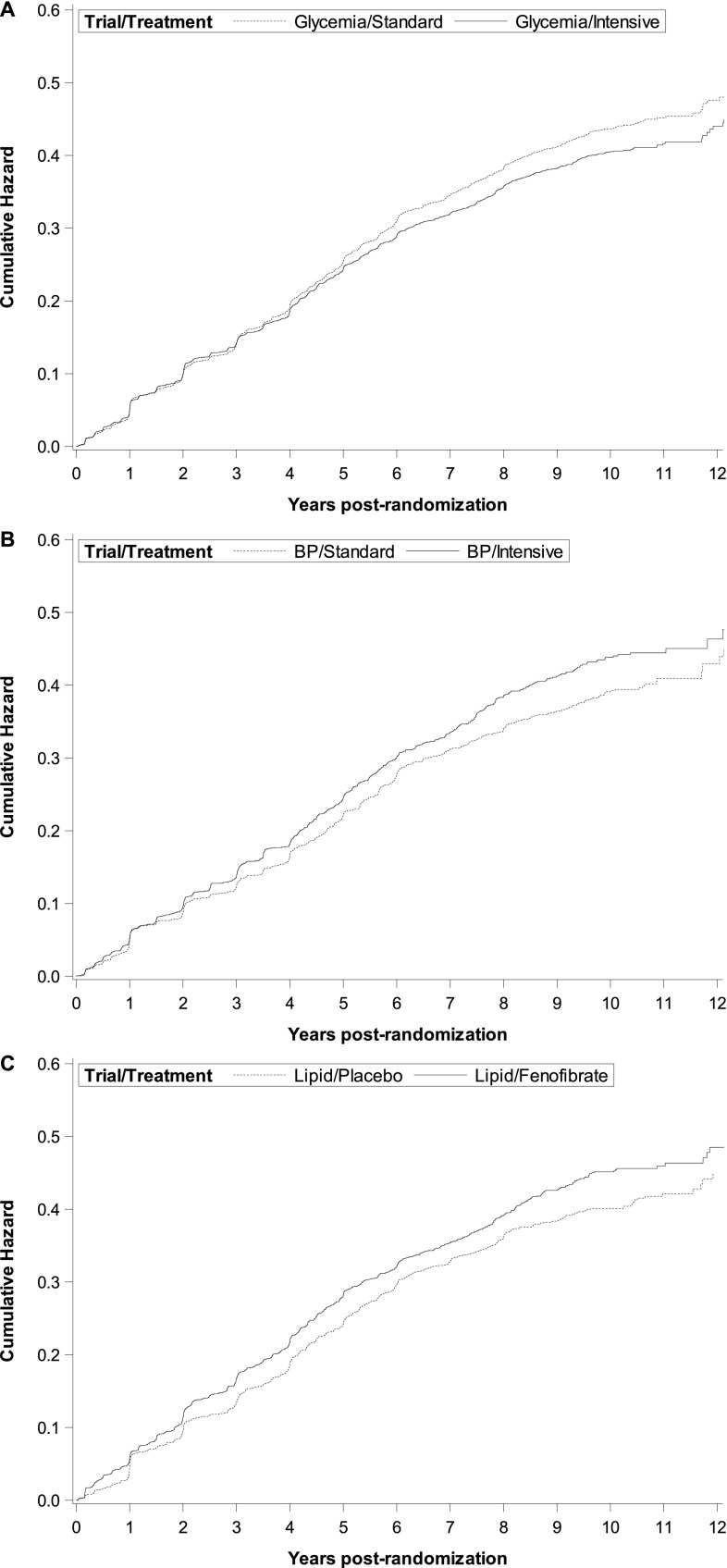
Figure 2, A–C illustrates the cumulative incidence for the composite outcome, stratified by treatment groups. Table 2 displays the univariable and multivariable results for the effects of the three treatment trials on the composite and secondary kidney outcomes. Randomization to the intensive glycemic control arm reduced the incidence of the composite kidney outcome (hazard ratio [HR], 0.92; 95% confidence interval [95% CI], 0.86 to 0.98) but this effect was primarily driven by a reduction in incident macroalbuminuria (SHR, 0.68; 95% CI, 0.59 to 0.77). Post hoc analysis for “probable maintenance dialysis” in the glycemia trial censored participants with a final serum creatinine <2.0, yielding a univariable SHR of 0.85 (95% CI, 0.57 to 1.28).
Figure 2.
Cumulative incidence of the primary composite kidney outcome (incident macroalbuminuria [UACR>300 μg/mg], doubling of creatinine, incident dialysis or all-cause mortality) according to randomization arm. (A) Glycemia trial (HbA1c: intensive arm <6.0% versus standard arm 7.0%–7.9%) with the intensive arm showing reduced risk, (B) BP trial (systolic BP: intensive arm <120 mm Hg versus standard arm <140 mm Hg) with the intensive arm showing higher risk, and (C) lipid trial (fenofibrate versus placebo) with the fenofibrate arm showing higher risk.
Table 2.
Effects of intensive glycemic control, BP control, and fenofibrate on long-term kidney outcomes in 10,139 participants of the ACCORD study
| Outcome | Intensive Glycemic Control | Intensive BP Control | Fenofibrate Treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n=10,139 (5074 Intensive; 5065 Standard) | n=4682 (2345 Intensive; 2337 Standard) | n=5457 (2736 Fibrate; 2721 Placebo) | |||||||
| Event Number | Univariable HR/SHR (95% CI) | Multivariablea HR/SHR (95% CI) | Event Number | Univariable HR/SHR (95% CI) | Multivariablea HR/SHR (95% CI) | Event Number | Univariable HR/SHR (95% CI) | Multivariablea HR/SHR (95% CI) | |
| Composite kidney outcome | 1652 Int 1759 Std | 0.92 (0.86 to 0.99)d | 0.92 (0.86 to 0.98)d | 780 Int 721 Std | 1.15 (1.04 to 1.23)d | 1.16 (1.05 to 1.28)d | 1007 Feno 903 Plac | 1.15 (1.05 to 1.26)d | 1.16 (1.06 to 1.27)d |
| Macroalbuminuriab,c | 421 Int 567 Std | 0.72 (0.64 to 0.82)d | 0.68 (0.59 to 0.77)d | 212 Int 219 Std | 1.00 (0.83 to 1.21) | 0.97 (0.79 to 1.17) | 304 Feno 253 Plac | 1.19 (1.00 to 1.40) | 1.11 (0.93 to 1.33) |
| Doubling of creatininec | 490 Int 464 Std | 1.05 (0.93 to 1.20) | 1.09 (0.94 to 1.23) | 245 Int 170 Std | 1.52 (1.25 to 1.85)d | 1.64 (1.30 to 2.06)d | 344 Feno 195 Plac | 1.83 (1.54 to 2.18)d | 2.00 (1.61 to 2.49)d |
| Dialysisc | 161 Int 190 Std | 0.84 (0.68 to 1.04) | 0.92 (0.72 to 1.16) | 72 Int 81 Std | 0.91 (0.67 to 1.25) | 0.90 (0.63 to 1.28) | 98 Feno 100 Plac | 0.97 (0.74 to 1.29) | 1.09 (0.80 to 1.50) |
| All-cause mortality | 952 Int 953 Std | 1.00 (0.92 to 1.09) | 1.00 (0.91 to 1.10) | 406 Int 403 Std | 1.03 (0.90 to 1.18) | 1.01 (0.88 to 1.17) | 537 Feno 559 Plac | 0.95 (0.84 to 1.06) | 0.96 (0.85 to 1.08) |
ACCORD, Action to Control Cardiovascular Risk in Diabetes; HR, hazard ratio; SHR, subhazard ratio; 95% CI, 95% confidence interval; Int, intensive; Std, standard; Feno, fenofibrate; Plac, placebo.
Multivariable analyses are adjusted for: baseline age, sex, ethnicity, study site, randomization to tight BP group, randomization to fenofibrate group, previous cardiovascular event, eGFR, urine albumin-to-creatinine ratio, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, hemoglobin A1c, and systolic BP.
Denominators for macroalbuminuria outcome: 4410 intensive glycemic, 4431 standard glycemic; 2065 intensive BP, 2040 standard BP; 2388 fenofibrate, 2348 placebo.
Models for nonfatal components of the composite outcome include death as a competing risk.
Statistically significant results.
Randomization to intensive BP control resulted in an increased risk for the composite kidney outcome (HR, 1.16; 95% CI, 1.05 to 1.28); however, doubling of serum creatinine was the only secondary outcome to be affected (SHR, 1.64; 95% CI, 1.30 to 2.06). Similar results were obtained via randomization to fenofibrate versus placebo (HR, 1.16; 95% CI, 1.06 to 1.27) with a strong effect from increased risk for doubling of serum creatinine (HR, 2.00; 95% CI, 1.61 to 2.49). Post hoc analyses for the composite kidney outcome excluding death in the definition did not significantly change the results.
Post hoc analyses for a differential glycemic intervention effect was marginally significant (P=0.05), whereas the test for a differential effect in the BP or lipid trials was significant (P=0.01). During active treatment, there was almost no difference in incidence of the primary kidney outcome between intensive and standard glycemic arms (HR, 0.99; 95% CI, 0.91 to 1.06), whereas in the post-trial follow-up, the observed incidence was reduced in intensive arm participants (HR, 0.83; 95% CI, 0.71 to 0.97). In the BP trial, an increased incidence of the primary kidney outcome in the intensive versus standard arm observed during the active trial period (HR, 1.12; 95% CI, 1.00 to 1.26) became larger during the post-trial follow-up (HR, 1.41; 95% CI, 1.11 to 1.79). Similarly, in the lipid trial, the increased incidence in the fibrate versus placebo arm during the active phase (HR, 1.16; 95% CI, 1.05 to 1.29) became larger during post-trial follow-up as well (HR, 1.36; 95% CI, 1.10 to 1.69).
There was evidence for effect modification by sex in the glycemia trial for the primary composite kidney outcome (P=0.06), incident macroalbuminuria (P=0.02), and dialysis outcomes (P=0.04), and in the BP and lipid trials for the primary composite kidney outcome, macroalbuminuria and dialysis (P<0.1). There was also evidence for effect modification by race in the BP and lipid trials for the primary composite kidney outcome (P=0.01), doubling of serum creatinine (P<0.01), and all-cause mortality (P=0.01). Results of the stratified analyses are shown in Tables 3–5.
Table 3.
Incidence of long-term kidney outcomes according to initial randomization to intensive glycemic control (hemoglobin A1c <6.0%), stratified by sex
| Outcomea | Women | Men | ||
|---|---|---|---|---|
| n=3825 (1917 Intensive; 1908 Standard) | n=6146 (3075 Intensive; 3071 Standard) | |||
| Event Number | HR/SHR (95% CI) | Event Number | HR/SHR (95% CI) | |
| Composite outcome | 579 Int | 1.0 (0.89 to 1.12) | 1036 Int | 0.88 (0.81 to 0.95)c |
| 589 Std | 1131 Std | |||
| Macroalbuminuriab | 150 Int | 0.83 (0.66 to 1.05) | 258 Int | 0.61 (0.51 to 0.71)c |
| 170 Std | 394 Std | |||
| Dialysis | 67 Int | 1.11 (0.76 to 1.62) | 94 Int | 0.83 (0.61 to 1.13) |
| 57 Std | 129 Std | |||
HR, hazard ratio; SHR, subhazard ratio; 95% CI, 95% confidence interval; Int, intensive; Std, standard.
HRs and 95% CIs are for fully adjusted multivariable analyses.
Denominators for macroalbuminuria outcome among women: 1664 intensive, 1689 standard; among men: 2688 intensive, 2704 standard.
Statistically significant results.
Table 5.
Incidence of long-term kidney outcomes according to initial randomization to fenofibrate versus placebo (in addition to statin therapy), stratified by sex
| Outcomea | Women | Men | White Race | Nonwhite Race | ||||
|---|---|---|---|---|---|---|---|---|
| n=1634 (821 Fibrate, 813 Placebo) | n=3721 (1864 Fibrate, 1857 Placebo | n=3676 (1858 Fibrate, 1818 Placebo) | n=1679 (827 Fibrate, 852 Placebo) | |||||
| Event Number | HR/SHR (95% CI) | Event Number | HR/SHR (95% CI) | Event Number | HR/SHR (95% CI) | Event Number | HR/SHR (95% CI) | |
| Composite kidney outcome | 284 Feno | 1.32 (1.11 to 1.57)c | 703 Feno | 1.10 (0.99 to 1.22) | 680 Feno | 1.08 (0.97 to 1.20) | 307 Feno | 1.36 (1.15 to 1.61)c |
| 232 Plac | 647 Plac | 629 Plac | 250 Plac | |||||
| Macroalbuminuriab | 88 Feno | 1.51 (1.07 to 2.14)c | 212 Feno | 1.01 (0.82 to 1.24) | — | — | ||
| 57 Plac | 190 Plac | |||||||
| Doubling of creatinine | — | — | 220 Feno | 1.82 (1.46 to 2.28)c | 111 Feno | 2.08 (1.51 to 2.86)c | ||
| 121 Plac | 63 Plac | |||||||
| Dialysis | 31 Feno | 1.03 (0.61 to 1.75) | 67 Feno | 1.12 (0.76 to 1.66) | — | — | ||
| 31 Plac | 69 Plac | |||||||
| All-cause mortality | — | — | 379 Feno 416 Plac | 0.88 (0.76, 1.01) | 152 Feno | 1.26 (1.0, 1.60) | ||
| 128 Plac | ||||||||
HR, hazard ratio; SHR, subhazard ratio; 95% CI, 95% confidence interval; Feno, fenofibrate; Plac, placebo; —, analysis not performed because interaction term was not significant.
HRs and 95% CIs are for fully adjusted multivariable analyses using contrast statements from models with participants from both subtrials.
Denominators for macroalbuminuria outcome among women: 709 fenofibrate, 713 placebo; among men: 1645 fenofibrate, 1603 placebo; among whites: 1646 fenofibrate, 1587 placebo; among nonwhites: 708 fenofibrate, 729 placebo.
Statistically significant results.
Table 4.
Incidence of long-term kidney outcomes according to initial randomization to intensive BP control (systolic BP <120 mm Hg), stratified by sex and also by white race
| Outcomea | Women | Men | White Race | Nonwhite Race | ||||
|---|---|---|---|---|---|---|---|---|
| n=2191 (1089 Intensive; 1102 Standard) | n=2425 (1219 Intensive; 1206 Standard) | n=2808 (1382 Intensive, 1426 Standard) | n=3487 | |||||
| Event Number | HR/SHR (95% CI) | Event Number | HR/SHR (95% CI) | Event Number | HR/SHR (95% CI) | Event Number | HR/SHR (95% CI) | |
| Composite kidney outcome | 352 Int | 1.27 (1.09 to 1.49)c | 417 Int | 1.07 (0.94 to 1.23) | 503 Int | 1.11 (0.98 to 1.26) | 266 Int | 1.26 (1.06 to 1.51)c |
| 300 Std | 400 Std | 456 Std | 244 Std | |||||
| Macroalbuminuriab | 86 Int | 0.86 (0.64 to 1.18) | 126 Int, | 1.04 (0.81 to 1.34) | — | — | ||
| 89 Std | 124 Std | |||||||
| Doubling of creatinine | — | — | 169 Int | 2.13 (1.63 to 2.77)c | 72 Int | 1.17 (0.84 to 1.63) | ||
| 86 Std | 70 Std | |||||||
| Dialysis | 28 Int | 0.90 (0.52 to 1.56) | 43 Int | 0.89 (0.55 to 1.45) | — | — | ||
| 34 Std | 44 Std | |||||||
| All-cause mortality | — | — | 265 Int | 0.92 (0.78 to 1.09) | 133 Int | 1.24 (0.97 to 1.59) | ||
| 282 Std | 114 Std | |||||||
HR, hazard ratio; SHR, subhazard ratio; 95% CI, 95% confidence interval; Int, intensive; Std, standard; —, analysis not performed because interaction term was not significant.
HRs and 95% CIs are for fully adjusted multivariable analyses using contrast statements from models with participants from both subtrials.
Denominators for macroalbuminuria outcome among women: 965 intensive, 966 standard; among men: 1081 intensive, 1063 standard; among whites: 1249 intensive, 1274 standard; among nonwhites: 797 intensive, 755 standard.
Statistically significant results.
Discussion
This long-term follow-up study of >10,000 individuals with type 2 diabetes at high risk for cardiovascular events provides further data against targeting intensive glycemic and BP control or the use of fibrates in this population. Specifically, although intensive glycemic control ameliorated the risk for incident macroalbuminuria, there was a dearth of evidence to support an effect on creatinine doubling or incident dialysis. Moreover, we found probable harm from intensive BP control or fibrate use on these kidney outcomes.
Intensive Glycemic Control
To put these findings into context, it is important to review in-trial and long term follow-up results from the Diabetes Control and Complications Trial (DCCT) and United Kingdom Prospective Diabetes Study (UKPDS), which provided clear evidence that improved glycemic control significantly reduced the risk for progression of both albuminuria and worsening eGFR (9,28–30). Although these landmark studies transformed treatment of diabetes and demonstrated an enduring effect of controlled glycemia on microvascular outcomes over decades, it is important to underscore the trial designs and characteristics of these study populations. DCCT participants had type 1 diabetes and a mean age of 26 years; HbA1c was 7% for the intensive treatment group versus 9% for the conventional group. The UKPDS studied older people (mean age 54 years) with type 2 diabetes but excluded congestive heart failure or history of multiple vascular events, and HbA1c was 7.0% in the intensive group compared with 7.9% in the conventional group. In contrast, ACCORD trial participant mean age was 63 years, most had a history of macrovascular disease, and HbA1c was 6.4% for the intensive group versus 7.5% for the conventional group. Consideration of these differences dispel much of the quandary over the differences in findings between these distinct patient populations.
Discrepant findings from the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial, a cohort more similar to that of the ACCORD trial, are more difficult to explain (11,31). Although the ADVANCE trial found that intensive glycemia conveyed a decrease in incident macroalbuminuria (HR, 0.70; 95% CI, 0.57 to 0.85), an estimate very similar to our results, they also found a reduced risk for ESKD after a mean of 9.9 years (HR, 0.54; 95% CI, 0.34 to 0.85). A third trial (97% men), the Veterans Affairs Diabetes Trial (VADT), did not find an independent effect of intensive glycemic control on either progressive albuminuria or decline in eGFR or ESKD (12,32); however, the intensive control group did have a slightly greater (albeit statistically insignificant) percent with eGFR>60 ml/min per 1.73 m2 (32). An important distinction between these three cohorts is the difference in severity of diabetes. Participants in the ADVANCE study had a baseline hemoglobin A1c of 7.2% versus 8.1% and 9.4% in the ACCORD and VADT trials, respectively (33). Only 1.5% of participants in the ADVANCE trial required insulin at baseline, versus 35% in the ACCORD trial and 52% in VADT.
Intensive BP Control
Randomized trials searching for the ideal BP goal for patients with DKD have also generated inconsistent results. Although it is broadly accepted that macro- and microvascular risk is reduced with BPs <140/90 mm Hg (34,35), conflicting findings exist with reductions below this threshold (14,36,37). During the ACCORD study period, targeting a systolic BP to <120 mm Hg yielded a reduction in incident macroalbuminuria 4.7 years after randomization, but only in participants with baseline microalbuminuria, and had no effect on the composite outcome of doubling of serum creatinine, elevated serum creatinine ≥3.2 mg/dl, or need for dialysis (14). Our follow-up study of these participants 7.7 years after randomization demonstrates no benefit to sustained incident macroalbuminuria, and more importantly, a harm to the composite kidney outcome, predominantly carried by doubling of creatinine. Moreover, a separate analysis of ACCORD trial data found that in participants with baseline CKD (UACR≥30 μg/mg or eGFR<60 ml/min per 1.73 m2), randomization to intensive BP control did not yield significant benefit to cerebrovascular outcomes as it did in participants without baseline CKD (38).
In line with our findings are secondary kidney outcomes from the Systolic Blood Pressure Intervention Trial, a study of similar design to the ACCORD BP trial, conducted in participants without diabetes (39). Although randomization to systolic BP <120 mm Hg did not significantly affect the composite or individual kidney outcomes in participants with baseline CKD, in those without baseline CKD there was an increased risk for ≥30% decline in eGFR to <60 ml/min per 1.73 m2 (HR, 3.49; 95% CI, 2.44 to 5.10). There was also a three-fold increase in AKI requiring hospitalization in the entire cohort (P<0.001). Also in support of our results was the lack of significant benefit of intensive BP control to the primary outcome in the subgroup with baseline CKD (39).
Fenofibrate
The ACCORD lipid trial and the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Studies have both documented a reversible increase in serum creatinine with fibrates over a short-term period (17,19). In a washout substudy of FIELD participants, there was a statistically significant smaller decline in eGFR from baseline to an 8-week postwashout visit in the fibrate versus placebo groups (1.9 versus 6.9 ml/min per 1.73 m2, respectively) (17). However, this change in eGFR occurred over a mean treatment time of 5 years, calling into question the clinical significance of this change when viewed as an annual rate of change. The ACCORD lipid trial also found that withdrawal of fibrate therapy resulted in a reduction in creatinine after 51 days (19). Although there are probably reversible physiologic effects from fibrates on serum creatinine levels, there may also be long-term, irreversible consequences for DKD. Our finding that the effect of fibrates on the composite kidney outcome became larger during the post-trial follow-up period further substantiates this notion.
Strengths and Limitations
A problematic issue in our study was the self-report of “need for dialysis” without adjudication or information regarding whether this need was sustained. Because of study design constraints, we also analyzed doubling of serum creatinine using a single rather than multiple creatinine values. Although the in-trial protocol measured serum creatinine every 4 months in lipid trial participants, creatinine was only measured annually after the first year of follow-up in BP trial participants; furthermore, in the extension phase, it was only measured a maximum of three other occasions over no more than five additional years. Another limitation was the use of a single, random UACR (40), which can result in misclassification of microalbuminuria and a higher risk of a type I error (41). Strengths of the study include the large sample size, the randomized controlled design, and intention-to-treat analysis. In addition, the use of a competing risk approach allows for a less biased and more easily interpreted risk assessment for kidney outcomes, which are always associated with increased risk of death (42).
In summary, this study shows a benefit of intensive glycemic control primarily on incident macroalbuminuria, and an increased risk of harm from intensive BP control and fenofibrate therapy on sustained doubling of serum creatinine. It is important to consider the effect of intensive therapies on microvascular outcomes along with macrovascular outcomes. The original ACCORD trial and its extension phase, the ACCORDION trial, both demonstrated a lack of benefit from intensive glycemic control, intensive BP control, or fibrate use, on the risk for incident cardiovascular events and death in people with type 2 diabetes at high risk for cardiovascular disease. The lack of substantive benefit from these interventions on micro- and macrovascular outcomes, and now evidence of harm on kidney outcomes, should be considered when revising current guidelines particularly for BP control and lipid therapies.
Disclosures
J.B.B. has received contracted consulting fees, paid to his institution, and travel support from Adocia, AstraZeneca, Dexcom, Elcelyx Therapeutics, Eli Lilly, Intarcia Therapeutics, Lexicon, Metavention, NovaTarg, Novo Nordisk, Sanofi, Senseonics, and vTv Therapeutics; and grant support from AstraZeneca, Boehringer Ingelheim, Johnson & Johnson, Lexicon, Novo Nordisk, Sanofi, Theracos, and vTv Therapeutics. He holds stock options in Mellitus Health and PhaseBio and served on the board of the AstraZeneca HealthCare Foundation. He is supported by a grant from the National Institutes of Health (UL1TR002489). F.I.-B. is a consultant for Novo Nordisk, Covance, Bayer, and Sanofi and holds grants from Novo Nordisk and the National Institutes of Health. J.C.M. received a research grant from Abbott Laboratories to study kidney function in the ACCORD lipid trial. A.K.M., R.J.S., C.F.P., V.P., D.L.S., L.K., and T.E.C. have no relevant disclosures.
Acknowledgments
We would like to thank the participants and their families for their participation. The Action to Control Cardiovascular Risk in Diabetes Study was supported by National Heart, Lung, and Blood Institute contracts N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAA #Y1-HC-9035, and IAA #Y1-HC-1010. Other components of the National Institutes of Health, including the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute on Aging contributed funding. General Clinical Research Centers provided support at many sites. Action to Control Cardiovascular Risk in Diabetes Follow-On Study activities were supported by National Heart, Lung, and Blood Institute contract HHSN268201100027C.
The contents of this article do not represent the views of the National Institutes of Health or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “ACCORDION: Ensuring That We Hear the Music Clearly,” on pages 1621–1623.
References
- 1.Kainz A, Hronsky M, Stel VS, Jager KJ, Geroldinger A, Dunkler D, Heinze G, Tripepi G, Oberbauer R: Prediction of prevalence of chronic kidney disease in diabetic patients in countries of the European Union up to 2025. Nephrol Dial Transplant 30[Suppl 4]: iv113–iv118, 2015 [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System : USRDS 2016 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2016 [Google Scholar]
- 3.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH: Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 24: 302–308, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG; Chronic Kidney Disease Prognosis Consortium : Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 380: 1662–1673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J; ADVANCE Collaborative Group : Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20: 1813–1821, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Packham DK, Alves TP, Dwyer JP, Atkins R, de Zeeuw D, Cooper M, Shahinfar S, Lewis JB, Lambers Heerspink HJ: Relative incidence of ESRD versus cardiovascular mortality in proteinuric type 2 diabetes and nephropathy: Results from the DIAMETRIC (Diabetes Mellitus Treatment for Renal Insufficiency Consortium) database. Am J Kidney Dis 59: 75–83, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Sabe MA, Claggett B, Burdmann EA, Desai AS, Ivanovich P, Kewalramani R, Lewis EF, McMurray JJ, Olson KA, Parfrey P, Solomon SD, Pfeffer MA: Coronary artery disease is a predictor of progression to dialysis in patients with chronic kidney disease, type 2 diabetes mellitus, and anemia: An analysis of the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT). J Am Heart Assoc 5: e002850, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsioufis C, Kokkinos P, Macmanus C, Thomopoulos C, Faselis C, Doumas M, Stefanadis C, Papademetriou V: Left ventricular hypertrophy as a determinant of renal outcome in patients with high cardiovascular risk. J Hypertens 28: 2299–2308, 2010 [DOI] [PubMed] [Google Scholar]
- 9.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B; DCCT/EDIC Research Group : Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 365: 2366–2376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilous R: Microvascular disease: What does the UKPDS tell us about diabetic nephropathy? Diabet Med 25[Suppl 2]: 25–29, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, Li Q, MacMahon S, Cooper ME, Hamet P, Marre M, Mogensen CE, Poulter N, Mancia G, Cass A, Patel A, Zoungas S; ADVANCE Collaborative Group : Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int 83: 517–523, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Agrawal L, Azad N, Emanuele NV, Bahn GD, Kaufman DG, Moritz TE, Duckworth WC, Abraira C; Veterans Affairs Diabetes Trial (VADT) Study Group : Observation on renal outcomes in the Veterans Affairs Diabetes Trial. Diabetes Care 34: 2090–2094, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr., Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A, O’Connor P, Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I; ACCORD trial group : Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the ACCORD randomised trial. Lancet 376: 419–430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ismail-Beigi F, Craven TE, O’Connor PJ, Karl D, Calles-Escandon J, Hramiak I, Genuth S, Cushman WC, Gerstein HC, Probstfield JL, Katz L, Schubart U; ACCORD Study Group : Combined intensive blood pressure and glycemic control does not produce an additive benefit on microvascular outcomes in type 2 diabetic patients. Kidney Int 81: 586–594, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, Arima H, Monaghan H, Joshi R, Colagiuri S, Cooper ME, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Lisheng L, Mancia G, Marre M, Matthews DR, Mogensen CE, Perkovic V, Poulter N, Rodgers A, Williams B, MacMahon S, Patel A, Woodward M; ADVANCE-ON Collaborative Group : Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 371: 1392–1406, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Charlton-Menys V, DeMicco DA, Fuller JH; CARDS Investigators : Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: An analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 54: 810–819, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, Sullivan DR, Jenkins AJ, O’Connell RL, Whiting MJ, Glasziou PP, Simes RJ, Kesäniemi YA, Gebski VJ, Scott RS, Keech AC; Fenofibrate Intervention and Event Lowering in Diabetes Study investigators : Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 54: 280–290, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Bonds DE, Craven TE, Buse J, Crouse JR, Cuddihy R, Elam M, Ginsberg HN, Kirchner K, Marcovina S, Mychaleckyj JC, O’Connor PJ, Sperl-Hillen JA: Fenofibrate-associated changes in renal function and relationship to clinical outcomes among individuals with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) experience. Diabetologia 55: 1641–1650, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mychaleckyj JC, Craven T, Nayak U, Buse J, Crouse JR, Elam M, Kirchner K, Lorber D, Marcovina S, Sivitz W, Sperl-Hillen J, Bonds DE, Ginsberg HN: Reversibility of fenofibrate therapy-induced renal function impairment in ACCORD type 2 diabetic participants. Diabetes Care 35: 1008–1014, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC Jr., Grimm RH Jr., Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD; ACCORD Study Group : Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: Design and methods. Am J Cardiol 99[Suppl 12A]: 21i–33i, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Gerstein HC, Riddle MC, Kendall DM, Cohen RM, Goland R, Feinglos MN, Kirk JK, Hamilton BP, Ismail-Beigi F, Feeney P; ACCORD Study Group : Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 99[Suppl12A]: 34i–43i, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F; ACCORD Study Group : Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 362: 1575–1585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC Jr., Cushman WC, Simons-Morton DG, Byington RP; ACCORD Study Group : Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 362: 1563–1574, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerstein HC, Miller ME, Byington RP, Goff DC Jr., Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr., Probstfield JL, Simons-Morton DG, Friedewald WT; Action to Control Cardiovascular Risk in Diabetes Study Group : Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ACCORD Study Group : Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care 39: 701–708, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 28.The Diabetes Control and Complications (DCCT) Research Group : Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int 47: 1703–1720, 1995 [DOI] [PubMed] [Google Scholar]
- 29.UK Prospective Diabetes Study (UKPDS) Group : Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837–853, 1998 [PubMed] [Google Scholar]
- 30.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577–1589, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Wong MG, Perkovic V, Chalmers J, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, Heller S, MacMahon S, Mancia G, Marre M, Matthews D, Neal B, Poulter N, Rodgers A, Williams B, Zoungas S; ADVANCE-ON Collaborative Group : Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care 39: 694–700, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Agrawal L, Azad N, Bahn GD, Ge L, Reaven PD, Hayward RA, Reda DJ, Emanuele NV; VADT Study Group : Long-term follow-up of intensive glycaemic control on renal outcomes in the Veterans Affairs Diabetes Trial (VADT). Diabetologia 61: 295–299, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS; American Diabetes Association; American College of Cardiology Foundation; American Heart Association : Intensive glycemic control and the prevention of cardiovascular events: Implications of the ACCORD, ADVANCE, and VA diabetes trials: A position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation 119: 351–357, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Schrier RW, Estacio RO, Esler A, Mehler P: Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 61: 1086–1097, 2002 [DOI] [PubMed] [Google Scholar]
- 35.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr., Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr., Narva AS, Ortiz E: 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507–520, 2014 [DOI] [PubMed] [Google Scholar]
- 36.de Galan BE, Perkovic V, Ninomiya T, Pillai A, Patel A, Cass A, Neal B, Poulter N, Harrap S, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Glasziou P, Grobbee DE, MacMahon S, Chalmers J; ADVANCE Collaborative Group : Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 20: 883–892, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taler SJ, Agarwal R, Bakris GL, Flynn JT, Nilsson PM, Rahman M, Sanders PW, Textor SC, Weir MR, Townsend RR: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis 62: 201–213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papademetriou V, Zaheer M, Doumas M, Lovato L, Applegate WB, Tsioufis C, Mottle A, Punthakee Z, Cushman WC; ACCORD Study Group : Cardiovascular outcomes in action to control cardiovascular risk in diabetes: Impact of blood pressure level and presence of kidney disease. Am J Nephrol 43: 271–280, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group : A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saydah SH, Pavkov ME, Zhang C, Lacher DA, Eberhardt MS, Burrows NR, Narva AS, Eggers PW, Williams DE: Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 59: 675–683, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smulders YM, Slaats EH, Rakic M, Smulders FT, Stehouwer CD, Silberbusch J: Short-term variability and sampling distribution of various parameters of urinary albumin excretion in patients with non-insulin-dependent diabetes mellitus. J Lab Clin Med 132: 39–46, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Jiang Y, Fine JP, Mottl AK: Competing risk of death with end-stage renal disease in diabetic kidney disease. Adv Chronic Kidney Dis 25: 133–140, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Elam MB, Ginsberg HN, Lovato LC, Corson M, Largay J, Leiter LA, Lopez C, O’Connor PJ, Sweeney ME, Weiss D, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm R, Ismail-Beigi F, Goff DC Jr., Fleg JL, Rosenberg Y, Byington RP; ACCORDION Study Investigators : Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol 2: 370–380, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]