Abstract
Background and objectives
Current therapies for hyponatremia have variable effectiveness and tolerability, and in certain instances, they are very expensive. We examined the effectiveness, safety, and tolerability of urea for the treatment of inpatient hyponatremia.
Design, setting, participants, & measurements
We identified all patients hospitalized at the University of Pittsburgh Medical Center between July 2016 and August 2017 with hyponatremia (plasma sodium <135 mEq/L) who received urea, including a subgroup of patients who received urea as the sole drug therapy for hyponatremia (urea-only treated). We matched urea only–treated patients to a group of patients with hyponatremia who did not receive urea (urea untreated) and compared changes in plasma sodium at 24 hours and the end of therapy as well as the proportion of patients who achieved plasma sodium ≥135 mEq/L. We abstracted data on adverse events and reported side effects of urea.
Results
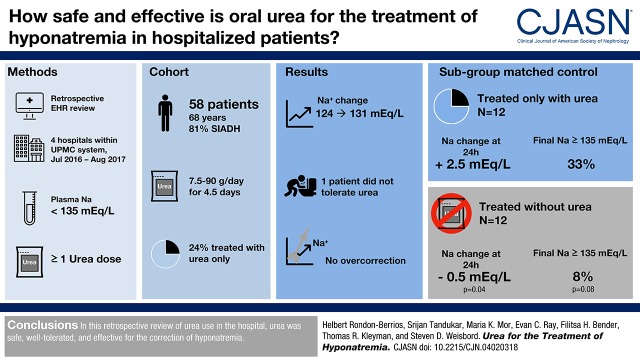
Fifty-eight patients received urea (7.5–90 g/d) over a median of 4.5 (interquartile range, 3–8) days and showed an increase in plasma sodium from 124 mEq/L (interquartile range, 122–126) to 131 mEq/L (interquartile range, 127–134; P<0.001). Among 12 urea only–treated patients, plasma sodium increased from 125 mEq/L (interquartile range, 122–127) to 131 mEq/L (interquartile range, 129–136; P=0.001) by the end of urea therapy. There was a larger increase in plasma sodium at 24 hours in urea only–treated patients compared with urea-untreated patients (2.5 mEq/L; interquartile range, 0–4.5 versus −0.5 mEq/L; interquartile range, −2.5 to 1.5; P=0.04), with no difference in change in plasma sodium by the end of therapy (6 mEq/L; interquartile range, 3.5–10 versus 5.5 mEq/L; interquartile range, 3–7.5; P=0.51). A greater proportion of urea only–treated patients achieved normonatremia, but this difference was not statistically significant (33% versus 8%; P=0.08). No patients experienced overly rapid correction of plasma sodium, and no serious adverse events were reported.
Conclusions
Urea seems effective and safe for the treatment of inpatient hyponatremia, and it is well tolerated.
Keywords: hyponatremia, urea, inappropriate ADH syndrome
Visual Abstract
Introduction
Hyponatremia, defined as a plasma sodium <135 mEq/L, is the most common electrolyte disorder, and it is associated with increased mortality (1) and health resource utilization (2) in hospitalized patients. It has also been recently recognized that mild and seemingly asymptomatic hyponatremia is associated increased morbidity, including neurocognitive deficits, gait disturbances, falls, bone fractures, and osteoporosis (3). Although standard therapeutic interventions for hyponatremia, including fluid restriction and oral sodium chloride tablets, are commonly used (4), evidence of their efficacy from clinical trials is lacking. Moreover, patient adherence to these treatments, particularly fluid restriction, is commonly suboptimal (3). The discovery of vasopressin antagonists provided a new drug class targeting elevated vasopressin levels that mediate most forms of hyponatremia (5). Despite demonstrated efficacy in clinical trials (6), the use of vasopressin antagonists has been limited by their very high cost as well as safety concerns related to liver disease and the potential for overly rapid correction of plasma sodium (7). Thus, despite the frequency of serious adverse events associated with hyponatremia, there is a paucity of definitively effective, safe, and reasonably priced treatments that do not pose challenges with regard to patient adherence.
Patient series conducted in Europe (8–13) have shown that oral urea, an osmotic agent that increases urinary free water excretion, is safe and effective for the treatment of hyponatremia. However, urea has not been available for the treatment of hyponatremia in the United States until very recently, when Ure-Na, a novel commercial formulation, was introduced. This oral preparation of urea is supplied as a powder packet that is mixed with water or juice. The US Food and Drug Administration considers urea a medical food (generally regarded as safe category) (14) and therefore, does not require a medical prescription. The University of Pittsburgh Medical Center added this novel formulation of oral urea to its inpatient formulary in late July 2016. Herein, we describe the first-year experience of its use for the treatment of inpatient hyponatremia at our institution.
Materials and Methods
Overall Study Cohort
We used the electronic medical record to identify all adults hospitalized at any of four University of Pittsburgh Medical Center hospitals between July 2016 and August 2017 who were diagnosed with hyponatremia (plasma sodium <135 mEq/L) at the time of admission or during their hospitalization and who received one or more doses of oral urea during the hospitalization. For this overall cohort, we recorded baseline demographic and clinical characteristics and ascertained the etiology of hyponatremia on the basis of an assessment of the clinical data available in the medical record. In cases of discordance between the cause of hyponatremia identified by our assessment and the documented etiology in the medical record, we considered the etiology to be that on the basis of our review. In instances in which we could not definitively determine the etiology on our record review, we considered the etiology to be what was documented in the medical record. We documented the presence of symptoms deemed potentially related to hyponatremia. We abstracted all plasma sodium, BUN, plasma creatinine, urine osmolality, and urine sodium measurements during the hospitalization. Plasma sodium measurements were recorded along with simultaneous plasma glucose when available, and the Katz correction (i.e., adding 1.6 mEq/L to plasma sodium for every 100 mg/dl of plasma glucose over 100 mg/dl) was used to correct for significant hyperglycemia when present. When estimating change in plasma sodium at 24 hours after the start of treatment, we recorded the value that was closest to the 24-hour time point. We also recorded the daily dose and duration of treatment with urea, and we documented all patient-reported side effects and adverse events associated with this therapy, including overly rapid correction of plasma sodium, which is defined as an increase in plasma sodium >10 mEq/L in 24 hours or >8 mEq/L in 24 hours for patients at high risk for osmotic demyelination syndrome (i.e., initial plasma sodium <105 mEq/L, history of cirrhosis, alcoholism, malnutrition, or plasma potassium <3.5 mEq/L) (15). We recorded all other treatments prescribed for hyponatremia during the hospitalization. The institutional review board of the University of Pittsburgh approved all study procedures.
Urea Only–Treated and Urea-Untreated Patients
To explore the independent effects of urea on plasma sodium, we identified a subgroup of patients from our overall cohort who had the syndrome of inappropriate antidiuretic hormone secretion (SIADH) as the only cause of hyponatremia and who received urea as the sole drug therapy for hyponatremia (urea-only treated). These patients were confirmed to have received urea as the only drug treatment if there was no evidence that sodium chloride tablets, loop diuretics, vasopressin antagonists, intravenous normal saline, intravenous hypertonic saline, or potassium chloride had been simultaneously prescribed during the hospitalization for the treatment of hyponatremia. We also identified a group of patients who were hospitalized at one of the same four hospitals between July 2015 and July 2016 (before oral urea was available for use within the health system) with a diagnosis of hyponatremia due to SIADH (urea-untreated patients). We matched urea only–treated to urea-untreated patients (1:1) on sex and baseline plasma sodium (within 3 mEq/L). For urea-untreated patients, we abstracted data on all treatments used for hyponatremia and recorded all plasma sodium, urine osmolality, and urine sodium measurements from the time of onset of hyponatremia until hospital discharge. We also recorded the length of hospital stay after initiation of therapy for hyponatremia in both groups (i.e., urea in urea only–treated patients; other drug therapies in urea-untreated patients).
Statistical Analyses
We report continuous variables as medians and interquartile ranges (IQRs) and categorical variables as numbers (percentages). We used the Wilcoxon signed rank test to compare plasma sodium at baseline with plasma sodium at the conclusion of therapy in the overall cohort of patients and within urea only–treated patients. We also used the Wilcoxon signed rank test to compare the change in plasma sodium at 24 hours and the conclusion of therapy for hyponatremia between matched urea only–treated and urea-untreated patients. We used the McNemar test to compare the proportion of urea only–treated and urea-untreated patients who reached a normal plasma sodium (defined as a value ≥135 mEq/L) at the end of therapy. We analyzed differences in length of hospital stay between urea only–treated and urea-untreated patients using a discrete proportional hazards model, which modeled the length of the hospital stay as the time until discharge using standard survival techniques. We used this method that allowed for ties in the data due to patients being discharged on the same day, because length of stay was measured discretely (i.e., at the level of the day) rather than continuously (i.e., at the level of minutes), resulting in multiple ties in the data (16). All P values are two sided, with significance evaluated at the 0.05 level. We used SAS version 9.4 to conduct all analyses.
Results
Overall Study Cohort and Effect of Urea Therapy
We identified 58 hospitalized patients with hyponatremia who received oral urea. The median age of these patients was 68 years old (IQR, 55–79), and 35 (60%) were men (Supplemental Tables 1–3, Table 1). The cause of hyponatremia was SIADH in 47 (81%) patients, whereas more than one etiology was reported in 11 (19%) patients. The etiologies of SIADH included medications in 18 (31%) patients, intracranial disorders in 13 (22%) patients, and idiopathic in 13 (22%) patients. Forty-six (79%) patients had no apparent symptoms. No patients had severe symptoms attributed to hyponatremia (e.g., seizures or coma).
Table 1.
Baseline characteristics of patients admitted to the University of Pittsburgh Medical Center with hyponatremia and treated with urea between July 2016 and August 2017
| Characteristic | N=58 |
|---|---|
| Demographic characteristics | |
| Median age [IQR], yr | 68 [55–79] |
| Men, N (%) | 35 (60.3) |
| Clinical and treatment characteristics | |
| Etiology of hyponatremia, N (%) | |
| SIADHb | 47 (81) |
| Medications | 18 (31) |
| Idiopathic | 13 (22) |
| Intracranial disorders | 13 (22) |
| Malignancy | 10 (17) |
| Pulmonary disorders | 8 (14) |
| Pain | 7 (12) |
| Other | 2 (3) |
| Thiazide diuretics | 7 (12) |
| Heart failure | 6 (10) |
| Hypovolemia | 6 (10) |
| Kidney disease | 4 (7) |
| Adrenal insufficiency | 2 (3) |
| Cirrhosis | 1 (2) |
| Other therapies for hyponatremia, N (%) | |
| Fluid restriction | 51 (88) |
| Sodium chloride tablets | 22 (38) |
| Loop diuretics | 19 (33) |
| Normal saline | 12 (21) |
| Vasopressin antagonists | 6 (10) |
| Hypertonic saline 3% | 4 (7) |
| Potassium chloride tablets | 3 (5) |
| Glucocorticoids | 1 (2) |
Etiologies of hyponatremia and therapies for hyponatremia are not mutually exclusive. IQR, interquartile range; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
Among these 58 patients, urea was administered at a dose that ranged from 7.5 to 90 g/d for a median duration of 4.5 days (IQR, 3–8). Although most patients received a stable dose of urea, nine (16%) patients received varying doses (Supplemental Table 4). Fifty-one of 58 (88%) patients were also prescribed fluid restriction ranging from 0.8 to 1.5 L/d, whereas 22 (38%) patients received sodium chloride tablets. No significant hyperglycemia was encountered that required correction of plasma sodium values. The plasma sodium increased from a baseline of 124 mEq/L (IQR, 122–126) to 131 mEq/L (IQR, 127–134) at the end of treatment (P<0.001). One of these 58 (2%) patients discontinued urea, reportedly due to dysgeusia. There were no documented adverse events associated with urea therapy, and no patients showed overly rapid correction of plasma sodium.
Comparison of Urea Only–Treated and Urea-Untreated Patients
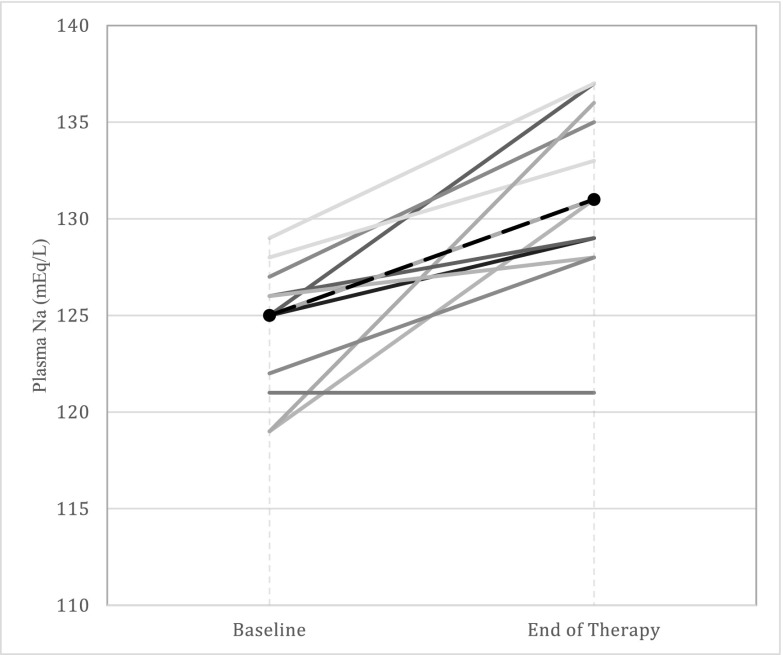
We identified 14 (24%) patients from our overall cohort who received urea as the sole drug therapy for hyponatremia, and within this group, we selected 12 (21%) patients whose hyponatremia was due solely to SIADH (urea only–treated patients). The median age of these patients was 72 years old (IQR, 64–78), eight (67%) were men, and 11 (92%) were prescribed fluid restriction (0.8–1.5 L/d) in addition to urea (Supplemental Table 2). Among urea only–treated patients, plasma sodium increased by 2.5 mEq/L (IQR, 0–4.5) over the first 24 hours of therapy (P=0.02) and from a baseline of 125 mEq/L (IQR, 122–127) to 131 mEq/L (IQR, 129–136) at the end of urea therapy (P=0.001) (Figure 1, Table 2).
Figure 1.
Increase in plasma sodium (Na) from baseline to the completion of therapy among urea only–treated patients.
Table 2.
Baseline and post-treatment laboratory parameters among urea only–treated patients
| Laboratory Parameter | Baseline | 24 h | End of Therapy |
|---|---|---|---|
| Plasma sodium, mEq/L | 125 [122–127] | 127 [124–129]; P=0.02 | 131 [129–136]; P=0.001 |
| BUN, mg/dl | 16 [10–20] | 24 [20–35]; P=0.001 | 42 [26–53]; P<0.001 |
| Serum creatinine, mg/dl | 0.8 [0.75–1] | 0.8 [0.75–1]; P=0.91 | 0.9 [0.75–1]; P=0.59 |
| Urine osmolality, mOsm/kg | 365 [361–561]a | 450 [359–522.5]b; P=0.12 | 546 [542–602]c; P=0.13 |
| Urine sodium, mEq/L | 75 [42–105]a | NA | 50 [37–76]c; P=0.06 |
Data are presented as median [interquartile range]. P values represent comparison with baseline values. NA, not available.
One missing value.
Eight missing values.
Seven missing values.
Among these patients, we observed an increase in BUN from 16 mg/dl (IQR, 10–20) at baseline to 42 mg/dl (IQR, 26–53) at the conclusion of treatment, which corresponded to a median change of 6.5 mg/dl (IQR, 3.5–10) per day (Table 3). Additionally, urine osmolality increased from 365 mOsm/kg (IQR, 361–561) to 546 mOsm/kg (IQR, 542–602; P=0.13), whereas urine sodium decreased from 75 mEq/L (IQR, 42–105) to 50 mEq/L (IQR, 37–76; P=0.06). We observed no change in serum creatinine with urea therapy (0.8 mg/dl [IQR, 0.75–1] at baseline versus 0.9 mg/dl [IQR, 0.75–1] at the end of therapy; P=0.59) (Table 2).
Table 3.
Comparison of outcomes between urea only–treated and urea-untreated patients
| Outcome | Urea-Only Treated, n=12 | Urea Untreated, n=12 | P Value |
|---|---|---|---|
| Baseline plasma sodium | 125 [122–127] | 123 [121–125] | NA |
| Change in plasma sodium by 24 h, mEq/L | 2.5 [0–4.5] | −0.5 [−2.5 to 1.5] | 0.04 |
| Change in plasma sodium by end of therapy, mEq/L | 6 [3.5–10] | 5.5 [3–7.5] | 0.51 |
| Normalization of plasma sodium, N (%) | 4 (33) | 1 (8) | 0.08 |
| LOS, d | 6 [3.5–7] | 6 [4–6] | 0.74 |
Data are presented as median [interquartile range] or N (%). NA, not applicable, because urea-treated and untreated patients were matched for baseline plasma sodium; LOS, length of hospital stay.
We matched these 12 urea only–treated patients to 12 urea-untreated patients. The median age of the urea-untreated patients was 84 years old (IQR, 61–88), which was higher but not statistically different from urea only–treated patients (84 versus 72; P=0.41) (Supplemental Table 3). Among urea-untreated patients, six (50%) received sodium chloride tablets, three (25%) received potassium chloride, two (17%) received loop diuretics, one (8%) received a vasopressin antagonist, one (8%) received hypertonic saline, and all were prescribed fluid restriction (Supplemental Table 3).
Compared with urea-untreated patients, urea only–treated patients showed a statistically significant increase in plasma sodium in the first 24 hours of therapy (2.5 mEq/L [IQR, 0–4.5] versus −0.5 mEq/L [IQR, −2.5 to 1.5]; P=0.04) (Table 3). There was no difference in the change in plasma sodium over the full course of hyponatremia treatment between urea only–treated patients and urea-untreated patients (6 mEq/L [IQR, 3.5–10] versus 5.5 mEq/L [IQR, 3–7.5]; P=0.51) or the length of hospital stay (6 days [IQR, 3.5–7] versus 6 days [IQR, 4–6]; P=0.74). A greater proportion of urea only–treated patients achieved a normal plasma sodium at the end of therapy compared with urea-untreated patients, but this difference did not meet the level of statistical significance (33% versus 8%; P=0.08) (Table 3).
Discussion
The findings of our study show that a formulation of oral urea that has recently become available for use in the United States seems to be effective for the treatment of hyponatremia in the inpatient setting. Furthermore, this agent was safe and well tolerated by patients with no documented serious adverse events or instances in which the rate of correction of plasma sodium was overly rapid. These findings have important implications for patient care in the United States.
At present, there are notable barriers to the effective treatment of hyponatremia using currently available interventions. The cornerstone of therapy for this common electrolyte disturbance is restriction of fluid intake, in many cases to a threshold of ≤800 ml/d (15,17). Although commonly used to increase plasma sodium, strict fluid restriction not only has very limited efficacy (4), but it is also frequently not sustainable. Accordingly, the identification of treatments for which patient adherence is likely to be high is an important therapeutic priority. Our study showed that this formulation of urea seems to be well tolerated by patients over a short course of therapy. Although future studies of patients who are prescribed this preparation of urea for longer periods in the outpatient setting are needed to determine patient adherence, our findings provide preliminary support for the potential role of this agent.
There are also important clinical issues and financial challenges associated with other currently available therapies. Sodium chloride tablets are frequently prescribed in combination with fluid restriction and/or loop diuretics to raise the plasma sodium. However, the need for concomitant fluid restriction and/or loop diuretics likely affects long-term adherence to this therapy. Unlike salt tablets, urea poses no risk for volume expansion and generally allows for more liberal fluid restriction (e.g., 1.5–2 L/d) (18); hence, it would be expected to be associated with greater patient adherence. In the last decade, vasopressin antagonists have emerged as an effective therapy for hyponatremia (6). However, important safety concerns associated with these medications include an elevated risk for liver injury when used at high doses as well as an increased risk of overly rapid correction of plasma sodium (7). Additionally, the retail price of vasopressin antagonists (e.g., $438.29 for a single 15-mg tablet of tolvaptan) (19) precludes their widespread use. Comparatively, the cost of a single 15-g dose of urea is just $3.74 (20), which strongly suggests that it is a considerably more cost effective than vasopressin antagonists.
Prior studies in Europe have examined the efficacy of oral urea for the treatment of hyponatremia. For example, Decaux and Genette (10) showed that urea raised the plasma sodium by 21 mEq/L in seven outpatients with chronic hyponatremia over a period of 7 days. Other studies have shown similar results (8,9,11–13). However, these studies examined outpatients predominantly, lacked a control group, and administered a European formulation of urea that is unavailable for use in the United States. Nonetheless, our findings build on these past studies by describing the effectiveness, safety, and tolerability of a formulation of oral urea now available for use by practitioners in the United States.
We observed a modest but expected elevation of BUN in the urea only–treated group, which should not be interpreted as a reduction in kidney function but rather, is the result of normal urea metabolism. We also noted an increase in urine osmolality and a decrease in urine sodium concentration with urea, which although not statistically significant, are physiologic effects that have been reported in prior studies (11,21). These changes in urine osmolality and urine sodium likely reflect the effects of urea on solute and free water excretion; in this regard, urea has been shown to have a direct antinatriuretic effect leading to positive sodium mass balance (22). This effect of urea on urinary sodium excretion is significant, because the pathogenesis of hyponatremia in SIADH not only involves dilution from water retention but also urinary sodium loss as compensation for the mild volume expansion that occurs in this disorder (23).
Overly rapid correction of plasma sodium is a potential side effect of all current therapies for hyponatremia. It is typically observed with severe hyponatremia (i.e., plasma sodium <120 mEq/L), and in this setting, it is seen in most patients receiving a vasopressin antagonist and about 10% of patients receiving hypertonic saline (24). Excessively rapid correction of plasma sodium with urea has also been reported in a series of patients with severe hyponatremia; nearly one third of patients experienced plasma sodium correction >12 mEq/L per day without any cases of osmotic demyelination syndrome (9). We observed no overcorrection of hyponatremia associated with the use of urea in our cohort, with the caveat that only nine (16%) patients had an initial plasma sodium <120 mEq/L.
It is important to note that urea was used inappropriately in a small number of patients in our cohort. The use of urea is contraindicated in patients with hypovolemic hyponatremia, patients with hyponatremia associated with adrenal insufficiency, and patients with drug-induced hyponatremia (including SIADH) when the offending medication can be safely discontinued. Furthermore, urea is relatively contraindicated in patients with cirrhosis (25,26) given the potential for it to be metabolized into ammonium by urease-producing bacteria in the colon, which can lead to hyperammonemia (27). Because our study was retrospective, we played no role in the prescribing of urea to patients included in our cohort. However, it is essential for clinical providers to recognize that the primary clinical indication for the administration of urea in hyponatremia is SIADH (18), although it can also be used in the setting of heart failure (28). Our finding of seemingly inappropriate use of urea in certain instances underscores the importance of educating providers about the appropriate indications for this therapeutic agent in hyponatremia.
Our study has several limitations. First, this was a retrospective study. Therefore, the observed differences in treatment effects between groups may be cofounded and may not necessarily causal. For instance, although we matched the urea only–treated and urea-untreated groups on sex and baseline plasma sodium concentration, we were not able to match for other factors (e.g., baseline urine osmolality, etiology of SIADH, and variability and timing of treatment used in urea-untreated group) that may account for some of the differences in the change in plasma sodium. Second, our analysis relied on data documented in the medical record, which is susceptible to missing information. For example, 43% of patients did not have urine osmolality or urine sodium concentration measured at the end of therapy, and therefore, determinations of the effects of urea on these parameters were limited. Similarly, 45% of plasma sodium measurements did not have a simultaneous plasma glucose measured. However, in those instances in which these plasma tests were performed simultaneously, we did not observe plasma glucose levels that would require correction of plasma sodium. Third, measurements of plasma sodium at 24 hours were not obtained at exactly 24 hours. However, the large majority (72%) were obtained within 3 hours before or after the 24-hour time point. Fourth, our study population was small, decreasing the power of our comparisons. Nonetheless, our study is the second largest study on the use of urea to date, and it is the first to report on its use in the United States. Fifth, the duration of urea therapy was short, because our analyses focused on the effects of this medication among hospitalized patients. Future studies will need to evaluate the longer-term effects of this urea preparation on patient-centered outcomes in the outpatient setting. Sixth, we did not assess the comparative costs of urea therapy. Economic analyses that delineate its potential financial benefits compared with other treatments will help inform its role in the treatment of this very common electrolyte disorder.
In conclusion, we showed that a formulation of oral urea now available in the United States seems to be effective for the treatment of inpatient hyponatremia and that it is safe and well tolerated. Randomized trials that assess the efficacy, safety, tolerability, and costs of this preparation of urea in larger numbers of hospitalized and ambulatory patients are needed to establish the precise therapeutic role of this agent for the management of hyponatremia.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by grants K08 DK110332 (to E.C.R.) and P30 DK079307 (to T.R.K.) from the National Institutes of Health.
Preliminary findings of this study were previously reported at the annual meeting of the National Kidney Foundation in Austin, Texas April 10–14, 2018.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04020318/-/DCSupplemental.
References
- 1.Waikar SS, Mount DB, Curhan GC: Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 122: 857–865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deitelzweig S, Amin A, Christian R, Friend K, Lin J, Lowe TJ: Health care utilization, costs, and readmission rates associated with hyponatremia. Hosp Pract (1995) 41: 89–95, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Rondon-Berrios H, Berl T: Mild chronic hyponatremia in the ambulatory setting: Significance and management. Clin J Am Soc Nephrol 10: 2268–2278, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg A, Verbalis JG, Amin AN, Burst VR, Chiodo JA 3rd, Chiong JR, Dasta JF, Friend KE, Hauptman PJ, Peri A, Sigal SH: Current treatment practice and outcomes. Report of the hyponatremia registry. Kidney Int 88: 167–177, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berl T: Vasopressin antagonists. N Engl J Med 373: 981, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C; SALT Investigators : Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355: 2099–2112, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Rondon-Berrios H, Berl T: Vasopressin receptor antagonists: Characteristics and clinical role. Best Pract Res Clin Endocrinol Metab 30: 289–303, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Coussement J, Danguy C, Zouaoui-Boudjeltia K, Defrance P, Bankir L, Biston P, Piagnerelli M: Treatment of the syndrome of inappropriate secretion of antidiuretic hormone with urea in critically ill patients. Am J Nephrol 35: 265–270, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Decaux G, Andres C, Gankam Kengne F, Soupart A: Treatment of euvolemic hyponatremia in the intensive care unit by urea. Crit Care 14: R184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decaux G, Genette F: Urea for long-term treatment of syndrome of inappropriate secretion of antidiuretic hormone. Br Med J (Clin Res Ed) 283: 1081–1083, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decaux G, Unger J, Brimioulle S, Mockel J: Hyponatremia in the syndrome of inappropriate secretion of antidiuretic hormone. Rapid correction with urea, sodium chloride, and water restriction therapy. JAMA 247: 471–474, 1982 [PubMed] [Google Scholar]
- 12.Pierrakos C, Taccone FS, Decaux G, Vincent JL, Brimioulle S: Urea for treatment of acute SIADH in patients with subarachnoid hemorrhage: A single-center experience. Ann Intensive Care 2: 13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soupart A, Coffernils M, Couturier B, Gankam-Kengne F, Decaux G: Efficacy and tolerance of urea compared with vaptans for long-term treatment of patients with SIADH. Clin J Am Soc Nephrol 7: 742–747, 2012 [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration: Select Committee on GRAS Substances (SCOGS) Opinion: Urea, 1978. Available at: http://wayback.archive-it.org/7993/20171031064318/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm261338.htm. Accessed March 6, 2018
- 15.Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, Thompson CJ: Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med 126[Suppl 1]: S1–S42, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Allison P: Discrete-time methods for the analysis of event histories. Sociol Methodol 13: 61–98, 1982 [Google Scholar]
- 17.Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai C, Joannidis M, Soupart A, Zietse R, Haller M, van der Veer S, Van Biesen W, Nagler E; Hyponatraemia Guideline Development Group : Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol Dial Transplant 29[Suppl 2]: i1–i39, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Decaux G, Gankam Kengne F, Couturier B, Vandergheynst F, Musch W, Soupart A: Actual therapeutic indication of an old drug: Urea for treatment of severely symptomatic and mild chronic hyponatremia related to SIADH. J Clin Med 3: 1043–1049, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GoodRx: Tolvaptan (Samsca). 2018. Available at: https://www.goodrx.com/tolvaptan?form=tablet&dosage=15mg&quantity=1&days_supply=&label_override=Samsca. Accessed March 6, 2018
- 20.Ure-Na: Buy Ure-Na. 2018. Available at: http://www.ure-na.com/category-s/101.htm. Accessed March 6, 2018
- 21.Decaux G, Brimioulle S, Genette F, Mockel J: Treatment of the syndrome of inappropriate secretion of antidiuretic hormone by urea. Am J Med 69: 99–106, 1980 [DOI] [PubMed] [Google Scholar]
- 22.Verbalis JG, Baldwin EF, Neish PN, Robinson AG: Effect of protein intake and urea on sodium excretion during inappropriate antidiuresis in rats. Metabolism 37: 46–54, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Verbalis JG: Whole-body volume regulation and escape from antidiuresis. Am J Med 119[Suppl 1]: S21–S29, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Sterns RH, Silver SM, Hix JK: Urea for hyponatremia? Kidney Int 87: 268–270, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Decaux G, Mols P, Cauchi P, Delwiche F: Use of urea for treatment of water retention in hyponatraemic cirrhosis with ascites resistant to diuretics. Br Med J (Clin Res Ed) 290: 1782–1783, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Decaux G, Mols P, Cauchie P, Flamion B, Delwiche F: Treatment of hyponatremic cirrhosis with ascites resistant to diuretics by urea. Nephron 44: 337–343, 1986 [DOI] [PubMed] [Google Scholar]
- 27.Phillips GB, Schwartz R, Gabuzda GJ Jr., Davidson CS: The syndrome of impending hepatic coma in patients with cirrhosis of the liver given certain nitrogenous substances. N Engl J Med 247: 239–246, 1952 [DOI] [PubMed] [Google Scholar]
- 28.Cauchie P, Vincken W, Decaux G: Urea treatment for water retention in hyponatremic congestive heart failure. Int J Cardiol 17: 102–104, 1987 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.