Abstract
Background and objectives
In the general population, the quality of the patient experience with their primary care physician may influence health outcomes but this has not been evaluated in CKD. This is relevant for the growing Hispanic CKD population, which potentially faces challenges to the quality of the patient experience related to language or cultural factors. We evaluated the association between the patient experience with their primary care physician and outcomes in Hispanics with CKD.
Design, setting, participants, & measurements
This prospective observational study included 252 English- and Spanish-speaking Hispanics with entry eGFR of 20–70 ml/min per 1.73 m2, enrolled in the Hispanic Chronic Renal Insufficiency Cohort study between 2005 and 2008. Patient experience with their primary care physician was assessed by the Ambulatory Care Experiences Survey subscales: communication quality, whole-person orientation, health promotion, interpersonal treatment, and trust. Poisson and proportional hazards models were used to assess the association between the patient experience and outcomes, which included hospitalization, ESKD, and all-cause death.
Results
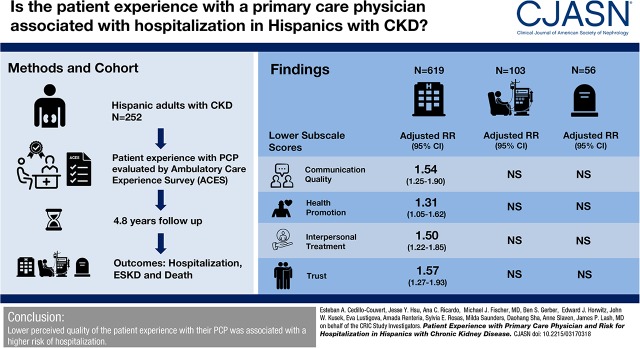
Participants had a mean age of 56 years, 38% were women, 80% were primary Spanish speakers, and had a mean eGFR of 38 ml/min per 1.73 m2. Over 4.8 years (median) follow-up, there were 619 hospitalizations, 103 ESKD events, and 56 deaths. As compared with higher subscale scores, lower scores on four of the five subscales were associated with a higher adjusted rate ratio (RR) for all-cause hospitalization (communication quality: RR, 1.54; 95% confidence interval [95% CI], 1.25 to 1.90; health promotion: RR, 1.31; 95% CI, 1.05 to 1.62; interpersonal treatment: RR, 1.50; 95% CI, 1.22 to 1.85; and trust: RR, 1.57; 95% CI, 1.27 to 1.93). There was no significant association of subscales with incident ESKD or all-cause death.
Conclusions
Lower perceived quality of the patient experience with their primary care physician was associated with a higher risk of hospitalization.
Keywords: Humans; Female; Middle Aged; glomerular filtration rate; Prospective Studies; Language; Physicians, Primary Care; Follow-Up Studies; Proportional Hazards Models; Renal Insufficiency, Chronic; Kidney Failure, Chronic; Risk; Cause of Death; Hispanic Americans; hospitalization; Ambulatory Care; Surveys and Questionnaires; Health Promotion
Visual Abstract
Introduction
According to the US Renal Data System (USRDS), Medicare patients with CKD experience a 150% higher hospitalization rate than those without CKD, generating over $17 billion in expenses in 2014 (1). Prior studies have demonstrated the association of sociodemographic and clinical factors on hospitalizations (i.e., age, income, severity of CKD, cardiovascular disease, and anemia) (2–4). Although a recent Canadian study reported that in patients with CKD, continuity of the relationship with a primary care provider was associated with reduced risk of hospitalization (5), the quality of the experience with their primary care physician from a patient’s perspective has not been evaluated.
The influence of the patient experience with their primary care physician on hospitalization is of particular relevance to the Hispanic population with CKD. Hispanics are the largest minority in the United States. It is estimated that 57.5 million Hispanics currently reside in the United States and this number is projected to double in the next 20 years (6). Consequently, there will be continued growth of the Hispanic CKD population, which is currently estimated at 8.6 million (7). Furthermore, this population may face challenges to the quality of the patient–provider interactions related to language (i.e., low English proficiency) (8,9) and cultural factors (e.g., values and beliefs) (10–12).
Although there are limited data comparing rates of hospitalization in Hispanics with nondialysis requiring CKD with non-Hispanic whites, in the general population, there is strong evidence that rates of preventable hospitalizations are higher in Hispanics than in non-Hispanic whites in the United States (13). Therefore, evaluating the patient–physician interaction may provide important insights into potentially modifiable factors influencing health care utilization in this growing population. The purpose of this study is to evaluate the association of the patient experience with their primary care physician with hospitalization, incident ESKD, and death in Hispanics with CKD enrolled in a long-term observational study, the Hispanic Chronic Renal Insufficiency Cohort (CRIC) study.
Materials and Methods
Study Population
The Hispanic CRIC study is an ongoing ancillary study to the prospective, multicenter CRIC study. The design, methods, and baseline characteristics have been published previously (14,15). Eligibility and exclusion criteria for the Hispanic CRIC study were identical to the parent CRIC study. The study recruited 327 Hispanics with an eGFR of 20–70 ml/min per 1.73 m2 from October 2005 to June 2008. Participants were recruited from university-based, community-based, and private health clinics in the Chicago area (16). The study protocol was approved by the Institutional Review Board at the University of Illinois (IRB number 2005-0528) and is in accordance with the Declaration of Helsinki. All participants provided informed consent. The first question of the Ambulatory Care Experience Survey (ACES) is “Is there one particular doctor that you think of as your regular personal doctor?” Individuals who answered yes to this question were included in this analysis (n=252) and those who answered no were excluded (n=37). The remaining 38 Hispanic CRIC study participants did not complete the questionnaire.
Predictors
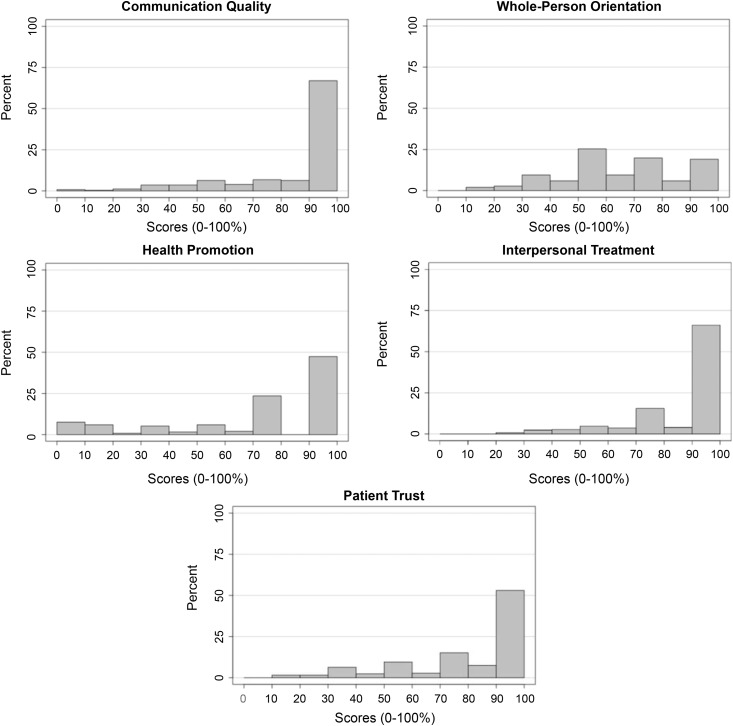
The ACES measures the quality of the patient’s experience with their primary care physician using the Institute of Medicine definition of primary care as the underlying conceptual model for measurement (17). This survey, which has been validated in English and Spanish, has excellent psychometric properties in a wide range of patient populations, achieving a Cronbach α of at least 0.70 (18,19). It has been used in a number of research studies (17,20–23) and was used to develop the Consumer Assessment of Health Care Providers and Systems Clinician/Group Survey, which is considered to be the national standard for ambulatory care patient experience measurement (24). The ACES was administered to participants at a baseline visit. Bilingual culturally congruent coordinators assisted participants who were unable to complete the survey because of limitations with literacy or vision. The survey has five summary measures: communication quality, whole-person orientation, health promotion, interpersonal treatment, and patient trust (Supplemental Table 1). Subscale scores range from 0 to 100 points, with higher scores indicating more favorable experiences. For subscale scores that were left-skewed with ceiling effect (communication and interpersonal treatment; Figure 1), a low subscale score was defined as a score <100. For all other subscales, a low subscale score was defined as below the median.
Figure 1.
The distribution of ACES scores varied by subscale.
Outcome Measures
The following outcomes were evaluated: (1) all-cause, cardiovascular, and noncardiovascular hospitalization; (2) incident ESKD requiring dialysis or kidney transplantation; and (3) death from any cause. Hospitalizations were ascertained every 6 months by self-report and confirmed by hospital queries. Hospitalizations were categorized using the Agency for Healthcare Research and Quality (AHRQ) Clinical Classifications Software categorization scheme on the basis of the first position International Classification of Diseases, Ninth Revision code (25). Any hospitalization within “diseases of the circulatory system” category was designated as cardiovascular, and all others as noncardiovascular. Ascertainment of ESKD was supplemented by crosslinkage with USRDS. Deaths were ascertained from reports by next of kin, death certificates, hospital records, and linkage with the Social Security Death Master File. Participation retention was 90%. For the outcome of hospitalization, in participants who were lost to follow-up, data were censored at the time of the last visit. For the outcomes of ESKD and death, data were censored at the time of ESKD or death (using crosslinkage to USRDS or the Death Master File) or on May 2014.
Covariates
Detailed information for sociodemographic characteristics (age, sex, education, health insurance), place of birth (United States versus not United States), medical history, and medications were obtained at baseline. Health insurance was categorized as Medicare, Medicaid, private/commercial, unknown/incomplete, and no health insurance. Language preference (English versus Spanish) was determined by the language chosen for form completion. Prior nephrology contact was determined at baseline with the question: “Have you ever seen a nephrologist or kidney doctor?” Anthropometric measures (height, weight, body mass index) and BP were measured by coordinators using standard, validated protocols. Diabetes was defined as fasting glucose level ≥126 mg/dl or use of insulin or oral hypoglycemic medications; hypertension was defined as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or use of antihypertensive medications. GFR was estimated annually using a CRIC-specific equation that includes serum creatinine level, cystatin C level, age, sex, and race (26). We also measured 24-hour urine albumin. Self-reported medication adherence was categorized as high, medium, or low, using a previously described approach (27). Depressive symptoms were assessed using the Beck Depression Inventory (BDI) (28) and a score ≥11 was considered indicative of depressive symptoms (29). Health-related quality of life was assessed using the Kidney Disease Quality of Life-36 survey (KDQOL-36) (30,31).
Statistical Analyses
Descriptive statistics for demographic and clinical characteristics were summarized as mean (SD) or median (interquartile range) for continuous variables and percentages for categorical variables. Chi-squared test, two-sample t test, or Kruskal–Wallis test were used to compare categorical and continuous variables as appropriate. Poisson regression with an offset of follow-up years was used to evaluate the association between ACES subscale scores and the total number of hospitalization events. (32) Cox proportional hazards models were used to examine the association between subscale scores and ESKD (cause-specific hazards models with competing events treated as censoring) and all-cause death. For analyses of incident ESKD, death was treated as a competing event. We adjusted for clinical center and the following potential confounders which were selected a priori, on the basis of prior literature: sociodemographics (age, sex, education, income, and primary language), nephrology care, clinical factors (diabetes, hypertension, cardiovascular disease, eGFR, and proteinuria), KDQOL-36 score, and BDI score (1–4).
For the outcome of hospitalization, we conducted the following sensitivity analyses: (1) we utilized AHRQ criteria to assess hospitalizations for an ambulatory care-sensitive condition (33); (2) we added insurance status and medication adherence separately to the final multivariable model; and (3) we adjusted for time-updated BP and hemoglobin A1c (HbA1c). All analyses were performed using SAS, version 9.4 (SAS Institute).
Results
For the 252 participants who reported having a primary physician, the mean age was 56 years, 62% were men, 67% had less than a high school education, 30% had no health insurance, 81% were primary Spanish speakers, the mean eGFR was 38±13 ml/min per 1.73 m2, and the median proteinuria was 0.8 g/d. Figure 1 provides the distribution of subscale scores. Table 1 describes baseline characteristics by low versus high ACES subscale scores. Compared with those with higher communication quality subscale scores, individuals with lower scores were younger and less likely to self-report high medication adherence, and less likely to have hypertension, but were more likely to have lower mental quality of life Mental Component Summary (MCS) (MCS score ≤50), and depressive symptoms (BDI score ≥11). For the whole-person domain, individuals with lower scores were less likely to have hypertension compared with individuals with higher scores. Compared with those with higher health promotion domain subscale scores, individuals with lower scores were more likely to have at least a high school education, Medicaid, be born in the United States, and less likely to have diabetes. Compared with those with higher interpersonal treatment scores, those with lower scores were younger and more likely to have at least a high school education and MCS score ≤50, but less likely to self-report high medication adherence, health insurance, and diabetes. For the patient trust domain, individuals with lower scores were more likely to be younger and have at least a high school education and lower quality of life Physical Component Summary (PCS) (PCS and MCS scores ≤50). The survey was not completed by 38 individuals. Compared with those who completed the survey, those who did not complete the form were older (mean age 59 years in non-responders versus 56 years in responders), less likely to have completed high school (24% in non-responders versus 33% in responders), and had higher median albuminuria (1.2 [IQR, 0.3–3.7] in non-responders versus 0.6 [IQR, 0.1-3.4] g/24 h in responders). However, they were similar in terms of sex (women: 37% in non-responders versus 38% in responders), health insurance coverage (74% in non-responders versus 70% in responders), and mean eGFR (35 ml/min per 1.73 m2 in non-responders versus 37 ml/min per 1.73 m2 in responders).
Table 1.
Baseline characteristics by high versus low patient experience subscale scores
| Variable | Communication Quality | Whole-Person Orientation | Health Promotion | Interpersonal Treatment | Patient Trust | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low, n=103 | High, n=148 | Low, n=115 | High, n=137 | Low, n=83 | High, n=168 | Low, n=111 | High, n=140 | Low, n=118 | High, n=133 | |
| Age, yr | 54±12 | 58±11a | 55±12 | 57±11 | 55±13 | 57±11 | 54±12 | 58±11b | 54±12 | 58±10b |
| Women | 39% | 30% | 40% | 39% | 38% | 41% | 37% | 41% | 41% | 38% |
| Income <$20,000 | 79% | 75% | 81% | 74% | 73% | 79% | 74% | 79% | 74% | 80% |
| Education <high school | 64% | 65% | 64% | 64% | 55% | 69%a | 57% | 71%a | 58% | 71%a |
| Health insurance | ||||||||||
| Medicare | 26% | 31% | 26% | 31% | 22% | 33%a | 21% | 36% | 25% | 33% |
| Medicaid | 19% | 15% | 17% | 16% | 27% | 11%a | 18% | 16% | 18% | 16% |
| Private/commercial | 16% | 17% | 15% | 18% | 15% | 18%a | 17% | 17% | 19% | 14% |
| Unknown/incomplete | 8% | 11% | 8% | 12% | 11% | 10%a | 11% | 10% | 11.1% | 9% |
| None | 31% | 25% | 33% | 23% | 25% | 29%a | 34% | 22% | 27% | 28% |
| Language preference, Spanish | 85% | 79% | 82% | 80% | 76% | 84% | 84% | 79% | 81% | 81% |
| Born in United States | 14% | 16% | 17% | 14% | 22% | 12%a | 14% | 16% | 16% | 14% |
| Current smoker | 6% | 8% | 4% | 10% | 4% | 9% | 5% | 9% | 7% | 8% |
| Diabetes | 64% | 71% | 64% | 71% | 58% | 73%a | 61% | 74%a | 64% | 72% |
| Hypertension | 85% | 95%a | 87% | 94%a | 88% | 92% | 88% | 93% | 88% | 93% |
| Cardiovascular disease | 29% | 20% | 21% | 26% | 22% | 24% | 23% | 24% | 23% | 24% |
| Nephrology care | 55% | 46% | 50% | 49% | 48% | 51% | 54% | 46% | 53% | 47% |
| Medication adherence, high | 51% | 64%a | 55% | 62% | 52% | 62% | 48% | 67%b | 53% | 64% |
| Medication adherence, medium | 23% | 23%a | 24% | 22% | 23% | 23% | 24% | 22%b | 25% | 21% |
| Medication adherence, low | 51% | 64%a | 55% | 62% | 52% | 62% | 48% | 67%b | 53% | 64% |
| Physical Component Summary score <50 | 83% | 77% | 84% | 76% | 84% | 77% | 82% | 78% | 85% | 74%a |
| Mental Component Summary score <50 | 76% | 48%b | 64% | 55% | 67% | 56% | 66% | 54% | 70% | 50%† |
| Beck Depression Inventory score ≥11 | 58% | 41%b | 51% | 44% | 51% | 46% | 53% | 44% | 53% | 44% |
| eGFR, ml/min per 1.73 m2 | 37 [29–48] | 37 [30–46] | 38 [31–48] | 35 [28–45] | 38 [32–48] | 37 [27–45] | 36 [27–47] | 38 [30–46] | 37 [27–48] | 37.3 [30.4–46.1] |
| Albumin, g/24 h | 0.8 [0.1–2.9] | 0.4 [0–3.4) | 0.4 [0–2.4] | 0.6 [0.1–3.7] | 0.4 [0–2.6] | 0.6 (0.1–3.3) | 1.2 [0.1–3.5] | 0.4 [0–2.4] | 0.9 [0–3.0] | 0.4 [0–3.3] |
| ACEI/ARB use | 69% | 72% | 72% | 69% | 68% | 72% | 68% | 73% | 72% | 69.7% |
Values for categorical variables are given as percentage; values for continuous variables are given as mean±SD or median [interquartile range]. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
P<0.05.
P<0.01.
Median follow-up was 4.8 years. We identified 619 hospitalizations (0–21 per participant), of which 159 were cardiovascular and 460 were noncardiovascular. In addition, there were 103 ESKD events and 56 deaths.
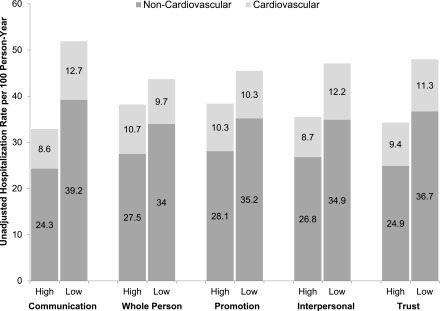
Rates of hospitalization by low versus high subscale scores are shown in Figure 2. As compared with higher scores, lower communication quality, health promotion, interpersonal treatment, and trust subscale scores were each associated with a higher adjusted rate ratio for all-cause hospitalization (Table 2) after adjustment for sociodemographic and clinical factors. There was no association between lower whole-person orientation subscale score and hospitalization. The results for cardiovascular and non-cardiovascular hospitalizations followed a similar pattern. Because of missing data, 54 individuals were excluded from the final regression model (income n=22, albuminuria n=33).
Figure 2.
Individuals with lower ACES subscales scores experienced higher hospitalization rates.
Table 2.
Association of low patient experience subscale scores with rates of hospitalizations
| Hospitalization Type | Subscales | Events, n | Rate per 100 person-yr | Adjusted Rate Ratio (95% CI) | Adjusted Absolute Risk Reduction per 100 person-yr (95% CI) |
|---|---|---|---|---|---|
| All-cause | Communication low | 282 | 51.9 | 1.54 (1.25 to 1.90) | −19.7 (−38.8 to −0.5) |
| Communication high | 257 | 32.8 | Reference | ||
| Whole-person orientation low | 262 | 43.7 | 1.20 (0.97 to 1.47) | −8.2 (−26.5 to 10.2) | |
| Whole-person orientation high | 279 | 38.2 | Reference | ||
| Health promotion low | 195 | 45.5 | 1.31 (1.05 to 1.62) | −12.4 (−31.7 to 6.8) | |
| Health promotion high | 344 | 38.3 | Reference | ||
| Interpersonal treatment low | 278 | 47.1 | 1.50 (1.22 to 1.85) | −18.7 (−38.0 to 0.5) | |
| Interpersonal treatment high | 261 | 35.5 | Reference | ||
| Trust low | 298 | 47.9 | 1.57 (1.27 to 1.93) | −19.7 (−37.9 to −1.6) | |
| Trust high | 241 | 34.2 | Reference | ||
| Noncardiovascular | Communication low | 213 | 39.2 | 1.50 (1.18 to 1.91) | −10.5 (−24.5 to 3.5) |
| Communication high | 190 | 24.3 | Reference | ||
| Whole-person orientation low | 204 | 34.0 | 1.25 (0.98 to 1.58) | −5.7 (−19.4 to 7.9) | |
| Whole-person orientation high | 201 | 27.5 | Reference | ||
| Health promotion low | 151 | 35.2 | 1.36 (1.06 to 1.74) | −8.1 (−22.4 to 6.2) | |
| Health promotion high | 252 | 28.1 | Reference | ||
| Interpersonal treatment low | 206 | 34.9 | 1.47 (1.16 to 1.87) | −10.2 (−24.5 to 4.0) | |
| Interpersonal treatment high | 197 | 26.8 | Reference | ||
| Trust low | 228 | 36.7 | 1.64 (1.29 to 2.09) | −12.6 (−26.2 to 1.1) | |
| Trust high | 175 | 24.9 | Reference | ||
| Cardiovascular | Communication low | 69 | 12.7 | 1.71 (1.12 to 2.62) | −9.8 (−23.1 to 3.6) |
| Communication high | 67 | 8.6 | Reference | ||
| Whole-person orientation low | 58 | 9.7 | 1.06 (0.69 to 1.62) | −1.0 (−13.1 to 11.1) | |
| Whole-person orientation high | 78 | 10.7 | Reference | ||
| Health promotion low | 44 | 10.3 | 1.18 (0.75 to 1.85) | −2.9 (−15.5 to 9.6) | |
| Health promotion high | 92 | 10.3 | Reference | ||
| Interpersonal treatment low | 72 | 12.2 | 1.58 (1.04 to 2.40) | −8.1 (−20.8 to 4.6) | |
| Interpersonal treatment high | 64 | 8.7 | Reference | ||
| Trust low | 70 | 11.3 | 1.34 (0.89 to 2.04) | −5.0 (−16.8 to 6.8) | |
| Trust high | 66 | 9.4 | Reference |
Adjusted for demographic factors (age, sex, income, education, Spanish language preference), nephrology care and clinical factors (diabetes, hypertension, any cardiovascular disease, Kidney Disease Quality of Life-36 survey score, Beck Depression Inventory score, eGFR, and albuminuria). 95% CI, 95% confidence interval.
Rates for incident ESKD and all-cause death were 3.7 (2.9–4.8) and 8.7 (7.1–10.5) per 100 patient-years, respectively. In fully adjusted models, there was no significant association between lower subscale scores and incident ESKD or death (Table 3).
Table 3.
Association of low patient experience subscale scores with incident ESKD and all-cause death
| Outcome | Subscales | Events, n | Rate per 100 person-yr | Hazard Ratio (95% CI) | Adjusted Absolute Risk Reduction per 100 person-yr (95% CI) |
|---|---|---|---|---|---|
| ESKD | Communication low | 36 | 8.2 | 1.58 (0.93 to 2.71) | −0.8 (−4.1 to 2.5) |
| Communication high | 55 | 9.0 | Reference | ||
| Whole-person orientation low | 35 | 6.9 | 1.01 (0.59 to 1.71) | −0.1 (−3.4 to 3.2) | |
| Whole-person orientation high | 56 | 10.3 | Reference | ||
| Health promotion low | 24 | 6.6 | 1.32 (0.74 to 2.37) | −0.5 (−3.9 to 2.9) | |
| Health promotion high | 67 | 9.7 | Reference | ||
| Interpersonal treatment low | 42 | 9.1 | 1.15 (0.68 to 1.94) | −0.2 (−3.5 to 3.1) | |
| Interpersonal treatment high | 49 | 8.3 | Reference | ||
| Trust low | 44 | 8.9 | 1.25 (0.74 to 2.11) | −0.4 (−3.7 to 2.8) | |
| Trust high | 47 | 8.5 | Reference | ||
| All-cause death | Communication low | 20 | 3.7 | 0.90 (0.45 to 1.82) | 0.3 (−6.9 to 7.6) |
| Communication high | 26 | 3.3 | Reference | ||
| Whole-person orientation low | 18 | 3.0 | 0.87 (0.43 to 1.78) | 0.8 (−6.4 to 8.0) | |
| Whole-person orientation high | 28 | 3.8 | Reference | ||
| Health promotion low | 11 | 2.6 | 0.62 (0.27 to 1.44) | 2.1 (−4.8 to 9.0) | |
| Health promotion high | 35 | 3.9 | Reference | ||
| Interpersonal treatment low | 18 | 3.0 | 0.81 (0.40 to 1.64) | 1.0 (−6.2 to 8.1) | |
| Interpersonal treatment high | 28 | 3.8 | Reference | ||
| Trust low | 22 | 3.5 | 0.92 (0.47 to 1.79) | 0.4 (−6.9 to 7.7) | |
| Trust high | 24 | 3.4 | Reference |
Adjusted for demographic factors (age, sex, income, education, Spanish language preference) and clinical factors (diabetes, hypertension, any cardiovascular disease, Kidney Disease Quality of Life-36 survey score, Beck Depression Inventory score, eGFR, and proteinuria). 95% CI, 95% confidence interval.
Sensitivity Analyses
For the outcome of hospitalization because of ambulatory care-sensitive conditions, the results were in the same direction as in the main analysis. However, only the association with the interpersonal subscale was statistically significant (Supplemental Table 2). After adding health insurance to the fully adjusted model, the association between subscale scores and hospitalization was unchanged (Supplemental Table 3). Similarly, findings were largely unchanged after adjusting for baseline medication adherence, as well as time-updated systolic BP and HbA1c (Supplemental Table 3).
Discussion
In this cohort of Hispanics with CKD, lower patient–physician interaction subscales scores were independently associated with higher risk of hospitalization but not with incident ESKD or death. To the best of our knowledge, this represents the first longitudinal study evaluating the association between the patient experience with their primary care physician and outcomes in Hispanic patients with CKD.
Our findings regarding the association between lower subscale scores and hospitalization risk are of particular significance in view of the high risk for hospitalization among patients with CKD (1). Hispanic CRIC participants experienced rates of hospitalizations which were more than two-fold higher than in Medicare patients without CKD but comparable to those with CKD as reported by the USRDS (1). Previous studies have demonstrated the association of sociodemographic and clinical factors on risk for hospitalization (2–4). However, the association of the patient experience with their primary care physician is of critical importance given the growing interest in patient-centered care, defined by the Institute of Medicine as care which is “responsive to individual patient preferences, needs, and values” (34). Furthermore, recent publications have emphasized the need to improve the provision of patient-centered care for patients with CKD (35), as well as the importance of diversification of the workforce to facilitate culturally congruent care (36).
Our findings are of particular relevance in view of the growth of the United States Hispanic population and their increased burden of ESKD. USRDS data indicate that incident ESKD rates are 1.35 times higher in Hispanics than in non-Hispanics (1). Overall, we found Hispanics with CKD rated their experience with their primary care physician highly and scores were very similar to those observed in large and diverse samples of patients without CKD (17). Furthermore, we found that individuals with lower scores were younger and more likely to have depressive symptoms and low mental health-related quality of life. These findings are consistent with a prior study of predominately non-Hispanics with coronary disease, which found that individuals with depressive symptoms had poor patient–physician communication (37). It is possible that self-reports of lower quality patient–physician interaction may be partly related to the psychologic state of the patient, unmet expectations, and less satisfaction with care (38,39).
Prior studies have suggested that language and cultural barriers may have a negative influence on Spanish-speaking patients’ experiences of care (9,40,41). Fernandez et al. (41) found that greater physician Spanish language fluency and cultural competence were associated with better patient-reported interpersonal processes of care. Of note, we did not find a significant association between the participant’s language preference and subscale scores. Furthermore, we adjusted for language preference in our multivariable regression models and the association with hospitalization risk was not attenuated. However, we were not able to assess patient–provider language concordance or provider cultural competency. There is evidence suggesting that specific cultural factors may influence the patient–physician relationship in Hispanics (10–12). For example, some have suggested that values such as personalismo (establishing a personal relationship with a health care provider) and familismo (family loyalty) are of particular importance in providing culturally competent health care for Hispanics (10,12).
There are several potential explanations for the association between lower quality of the patient experience and increased hospitalization risk. Other studies have found the quality of the patient experience with their primary care physician to be associated with better adherence and disease control (42–44), both of which have been associated with lower risk for hospitalization (45,46). Consistent with this, we found that individuals with lower communication quality and interpersonal treatment subscale scores were less likely to be adherent to medications. However, the association between subscale scores and hospitalization was unchanged when adherence was added to the model. To address the role of disease control, we conducted a sensitivity analysis adjusting for time-updated BP and HbA1c and found that the strength of the association was not attenuated. These findings suggest that other factors may be responsible for the observed associations. In addition, it is possible that patients with higher satisfaction with their physician interaction may be more likely to interact with their physician when acute medical issues arise and therefore avoid the need for hospitalization. It is also possible that higher quality of patient–physician interaction may facilitate patient activation, which is defined as the knowledge, skills, and confidence a person has in managing their own health and health care (47). Several studies have found lower level of activation to be associated with increased emergency department visits and hospitalizations (48). Furthermore, individuals with lower physician trust may have less general confidence in the health care system, receive less effective health care and less support for self-care, and consequently experience more urgent disease exacerbations and hospitalizations.
In a sensitivity analysis, we found that a lower interpersonal treatment score was associated with higher risk for ambulatory care-sensitive hospitalization, which is considered a measure of the effectiveness of ambulatory care. This finding has important implications in terms of patient burden and costs and suggests the need to evaluate interventions designed to improve the quality of the patient–physician interaction as a potential strategy to reduce the risk for hospitalization in Hispanics with CKD. There is evidence that the quality of communication between patients and their physicians is potentially modifiable (49,50). In a systematic review of randomized controlled trials evaluating interventions designed to improve physician communication behaviors, Rao et al. (49) found that physicians randomized to a communication intervention were more likely to receive higher ratings of their overall communication style compared with controls. Similarly, patients who received a communication training intervention participated more during the health care visit and obtained more information from their physician (49,50).
In contrast, we did not observe a significant association between satisfaction with the patient–physician interaction and incident ESKD. This may be because risk reduction for CKD progression requires long-term disease control, whereas short-term management strategies that are facilitated by a strong relationship with the primary care physician may be more effective in reducing hospitalizations. In addition, we did not assess the quality of the patient–physician interaction with the nephrologist, which could potentially have a more significant influence on CKD progression.The strengths of this study include its prospective design, long-term follow-up, detailed characterization of a wide range of factors, and utilization of a validated survey that provides a comprehensive assessment of the participant’s experience with their primary care physician. However, our study has several limitations. Because the majority of Hispanic participants were recruited in Chicago area, findings from this study may not be fully generalizable to the entire population of Hispanics with CKD in the United States. Nonetheless, the composition of Hispanic participants is reflective of the heterogeneity of the United States Hispanic population in terms of country of origin, education, income, and primary language (15). In addition, the wording of the first item of the ACES precludes the evaluation of nonphysician providers and does not distinguish between care provided by a primary care physician or a specialist. Furthermore, we did not have detailed information regarding sources and characteristics of participants’ health care. However, we had detailed information regarding insurance coverage and found that the strength of the association with risk of hospitalization remained robust after adjustment for this factor. Another limitation was that the ACES was not administered to non-Hispanics and for this reason, we are not able to make racial/ethnic comparisons. Furthermore, as mentioned earlier, we were not able to assess patient–provider language concordance or provider cultural competency. Finally, in view of the observational study design, causal relationships cannot be established.
In conclusion, we found that lower perceived quality of patient–physician interaction was associated with higher risk of hospitalization in Hispanics with CKD. Further studies are required to confirm this association and implement strategies designed to improve the quality of the patient experience with their primary care physician with the long-term objective of providing patient-centered care for Hispanics with CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
Funding for the Chronic Renal Insufficiency Cohort (CRIC) study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grant numbers U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award, the National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) (UL1TR000003), Johns Hopkins University (UL1TR-000424), University of Maryland (GCRC M01 RR-16500), Clinical and Translational Science Collaborative of Cleveland (UL1TR000439) from the NCATS component of the National Institutes of Health and NIH roadmap for Medical Research, the Michigan Institute for Clinical and Health Research (UL1TR000433), University of Illinois at Chicago CTSA (UL1RR029879), Tulane Center of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases (P20 GM109036), and the Kaiser Permanente NIH/National Center for Research Resources University of California San Francisco-Clinical and Translational Science Institute (UL1RR-024131). J.P.L. is funded by the NIDDK (K24DK092290, R01-DK072231). E.A.C.-C. is funded by a Research Supplement to Promote Diversity in Health-Related Research (U01-DK060980). A.C.R. is funded by the NIDDK (K23DK094829). M.S. is also funded by the NIDDK (K23DK10311).
This work was presented at the 2016 American Society of Nephrology Kidney Week November 17–20, 2016, in Chicago, IL.
The CRIC Study Group investigators include Lawrence J. Appel (Johns Hopkins University), Harold L. Feldman (Perelman School of Medicine, University of Pennsylvania), Alan S. Go (Kaiser Permanente Division of Research, Oakland, California), Jiang He (Tulane University), John W. Kusek (National Institute for Diabetes and Digestive and Kidney Diseases), Mahboob Rahman (Case Western Reserve University School of Medicine), Akinlolu Ojo (University of Arizona Health Sciences), and Raymond Townsend (Hospital of the University of Pennsylvania).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03170318/-/DCSupplemental.
See related editorial, “Better Patient Ambulatory Care Experience: Does It Translate into Improved Outcomes among Patients with CKD?,” on pages 1619–1620.
References
- 1.US Renal Data System: United States Renal Data System 2016 Annual Data Report. Available at: https://www.usrds.org/2016/view/Default.aspx. Accessed April 30, 2017
- 2.Holland DC, Lam M. Predictors of hospitalization and death among pre-dialysis patients: A retrospective cohort study. Nephrol Dial Transplant 15: 650–658, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Mix T-CH, St peter WL, Ebben J, Xue J, Pereira BJ, Kausz AT, Collins AJ: Hospitalization during advancing chronic kidney disease. Am J Kidney Dis 42: 972–981, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Xie Y, Bowe B, Xian H, Balasubramanian S, Al-Aly Z: Rate of kidney function decline and risk of hospitalizations in stage 3A CKD. Clin J Am Soc Nephrol 10: 1946–1955, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiebe N, Klarenbach SW, Allan GM, Manns BJ, Pelletier R, James MT, Bello A, Hemmelgarn BR, Tonelli M; Alberta Kidney Disease Network : Potentially preventable hospitalization as a complication of CKD: A cohort study. Am J Kidney Dis 64: 230–238, 2014 [DOI] [PubMed] [Google Scholar]
- 6.US Census Bureau: Facts for Features: Hispanic Heritage Month, 2017. Available at: https://www.census.gov/newsroom/facts-for-features/2017/hispanic-heritage.html. Accessed October 4, 2017
- 7.Ricardo AC, Flessner MF, Eckfeldt JH, Eggers PW, Franceschini N, Go AS, Gotman NM, Kramer HJ, Kusek JW, Loehr LR, Melamed ML, Peralta CA, Raij L, Rosas SE, Talavera GA, Lash JP: Prevalence and correlates of CKD in hispanics/latinos in the United States. Clin J Am Soc Nephrol 10: 1757–1766, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmins CL: The impact of language barriers on the health care of Latinos in the United States: A review of the literature and guidelines for practice. J Midwifery Womens Health 47: 80–96, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Carrasquillo O, Orav EJ, Brennan TA, Burstin HR: Impact of language barriers on patient satisfaction in an emergency department. J Gen Intern Med 14: 82–87, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Quesada GM, Heller PL: Sociocultural barriers to medical care among Mexican Americans in Texas: A summary report of research conducted by the Southwest Medical Sociology Ad Hoc Committee. Med Care 15[Suppl 5]: 93–101, 1977 [DOI] [PubMed] [Google Scholar]
- 11.Hoppe SK, Heller PL: Alienation, familism and the utilization of health services by Mexican Americans. J Health Soc Behav 16: 304–314, 1975 [PubMed] [Google Scholar]
- 12.Flores G: Culture and the patient-physician relationship: Achieving cultural competency in health care. J Pediatr 136: 14–23, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Moy E, Chang E, Barrett M; Centers for Disease Control and Prevention (CDC) : Potentially preventable hospitalizations - United States, 2001-2009. MMWR Suppl 62: 139–143, 2013 [PubMed] [Google Scholar]
- 14.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JP; CRIC and H-CRIC Study Groups : CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis 58: 214–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lora CM, Ricardo AC, Brecklin CS, Fischer MJ, Rosman RT, Carmona E, Lopez A, Balaram M, Nessel L, Tao KK, Xie D, Kusek JW, Go AS, Lash JP: Recruitment of Hispanics into an observational study of chronic kidney disease: The Hispanic Chronic Renal Insufficiency Cohort Study experience. Contemp Clin Trials 33: 1238–1244, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safran DG, Karp M, Coltin K, Chang H, Li A, Ogren J, Rogers WH: Measuring patients’ experiences with individual primary care physicians. Results of a statewide demonstration project. J Gen Intern Med 21: 13–21, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safran DG, Kosinski M, Tarlov AR, Rogers WH, Taira DH, Lieberman N, Ware JE: The Primary Care Assessment Survey: Tests of data quality and measurement performance. Med Care 36: 728–739, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Benachi Sandoval N, Castillo Martínez A, Vilaseca Llobet JM, Torres Belmonte S, Risco Vilarasau E: [Validation of the spanish version of the primary care assessment survey questionnaire]. Rev Panam Salud Publica 31: 32–39, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez HP, von Glahn T, Rogers WH, Chang H, Fanjiang G, Safran DG: Evaluating patients’ experiences with individual physicians: A randomized trial of mail, internet, and interactive voice response telephone administration of surveys. Med Care 44: 167–174, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez HP, Rogers WH, Marshall RE, Safran DG: The effects of primary care physician visit continuity on patients’ experiences with care. J Gen Intern Med 22: 787–793, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sequist TD, Schneider EC, Anastario M, Odigie EG, Marshall R, Rogers WH, Safran DG: Quality monitoring of physicians: Linking patients’ experiences of care to clinical quality and outcomes. J Gen Intern Med 23: 1784–1790, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sequist TD, von Glahn T, Li A, Rogers WH, Safran DG: Statewide evaluation of measuring physician delivery of self-management support in chronic disease care. J Gen Intern Med 24: 939–945, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Network for Regional Healthcare Improvement: Short Form Patient Experience Survey – Research Findings, October 2015. Available at: http://www.nrhi.org/uploads/research-findings-patient-experience.pdf. Accessed April 23, 2018
- 25.Healthcare Cost and Utilization Project: Clinical Classifications Software (CCS) for ICD-9-CM. Available at: www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed February 27, 2018
- 26.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI; CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cedillo-Couvert EA, Ricardo AC, Chen J, Cohan J, Fischer MJ, Krousel-Wood M, Kusek JW, Lederer S, Lustigova E, Ojo A, Porter AC, Sharp LK, Sondheimer J, Diamantidis C, Wang X, Roy J, Lash JP; CRIC Study Investigators : Self-reported medication adherence and CKD progression. Kidney Int Rep 3: 645–651, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craven JL, Rodin GM, Littlefield C: The Beck Depression Inventory as a screening device for major depression in renal dialysis patients. Int J Psychiatry Med 18: 365–374, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ: Validation of depression screening scales in patients with CKD. Am J Kidney Dis 54: 433–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB: Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 3: 329–338, 1994 [DOI] [PubMed] [Google Scholar]
- 31.RAND Health: Kidney Disease Quality of Life Instrument (KDQOL). Available at: https://www.rand.org/health/surveys_tools/kdqol.html. Accessed February 14, 2018
- 32.Yang W, Jepson C, Xie D, Roy JA, Shou H, Hsu JY, Anderson AH, Landis JR, He J, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Statistical methods for recurrent event analysis in cohort studies of CKD. Clin J Am Soc Nephrol 12: 2066–2073, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agency for Healthcare Research and Quality: Prevention Quality Indicators Overview: Available at: http://qualityindicators.ahrq.gov/Modules/pqi_resources.aspx. Accessed April 22, 2018
- 34.Institute of Medicine (US) Committee on Quality of Health Care in America : Crossing the Quality Chasm: A New Health System for the 21st Century, Washington, DC, National Academies Press (US), 2001 [PubMed] [Google Scholar]
- 35.O’Hare AM. Patient-centered care in renal medicine: Five strategies to meet the challenge. Am J Kidney Dis 71: 732–736, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Valentijn PP, Pereira FA, Ruospo M, Palmer SC, Hegbrant J, Sterner CW, Vrijhoef HJM, Ruwaard D, Strippoli GFM: Person-centered integrated care for chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 13: 375–386, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schenker Y, Stewart A, Na B, Whooley MA: Depressive symptoms and perceived doctor-patient communication in the Heart and Soul study. J Gen Intern Med 24: 550–556, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson JL, Kroenke K: The effect of unmet expectations among adults presenting with physical symptoms. Ann Intern Med 134: 889–897, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Jackson JL, Chamberlin J, Kroenke K. Predictors of patient satisfaction. Soc Sci Med 52(4): 609–620, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Morales LS, Cunningham WE, Brown JA, Liu H, Hays RD: Are Latinos less satisfied with communication by health care providers? J Gen Intern Med 14: 409–417, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez A, Schillinger D, Grumbach K, Rosenthal A, Stewart AL, Wang F, Pérez-Stable EJ: Physician language ability and cultural competence. An exploratory study of communication with Spanish-speaking patients. J Gen Intern Med 19: 167–174, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zolnierek KBH, Dimatteo MR: Physician communication and patient adherence to treatment: A meta-analysis. Med Care 47: 826–834, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orth JE, Stiles WB, Scherwitz L, Hennrikus D, Vallbona C: Patient exposition and provider explanation in routine interviews and hypertensive patients’ blood pressure control. Health Psychol 6: 29–42, 1987 [DOI] [PubMed] [Google Scholar]
- 44.Safran DG, Taira DA, Rogers WH, Kosinski M, Ware JE, Tarlov AR: Linking primary care performance to outcomes of care. J Fam Pract 47: 213–220, 1998 [PubMed] [Google Scholar]
- 45.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ: Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 166: 1836–1841, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Kornum JB, Thomsen RW, Riis A, Lervang H-H, Schønheyder HC, Sørensen HT: Diabetes, glycemic control, and risk of hospitalization with pneumonia: A population-based case-control study. Diabetes Care 31: 1541–1545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hibbard JH, Mahoney ER, Stockard J, Tusler M: Development and testing of a short form of the patient activation measure. Health Serv Res 40: 1918–1930, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinney RL, Lemon SC, Person SD, Pagoto SL, Saczynski JS: The association between patient activation and medication adherence, hospitalization, and emergency room utilization in patients with chronic illnesses: A systematic review. Patient Educ Couns 98: 545–552, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Rao JK, Anderson LA, Inui TS, Frankel RM: Communication interventions make a difference in conversations between physicians and patients: A systematic review of the evidence. Med Care 45: 340–349, 2007 [DOI] [PubMed] [Google Scholar]
- 50.D’Agostino TA, Atkinson TM, Latella LE, Rogers M, Morrissey D, DeRosa AP, Parker PA: Promoting patient participation in healthcare interactions through communication skills training: A systematic review. Patient Educ Couns 100: 1247–1257, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.