Abstract
The ability of the immune system to differentiate self from nonself is critical in determining the immune response to antigens expressed on transplanted tissue. Even with conventional immunosuppression, acceptance of the allograft is an active process often determined by the presence of regulatory T cells (Tregs). Tregs classically are CD4+ cells that constitutively express high levels of the IL-2 receptor α chain CD25, along with the transcription factor Foxp3. The use of Tregs in the field of solid organ transplantation is related specifically to the objective of achieving tolerance, with the goal of reducing or eliminating immunosuppressive drugs as well as maintaining tissue repair and managing acute rejection. A key issue in clinical use of Tregs is how to effectively expand the number of Tregs, either through increasing numbers of endogenous Tregs or by the direct infusion of exogenously expanded Tregs. In order to realize the benefits of Treg therapy in solid organ transplantation, a number of outstanding challenges need to be overcome, including assuring an effective expansion of Tregs, improving long-term Treg stability and reduction of risk-related to off-target, nonspecific, immunosuppressive effects related specially to cancer.
Keywords: Allografts, Goals, IL2RA protein, human, immunosuppression, Interleukin-2 Receptor Alpha Subunit, Interleukins, kidney, kidney transplantation, Neoplasms, Receptors, Interleukin-2, T-lymphocytes, Regulatory, tolerance, transcription factors, transplantation
Introduction
The ability of the immune system to differentiate self from nonself is critical in determining the immune response to antigens expressed on transplanted tissue. The immune system responds to the antigens through the interaction of T cells with the MHC.
According to animal model studies (1), acceptance/tolerance of an allograft is an active process often determined by the presence of regulatory T cells (Tregs). Therefore, numerous studies have focused on ways of either expanding endogenously occurring Tregs or using exogenously expanded Tregs to achieve acceptance of the allograft while avoiding the complications of long-term immunosuppression (2).
Types of Tregs: Phenotyping
Regulatory T cells are broadly classified as thymus derived (natural) Tregs, or peripheral inducible Tregs. Inducible Tregs can be generated from natural Tregs or naïve CD4+CD25− cells upon T cell receptor stimulation in the presence of cytokines such as TGF-β and IL-2 (3,4).
CD4+ Treg cells constitutively express high levels of the IL-2 receptor α chain CD25, together with the transcription factor Foxp3, whose methylation status is a reliable marker for identifying a stable population of these cells (5). The surface marker CD127 is inversely correlated with Foxp3 expression (6). Multiple other cellular markers have been shown to be expressed by Tregs (Table 1) (7,8).
Table 1.
Additional markers defining Treg subpopulations
| Marker | Positive (+) /Negative (−) | Related to |
|---|---|---|
| CD45RA | + | Naïve cells |
| CD45RO | + | Memory cells |
| ICOS | + | Production of IL-10 or TGF-β |
| CD39/73 | + | cAMP- or adenosine-mediated suppression |
| LAP | + | LAP/TGF-β complex in activated cells |
| CD103 | + | Effector and memory cells |
| GITR | + | Costimulatory |
| CD31 | + | Adhesion and transmigration |
| CD147 | + | Activated Treg |
| CCR4 | + | Migrate to the graft |
| CD49d | − | Part of lymphocyte homing receptor, useful for purification Tregs with CD127lo |
| CD62L | +/Low | Naïve cells/ effector and memory cells |
| CD161 | + | Proinflammatory potential |
| CTLA-4 | + | Coinhibitory molecule |
| CD71 | + | Activated Treg |
| GARP | + | Binds to TGF-β in activated cells |
| CD137 | + | Antigen-specific T cells |
ICOS, inducible costimulator; LAP, latency-associated peptide; GITR, glucocorticoid-induced TNF receptor; CCR4, C-C chemokine receptor type 4; CTLA-4, cytotoxic T lymphocyte antigen-4; GARP, glycoprotein A repetitions predominant.
CD4+CD25+FOXP3+ Tregs constitute 5%–10% of all circulating CD4+ cells (9).
Mechanism of Actions, Interactions with Other Cell Types
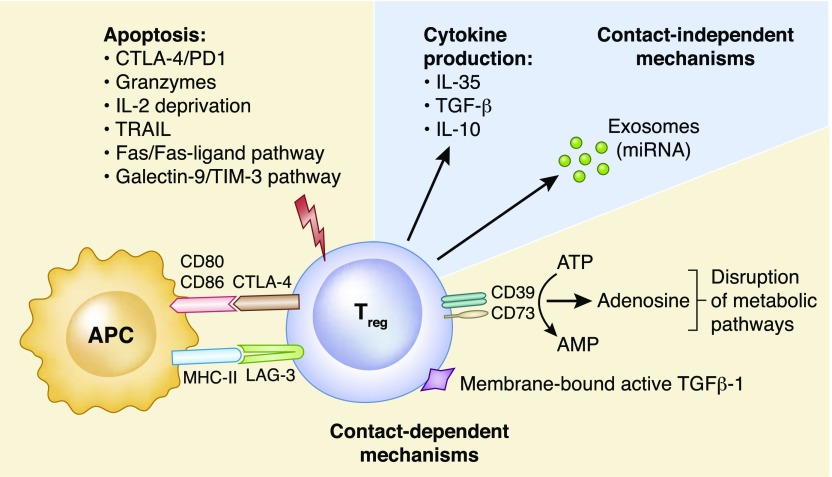
Contact dependent and independent mechanisms have been shown to contribute to the suppressive activity of regulatory T cells (Figure 1) and may act simultaneously depending on the context (2,7).
Figure 1.
Mechanisms of Treg suppression. Contact-dependent mechanisms: Induction of apoptosis via engagement of cytotoxic T lymphocyte antigen-4 (CTLA-4) and programed cell death 1 (PD1), granzyme A/B, TNF-related apoptosis-inducing ligand (TRAIL), the Fas/Fas-ligand pathway, the galectin-9/T cell immunoglobulin and mucin domain-3 (TIM-3) pathway and through IL-2 deprivation. Interaction of CTLA-4 with its ligand CD80/86 on antigen presenting cells (APCs) delivers a negative signal that prevents T cell activation. Cell surface lymphocyte activation gene 3 (LAG-3) binds to MHC class II molecules preventing the maturation and the ability of APCs to activate effector T cells. Expression of membrane-bound active TGFβ-1. Disruption of metabolic pathways through the production of adenosine from the ectoenzymes CD39/CD73-mediated degradation of extracellular ATP to AMP. ATP-derived adenosine increases intracellular cAMP levels on Tregs that is transferred to T effector cells through gap junctions, leading to the upregulation of inducible cAMP early repressor (ICER) and in turn, the inhibition of nuclear factor of activated T-cells (NFAT) and IL-2 transcription. Contact-independent mechanisms: Anti-inflammatory cytokine production (IL-10, IL-35, and TGF-β) has been linked with inhibition of T cell activation in vivo. Transfer of micro ribonucleic acid (miRNA) through exosomes can silence specific genes in T effector cells, preventing proliferation and cytokine production.
The ability of Tregs to cause “linked” or “bystander” suppression of effector T cells with different antigen specificities, or confer tolerance to T cells that recognize a third-party antigen (“infectious tolerance”), are other ways of amplifying and extending their suppressor function (10,11). Finally, although Tregs can suppress the function of activated CD4+ and CD8+ T cells, B cells, macrophages, and dendritic cells, memory T cells are less responsive to their suppressive effect (12).
Expansion of Natural Tregs
Within a clinical context it is important to consider the effects of immunosuppressive drugs on Tregs. Treatment with calcineurin inhibitors decreases Treg viability and proliferation (13). The effects of mycophenolic acid on Tregs appear to be more variable (14–16) whereas glucocorticoids appear not to affect Tregs, although one study has described steroid-related IL-2–dependent expansion of Tregs (17). The mTOR inhibitors have been shown to promote the differentiation and expansion of Tregs (18) as well as increase Foxp3 expression (19), although this effect may decline over time (20). In certain scenarios, induction therapy with thymoglobulin has been shown to favorably shift the Treg to T effector ratio (21,22). Because of the high expression of CD25 on Tregs, it is thought that anti-CD25 (basiliximab) therapy may have a deleterious effect on Treg populations (23). The use of alemtuzumab has also been shown to lead to the generation/expansion of Tregs (20). Unsurprisingly, because of the importance of the B7:CD28 interaction for the maintenance and generation of Tregs (24), the use of costimulatory molecule blocker belatacept seems to reduce their number (25,26).
Interestingly, two nonimmunosuppressive drugs have been shown to increase Treg populations. Metformin promotes phosphorylation of p-STAT5 and FOXP3 (27). Erythropoietin, on the other hand, is reported to inhibit proliferation of conventional T cells, while simultaneously sparing Treg proliferation (28). Finally, the use of low-dose recombinant IL-2 has been considered as a potential means of expanding Tregs (29), although it appears to have a narrow therapeutic window because of the risk of stimulating natural killer cell activity (30).
Inducible Tregs: Types, Ways of Expansion, and Stability
Most protocols for Treg isolation from peripheral blood utilize leukapheresis followed by serial enrichment for a CD25+ population. The observations that ex vivo–expanded Tregs are more suppressive compared with freshly isolated Tregs (31) justifies the need of expansion.
This expansion can be done in an antigen-specific or -nonspecific way. Donor alloantigen-specific Tregs have been shown to be more effective than polyclonal cells; however, polyclonal cells are easier to produce (7). Protocols for nonspecific stimulation involve T cell receptor (CD3) stimulation with CD28 costimulation (32). Specific stimulation uses exposing Tregs to alloantigens or indirect presentation of donor peptide on recipient dendritic cells in the presence of IL-2 (33). Additional activation with antigen and IL-12 induces more potent, antigen-specific Th1-like Tregs that express T-bet and IFN-γ (34).
Finally, Tregs can be genetically engineered for alloantigen specificity (35). In order to avoid contamination with T effector cells during the expansion procedure, Treg friendly rapamycin has been used in the manufacturing process (36). An alternative approach to increase Treg specificity would be to find ways to optimize Treg homing to the allograft (37). Additionally, long-term stability of Tregs is also a concern, as some Tregs have shown the ability to switch to an effector phenotype (38).
Clinical Use of Tregs
The indications for the use of Tregs in the field of solid organ transplantation are related to the objective of achieving tolerance while maintaining tissue repair and managing acute rejection.
The first objective is described in this review, but with respect to tissue repair, Tregs may play an important role during repair in the kidney with ischemic–reperfusion injury. Kidney-infiltrating Tregs have been shown to play a direct role in promoting healing, probably by negative modulation of the proinflammatory cytokines produced by other T cells (39). In terms of acute rejection, it is conceivable that conventional recipient-derived T cells contribute to graft tolerance if they acquire a resting tissue-resident phenotype (40). Interestingly, a recent study has shown that inducible Treg treatment in a murine model decreased serum donor-specific antibody levels and deposition of IgG within allografts, indicating a potential use in the treatment of humoral rejection (41).
With regards to the number of Tregs necessary to achieve tolerance, more important than the total dose administered may be increase Tregs to at least 30% of T effectors (3). Tang and Lee (42) have calculated that if polyclonal Tregs were administered after lymphodepletion with thymoglobulin, a dose of 3–5×109 could effectively increase the Treg percentage. The clinical trials related to Treg and kidney transplantation that are ongoing are presented in Table 2 according to the information obtained from Clinicaltrials.gov (43–54).
Table 2.
Clinical trials related to Treg and kidney transplantation that are ongoing
| Study | Status | Location |
|---|---|---|
| Treg adoptive therapy for subclinical inflammation in kidney transplantation (43) | Active, not recruiting | University of California, San Francisco, California |
| Treg therapy in subclinical inflammation in kidney transplantation (44) | Recruiting | University of Alabama at Birmingham, Birmingham, Alabama |
| Cedars-Sinai Medical Center, Los Angeles, California | ||
| University of California, San Francisco, San Francisco, California | ||
| (and three more...) | ||
| Rapamycin and regulatory T cells in kidney transplantation (45) | Completed | Policlinico Fondazione IRCCS “San Matteo,” Pavia, Italy |
| Infusion of T-Regulatory cells in kidney transplant recipients (The ONE Study) (46) | Active, not recruiting | Massachusetts General Hospital Boston, Massachusetts |
| A pilot study using autologous regulatory T cell infusion zortress (Everolimus) in renal transplant recipients (47) | Not yet recruiting | University of Kentucky Medical Center Lexington, Kentucky |
| Effect of different therapeutic strategies on regulatory T cells in kidney transplantation (48) | Completed | Policlinico Fondazione IRCCS “San Matteo” Pavia, Italy |
| Donor-alloantigen-reactive regulatory T cell (darTreg) therapy in renal transplantation (The ONE Study) (49) | Recruiting | University of California San Francisco - Transplant Department, San Francisco, California |
| Effects of sevoflurane and desflurane on Treg (50) | Completed | Ramathibodi Hospital, Ratchathewi, Bangkok, Thailand |
| Trial of adoptive immunotherapy with TRACT to prevent rejection in living donor kidney transplant recipients (51) | Active, not recruiting | Northwestern University Comprehensive Transplant Center, Chicago, Illinois |
| Role of T helper 17 and regulatory T cells in delayed graft function (52) | Active, not recruiting | McGill University Health Center, Montreal, Quebec, Canada |
| Ultra-low-dose subcutaneous IL-2 in renal transplantation (53) | Active, not recruiting | Brigham and Women’s Hospital, Boston |
| The differential effects of 3 different immunosuppressive (54) | Completed | Northwestern Memorial Hospital, Chicago, Illinois |
TRACT, T-reg Adoptive Cell Transfer.
Safety: Need for Tumor Surveillance
Numerous studies have shown that patients with cancer with a poorer prognosis have an increased number of Tregs in the circulation, as well in the lymphoid tissues and tumor microenvironment. However, induction or infusion of Tregs in patients with a chronic inflammation process may restrict cancer initiation through reducing local expression of cancer-driving proinflammatory cytokines (55).
Accordingly, it may be best to develop a screening protocol in order to rule out an established tumor before Treg infusion, so as to expand Tregs that can express markers for migration only to inflammatory sites.
Monitoring the Effect of Tregs on the Immune Response
It cannot be assumed that tolerance induced by Tregs is totally stable and one of the real challenges will be to develop a means of monitoring the immune response. Some studies have labeled expanded Tregs with deuterium to monitor the adoptively transferred cells by flow cytometry (56). However, performing protocol kidney biopsies after Treg infusion to assess changes in inflammation (57) and monitoring of donor-specific antibodies may be necessary.
Finally, because metabolic alterations represent a state of immediate cellular responses to stresses, metabolomic measurements may be useful in this context (58).
In conclusion, a more realistic intermediate goal for Treg therapy in solid organ recipients is the reduction of immunosuppressive treatment and defining more individualized immunosuppressive protocols rather than tolerance. In order to achieve this goal, it will be necessary to assure effective expansion of Tregs, improve their long-term stability, and reduce the risk of nonspecific immunosuppression, related specially to cancer, by optimizing Treg homing to the allograft.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Duran-Struuck R, Sondermeijer HP, Bühler L, Alonso-Guallart P, Zitsman J, Kato Y, Wu A, McMurchy AN, Woodland D, Griesemer A, Martinez M, Boskovic S, Kawai T, Cosimi AB, Wuu CS, Slate A, Mapara M, Baker S, Tokarz R, D’Agati V, Hammer S, Pereira M, Lipkin WI, Wekerle T, Levings M, Sykes M: Effect of ex vivo-expanded recipient regulatory T cells on hematopoietic chimerism and kidney allograft tolerance across MHC barriers in cynomolgus macaques. Transplantation 101: 274–283, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang Q, Vincenti F: Transplant trials with Tregs: Perils and promises. J Clin Invest 127: 2505–2512, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Povoleri GA, Scottà C, Nova-Lamperti EA, John S, Lombardi G, Afzali B: Thymic versus induced regulatory T cells - who regulates the regulators? Front Immunol 4: 169, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwang NA, Leventhal JR: Cell therapy in kidney transplantation: Focus on regulatory T cells. J Am Soc Nephrol 28: 1960–1972, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J: Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 5: e38, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA: CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203: 1701–1711, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason GM, Lowe K, Melchiotti R, Ellis R, de Rinaldis E, Peakman M, Heck S, Lombardi G, Tree TI: Phenotypic complexity of the human regulatory T cell compartment revealed by mass cytometry. J Immunol 195: 2030–2037, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Lam AJ, Hoeppli RE, Levings MK: Harnessing advances in T regulatory cell biology for cellular therapy in transplantation. Transplantation 101: 2277–2287, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Vaikunthanathan T, Safinia N, Boardman D, Lechler RI, Lombardi G: Regulatory T cells: Tolerance induction in solid organ transplantation. Clin Exp Immunol 189: 197–210, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldmann H, Adams E, Fairchild P, Cobbold S: Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol Rev 212: 301–313, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Mohr Gregoriussen AM, Bohr HG: A novel model on DST-induced transplantation tolerance by the transfer of self-specific donor tTregs to a haplotype-matched organ recipient. Front Immunol 8: 9, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall BM, Pearce NW, Gurley KE, Dorsch SE: Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. III. Further characterization of the CD4+ suppressor cell and its mechanisms of action. J Exp Med 171: 141–157, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scottà C, Fanelli G, Hoong SJ, Romano M, Lamperti EN, Sukthankar M, Guggino G, Fazekasova H, Ratnasothy K, Becker PD, Afzali B, Lechler RI, Lombardi G: Impact of immunosuppressive drugs on the therapeutic efficacy of ex vivo expanded human regulatory T cells. Haematologica 101: 91–100, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demirkiran A, Sewgobind VD, van der Weijde J, Kok A, Baan CC, Kwekkeboom J, Tilanus HW, Metselaar HJ, van der Laan LJ: Conversion from calcineurin inhibitor to mycophenolate mofetil-based immunosuppression changes the frequency and phenotype of CD4+FOXP3+ regulatory T cells. Transplantation 87: 1062–1068, 2009 [DOI] [PubMed] [Google Scholar]
- 15.He X, Smeets RL, Koenen HJ, Vink PM, Wagenaars J, Boots AM, Joosten I: Mycophenolic acid-mediated suppression of human CD4+ T cells: More than mere guanine nucleotide deprivation. Am J Transplant 11: 439–449, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Wu T, Zhang L, Xu K, Sun C, Lei T, Peng J, Liu G, Wang R, Zhao Y: Immunosuppressive drugs on inducing Ag-specific CD4(+)CD25(+)Foxp3(+) Treg cells during immune response in vivo. Transpl Immunol 27: 30–38, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, Howard OM: Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol 36: 2139–2149, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Gallon L, Traitanon O, Yu Y, Shi B, Leventhal JR, Miller J, Mas V, L X, Mathew JM: Differential effects of calcineurin and mammalian target of rapamycin inhibitors on alloreactive Th1, Th17, and regulatory T cells. Transplantation 99: 1774–1784, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Kim KW, Chung BH, Kim BM, Cho ML, Yang CW: The effect of mammalian target of rapamycin inhibition on T helper type 17 and regulatory T cell differentiation in vitro and in vivo in kidney transplant recipients. Immunology 144: 68–78, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloom DD, Chang Z, Fechner JH, Dar W, Polster SP, Pascual J, Turka LA, Knechtle SJ: CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant 8: 793–802, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, Ames S, Lerner S, Ebcioglu Z, Nair V, Dinavahi R, Sehgal V, Heeger P, Schroppel B, Murphy B: Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant 10: 2132–2141, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Q, Leung J, Melli K, Lay K, Chuu EL, Liu W, Bluestone JA, Kang SM, Peddi VR, Vincenti F: Altered balance between effector T cells and FOXP3+ HELIOS+ regulatory T cells after thymoglobulin induction in kidney transplant recipients. Transpl Int 25: 1257–1267, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Bluestone JA, Liu W, Yabu JM, Laszik ZG, Putnam A, Belingheri M, Gross DM, Townsend RM, Vincenti F: The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant 8: 2086–2096, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA: Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol 171: 3348–3352, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Riella LV, Liu T, Yang J, Chock S, Shimizu T, Mfarrej B, Batal I, Xiao X, Sayegh MH, Chandraker A: Deleterious effect of CTLA4-Ig on a Treg-dependent transplant model. Am J Transplant 12: 846–855, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Alvarez Salazar EK, Cortés-Hernández A, Alemán-Muench GR, Alberú J, Rodríguez-Aguilera JR, Recillas-Targa F, Chagoya de Sánchez V, Cuevas E, Mancilla-Urrea E, Pérez García M, Mondragón-Ramírez G, Vilatobá M, Bostock I, Hernández-Méndez E, De Rungs D, García-Zepeda EA, Soldevila G: Methylation of FOXP3 TSDR underlies the impaired suppressive function of Tregs from long-term belatacept-treated kidney transplant patients. Front Immunol 8: 219, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SY, Lee SH, Yang EJ, Kim EK, Kim JK, Shin DY, Cho ML: Metformin ameliorates inflammatory bowel disease by suppression of the STAT3 signaling pathway and regulation of the between Th17/Treg balance. PLoS One 10: e0135858, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purroy C, Fairchild RL, Tanaka T, Baldwin WM 3rd, Manrique J, Madsen JC, Colvin RB, Alessandrini A, Blazar BR, Fribourg M, Donadei C, Maggiore U, Heeger PS, Cravedi P: Erythropoietin receptor-mediated molecular crosstalk promotes T cell immunoregulation and transplant survival. J Am Soc Nephrol 28: 2377–2392, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, Fonfrede M, Rosenzwajg M, Bernard C, Klatzmann D: Low-dose interleukin 2 in patients with type 1 diabetes: A phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 1: 295–305, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Gasteiger G, Hemmers S, Firth MA, Le Floc’h A, Huse M, Sun JC, Rudensky AY: IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J Exp Med 210: 1167–1178, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chai JG, Coe D, Chen D, Simpson E, Dyson J, Scott D: In vitro expansion improves in vivo regulation by CD4+CD25+ regulatory T cells. J Immunol 180: 858–869, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M: Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood 104: 895–903, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Veerapathran A, Pidala J, Beato F, Yu XZ, Anasetti C: Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood 118: 5671–5680, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verma ND, Hall BM, Plain KM, Robinson CM, Boyd R, Tran GT, Wang C, Bishop GA, Hodgkinson SJ: Interleukin-12 (IL-12p70) promotes induction of highly potent Th1-Like CD4(+)CD25(+) T regulatory cells that inhibit allograft rejection in unmodified recipients. Front Immunol 5: 190, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, Broady R, Levings MK: Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest 126: 1413–1424, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan J, Feng L, Sun G, Chen P, Zhou Y, Xia M, Li H, Li Y: Interplay between mTOR and STAT5 signaling modulates the balance between regulatory and effective T cells. Immunobiology 220: 510–517, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Lamarche C, Levings MK: Guiding regulatory T cells to the allograft. Curr Opin Organ Transplant 23: 106–113, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Sawant DV, Vignali DA: Once a Treg, always a Treg? Immunol Rev 259: 173–191, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, Crow MT, King LS, Rabb H: Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int 76: 717–729, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Prosser AC, Kallies A, Lucas M: Tissue-resident lymphocytes in solid organ transplantation: Innocent passengers or the key to organ transplant survival? Transplantation 102: 378–386, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Liao T, Xue Y, Zhao D, Li S, Liu M, Chen J, Brand DD, Zheng H, Zhang Y, Zheng SG, Sun Q: In vivo attenuation of antibody-mediated acute renal allograft rejection by ex vivo TGF-β-induced CD4+Foxp3+ regulatory T cells. Front Immunol 8: 1334, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang Q, Lee K: Regulatory T-cell therapy for transplantation: How many cells do we need? Curr Opin Organ Transplant 17: 349–354, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Vincenti F: Treg adoptive therapy for subclinical inflammation in kidney transplantation. . Available at: https://clinicaltrials.gov/ct2/show/NCT02088931?term=Treg+adoptive+therapy+for+subclinical+inflammation+in+kidney+transplantation&rank=1. Accessed May 14, 2018 [Google Scholar]
- 44.Vincenti F: . Treg therapy in subclinical inflammation in kidney transplantation. Available at: https://clinicaltrials.gov/ct2/show/NCT02711826?term=Treg+therapy+in+subclinical+inflammation+in+kidney+transplantation&rank=1. Accessed May 14, 2018 [Google Scholar]
- 45.Dal Canton A: . Rapamycin and regulatory T cells in kidney Transplantation. Available at: https://clinicaltrials.gov/ct2/show/NCT01014234?term=Rapamycin+and+regulatory+T+cells+in+kidney+transplantation&rank=1. Accessed May 14, 2018 [Google Scholar]
- 46.Markmann J: . Infusion of T-Regulatory cells in kidney transplant recipients (The ONE Study). Available at: https://clinicaltrials.gov/ct2/show/NCT02091232?term=Infusion+of+T-Regulatory+cells+in+kidney+transplant+recipients+%28The+ONE+Study%29&rank=1. Accessed May 14, 2018 [Google Scholar]
- 47.Gedaly R: . A pilot study using autologous regulatory T cell infusion zortress (Everolimus) in renal transplant recipients. Available at: https://clinicaltrials.gov/ct2/show/NCT03284242?term=A+pilot+study+using+autologous+regulatory+T+cell+infusion+zortress+%28Everolimus%29+in+renal+transplant+recipients&rank=1. Accessed May 14, 2018 [Google Scholar]
- 48.Dal Canton A: . Effect of different therapeutic strategies on regulatory T cells in kidney transplantation. Available at: https://clinicaltrials.gov/ct2/show/NCT01640743?term=Effect+of+different+therapeutic+strategies+on+regulatory+T+cells+in+kidney+transplantation&rank=1. Accessed May 14, 2018 [Google Scholar]
- 49.Feng S, Bluestone J, Kang S-M, Tang Q: . Donor-alloantigen-reactive regulatory T cell (darTreg) therapy in renal transplantation (The ONE Study). Available at: https://clinicaltrials.gov/ct2/show/NCT02244801?term=Donor-alloantigen-reactive+regulatory+T+cell+%28darTreg%29+therapy+in+renal+transplantation+%28The+ONE+Study%29&rank=1. Accessed May 14, 2018 [Google Scholar]
- 50.Chutipongtanate A: . Effects of sevoflurane and desflurane on Treg. Available at: https://clinicaltrials.gov/ct2/show/NCT02559297?term=Effects+of+sevoflurane+and+desflurane+on+Treg&rank=1. Accessed May 14, 2018 [Google Scholar]
- 51.Skaro A: . Trial of adoptive immunotherapy with TRACT to prevent rejection in living donor kidney transplant recipients. Available at: https://clinicaltrials.gov/ct2/show/NCT02145325?term=Trial+of+adoptive+immunotherapy+with+TRACT+to+prevent+rejection+in+living+donor+kidney+transplant+recipients&rank=1. Accessed May 14, 2018 [Google Scholar]
- 52.Paraskevas S: . Role of T helper 17 and regulatory T cells in delayed graft function. Available at: https://clinicaltrials.gov/ct2/show/NCT01232816?term=Role+of+T+helper+17+and+regulatory+T+cells+in+delayed+graft+function&rank=1. Accessed May 14, 2018 [Google Scholar]
- 53.Chandraker AK: . Ultra-low-dose subcutaneous IL-2 in renal Transplantation. Available at: https://clinicaltrials.gov/ct2/show/NCT02417870?term=Ultra-low-dose+subcutaneous+IL-2+in+renal+transplantation&rank=1. Accessed May 14, 2018 [Google Scholar]
- 54.Gallon L: . The Differential Effects of 3 Different Immunosuppressive. Available at: https://clinicaltrials.gov/ct2/show/NCT00729248?term=The+Differential+Effects+of+3+Different+Immunosuppressive&rank=1. Accessed May 14, 2018 [Google Scholar]
- 55.Wolf D, Sopper S, Pircher A, Gastl G, Wolf AM: Treg(s) in cancer: Friends or foe? J Cell Physiol 230: 2598–2605, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q: Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 7: 315ra189, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chandran S, Tang Q, Sarwal M, Laszik ZG, Putnam AL, Lee K, Leung J, Nguyen V, Sigdel T, Tavares EC, Yang JYC, Hellerstein M, Fitch M, Bluestone JA, Vincenti F: Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. Am J Transplant 17: 2945–2954, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanimine N, Turka LA, Priyadharshini B: Cell Immunometabolism in Transplantation. Transplantation 102: 230–239, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]