Abstract
Background and objectives
Patients are informed of the risk of kidney biopsy–related complications using data from nonhospitalized patients, which may underestimate the risk for hospitalized patients. We evaluated the rate and risk factors of kidney biopsy–related complications in hospitalized patients with acute kidney disease (AKD) to better estimate the risk in this population.
Design, setting, participants, & measurements
We used data from the Yale biopsy cohort to evaluate rates of kidney biopsy–related complications including adjudicated procedure-related bleeding requiring blood transfusions or angiographic interventions, medium- or large-sized hematomas, reimaging after biopsy including abdominal ultrasonography or computed tomography, and death in hospitalized patients with AKD (including AKI). We evaluated univariable and multivariable association of risk factors with transfusions. We compared rates of complications between hospitalized and nonhospitalized patients.
Results
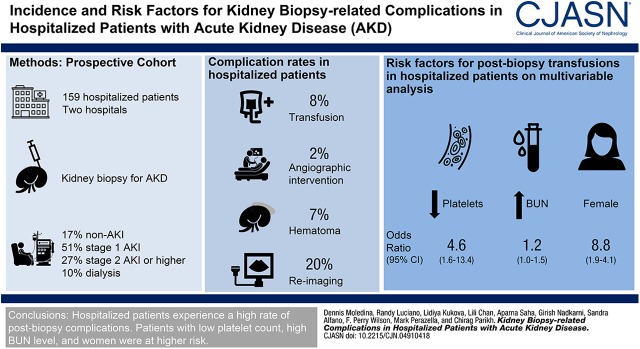
Between 2015 and 2017, 159 hospitalized patients underwent a kidney biopsy for AKD evaluation, of which 80 (51%) had stage 1 AKI, 42 (27%) had stage 2 (or higher) AKI, and 27 (17%) had AKD (without AKI). Of these, 12 (8%; 95% confidence interval [95% CI], 5% to 15%) required a transfusion, three (2%; 95% CI, 1% to 5%) required an intervention, 11 (7%; 95% CI, 4% to 12%) had hematoma, and 31 (20%; 95% CI, 14% to 26%) required reimaging after biopsy. Of the four (3%; 95% CI, 1% to 6%) deaths during hospitalization, none were related to the biopsy. Female sex, lower platelet count, and higher BUN were associated with postbiopsy transfusions on univariable and multivariable analyses. Trainee as proceduralist and larger needle gauge were associated with transfusions in univariable, but not multivariable, analysis. Nonhospitalized patients had lower rates of transfusion than hospitalized patients, although the latter also had lower prebiopsy hemoglobin and greater surveillance after biopsy.
Conclusions
Hospitalized patients experience higher risk of postbiopsy complications than previously reported and several factors, such as lower platelet count, female sex, and higher BUN, are associated with this risk.
Keywords: interventional nephrology; kidney biopsy; kidney failure; risk factors; ultrasonography; kidney pathology; blood transfusion; female; adult; prospective studies; multivariable analysis; Blood Urea Nitrogen; Confidence Intervals; Platelet Count; Angiography; Acute Kidney Injury; biopsy; Tomography, X-Ray Computed; Hematoma; hospitalization; Hemoglobins; Cohort Studies
Visual Abstract
Introduction
The percutaneous kidney biopsy is an essential tool for the evaluation of patients with unexplained loss of kidney function and the gold standard in diagnosis of multiple kidney disorders (1). With more recent research initiatives, such as the Kidney Precision Medicine Project and Nephrotic Syndrome Study Network, that seek to obtain kidney tissue in the hopes of discovering novel therapies for kidney diseases, the kidney biopsy now also plays an important role in research (2). The expansion of the clinical and research use of this procedure necessitates corresponding improvements in the evaluation and mitigation of the risk of procedure-related complications for future patients and research participants.
Over the years, enhancements to the kidney biopsy technique, such as the introduction of the automated biopsy device, real-time image guidance, and use of smaller gauge needles, have led to the development of a safer and more efficient method of obtaining kidney tissue (3,4). The procedure is considered low-risk and, on the basis of published data, patients are often informed of the chance of procedure-related bleeding requiring a blood transfusion in approximately 1% cases and angiographic intervention in 0.6% (5). However, these data are primarily obtained from biopsies in stable ambulatory patients, who tend to have a lower rate of complications; data on postprocedural complications in hospitalized individuals with acute kidney disease (AKD) are sparse. Factors that are associated with higher risk of postprocedure complications according to prior studies include higher BUN, lower platelet count, and lower hemoglobin level (5). The incidence of these factors is expected to be greater in hospitalized patients due to associated illnesses and comorbidities. It is therefore important to determine whether hospitalized individuals face a higher risk when undergoing a kidney biopsy. Such an evaluation will guide patient selection for clinical and research purposes, and also improve the informed consent process.
The goals of our study were to determine the rate and risk factors of biopsy-related complications in hospitalized patients with AKD. We used data from the prospective Yale biopsy cohort, which enrolled patients who underwent a kidney biopsy at two academic medical centers affiliated with Yale University. We compared the data obtained from the Yale cohort to the Nationwide Inpatient Sample (NIS). We also compared complication rates from hospitalized patients to nonhospitalized patients.
Materials and Methods
Participants, Settings, and Study Design
We included participants enrolled in the Yale biopsy cohort between January of 2015 and December of 2017 (6). Adult patients were approached to participate if they were scheduled to undergo a clinically indicated native kidney biopsy at either of two Yale-affiliated hospitals (York Street and St. Raphael’s campuses of Yale New Haven Hospital). Patients received an ultrasound- or computed tomography (CT)–guided kidney biopsy. Patients scheduled for a biopsy of a transplanted kidney or a kidney mass were excluded.
In this substudy, we included participants who underwent a kidney biopsy for evaluation of AKD. We excluded participants who underwent a biopsy for indications other than AKD or did not undergo a kidney biopsy after enrollment. We defined AKD according to the Kidney Disease Improving Global Outcomes criteria (7,8). AKD includes AKI, but the rise in creatinine can occur within 3 months. All participants provided written informed consent. This study was approved by the Yale Human Investigation Committee.
Outcomes and Predictors
We evaluated all participants for the following complications: biopsy-related bleeding requiring packed red blood cell transfusion or angiographic intervention; hematoma identified on an imaging study, and need for reimaging study after the biopsy within 7 days; and death within 30 days of the biopsy. All major complications were reviewed by D.G. Moledina and a complication was deemed biopsy-related on the basis of presence of ipsilateral hematoma, temporal association with procedure, absence of alternative explanation for drop in hemoglobin, or provider documentation (Supplemental Table 1). Patients who received transfusions deemed unrelated to the biopsy were considered as having no transfusions. We used transfusions as our primary outcome. In a supplementary analysis, we tested the outcome of any complication including presence of transfusion, angiographic interventions, or hematoma.
We defined the presence of hematoma as a hematoma on imaging that was at least 5 cm in any dimension or described as moderate, medium, or large (9). By our center’s protocol, all patients are imaged immediately after the biopsy to evaluate for postprocedural hematoma. We evaluated need for a second ultrasound or CT imaging study to check for postprocedure bleeding within 7 days after the biopsy.
For nonhospitalized patients, we collected data on their health care contact within 7 days after a kidney biopsy. We noted that 95 (98%) of 97 outpatients had documented follow-up in our electronic health record (EHR). As risk factors of transfusions after biopsy, we evaluated factors known to be associated with higher risk of blood transfusions on the basis of review of the literature (5,10).
Sources of Data
Yale Biopsy Cohort.
We collected demographic, clinical history, and laboratory results from chart review of the Epic (Epic Inc., Verona, WI) EHR and through the Yale Joint Data Analytics Team’s Helix data repository. We also reviewed charts to evaluate whether complications were related to the kidney biopsy procedure and to evaluate indications for second imaging study after a kidney biopsy procedure.
NIS.
We used data from the NIS from 2012 to 2014. The NIS is a nationally representative sample of hospitalized patients created by stratified probability sampling of State Inpatient Databases (11). We included hospitalizations with International Classification of Diseases, Ninth Revision, Clinical Modification procedure code for kidney biopsy and diagnosis code for AKI (Supplemental Table 2). We excluded hospitalizations with diagnosis of kidney transplant or kidney malignancy. We identified hospitalizations with codes for blood transfusion, hematoma, angiogram of the renal artery, and mortality.
Statistical Analyses
We present data as median (interquartile range) or count (percentage). We compared continuous and categoric variables using the Wilcoxon rank-sum test and chi-squared test or Fisher’s exact test, respectively. We performed univariable comparison of prebiopsy risk factors with postbiopsy complications using the Wilcoxon rank-sum test. To select variables in a multivariable model, we used a stepwise backward regression method with threshold for exclusion of P>0.05. For variables with high collinearity, we included them in distinct models. We evaluated model performance using area under the receiver operating characteristic curve and its 95% confidence interval (95% CI), tested model calibration using Hosmer–Lemeshow goodness-of-fit tests, and compared models using likelihood ratio test. To estimate the 95% CI of observed rate of biopsy complications, we used the Wilson method as described by Brown et al. (12) assuming binomial distribution. We used Stata Statistical Software: Release 14 (StataCorp LP, College Station, TX) for all analyses. All statistical tests were two-sided with a significance level of 0.05.
Results
Baseline Characteristics
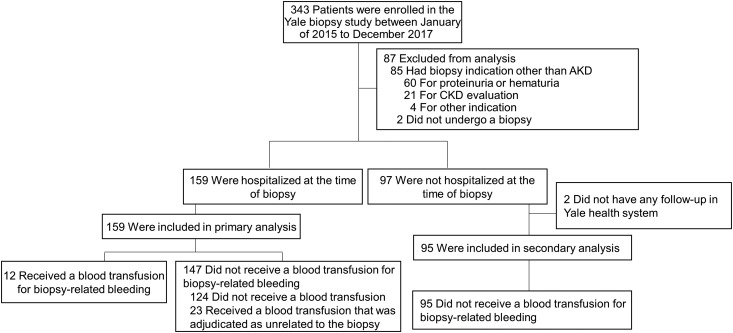
Of the 343 participants of the Yale biopsy cohort, 256 underwent a kidney biopsy for evaluation of AKD, and 159 (62%) were hospitalized at the time of their kidney biopsy and were included in the primary analysis (Figure 1). Baseline characteristics of study participants are presented in Table 1.
Figure 1.
STARD flow diagram of patients in the Yale biopsy cohort showing participants included in final analysis. We defined acute kidney disease (AKD) according to the Kidney Disease Improving Global Outcomes criteria. AKD includes (1) AKI (rise in serum creatinine ≥0.3 mg/dl in 48 hours or ≥50% rise in 7 days), (2) new kidney failure (GFR<60 for <3 months), or (3) slowly progressive kidney failure (≥35% drop in GFR in 3 months, or 50% serum creatinine rise in <3 months). 95% CI, 95% confidence interval; STARD, Standards for the Reporting of Diagnostic Accuracy Studies.
Table 1.
Baseline characteristics of hospitalized participants with acute kidney disease in the Yale biopsy cohort
| Characteristic | Participants (n=159) |
|---|---|
| Demographics | |
| Age, yr | 59 (47, 68) |
| Female | 68 (43%) |
| Black race | 37 (23%) |
| Diabetes | 63 (40%) |
| Hypertension | 106 (67%) |
| Cirrhosis | 20 (13%) |
| CKD | 91 (62%) |
| BMI, kg/m2 | 28 (25, 33) |
| BMI>35 kg/m2 | 32 (20%) |
| Baseline laboratory features | |
| Baseline creatinine, mg/dl | 1.3 (0.9, 1.9) |
| Baseline GFR, ml/min | 46 (30, 74) |
| Baseline protein-to-creatinine ratio, mg/mg | 2.0 (0.6, 5.1) |
| Dipstick protein ≥3 | 111 (73%) |
| Features at biopsy | |
| AKI | 132 (83%) |
| Stage 1 | 80 (51%) |
| Stage 2 or higher | 42 (27%) |
| Dialysis | 16 (10%) |
| Acute kidney disease (excluding AKI) | 27 (17%) |
| Creatinine, mg/dl | 4.4 (3.2, 6.8) |
| BUN, mg/dl | 52 (35, 76) |
| BUN>60 mg/dl | 65 (41%) |
| Hemoglobin, g/dl | 9.2 (8.0, 10.2) |
| Hemoglobin<10 g/dl | 116 (73%) |
| Platelets per mm3 | 202 (142, 278) |
| Location | |
| Floor | 143 (90%) |
| Intensive care unit | 16 (10%) |
| Campus | |
| York St. | 128 (81%) |
| St. Raphael’s | 31 (19%) |
| Procedural factors | |
| Passes | 3 (2, 3) |
| Fellow as primary proceduralist (versus Attending) | 103 (65%) |
| Nephrology (versus Radiology) | 104 (65%) |
| Needle gauge 16 (versus 18) | 88 (55%) |
| Ultrasound-guided (versus CT) | 118 (74%) |
| Primary histologic diagnosis | |
| Acute interstitial nephritis | 24 (15%) |
| Acute tubular necrosis | 30 (19%) |
| Diabetes | 28 (18%) |
| Glomerular disease | 37 (23%) |
| Arterionephrosclerosis | 17 (11%) |
| Other | 23 (14%) |
N (%) or median (interquartile range) seen. BMI, body mass index; CT, computerized tomography.
Procedure-Related Complications after Kidney Biopsy
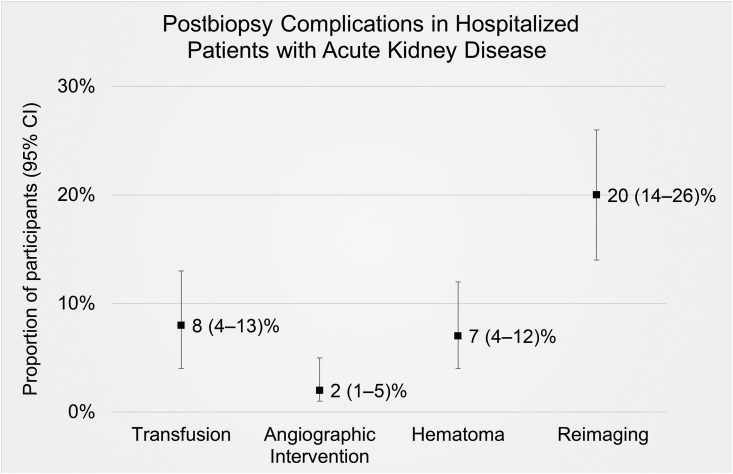
After the biopsy, 12 (8%) participants required a transfusion, three (2%) required an angiographic intervention, and 11 (7%) had a hematoma (Figure 2, Table 2). Within 30 days of the kidney biopsy, four (3%) participants died; however, none of the deaths were deemed related to the kidney biopsy procedure (Supplemental Table 1).
Figure 2.
Proportion (and its 95% confidence interval) of hospitalized participants with acute kidney disease experiencing postbiopsy complications.
Table 2.
Complications after kidney biopsy in hospitalized patients with acute kidney disease
| Complication | N (% [95% CI of Proportion]) or Median (Interquartile Range) |
|---|---|
| Total | 159 |
| Blood transfusion for biopsy-related bleedinga | 12 (8 [4 to 13]) |
| Angiographic intervention to stop bleeding | 3 (2 [1 to 5]) |
| Hematomab | 11 (7 [4 to 12]) |
| Death in 30 dc | 4 (3 [1 to 6]) |
| Reimaging after biopsy to check for bleedingd | 31 (20 [14 to 26]) |
| Drop in hemoglobin, g/dl | 0.8 (0.4, 1.3) |
| Hemoglobin drop >1 | 60 (39 [31 to 47]) |
| Hemoglobin drop >2 | 15 (10 [6 to 15]) |
Data presented as N [% (95% confidence interval of proportion)]. Fifteen (10%) patients experienced any complication defined as requirement of either an angiographic intervention or transfusion, or presence of hematoma.
All transfusion events were reviewed to determine their relationship with the kidney biopsy procedure (see Supplemental Table 1).
Hematoma described as medium, large, or at least 5 cm in any dimension.
None of the deaths were due to a biopsy-related complication.
Reimaging includes patients that required a second abdominal ultrasound or computed tomography imaging study to evaluate for bleeding within 7 d of a kidney biopsy.
Blood transfusions occurred 13 (4–41) hours after the kidney biopsy. Patients who required a transfusion had a lower hemoglobin after biopsy, greater drop in hemoglobin, and more hematomas (Table 3). All interventions occurred in patients who required a transfusion, whereas the occurrence of death was comparable between the two groups. We also noted that patients who required a transfusion had more frequent hemoglobin level testing and imaging studies after a biopsy.
Table 3.
Univariable association of risk factors with transfusions in hospitalized patients with acute kidney disease
| Characteristic | Missing | Transfusion n=12 | No Transfusion n=147 | P Valuea |
|---|---|---|---|---|
| Demographics | ||||
| Age, yr | 0 | 56 (46, 70) | 59 (48, 68) | 0.75 |
| Female | 0 | 9 (75%) | 59 (40%) | 0.02 |
| Black race | 0 | 4 (33%) | 33 (22%) | 0.39 |
| Diabetes | 2 | 2 (18%) | 61 (42%) | 0.12 |
| Hypertension | 0 | 6 (50%) | 100 (68%) | 0.20 |
| Cirrhosis | 2 | 3 (27%) | 17 (12%) | 0.13 |
| CKD | 12 | 7 (58%) | 84 (62%) | 0.79 |
| Weight, kg | 0 | 78 (64, 91) | 86 (70, 101) | 0.22 |
| Height, cm | 0 | 168 (159, 171) | 170 (163, 178) | 0.18 |
| BMI, kg/m2 | 0 | 27 (25, 33) | 28 (25, 33) | 0.56 |
| BMI>35 kg/m2 | 0 | 2 (17%) | 30 (20%) | 0.76 |
| Baseline laboratory features | ||||
| Baseline creatinine, mg/dl | 12 | 1.3 (0.8, 1.7) | 1.3 (1.0, 1.9) | 0.54 |
| Baseline GFR, ml/min | 12 | 48 (31, 71) | 46 (29, 74) | 0.97 |
| Baseline protein-to-creatinine ratio, mg/mg | 30 | 4.7 (1.0, 6.6) | 2.0 (0.5, 4.8) | 0.44 |
| Dipstick protein ≥3 | 6 | 11 (92%) | 100 (71%) | 0.12 |
| Features at biopsy | ||||
| AKI | 0 | 10 (83%) | 122 (83%) | 0.98 |
| Stage 1 | 3 | 7 (58%) | 73 (51%) | 0.61 |
| Stage 2 or higher | 3 | 2 (17%) | 40 (28%) | 0.40 |
| Dialysis | 1 | 2 (17%) | 14 (10%) | 0.44 |
| Acute kidney disease (excluding AKI) | 0 | 2 (17%) | 25 (17%) | 0.98 |
| Creatinine, mg/dl | 0 | 6.3 (3.9, 9.0) | 4.4 (3.2, 6.4) | 0.20 |
| BUN, mg/dl | 0 | 66 (57, 106) | 51 (34, 76) | 0.04 |
| BUN>60 mg/dl | 0 | 8 (67%) | 57 (39%) | 0.06 |
| Hemoglobin, g/dl | 0 | 8.5 (7.4, 8.7) | 9.4 (8.1, 10.4) | 0.008 |
| Hemoglobin<10 g/dl | 0 | 12 (100%) | 104 (71%) | 0.03 |
| Platelets per mm3 | 0 | 112 (86, 189) | 209 (150, 285) | 0.003 |
| Platelets<120,000 per mm3 | 0 | 7 (23%) | 5 (4%) | 0.002 |
| PTT before biopsy | 24 | 30 (28, 40) | 28 (25, 33) | 0.13 |
| INR before biopsy | 16 | 1.1 (1.0, 1.3) | 1.1 (0.9, 1.2) | 0.25 |
| Antiplatelet drugs (7 d before biopsy) | 16 | 1 (9%) | 18 (14%) | 0.67 |
| Anticoagulant drugs (7 d before biopsy) | 0 | 6 (50%) | 78 (53%) | 0.84 |
| BP | ||||
| Systolic BP | 23 | 143 (136, 153) | 135 (121, 150) | 0.50 |
| Diastolic BP | 23 | 81 (62, 87) | 77 (67, 82) | 0.87 |
| Mean arterial pressure | 23 | 97 (92, 103) | 97 (84, 105) | 0.89 |
| Systolic>140 mm of Hg | 23 | 5 (56%) | 53 (42%) | 0.50 |
| Diastolic>90 mm of Hg | 23 | 0 (0%) | 13 (10%) | 0.60 |
| Location | 0.02 | |||
| Floor | 0 | 8 (67%) | 135 (92%) | |
| Intensive care unit | 0 | 4 (33%) | 12 (8%) | |
| York St. (versus St. Raphael’s campus) | 0 | 12 (100%) | 116 (79%) | 0.08 |
| Procedural Factors | ||||
| Passes | 13 | 2 (3, 3) | 3 (3, 2) | 0.95 |
| Fellow as primary proceduralist (versus Attending) | 0 | 11 (92%) | 92 (63%) | 0.04 |
| Nephrology (versus Radiology) | 0 | 11 (92%) | 93 (63%) | 0.05 |
| Needle gauge 16 (versus 18) | 0 | 10 (83%) | 78 (53%) | 0.04 |
| Ultrasound-guided (versus CT) | 0 | 11 (92%) | 107 (73%) | 0.15 |
| Desmopressin within 4 h of biopsy | 0 | 7 (58%) | 117 (80%) | 0.09 |
| Postprocedural findings and complications | ||||
| Hemoglobin after biopsy, g/dl | 4 | 6.6 (6.3, 7.0) | 8.4 (7.4, 9.5) | <0.001 |
| Drop in hemoglobin, g/dl | 4 | 1.4 (0.7, 2.1) | 0.7 (0.4, 1.3) | 0.003 |
| Percentage drop in hemoglobin | 4 | 16.4 (9.0, 23.9) | 7.5 (3.8, 13.8) | 0.003 |
| Number of hemoglobin measurements | 4 | 12 (9, 17) | 6 (3, 8) | <0.001 |
| Reimaging after biopsy | 0 | 9 (75%) | 22 (15%) | <0.001 |
| Time to reimaging, h | 12.9 (1.9, 40.0) | 29.9 (19.7, 72.7) | 0.14 | |
| Hematoma | 14 | 8 (73%) | 3 (2%) | <0.001 |
| Angiographic intervention after biopsy | 0 | 3 (25%) | 0 (0%) | <0.001 |
| Death | 0 | 1 (8%) | 3 (2%) | 0.18 |
| Primary histologic diagnosis | 0 | 0.22 | ||
| Acute interstitial nephritis | 0 | 0 (0%) | 24 (16%) | |
| Acute tubular necrosis | 0 | 4 (33%) | 26 (18%) | |
| Diabetes | 0 | 1 (8%) | 27 (18%) | |
| Glomerular disease | 0 | 4 (33%) | 33 (22%) | |
| Arterionephrosclerosis | 0 | 0 (0%) | 17 (12%) | |
| Other | 0 | 3 (25%) | 20 (14%) | |
| Other biopsy features | ||||
| IFTA>40% on the basis of biopsy report | 15 | 1 (10%) | 41 (31%) | 0.17 |
| Glomerulosclerosis on the basis of biopsy, % | 12 | 9 (0, 17) | 15 (0, 42) | 0.23 |
Data are presented as median (interquartile range) or n (%). BMI, body mass index; PTT, partial thromboplastin time; INR, international normalized ratio; CT, computerized tomography; IFTA, interstitial fibrosis and tubular atrophy.
Wilcoxon rank-sum test, chi-squared, or Fisher’s exact test.
All patients were checked immediately after a kidney biopsy for presence of hematoma. A second abdominal ultrasound or CT imaging study was performed in 31 (20%) participants, 26 (19–73) hours after the biopsy. In 23 (74%) participants, these studies were performed to evaluate for bleeding (for indications such as “hemoglobin drop,” “evaluate for hematoma,” “concern for bleeding”), including follow-up studies in five who had a hematoma immediately after the biopsy, four (13%) for abdominal or flank pain, and four (13%) for reasons unrelated to the biopsy. Of the 11 participants with a hematoma, six were identified immediately after the biopsy, whereas five were identified 40 (2–151) hours after biopsy. In eight (66%) of 12 participants, the transfusion was initiated after a hematoma was identified.
Association of Risk Factors with Transfusions
The univariable association of various factors with transfusions is presented in Table 3. Women, participants with lower platelet and hemoglobin levels, those who underwent a biopsy with larger needle gauge, and those who were admitted to an intensive care unit required more transfusions. However, cirrhosis, biopsy passes, imaging modality, international normalized ratio, partial thromboplastin time, antiplatelet or anticoagulant drug use, or BP before biopsy were not associated with transfusions. We found similar results in a supplementary analysis using outcome of any complication (Supplemental Table 3). We found that platelet count, sex, and BUN level were independently associated with transfusions after biopsy (Table 4). Although desmopressin was not associated with transfusions in univariable analysis, it was associated with a lower risk of transfusions on controlling for BUN level (odds ratio, 0.24; 95% CI, 0.06 to 0.88).
Table 4.
Risk factors associated with blood transfusion after kidney biopsy in hospitalized patients with acute kidney disease
| Variable | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Platelet count | 3.3 (1.3 to 8.1) | 5.0 (1.7 to 14.2) | 4.6 (1.6 to 13.4) |
| Female | N/A | 8.4 (1.9 to 37.2) | 8.8 (1.9 to 41.0) |
| BUN | N/A | N/A | 1.2 (1.0 to 1.5) |
| AUC | 0.76 (0.61 to 0.91) | 0.83 (0.70 to 0.95) | 0.86 (0.76 to 0.97) |
| P value | N/A | 0.002 | 0.03 |
Odds ratio (95% confidence interval) per 10,000/mm3 decrease in platelet count, female sex (versus male), and 10 mg/dl increase in BUN. N/A, not applicable; AUC, area under the receiver operating characteristic curve.
Needle Gauge and Biopsy Complications
Participants who underwent biopsies with 16-gauge (G) needles had a trend toward a higher rate of complications as compared with 18G needles (Supplemental Table 4). The number of glomeruli obtained, number of biopsies judged inadequate by the pathologist, or biopsies with over ten or 25 glomeruli were similar between 16G and 18G biopsies. Needle gauge and department of proceduralist had a high degree of collinearity (R2=0.76), such that most biopsies with 16G were performed by Nephrology (versus Radiology). Therefore, we included them in separate multivariable models. Both needle gauge and department of proceduralist were associated with risk of transfusions in multivariable models controlling for platelet count, sex, and BUN (Supplemental Table 5). However, the association of needle gauge and department of proceduralist was no longer significant after further controlling for the level of training of the proceduralist.
National Trends of Complications after Kidney Biopsy
We compared Yale data with national trends using data from the NIS. We identified 53,315 hospitalizations with kidney biopsies and AKI in the NIS between 2012 and 2014. Both the Yale and NIS cohorts had similar rates of transfusion during hospitalization, angiographic interventions, and deaths during hospitalization (Supplemental Figure 1, Supplemental Table 6).
Complications in Nonhospitalized Kidney Biopsy Patients
Of the 256 participants with AKD, 97 (38%) were not hospitalized at the time of their kidney biopsy (Supplemental Figure 2). Nonhospitalized patients had a higher platelet count and lower BUN (Supplemental Table 7). None of the nonhospitalized patients required a transfusion, required an intervention to stop bleeding, or died within 30 days after a kidney biopsy (Supplemental Table 8). However, there was no difference in rates of hematoma or drop in hemoglobin between hospitalized and nonhospitalized patients. Nonhospitalized patients had fewer reimaging studies to evaluate for bleeding and fewer hemoglobin measurements. Of the 95 (98%) with available follow-up, eight (8%) sought medical attention for biopsy-related reasons after being discharged (five presented to the emergency department, two called their nephrologist, and one presented to the clinic), six participants received imaging studies to evaluate for bleeding, and five required admission to the hospital.
Discussion
We evaluated the rate and risk factors of procedure-related bleeding after a kidney biopsy in hospitalized patients with AKD. First, contrary to the low numbers reported in studies looking at nonhospitalized patients without AKD, we report higher bleeding risk in hospitalized patients who underwent a kidney biopsy. Second, we found that platelet count, BUN, and sex were independently associated with transfusions after a kidney biopsy. Third, we noted that hospitalized patients had a higher rate of complications than nonhospitalized patients. Our findings could inform kidney biopsy–related decisions both in clinical and research settings.
Currently, patients receiving a kidney biopsy are often informed of procedure-related bleeding requiring a blood transfusion in approximately 1% cases and angiographic intervention in 0.6% on the basis of studies that primarily included nonhospitalized patients (5). In contrast, our results show that 8% of hospitalized patients require a blood transfusion and 2% required an angiographic intervention because of biopsy-related bleeding. Our overall transfusion rate, including both hospitalized and nonhospitalized patients, was 5%, which is comparable to that reported in the study by Korbet et al. where 5.3% of patients required a transfusion. However, this study did not report the fraction of patients who were hospitalized (10). Our local complication rate in hospitalized patients was also comparable to national data from the NIS. The rates of angiographic interventions after kidney biopsy were similar in both cohorts. Although we did not have data on transfusions attributable to the biopsy in the NIS, we found that the overall rate of transfusion during hospitalization was comparable.
Hospitalized patients may be at higher risk of transfusions due to greater presence of risk factors of bleeding, lower prebiopsy hemoglobin, or greater surveillance. First, hospitalized patients had lower platelet count and higher BUN than nonhospitalized patients, which could have increased their bleeding risk. However, the observation of comparable rates of hematoma and drop in hemoglobin between the two groups does not support this possibility. Second, hospitalized patients had a lower prebiopsy hemoglobin and were thus more likely to drop below the threshold for transfusion after a biopsy. Third, hospitalized patients received greater surveillance in the form of higher number of hemoglobin and imaging checks after the biopsy, which could increase the likelihood of detection of presence of hematoma or hemoglobin value below transfusion threshold in these patients.
We evaluated several risk factors shown to be associated with higher risk of complications in previous studies. A meta-analysis found that patients in studies with lower hemoglobin and AKI required more transfusions (5). Another study demonstrated that lower platelet count was associated with higher procedure-related complications (13). Similar to previous studies, we found that lower platelet count, hemoglobin level, female sex, higher BUN, and intensive care unit admission were associated with higher risk of transfusion in univariable analysis (5,13). In a multivariable model, we found that platelet count, female sex, and BUN were independent risk factors for transfusion. Some known risk factors of postprocedure bleeding, including higher prothrombin time, partial thromboplastin time, and BP, were not found to be associated with transfusion risk in our study (10,13,14). Because clinicians performing the biopsy were aware of these risk factors, they could have either excluded patients or corrected these abnormalities before performing the biopsy, resulting in null association observed by us.
The major consideration in selection of needle gauge for a kidney biopsy is the balance between the risk of bleeding versus the benefit of obtaining additional tissue. Although a needle with larger diameter (smaller numeric gauge) provides more tissue, it also increases risk of complications (15). Two studies showed an increase occurrence of hematoma in patients whose biopsies were performed with a 16G (versus 18G) needle (9,16). Although we found a higher rate of complications in those who received a kidney biopsy with 16G needles, this association was NS after controlling for level of training of proceduralist.
We chose to study postbiopsy complications on the basis of their unequivocal effect on cost and quality of care (17,18). Blood transfusions may acutely lead to life-threatening transfusion reactions. They may also increase the risk of sensitization in patients who may need a kidney transplant in the future (19–21). In addition, blood transfusions are associated with higher cost to hospitals, patients, and payers (22). Moreover, there is often a shortage of blood products and blood transfusions may not be appropriate for certain patients such as Jehovah’s witnesses (19,23). Although we evaluated other major complications in our study, we could not evaluate the independent risk factors of these complications because there were no deaths attributable to the biopsy in our study and only three patients required an intervention to stop bleeding.
Our findings could guide physicians in their recommendation of the kidney biopsy procedure to future patients. These findings could also help inform researchers and potential participants of studies performing research kidney biopsies, such as the Kidney Precision Medicine Project and Nephrotic Syndrome Study Network, by providing accurate and personalized risk estimates for study planning and protocol development, informed consent, and monitoring the rate of complications after research biopsies. Careful selection of patients on the basis of our data for both clinical and research biopsies could reduce risk of complications, reduce expenses, and improve quality of care.
Our study has several strengths. First, this is the first study to evaluate risk of kidney biopsy–related complications specifically in hospitalized patients with AKD. Second, we compared local data from our center to the NIS and found similar rates of complications. Third, we systematically evaluated >20 potential risk factors to obtain a multivariable model that could help personalize the consent process before a kidney biopsy. Fourth, we reviewed charts for all participants with complications to establish their association with the biopsy. Our study also has some limitations. First, this is a study from the Yale health care system spanning two hospitals; the generalizability of our findings will need to be tested in a larger, multicenter study. Second, the NIS does not have data on platelet count, hemoglobin level, or needle gauge, which limited our ability to validate the model we created. The NIS also did not have data on whether the transfusions were before or after the biopsy. Third, we cannot say with certainty whether modifying the risk factors described will reduce complications because this is an observational study. Fourth, we did not have data on bleeding time of patients before the biopsy, which was shown to be associated with higher risk of complications in a prior study (10). Fifth, we did not specifically contact nonhospitalized patients to evaluate the occurrence of postbiopsy complications but relied on EHR for this, which could have led us to miss patients that sought care outside our EHR system.
In conclusion, we found that hospitalized patients receiving a kidney biopsy for AKD have a higher risk of requiring a blood transfusion after the procedure. Women, and patients with lower platelet count and higher BUN, tend to have greater risk of complications.
Disclosures
None.
Supplementary Material
Acknowledgments
We wish to thank the participants of the Yale biopsy study, without whom this study would not have been possible.
This work was supported by the National Institutes of Health (NIH) K24DK090203 to C.R.P. D.G.M. is supported by T32DK007276 from the NIH, by the Robert E. Leet and Clara Guthrie Patterson Trust Mentored Clinical Research Award, and by the American Heart Association (18CDA34060118). F.P.W. is supported by K23DK097201 and R01DK113191, L.C. by T32DK007757, and G.N. by K23DK107908 from the NIH. C.R.P., D.G.M., F.P.W., R.L.L., and S.A. are funded by the National Institute of Diabetes and Digestive and Kidney Diseases–sponsored Kidney Precision Medicine Program (UG3-DK114866).
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. These organizations were not involved in study design, analysis, interpretation, or manuscript creation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04910418/-/DCSupplemental.
See related editorial, “Kidney Biopsy in Hospitalized Patients with Acute Kidney Disease: Is There an Increased Risk?,” on pages 1617–1618.
References
- 1.Waikar SS, McMahon GM: Expanding the role for kidney biopsies in acute kidney injury. Semin Nephrol 38: 12–20, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Diabetes and Digestive and Kidney Diseases: Kidney precision medicine project, 2016. Available at: https://www.niddk.nih.gov/research-funding/research-programs/kidney-precision-medicine-project-kpmp. Accessed July 24, 2017
- 3.Kim D, Kim H, Shin G, Ku S, Ma K, Shin S, Gi H, Lee E, Yim H: A randomized, prospective, comparative study of manual and automated renal biopsies. Am J Kidney Dis 32: 426–431, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Maya ID, Maddela P, Barker J, Allon M: Percutaneous renal biopsy: Comparison of blind and real-time ultrasound-guided technique. Semin Dial 20: 355–358, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Corapi KM, Chen JL, Balk EM, Gordon CE: Bleeding complications of native kidney biopsy: A systematic review and meta-analysis. Am J Kidney Dis 60: 62–73, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Moledina DG, Cheung B, Kukova L, Luciano RL, Peixoto AJ, Wilson FP, Alfano S, Parikh CR: A survey of patient attitudes toward participation in biopsy-based kidney research. Kidney Int Rep 3: 412–416, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, Tolwani AJ, Waikar SS, Weisbord SD: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 61: 649–672, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 9.Cui S, Heller HT, Waikar SS, McMahon GM: Needle size and the risk of kidney biopsy bleeding complications. Kidney Int Rep 1: 324–326, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korbet SM, Volpini KC, Whittier WL: Percutaneous renal biopsy of native kidneys: A single-center experience of 1,055 biopsies. Am J Nephrol 39: 153–162, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Healthcare Cost and Utilization Project (HCUP), 2018: Available at: https://www.hcup-us.ahrq.gov/overview.jsp. Accessed April 16, 2018
- 12.Brown LD, Cai TT, DasGupta A: Interval estimation for a binomial proportion. Stat Sci 16: 101–133, 2001 [Google Scholar]
- 13.Torres Munoz A, Valdez-Ortiz R, Gonzalez-Parra C, Espinoza-Davila E, Morales-Buenrostro LE, Correa-Rotter R: Percutaneous renal biopsy of native kidneys: Efficiency, safety and risk factors associated with major complications. Arch Med Sci 7(5): 823–831, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kriegshauser JS, Patel MD, Young SW, Chen F, Eversman WG, Chang YH: Risk of bleeding after native renal biopsy as a function of preprocedural systolic and diastolic blood pressure. J Vasc Interv Radiol 26: 206–212, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Roth R, Parikh S, Makey D, Foster J, Rozenblit G, Satoskar A, Nadasdy G, Von Visger J, Hebert L, Rovin BH, Nadasdy T, Brodsky SV: When size matters: Diagnostic value of kidney biopsy according to the gauge of the biopsy needle. Am J Nephrol 37: 249–254, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Simard-Meilleur MC, Troyanov S, Roy L, Dalaire E, Brachemi S: Risk factors and timing of native kidney biopsy complications. Nephron Extra 4: 42–49, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whittier WL, Korbet SM: Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol 15: 142–147, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Manno C, Strippoli GF, Arnesano L, Bonifati C, Campobasso N, Gesualdo L, Schena FP: Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int 66: 1570–1577, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP: Transfusion medicine. First of two parts--blood transfusion. N Engl J Med 340: 438–447, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Leffell MS, Kim D, Vega RM, Zachary AA, Petersen J, Hart JM, Rossert J, Bradbury BD: Red blood cell transfusions and the risk of allosensitization in patients awaiting primary kidney transplantation. Transplantation 97: 525–533, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Spahn DR, Goodnough LT: Alternatives to blood transfusion. Lancet 381: 1855–1865, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toner RW, Pizzi L, Leas B, Ballas SK, Quigley A, Goldfarb NI: Costs to hospitals of acquiring and processing blood in the US: A survey of hospital-based blood banks and transfusion services. Appl Health Econ Health Policy 9: 29–37, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Nagurney A, Masoumi AH, Yu M: Supply chain network operations management of a blood banking system with cost and risk minimization. Computational Management Science. 9: 205–231, 2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.