Abstract
For warfarin-treated patients with atrial fibrillation (AF) at low thromboembolic risk, recent studies have shown harm associated with periprocedural bridging using low–molecular-weight heparin. Clinician surveys have indicated a preference toward excessive bridging, especially among noncardiologists; however, little is known about actual practice patterns in these patients. We performed a retrospective evaluation of bridging in the setting of gastrointestinal endoscopy. We identified 938 patients with AF on warfarin who underwent esophagogastroduodenoscopy or colonoscopy between 2012 and 2016 at a tertiary health center. Urgent, inpatient, or advanced endoscopic procedures were excluded. Clinical variables were abstracted using a predefined data dictionary. Values were expressed as means and compared using a t test or a chi-squared test as appropriate. Three hundred seventy-four patients met criteria for analysis. Twenty-five percent of these patients received bridging therapy, including 11% of patients with CHADS2 scores of 0 to 2 without valvular AF or previous venous thromboembolism. Of the clinical variables assessed, CHADS2, CHA2DS2-VASc, and a history of stroke were the strongest predictors of bridging. Cardiologists were also significantly less likely to prescribe bridging than noncardiology providers (18% vs 30%, p = 0.011); this effect was significant when controlling for CHADS2, CHA2DS2-VASc, or stroke history. In conclusion, patients with AF on warfarin receive excessive low–molecular-weight heparin bridging in the setting of endoscopy; the lower rates of bridging observed among cardiologists suggests a need for their increased involvement in this decision making.
Background
A growing number of patients currently take warfarin for chronic atrial fibrillation (AF), many of whom require interruption of anticoagulation for procedures.1,2 In this setting, it is a common practice to prescribe bridging therapy with low–molecular-weight heparin (LMWH) to reduce thromboembolic risk. Based on previous studies and the landmark BRIDGE trial, perioperative bridging with LMWH in patients with nonvalvular AF on warfarin appears to result in increased risk of bleeding without reduction in thromboembolism.3–9 Despite these practice-changing findings, 2 subsequent surveys demonstrated a preference toward excessive bridging among clinicians, even in those at low thromboembolic risk; in these surveys, cardiologists were less likely to prescribe bridging compared with other specialists.10,11 Given these survey results, we wished to determine actual practice patterns for LMWH bridging in warfarin-treated patients with AF in the setting of gastrointestinal endoscopy, particularly in those at low thromboembolic risk. Based on previous surveys, we hypothesized that rates of LMWH bridging would be excessive in patients with low thromboembolic risk and that noncardiologists would prescribe LMWH more frequently than cardiology providers. We also hypothesized that stroke risk factors would significantly influence this decision making.
Methods
Our primary objective was to identify rates of bridging with LMWH both in our cohort and in the subset of patients at low thromboembolic risk; low risk was defined as having nonvalvular AF with a CHADS2 score of 0 to 2, a cutoff that was accepted in previous surveys and available guidelines.2,8 Our secondary analyses included an assessment of the predictive values of stroke risk factors (CHADS2, CHA2DS2-VASc, and previous stroke) and the specialty of the responsible clinician on the decision to bridge. We additionally analyzed rates of bridging in patients with mechanical valves and those on antiplatelet agents such as aspirin, clopidogrel, ticegrelor, or prasugrel.
To accomplish this, we conducted a retrospective chart review to identify all patients with AF on chronic warfarin therapy who underwent elective, outpatient esophagogastroduodenoscopy or colonoscopy requiring warfarin discontinuation between 2012 and 2016 at the University of Michigan. First, we searched the entire electronic medical record using DataDirect to identify patients with AF who had undergone esophagogastroduodenoscopy or colonoscopy. We identified patients with AF using ICD-9 and ICD-10 codes found in the problem list, medical history, or professional billing (inpatient and outpatient) and identified patients who underwent endoscopy using CPT codes. See Appendix S1 for the full list of ICD and CPT codes. Chronic warfarin use was defined as continuous use for 30 or more days before endoscopy. Patients were excluded if they had undergone inpatient endoscopy or advanced endoscopic procedures (see Appendix S1 for the full list of excluded procedures), or if warfarin was not held for procedure. Patients who had undergone left ventricular assist device placement were also excluded as they inherently required bridging. Clinical, laboratory, and endoscopic data were extracted manually through a thorough chart review. Stroke risk scores (CHADS2 and CHA2DS2-VASc) were calculated based on these data. Details of the perioperative anticoagulation plan were collected, including whether the patient received bridging with LMWH and the specialty of the clinician responsible for making the decision. This was done via a thorough review of outpatient and telephone notes.
Descriptive statistics were calculated as means (standard deviation) or medians (interquartile range) and compared using chi-square tests or t testing as appropriate. We developed 3 multivariable logistic regression models for the outcome of LMWH bridging use, which adjusted for clinician specialty, the date of endoscopy, and each individual stroke risk factor (CHADS2, CHA2DS2-VASc, and history of stroke). Analyses were performed using Stata version 14.2 (College Station, Texas).
Results
Our final study population included 374 patients with a mean age of 67.3, 68% of whom were men (Figure 1). For patients in whom warfarin was stopped (n = 340), bridging therapy was prescribed to 25%. After excluding patients with previous venous thromboembolism or valve replacement, 18% of patients received LMWH bridging (Table 1); of these patients, 11.3% of those with CHADS2 0 to 2 received bridging compared with 31.9% with scores of 3 or higher (p <0.001). Bridging was used in 44 of 164 of patients (27%) before and 42 of 210 of patients (20%) after the BRIDGE trial (p = 0.119). All patients with mechanical aortic and mitral valves received LMWH bridging (Table 2).
Figure 1.
Patient inclusion breakdown.
Table 1.
Use of bridging based on prior risk factors (% in rows)
| Bridging | ||
|---|---|---|
| Yes | No | |
| All Patients (n = 340) | 86 (25%) | 254 (75%) |
| Excluding Valve Replacement & Prior Venous Thromboembolism (n = 277) | 50 (18%) | 227 (82%) |
| Excluding Prior Stroke & Prior Venous Thromboembolism (n = 258) | 38 (15%) | 220 (85%) |
| Excluding Valve Replacement, Prior Stroke, & Prior Venous Thromboembolism (n = 238) | 29 (12%) | 209 (88%) |
Table 2.
Demographics, comorbidities, and procedures by bridging use
| Bridging | P-value | ||
|---|---|---|---|
| Yes (n = 86) | No (n = 254) | ||
| Mean Age (SD) (years) | 67.0 (14.7) | 67.5 (14.6) | 0.746 |
| Men | 48 (56%) | 184 (72%) | 0.004 |
| White | 77 (90%) | 233 (92%) | 0.703 |
| Black | 4 (5%) | 13 (5%) | |
| Mean CHADS2 (SD) | 2.7 (1.3) | 2.0 (1.0) | <0.001 |
| Mean CHA2DS2-VASc (SD) | 4.1 (1.8) | 3.4 (1.4) | <0.001 |
| Prior Stroke | 33 (38%) | 19 (8%) | <0.001 |
| Mean HAS-BLED (SD) | 1.5 (0.9) | 1.3 (0.8) | 0.065 |
| Staff Making Anticoagulation Decision (% in row) | |||
| ■ Cardiology | 22 (18%) | 104 (82%) | 0.012 |
| ■ Gastroenterology | 29 (35%) | 54 (65%) | |
| ■ Primary Care Provider | 31 (30%) | 72 (70%) | |
| ■ Other | 4 (15%) | 23 (85%) | |
| Staff Making Anticoagulation Decision (% in row) | |||
| ■ Cardiology | 22 (18%) | 104 (82%) | 0.011 |
| ■ Non-Cardiology | 64 (30%) | 150 (70%) | |
| Esophagogastroduodenoscopy | 24 (29%) | 57 (23%) | 0.340 |
| Colonoscopy | 62 (72%) | 193 (77%) | |
| Mechanical Valve (% in row) | 9 (100%) | 0 (0%) | n/a |
| • Aortic Mech Valve | 7/7 (100%) | 0/7 (0%) | n/a |
| • Mitral Mech Valve | 2/2 (100%) | 0/2 (0%) | |
| Number of Antiplatelet Agents (% in row) | |||
| • None | 54 (25%) | 160 (75%) | 0.722 |
| • One | 31 (25%) | 93 (75%) | |
| • Two | 1 (50%) | 1 (50%) | |
Note: Antiplatelet agents included aspirin, clopidogrel, prasugrel, and ticegrelor.
In bivariate analyses, CHADS2, CHA2DS2-VASc, and history of stroke were significantly associated with bridging therapy (Table 2). Each of these predictors remained statistically significant in multivariate regression models when controlling for the date of endoscopy and the specialist making the decision of these models; a history of stroke was the strongest predictor (Figure 2). Additionally, a history of stroke was a significantly better predictor of bridging than the number of other stroke risk factors (e.g., hypertension, age, diabetes) (p = 0.001), an effect that persisted regardless of the number of risk factors (Figure 3). Lastly, cardiologists were significantly less likely to prescribe bridging therapy than other specialists (Table 2). This effect remained significant in logistic regression when controlled for CHADS2, CHA2DS2-VASc, and history of stroke (Figure 2).
Figure 2.

Multivariate model from predicting bridging use.
Figure 3.
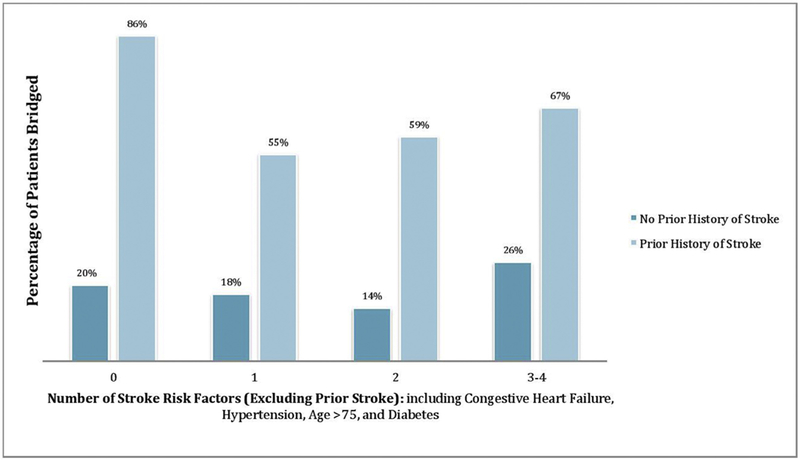
Bridging rates based on stroke risk factors.
Discussion
We describe the use of bridging anticoagulation for warfarin-treated patients with AF who underwent elective gastrointestinal endoscopy, the most common procedure included in the BRIDGE trial. The use of LMWH bridging was in excess of what would have been expected based on patient attributes and guidelines available at the time. This was true even for patients with nonvalvular AF at low thromboembolic risk (CHADS2 score of 0 to 2), a population with no clear indication for bridging. We also confirmed the findings from our recent provider survey that specialists outside of cardiology are more likely to prescribe bridging LMWH than cardiologists.10–12
Similar to previous survey data, stroke risk factors such as previous stroke, CHADS2, and CHA2DS2-VASc appear to weigh significantly into this decision making; this occurs despite an absence of evidence that stroke risk scores accurately risk-stratify patients with respect to bridging. The effect of previous stroke was particularly striking, with these patients being more than 8 times more likely to receive bridging therapy. Although patients with recent stroke were excluded from the BRIDGE trial, there were no patients in our study who had experienced stroke within 3 months of their procedure; thus, none of our patients would have been excluded from the BRIDGE trial based on that criterion.
All patients with mechanical aortic and mitral valve replacements received bridging, which is in accordance with available guidelines.2,8 Interestingly, there did not appear to be a significant relationship between the number of antiplatelet agents prescribed and the decision to bridge.
Most clinically relevant was the effect of the specialist responsible for the decision making, with cardiologists being nearly half as likely to prescribe bridging therapy compared with their noncardiology colleagues. It is unclear why this discrepancy exists. It may be due to the emphasis on anticoagulation management and related trials among cardiology providers; however, the BRIDGE trial results were presented at the 2015 International Society for Thrombosis and Haemostasis conference, not a cardiology meeting.8 Our data also demonstrate that despite more evidence-based use of bridging anticoagulation, cardiology providers are only responsible for the decision to bridge in 37% of cases. Efforts to improve the evidence-based management in the periprocedure setting are needed and may include a closer involvement of cardiology providers or anticoagulation clinics.12
There are several potential limitations to this study. Although this study included patients from both before and after the BRIDGE trial era, the use of bridging anticoagulation remained elevated in both time periods. Further longitudinal evaluation is needed to understand practice patterns following this seminal study. Second, these results represent the practice pattern at a single institution. However, they mirror the findings from 2 multicenter provider surveys.10–12 Finally, as with all retrospective, observational studies, a potential for unmeasured confounding bias exists.
In conclusion, patients with AF on chronic warfarin therapy are prescribed bridging anticoagulation more often than they should be around the time of gastrointestinal endoscopy procedures. A history of stroke and a noncardiology provider are 2 strong predictors of LMWH bridging administration. Given the potential for significant harm associated with inappropriate bridging, system-wide efforts should be explored to encourage more evidence-based use of periprocedural anticoagulation.
Supplementary Material
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary Data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.amjcard.2018.02.043.
References
- 1.Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes 2012;5:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douketis JD, Berger PB, Dunn AS, Jaffer AK, Spyropoulos AC, Becker RC, Ansell J. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2008;133(6 suppl):299S–339S. [DOI] [PubMed] [Google Scholar]
- 3.Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010;8:884–890. [DOI] [PubMed] [Google Scholar]
- 4.Wysokinski WE, McBane RD, Daniels PR, Litin SC, Hodge DO, Dowling NF, Heit JA. Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation. Mayo Clin Proc 2008;83:639–645. [DOI] [PubMed] [Google Scholar]
- 5.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 6.Olesen JB, Torp-Pedersen C, Hansen ML, Lip GYH. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0–1: a nationwide cohort study. Thromb Haemost 2012;107:1172–1179. [DOI] [PubMed] [Google Scholar]
- 7.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC Jr, Priori SG, Estes NA 3rd, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Ohman EM, Stevenson WG, Tarkington LG, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2011;123:e269–e367. [DOI] [PubMed] [Google Scholar]
- 8.Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, Dunn AS, Kunz R. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 suppl):e326S–e350S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, Garcia DA, Jacobson A, Jaffer AK, Kong DF, Schulman S, Turpie AG, Hasselblad V, Ortel TL; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015;373:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaker GC, Theriot P, Binder LG, Dobesh PP, Cuker A, Doherty JU. Management of periprocedural anticoagulation: a survey of contemporary practice. J Am Coll Cardiol 2016;68:217–226. [DOI] [PubMed] [Google Scholar]
- 11.Barnes GD, Kurlander J, Haymart B, Kaatz S, Saini S, Froehlich JB. Bridging anticoagulation before colonoscopy: results of a multispecialty clinician survey. JAMA Cardiol 2016;1:1076–1077. [DOI] [PubMed] [Google Scholar]
- 12.Barnes GD, Nallamothu BK, Sales AE, Froehlich JB. Reimagining anticoagulation clinics in the era of direct oral anticoagulants. Circ Cardiovasc Qual Outcomes 2016;9:182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


