Abstract
The thalamus has long been recognized for its role in relaying sensory information from the periphery, a function accomplished by its “first-order” nuclei. However, a second category of thalamic nuclei, termed “higher- order” nuclei, have been shown instead to mediate communication between cortical areas. The nucleus reuniens of the midline thalamus (RE) is a higher-order nucleus known to act as a conduit of reciprocal communication between the medial prefrontal cortex (mPFC) and hippocampus. While anatomical and behavioural studies of RE are numerous, circuit- based electrophysiological studies, particularly those examining the impact of cortical input and the thalamic reticular nucleus (TRN) on RE neuron firing, are sparse. To characterize RE neuron firing properties and dissect the circuit dynamics of the infralimbic subdivision of the mPFC (ilPFC), the TRN and RE, we used in vivo, extracellular, single- unit recordings in male Sprague Dawley rats and manipulated neural activity using targeted pharmacological manipulations, electrical stimulation and a projection-s pecific implementation of designer receptors exclusively activated by designer drugs (DREADDs). We show that ilPFC inhibition reduces multiple burst firing parameters in RE, whereas ilPFC stimulation drives burst firing and dampens tonic firing. In addition, TRN inhibition reduces the number of spontaneously active neurons in RE. Finally, inhibition of ilPFC terminals in RE selectively enhances a subset of burst firing parameters. These findings demonstrate that ilPFC input, both via direct projections and via the TRN, can modulate RE neuron firing pattern in nuanced and complex ways. They also highlight the ilPFC- TRN- RE circuit as a likely critical component of prefrontal–hippocampal interactions.
Keywords: corticothalamic circuits, limbic thalamus, thalamic bursting, thalamic reticular nucleus
1. INTRODUCTION
The thalamus has long been recognized for its role as a way station for sensory information bound for cortex, a function accomplished by its “first-order” nuclei (Sherman, 2016; Sherman & Guillery, 2001). However, the thalamus also contains subregions that facilitate information transfer between cortical areas, termed “higher- order” nuclei. This transthalamic route between cortical areas is thought to allow for enhanced modulation or gating of information not available to cortico–cortical pathways (Guillery & Sherman, 2002; Mitchell, 2015; Saalmann, Pinsk, Wang, Li, & Kastner, 2012), but higher- order thalamic nuclei are much less well- studied compared to first-order nuclei. In particular, it is still unclear precisely how corticothalamic input modulates thalamocortical cell firing in higher- order nuclei. Corticothalamic input to both first-o rder and higher- order thalamic nuclei consists of direct, monosynaptic connections and feedforward inhibition from the thalamic reticular nucleus (TRN) and extrathalamic sources (Halassa & Acsády, 2016; Sherman & Guillery, 2001). This circuity is complex and corticothalamic input is functionally heterogenous across cortical areas, highlighting the importance of characterizing the circuit dynamics of these connections in different parts of the corticothalamic network (Crandall, Cruikshank, & Connors, 2015; Halassa et al., 2014).
The nucleus reuniens of the midline thalamus is a higher- order thalamic nucleus that represents a critical mediator of communication between the mPFC and hippocampus. Previous studies have shown that RE is involved in several different classes of behaviour that share in common a dependence on the coordinated action of these two regions (Cassel et al., 2013), including spatial navigation (Ito, Zhang, Witter, Moser, & Moser, 2015; Jankowski et al., 2014), working memory (Barker & Warburton, 2018; Cholvin et al., 2013; Duan et al., 2015; Hallock, Wang, Shaw, & Griffin, 2013; Layfield, Patel, Hallock, & Griffin, 2015; Maisson, Gemzik, & Griffin, 2018; Viena, Linley, & Vertes, 2018), executive function (Linley, Gallo, & Vertes, 2016; Prasad, Abela, & Chudasama, 2017; Prasad, Macgregor, & Chudasama, 2013) fear learning (Davoodi, Motamedi, Akbari, Ghanbarian, & Jila, 2011; Ramanathan, Jin, Ressler, & Maren, 2018; Rangel, Baldo, & Canteras, 2018; Sierra et al., 2017; Troyner, Bicca, & Bertoglio, 2018; Vetere et al., 2017; Xu & Südhof, 2013), mood regulation (Kafetzopoulos et al., 2017) and threat processing (Salay, Ishiko, & Huberman, 2018). RE inputs to hippocampus selectively target apical tufted dendrites and interneurons in the stratum-l acunosum moleculare of the dorsal and ventral CA1, as well as the molecular layer of the subiculum, and these connections are reciprocal (Çavdar et al., 2008; Varela, Kumar, Yang, & Wilson, 2014; Vertes, Linley, & Hoover, 2015). Corticothalamic projections to RE appear to follow the basic organizing principles described for other thalamic nuclei: neurons from layers 5 and 6 of the medial prefrontal cortex (mPFC) send monosynaptic connections to RE (McKenna & Vertes, 2004; Varela et al., 2014) and layer 6 ilPFC neurons send collaterals to the antero- medial TRN (Cornwall, Cooper, & Phillipson, 1990), the same TRN subregion that projects to RE (Kolmac & Mitrofanis, 1997).
Despite the well- established importance of RE in a variety of behaviours and the thorough characterization of its anatomical connections, studies examining the baseline firing properties of RE neurons and their modulation by afferent structures are sparse (Walsh, Brown, & Randall, 2017). In particular, it is unknown how mPFC controls the firing of RE neurons and whether corticothalamic input from mPFC to RE is similar or different to that in other parts of the thalamus. Cortical input is likely to be dynamic and critical to gating RE output in a variety of behavioural contexts. Our goal was to assess the combined and separate contributions of direct and indirect ilPFC- RE pathways to controlling RE neuron firing. We focus on inputs to RE from the infralimbic subdivision of the mPFC (ilPFC), because we have previously demonstrated that inhibition of ilPFC enhances dopamine (DA) neuron firing in the ventral tegmental area (VTA) via the ventral subiculum, likely by disinhibiting RE (Patton, Bizup, & Grace, 2013; Zimmerman & Grace, 2016). We used in vivo, extracellular, single-u nit recordings in the anesthetized rat and manipulated neural activity using targeted pharmacological manipulations, electrical stimulation and a projection- specific implementation of designer receptors exclusively activated by designer drugs (DREADDs). Our findings show that ilPFC can robustly modulate multiple aspects of RE neuron firing across diverse timescales.
2. METHODS
2.1. Animals
All experiments were performed in accordance with the guidelines outlined in the United States Public Health Service Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. All experiments were performed in young adult (>65 days old) male Sprague Dawley rats (59 rats total; 300–500 g; Envigo, Frederick, MD).
2.2. In vivo electrophysiology in anesthetized rats
Rats were anesthetized with an initial dose of chloral hydrate (400 mg/kg i.p., Sigma) and supplemented periodically (i.p.) to maintain suppression of the hindlimb withdrawal reflex. Rats were placed in a stereotaxic frame (Kopf) and body temperature was maintained at 37°C with a temperature- controlled heating pad and rectal probe. Extracellular recordings were performed using single glass microelectrodes (impedance 6–8 M) filled with a 2% Chicago Sky Blue solution (Sigma) in 2 M NaCl. This impedance ensured waveforms from single neurons could be clearly resolved with a very high signal-to- noise ratio and without contamination from neighbouring neurons. Following a craniotomy electrodes were lowered into the RE in vertical tracks at 0.2 mm intervals in the x-y plane via hydraulic micropositioner. Before each track a short period of stabilization was incorporated to minimize the impact of the brain compression secondary to electrode movement on unit activity. The sampling area comprised a block of tissue including the RE from bregma/dural surface (in mm) AP: −1.4 to −2.2, ML: 0.1–0.5, and DV: −5.5 to −7.5. Sampling order in the AP direction was counterbalanced across animals. Following an additional short period of stabilization to ensure that the lowering of the electrode was not interfering with the firing of each neuron, every spontaneously active neuron encountered in this block of tissue was recorded for at least 3 m, and given the typical 1–2 Hz firing rate of RE neurons this resulted in ~270 spikes being included in the analysis of firing properties for each neuron. Individual neurons were recorded with broad filter settings (low pass, 10 Hz; high pass, 16 kHz). Immediately following recordings, the recording site was marked via electrophoretic ejection of Chicago Sky Blue from the tip of the recording electrode. All neurons included in the analysis were confirmed to be located in the RE by referencing their position to the marked recording site.
2.3. Intracranial infusions and electrical stimulation
For intracranial infusions a guide cannula (23 gauge) was placed above the ilPFC or TRN at the following coordinates from bregma/skull surface (in mm): ilPFC AP: 2.7; ML: 0.5; DV: −3.5; TRN AP: −1.4, ML: 1.6, DV: −5.0, according to the Paxinos and Watson brain atlas (Paxinos & Watson, 2013). Subsequently an infusion cannula (33 gauge) was inserted into the guide cannula, extending 2 mm beyond the tip of the guide cannula. Pharmacological agents dissolved in Dulbecco’s phosphate buffered saline (dPBS; Sigma) or dPBS vehicle only were administered through the infusion cannula at a rate of 0.5 μl/min. The guide cannula was left in place for 3 m following infusions to allow for adequate diffusion of drug. Drug doses were as follows for all experiments: ilPFC: Tetrodotoxin (TTX; Sigma) 1 M in 0.5 μl; TRN: muscimol, BODIPY® TMR- X conjugate (Thermofisher) 0.8 mM in 0.2 μl. All pharmacological agents were injected at doses reported previously to induce specific behavioural and/or neurochemical effects (Allen et al., 2008; Lodge & Grace, 2007; Patton et al., 2013; Valenti, Lodge, & Grace, 2011). Rats received only one injection per region and individual RE cells were recorded from 5 m to 2.5 hr after infusions.
To visualize the spread of fluorescent muscimol, animals were transcardially perfused with 0.85% saline followed by 4% paraformaldehyde. Brains were post-fixed in 4% paraformaldehyde overnight and cryoprotected (25% w/v sucrose in PBS) until saturated. Coronal sections (60 μm thickness) were taken. During image acquisition exposure time was optimized for image quality. The spread of the drug was then qualitatively assessed on several sections and if more than 30% of the area of fluorescence was outside the area of the TRN the animal was excluded from further analyses.
For electrical stimulation, a concentric bipolar stimulating electrode was lowered into ilPFC at the following coordinates from bregma/skull surface (in mm): ilPFC AP: 2.7; ML: 0.5; DV: −4.5. Single pulses (1 mA, 0.25 msec pulse- width) were delivered at 0.5 hz. Spontaneously active neurons, regardless of initial firing pattern, were recorded for 3 m before 5 pulses were delivered. If the firing pattern of the neuron changed in any fashion in response to these initial pulses, more pulses were delivered (~15–25) until the firing pattern of the neuron stabilized, that is, repeated stimuli no longer resulted in any appreciable changes in neuronal firing pattern. Spontaneous activity was also recorded for several minutes after stimulation to assess for persistent effects of stimulation on spontaneous firing pattern. Multiple neurons were recorded in the same animal, but the effects of stimulation did not differ qualitatively in neurons recorded early versus late in recordings.
2.4. Viral constructs
To achieve hM4Di DREADDs expression in ilPFC, we used an AAV vector (rAAV2-hSyn-HA-hM4D(Gi)-IRES-mCitrine) containing a synapsin promoter, as well as a N- terminal HA tag and mCitrine reporter. A vector lacking the DREADDs gene (rAAV2-hSyn-EGFP) was used for control experiments. Both vectors were obtained from the UNC Vector Core, Chapel Hill, NC.
2.5. Survival surgeries for virus injections
All survival surgeries were performed under general anaesthesia in a sterile environment. Briefly, rats were anesthetized with isoflurane (induction: 5%; maintenance: 1%–3% in oxygen) and placed in a stereotaxic apparatus using blunt, atraumatic ear bars. 400 nl of virus was then injected unilaterally into the right ilPFC via a pulled glass pipette (38– 42 μm tip diameter) and pneumatic pressure over the course of 15 m. The pipette was left in place for 15 m after the infusion to allow for adequate diffusion. The wound was then closed and antibiotic cream was applied to the wound edge, and the rat was removed from the stereotaxic frame and monitored closely until conscious. Rats received analgesia (Medigel containing carpofen, 5 mg/kg/day, p.o.) 24 hr before and 24 hr after surgery, and immediately following surgery (carpofen, 5 mg/kg, s.c.). Rats were allowed 12 weeks to achieve sufficient construct expression in terminals in RE before experiments.
2.6. In vivo electrophysiology in DREADD- expressing animals
Animals were prepared for recording and the RE was sampled as described above. Extracellular recordings were performed using a single glass microelectrode glued to a pulled glass pipette (20 μm tip diameter) filled with dPBS vehicle or CNO (Tocris, Bristol, UK). The recording electrode tip and infusion pipette tip were separated by ~150 μm. Spontaneously active neurons were recorded for 3 m before vehicle or CNO (dissolved in dBPS; 100 μM in 60 nl) application, during the entirety of vehicle or CNO application (30 to 60 s), and for at least 3 m after vehicle or CNO application. Multiple neurons were recorded in the same animal and separated by at least 200 μm. Each neuron received only one exposure to vehicle or CNO.
2.7. Localization of DREADD expression
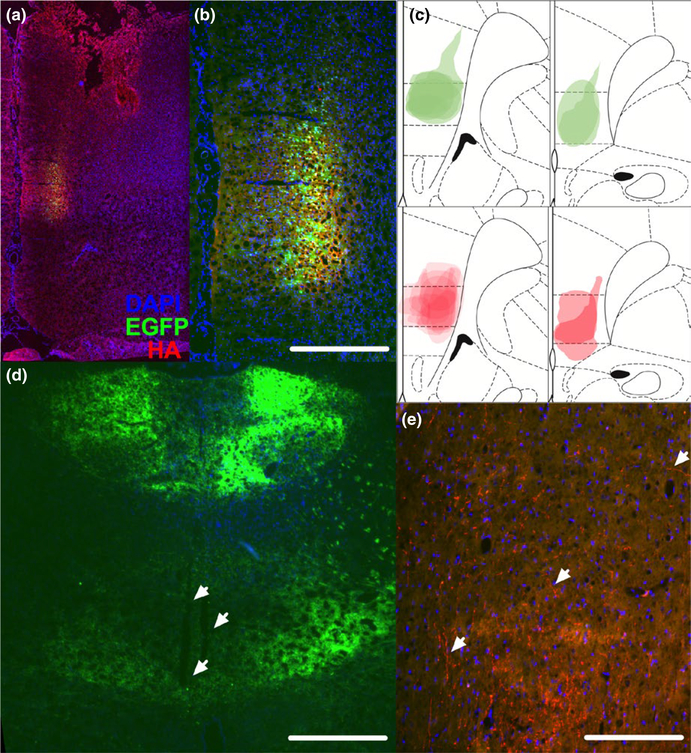
Immediately following electrophysiological recordings animals were perfused transcardially with 0.85% saline followed by 4% paraformaldehyde. Brains were postfixed in 4% paraformaldehyde overnight and cryoprotected (25% w/v sucrose in PBS) until saturated. Coronal sections (10 μm thickness) were taken and mounted onto glass slides. Immunohistochemistry for HA was then performed in the following manner. Tissue was blocked in a solution of 1× PBS containing 0.3% Triton X-1 00 and 3% normal goat serum, then incubated in primary antibody (rabbit anti-H A, 1:500, Cell Signaling, 3724, Danvers, MA) overnight at room temperature. Slides were then washed and incubated with a secondary fluorescent antibody (goat anti- rabbit 594, 1:500, Abcam, ab150080, Cambridge, MA) for 3 hr. Slices were counterstained with DAPI and coverslipped with ProLong Gold. Viral spread of DREADDs- expressing constructs was qualitatively assessed in ilPFC on several sections using anti- HA fluorescence and if more than 30% of the area of fluorescence was outside the ilPFC the animal was excluded from future analyses. Terminal expression in RE was also confirmed using anti- HA fluorescence. Viral spread and terminal expression were assessed in EGFP-o nly animals using endogenous fluorescence. During image acquisition exposure time was optimized for image quality. All antibodies were previously validated for specificity, as described on the manufacturer’s websites and the Journal of Comparative Neurology antibody database.
2.8. Analysis parameters for RE neuron firing properties
For the purposes of calculating firing rate, all of the spikes fired within a burst were considered as separate events. For example, the firing rate for a 10 s period of recording interval to end burst: 10 ms, minimum interval between burst: 200 ms, minimum duration of burst: 2 ms, minimum number of spikes within a burst: 2 (Kim et al., 2011). A 6- ms interspike interval (ISI) was used for the maximum interval to start a burst after an examination of ISI distributions in our data revealed that some putative burst spikes were occurring at intervals >4 ms, which is the typical but conservative cutoff used to classify thalamic bursts (Guido, Lu, & Sherman, 1992; Lu, Guido, & Sherman, 1992; Ramcharan, Gnadt, & Sherman, 2005). We also verified that bursts conformed to other previously established criteria for thalamic burst firing; that is, a preceding >100 ms of no spikes and a gradual lengthening of the ISI within a burst (Guido et al., 1992). For mean spikes per burst analysis, neurons in which no bursts occurred were excluded from analysis. The average number of spontaneously active RE neurons per electrode track, that is, “cells/track” was calculated for each individual animal.
2.9. Experimental design and statistical analysis
Electrophysiological analysis of RE neuron activity was performed using commercially available software (LabChart and NeuroExplorer). All statistics were calculated using the GraphPad Prism software program (GraphPad Software). In our studies, we found that burst firing propensity (i.e. per cent spikes fired in bursts) in RE neurons, even recorded in the same animal in close spatial and temporal proximity, was highly variable and not normally distributed. However, our measurement of mean spikes per burst provides a less- variable indicator of burst firing propensity that has been shown to depend on the same intracellular mechanisms as burst firing (Guido et al., 1992). While single- unit, extracellular recording data from RE neurons are sparse, the sample sizes for RE neurons recordings throughout the current work were based on previous reports examining analogous circuits and/or similar experimental approaches (Kim et al., 2011; Lara- Vásquez, Espinosa, Durán, Stockle, & Fuentealba, 2016; Mahler et al., 2014; Ramcharan et al., 2005; Whitt, Masri, Pulimood, & Keller, 2013). Experimental design for each experiment is as follows.
2.9.1. ilPFC inhibition experiment
About 21 total rats were included in this experiment. A total of 138 neurons were found to lie within RE and were included in the analysis. Within each animal, several neurons were recorded before TTX infusion, then a single drug or vehicle infusion was performed in the ilPFC, then several more neurons were recorded. Control animals received only vehicle (i.e. dPBS) infusion. Therefore, neurons were recorded under four different conditions: (a) before vehicle injection, (b) after vehicle injection, (c) before TTX injection and (d) after TTX injection. Note that conditions 1 and 3 are identical. TTX was utilized here to maintain consistency with our prior report assessing the involvement of RE on VTA DA neuron firing (Zimmerman & Grace, 2016), and because we have utilized TTX in ilPFC in multiple prior reports and observed robust effects (Moreines, Owrutsky, & Grace, 2017; Patton et al., 2013). Neurons exposed to the same experimental conditions were pooled across animals for statistical analyses. Reported sample sizes indicate the number of cells recorded in total from all animals. Significance testing was performed using the Mann–Whitney U test.
2.9.2. ilPFC stimulation experiment
Three rats were included in the experiment. Eight neurons from these rats were found to lie within RE and were included in the analysis. Each neuron was recorded before, during and after acute electrical stimulation. All RE neurons from all animals were pooled for analyses.
2.9.3. TRN inhibition experiment
About 17 rats were included in the experiment. A total of 132 neurons from these rats were found to lie within RE and were included in the analysis. The experimental design here was identical to that described above in the “ilPFC Inhibition Experiment”, but fluorescently conjugated muscimol in TRN was used instead of TTX in ilPFC. Fluorescently conjugated muscimol (Allen et al., 2008) was used to achieve neuronal inhibition, which (a) would not be expected to affect fibres of passage and (b) permitted visualization of the extent of drug spread—a critical point given the unusual shape of TRN in the coronal plane and its close proximity to RE (Paxinos & Watson, 2013). Significance testing was performed using the Mann–Whitney U test.
2.9.4. DREADDs experiment
About 18 rats were included in the experiment. A total of 75 neurons from these rats were found to lie within RE and were included in the analysis. Multiple neurons were recorded in the same animal. Each neuron was recorded before, during and after vehicle or CNO infusion, as described above. This resulted in cells exposed to four different manipulations as follows: animals expressing EGFP + vehicle injection (EGFP + VEH), animals expressing EGFP + CNO injection (EGFP + CNO), animals expressing hM4Di + vehicle injection (hM4Di + VEH) and animals expressing hM4Di + CNO injection (hM4Di + CNO). Significance testing was performed using the Wilcoxon matched pairs signed- rank test and confidence intervals of the mean paired difference reported.
3. RESULTS
3.1. Baseline firing properties of RE neurons
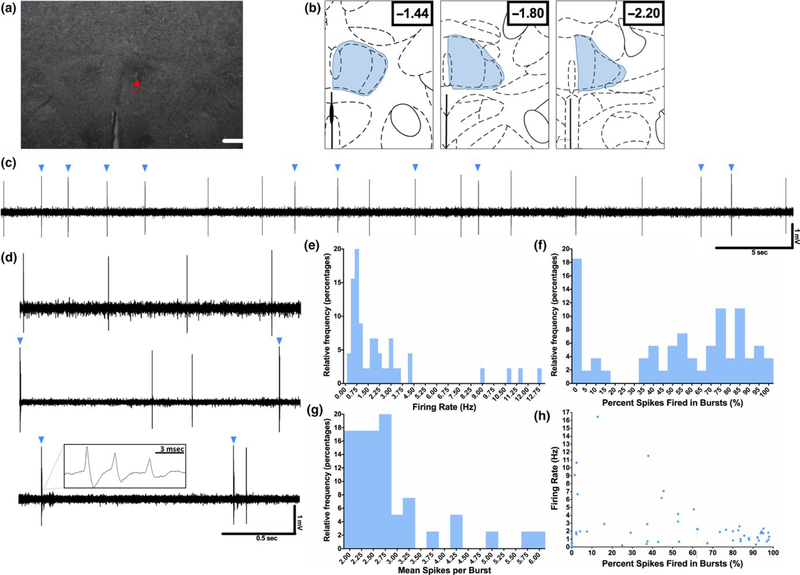
Extracellular recordings of spontaneously active RE neurons were performed in anesthetized rats (Figure 1a- b). Each neuron was recorded for 3 m and firing rate and multiple burst firing parameters were measured. Burst firing was defined using previously established criteria (Guido et al., 1992; Kim et al., 2011) and based on independent assessment of interspike intervals in the recorded sample. The majority of RE neurons recorded exhibited both tonic and burst firing patterns, most often intermixed in the same neuron (Figure 1c–d). The mean firing rate of RE neurons was 2.16 ± 0.36 Hz while the median firing rate was 1.60 Hz (Figure 1e). Of the recorded neurons, >75% exhibited a firing rate at or below 3.0 Hz, while firing rates in the remaining minority of neurons ranged from 4–12 Hz. The mean percentage of spikes fired in bursts was 53.6 ± 4.8%, while the median was 61.4% (Figure 1f). Of the recorded neurons, 18% did not exhibit any burst firing, while the remaining neurons exhibited a percentage of spikes fired in bursts ranging from <5% to >95%. In addition, the mean spikes per burst in RE neurons was 2.9 ± 0.1 spikes, while the median was 2.68 spikes (Figure 1g). Although observations suggested that neurons with higher firing rates tended to exhibit a lower percentage of spikes fired in bursts (Figure 1h), these parameters did not show a significant degree of correlation (F(1,46) = 3.44, p = 0.15, 95% CI = −0.04–0.001; R2 = 0.07).
FIGURE 1.
Baseline firing properties of RE neurons. Extracellular recordings of spontaneously active RE neurons were performed in anesthetized rats under baseline conditions. (a) Representative image of the location of an example neuron recorded in RE. The location of this neuron was marked following recording by iontophoretic application of Chicago Sky Blue dye (dashed arrow). Scale bar = 900 μm. (b) Schematic depiction of the area within RE from which all RE neurons throughout the manuscript were sampled (blue shading). The sampling area comprised a block of tissue including the RE from bregma/dural surface (in mm) AP: −1.4 to −2.2, ML: 0.1–0.5, and DV: −5.5 to −7.5. All neurons included in the analysis were confirmed to be located in the RE by referencing their position to a marked recording site generated via electrophoretic ejection of Chicago Sky Blue from the tip of the recording electrode. (c) Representative trace demonstrating the typical firing pattern of an RE neuron, with mixed tonic and burst (blue triangles) firing. (d) Three representative traces demonstrating the variable amount of burst firing present in the RE neuron population, from completely absent (top), to mixed (middle), to nearly all bursting (bottom). Inset in bottom panel demonstrates typical RE burst architecture. (e–g) Relative frequency histograms of firing rate, the percentage of spikes fired in bursts and the mean spikes per burst for all recorded RE cells. Bin sizes for each column are as follows: e = 0.25 Hz, f = 5%, g = 0.25 spikes/burst. (h) Firing rate plotted against the percentage of spikes fired in bursts. These parameters were not significantly correlated in this group of recorded neurons, although only slowly firing neurons tended to show high levels of bursting. n = 48 cells for panels e–h
3.2. Inhibition of ilPFC reduces burst firing in RE neurons
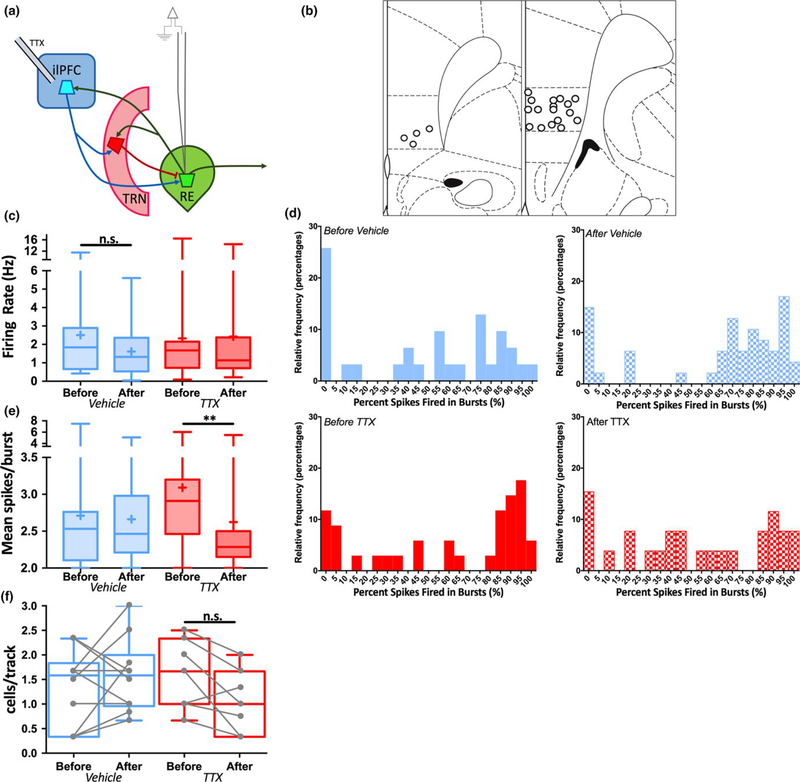
Our previous report (Zimmerman & Grace, 2016) implicated ilPFC- RE communication in controlling VTA DA neuron population activity. However, the circuit dynamics underlying ilPFC- RE communication have not been studied in detail. To address this question spontaneous activity in RE cells was recorded in anesthetized rats before and after acute infusion of TTX into ilPFC (1 μmol/L in 0.5 μl), in which several neurons were recorded before and after TTX infusion in the same animal (Figure 2a–b). Data from all neurons recorded in all animals were pooled. Firing rate and burst firing parameters were assessed in each neuron recorded. To assess for effects of time under anaesthesia and prior sampling in RE, RE neurons were recorded in animals in which dPBS vehicle was infused into ilPFC. Following TTX or vehicle infusion in ilPFC no differences were observed in firing rate in RE neurons recorded after infusion compared to RE neurons recorded before infusion (Figure 2c; Mann–Whitney, VEH: U = 622, p = 0.28, 95% CI = −0.8355–0.304, TTX: U = 390.5, p = 0.44, 95% CI = −29.05–7.04). However, following the same manipulation there was a reduction in burst firing propensity across the population of RE neurons sampled (Figure 2d). In addition, the average number of spikes within a burst for each neuron was measured (Figure 2e), a parameter that depends on the amplitude of low- threshold calcium spikes within a neuron (Sherman, 1996). Following TTX infusion (but not vehicle), there was a large, statistically significant decrease in the average number of spikes within a burst (Mann–Whitney, VEH: U = 529.5, p = 0.59, 95% CI = −0.1733–0.2816, TTX: U = 197.5, p = 0.005, 95% CI = −0.7258–0.09293). Finally, no change was observed in the number of spontaneously active RE neurons per track following either vehicle or TTX infusion (Figure 2f; Mann–Whitney, VEH: U = 41.5, p = 0.54, 95% CI = −0.67–1.167, TTX: U = 14, p = 0.19, 95% CI = −1.5–0.34). These data suggest that ilPFC contributes to the maintenance of burst firing in RE neurons.
FIGURE 2.
Inhibition of ilPFC reduces burst firing in RE neurons. Spontaneous activity was recorded in multiple RE neurons in multiple animals both before and after acute infusion of TTX (1 μM in 0.5 μl) or dPBS vehicle into ilPFC. (a) Schematic of experimental design. (b) Representation of histological placements of infusion cannulae into ilPFC. (c) The firing rate of RE neurons plotted as “box- and-w hiskers” plots, here and in subsequent figures representing highest and lowest values (highest and lowest horizontal lines), interquartile range (rectangle), mean (“+” symbol) and median (horizontal line in rectangle). Firing rates recorded before vehicle or TTX infusion did not differ from those recorded after infusion. (d) Relative frequency distribution histograms depicting the percentage of spikes fired in bursts in neurons recorded either before or after vehicle (top) or TTX (bottom) infusion into ilPFC. Burst firing propensity was reduced in the population of RE neurons recorded after TTX infusion. Bin size for each column is 5% of spikes fired in bursts. (e) The mean spikes per burst of RE neurons recorded after TTX infusion was decreased compared to those recorded before infusion. (f) The number of spontaneously active RE neurons per electrode track did not differ following vehicle or TTX infusion. **p < 0.01 (Mann–Whitney U test). Before Vehicle n = 31 cells from 12 animals, After Vehicle n = 47 cells, Before TTX n = 34 cells from 9 animals, After TTX n = 26 cells
3.3. Electrical Stimulation of ilPFC induces burst firing and reduces tonic firing in RE neurons
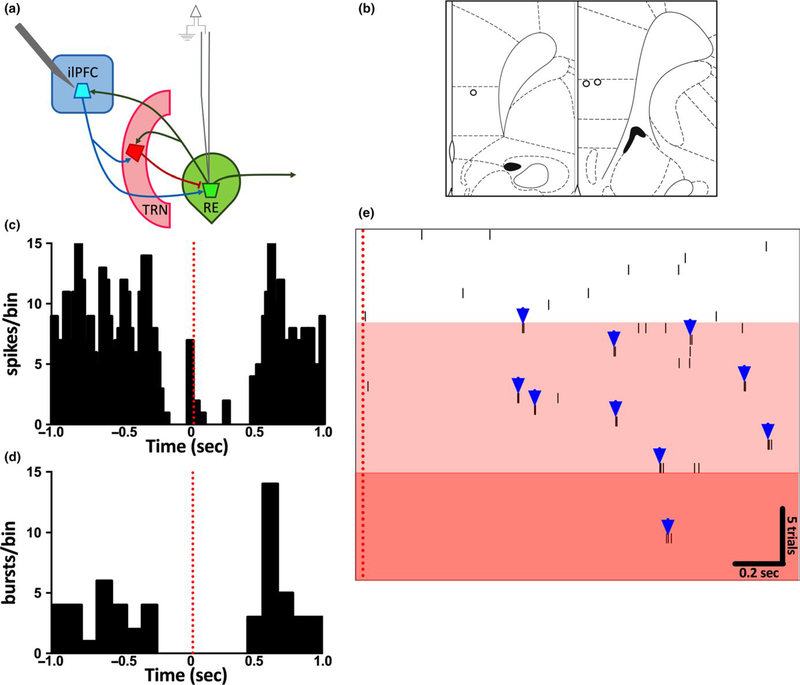
Direct ilPFC projections to RE are glutamatergic, but ilPFC influence on RE likely also depends on feedforward inhibition arising from well- described ilPFC projections to the TRN (Çavdar et al., 2008; Cornwall et al., 1990), but see (Vertes, 2003). To examine the combined contribution of these pathways in controlling RE neuron firing, spontaneous activity in single RE neurons was recorded before, during, and after electrical stimulation of ilPFC (0.5 hz, 1 mA) using a concentric bipolar electrode (Figure 3). Several successive trials were performed in each neuron and multiple neurons were recorded in the same animal. In 75% of neurons recorded, individual pulses to ilPFC resulted initially in cessation of all firing, followed ~0.6 s later by rebound spiking, predominantly consisting of bursting (Figure 3c–d). In responsive neurons, several successive pulses (i.e. ~15–25) cessation of firing altogether. This effect of stimulation subresulted in a gradual loss of tonic firing and predominance of sided within 1 m following cessation of stimuli. These findburst firing (Figure 3e), in most cases leading to a complete ings suggest that ilPFC stimulation is sufficient to entrain bursting in, and even possibly silence altogether, RE neurons. Furthermore, these effects are likely mediated by feedforward inhibition from the ilPFC- TRN pathway (Halassa et al., 2011).
FIGURE 3.
Electrical Stimulation of ilPFC induces burst firing and attenuates tonic firing in RE neurons. Spontaneously active RE neurons were recorded both before and during electrical stimulation of ilPFC (0.5 hz, 1 mA) via a concentric bipolar stimulating electrode. Several successive trials were performed in each neuron. (a) Schematic of experimental design. (b) Representation of histological placements of stimulating electrodes in recorded animals. (c) Cumulative perievent histogram of firing rate for a subset of trials in all RE neurons recorded (n = 10 trials/cell) demonstrating inhibition immediately following ilPFC stimulation (red dotted line). (d) Cumulative perievent histogram of burst firing plotted from the same traces as in C demonstrating inhibition immediately following ilPFC stimulation (red dotted line), followed by rebound bursting ~0.6sec poststimulation. (e) Perievent raster of 30 successive trials within a single RE neuron. Electrical stimulation of ilPFC (red dotted line) in successive trials gradually converted the firing pattern of this neuron from tonic to burst firing (white vs. light red shaded areas). After 21 trials, the neuron nearly ceased firing (darker red area). n = 8 cells from three animals
3.4. Inhibition of TRN decreases the number of spontaneously active RE neurons
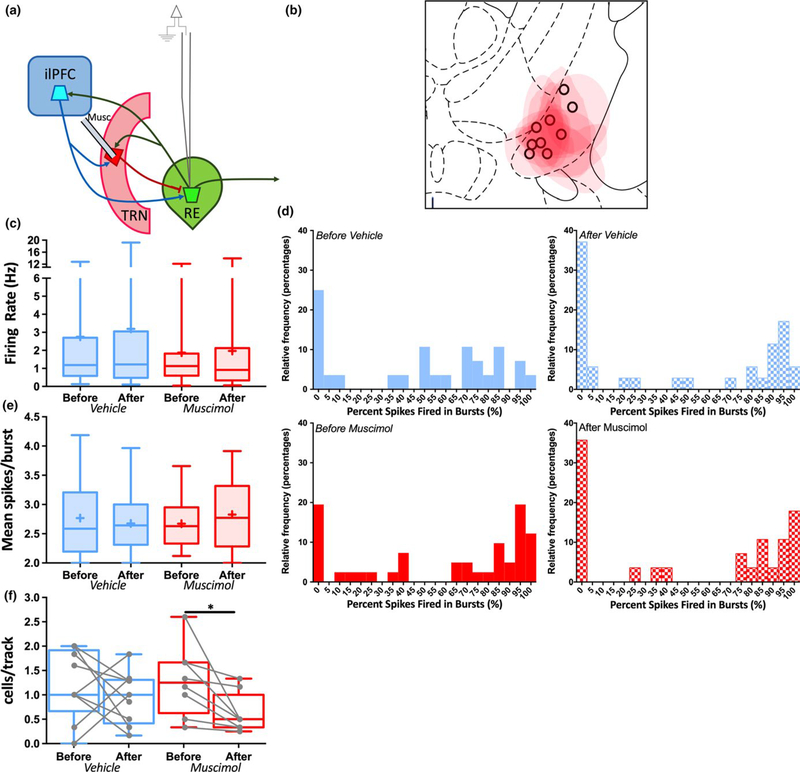
Given the likely impact of feedforward inhibition from the ilPFC-T RN pathway on RE neuron firing, the effects of manipulating TRN itself were investigated (Figure 4). The portion of TRN that most strongly projects to RE was targeted with fluorescently conjugated muscimol injections (Çavdar et al., 2008; Cornwall et al., 1990; Kolmac & Mitrofanis, 1997). The experimental design and analyses for these experiments were identical to those in the ilPFC inhibition experiment described above. Following fluorescently conjugated muscimol infusion into TRN, no changes were observed in the firing rate of RE neurons recorded after vehicle or muscimol infusion compared to those recorded before (Figure 4c; Mann–Whitney, VEH: U = 471, p = 0.80, 95% CI = −0.7047–0.7253, TTX: U = 529, p = 0.59, 95% CI = −0.5863–0.3439). Similarly, no change in burst firing propensity (Figure 4d) or mean spikes within a burst (Figure 4e; Mann–Whitney, VEH: U = 249.5, p = 0.76, 95% CI = −0.3751–0.3206, TTX: U = 258, p = 0.45, 95% CI = −0.1663–0.4583) were observed. However, muscimol infusion produced a substantial and statistically significant reduction in the CI = −1.296–0.041). These data suggest that, when manumber of spontaneously active RE neurons encountered nipulated in isolation, TRN input to RE has a qualitatively (Figure 4f; Mann–Whitney, VEH: U = 31.5, p = 0.45, distinct role compared to feedforward inhibition from cor95% CI = −0.83–0.33, TTX: U = 13.5, p = 0.038, 95% ticothalamic inputs.
FIGURE 4.
TRN inhibition decreases the number of spontaneously active RE neurons. Spontaneous activity was recorded in multiple RE neurons in multiple animals both before and after acute infusion of fluorescently tagged muscimol (0.8 μM in 0.2 μl) or dPBS vehicle into TRN (a) Schematic of experimental design. (b) Representation of histological placements of infusion cannulae for vehicle groups (black circles) and maximum extent of fluorescence for muscimol groups (red shading) in TRN. (c) The firing rate of RE neurons recorded before vehicle or muscimol infusion did not differ from those recorded after infusion. (d) Relative frequency distribution histograms depicting the percentage of spikes fired in bursts in neurons recorded either before or after vehicle (top) or muscimol (bottom) infusion into TRN. There were no differences in burst firing propensity before and after vehicle or muscimol infusion. Bin size for each column is 5% of spikes fired in bursts. (e) The mean spikes per burst of RE neurons recorded before vehicle or muscimol infusion did not differ from those recorded after infusion. (f) The number of spontaneously active RE neurons per electrode track recorded after muscimol infusion was decreased compared to those recorded before infusion. *p < 0.05 (Mann– Whitney U test). Before Vehicle n = 28 cells from nine animals, After Vehicle n = 35 cells, Before Muscimol n = 41 cells from eight animals, After Muscimol n = 28 cells
3.5. Inhibition of ilPFC terminals in RE enhances burst firing in RE neurons
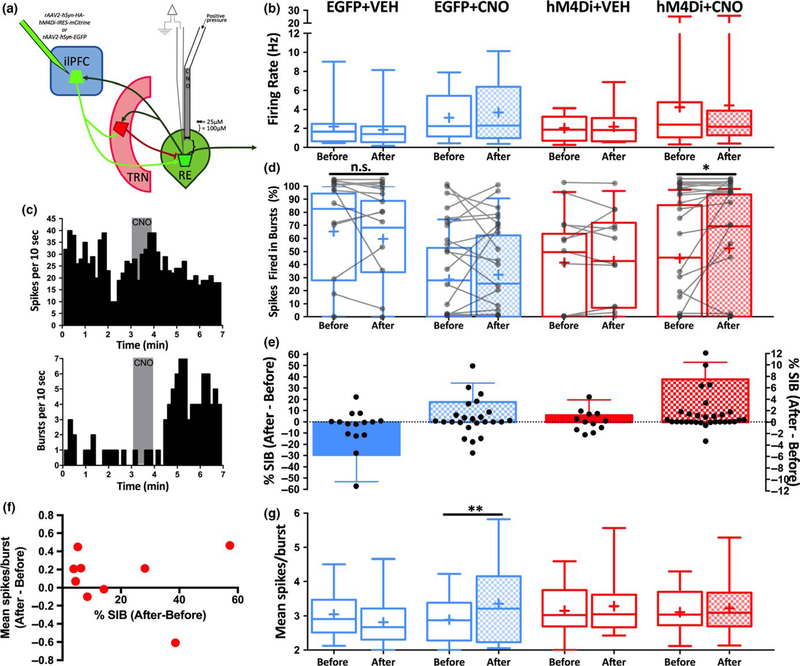
The findings described above are consistent with the ilPFC influencing the firing pattern of RE neurons via feedforward inhibition. However, ilPFC also sends monosynaptic, glutamatergic projections to RE. In order to assess the role of these projections in controlling RE neuron firing, a projection- specific approach utilizing DREADDs (Ge et al., 2017; Lichtenberg et al., 2017; Mahler et al., 2014, 2018; Malvaez, Shieh, Murphy, Greenfield, & Wassum, 2018; McGlinchey & Aston- Jones, 2018; Stachniak, Ghosh, & Sternson, 2014) was used. AAV vector constructs containing the inhibitory DREADD hM4Di fused to EGFP (rAAV2-hSyn-HA-hM4Di-IRES-mCitrine) or EGFP only controls (rAAV2-hSyn-EGFP) were injected into ilPFC (Figure 5a–b). After recordings, the maximal extent of EGFP expression across EGFP- only animals and anti- HA immunoreactivity across hM4Di animals was assessed to ensure localization to the ilPFC (Figure 5c). After allowing sufficient time for construct expression in ilPFC terminals in RE (i.e. 12 weeks; Figure 5d–e, white arrowheads), individual RE neurons were recorded before and after local microinfusion of CNO (60 nl of 100 μM) or dPBS vehicle control (VEH) via a combined glass injection pipette- recording electrode (Figure 6a). Following acute CNO or VEH application, no effect on firing rate was found in any group (Figure 6b). However, following CNO application onto RE neurons in animals expressing hM4Di (Figure 6c–e) there was a statistically significant enhancement of the percentage of spikes fired in bursts (Figure 6d; Wilcoxon matched pairs signed- rank test, p = 0.02, 95% CI = 0–3.843). This effect was observed in a subset of RE neurons (12/30 neurons, 40% of recorded neurons) and appeared most prominent in neurons exhibiting a low percentage of spikes fired in bursts before CNO application. This effect was observed in some individual neurons recorded in animals expressing EGFP only or following vehicle injection (Figure 6d), but there were no statistically significant effects in any of these groups as a whole (Wilcoxon matched pairs signed-r ank test, EGFP + VEH: p = 0.38, 95% CI = −10.75–1.69; EGFP + CNO: p = 0.89, 95% CI = −6.27–3.33, hM4Di + VEH: p = 0.99, 95% CI = −9.86–7.30). Notably, neurons in the hM4Di + CNO group that exhibited an enhancement in bursting following CNO application did not show a consistent change in the mean number of spikes fired within a burst (Figure 6f). Finally, a statistically significant increase in the average number of spikes within a burst following CNO application in EGFP animals was found (Wilcoxon matched pairs signed- rank test, p = 0.005, 95% CI = −0.052–0.77) but no differences in this parameter were observed in any other group (Figure 6g). Taken together, these findings suggest that the direct ilPFC-RE projection provides an input necessary to maintain certain characteristics of burst firing in RE neurons.
FIGURE 5.
Histological validation of viral vector expression in ilPFC and RE AAV vector constructs containing the inhibitory DREADD hM4Di (rAAV2-hSyn-HA-hM4Di-IRES-mCitrine) or EGFP only controls (rAAV2-hSyn-EGFP) were infused into ilPFC. Blue = DAPI, Green = EGFP, Red = anti- HA antibody throughout, as in A. (a) Representative coronal section demonstrating expression restricted to ilPFC. (b) Higher magnification image of section picture in A. Scale bar = 300 μm. (c) Superimposed traces of the maximal extent of EGFP expression across EGFP- only animals (green, top) anti- HA immunoreactivity across hM4Di animals (red, bottom) from all animals recorded. (d) Low-m agnification image demonstrating terminal expression throughout the midline thalamus in a EGFP animal with recording electrode tracks present in RE (white arrows). Scale bar = 900 μm. (e) Representative image of HA+ terminal labelling in RE (white arrows). Scale bar = 150 μm
FIGURE 6.
Inhibition of ilPFC terminals in RE enhances burst firing in RE neurons. Individual RE neurons in rats expressing hM4Di or EGFP only in ilPFC and ilPFC terminals in RE were recorded before, during and after local microinfusion of CNO (60 nl of 100 μM) or dPBS vehicle control (VEH) via a combined glass injection pipette- recording electrode. (a) Schematic diagram demonstrating the virus injection site, relevant circuitry and combined glass injection pipette- recording electrode dimensions. (b) Acute CNO or VEH application had no effect on firing rate in any group. For panels b,d,e,g groups as indicated in panel b. “Before” is the firing parameter measured for 3 m before vehicle or CNO application, and “After” is the firing parameter measured for 3 m after vehicle or CNO application. (c) Cumulative perievent histograms of firing rate (top) and burst firing (bottom) for a single RE neuron in an hM4Di animal before, during (grey box) and after CNO application. (d) The percentage of spikes fired in bursts presented as individual neurons (grey lines) and grouped box- and-w hiskers plots before and after acute CNO application. CNO application enhanced burst firing in RE neurons of animals expressing hM4Di, but this effect was not consistently observed in any other group. (e) Data from D presented as the difference in percentage of spikes fired in bursts (% SIB) before and after vehicle or CNO application. Individual values plotted in black circles on left y- axis and group means + standard error of the mean plotted in bars on right y- axis, for clarity. (f) Data from a subset of neurons in the hM4Di+CNO group that exhibited an increase in burst firing following CNO application, demonstrating inconsistent changes in mean spikes/burst. (g) Acute CNO enhanced the mean number of spikes within a burst in the EGFP + CNO group, but not in any other group. *p < 0.05, **p < 0.01 (Wilcoxon paired signed-r ank test). EGFP + VEH n = 15 neurons from 3 animals, EGFP + CNO n = 18 neurons from four animals, hM4Di+VEH n = 12 neurons from two animals, hM4Di+CNO n = 30 neurons from nine animals
4. DISCUSSION
These findings characterize RE neuron firing patterns in an intact preparation and describe the circuit dynamics underlying ilPFC- RE communication. Pharmacological inhibition of ilPFC, while not affecting firing rate, was found to reduce the incidence of burst firing across the population of RE neurons and also reduce the mean number of spikes within a burst. In contrast, electrical stimulation of ilPFC acutely drove burst firing, and repeated stimulation was in many RE neurons able to silence almost all spontaneous activity. With respect to the distinct contributions of the TRN and monosynaptic ilPFC-R E projection to RE neuron firing, pharmacological inhibition of TRN did not affect any of the measured RE neuron firing parameters, but did reduce the number of spontaneously active RE neurons encountered in the recordings. Finally, inhibition of the direct ilPFC- RE pathway acutely enhanced burst firing in RE neurons.
The effects of acute inhibition or stimulation of ilPFC that were observed selectively affected burst firing parameters. This is potentially consistent with prior studies that have shown that TRN- mediated feedforward inhibition driven by cortex is powerful, often overwhelming the monosynaptic corticothalamic projection (Beierlein & Connors, 2002; Crandall et al., 2015; Halassa et al., 2011; Paz et al., 2011; Swadlow & Weyand, 1987). Indeed, repeated stimulation of ilPFC completely silenced 75% of RE neurons after 20–22 stimuli. Following ilPFC inhibition (albeit on a much longer timescale compared to electrical stimulation), there was a reduction in the number of spikes within a burst, a parameter that depends on the amplitude of the T- type calcium channel- mediated current (i.e. the “low threshold spike”) that underlies thalamic burst firing (Sherman, 2001). It should be noted that ilPFC inhibition could be exerting other effects independent of membrane potential that would impact the number of spikes fired in a burst. In addition, it should be noted that the manipulations of ilPFC were not projection specific, and therefore these effects could be mediated in part by feedforward inhibition arising from extrathalamic sources, such as the zona incerta (Halassa & Acsády, 2016; Sesack, Deutch, Roth, & Bunney, 1989). However, these manipulations provide the advantage of assessing the impact of ilPFC on the RE via a combined contribution of both direct and indirect pathways. Thus, these findings demonstrate that the predominant influence of ilPFC on RE neuron firing is on firing pattern.
The effects of targeted manipulation of two monosynaptic inputs to RE neurons were also evaluated: the subregion of TRN known to project to RE and ilPFC terminals in RE. Surprisingly, acute pharmacological infusion of fluorescently conjugated muscimol into TRN did not impact firing rate or any of the measured burst firing parameters in RE neurons, but did reduce the number of spontaneously active neurons encountered in RE. These findings could be attributable to an unanticipated effect of muscimol on TRN neurons, given the unique GABAergic signalling known to exist in the TRN in some in vitro preparations (Sun et al., 2012). However, they could also suggest that in the anesthetized state, closed- loop TRN input to RE might predominate over the comparatively less robust glutamatergic input. This would produce a pacemaker- like activity that, when removed, results in a portion of target neurons exhibiting no spontaneous firing, as opposed to a graded change in bursting propensity. Furthermore, the effect of inhibiting TRN itself was different from that of inhibiting ilPFC, possibly because ilPFC drives TRN firing in a way that is distinct from the intrinsic firing of TRN neurons themselves (Crandall et al., 2015).
In contrast to the effects of inhibiting ilPFC cell bodies on RE neuron firing, projection- specific inhibition of monosynaptic ilPFC inputs to RE induced an acute enhancement of burst firing in a subset of RE neurons. This effect could be attributable to the removal of a glutamatergic input causing a hyperpolarization of RE neurons, leading to more de- inactivation of T-type calcium channels and a larger low- threshold spike (Sherman & Guillery, 2001), although this was not directly tested in our experiments. However, this manipulation did not enhance the mean number of spikes per burst, which was reduced in parallel with a reduction in bursting seen following ilPFC inhibition. This effect suggests that inhibition of the glutamatergic ilPFC- RE input has distinct effects on RE cell membrane properties compared to that of removing all ilPFC input (feedforward or otherwise), implying the potential for highly nuanced control of RE cell firing depending on the specific inputs that are engaged. It should be noted that individual neurons in other treatment groups did exhibit changes in burst firing following application of CNO or vehicle. In particular, burst firing was often modulated up or down following CNO application in the EGFP + CNO group. Several previous studies have characterized off- target effects of CNO (MacLaren et al., 2016; Padovan- Hernandez & Knackstedt, 2018; Roth, 2016; Saloman et al., 2016). However, in this study no effects were apparent in any group other than the hM4Di + CNO group when examined as a population, and the combined effects of hM4Di+CNO appear to be distinct from those of CNO by itself. In addition, it should be noted that while we did not electrophysiologically validate the effects of CNO on hM4Di- expressing axons in RE, this experimental approach has been electrophysiologically validated in several previous in vitro and in vivo studies in other projections (Ge et al., 2017; Lichtenberg et al., 2017; Mahler et al., 2014, 2018; Malvaez et al., 2018; McGlinchey & Aston-J ones, 2018; Stachniak et al., 2014).
One factor to be considered is that all recordings were performed in anesthetized rats. Anaesthesia is known to affect the firing properties of thalamic neurons, including burst firing, in a state-dependent manner (Alkire, Hudetz, & Tononi, 2008), that is, bursting has traditionally been shown to be enhanced in states of sleep or anaesthesia (Bezdudnaya et al., 2006; Sherman & Guillery, 2001). However, the current findings are likely to be relevant to the awake state for several reasons. Thalamic burst firing has been described in sleep and wakefulness across species (Jeanmonod, Magnin, & Morel, 1996; Kim, Ohara, & Lenz, 2009; Nicolelis & Fanselow, 2002; Ramcharan et al., 2005; Swadlow & Gusev, 2001), suggesting that it could be used as an important mode of information transfer in the brain in various states of consciousness. Therefore, the circuit dynamics described here could potentially be highly relevant, as anaesthesia is likely to impact baseline activity but it should not qualitatively impact the effects of pathway activation or inhibition. In addition, higher-o rder thalamic relays, such as RE, have been shown to burst more than first- order relays, suggesting that burst firing might have an even greater functional relevance in these thalamic nuclei (Ramcharan et al., 2005; Wei, Bonjean, Petry, Sejnowski, & Bickford, 2011). Finally, the firing pattern of individual neurons recorded in higher-order thalamic relays, including RE, is highly heterogeneous across preparations (Ito et al., 2015; Kim et al., 2011; Lara- Vasquez, Espinosa, Durn, Stockle, & Fuentealba, 2016; Morales, Ramcharan, Sundararaman, Morgera, & Vertes, 2007; Ramcharan et al., 2005; Walsh et al., 2017; Zhang, Yoshida, Katz, & Lisman, 2012), and the current findings reflect this, permitting an examination of the effects of these manipulations on an RE neuron population with various baseline firing properties.
Corticothalamic input plays a key role in modulating thalamocortical neuron firing pattern (Briggs & Usrey, 2008; Crandall et al., 2015; Godwin, Vaughan, & Sherman, 1996), possibly permitting higher efficiency of information transfer and nuanced population- level coding (Behuret, Deleuze, & Bal, 2015; Mukherjee & Kaplan, 1995; Whitmire, Waiblinger, Schwarz, & Stanley, 2016; Wolfart, Debay, Le Masson, Destexhe, & Bal, 2005). The current findings demonstrate that the ilPFC can modulate multiple RE neuron burst firing parameters, including per cent of spikes fired in bursts and the mean number of spikes within a burst, both in tandem and independent via the monosynaptic ilPFC- RE projection. The number of spikes within a burst has recently been demonstrated to be a robust, independent mode of information transfer in thalamic neuron models (Elijah, Samengo, & Montemurro, 2015). In addition, the firing pattern of thalamocortical neurons can determine the manner by which these neurons engage cortical principle neurons and interneurons (Bayazitov, Westmoreland, & Zakharenko, 2013; Bruno & Sakmann, 2006; Hu & Agmon, 2016; LeBlanc et al., 2017; Swadlow & Gusev, 2001). In the case of RE in particular, the modulation of RE neuron firing by ilPFC could heavily influence how RE engages its downstream targets in the hippocampus, which include pyramidal neurons and interneurons (Dolleman- Van der Weel & Witter, 2000). Future studies examining the influence of firing pattern in RE on engagement of these circuits will be critical, given the unique circuit organization of the hippocampus compared to other cortical regions targeted by thalamic input.
We have previously demonstrated that ilPFC (Patton et al., 2013), via the ilPFC-R E- ventral subiculum circuit (Zimmerman & Grace, 2016), controls the proportion of DA neurons in the VTA that are spontaneously active (“population activity”), a critical signalling parameter in the DA system (Grace, 2016). Specifically, we showed that inhibition of the ilPFC paradoxically enhanced VTA DA neuron population activity, but concomitant inhibition of RE prevented this effect. These findings suggested that inhibition of ilPFC disinhibits RE, but the paucity of studies examining the influence of ilPFC on RE neuron firing prevented any definitive conclusions. The current work introduces the possibility that inhibition of ilPFC shifts the firing pattern of the RE neuron population from bursting to tonic firing, while stimulation of ilPFC was capable of silencing RE neuron firing. The loss of this inhibition would depolarize RE neuron membrane potential, allowing for other inputs to exert more influence on RE neuron firing. From a clinical perspective disruption of corticothalamic communication in these circuits, as has been observed in schizophrenia (Anticevic et al., 2014, 2015; Woodward & Heckers, 2016; Woodward, Karbasforoushan, & Heckers, 2012), could lead to deficits in prefrontal–hippocampal- dependent behaviours (Krol, Wimmer, Halassa, & Feng, 2018; Pratt, Morris, & Dawson, 2018; Reagh, Murray, & Yassa, 2017) and a dysregulated, hyperdopaminergic state, both of which may play a role in the disease.
ACKNOWLEDGEMENTS
We thank Elena Vazey, Kathryn Gill, Christy Smolak, Nicole MacMurdo Fabio Ferrarelli, Robert Kass, Michael Beierlein, Robert Vertes and all members of the Grace lab for their helpful advice and technical assistance.
Abbreviations:
- DA
clozapine-n-oxide
- DA
dopamine
- DREADDs
designer receptors exclusively activated by designer drugs
- ilPFC
infralimbic prefrontal cortex
- mPFC
medial prefrontal cortex
- RE
nucleus reuniens
- TRN
thalamic reticular nucleus
- VTA
ventral tegmental area
Footnotes
CONFLICT OF INTEREST
ECZ declares no competing financial interests. AAG reports the following: Johnson & Johnson, Lundbeck, Pfizer, GSK, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, Janssen, Alkermes, Newron.
DATA ACCESSIBILITY
Data will be made available upon request, please contact Anthony A. Grace.
REFERENCES
- Alkire MT, Hudetz AG, & Tononi G (2008). Consciousness and anesthesia. Science, 322, 876–880. 10.1126/science.1149213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, & Brown TH (2008). Imaging the spread of reversible brain inactivations using fluorescent muscimol. Journal of Neuroscience Methods, 171, 30–38. 10.1016/j.jneumeth.2008.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, … Glahn DC (2014). Characterizing thalamo- cortical disturbances in schizophrenia and bipolar illness. Cerebral Cortex, 24, 3116–3130. 10.1093/cercor/bht165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, … Cannon TD (2015). Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry, 72, 882–891. 10.1001/jamapsychiatry.2015.0566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GRI, & Warburton EC (2018). A critical role for the nucleus reuniens in long-t erm, but not short-term associative recogni- tion memory formation. Journal of Neuroscience, 38, 3208–3217. 10.1523/JNEUROSCI.1802-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayazitov IT, Westmoreland JJ, & Zakharenko SS (2013). Forward suppression in the auditory cortex is caused by the Cav3.1 calcium channel-m ediated switch from bursting to tonic firing at thalamocortical projections. Journal of Neuroscience, 33, 18940–18950. 10.1523/JNEUROSCI.3335-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behuret S, Deleuze C, & Bal T (2015). Corticothalamic synaptic noise as a mechanism for selective attention in thalamic neurons. Frontiers in Neural Circuits, 9, 11633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, & Connors BW (2002). Short- term dynamics of thalamocortical and intracortical synapses onto layer 6 neurons in neocortex. Journal of Neurophysiology, 88, 1924–1932. 10.1152/jn.2002.88.4.1924 [DOI] [PubMed] [Google Scholar]
- Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso JM, & Swadlow HA (2006). Thalamic burst mode and inattention in the awake LGNd. Neuron, 49, 421–432. 10.1016/j.neuron.2006.01.010 [DOI] [PubMed] [Google Scholar]
- Briggs F, & Usrey WM (2008). Emerging views of corticothalamic function. Current Opinion in Neurobiology, 18, 403–407. 10.1016/j.conb.2008.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, & Sakmann B (2006). Cortex is driven by weak but synchronously active thalamocortical synapses. Science, 312, 1622–1627. 10.1126/science.1124593 [DOI] [PubMed] [Google Scholar]
- Cassel J-C, Pereira de Vasconcelos A, Loureiro M, Cholvin T, Dalrymple-Alford JC, & Vertes RP (2013). The reuniens and rhomboid nuclei: Neuroanatomy, electrophysiological characteristics and behavioral implications. Progress in Neurobiology, 111, 34–52. 10.1016/j.pneurobio.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çavdar S, Onat FY, Çakmak YÖ, Yananli HR, Gülçebi M, & Aker R (2008). The pathways connecting the hippocampal formation, the thalamic reuniens nucleus and the thalamic reticular nucleus in the rat. Journal of Anatomy, 212, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, De Sa Nogueira D, … Cassel J-C (2013). The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. Journal of Neuroscience, 33, 8772–8783. 10.1523/JNEUROSCI.0771-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, & Phillipson OT (1990). Projections to the rostral reticular thalamic nucleus in the rat. Experimental Brain Research, 80, 157–171. [DOI] [PubMed] [Google Scholar]
- Crandall SR, Cruikshank SJ, & Connors BW (2015). A corticothalamic switch: Controlling the thalamus with dynamic synapses. Neuron, 86, 768–782. 10.1016/j.neuron.2015.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodi FG, Motamedi F, Akbari E, Ghanbarian E, & Jila B (2011). Effect of reversible inactivation of reuniens nucleus on memory processing in passive avoidance task. Behavioral Brain Research, 221, 1–6. 10.1016/j.bbr.2011.02.020 [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, & Witter MP (2000). Nucleus reuniens thalami innervates gamma aminobutyric acid positive cells in hippocampal field CA1 of the rat. Neuroscience Letters, 278, 145–148. 10.1016/S0304-3940(99)00935-0 [DOI] [PubMed] [Google Scholar]
- Duan AR, Varela C, Zhang Y, Shen Y, Xiong L, Wilson MA, & Lisman J (2015). Delta frequency optogenetic stimulation of the thalamic nucleus reuniens is sufficient to produce working memory deficits: Relevance to schizophrenia. Biological Psychiatry, 77, 1098–1107. 10.1016/j.biopsych.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elijah DH, Samengo I, & Montemurro MA (2015). Thalamic neuron models encode stimulus information by burst-s ize modulation. Frontiers in Computational Neuroscience, 9, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge F, Wang N, Cui C, Li Y, Liu Y, Ma Y, … Sun X (2017). Glutamatergic projections from the entorhinal cortex to dorsal dentate gyrus mediate context-i nduced reinstatement of heroin seeking. Neuropsychopharmacology, 42, 1860–1870. 10.1038/npp.2017.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin DW, Vaughan JW, & Sherman SM (1996). Metabotropic glutamate receptors switch visual response mode of lateral geniculate nucleus cells from burst to tonic. Journal of Neurophysiology, 76, 1800–1816. 10.1152/jn.1996.76.3.1800 [DOI] [PubMed] [Google Scholar]
- Grace AA (2016). Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nature Reviews Neuroscience, 17, 524–532. 10.1038/nrn.2016.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W, Lu SM, & Sherman SM (1992). Relative contributions of burst and tonic responses to the receptive field properties of lateral geniculate neurons in the cat. Journal of Neurophysiology, 68, 2199–2211. 10.1152/jn.1992.68.6.2199 [DOI] [PubMed] [Google Scholar]
- Guillery RW, & Sherman SM (2002). Thalamic relay functions and their role in corticocortical communication. Neuron, 33, 163–175. 10.1016/S0896-6273(01)00582-7 [DOI] [PubMed] [Google Scholar]
- Halassa MM, & Acsády L (2016). Thalamic inhibition: Diverse sources, diverse scales. Trends in Neurosciences, 39, 680–693. 10.1016/j.tins.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, Zikopoulos B, … Wilson MA (2014). State-d ependent architecture of thalamic reticular subnetworks. Cell, 158, 808–821. 10.1016/j.cell.2014.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, & Moore CI (2011). Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nature Neuroscience, 14, 1118–1120. 10.1038/nn.2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Wang A, Shaw CL, & Griffin AL (2013). Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working- memory dependent tactile- visual conditional discrimination task. Behavioral Neuroscience, 127, 860–866. 10.1037/a0034653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, & Agmon A (2016). Differential excitation of distally versus proximally targeting cortical interneurons by unitary thalamocortical bursts. Journal of Neuroscience, 36, 6906–6916. 10.1523/JNEUROSCI.0739-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Zhang S-J, Witter MP, Moser EI, & Moser M-B (2015). A prefrontal- thalamo- hippocampal circuit for goal- directed spatial navigation. Nature, 522, 50–55. 10.1038/nature14396 [DOI] [PubMed] [Google Scholar]
- Jankowski MM, Islam MN, Wright NF, Vann SD, Erichsen JT, Aggleton JP, & O’Mara SM (2014). Nucleus reuniens of the thalamus contains head direction cells. eLife Sciences, 3, 1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanmonod D, Magnin M, & Morel A (1996). Low- threshold calcium spike bursts in the human thalamus. Common physiopathology for sensory, motor and limbic positive symptoms. Brain, 119(Pt 2), 363–375. 10.1093/brain/119.2.363 [DOI] [PubMed] [Google Scholar]
- Kafetzopoulos V, Kokras N, Sotiropoulos I, Oliveira JF, LeiteAlmeida H, Vasalou A, … Dalla C (2017). The nucleus reuniens: A key node in the neurocircuitry of stress and depression. Molecular Psychiatry, 21, 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Ohara S, & Lenz FA (2009). Mental arithmetic leads to multiple discrete changes from baseline in the firing patterns of human thalamic neurons. Journal of Neurophysiology, 101, 2107–2119. 10.1152/jn.91087.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Woo J, Park Y-G, Chae S, Jo S, Choi JW, … Kim D (2011). Thalamic T-type Ca2+ channels mediate frontal lobe dysfunctions caused by a hypoxia- like damage in the prefrontal cortex. Journal of Neuroscience, 31, 4063–4073. 10.1523/JNEUROSCI.4493-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmac CI, & Mitrofanis J (1997). Organisation of the reticular thalamic projection to the intralaminar and midline nuclei in rats. The Journal of Comparative Neurology, 377, 165–178. 10.1002/(ISSN)1096-9861 [DOI] [PubMed] [Google Scholar]
- Krol A, Wimmer RD, Halassa MM, & Feng G (2018). Thalamic reticular dysfunction as a circuit endophenotype in neurodevelopmental disorders. Neuron, 98, 282–295. 10.1016/j.neuron.2018.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Vásquez A, Espinosa N, Durán E, Stockle M, & Fuentealba P (2016). Midline thalamic neurons are differentially engaged during hippocampus network oscillations. Scientific Reports, 6, 29807 10.1038/srep29807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Vasquez A, Espinosa N, Durn E, Stockle M, & Fuentealba P (2016). Midline thalamic neurons are differentially engaged during hippocampus network oscillations. Scientific Reports, 6, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layfield DM, Patel M, Hallock H, & Griffin AL (2015). Inactivation of the nucleus reuniens/rhomboid causes a delay-d ependent impairment of spatial working memory. Neurobiology of Learning and Memory, 125, 163–167. 10.1016/j.nlm.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc BW, Cross B, Smith KA, Roach C, Xia J, Chao Y-C, … Saab CY (2017). Thalamic bursts down-r egulate cortical theta and nociceptive behavior. Scientific Reports, 7, 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg NT, Pennington ZT, Holley SM, Greenfield VY, Cepeda C, Levine MS, & Wassum KM (2017). Basolateral amygdala to orbitofrontal cortex projections enable cue- triggered reward expectations. Journal of Neuroscience, 37, 8374–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley SB, Gallo MM, & Vertes RP (2016). Lesions of the ventral midline thalamus produce deficits in reversal learning and attention on an odor texture set shifting task. Brain Research, 1649, 110–122. 10.1016/j.brainres.2016.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, & Grace AA (2007). Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. Journal of Neuroscience, 27, 11424–11430. 10.1523/JNEUROSCI.2847-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Guido W, & Sherman SM (1992). Effects of membrane voltage on receptive field properties of lateral geniculate neurons in the cat: Contributions of the low-t hreshold Ca2 + conductance. Journal of Neurophysiology, 68, 2185–2198. 10.1152/jn.1992.68.6.2185 [DOI] [PubMed] [Google Scholar]
- MacLaren DAA, Browne RW, Shaw JK, Radhakrishnan SK, Khare P, España RA, & Clark SD (2016). lozapine N-o xide administration produces behavioral effects in long–evans rats: Implications for designing DREADD experiments. eNeuro, 3, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Brodnik ZD, Cox BM, Buchta WC, Bentzley BS, Cope ZA, … Aston-Jones G (2018). Chemogenetic manipulations of ventral tegmental area dopamine neurons reveal multifaceted roles in cocaine abuse. bioRxiv, 246595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, … Aston-Jones G (2014). Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nature Neuroscience, 17, 577–585. 10.1038/nn.3664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisson DJN, Gemzik ZM, & Griffin AL (2018). Optogenetic suppression of the Nucleus Reuniens selectively impairs encoding during spatial working memory - PubMed - NCBI. Neurobiology of Learning and Memory, 155, 78–85. 10.1016/j.nlm.2018.06.010 [DOI] [PubMed] [Google Scholar]
- Malvaez M, Shieh C, Murphy MD, Greenfield VY, & Wassum KM (2018). Distinct cortical- amygdala projections drive reward value encoding and retrieval. bioRxiv, 299958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EM, & Aston-Jones G (2018). Dorsal hippocampus drives context- induced cocaine seeking via inputs to lateral septum. Neuropsychopharmacology, 43, 987–1000. 10.1038/npp.2017.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, & Vertes RP (2004). Afferent projections to nucleus reuniens of the thalamus. The Journal of Comparative Neurology, 480, 115–142. 10.1002/(ISSN)1096-9861 [DOI] [PubMed] [Google Scholar]
- Mitchell AS (2015). The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision- making. Neuroscience & Biobehavioral Reviews, 54, 76–88. 10.1016/j.neubiorev.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Morales GJ, Ramcharan EJ, Sundararaman N, Morgera SD, & Vertes RP (2007). Analysis of the actions of nucleus reuniens and the entorhinal cortex on EEG and evoked population behavior of the hippocampus. Conference Proceedings IEEE Engineering in Medicine and Biology Society, 2007, 2480–2484. [DOI] [PubMed] [Google Scholar]
- Moreines JL, Owrutsky ZL, & Grace AA (2017). Involvement of infralimbic prefrontal cortex but not lateral habenula in dopamine attenuation after chronic mild stress. Neuropsychopharmacology, 42, 904–913. 10.1038/npp.2016.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, & Kaplan E (1995). Dynamics of neurons in the cat lateral geniculate nucleus: In vivo electrophysiology and computational modeling. Journal of Neurophysiology, 74, 1222–1243. 10.1152/jn.1995.74.3.1222 [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, & Fanselow EE (2002). Dynamic shifting in thalamocortical processing during different behavioural states. Philosophical Transactions of the Royal Society B, 357, 1753–1758. 10.1098/rstb.2002.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovan-Hernandez Y, & Knackstedt LA (2018). Dose- dependent reduction in cocaine-i nduced locomotion by Clozapine-N- Oxide in rats with a history of cocaine self- administration - PubMed - NCBI. Neuroscience Letters, 674, 132–135. 10.1016/j.neulet.2018.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton MH, Bizup BT, & Grace AA (2013). The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. Journal of Neuroscience, 33, 16865–16873. 10.1523/JNEUROSCI.2449-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (2013). The rat brain in stereotaxic coordinates (7th ed.). New York, NY: Academic Press. [Google Scholar]
- Paz JT, Bryant AS, Peng K, Fenno L, Yizhar O, Frankel WN, … Huguenard JR (2011). A new mode of corticothalamic transmission revealed in the Gria4(- /- ) model of absence epilepsy. Nature Neuroscience, 14, 1167–1173. 10.1038/nn.2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad JA, Abela AR, & Chudasama Y (2017). Midline thalamic reuniens lesions improve executive behaviors. Neuroscience, 345, 77–88. 10.1016/j.neuroscience.2016.01.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad JA, Macgregor EM, & Chudasama Y (2013). Lesions of the thalamic reuniens cause impulsive but not compulsive responses. Brain Structure and Function, 218, 85–96. 10.1007/s00429-012-0378-5 [DOI] [PubMed] [Google Scholar]
- Pratt JA, Morris B, & Dawson N (2018). Deconstructing schizophrenia: Advances in preclinical models for biomarker identification. Behavioral Neurobiology of Schizophrenia and Its Treatment, Current Topics in Behavioral Neurosciences, 8, 1–29. [DOI] [PubMed] [Google Scholar]
- Ramanathan KR, Jin J, Ressler RL, & Maren S (2018). Nucleus reuniens is required for encoding and retrieving precise contextual fear memories in rats. bioRxiv, 340190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, & Sherman SM (2005). Higher-o rder thalamic relays burst more than first-o rder relays. Proceedings of the National Academy of Sciences of the United States of America, 102, 12236–12241. 10.1073/pnas.0502843102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel MJ, Jr, Baldo MVC, & Canteras NS (2018). Influence of the anteromedial thalamus on social defeat-a ssociated contextual fear memory. Behavioral Brain Research, 339, 269–277. 10.1016/j.bbr.2017.10.038 [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Murray EA, & Yassa MA (2017). Repetition reveals ups and downs of hippocampal, thalamic, and neocortical engagement during mnemonic decisions. Hippocampus, 27, 169–183. 10.1002/hipo.22681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL (2016). DREADDs for neuroscientists. Neuron, 89, 683–694. 10.1016/j.neuron.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, & Kastner S (2012). The pulvinar regulates information transmission between cortical areas based on attention demands. Science, 337, 753–756. 10.1126/science.1223082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salay LD, Ishiko N, & Huberman AD (2018). A midline thalamic circuit determines reactions to visual threat. Nature, 557, 183–189. 10.1038/s41586-018-0078-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloman JL, Scheff NN, Snyder LM, Ross SE, Davis BM, & Gold MS (2016). Gi- DREADD expression in peripheral nerves produces ligand- dependent analgesia, as well as ligand-i ndependent functional changes in sensory neurons. Journal of Neuroscience, 36, 10769–10781. 10.1523/JNEUROSCI.3480-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, & Bunney BS (1989). Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. The Journal of Comparative Neurology, 290, 213–242. 10.1002/(ISSN)1096-9861 [DOI] [PubMed] [Google Scholar]
- Sherman SM (1996). Dual response modes in lateral geniculate neurons: Mechanisms and functions. Visual Neuroscience, 13, 205–213. 10.1017/S0952523800007446 [DOI] [PubMed] [Google Scholar]
- Sherman SM (2001). Tonic and burst firing: Dual modes of thalamocortical relay. Trends in Neurosciences, 24, 122–126. 10.1016/S0166-2236(00)01714-8 [DOI] [PubMed] [Google Scholar]
- Sherman SM (2016). Thalamus plays a central role in ongoing cortical functioning. Nature Neuroscience, 16, 533–541. 10.1038/nn.4269 [DOI] [PubMed] [Google Scholar]
- Sherman SM, & Guillery RW (2001). Exploring the thalamus. Boston, MA: MIT Press. [Google Scholar]
- Sierra RO, Pedraza LK, Zanona QK, Santana F, Boos FZ, Crestani AP, … Quillfeldt JA (2017). Reconsolidation- induced rescue of a remote fear memory blocked by an early cortical inhibition: Involvement of the anterior cingulate cortex and the mediation by the thalamic nucleus reuniens. Hippocampus, 27, 596–607. 10.1002/hipo.22715 [DOI] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, & Sternson SM (2014). Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron, 82, 797–808. 10.1016/j.neuron.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y-G, Wu C-S, Renger JJ, Uebele VN, Lu H-C, & Beierlein M (2012). GABAergic synaptic transmission triggers action potentials in thalamic reticular nucleus neurons. Journal of Neuroscience, 32, 7782–7790. 10.1523/JNEUROSCI.0839-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, & Gusev AG (2001). The impact of “bursting” thalamic impulses at a neocortical synapse. Nature Neuroscience, 4, 402–408. 10.1038/86054 [DOI] [PubMed] [Google Scholar]
- Swadlow HA, & Weyand TG (1987). Corticogeniculate neurons, corticotectal neurons, and suspected interneurons in visual cortex of awake rabbits: Receptive-f ield properties, axonal properties, and effects of EEG arousal. Journal of Neurophysiology, 57, 977–1001. 10.1152/jn.1987.57.4.977 [DOI] [PubMed] [Google Scholar]
- Troyner F, Bicca MA, & Bertoglio LJ (2018). Nucleus reuniens of the thalamus controls fear memory intensity, specificity and long- term maintenance during consolidation- PubMed - NCBI. Hippocampus, 28, 602–616. [DOI] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, & Grace AA (2011). Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. Journal of Neuroscience, 31, 4280–4289. 10.1523/JNEUROSCI.5310-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Kumar S, Yang JY, & Wilson MA (2014). Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Structure and Function, 219, 911–929. 10.1007/s00429-013-0543-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2003). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse (New York, N. Y.), 51, 32–58. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, & Hoover WB (2015). Limbic circuitry of the midline thalamus. Neuroscience & Biobehavioral Reviews, 54, 89–107. 10.1016/j.neubiorev.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetere G, Kenney JW, Tran LM, Xia F, Steadman PE, Parkinson J, … Frankland PW (2017). Chemogenetic Interrogation of a Brain- wide Fear Memory Network in Mice. Neuron, 94, 363–374 e364. 10.1016/j.neuron.2017.03.037 [DOI] [PubMed] [Google Scholar]
- Viena TD, Linley SB, & Vertes RP (2018). Inactivation of nucleus reuniens impairs spatial working memory and behavioral flexibility in the rat. Hippocampus, 28, 297–311. 10.1002/hipo.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DA, Brown JT, & Randall AD (2017). In vitro characterization of cell- level neurophysiological diversity in the rostral nucleus reuniens of adult mice. The Journal of Physiology, 595, 3549–3572. 10.1113/JP273915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Bonjean M, Petry HM, Sejnowski TJ, & Bickford ME (2011). Thalamic burst firing propensity: A comparison of the dorsal lateral geniculate and pulvinar nuclei in the tree shrew. Journal of Neuroscience, 31, 17287–17299. 10.1523/JNEUROSCI.6431-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire CJ, Waiblinger C, Schwarz C, & Stanley GB (2016). Information coding through adaptive gating of synchronized thalamic bursting. Cell Reports, 14, 795–807. 10.1016/j.celrep.2015.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt JL, Masri R, Pulimood NS, & Keller A (2013). Pathological activity in mediodorsal thalamus of rats with spinal cord injury pain. Journal of Neuroscience, 33, 3915–3926. 10.1523/JNEUROSCI.2639-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J, Debay D, Le Masson G, Destexhe A, & Bal T (2005). Synaptic background activity controls spike transfer from thalamus to cortex. Nature Neuroscience, 8, 1760–1767. 10.1038/nn1591 [DOI] [PubMed] [Google Scholar]
- Woodward ND, & Heckers S (2016). Mapping Thalamocortical Functional Connectivity in Chronic and Early Stages of Psychotic Disorders. Biological Psychiatry, 79, 1016–1025. 10.1016/j.biopsych.2015.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, & Heckers S (2012). Thalamocortical dysconnectivity in schizophrenia. American Journal of Psychiatry, 169, 1092–1099. 10.1176/appi.ajp.2012.12010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, & Südhof TC (2013). A neural circuit for memory specificity and generalization. Science, 339, 1290–1295. 10.1126/science.1229534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yoshida T, Katz DB, & Lisman JE (2012). NMDAR antagonist action in thalamus imposes δ oscillations on the hippocampus. Journal of Neurophysiology, 107, 3181–3189. 10.1152/jn.00072.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman EC, & Grace AA (2016). The nucleus reuniens of the midline thalamus gates prefrontal- hippocampal modulation of ventral tegmental area dopamine neuron activity. Journal of Neuroscience, 36, 8977–8984. 10.1523/JNEUROSCI.1402-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]