Abstract
Orthopedic tissues respond to mechanical loads to maintain normal homeostasis and in response to injury. As the body of work on this continues to grow, it is important to synthesize the recent studies across tissues and specialties with one another and with past studies. Hence, this review highlights the knowledge gained since 2000, with only few exceptions, concerning the effects of mechanical load and biologics on remodeling and repair of orthopedic tissue.
This review is separated into 4 sections: tendon and ligament, meniscus, cartilage, and bone. Each section begins with a brief anatomical description followed by discussions of remodeling and repair and concludes with a concise presentation of information regarding repair enhancement through biologics. In addition to summarizing recent work, this review provides insights for future directions and, through the combined discussion of mechanics and biologics, opportunities for translation to clinical use. This is Part I, which will discuss 1) tendon and ligament and 2) meniscus. Look for Part II (on cartilage and bone) in the March 2011 issue.
“…the extrafibrillar matrix …allows relative sliding of collagen bundles and carries blood vessels and nerves throughout the tissue.1 ”
Tendon and Ligament
Anatomy
Tendons and ligaments are hierarchical structures primarily composed of collagen I molecules bound together into fibrils. Fibrils generally are organized in parallel bundles, or fibers, which are grouped into fascicles that are the macroscopic subunits of the tissue. Water, cells, proteoglycans, and other glycoproteins along with loose connective tissue make up the extrafibrillar matrix that allows relative sliding of collagen bundles and carries blood vessels and nerves throughout the tissue.1 While it is believed that the cells sparsely interspersed within the collagen fibers sense tissue strains and mediate tissue remodeling, the transfer of strain from the macroscopic to microscopic level is only partially understood.2 In addition, the structure, composition, and properties of both tendons and ligaments vary along their lengths (most notably at their bony insertions), across anatomical sites, and between each other.1
Remodeling
Research before the 21st century indicates that, like other orthopedic tissues, tendons and ligaments positively respond to moderate loading or activity levels, whereas disuse/immobilization or overloading tends to lead to pathology and deteriorated properties.3 Recent work generally supports these findings with more sophisticated measurement tools. For example, advances in imaging techniques and microdialysis have enabled interesting in vivo human studies of tendon remodeling. Multiple studies show that blood flow is significantly increased to peritendinous tissue during and immediately following exercise in order to satisfy increases in local oxygen consumption.4 These changes are associated with further metabolic activity5, including increased intratendinous glucose uptake during exercise6 (Figure 1) as well as increased collagen synthesis7 and proteolytic activity for extended periods following exercise. Taken together, these data suggest that loading of tendon elevates tenocyte metabolism, potentially activating tissue remodeling. In fact, numerous in vivo human studies using ultrasound and electromyography suggest that the end-effect may be increased tendon cross-sectional area, which may translate to increases in tendon stiffness.8 Additional experiments demonstrate that improvements in tendon modulus are possible, especially in counteracting declines due to age9 and microgravity.10 Still, improvements in mechanical properties are not consistently observed, suggesting that the optimal loading parameters remain unknown.
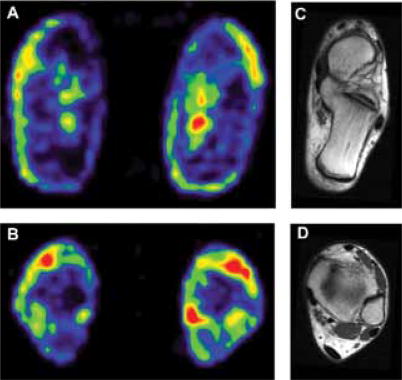
Figure 1.
Positron emission tomography scans of the lower leg at the level of the Achilles tendon insertion into the calcaneus (A) and more proximally (B) demonstrate increased uptake of [18F]-2-fluoro-2-deoxy-D-glucose in the exercised (right images) versus resting leg (left images) immediately following voluntary plantar flexor contractions. Corresponding magnetic resonance imaging scans show locations of (C) the tendon insertion site (dark crescent shape at end of calcaneus) and (D) the tendon proper (dark oval at bottom).6 Reprinted with permission from American Physiological Society.
In addition to human data, animal studies have continued to refine our understanding of the underlying mechanisms of mechanically induced remodeling. Excessive loading of rotator cuff tendons in the form of overuse can lead to histologic and mechanical degenerative changes,11 and these findings have subsequently been correlated with increased expression of cartilaginous markers12; insulinlike growth factor 1 (IGF-1);13 nitric oxide synthase;14 and angiogenic, inflammatory, and apoptotic factors15,16 (Figure 2). Other in vivo studies have similarly implicated load with decreased material properties and formation of microdamage17,18 along with upregulation of factors involved in angiogenesis, remodeling,19 pain, and inflammation.20 These data are supported by in vitro studies showing accumulation of microtears from subfailure cyclic loading21 as well as stretch-induced expression changes in myriad growth factors; inflammatory, pain, and apoptotic mediators; and proteases.22–25 These observations match changes clinically associated with tendinopathy,26,27 thereby supporting theories of an overload etiology.
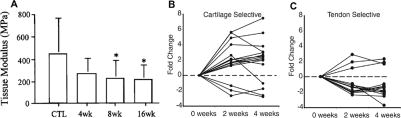
Figure 2.
Effects of overuse activity on the supraspinatus tendon in a rat model. Decreases in tendon modulus (A) and changes in expression of 17 cartilage-specific genes (B) and 12 tendon-specific genes (C) in overuse animals compared to controls indicate tendon weakening and potential metaplasia of the supraspinatus into a more fibrocartilaginous phenotype with overuse.11,12 Reprinted with permission from Elsevier and John Wiley and Sons.
In contrast, there also is evidence that disuse can lead to pathologic degeneration.28 Immobilization and stress deprivation lead to mechanical deficiencies in the tendon proper and its insertion sites.29,30 Similar observations made in vitro31 seem to be mediated by a rapid and sustained increase in matrix metalloproteinase 3 (MMP-3) and MMP-13 activity with unloading.32 MMPs are a broad family of proteases capable of digesting various extracellular proteins, including collagen, with varying efficiencies and are counterbalanced by tissue inhibitors of metalloproteinases (TIMPs).33 Gene analysis and protein quantification studies show that MMPs and TIMPs are clearly regulated by mechanical loading, but their coordinate functions during tendon remodeling are complex and remain to be elucidated.34 Nevertheless, tendon unloading seems to consistently upregulate MMP expression as well as decrease TIMP/MMP ratios, suggesting an overall catabolic tissue environment.35 These findings, together with the overuse data, suggest that both excessive and diminished load levels lead to deleterious effects on tendons and ligaments and that an optimum load range exists to maintain healthy tissue.
The structural changes underlying the altered mechanical properties seen with tendon remodeling in response to load are still unknown. Neither changes in total collagen content3,31 nor fibril diameters36 conclusively correlate with observed mechanical differences, indicating that more sensitive assays of tendon hierarchical composition are required to determine the structure–function relationships involved in tissue remodeling.
While most attention has been given to tension, compressive loading has significant effects on tendons and ligaments as well. This is most evident in flexor tendons and tissues that wrap around bony structures, creating compressive regions of fibrocartilagenous composition. In fact, removal of these compressive loads through tendon translocation leads to drastic tissue remodeling.37 Finally, there is growing evidence that load-induced fluid flow in tendon38 also may contribute to collagen fiber alignment and tissue remodeling.39
Repair
The effect of load on healing varies significantly with tissue anatomy (eg, intrasynovial vs extrasynovial), injury site (eg, midsubstance vs insertion), and the particular healing phases during which the load is applied. In animal studies, structural properties are decreased after healing with immobilization or intramuscular botulinum toxin injection in the Achilles and supraspinatus tendons.40,41 Additionally, material and structural properties of healing ligament are worse with hindlimb unloading, which may be the result of diminished remodeling.42,43 In contrast to these findings, immobilization produces improved collagen organization and mechanical properties over cage activity and exercise in the rotator cuff insertion site.44 However, insertional repair on flexor digitorum profundus tendons exhibited improved structural and material properties with passive stretching45 versus immobilization.
Results are similarly confusing when considering the effects of elevated loads. Exercise following immobilized repair of the supraspinatus resulted in inferior mechanics,46 whereas in flexor tendons, which have a long history of improved repair with loading, increasing the level of force had no effect on mechanical properties of the repair.47 Furthermore, possible excessive loading of the healing medial collateral ligament due to a combined medial collateral/anterior cruciate ligament reconstruction resulted in inferior repair tissue.48 Still, mechanical load seems to provide a protective role against collagen degradation,49 possibly enabling more productive remodeling through preferential elimination of unstressed fibers.50 In the case of cyclic loading, frequency may be important, as it is hypothesized to be the mechanism underlying the benefits of eccentric loading in physical therapy.51
In summary, as also seen in remodeling of normal tissue, there is likely a “U”-shaped relationship between healing and mechanical loading. That is, both too little load and too much load appear to be detrimental to tendon healing, suggesting some moderate load will lead to optimal repair. However, the dynamics of the healing process and the significant differences between tissues and regions of even the same tissue seem to limit generalization of results and focus interpretations to the specific tissue being tested.
Biologic Enhancement
Repair enhancements have focused roughly on exogenous application of stem cells52 and the effects of various biochemicals, such as cytokines, growth factors, and proteases.53,54 There have been a small number of investigations into the interaction of these mediators with mechanical loading during healing. Insulinlike growth factor and growth hormone (GH) delivered systemically improved mechanical properties and collagen synthesis for both cage activity and hindlimb unloaded animals, with nearly full compensation of the deficits of unloading.55 Growth hormone increased collagen I synthesis in human tendon, and its effects were unaffected by moderate exercise,56 although in rats, GH treatment alone did not translate into improved repair properties.55 Furthermore, platelet-rich plasma has been used recently for repair augmentation,57 though fundamental data on its role remain limited. In rat Achilles tendon, mechanical loading has been shown to be necessary for the realization of long-term benefits of platelet-rich plasma injection.58
“…mechanical load seems to provide a protective role against collagen degradation,49 possibly enabling more productive remodeling through preferential elimination of unstressed fibers.50 ”
Interesting theories regarding the role of neuronal activity during healing also have been developed in the past decade. Evidence of nerve ingrowth into tendon fascicles during inflammatory and proliferative stages of healing along with increases in substance P (SP), calcitonin gene-related peptide (CGRP), vasoactive intestinal peptide (VIP), and neuropeptide Y (NPY) have indicated the active role of nerves in healing.59 In addition, SP, NPY, and VIP have been administered to healing ligaments, resulting in improved repair strength and collagen organization.60,61 The mechanisms for these improvements are likely due to the proliferative effect of these neuropeptides on fibroblasts and capillaries60 as opposed to paradoxical inhibition of healing-associated growth factors or matrix synthesis.62 In relation to mechanical loading, the detrimental effects of immobilization have been associated with decreased expression of SP and CGRP receptors.63
Meniscus
Anatomy
The meniscus is a highly heterogeneous tissue, likely as a result of adaptations to its unique functional demands. The fibrous outer portion is composed primarily of circumferentially aligned type I collagen fibers that resist tensile loads, while the cartilaginous inner portion has more type II collagen and aggregating proteoglycans to support compression. Meniscal cells also vary significantly with abundant fibroblast-like cells near the periphery and less numerous chondrocyte-like cells in the deeper sections.64 As a result of these anatomical and biological heterogeneities, predicted cellular strains due to tissue-level deformations and gene expression are highly region dependent.65,66
Remodeling
The primary in vivo investigations of load-induced remodeling of meniscal tissue, not published during the past decade, focus on immobilization and report detrimental effects,67,68 whereas the predominance of contemporary papers examine meniscal tissue explants and isolated cells. Studies over a range of dynamic loads on tissue explants depict contradictory results regarding protein synthesis, with observations of increases,69 mixed or no changes,70 or decreases71 in extracellular matrix (ECM) synthesis. There is some evidence that complete unloading may lead to catabolic tissue activity,72 but these data also are inconsistent.73 Nevertheless, there is general consensus that supraphysiologic cyclic loading (20% strain or 0.1 MPa74) may induce catabolism through upregulation of proteases modulated by interleukin 1 (IL-1), which is, in turn, dependent on nitric oxide production through nitric oxide synthase.70,73,75
Cell-based studies provide a different story. Whether tensile stretch stimulates similar increases in proinflammatory gene expression76 or, conversely, a strong inhibitory effect on IL-1–modulated genes77 is unclear. In addition, application of elevated hydrostatic pressures results in increased anabolic rather than catabolic activity.78 One reason for this discrepancy with tissue-level experiments may be that in vivo microscopic cellular strains are possibly significantly larger than corresponding macroscopic tissue strains,79 therefore suggesting that the strains used in the aforementioned cellular studies are not consistent with the strains applied to tissue explants.
“ …the detrimental effects of immobilization have been associated with decreased expression of substance P and calcitonin gene-related peptide.63”
Repair
While injuries occurring in the more vascular periphery heal well, those in the inner region do not.64 Possibly owing to the extremely poor repair potential of the inner meniscus regardless of mechanical stimulation,80 there have been few investigations of the interplay between load and meniscal healing. Still, the few animal models that exist indicate that mobilization, as compared with immobilization, reduces glycosaminoglycan degradation and expression of proinflammatory mediators81 while increasing blood flow within the meniscus.82
Biologic Enhancement
Possibilities for improving the intrinsic healing response of the meniscus include introduction of engineered tissues83 and manipulation of the vast number of growth factors that have been shown to affect meniscal cells.84 Most studies of integrative repair have used in vitro models85 and demonstrated that inhibiting IL-1, tumor necrosis factor α (TNF-α), or MMPs can enhance healing and integration86 (Figure 3). Despite evidence that this also may be accomplished through mechanical loading,72 no study has directly investigated this effect on repair. In terms of combined biologic and mechanical enhancement of repair, static hydrostatic pressure on meniscal cell-seeded scaffolds has a synergistic anabolic effect with transforming growth factor β1 (TGF-β1),87 yet static compression of tissue explants seems to counteract any benefits of TGF-β1, IGF-1, platelet-derived growth factor, or basic fibroblast growth factor application.88
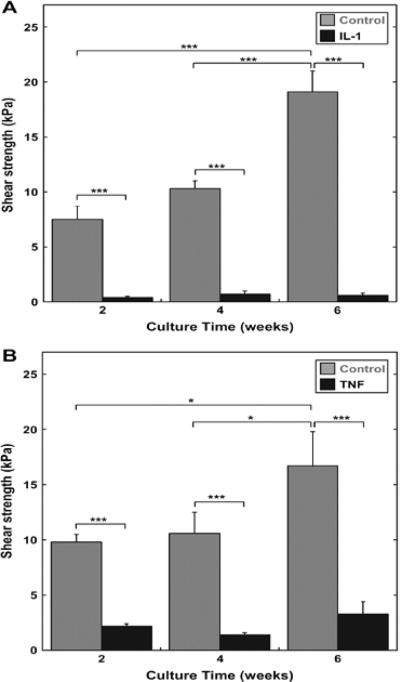
Figure 3.
Potential biologic targets for improved meniscal healing. Meniscal explants cultured with 10 ng/mL interleukin 1 (A) or with 10 ng/mL tumor necrosis factor α (B) show severely reduced push-out strength in an in vitro integrative repair model.86 Reprinted with permission from Elsevier.
Footnotes
Authors’ Disclosure Statement
The authors report no actual or potential conflict of interest in relation to this article.
Contributor Information
Spencer E. Szczesny, McKay Orthopaedic Research Laboratory, Department of Orthopaedic Surgery, and Bioengineering Department, School of Engineering and Applied Science, University of Pennsylvania, Philadelphia, Pennsylvania.
Chang Soo Lee, McKay Orthopaedic Research Laboratory, Department of Orthopaedic Surgery, University of Pennsylvania, Philadelphia, Pennsylvania, and Assistant Professor, Department of Orthopaedic Surgery, Inje University Ilsanpaik Hospital, Goyang-si, Gyeonggi-do, Korea.
Louis J. Soslowsky, McKay Orthopaedic Research Laboratory, Department of Orthopaedic Surgery, and Professor of Bioengineering, Bioengineering Department, School of Engineering and Applied Science, University of Pennsylvania, Philadelphia, Pennsylvania.
References
- 1.Jozsa LG, Kannus P. Human Tendons: Anatomy, Physiology, and Pathology. Champaign, IL: Human Kinetics; 1997. [Google Scholar]
- 2.Wall ME, Weinhold PS, Siu T, Brown TD, Banes AJ. Comparison of cellular strain with applied substrate strain in vitro. J Biomech. 2007;40(1):173–181. doi: 10.1016/j.jbiomech.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan CI, Marsh RL. Effects of exercise on the biomechanical, biochemical and structural properties of tendons. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(4):1101–1107. doi: 10.1016/s1095-6433(02)00139-3. [DOI] [PubMed] [Google Scholar]
- 4.Kubo K, Ikebukuro T, Tsunoda N, Kanehisa H. Changes in oxygen consumption of human muscle and tendon following repeat muscle contractions. Eur J Appl Physiol. 2008;104(5):859–866. doi: 10.1007/s00421-008-0841-4. [DOI] [PubMed] [Google Scholar]
- 5.Kjaer M, Langberg H, Miller BF, et al. Metabolic activity and collagen turnover in human tendon in response to physical activity. J Musculoskelet Neuronal Interact. 2005;5(1):41–52. [PubMed] [Google Scholar]
- 6.Bojsen-Moller J, Kalliokoski KK, Seppanen M, Kjaer M, Magnusson SP. Low-intensity tensile loading increases intratendinous glucose uptake in the Achilles tendon. J Appl Physiol. 2006;101(1):196–201. doi: 10.1152/japplphysiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- 7.Langberg H, Ellingsgaard H, Madsen T, et al. Eccentric rehabilitation exercise increases peritendinous type I collagen synthesis in humans with Achilles tendinosis. Scand J Med Sci Sports. 2007;17(1):61–66. doi: 10.1111/j.1600-0838.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 8.Kubo K, Ikebukuro T, Yata H, Tsunoda N, Kanehisa H. Time course of changes in muscle and tendon properties during strength training and detraining. J Strength Cond Res. 2010;24(2):322–331. doi: 10.1519/JSC.0b013e3181c865e2. [DOI] [PubMed] [Google Scholar]
- 9.Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol. 2003;548(pt 3):971–981. doi: 10.1113/jphysiol.2002.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves ND, Maganaris CN, Ferretti G, Narici MV. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J Appl Physiol. 2005;98(6):2278–2286. doi: 10.1152/japplphysiol.01266.2004. [DOI] [PubMed] [Google Scholar]
- 11.Soslowsky LJ, Thomopoulos S, Tun S, et al. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9(2):79–84. [PubMed] [Google Scholar]
- 12.Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ. Rat supraspinatus tendon expresses cartilage markers with overuse. J Orthop Res. 2007;25(5):617–624. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- 13.Scott A, Cook JL, Hart DA, Walker DC, Duronio V, Khan KM. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007;56(3):871–881. doi: 10.1002/art.22426. [DOI] [PubMed] [Google Scholar]
- 14.Szomor ZL, Appleyard RC, Murrell GA. Overexpression of nitric oxide synthases in tendon overuse. J Orthop Res. 2006;24(1):80–86. doi: 10.1002/jor.20009. [DOI] [PubMed] [Google Scholar]
- 15.Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GA. Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Joint Surg Br. 2009;91(3):417–424. doi: 10.1302/0301-620X.91B3.21652. [DOI] [PubMed] [Google Scholar]
- 16.Perry SM, McIlhenny SE, Hoffman MC, Soslowsky LJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J Shoulder Elbow Surg. 2005;14(1 suppl S):79S–83S. doi: 10.1016/j.jse.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Fung DT, Wang VM, Andarawis-Puri N, et al. Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech. 2010;43(2):274–279. doi: 10.1016/j.jbiomech.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakama LH, King KB, Abrahamsson S, Rempel DM. Effect of repetition rate on the formation of microtears in tendon in an in vivo cyclical loading model. J Orthop Res. 2007;25(9):1176–1184. doi: 10.1002/jor.20408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakama LH, King KB, Abrahamsson S, Rempel DM. VEGF, VEGFR-1, and CTGF cell densities in tendon are increased with cyclical loading: an in vivo tendinopathy model. J Orthop Res. 2006;24(3):393–400. doi: 10.1002/jor.20053. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Wang JH. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J Orthop Res. 2010;28(2):198–203. doi: 10.1002/jor.20962. [DOI] [PubMed] [Google Scholar]
- 21.Fung DT, Wang VM, Laudier DM, et al. Subrupture tendon fatigue damage. J Orthop Res. 2009;27(2):264–273. doi: 10.1002/jor.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. Eur J Appl Physiol. 2001;86(1):48–52. doi: 10.1007/s004210100502. [DOI] [PubMed] [Google Scholar]
- 23.Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching of human patellar tendon fibroblasts: activation of JNK and modulation of apoptosis. Knee Surg Sports Traumatol Arthrosc. 2003;11(2):122–129. doi: 10.1007/s00167-002-0322-y. [DOI] [PubMed] [Google Scholar]
- 24.Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166–172. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou D, Lee HS, Villarreal F, et al. Differential MMP-2 activity of ligament cells under mechanical stretch injury: an in vitro study on human ACL and MCL fibroblasts. J Orthop Res. 2005;23(4):949–957. doi: 10.1016/j.orthres.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Fenwick SA, Hazleman BL, Riley GP. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002;4(4):252–260. doi: 10.1186/ar416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop. 2003;(415):111–120. doi: 10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed] [Google Scholar]
- 28.Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007;88(4):217–226. doi: 10.1111/j.1365-2613.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res. 2007;25(9):1154–1163. doi: 10.1002/jor.20418. [DOI] [PubMed] [Google Scholar]
- 30.Uchida H, Tohyama H, Nagashima K, et al. Stress deprivation simultaneously induces over-expression of interleukin-1beta, tumor necrosis factor-alpha, and transforming growth factor-beta in fibroblasts and mechanical deterioration of the tissue in the patellar tendon. J Biomech. 2005;38(4):791–798. doi: 10.1016/j.jbiomech.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Abreu EL, Leigh D, Derwin KA. Effect of altered mechanical load conditions on the structure and function of cultured tendon fascicles. J Orthop Res. 2008;26(3):364–373. doi: 10.1002/jor.20520. [DOI] [PubMed] [Google Scholar]
- 32.Leigh DR, Abreu EL, Derwin KA. Changes in gene expression of individual matrix metalloproteinases differ in response to mechanical unloading of tendon fascicles in explant culture. J Orthop Res. 2008;26(10):1306–1312. doi: 10.1002/jor.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29(5):290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84(2):649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 35.Gardner K, Arnoczky SP, Caballero O, Lavagnino M. The effect of stress-deprivation and cyclic loading on the TIMP/MMP ratio in tendon cells: an in vitro experimental study. Disabil Rehabil. 2008;30(20–22):1523–1529. doi: 10.1080/09638280701785395. [DOI] [PubMed] [Google Scholar]
- 36.Hansen P, Haraldsson BT, Aagaard P, et al. Lower strength of the human posterior patellar tendon seems unrelated to mature collagen crosslinking and fibril morphology. J Appl Physiol. 2010;108(1):47–52. doi: 10.1152/japplphysiol.00944.2009. [DOI] [PubMed] [Google Scholar]
- 37.Malaviya P, Butler DL, Boivin GP, et al. An in vivo model for load-modulated remodeling in the rabbit flexor tendon. J Orthop Res. 2000;18(1):116–125. doi: 10.1002/jor.1100180117. [DOI] [PubMed] [Google Scholar]
- 38.Helmer KG, Nair G, Cannella M, Grigg P. Water movement in tendon in response to a repeated static tensile load using one-dimensional magnetic resonance imaging. J Biomech Eng. 2006;128(5):733–741. doi: 10.1115/1.2244573. [DOI] [PubMed] [Google Scholar]
- 39.Ng CP, Swartz MA. Mechanisms of interstitial flow-induced remodeling of fibroblast-collagen cultures. Ann Biomed Eng. 2006;34(3):446–454. doi: 10.1007/s10439-005-9067-3. [DOI] [PubMed] [Google Scholar]
- 40.Eliasson P, Andersson T, Aspenberg P. Rat Achilles tendon healing: mechanical loading and gene expression. J Appl Physiol. 2009;107(2):399–407. doi: 10.1152/japplphysiol.91563.2008. [DOI] [PubMed] [Google Scholar]
- 41.Galatz LM, Charlton N, Das R, Kim HM, Havlioglu N, Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg. 2009;18(5):669–675. doi: 10.1016/j.jse.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Martinez DA, Vailas AC, Vanderby R, Jr, Grindeland RE. Temporal extracellular matrix adaptations in ligament during wound healing and hindlimb unloading. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1552–1560. doi: 10.1152/ajpregu.00423.2007. [DOI] [PubMed] [Google Scholar]
- 43.Provenzano PP, Martinez DA, Grindeland RE, et al. Hindlimb unloading alters ligament healing. J Appl Physiol. 2003;94(1):314–324. doi: 10.1152/japplphysiol.00340.2002. [DOI] [PubMed] [Google Scholar]
- 44.Gimbel JA, Van Kleunen JP, Williams GR, Thomopoulos S, Soslowsky LJ. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007;129(3):400–404. doi: 10.1115/1.2721075. [DOI] [PubMed] [Google Scholar]
- 45.Thomopoulos S, Zampiakis E, Das R, Silva MJ, Gelberman RH. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J Orthop Res. 2008;26(12):1611–1617. doi: 10.1002/jor.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peltz CD, Sarver JJ, Dourte LM, Wurgler-Hauri CC, Williams GR, Soslowsky LJ. Exercise following a short immobilization period is detrimental to tendon properties and joint mechanics in a rat rotator cuff injury model. J Orthop Res. 2010;28(7):841–845. doi: 10.1002/jor.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyer MI, Gelberman RH, Burns ME, Dinopoulos H, Hofem R, Silva MJ. Intrasynovial flexor tendon repair. An experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J Bone Joint Surg Am. 2001;83(6):891–899. [PubMed] [Google Scholar]
- 48.Abramowitch SD, Yagi M, Tsuda E, Woo SL. The healing medial collateral ligament following a combined anterior cruciate and medial collateral ligament injury—a biomechanical study in a goat model. J Orthop Res. 2003;21(6):1124–1130. doi: 10.1016/S0736-0266(03)00080-9. [DOI] [PubMed] [Google Scholar]
- 49.Marsolais D, Duchesne E, Cote CH, Frenette J. Inflammatory cells do not decrease the ultimate tensile strength of intact tendons in vivo and in vitro: protective role of mechanical loading. J Appl Physiol. 2007;102(1):11–17. doi: 10.1152/japplphysiol.00162.2006. [DOI] [PubMed] [Google Scholar]
- 50.Bhole AP, Flynn BP, Liles M, Saeidi N, Dimarzio CA, Ruberti JW. Mechanical strain enhances survivability of collagen micronetworks in the presence of collagenase: implications for load-bearing matrix growth and stability. Philos Transact A Math Phys Eng Sci. 2009;367(1902):3339–3362. doi: 10.1098/rsta.2009.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rees JD, Lichtwark GA, Wolman RL, Wilson AM. The mechanism for efficacy of eccentric loading in Achilles tendon injury; an in vivo study in humans. Rheumatology (Oxford) 2008;47(10):1493–1497. doi: 10.1093/rheumatology/ken262. [DOI] [PubMed] [Google Scholar]
- 52.Chong AK, Chang J, Go JC. Mesenchymal stem cells and tendon healing. Front Biosci. 2009;14:4598–4605. doi: 10.2741/3552. [DOI] [PubMed] [Google Scholar]
- 53.Bedi A, Kovacevic D, Hettrich C, et al. The effect of matrix metalloproteinase inhibition on tendon-to-bone healing in a rotator cuff repair model. J Shoulder Elbow Surg. 2010;19(3):384–391. doi: 10.1016/j.jse.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 54.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33(5):381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 55.Provenzano PP, Alejandro-Osorio AL, Grorud KW, et al. Systemic administration of IGF-I enhances healing in collagenous extracellular matrices: evaluation of loaded and unloaded ligaments. BMC Physiol. 2007;7:2. doi: 10.1186/1472-6793-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doessing S, Heinemeier KM, Holm L, et al. Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J Physiol. 2010;588(pt 2):341–351. doi: 10.1113/jphysiol.2009.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 58.Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop. 2006;77(5):806–812. doi: 10.1080/17453670610013033. [DOI] [PubMed] [Google Scholar]
- 59.Ackermann PW, Salo PT, Hart DA. Neuronal pathways in tendon healing. Front Biosci. 2009;14:5165–5187. doi: 10.2741/3593. [DOI] [PubMed] [Google Scholar]
- 60.Burssens P, Steyaert A, Forsyth R, van Ovost EJ, Depaepe Y, Verdonk R. Exogenously administered substance P and neutral endopeptidase inhibitors stimulate fibroblast proliferation, angiogenesis and collagen organization during Achilles tendon healing. Foot Ankle Int. 2005;26(10):832–839. doi: 10.1177/107110070502601008. [DOI] [PubMed] [Google Scholar]
- 61.Grorud KW, Jensen KT, Provenzano PP, Vanderby R., Jr Adjuvant neuropeptides can improve neuropathic ligament healing in a rat model. J Orthop Res. 2007;25(6):703–712. doi: 10.1002/jor.20335. [DOI] [PubMed] [Google Scholar]
- 62.Salo P, Bray R, Seerattan R, Reno C, McDougall J, Hart DA. Neuropeptides regulate expression of matrix molecule, growth factor and inflammatory mediator mRNA in explants of normal and healing medial collateral ligament. Regul Pept. 2007;142(1–2):1–6. doi: 10.1016/j.regpep.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Bring DK, Reno C, Renstrom P, Salo P, Hart DA, Ackermann PW. Joint immobilization reduces the expression of sensory neuropeptide receptors and impairs healing after tendon rupture in a rat model. J Orthop Res. 2009;27(2):274–280. doi: 10.1002/jor.20657. [DOI] [PubMed] [Google Scholar]
- 64.Rodeo SA, Kawamura S. Form and function of the meniscus. In: Einhorn TA, O’Keefe RJ, Buckwalter JA, editors. Orthopaedic Basic Science: Foundations of Clinical Practice. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2007. pp. 175–189. [Google Scholar]
- 65.Gupta T, Haut Donahue TL. Role of cell location and morphology in the mechanical environment around meniscal cells. Acta Biomater. 2006;2(5):483–492. doi: 10.1016/j.actbio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Upton ML, Hennerbichler A, Fermor B, Guilak F, Weinberg JB, Setton LA. Biaxial strain effects on cells from the inner and outer regions of the meniscus. Connect Tissue Res. 2006;47(4):207–214. doi: 10.1080/03008200600846663. [DOI] [PubMed] [Google Scholar]
- 67.Akeson WH, Woo SL, Amiel D, Coutts RD, Daniel D. The connective tissue response to immobility: biochemical changes in periarticular connective tissue of the immobilized rabbit knee. Clin Orthop. 1973;(93):356–362. doi: 10.1097/00003086-197306000-00039. [DOI] [PubMed] [Google Scholar]
- 68.Klein L, Player JS, Heiple KG, Bahniuk E, Goldberg VM. Isotopic evidence for resorption of soft tissues and bone in immobilized dogs. J Bone Joint Surg Am. 1982;64(2):225–230. [PubMed] [Google Scholar]
- 69.Shin SJ, Fermor B, Weinberg JB, Pisetsky DS, Guilak F. Regulation of matrix turnover in meniscal explants: role of mechanical stress, interleukin-1, and nitric oxide. J Appl Physiol. 2003;95(1):308–313. doi: 10.1152/japplphysiol.00131.2003. [DOI] [PubMed] [Google Scholar]
- 70.Zielinska B, Killian M, Kadmiel M, Nelsen M, Haut Donahue TL. Meniscal tissue explants response depends on level of dynamic compressive strain. Osteoarthritis Cartilage. 2009;17(6):754–760. doi: 10.1016/j.joca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 71.Upton ML, Chen J, Guilak F, Setton LA. Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res. 2003;21(6):963–969. doi: 10.1016/S0736-0266(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 72.Natsu-Ume T, Majima T, Reno C, Shrive NG, Frank CB, Hart DA. Menisci of the rabbit knee require mechanical loading to maintain homeostasis: cyclic hydrostatic compression in vitro prevents derepression of catabolic genes. J Orthop Sci. 2005;10(4):396–405. doi: 10.1007/s00776-005-0912-x. [DOI] [PubMed] [Google Scholar]
- 73.Gupta T, Zielinska B, McHenry J, Kadmiel M, Haut Donahue TL. IL-1 and iNOS gene expression and NO synthesis in the superior region of meniscal explants are dependent on the magnitude of compressive strains. Osteoarthritis Cartilage. 2008;16(10):1213–1219. doi: 10.1016/j.joca.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 74.McHenry JA, Zielinska B, Donahue TL. Proteoglycan breakdown of meniscal explants following dynamic compression using a novel bioreactor. Ann Biomed Eng. 2006;34(11):1758–1766. doi: 10.1007/s10439-006-9178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hennerbichler A, Fermor B, Hennerbichler D, Weinberg JB, Guilak F. Regional differences in prostaglandin E2 and nitric oxide production in the knee meniscus in response to dynamic compression. Biochem Biophys Res Commun. 2007;358(4):1047–1053. doi: 10.1016/j.bbrc.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fermor B, Jeffcoat D, Hennerbichler A, Pisetsky DS, Weinberg JB, Guilak F. The effects of cyclic mechanical strain and tumor necrosis factor alpha on the response of cells of the meniscus. Osteoarthritis Cartilage. 2004;12(12):956–962. doi: 10.1016/j.joca.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 77.Ferretti M, Madhavan S, Deschner J, Rath-Deschner B, Wypasek E, Agarwal S. Dynamic biophysical strain modulates proinflammatory gene induction in meniscal fibrochondrocytes. Am J Physiol Cell Physiol. 2006;290(6):C1610–C1615. doi: 10.1152/ajpcell.00529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzuki T, Toyoda T, Suzuki H, Hisamori N, Matsumoto H, Toyama Y. Hydrostatic pressure modulates mRNA expressions for matrix proteins in human meniscal cells. Biorheology. 2006;43(5):611–622. [PubMed] [Google Scholar]
- 79.Upton ML, Gilchrist CL, Guilak F, Setton LA. Transfer of macroscale tissue strain to microscale cell regions in the deformed meniscus. Biophys J. 2008;95(4):2116–2124. doi: 10.1529/biophysj.107.126938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guisasola I, Vaquero J, Forriol F. Knee immobilization on meniscal healing after suture: an experimental study in sheep. Clin Orthop. 2002;(395):227–233. doi: 10.1097/00003086-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 81.Ferretti M, Srinivasan A, Deschner J, et al. Anti-inflammatory effects of continuous passive motion on meniscal fibrocartilage. J Orthop Res. 2005;23(5):1165–1171. doi: 10.1016/j.orthres.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bray RC, Smith JA, Eng MK, Leonard CA, Sutherland CA, Salo PT. Vascular response of the meniscus to injury: effects of immobilization. J Orthop Res. 2001;19(3):384–390. doi: 10.1016/S0736-0266(00)00037-1. [DOI] [PubMed] [Google Scholar]
- 83.Hoben GM, Athanasiou KA. Meniscal repair with fibrocartilage engineering. Sports Med Arthrosc. 2006;14(3):129–137. doi: 10.1097/00132585-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 84.Sanchez-Adams J, Athanasiou KA. The knee meniscus: a complex tissue of diverse cells. Cell Mol Bioeng. 2009;2(3):332–340. [Google Scholar]
- 85.McNulty AL, Guilak F. Integrative repair of the meniscus: lessons from in vitro studies. Biorheology. 2008;45(3–4):487–500. [PMC free article] [PubMed] [Google Scholar]
- 86.Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Interleukin-1 and tumor necrosis factor alpha inhibit repair of the porcine meniscus in vitro. Osteoarthritis Cartilage. 2007;15(9):1053–1060. doi: 10.1016/j.joca.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gunja NJ, Uthamanthil RK, Athanasiou KA. Effects of TGF-beta1 and hydrostatic pressure on meniscus cell-seeded scaffolds. Biomaterials. 2009;30(4):565–573. doi: 10.1016/j.biomaterials.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage. 2004;12(9):736–744. doi: 10.1016/j.joca.2004.05.007. [DOI] [PubMed] [Google Scholar]