Abstract
Recently, the field of stem cell-based regeneration has turned its attention toward chemical approaches for controlling the pluripotency and differentiation of embryonic stem cells (ESCs) using drug-like small molecule modulators. Growth factor receptors or their associated downstream kinases that regulate intracellular signaling pathways during differentiation are typically the targets for these molecules. The glycocalyx, which plays an essential role in actuating responses to growth factors at the cellular boundary, offers an underexplored opportunity for intervention using small molecules to influence differentiation. Here, we show that surfen, an antagonist of cell-surface glycosaminoglycans required for growth factor association with cognate receptors, acts as a potent and general inhibitor of differentiation and promoter of pluripotency in mouse ESCs. This finding shows that drugging the stem cell Glycome with small molecules to silence differentiation cues can provide a powerful new alternative to existing techniques for controlling stem cell fate.
Keywords: Pluripotency, Differentiation, Heparan sulfate, Embryonic stem cell, Carbohydrate, Fibroblast growth factors
Introduction
The defining traits of pluripotent stem cells (PSCs), which include embryonic stem cells (ESCs) isolated from the inner cell mass of blastocysts and induced pluripotent stem cells (iPSCs) derived from somatic cells through cellular reprograming, are their high self-renewal capacity and their ability to produce cell types of all three germ layers [1, 2]. Chemical approaches for the generation and maintenance of PSCs are attractive [3, 4], as they offer important advantages over methods relying on gene manipulation [5], or the use of cytokines [6] and growth factors [7] to confine cells in their pluripotent state. The use of small molecules alleviates safety concerns regarding permanent modifications to the genome of the target cells, while offering increased chemical stability, low cost of production, and better pharmacokinetic profiles for in vivo applications compared to biologics. Whereas the procedures for assessing the toxicity of small molecules are well-established as part of the U. S. Federal Drug Administration approval process, genetic targeting as a therapeutic strategy is not. Chemical modulation can offer the ability to fine-tune the resulting effects based on reversibility or dosage. Genetic instability has been reported for cells following genetic targeting, especially in iPSCs [8]. Compounds have now been discovered that promote cellular reprograming toward the pluripotent state or regulate germ layer specification [9]. Whereas the field of small molecule-based modulators of cellular differentiation has focused primarily on targeting the activity of receptor and intracellular kinases or epigenetic enzymes controlling gene expression [10], glycan interactions at the cell-matrix interface regulating the activation of receptors upstream of these pathways have received much less attention.
Cell surface glycans are essential for proper embryonic development; and stem cell differentiation is often accompanied by alterations in glycosylation patterns [11] (Fig. 1). In fact, unique glycan structures, such as the stage specific embryonic antigens (SSEAs) 1 and 3 can be used as indicators of pluripotency in murine and human ESCs, respectively [12]. At the same time, elimination of specific cell surface glycan structures caused by mutations in glycan biosynthesis genes results in embryonic lethality or severe congenital disorders [13]. Proteoglycans (PGs)—membrane-associated proteins modified with long chains of sulfated polysaccharides, called glycosaminoglycans (GAGs)—are representatives of one such family of essential glycoconjugate regulators of cellular differentiation. Through their GAG chains, PGs help mediate the association of growth factors with cell-surface receptors into ternary complexes that initiate intracellular signaling cascades and gene expression (Fig. 1) [14]. Murine embryonic stem cells (mESC) mutants missing the exostosin 1(Ext1) gene that encodes a glycosyltransferase involved in the elongation of heparan sulfate (HS) GAG chains, lack the ability to signal through growth factors of the fibroblast growth factor (FGF), transforming growth factor (TGF), or Wnt families and, as a result, are restricted in their capacity to exit from the embryonic state [15–17]. As master modulators of key intra-cellular signaling pathways, GAGs constitute unique targets for interfering with extracellular differentiation cues and influencing stem cell states [18].
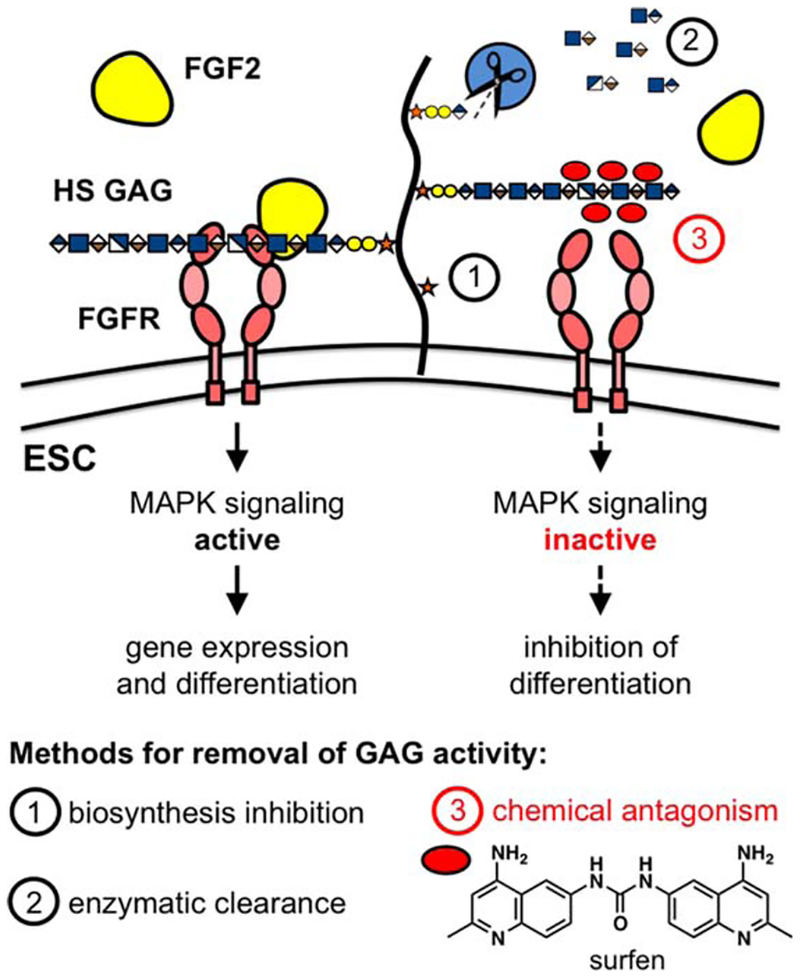
Figure 1.
Targeting glycosaminoglycans to influence embryonic stem cell fate. Top: HS GAGs are required for FGF2-dependent induction of the MAPK pathway and neural differentiation in murine embryonic stem cells. Deactivation of HS GAGs disrupts MAPK signaling and inhibits differentiation. Bottom: Small molecule antagonists provide an attractive alternative to genetic and enzymatic methods for the attenuation of HS GAG activity. Abbreviation: ESC, embryonic stem cell; FGFR, FGF receptor; GAG, glycosaminoglycan; HS GAG, heparan sulfate glycosaminoglycan; MAPK, mitogen-activated protein kinase.
It is well-known that mouse and human ESCs use different signaling pathways to maintain pluripotency [19]. For instance, while human ESCs require active FGF2 signaling to maintain pluripotency, mESCs can use FGF2 signaling for differentiation [18]. In this work, we focus our efforts on mESCs, where the role of HS GAG chains is well-studied providing a suitable model system to study how chemical modulators can affect pluripotency via glycan-mediated mechanisms.
Several methods have been developed for transient attenuation of GAG activity without imparting permanent genomic changes to the cell. Most commonly, the target GAG structures are enzymatically removed via depolymerization of the polysaccharide chains with bacterial lyases [20]. Whereas the substrate specificity of the enzyme determines which GAG classes will be eliminated (e.g., heparinases and chondroitinases respectively degrade HS or chondroitin sulfate chains), this process requires extended treatment of the cells with high concentrations of enzymes and the complete removal of inaccessible GAG structures can be challenging. As a consequence, heparinase treatment can inhibit endothelial differentiation in mESCs but fails to restrict them in a pluripotent state [21]. Alternatively, small molecule inhibitors can be used to interfere with the biosynthesis of GAGs [22–25], although they are yet to be tested as inhibitors of ESC differentiation.
For proper function, GAGs need to be modified with negatively charged sulfate groups that define growth factor and receptor binding sites. The inhibition of the general sulfate donor, PAPS (3’-phosphoadenosine-5’-phosphosulfate), with sodium chlorate leads to undersulfation of GAGs at the cell surface and loss of activity [26, 27]. While commonly used, chlorate treatment indiscriminately affects the sulfation of other glycan classes as well as proteins [28]. Such global and poorly characterized perturbations of sulfation may explain the contradictory reports on the effects of chlorate treatment on mESC differentiation [29, 30].
Direct antagonism of GAGs provides perhaps the most attractive opportunity for modulating signaling activity at the cell surface. Although small molecules can interact with GAGs [31], few have been tested for effects in stem cell differentiation. For instance, surfen, an aminoquinoline with heparin-neutralizing properties, was first reported in 1938 [32]. Since its discovery, surfen has been shown to inhibit HIV infection [33], vasculogenesis [34], or modulate T-cell activation [35], through the antagonism of HS GAG interactions with signaling and receptor proteins [36, 37]. In this study, we report that surfen effectively and reversibly restricts mESCs in their pluripotent state by attenuating the activity of their surface GAG structures in growth factor association and signaling.
Materials and Methods
mESC Culture
Oct4-GFP and Sox1-GFP (PrimCells) mESCs were maintained in gelatin-coated plastic tissue-culture dishes and mESC complete media: 0.01% leukemia inhibitory factor (LIF)/0.1% β-mercaptoethanol/1% L-glutamine/1% MEM-non-essential amino acids (NEAA)/10% fetal bovine serum (FBS)/knockout-dulbecco’s minimum essential media (KO-DMEM). Cells were passaged every other day by trypsinization (0.05% trypsin-EDTA) and splitting at 1:10, after washing with dulbecco’s phosphate buffered saline (DPBS). Unless otherwise indicated, all incubation conditions were conducted at 378C, 5% CO2.
mESC Differentiation (N2B27, Knockout Serum Replacement)
To induce differentiation, mESCs were seeded (10,000 cells/cm2) in 1 ml complete media overnight (D-1). After 24 hours of incubation, cells were washed twice with DPBS, and replenished with N2B27 media with or without compound (D0). N2B27 media was prepared by a 1:1 mixture of Neurobasal media and DMEM/F-12, 1:1,000 of B27 and 1:500 N2 supplements, as well as 1% L-glutamine, Penicillin/Streptomycin, and 0.1% β-mercaptoethanol. To induce spontaneous differentiation, cells were seeded similarly, and the differentiation media used was 15% knockout serum replacement (KSR)/1% NEAA/1% L-glutamine/0.1% β-mercaptoethanol/KO-DMEM. Media with or without additive was replenished every 1–2 days.
EB Formation and Differentiation
mESCs were detached and 20 μl drops of a 3 × 105 cells/ml suspension in mESC growth media (60,000 cells/EB) was plated using a multi-channel pipet onto the lids of 10 cm low-attachment plastic petri dishes (D-2). After 2 days, robust EBs were formed. About 30–40 EBs were used per experimental condition. The EBs were washed twice by resuspension in DPBS, and differentiation was initiated by resuspension of the EBs in N2B27 media onto low-attachment 10 cm dishes (D0). The media was replaced every other day by washing once with DPBS and resuspension in N2B27 with or without compound. After differentiation (D6), EBs were washed 2×, and dissociated into single cells by incubation with Accutase enzyme (RT, 5 minutes). Live flow cytometry analysis was performed after neutralization with 10% FBS/KO-DMEM, and gentle pipetting.
Flow Cytometry and Microscopy Analysis
For live cell analysis, mESCs or differentiated cells were detached from adherent culture with trypsin-EDTA, diluted in 10% FBS/KO-DMEM, and analyzed with a BD Accuri C6 flow cytometer. Cells were gated in a forward versus side scatter (FSC vs. SSC) plot to exclude cell debris and dead cells, and 15–20,000 events in the relevant gate was collected per sample. Analysis was performed with either Accuri C6 software or FlowJo (TreeStar) version 10.8. The FL-1 channel was used to monitor green fluorescent protein (GFP) expression. To determine % GFP-positive populations, mESCs cultured in complete media were first used to bisect FL-1 histograms into GFP-negative or positive populations. A Zeiss Axiovert epifluorescence microscope equipped with a black and white AxioCam camera was used to capture brightfield and fluorescence images. Live cell images for differentiation experiments were taken at the indicated day in the corresponding media before washing with DPBS. For immunostaining, cells were washed 2× DPBS, fixed with 4% p-formaldehyde (PFA)/PBS (room temperature, 10 minutes), blocked, and stained using primary antibodies (4°C, overnight) and fluorophore-labeled secondary antibodies (RT, 3 hours).
Western Blotting of Erk Phosphorylation
Oct4-GFP mESCs were used for all stimulation experiments. Cells were plated (1 × 105 cells/cm2) onto gelatinized six-well plates in mESC growth medium. After 8–12 hours, cells were washed with DPBS, and serum starved (~18 hours) by replacing with FBS-free mESC growth medium. Stimulation was performed by adding FGF2 (25 ng/ml) in FBS-free mESC media to cell monolayers for 10–15 minutes at 37°C, 5% CO2. The plate was then immediately incubated on ice, and total protein was extracted using 1× cell lysis buffer and scraping. After 10 minutes of incubation on ice, the mixture was centrifuged at 16,000g for 10 minutes (4°C) to pellet and remove insoluble components. The supernatant was subjected to a biscinchoninic acid (BCA) assay to quantify total protein levels, and upon normalization, 10 μg of protein was separated by SDS-PAGE (10% Tris-Glycine-sodium dodecyl sulfate- polyacryl-amide gel electrophoresis) and transferred onto a polyvinylidene fluoride (PVDF) membrane. Anti-phosphoErk and anti-Erk antibodies were used to probe for levels of phosphorylated and total Erk protein levels. Densitometry was performed using ImageJ.
Receptor Tyrosine Kinase Analysis
The instructions supplied with the Mouse Phospho-receptor tyrosine kinase (RTK) Array Kit (R&D Systems Cat. # ARY014) were closely followed as a protocol. Oct4-GFP mESCs were prepared as above (see Western blotting for Erk phosphorylation), except cells were seeded in a gelatinized T-75 flask. A total of 250 μg of whole cell lysate was used for each individual array, which includes duplicates spots of control and capture antibodies for different RTKs. Pixel density was determined via Adobe Photoshop (v 5.0) as the mean intensity of each capture antibody spot subtracted by the mean intensity of the PBS control spots.
RT-qPCR (Quantitative Reverse Transcription) Analysis
Primers were obtained from IDT Technologies. Total RNA was extracted from cells in adherent culture after washing 2× DPBS, and following manufacturer’s instructions for subsequent processing (Qiagen RNeasy Mini Kit). RNA purity and levels were assessed by UV analysis (NanoDrop), and lysates were stored at −20°C until ready for processing. Fifty nanograms of total RNA was used for cDNA synthesis, and gene expression was assessed using SYBR Green as a probe and an Applied Biosystems HT 7900 instrument.
Statistical Analysis
All mathematical analyses were performed using GraphPad Prism (v 6.0). The statistical significance of a single comparison was performed using the built-in analysis (Student’s t test), and multiple comparisons to a single control were conducted using the Dunnett’s test (multiple comparison t test). In general, each condition was conducted in duplicate in each experiment, and at least two independent biological replicates were used to derive conclusions. Thresholds for significance for all tests is set as *,p < .05; **, p .01; ***, p < .001; ****, p < .0001.
Results
Surfen Is a Potent, Reversible Inhibitor of Neural Differentiation and a Promoter of Pluripotency in mESCs
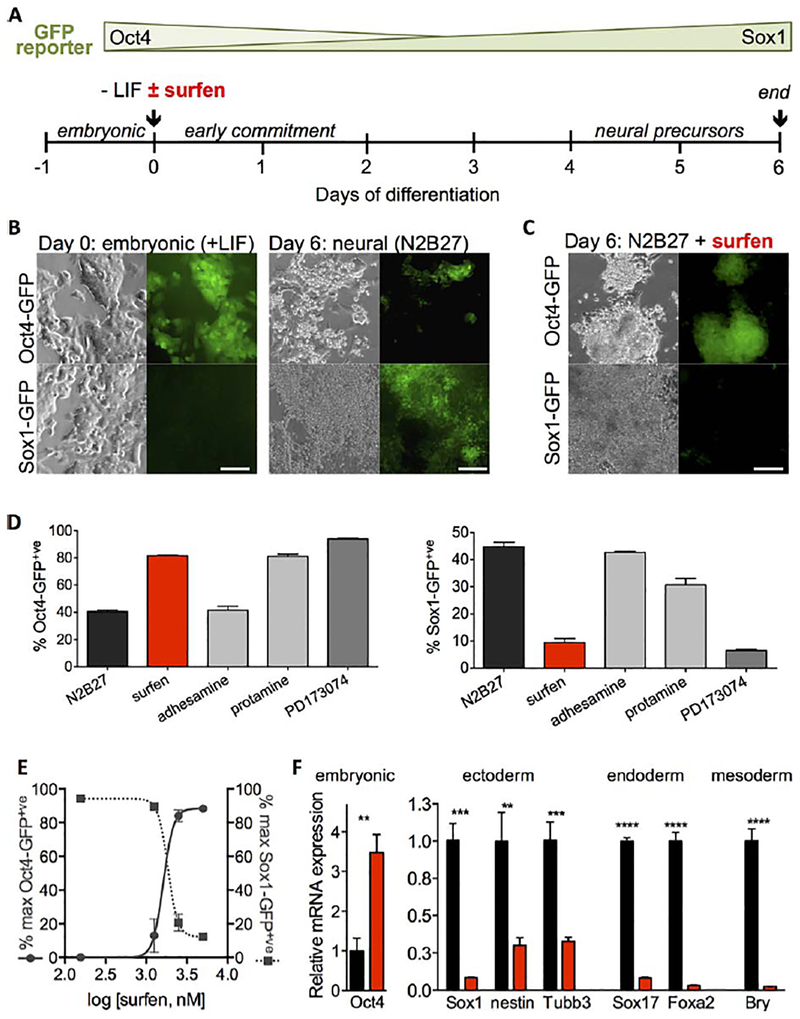
Cognizant of the profound effects of the genetic deletion of the Ext1 gene (vide supra), and the ensuing loss of surface HS expression, on the inability of mESCs to exit from the pluripotent state [15], we set out to investigate whether common GAG antagonists may chemically induce similar phenotype in wild type cells. We designed a dual endpoint flow cytometry assay that uses two GFP knock-in mESC lines to simultaneously evaluate both pluripotency and differentiation in the presence of GAG antagonists. We used an Oct4-GFP mESC line to monitor the expression of Oct4, which indicates stem cell pluripotency and stemness [38], and a Sox1-GFP mESC mutant (46C) [38] to assess neural commitment (Fig. 2A). Oct4-GFP mESCs express high levels of GFP when cultured under embryonic conditions in the presence of LIF and gradually lose GFP expression upon differentiation. Conversely, Sox1-GFP mESCs do not express GFP in embryonic culture but become GFP-positive upon acquisition of neural identity, which coincides with the expression of the early neuroectodermal marker, Sox1 [38]. Both cell lines exhibit a compact colony morphology when pluripotent, and lose this feature upon differentiation. Using a well-established protocol [39], we induced both reporter mESC lines in serum-free N2B27 media toward neuroectodermal differentiation and assessed the levels of Oct4 and Sox1 expression (Fig. 2B). At day 6 of differentiation, we observed ~20%–50% of Oct4-GFP mESCsremain GFP-positive, while ~40%–70% of Sox1-GFP mESCs have acquired GFP expression and neural phenotype in these surfen-untreated controls. Immunostaining of both Oct4-GFP and Sox1-GFP mESC lines, as well as wild-type E14Tg2a mESCs, also confirmed the loss of Oct4 and gain of Sox1 protein expression after 6 days of differentiation (Supporting Information Fig. S1).
Figure 2.
Dual endpoint GFP-reporter assay for evaluating heparin sulfate glycosaminoglycan (HS GAG) antagonists as inhibitors of neural differentiation in murine embryonic stem cells (mESCs). (A): Oct4-GFP and Sox1-GFP mESC reporter lines were used to evaluate the ability of HS GAG antagonists to inhibit neural differentiation and promote pluripotency over 6 days in N2B27 culture. (B): Live cell fluorescence micrographs show loss of pluripotency (Oct4) and acquisition of neural phenotype (Sox1) after 6 days of differentiation. Scale bar: 100 μm. (C): In the presence of surfen (5 μM), Oct4 and Sox1 expression profiles indicate that mESCs continue to maintain high levels pluripotency even after 6 days in differentiation. (D): Surfen shows enhanced ability to promote pluripotency and inhibit differentiation compared to other known HS modulators, protamine (10 μM), and adhesamine (5 μM). Surfen activity is comparable to that of PD173074 (1 μM), an ATP-competitive FGF receptor inhibitor. (E): Surfen inhibits differentiation in both Oct4-GFP and Sox1-GFP cell lines with IC50 ~2.0 μM. Each point represents technical duplicates (mean6SD), representative of two biological replicates. (F): Analysis of mRNA expression in Oct4-GFP mESCs on day 6 of treatment with surfen in N2B27 (5.0 μM) indicates cell arrest in the embryonic state. Relative mRNA expression is calculated from technical triplicates (mean6SD) normalized to untreated (N2B27) controls (defined as 1.0). This experiment is representative of two biological replicates. **, p <.0037; ***, p <.002; ****, p <.0001. Abbreviations: GFP, green fluorescent protein; LIF, leukemia inhibitory factor.
Whereas the current repertoire of GAG antagonists is rather small, we acquired and tested three commercially available molecules known to engage HS (surfen, adhesamine, and protamine) in our differentiation assay. Surfen and adhesamine have been reported to modulate FGF signaling, as well as cell adhesion and proliferation, respectively, through interaction with HS [36, 40]. Protamine is a high molecular weight cationic lysine and arginine-rich protein used as a neutralizing agent for the anticoagulant heparin (also a GAG) [41]. Initial evaluation of surfen (5.0 μM) via fluorescence microscopy indicated that it inhibited Sox1-GFP expression, while maintaining the colony morphology of mESCs and high Oct4 expression (Fig. 2C). To obtain a more quantitative analysis of differentiation in the presence of all three HS-binding molecules, we performed flow cytometry to assess cell populations on day 6 of differentiation (Supporting Information Fig. S2). For comparison, we also included PD173074, a small molecule FGF receptor (FGFR) antagonist shown previously to restrict mESCs in a pluripotent state, as a positive control (Fig. 2D) [42]. Whereas protamine (10 μM) maintained high levels of Oct4-GFP, it did not inhibit Sox1-GFP expression in our assay. Adhesamine (10 μM) showed no effect on either Sox1-GFP or Oct4-GFP expression compared to the untreated control. In contrast, surfen (5.0 μM) effectively inhibited neural differentiation (<10% Sox1-GFP positive cells), while maintaining the mESCs in a pluripotent state (>90% Oct4-GFP positive population) after 6 days in differentiation (Fig. 2D). Further increases in the population of pluripotent cells (~99%; not shown) can be achieved at higher concentration of the antagonist; however, changes to the cellular morphology become apparent at concentrations above 10 μM, presumably, due to aggregation of negatively charged culture medium components by surfen (Supporting Information Fig. S3). Nonetheless, 5.0 μM surfen consistently inhibited differentiation in both Oct4-GFP and Sox1-GFP mESCs (Supporting Information Fig. S4) at levels similar to the known FGFR inhibitor, PD173074. For comparison, we also evaluated the effects of heparinase and chlorate treatment on mESC differentiation, since both are commonly used to attenuate HS GAG activity at the cell surface (vide supra). In agreement with prior work, chlorate treatment led to an initial acceleration of neural differentiation of mESCs toward Sox1-GFP+ populations [29], with eventual return to high levels of Oct4-GFP expression after day 2 (Supporting Information Fig. S5). Although removal of surface HS with bacterial heparinase provided some inhibition of mESC differentiation, it required large amounts of costly enzyme (~500-fold difference in cost per experiment) and suffered from a high degree of variability and low efficiency compared to surfen treatment (Supporting Information Fig. S6).
Surfen inhibited Sox1 and promoted the maintenance of Oct4 expression under the neural differentiation conditions in a dose-dependent manner. Inhibition curves established using the GFP fluorescence of the reporter cell lines yielded similar IC50 values of ~2.0 μM, (Fig. 2E; Supporting Information Fig. S7) indicating that surfen equally inhibits neural differentiation and promotes pluripotency, or, in other words, that the reduction in neural induction is not caused by mESC differentiation into non-neural lineages, but rather by placing limits on their ability to exit from a pluripotent state.
Our observation that surfen maintains mESCs in a pluripotent state, is further supported by our flow cytometry data indicating high levels of expression of SSEA-1 (Supporting Information Fig. S8), and a low abundance of the neural marker, Sox1, on day 6 of differentiation in N2B27 in the presence of the inhibitor (Supporting Information Fig. S1). Quantitative RT-qPCR analysis (see Supporting Information Table 1 for primer sequences) showing high expression of Oct4 and low levels of neuroectodermal (Sox1, Tubb3, nestin), mesodermmal (Bry), and endodermal (Foxa2, Sox17) markers provides further evidence for inhibition of differentiation (Fig. 2F and Supporting Information Fig. S9). We further characterized the pluripotent nature of the surfen-treated cells, and determined by qRT-PCR analysis that these cells are indeed embryonic and not in the epiblast state, as evidenced by high expression of the embryonic marker Rex1, but low levels of the epiblast markers FGF5 and Nodal (Supporting Information Fig. S10) [43]. It is important to point out that surfen had no significant effect on cell proliferation rates, excluding the possibility that inhibition of differentiation is caused by a decrease in cell viability (Supporting Information Fig. S11).
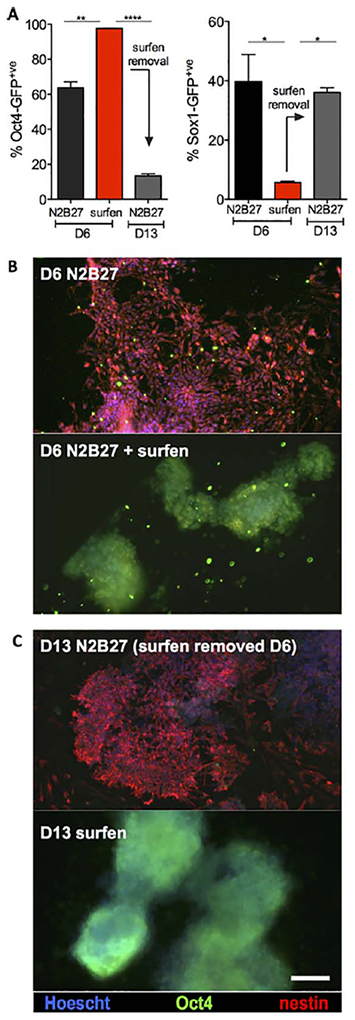
A key advantage of using small molecule modulators is the possibility to achieve transient and reversible change in cellular activity. We tested whether inhibition of differentiation by surfen can be reversed after its withdrawal. Sox1-GFP mESCs maintained in a pluripotent state during a 6-day challenge under neural differentiation conditions were replated in surfen-free N2B27 media (Fig. 3). After an additional 7 days in differentiation (D13), the cells underwent successful neural differentiation, as evidenced by loss of Oct4-GFP and increased Sox1-GFP fluorescence. (Fig. 3A) Cells immunostained at D6 of differentiation express high levels of Oct4 and low levels of nestin compared to non-treated cells (Fig. 3B), and the withdrawal of surfen allows increased nestin expression at D13 (Fig. 3C), whereas cells continuously treated with surfen for 13 days still display colony morphology, high levels of Oct4 and low levels of nestin (Supporting Information Figs. S12, S13).
Figure 3.
Surfen is a reversible inhibitor of differentiation. (A): After 6 days in neural differentiation in the presence of surfen (5 μM) cells remain pluripotent, as evidenced by high Oct4-GFP and low Sox1-GFP expression levels via flow cytometry. Differentiation resumes after removal of surfen at day 6 producing high levels of Sox1-GFP expression with concurrent loss of Oct4-GFP by day 13. %GFP+ve values are provided as technical duplicates (mean ± SD), representative of three biological replicates. *, p < .0335; **, p < .0048; ***, p .0001.(B): Immunostaining for neural markers, nestin, and β-III-tubulin in Oct4-GFP murine embryonic stem cells (mESCs) after 6 days of neural differentiation in N2B27 with or without surfen. (C): Nestin and b-III-tubulin expression in Oct4- GFP mESCs on day 13, 6 days after surfen removal. Scale bar: 100 μm. Abbreviation: GFP, green fluorescent protein.
Surfen Acts as a Broad-Spectrum Inhibitor of Signaling
Neuroectodermal commitment of mESCs to differentiation is known to initiate via autocrine activation of the mitogen-activated protein kinase (MAPK) signaling pathway by fibro-blast growth factors 2 and 4 (FGF2/4) [16]. HS GAGs are required for proper FGF function, acting as co-receptors responsible for the recruitment of FGFs to the cell surface and organization of the active growth factor-receptor complex [27, 44]. Auto-phosphorylation of the FGFR ensues, triggering downstream signaling events, including the phosphorylation of the Erk1/2 kinases, that ultimately result in gene expression and differentiation.
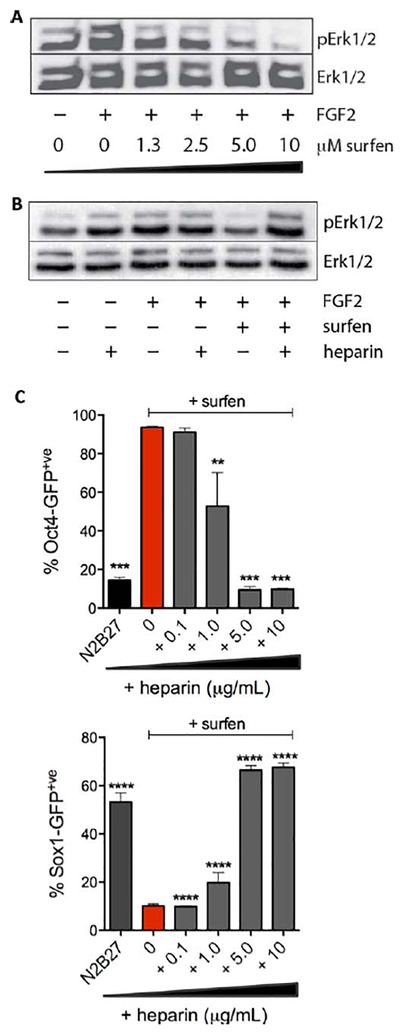
Western blot analysis of mESCs stimulated by FGF2 show robust levels of phospho-Erk1/2; however, this response is attenuated by surfen in a dose-dependent manner (Fig. 4A and Supporting Information Fig. S14). Surfen effectively inhibits binding of FGF2 to heparin in an enzyme-linked immunosorbent assay (ELISA) (Supporting Information Fig. S15) and MAPK signaling can be restored by the addition of exogenous heparin, which competes for cell surface HS-bound surfen molecules (Fig. 4B and Supporting Information Fig. S16). These observations provide evidence that surfen is likely inhibiting MAPK signaling by blocking FGF2 binding sites on cell surface HS. Consistent with Western blot data, flow cytometry analysis also indicates that heparin can restore differentiation following surfen treatment. Oct4-GFP mESCs treated with surfen (5.0 μM) and further titrated with increasing amounts of heparin showed that 5.0 μg/ml is sufficient to abrogate the effects of surfen, as evidenced by the low Oct4-GFP levels (Fig. 4C). The exit from pluripotency promoted by heparin in surfen-treated cells was observed to proceed to neural differentiation, as observed in enhanced Sox1-GFP levels (Fig. 4C).
Figure 4.
Surfen acts by inhibiting FGF2 signaling and its activity can be neutralized with soluble heparin. (A): Stimulation of Oct4- GFP murine embryonic stem cells (mESCs) with FGF2 in the presence of surfen leads to dose-dependent attenuation of Erk1/2 phosphorylation. (B): Erk1/2 phosphorylation in Oct4-GFP mESCs is recovered in the presence of heparin (5 μg/ml), a soluble competitor for cell surface heparan sulfate-bound surfen. (C): Added soluble heparin restores the ability of mESCs to undergo neural differentiation in the presence of surfen (5 μM). Dunnett’s multiple comparison test against surfen-treated control, ****, p < 0001. Shown are technical duplicates (mean ± SD), repeated with two biological replicates. Abbreviation: GFP, green fluorescent protein.
RTK Array
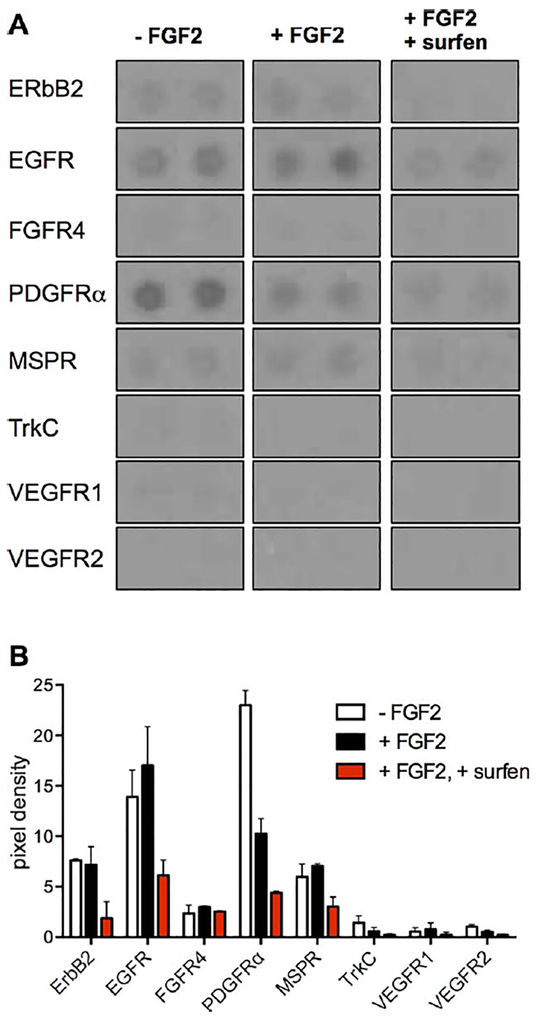
In addition to the MAPK pathway, HS is suspected to mediate a number of other signaling events associated with mESC differentiation [44]. Therefore, we also evaluated the effects of surfen toward other RTKs using an RTK array. (Fig. 5A) Oct4-GFP mESCs were stimulated with FGF2 (25 ng/ml) in the presence or absence of surfen (5.0 μM), and the resulting lysates were incubated identically with the capture array, which includes 39 specific RTK antibodies, allowing for the simultaneous detection of receptor tyrosine phosphorylation. (Fig. 5, Supporting Information Fig. S17) Surfen inhibited phosphorylation of numerous RTKs, including platelet-derived growth factor (PDGFRα) [45], ErbB2 [46], epidermal growth factor receptor (EGFR) [47], and macrophage-stimulating protein receptor (MSPR). (Fig. 5B) Interestingly, this broad inhibitory effect of surfen toward tyrosine kinase phosphorylation is similar to that caused by the Ext1 gene deletion in Sox1-GFP cells, which leads to the truncation of HS chains [48].
Figure 5.
Receptor tyrosine kinase (RTK) array analysis of embryonic Oct4-GFP murine embryonic stem cells in response to surfen treatment. Processed blots (A) and bar graphs (B) demonstrate that surfen inhibits phosphorylation of numerous RTKs. Bargraphs were generated using technical duplicates (means ± SD), and the experiment was performed once. Abbreviations: EGFR, epidermal growth factor receptor; GFP, green fluorescent protein; MSPR, macrophage-stimulating protein receptor.
Given the embryonic state of the cells, only low levels of pTrkC, and the vascular endothelial growth factor (VEGF) family receptors pVEGFR1, and pVEGFR2, were detected in the samples with or without FGF2 stimulation, compared to other RTKs. Despite the low abundance of these phospho-RTKs, surfen also caused a reduction in their phosphorylation. VEGF is known to interact with HS [49] and surfen has previously been reported to inhibit VEGF-mediated endothelial sprouting [37]. Quantitative phosphoproteomics studies of Ext1−/− endothelial cells similarly revealed that antagonism of HS inhibits phosphorylation of these RTKs [50].
Surfen Is a General Inhibitor of Differentiation
Having established the efficacy of surfen in inhibiting neural differentiation of mESCs in monolayer culture, we sought to evaluate its ability to maintain pluripotency in cells cultured in a three-dimensional format. EBs generated from the reporter cell lines via the hanging drop method were cultured in N2B27 neural induction medium in the presence of surfen for 6 days [51]. Fluorescence micrographs in Figure 6A clearly show suppression of GFP expression in Sox1-GFP mESCs in response to surfen (5 μM) compared to untreated cells, which was mirrored by high levels of fluorescence in the Oct4-GFP reporter cells. The preliminary visual assessment by microscopy of neural differentiation in EB culture was corroborated by flow cytometry analysis after EB dissociation (Fig. 6B).
Figure 6.
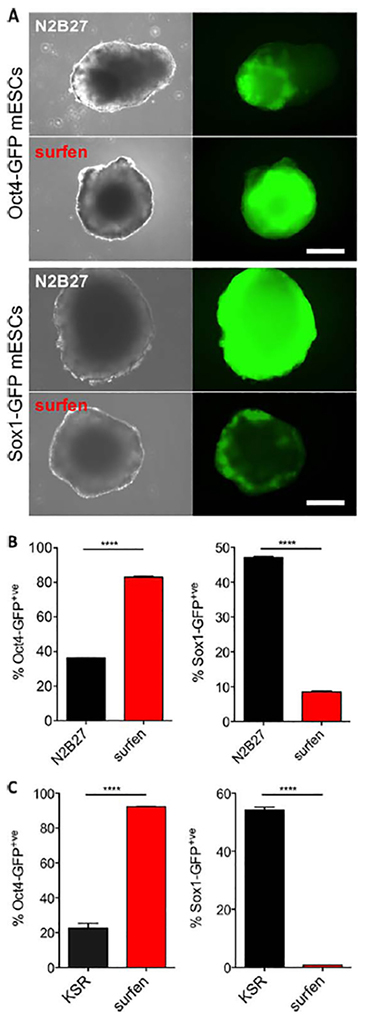
Surfen is a general inhibitor of neural and spontaneous differentiation in mESCs. Live cell fluorescence micrograph images (A) and flow cytometry analysis (B) show inhibition of neural differentiation and maintenance of pluripotency in EB culture 6 days after neural induction in N2B27 media. (C): Surfen maintains pluripotency in mESCs under spontaneous differentiation conditions 6 days after withdrawal of the leukemia inhibitory factor. Provided are technical duplicates (means ± SD), as a representative experiment of three biological replicates, ****, p < .0001. Scale bar: 100 μm. Abbreviations: GFP, green fluorescent protein; KSR, knockout serum replacement; mESCs, murine embryonic stem cells.
In culture, mESCs undergo spontaneous differentiation upon the withdrawal of LIF to generate cell types of all three (i.e., neuroectodermal, mesodermal, and endodermal) germ layer lineages. Accordingly, the reporter mESC lines cultured for 6 days in KSR (15%) differentiation media in the absence of LIF indicate a significant loss of Oct4 and increased levels of Sox1 expression and neural commitment (Fig. 6C). Inclusion of surfen (5 μM) resulted in ~80% GFP-positive population of Oct4-GFP reporter mESCs, indicating its ability to act as an effective general inhibitor under spontaneous differentiation conditions.
Discussion
ESCs continue to hold a significant promise for future biomedical applications. Small molecules can be used as reagents to control the pluripotency and differentiation of ESCs. To date, however, small molecules targeting the glycocalyx of stem cells to influence their fate are yet to be identified. Here, we report that surfen, a bis-aminoquinoline molecule, potently maintained pluripotencybisin mESCs, as shown by flow cytometry, qPCR, and immunostaining. (Figs. (2 and 3))
Surfen acts by binding negatively-charged HS molecules present on the glycocalyx of mESCs via its positively charged aminoquinoline groups [36]. By antagonizing interactions between GAGs and HS-binding cytokines, such as FGF2, surfen can attenuate signaling pathways downstream of FGF2 activation (e.g., Erk phosphorylation) (Figs. 4, 5). Surfen’s effects are dose-dependent and reversible, and the treated mESCs regain the ability to differentiate into neural precursor cells upon the withdrawal of surfen (Fig. 4). Given surfen’s ability to inhibit activation of signaling pathways similar to those observed in Ext1−/− mESCs [48], it presents a chemical means to induce a similar phenotype in mESCs. Interestingly, despite their abilities to bind HS, protamine and adhesamine, failed to maintain pluripotency in mESCs. This observation alludes to the fact that other molecular factors, such as shape complementarity, may be needed for proper antagonism. Indeed, surfen could align itself along the negatively charged sulfate and carboxyl groups of an HS disaccharide via its protonated quinolone rings [36]. This arrangement may be necessary for surfen’s observed effects, although more studies are required to properly determine this binding arrangement.
We wish to point out that the extrapolation of the effects of surfen on mESCs, toward human ESCs should be avoided. Human and mouse ESCs use distinct signaling pathways to control pluripotency and differentiation, and express different sets of receptors on their cell surfaces to do so [19]. Nonetheless, the role of GAGs in modulating mESC pluripotency is well documented and these cells provide a good model for establishing small molecules as modulators of stem cell fates through targeting of the cellular glycocalyx. This study thus provides a precedent for further exploration of the glycocalyx in the context human stem cell differentiation.
Conclusion
The cellular glycocalyx plays a defining role in facilitating the transfer of differentiation cues from the extracellular matrix to signaling receptors at the cell surface. As such, it harbors new exciting opportunities for intercepting these signals in order to influence the outcome of differentiation. We have now demonstrated the ability of surfen, a small molecule antagonist of cell-surface GAGs, to arrest ESCs in their pluripotent state by blocking the binding sites for growth factors within their glycocalyx. As a small molecule, surfen inhibits glycan-growth factor interactions in a reversible fashion, providing temporal control over signaling, gene expression, and differentiation. Surfen is a general inhibitor of GAG function; however, we anticipate that ongoing efforts in the molecular design and synthesis of chemical antagonists with enhanced selectivity toward unique subfamilies of GAG structures, or even other prominent classes of cell surface glycans involved in controlling stem cell pluripotency [52], will yield a new, expanded set of selective small molecule modulators of cellular signaling and differentiation.
Supplementary Material
Significance Statement.
The therapeutic potential of embryonic stem cells (ESCs) hinges on the ability to direct differentiation. Small molecules that enable precision control of stem cell states have become powerful tools in basic biology research and regenerative medicine. Although proteins and nucleic acids have been explored as targets in this area, cell surface glycans have not. In this study, a small molecule, surfen, that binds to heparan sulfate glycosaminoglycans, is utilized to maintain the pluripotency of ESCs. Surfen keeps ESCs pluripotent by antagonizing the interaction of glycosaminoglycans with growth factors and silencing downstream signaling pathways and gene expression. It is anticipated that this report will accelerate efforts in the design and synthesis of new molecules for targeting other glycans involved in controlling stem cell fate.
Acknowledgments
This work was supported by startup funds from UCSD, the NIH Pathway to Independence Award (NIBIB: 5 R00 EB013446–05) and the NIH Director’s New Innovator Award (1DP2HS087954–01). M. L. H. was partially supported by the NIH Pathway to Independence Award (NICHD: 1 K99 HD090292–01). M.L.H. and K.G. were supported in part by the Program of Excellence in Glycosciences (PEG, NHLBI: 4P01HL107150–06). C.J.F. was supported in part by the UCSD Molecular Biophysics Training Program (T32 GM08326). Quantitative PCR was performed with the support of the Genomics and Sequencing Core at the UCSD Center for AIDS Research (P30 AI032614), the VA San Diego Healthcare System, and the Veterans Medical Research Foundation. Fluorescence imaging was partially enabled by the UCSD Neuroscience Microscopy Shared Facility Grant (P30 NS047101). We gratefully acknowledge Drs. Ryan Weiss and Yitzhak Tor for providing compounds during the initial phase of this work and Dr. Jeffrey Esko for his critical evaluation of this study and valuable insights. We thank Pinyi Du of CFAR Genomics Center for assistance with PCR experiments.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Hochedlinger K, Jaenisch R. Nuclear transplantation, embryonic stem cells, and the potential for cell therapy. N Engl J Med 2003;349:275–286. [DOI] [PubMed] [Google Scholar]
- 2.Wernig M, Meissner A, Foreman R et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 2007; 448:318–324. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Li W, Laurent T et al. Small molecules, big roles- The chemical manipulation of stem cell fate and somatic cell reprogramming. J Cell Sci 2012;125:5609–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 2007;448:313–317. [DOI] [PubMed] [Google Scholar]
- 5.Ledermann B Embryonic stem cells and gene targeting. Exp Physiol 2000;85:603–613. [PubMed] [Google Scholar]
- 6.Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev 2015;26:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu C, Rosler E, Jiang J et al. Basic fibro-blast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. STEM CELLS 2005;23:315–323. [DOI] [PubMed] [Google Scholar]
- 8.Yoshihara M, Hayashizaki Y, Murakawa Y. Genomic instability of iPSCs: Challenges towards clinical applications. Stem Cell Rev 2017;13:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Do JT, Zhang Q et al. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci USA 2006;103: 17266–17271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Li K, Wei W et al. Chemical approaches to stem cell biology and therapeutics. Cell Stem Cell 2013;13:270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varki A, Freeze HH, Vacquier VD. Chapter 38: Glycans in development and systemic physiology In: Varki A, Cummings RD, Esko JD et al. eds. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2009. [PubMed] [Google Scholar]
- 12.Fox N, Damjanov I, Martinez-Hernandez A et al. Immunohistochemical localization of the early embryonic antigen (SSEA-1) in post-implantation mouse embryos and fetal and adult tissues. Dev Biol 1981;83:391–398. [DOI] [PubMed] [Google Scholar]
- 13.Freeze HH. Genetic defects in the human glycome. Nat Rev Genet 2006;7:537–551. [DOI] [PubMed] [Google Scholar]
- 14.Izumikawa T, Sato B, Kitagawa H. Chondroitin sulfate is indispensable for pluripotency and differentiation of mouse embryonic stem cells. Sci Rep 2014;4:3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin X, Wei G, Shi Z et al. Disruption of gastrulation and heparan sulfate biosynthesis. Dev Biol 2000;224:299–311. [DOI] [PubMed] [Google Scholar]
- 16.Johnson CE, Crawford BE, Stavridis M et al. Essential alterations of heparan sulfate during the differentiation of embryonic stem cells to Sox1-enhanced green fluorescent protein-expressing neural progenitor cells. STEM CELLS 2007;25:1913–1923. [DOI] [PubMed] [Google Scholar]
- 17.Kraushaar DC, Yamaguchi Y, Wang L. Heparan sulfate is required for embryonic stem cells to exit from self-renewal. J Biol Chem 2010;285:5907–5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraushaar DC, Dalton S, Wang L. Heparan sulfate: A key regulator of embryonic stem cell fate. Biol Chem 2013;394:741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnerch A, Cerdan C, Bhatia M. Distinguishing between mouse and human pluripotency regulation: The best laid plans of mice and men. STEM CELLS 2010; 28:419–430. [DOI] [PubMed] [Google Scholar]
- 20.Michaud P, Da Costa A, Courtois B et al. Polysaccharide lyases: Recent developments as biotechnological tools. Crit Rev Biotechnol 2003;23:233–266. [DOI] [PubMed] [Google Scholar]
- 21.Harfouche R, Hentschel DM, Piecewicz S et al. Glycome and transcriptome regulation of vasculogenesis. Circulation 2009;120:1883–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garud DR, Tran VM, Victor XV et al. Inhibition of heparan sulfate and chondroitin sul-fate proteoglycan biosynthesis. J Biol Chem 2008;283:28881–28887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegbahn A, Manner S, Persson A et al. Rules for priming and inhibition of glycosaminoglycan biosynthesis; probing the b4GalT7 active site. Chem Sci 2014;5:3501–3508. [Google Scholar]
- 24.Van Wijk XM, Oosterhof A, van den Broek SA et al. A 4-deoxy analogue of N-acetyl-D-glucosamine inhibits heparan sulphate expression and growth factor binding in vitro. Exp Cell Res 2010;316:2504–2512. [DOI] [PubMed] [Google Scholar]
- 25.Van Wijk XM, Lawrence R, Thijssen VL et al. A common sugar-nucleotide-mediated mechanism of inhibition of (glycosamino) glycan biosynthesis, as evidence by 6F-GalNAc (Ac3). FASEB J 2015;29:2993–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Humphries DE, Silbert JE. Chlorate: A reversible inhibitor of proteoglycan sulfation. Biochem Biophys Res Commun 1988;154: 365–371. [DOI] [PubMed] [Google Scholar]
- 27.Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science 1991;252:1705–1708. [DOI] [PubMed] [Google Scholar]
- 28.Baeuerle PA, Huttner WB. Chlorate: A potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun 1986; 141:870–877. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki N, Hirano T, Kobayashi K et al. Chemical inhibition of sulfation accelerates neural differentiation of mouse embryonic stem cells and human induced pluripotent stem cells. Biochem Biophys Res Commun 2010;401:480–486. [DOI] [PubMed] [Google Scholar]
- 30.Lanner F, Lee KL, Sohl M et al. Heparan sulfation-dependent fibroblast growth factor signaling maintains embryonic stem cells primed for differentiation in a heterogeneous state. STEM CELLS 2010;28:191–200. [DOI] [PubMed] [Google Scholar]
- 31.Choi S, Clements DJ, Pophristic V et al. The design and evaluation of heparin-binding foldamers. Angew Chem Int Ed Engl 2005;44: 6685–6689. [DOI] [PubMed] [Google Scholar]
- 32.Hunter DT Jr., Hill JM. Surfen: A quino-lone with oncogenic and heparin-neutralizing properties. Nature 1961;191:1378–1379. [DOI] [PubMed] [Google Scholar]
- 33.Roan NR, Sowinski S, Munch J et al. Aminoquinoline surfen inhibits the action of SEVI (semen-derived enhancer of viral infection). J Biol Chem 2010;285:1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu D, Fuster MM, Lawrence R et al. Heparan sulfate regulates VEGF165- and VEGF-mediated vascular hyperpermeability. J Biol Chem 2011;286:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warford J, Doucette CD, Hoskin DW et al. Murine T-cell activation is regulated by surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide). Biochem Biophys Res Commun 2014;443:524–530. [DOI] [PubMed] [Google Scholar]
- 36.Schuksz M, Fuster MM, Brown JR et al. Surfen, a small molecule antagonist of heparan sulfate. Proc Natl Acad Sci USA 2008; 105:13075–13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss RJ, Gordts PL, Le D et al. Small molecule antagonists of cell-surface heparan sulfate and heparin-protein interactions. Chem Sci 2015;6:5984–5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ying QL, Nichols J, Evans EP et al. Changing potency by spontaneous fusion. Nature 2002;416:545–548. [DOI] [PubMed] [Google Scholar]
- 39.Ying QL, Stavridis M, Griffiths D et al. Conversion of embryonic stem cells into neuroectodermal precursors in adherent mono-culture. Nat Biotechnol 2003;21:183–186. [DOI] [PubMed] [Google Scholar]
- 40.Yamazoe S, Shimogawa H, Sato S et al. A dumbbell-shaped small molecule that promotes cell adhesion and growth. Chem Biol 2009;16:773–782. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Rabenstein DL. Interaction of heparin with two synthetic peptides that neutralize the anticoagulant activity of heparin. Biochemistry 2006;45:15740–15747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ying QL, Wray J, Nichols J et al. The ground state of embryonic stem cell self-renewal. Nature 2008;453:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brons IGM, Smithers LE, Trotter MWB et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 2007; 448:191–195. [DOI] [PubMed] [Google Scholar]
- 44.Yayon A, Klagsbrun M, Esko JD et al. Cell surface, heparin-like molecules are required for binding of fibroblast growth factor to its high affinity receptor. Cell 1991;64:841–848. [DOI] [PubMed] [Google Scholar]
- 45.Feyzi E, Lustig F, Fager G et al. Characterization of heparin and heparan sulfate domains binding to the long splice variant of platelet-derived growth factor A chain. J Biol Chem 1997;272:5518–5524. [DOI] [PubMed] [Google Scholar]
- 46.Pankonin MS, Gallagher JT, Loeb JA. Specific structural features of heparan sulfate proteoglycans potentiate neuregulin-1 signaling. J Biol Chem 2005;280:383–388. [DOI] [PubMed] [Google Scholar]
- 47.Higashiyama S, Abraham JA, Miller J et al. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 1991;251:936–939. [DOI] [PubMed] [Google Scholar]
- 48.Pickford CE, Holley RJ, Rushton G et al. Specific glycosaminoglycans modulate neural specification of mouse embryonic stem cells. STEM CELLS 2011;29:629–640. [DOI] [PubMed] [Google Scholar]
- 49.Ono K, Hattori H, Takeshita S et al. Structural features in heparin that interact with VEGF165 and modulate its biological activity. Glycobiology 1999;9:705–711. [DOI] [PubMed] [Google Scholar]
- 50.Qiu H, Jiang J-L, Liu M et al. Quantitative phosphoproteomics analysis reveals broad regulatory role of heparan sulfate on endothelial signaling. Mol Cell Proteomics 2013;12:2160–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson M, Taylor AH, Jones EA et al. The culture of mouse embryonic stem cells and formation of embryoid bodies. Methods Mol Biol 2010;633:1–18. [DOI] [PubMed] [Google Scholar]
- 52.Wang YC, Stein JW, Lynch CL et al. Glycosyltransferase ST6GAL1 contributes to the regulation of pluripotency in human pluripotent stem cells. Sci Rep 2015;5:13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.