Abstract
Purpose
Methotrexate (MTX) treatment is associated with lower blood pressure (BP) and arterial stiffness in rheumatoid arthritis (RA). We investigated associations between single-nucleotide polymorphism (SNP) of the ATP-binding cassette efflux transporter gene ABCG2 (rs2231142), BP, and arterial stiffness in RA patients treated with MTX.
Patients and methods
Clinical and 24-hour peripheral and central BP, arterial wave reflection (Augmentation Index, AIx), arterial stiffness (Pulse Wave Velocity, PWV), and intracellular MTX polyglutamate (MTXPGs) concentrations were assessed in 56 RA patients on stable treatment with MTX using a repeated cross-sectional study design with measurements at baseline and after 8 months.
Results
Majority of the RA patients were homozygotes for the normal allele (CC, n=46) whereas 10 were rs2231142 heterozygotes (AC, n=10). MTXPGs concentrations were non-significantly higher in AC when compared to CC (144.3 vs 116.3 nmol/L packed RBCs, P=0.10). At baseline, the AC group had significantly lower age-adjusted clinical systolic BP (SBP) (P=0.01), 24-hour peripheral SBP (P=0.003), and central SBP (P=0.02) when compared to the CC group. However, AIx and PWV values were not significantly different between the two groups. When data from both visits were combined in a single analysis, and additionally adjusted for visit, gender, body mass index, and Disease Activity Score 28, the trend in SBP differences between-groups persisted but was no longer significant.
Conclusion
Future studies are required to test the hypothesis that this genetic polymorphism is associated with lower BP, arterial stiffness, and possibly, cardiovascular risk, in RA patients treated with MTX.
Keywords: methotrexate, disease-modifying antirheumatic drugs, blood pressure, augmentation index, rheumatoid arthritis, pulse wave velocity, single-nucleotide polymorphisms, ATP-binding cassette transporters, ABCG2
Introduction
The multidrug resistant ATP-binding cassette efflux transporter ABCG2, encoded by the ABCG2 gene in chromosome 4q22, is located in the membrane of various cells, including hepatocytes, renal cells, and enterocytes.1 Its main function is to reduce excessive exposure to toxins and drugs, including disease modifying anti-rheumatic drugs (DMARDs) such as methotrexate (MTX).2 ABCG2 transports not only MTX, but also its polyglutamate forms, specifically polyglutamate 2 and 3, and plays a key role in the absence of other transporters, such as ABCC2.3,4 The most common ABCG2 single nucleotide polymorphism (SNP) is rs2231142, where the C nucleotide is substituted for A at position number 421 (C421A), resulting in loss of ABCG2 function.4 Although studies have claimed that rs2231142 SNP does not influence the clinical response to MTX,5 a recent study showed that ABCG2 SNP influenced not only MTX treatment response but also its discontinuation.6 However, there is no conclusive evidence until now regarding the relationship between this SNP and MTX response rate in patients with rheumatoid arthritis (RA). We have recently reported that treatment with MTX is associated with lower blood pressure (BP) and pulse-wave velocity (PWV), a marker of arterial stiffness, in this group.7,8 These associations might explain, at least in part, the lower incidence of cardiovascular (CV) events in RA patients treated with MTX when compared to other DMARDs in epidemiological studies.9 Here, we seek to investigate the associations between the ABCG2 C421A SNP on red blood cell MTX polyglutamate concentrations (MTXPGs), BP, and arterial stiffness in RA patients treated with MTX in our RA cohort.
Methods
Patient recruitment and assessment have been described in detail elsewhere.7,8 Briefly, we studied a consecutive series of adult RA patients treated with MTX for at least 8 weeks. The study (registered in the Australian New Zealand Clinical Trials Registry, ACTRN12616001366448) was approved by the Southern Adelaide Clinical Human Research Ethics Committee (Ethics Approval Number: 76.14). Each participant gave an informed written consent before enrolling in the study.
MTXPGs, clinical and 24-hour peripheral and central BP, and markers of arterial stiffness (PWV and augmentation index, AIx) were assessed in two separate visits, at baseline and after 8-months.7,8 Genomic DNA was extracted from peripheral blood mononuclear cells. The concentration and purity of the samples was determined using a Nanodrop (Thermo Fisher Scientific, Waltham, MA, USA). The DNA was plated into a 96-well master plate at 5 ng/µL. The ABCG2 421C-A SNP (rs2231142) was determined by TaqMan® SNP genotyping assays using an ABI 7500 Fast Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA). After genotyping, and due to the relatively low frequency of the A allele, participants were classified based on their ABCG2 C421A status as: 1) loss of function, as determined by carriage of at least one copy of the A allele (ie., AA or AC genotype); or 2) normal function, or homozygote for the C allele (ie., CC genotype). Both the AC and CC groups were identified from within the same MTX-treated RA patient cohort, making the two genotype groups more likely to be comparable in terms of clinical and demographic characteristics.
Clinical peripheral SBP was the primary study end-point. Clinical peripheral diastolic BP (DBP), central BP, AIx, 24-hour peripheral and central BP, and PWV were secondary end-points. Based on our previous studies,7 a sample size of 56 RA patients treated with MTX provided 80% power (alpha=0.05) to detect a difference of 10 mmHg in SBP between the MTX and non-MTX groups. Variables were tested for normal distribution using the Kolmogorov-Smirnov test. Results are expressed as means ± SD, medians and interquartile ranges, or frequencies, as appropriate. Given the significant difference in age between the two genotype groups in this study, as well as the known impact of age on BP,10 AIx,11 and PWV,12 we calculated age-adjusted values prior to each comparison, and reported the age-adjusted means. We then combined the data from the baseline and 8-month visits and performed a between-genotype group comparison using a mixed effects model with adjustment for visit, age, gender, body mass index (BMI), and disease activity, as measured by the 28-joint Disease Activity Score (DAS28), which were included as fixed effects. The subject ID was included as a random intercept. Analyses were performed using Stata® software version 14.2 (StataCorp, TX, USA).
Results
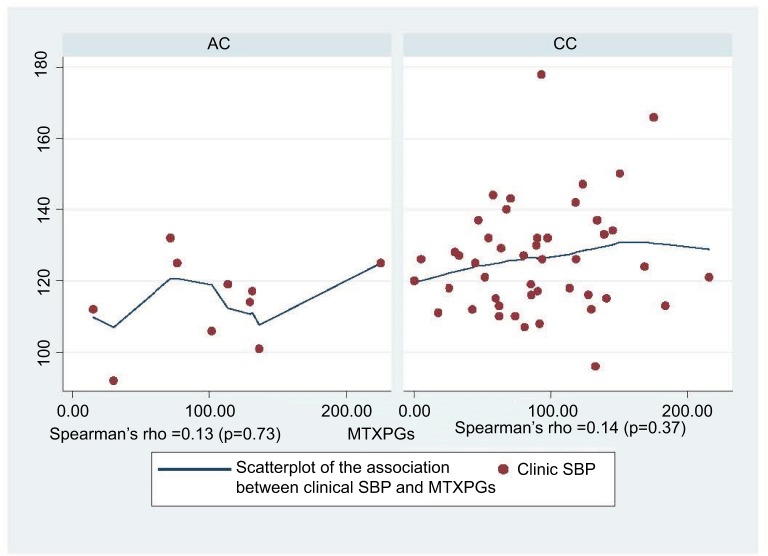
The majority of RA patients carried the CC genotype (n=46) whereas the remaining 10 carried the AC genotype. The CC group was significantly older than the AC group (52±18.7 vs 63±11 years, P=0.01; Table 1). The AC group had significantly lower age-adjusted peripheral and central SBP, and a trend towards lower peripheral and central DBP, when compared to the CC group (Table 1). The mean 24-hour-peripheral and central SBP values were also significantly lower in the AC group (Table 1). However, there were no significant between-group differences in AIx or PWV. After adjusting for MTX dose and duration, intracellular MTXPG concentrations were higher, albeit not significantly, in the AC group when compared to the CC group (Table 1). There was, however, a moderate positive correlation between clinical SBP and the level of MTXPGs in CC genotypes of ABCG2 SNP (Figure 1).
Table 1.
Baseline characteristics of subjects according to CC and AC genotyping of ABCG2 SNP
| CC genotype (n=46) | AC genotype (n=10) | P-value | |
|---|---|---|---|
|
| |||
| Age, years (mean ± SD) | 63±11 | 52±18.7 | 0.01b |
| Females, n (%) | 32 (69.6) | 7 (70.0) | 0.98 |
| Hypertension treatment, n (%) | 15 (32.6) | 1 (10.0) | 0.15 |
| BMI (kg/m3) | 27.4±0.62 | 25.7±1.4 | 0.27 |
| MTX dose (mg/week) | 14.5±0.56 | 14±1.2 | 0.72 |
| aMTXPGs (nmol/L packed RBCs) | 116.3±7 | 144.3±15.3 | 0.10 |
| CRP (mg/L) | 7±1.4 | 3.9±3.2 | 0.40 |
| DAS28 score | 2.8±1.1 | 2.6±0.2 | 0.55 |
| Stanford HAQ score | 0.69±0.08 | 0.89±0.18 | 0.33 |
| Clinical peripheral SBP (mmHg) | 127.2±1.5 | 117±3.5 | 0.01b |
| Clinical peripheral DBP (mmHg) | 74.1±1.1 | 69±2.5 | 0.07b |
| Clinical central SBP (mmHg) | 117.9±1.4 | 107±3.2 | 0.003c |
| Clinical central DBP (mmHg) | 76±1.1 | 70.8±2.5 | 0.06 |
| 24-hour peripheral SBP (mmHg) | 119.8±1.9 | 109.2±4 | 0.02b |
| 24-hour peripheral DBP (mmHg) | 70±1.2 | 65.7±2.5 | 0.13 |
| 24-hour central SBP (mmHg) | 116.4±1.4 | 108.9±3 | 0.02b |
| 24-hour central DBP (mmHg) | 76±0.90 | 73±1.9 | 0.18 |
| AIx (%) | 30.4±1.1 | 25.7±2.4 | 0.07b |
| AIx @ 75bpm (%) | 28.1±0.90 | 26.03±2 | 0.36 |
| 24-hour AIx @ 75bpm (%) | 29±0.75 | 26.6±1.6 | 0.19 |
| 24-hour PWV (m/second) | 9.01±0.09 | 9.04±0.20 | 0.91 |
Notes: All group means, except for age, are age-adjusted.
Means are adjusted for MTX dose and duration.
P-value≤0.05.
P-value≤0.01. Data presented as mean ± standard deviation unless otherwise indicated.
Abbreviations: RBCs, red blood cells; BMI, body mass index; MTX, Methotrexate; MTXPGs, MTX polyglutamates; SBP, systolic blood pressure; DBP, diastolic blood pressure; AIx, Augmentation index; PWV, pulse wave velocity; CRP, C-reactive protein.
Figure 1.
Association between clinical peripheral SBP and MTXPGs polyglutamate concentrations in MTX ABCG2 genotypes.
Abbreviations: clinical SBP, clinical peripheral systolic blood pressure; MTXPGs, methotrexate polyglutamates.
When data from both visits were combined in a mixed effects model with adjustment for age, gender, BMI, DAS28, and visit, there were no significant between-group differences in SBP, DPB, PWV, Alx, and MTXPGs (Table 2).
Table 2.
Adjusted mean (95% CI) differences in BP, AIx, PWV, and concentrations of MTXPGs between CC (n=46) and AC (n=10) polymorphisms of the MTX ABCG2 SNP using combined data from baseline and 8 months
| Model 1
|
Model 2
|
Model 3
|
||||
|---|---|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | |
|
| ||||||
| Clinic peripheral SBP (mmHg) | 6.2 (−3. 2, 15.5) | 0.19 | 5.7 (−3.7, 15.1) | 0.23 | 5.8 (−3.7, 15.4) | 0.23 |
| Clinic peripheral DBP (mmHg) | 1.9 (−4.4, 8.4) | 0.55 | 1.7 (−4.7, 8.05) | 0.61 | 1.4 (−5.03, 7.7) | 0.68 |
| Clinic central SBP (mmHg) | 7.3 (−1.7, 16.2) | 0.11 | 7.0 (−2.0, 16.0) | 0.13 | 7.02 (−2.1, 16.1) | 0.13 |
| Clinic central DBP (mmHg) | 2.7 (−3.7, 9.0) | 0.41 | 2.3 (−4.0, 8.7) | 0.47 | 1.9 (−4.4, 8.2) | 0.56 |
| AIx @75 (%) | −1.1 (−6.5, 4.2) | 0.68 | −0.92 (−6.3, 4.4) | 0.74 | −0.81 (−6.2, 4.6) | 0.77 |
| 24-hour peripheral SBP (mmHg) | 7.6 (−1.9, 17) | 0.12 | 7.1 (−2.3, 16.4) | 0.14 | 7.2 (−2.2, 16.6) | 0.13 |
| 24-hour peripheral DBP (mmHg) | 3.9 (−1.3, 9.1) | 0.14 | 3.5 (−1.6, 8.7) | 0.18 | 3.4 (−1.8, 8.6) | 0.20 |
| 24-hour central SBP (mmHg) | 4 (−2.9, 11.0) | 0.26 | 3.1 (−3.7, 9.8) | 0.37 | 3.4 (−3.3, 10.0) | 0.32 |
| 24-hour central DBP (mmHg) | 1.8 (−2.2, 6.0) | 0.38 | 1.4 (−2.7, 5.4) | 0.52 | 1.6 (−2.5, 5.6) | 0.45 |
| 24-hour AIx @75 (%) | 1.3 (−2.5, 5.2) | 0.50 | 1.3 (−2.5, 5.1) | 0.50 | 1.6 (−2.2, 5.4) | 0.42 |
| 24-hour PWV (m/second) | −0.04 (−0.74, 0.66) | 0.97 | −0.1 (−0.76, 0.63) | 0.85 | −0.1 (−76, 0.65) | 0.89 |
| MTXPGs (nmol/L) | 26.2 (−12.1, 64.5) | 0.18 | 27.7 (−10.6, 65.9) | 0.16 | 27.9 (−11.2, 67.0) | 0.16 |
Notes: Model 1 = adjusted for age and visit; Model 2 = adjusted for age, visit, gender, and BMI; Model 3 = adjusted for age, visit, gender, BMI, and DAS28.
Abbreviations: BMI, body mass index; MTX, Methotrexate; MTXPGs, MTX polyglutamates; BP, blood pressure; SBP, systolic BP; DBP, diastolic BP; AIx, Augmentation index; PWV, pulse wave velocity; DAS28, Disease Activity Score.
Discussion
This repeated cross-sectional study reported a significantly lower SBP, and a trend towards higher concentrations of MTXPGs, in RA patients with loss of function ABCG2 C421A genotypes when compared to patients who were homozygote for the normal allele. Although no longer significant during repeated assessment, the trend in between-group differences in SBP and DBP was maintained. We cannot rule out the possibility that the study was underpowered to detect these smaller, although clinically important, average differences observed in the repeated analysis. A larger group of AC genotype patients is therefore required to confirm our findings.
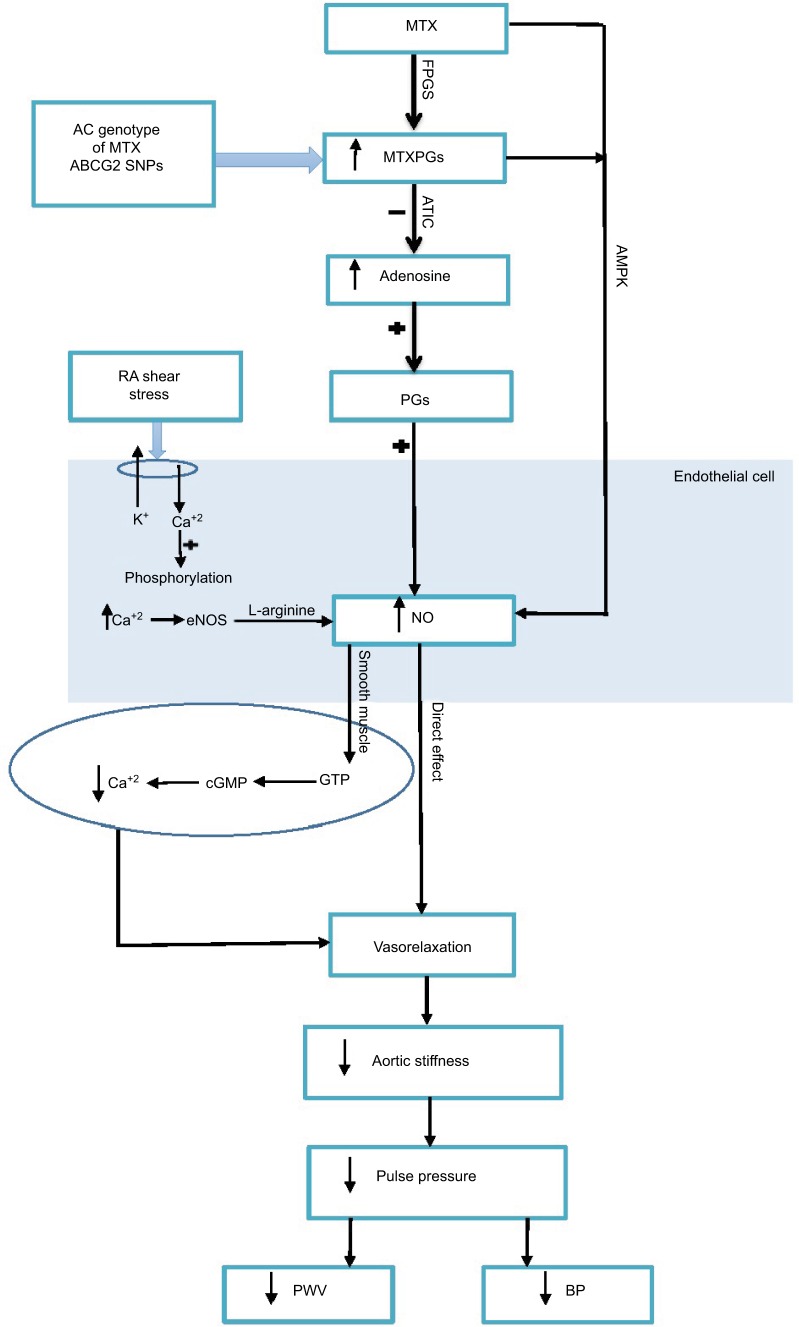
A few studies have failed to show that ABCG2 SNPs influence the clinical response to MTX.5,13 However, a recent study showed that ABCG2 SNPs are associated with MTX response in RA patients.6 Moreover, another study showed that A homozygotes have a reduced function of the ABCG2 transporter (ie., reduced efflux capability of MTX).14 This results in greater MTX accumulation and better MTX response.15 Furthermore, RA carriers of the AC genotype show better disease activity.16 Although this was attributed to the DMARD sulphasalazine, 94% of the RA cohort was treated with MTX. In agreement with the latter studies, this study found that the mutant allele of ABCG2 (AC) is associated with higher RBC accumulation of MTXPGs. The AC allele is associated with reduced function of the MTX transporter, hence greater MTXPG accumulation. Our preliminary results suggest that a higher concentration of MTXPGs, albeit not significant, might be associated with the reported differences in SBP between the AC and the CC groups. As suggested in our previous studies, MTXPGs inhibit aminoimidazole car-boxamide ribonucleotide transformylase.17 This leads to the accumulation of the substrate aminoimidazole carboxamide ribonucleotide and its metabolites which, in turn, inhibit adenosine deaminase and adenosine monophosphate deaminase.17 The accumulation of adenosine, with consequent BP lowering effects through peripheral and central mechanisms, might account for the reported differences in BP.18 Adenosine is known to cause vasodilation, either directly or through NO.19 Adenosine acts on the vascular endothelium via its receptors A1 and A2A and stimulates the release of PG, which enhances the release of nitric oxide (NO). Concurrently, adenosine acts centrally on the brain nucleus tractus, where adenosine activates endothelial NO synthase through A2A receptors. As a result, the level of NO is increased and thus, adenosine has a key role in modulating BP and arterial functions (Figure 2). The main strength of our study is the comprehensive repeated assessment of, clinical and over 24-hours, peripheral and central BP and markers of arterial stiffness. The limitations of our study include the lack of adenosine assessment and the possibility that the study might be underpowered to identify the somewhat smaller average differences in the repeated analysis. Due to inter-individual variability, genetic polymorphisms might provide essential information for a personalized approach to initiate the dose of MTX and subsequent monitoring. This MTX personalized approach could then be used to examine BP and arterial function effects of MTX on other high-risk patients with diseases other than RA—for example, designing randomized control trials for high-risk cardiac patients using MTX. Moreover, this genetic polymorphism could provide vital information in the field of molecular biology and genetic engineering. In particular, changing the sequence of the DNA intentionally is a known technique for introducing a mutation in a particular gene; it is also called site-specific mutagenesis. Researchers could use this finding in constructing novel MTX analogues that are characterized by combined anti-inflammatory and BP/arterial function effects. The CV-protective effects of MTX have a pharmacological attraction. MTX could be reused for managing CV risk in not only RA patients but also in the general population. It might provide useful information about the drug genetic variations that might assist physicians in personalizing the dose for high-risk patients. In conclusion, our study provides the first evidence of an association between genetic polymorphisms of MTX, and BP in RA patients. Further randomized-controlled studies are warranted to confirm these findings, and to establish whether these effects account for the reduced CV risk reported with MTX treatment.
Figure 2.
Potential mechanisms explaining the effects of MTX on BP and arterial function in RA.
Abbreviations: MTX, methotrexate; FPGs, Folylpolyglutamate synthetase enzyme; ATIC, 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase; NO, nitric oxide; eNOS, endothelial NO synthase; PG, prostaglandins; cGMP, cyclic guanosine-3′, 5-monophosphate; GTP, guanosine-5′-triphosphate; BP, blood pressure; PWV, pulse wave velocity; RA, rheumatoid arthritis.
Acknowledgments
Special thanks for the support by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University Riyadh, Saudi Arabia.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55(1):3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 2.van der Heijden JW, Oerlemans R, Tak PP, et al. Involvement of breast cancer resistance protein expression on rheumatoid arthritis synovial tissue macrophages in resistance to methotrexate and leflunomide. Arthritis Rheum. 2009;60(3):669–677. doi: 10.1002/art.24354. [DOI] [PubMed] [Google Scholar]
- 3.Vlaming ML, Pala Z, van Esch A, et al. Functionally overlapping roles of Abcg2 (Bcrp1) and Abcc2 (Mrp2) in the elimination of methotrexate and its main toxic metabolite 7-hydroxymethotrexate in vivo. Clin Cancer Res. 2009;15(9):3084–3093. doi: 10.1158/1078-0432.CCR-08-2940. [DOI] [PubMed] [Google Scholar]
- 4.Volk EL, Schneider E. Wild-type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporter. Cancer Res. 2003;63(17):5538–5543. [PubMed] [Google Scholar]
- 5.Kato T, Hamada A, Mori S, Saito H. Genetic polymorphisms in metabolic and cellular transport pathway of methotrexate impact clinical outcome of methotrexate monotherapy in Japanese patients with rheumatoid arthritis. Drug Metab Pharmacokinet. 2012;27(2):192–199. doi: 10.2133/dmpk.dmpk-11-rg-066. [DOI] [PubMed] [Google Scholar]
- 6.Jenko B, Lusa L, Tomsic M, Praprotnik S, Dolzan V. Clinical-pharmacogenetic predictive models for MTX discontinuation due to adverse events in rheumatoid arthritis. Pharmacogenomics J. 2017;17(5):412–418. doi: 10.1038/tpj.2016.36. [DOI] [PubMed] [Google Scholar]
- 7.Mangoni AA, Baghdadi LR, Shanahan EM, et al. Methotrexate, blood pressure and markers of arterial function in patients with rheumatoid arthritis: a repeated cross-sectional study. Ther Adv Musculoskelet Dis. 2017;9(9):213–229. doi: 10.1177/1759720X17719850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodman RJ, Baghdadi LR, Shanahan ME, Mangoni AA. The Temporal Relationship between Arterial Stiffening and Blood Pressure Is Modified by Methotrexate Treatment in Patients with Rheumatoid Arthritis. Front Physiol. 2017;8:593. doi: 10.3389/fphys.2017.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108(9):1362–1370. doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janner JH, Godtfredsen NS, Ladelund S, Vestbo J, Prescott E. The association between aortic augmentation index and cardiovascular risk factors in a large unselected population. J Hum Hypertens. 2012;26(8):476–484. doi: 10.1038/jhh.2011.59. [DOI] [PubMed] [Google Scholar]
- 11.Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74(11):2257–2262. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- 12.Alghatrif M, Strait JB, Morrell CH, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62(5):934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamp LK, Chapman PT, O’Donnell JL, et al. Polymorphisms within the folate pathway predict folate concentrations but are not associated with disease activity in rheumatoid arthritis patients on methotrexate. Pharmacogenet Genomics. 2010;20(6):367–376. doi: 10.1097/FPC.0b013e3283398a71. [DOI] [PubMed] [Google Scholar]
- 14.Dervieux T, Furst D, Lein DO, et al. Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis Rheum. 2004;50(9):2766–2774. doi: 10.1002/art.20460. [DOI] [PubMed] [Google Scholar]
- 15.Chan ES, Cronstein BN. Mechanisms of action of methotrexate. Bull Hosp Jt Dis. 2013;71(Suppl 1):S5–8. [PubMed] [Google Scholar]
- 16.Wiese MD, Alotaibi N, O’Doherty C, et al. Pharmacogenomics of NAT2 and ABCG2 influence the toxicity and efficacy of sulphasalazine containing DMARD regimens in early rheumatoid arthritis. Pharmacogenomics J. 2014;14(4):350–355. doi: 10.1038/tpj.2013.45. [DOI] [PubMed] [Google Scholar]
- 17.Baggott JE, Vaughn WH, Hudson BB. Inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase, adenosine deaminase and 5′-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochem J. 1986;236(1):193–200. doi: 10.1042/bj2360193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koupenova M, Johnston-Cox H, Ravid K. Regulation of atherosclerosis and associated risk factors by adenosine and adenosine receptors. Curr Atheroscler Rep. 2012;14(5):460–468. doi: 10.1007/s11883-012-0263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Fenton RA, Wheeler HB, et al. Adenosine A2a receptors increase arterial endothelial cell nitric oxide. J Surg Res. 1998;80(2):357–364. doi: 10.1006/jsre.1998.5439. [DOI] [PubMed] [Google Scholar]