Abstract
Background
With the advent of combination antiretroviral therapy (ART), people living with HIV have lived to older age. So they have experienced age-related illnesses and have taken non-antiretroviral (ARV) medications to manage these illnesses. The aims of this study were to investigate the use patterns of ARV agents in HIV-positive patients by age and to evaluate potential or contraindicated drug–drug interactions (DDIs) between ARV and non-ARV.
Methods
This study was retrospectively conducted with HIV-infected patients receiving ART medications between October 2011 and September 2017 at Chonbuk National University Hospital in South Korea. Data were collected by reviewing patients’ electronic medical charts.
Results
Among 207 patients diagnosed with HIV infection, 183 (86.9% males; 104 aged <50 years and 79 aged ≥50 years) were selected based on inclusion criteria. In 2017, the most frequently prescribed ART regimen was nucleoside reverse transcriptase inhibitors (NRTIs)/integrase strand transfer inhibitors (INSTIs; total, 66.3%; <50 years, 36.3%; ≥50 years, 30.0%) followed by NRTIs/protease inhibitors (PIs; total, 23.8%; <50 years, 15.0%; ≥50 years, 8.8%). In 2017, the most frequently prescribed NRTI combination was abacavir/lamivudine (total, 34.4%; <50 years, 20.6%; ≥50 years, 13.8%) followed by tenofovir alafenamide/emtricitabine (FTC; total, 31.3%; <50 years, 16.3%; ≥50 years, 15.0%) and tenofovir disoproxil fumarate/FTC (total, 28.1%; <50 years, 16.9%; ≥50 years, 11.3%). In 2017, elvitegravir (EVG)/cobicistat (COBI; total, 57.1%; <50 years, 30.4%; ≥50 years, 26.8%) was most frequently prescribed followed by dolutegravir (total, 32.1%; <50 years, 19.6%; ≥50 years, 12.5%). Potential or contraindicated DDIs between boosted PIs with ritonavir or EVG/COBI and coprescribed drugs occurred most frequently.
Conclusion
Currently, NRTIs/INSTIs is the most frequently prescribed ARV combination. Abacavir/lamivudine, tenofovir alafenamide/FTC, and tenofovir disoproxil fumarate/FTC are the most used NRTIs, and EVG/COBI followed by dolutegravir is the most prescribed INSTIs. Potential or contraindicated DDIs occur mainly between boosted PIs or EVG/COBI and non-ARV medications.
Keywords: human immunodeficiency virus, highly active antiretroviral therapy, drug utilization, drug interactions, Korea, elderly patients
Introduction
Since the advent of combination antiretroviral therapy (ART), the survival and quality of life of people living with HIV (PLWH) have steadily improved, thereby increasing the number of older PLWH.1,2 A 2013 estimate revealed that ~4.2 million PLWH across the world were >50 years old, and it is expected that this number will continue to increase.3 An aging population of PLWH is likely to experience age-associated illnesses such as cardiovascular diseases (CVD), cancers, osteoporosis, and cognitive impairment, similar to the general population. These age-related comorbidities may require chronic treatment. Consequently, it is necessary to not only suppress the viral load of HIV but also control age-related, non-AIDS-related illnesses for effectively managing an aging population of PLWH.
The combination of antiretroviral (ARV) and non-ARV medications in an aging population of PLWH may lead to adverse events (AEs), drug–drug interactions (DDIs), and poor drug adherence, all of which have negative effects on the efficacy and safety of ARV and non-ARV medications.4–8 In particular, the rate of incidence of DDIs is likely to rise in an aging population of PLWH due to polypharmacy for the treatment of multiple comorbidities along with the HIV infection.4,9–11 According to a retrospective clinical study involving HIV-positive patients aged ≥50 years, the average number of total prescribed medications was 14.2±5.9, and that of concomitant medications excluding ARVs was 11.6±5.7.7 Twenty-five contraindicated DDIs were found to occur in 20 (8.1%) HIV-infected patients.7 In other retrospective clinical studies, potential and contraindicated DDIs were found in 71 (62.8%) and 6 (5.3%) patients, respectively, out of 113 HIV-positive patients receiving comedications.9
The incidence rate of DDIs in HIV-positive patients may vary according to the ART regimens used. Potential DDIs may occur more frequently in HIV-positive patients on ritonavir (RTV)- or cobicistat (COBI)-boosted protease inhibitor (PI)-based or on non-nucleoside reverse transcriptase inhibitor (NNRTI)-based ART regimens.11,12 PI-based ART regimens are nine times as likely to induce potential DDIs as regimens without PIs.13 Additionally, patients treated with ART regimens containing NNRTIs were likely to experience about 4.3 times as many potential DDIs as their counterparts.13 Therefore, it is important that appropriate ART regimens that are less likely to interact with other comedications and less likely to affect non-AIDS illnesses are used, especially in older HIV-positive patients who are already suffering from comorbidities.
This study was aimed to investigate ARV usage patterns among HIV-positive patients in an age-wise manner and to evaluate the potential or contraindicated DDIs between ARVs and concomitantly prescribed drugs.
Methods
The Institutional Review Board (IRB) of Chonbuk National University Hospital granted ethical approval for this study (CUH 2017-11-028). The IRB waived the requirement for obtaining informed consent from the participants in this study since their data were deidentified and anonymously encoded prior to commencing analyses. This study was retrospectively conducted with the following categories of patients visiting Chonbuk National University Hospital, located in the city of Jeonju in North Jeolla Province of South Korea, between October 2011 and September 2017. The inclusion criteria were the following: 1) age ≥18 years; 2) diagnosis of HIV infection; and 3) having received ART at least once.
A retrospective chart review of the electronic medical records of selected HIV-infected patients was conducted, in which a trained hospital pharmacist collected the following information from paper case report forms: demographic characteristics (sex, age, weight, height, and body mass index), risk factors for HIV infection, prior HIV treatment, hepatitis B virus and hepatitis C virus positivity, comorbidities, prescribed medications for HIV infection and other diseases, and laboratory values (HIV-1 RNA copy, CD4+T-cell count, and estimated glomerular filtration rate [eGFR]).
Older adults living with HIV infection may suffer from more comorbidities and experience more rapid physical and cognitive aging than their normal counterparts do. Study of HIV-related literature reveals that the aging HIV-infected population is represented by patients aged ≥50 years.14 To compare the differences in the use of ARV drug regimens among individuals of different ages, the selected patients were divided into two groups, namely, patients <50 years and patients ≥50 years. In order to assess the usage patterns of HIV regimens in a year-wise manner during the study period, the regimens were categorized on the basis of the combination of two nucleoside reverse transcriptase inhibitors (NRTIs) and a third ARV agent based on recently published HIV treatment guidelines.15,16 The potential and contraindicated DDIs between ARV agents and concomitantly prescribed drugs during the study period were also investigated using the Liverpool HIV Drug Interactions website.17
All the analyses were conducted using SAS, version 9.3 (SAS Institute Inc., Cary, NC, USA). The mean and SD were used for continuous variables, whereas the frequencies (n) and percentages (%) were used for the categorical variables. The independent t-test was performed for comparing the differences in the means of the continuous variables, and the chi-squared test or Fisher’s exact test was also conducted for comparing the differences in the proportions of the categorical variables. P-values <0.05 were considered to be statistically significant.
Results
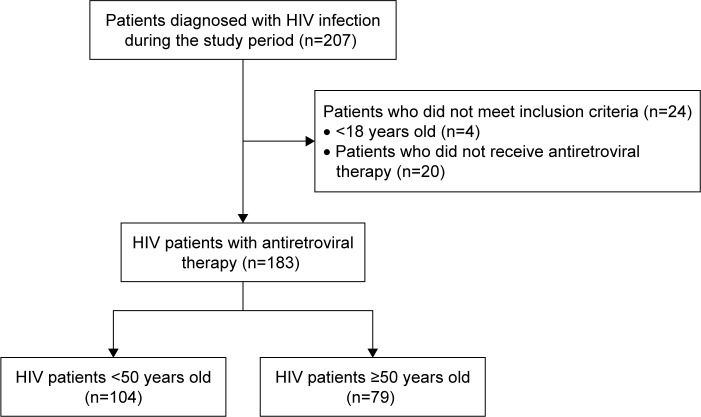
During the study period, 207 patients were diagnosed with HIV infection, of which 183 patients (104 patients aged <50 years and 79 patients aged ≥50 years) who met the aforementioned inclusion criteria were selected for the analysis (Figure 1). The baseline characteristics of the patients are presented in Table 1 and arranged according to their ages. The average age of all the patients was 47.3±12.4 years, and most of the patients (86.9%) were males. The eGFR levels of the patients aged <50 years were significantly higher than those of the patients aged ≥50 years. However, the incidence rates of diabetes mellitus (DM), hypertension (HTN), cancer, and benign prostatic hyperplasia were significantly higher in the patients aged ≥50 years than in patients aged <50 years.
Figure 1.
Flow diagram of steps in the selection of study subjects.
Table 1.
Baseline characteristics of the patients included in the study
| Variable | All patients (n=183) | <50 years (n=104) | ≥50 years (n=79) | P-value |
|---|---|---|---|---|
|
| ||||
| Age, mean (SD), years | 47.3 (12.4) | 38.7 (7.8) | 58.5 (7.2) | <0.001 |
| Sex, n (%) | ||||
| Male | 159 (86.9) | 89 (85.6) | 70 (88.6) | 0.547 |
| Female | 24 (13.1) | 15 (14.4) | 9 (11.4) | |
| BMI, mean (SD), kg/m2 | 22.3 (3.4) | 21.7 (3.5) | 23.1 (3.2) | 0.056 |
| Risk factors for HIV infection, n (%) | ||||
| Intravenous drug use | 1 (0.5) | 1 (1.0) | 0 (0.0) | |
| Hetero/homosexual | 37 (20.2) | 19 (18.3) | 18 (22.8) | 0.522 |
| Unknown | 145 (79.2) | 84 (80.8) | 61 (77.2) | |
| Previous HIV treatment, n (%) | ||||
| Naïve | 108 (59.0) | 66 (63.5) | 42 (53.2) | 0.161 |
| Experienced | 75 (41.0) | 38 (36.5) | 37 (46.8) | |
| HIV-1 RNA copy, mean (SD), copies/mL | 195,249 (93,942.4) | 292,763 (122,477.8) | 62,630 (14,359.5) | 0.107 |
| HIV-1 RNA copy, n (%) | ||||
| HIV-1 RNA copy<100,000 copies/mL | 136 (76.8) | 72 (70.6) | 64 (85.3) | |
| HIV-1 RNA copy≥100,000 copies/m | 41 (23.2) | 30 (29.4) | 11 (14.7) | 0.026 |
| CD4+T-cell count, mean (SD), cells/mm3 | 316.3 (223.3) | 296.9 (220.6) | 343.2 (225.6) | 0.175 |
| CD4+T-cell count, cells/mm3, n (%) | ||||
| <50 | 27 (15.3) | 18 (17.6) | 9 (12.2) | |
| ≥50 to <200 | 33 (18.8) | 19 (18.6) | 14 (18.9) | 0.601 |
| ≥200 | 116 (65.9) | 65 (63.7) | 51 (68.9) | |
| HBV positive, n (%) | 11 (6.0) | 4 (3.7) | 7 (8.9) | 0.212 |
| HCV positive, n (%) | 1 (0.5) | 1 (1.0) | 0 (0.0) | 1.000 |
| eGFR, mean (SD), mL/min/1.73 m2 | 108.0 (20.2) | 113.0 (21.2) | 100.5 (16.2) | 0.004 |
| Comorbidity, n (%) | ||||
| Diabetes mellitus | 28 (15.3) | 6 (5.8) | 22 (27.8) | <0.001 |
| Hypertension | 10 (5.5) | 1 (1.0) | 9 (11.4) | 0.003 |
| Dyslipidemia | 4 (2.2) | 1 (1.0) | 3 (3.8) | 0.317 |
| Cancer | 15 (8.2) | 4 (3.8) | 11 (13.9) | 0.014 |
| Asthma | 3 (1.6) | 1 (1.0) | 2 (2.5) | 0.579 |
| Chronic obstructive pulmonary disease | 1 (0.5) | 0 (0.0) | 1 (1.3) | 0.432 |
| Dementia, cognitive impairment | 5 (2.7) | 2 (1.9) | 3 (3.8) | 0.653 |
| Chronic kidney disease | 4 (2.2) | 3 (2.9) | 1 (1.3) | 0.635 |
| Benign prostatic hyperplasia | 9 (4.9) | 0 (0.0) | 9 (11.4) | <0.001 |
| Erectile dysfunction | 4 (2.2) | 2 (1.9) | 2 (2.5) | 1.000 |
| Myelodysplastic syndromes | 1 (0.5) | 0 (0.0) | 1 (1.3) | 0.432 |
| Gastritis, gastroesophageal reflux disease | 17 (9.3) | 7 (6.7) | 10 (12.7) | 0.171 |
| Thyroid disease | 1 (0.5) | 0 (0.0) | 1 (1.3) | 0.432 |
| Seizure | 4 (2.2) | 4 (3.8) | 0 (0.0) | 0.135 |
| Stroke | 7 (3.8) | 2 (1.9) | 5 (6.3) | 0.242 |
| Acute coronary syndrome | 2 (1.1) | 1 (1.0) | 1 (1.3) | 1.000 |
| Depression | 8 (4.4) | 4 (3.8) | 4 (5.1) | 1.000 |
| Opportunistic infections, n (%) | ||||
| Syphilis | 29 (15.8) | 15 (14.4) | 14 (17.7) | 0.545 |
| Pneumocystis pneumonia | 23 (12.6) | 14 (13.5) | 9 (11.4) | 0.676 |
| Candidiasis | 22 (12.0) | 15 (14.4) | 7 (8.9) | 0.252 |
| Varicella-Zoster virus | 14 (7.7) | 9 (8.7) | 5 (6.3) | 0.558 |
| Tuberculosis | 9 (4.9) | 5 (4.8) | 4 (5.1) | 0.937 |
| Cytomegalovirus | 9 (4.9) | 6 (5.8) | 3 (3.8) | 0.541 |
| Human papillomavirus | 9 (4.9) | 5 (4.8) | 4 (5.1) | 0.937 |
| Pneumonia | 9 (4.9) | 4 (3.8) | 5 (6.3) | 0.442 |
| Herpes simplex virus | 8 (4.4) | 6 (5.8) | 2 (2.5) | 0.289 |
| Mycobacterium avium complex | 4 (2.2) | 3 (2.9) | 1 (1.3) | 0.458 |
| Cryptococcosis | 4 (2.2) | 2 (1.9) | 2 (2.5) | 0.780 |
| JC virus | 2 (1.1) | 2 (1.9) | 0 (0.0) | 0.218 |
| Toxoplasmic encephalitis | 2 (1.1) | 0 (0.0) | 2 (2.5) | 0.103 |
| Kaposi’s sarcoma | 1 (0.5) | 1 (1.0) | 0 (0.0) | 0.382 |
| ARV regimens, n (%) | ||||
| NRTIs/NNRTIs | 37 (20.2) | 21 (20.2) | 16 (20.3) | |
| NRTIs/PIs | 100 (54.6) | 60 (57.7) | 40 (50.6) | 0.649 |
| NRTIs/INSTIs | 43 (23.5) | 21 (20.2) | 22 (27.8) | |
| NRTIs/NNRTIs/PIs | 3 (1.6) | 2 (1.9) | 1 (1.3) | |
Abbreviations: ARV, antiretroviral; BMI, body mass index; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HCV, hepatitis C virus; INSTIs, integrase strand transfer inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors.
The ARV drug combination regimens used during the study period are presented in a year-wise manner in Table 2. In 2011, the combination of NRTIs and PIs was most frequently prescribed in both groups of patients. Between 2011 and 2014, the prescription rate of this combination remained almost stable; however, the prescription rate gradually declined after 2014. The prescription rate of the combination of NRTIs and integrase strand transfer inhibitors (INSTIs) had tended to gradually increase ever since its first use in 2012, and this combination was the most frequently prescribed ARV drug regimen in 2017.
Table 2.
Antiretroviral drug combination regimens used during the study period
| Regimen | Age, years | Year, n (%)
|
||||||
|---|---|---|---|---|---|---|---|---|
| 2011 (n=53) | 2012 (n=71) | 2013 (n=89) | 2014 (n=115) | 2015 (n=130) | 2016 (n=154) | 2017 (n=160) | ||
|
| ||||||||
| NRTIs/NNRTIs | Total | 9 (17.0) | 11 (15.5) | 8 (9.0) | 13 (11.3) | 11 (8.5) | 23 (14.9) | 8 (5.0) |
| <50 | 5 (9.4) | 6 (8.5) | 3 (3.4) | 5 (4.3) | 4 (3.1) | 11 (7.1) | 3 (1.9) | |
| ≥50 | 4 (7.5) | 5 (7.0) | 5 (5.6) | 8 (7.0) | 7 (5.4) | 12 (7.8) | 5 (3.1) | |
|
| ||||||||
| NRTIs/PIs | Total | 40 (75.5) | 50 (70.4) | 61 (68.5) | 76 (66.1) | 67 (51.5) | 64 (41.6) | 38 (23.8) |
| <50 | 23 (43.4) | 30 (42.3) | 36 (40.5) | 45 (39.1) | 39 (30.0) | 40 (26.0) | 24 (15.0) | |
| ≥50 | 17 (32.1) | 20 (28.2) | 25 (28.1) | 31 (27.0) | 28 (21.5) | 24 (15.6) | 14 (8.8) | |
|
| ||||||||
| NRTIs/INSTIs | Total | – | 7 (9.9) | 17 (19.1) | 23 (20.0) | 45 (34.6) | 59 (38.3) | 106 (66.3) |
| <50 | – | – | 5 (5.6) | 7 (6.1) | 20 (15.4) | 30 (19.5) | 58 (36.3) | |
| ≥50 | – | 7 (9.9) | 12 (13.5) | 16 (13.9) | 25 (19.2) | 29 (18.8) | 48 (30.0) | |
|
| ||||||||
| NRTIs/NNRTIs/PIs | Total | 3 (5.7) | – | – | – | – | – | – |
| <50 | 2 (3.8) | – | – | – | – | – | – | |
| ≥50 | 1 (1.9) | – | – | – | – | – | – | |
|
| ||||||||
| NRTIs/PIs/INSTIs | Total | – | – | 1 (1.1) | 1 (0.9) | 2 (1.5) | 2 (1.3) | 1 (0.6) |
| <50 | – | – | – | – | 1 (0.8) | 1 (0.6) | – | |
| ≥50 | – | – | 1 (1.1) | 1 (0.9) | 1 (0.8) | 1 (0.6) | 1 (0.6) | |
|
| ||||||||
| NRTIs/NNRTIs/INSTIs | Total | – | – | – | – | – | – | 1 (0.6) |
| <50 | – | – | – | – | – | – | – | |
| ≥50 | – | – | – | – | – | – | 1 (0.6) | |
|
| ||||||||
| Non-NRTIs | Total | 1 (1.9) | 3 (4.2) | 2 (2.2) | 2 (1.7) | 5 (3.8) | 6 (3.9) | 6 (3.8) |
| <50 | 1 (1.9) | 2 (2.8) | 2 (2.2) | 2 (1.7) | 3 (2.3) | 3 (1.9) | 3 (1.9) | |
| ≥50 | – | 1 (1.4) | – | – | 2 (1.5) | 3 (1.9) | 3 (1.9) | |
Abbreviations: INSTIs, integrase strand transfer inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors.
The prescribed ARV drugs are presented in a year-wise manner in Table 3. Although zidovudine (ZDV)/lamivudine (3TC) was most frequently prescribed in 2011 (54.7%), its prescription rate gradually decreased by 2.5% in 2017. The prescription rate of abacavir (ABC)/3TC remained stable throughout the study period. The prescription rate of tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) exhibited a steady increase from 2012 (31.0%) to 2016 (60.4%). However, in 2017, this rate became almost half (28.1%) of that in 2016. The prescription rate of tenofovir alafenamide (TAF)/FTC, which was first used in 2017, was 31.3%, which may account for the decrease in the prescription rate of TDF/FTC. Rilpivirine (RPV) had been the most frequently prescribed NNRTI ever since its first use in 2015. Although efavirenz had been the most frequently prescribed NNRTI from 2011 to 2015, its prescription ended in 2017. Atazanavir and lopinavir (LPV)/RTV had been steadily prescribed until the introduction of elvitegravir (EVG) combined with COBI in 2015. In 2017, the prescription rate of boosted PIs with RTV or COBI was 97.8%. The prescription rates of dolutegravir (DTG) and EVG/COBI have been gradually increasing since their first use in 2015, whereas that of raltegravir (RAL) decreased after 2014.
Table 3.
NRTIs, NNRTIs, PIs, and INSTIs used during the study period
| Antiretroviral drug | Age, years
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 2011
|
2012
|
2013
|
2014
|
2015
|
2016
|
2017
|
||
| NRTIs | (n=53) | (n=71) | (n=89) | (n=115) | (n=130) | (n=154) | (n=160) | |
|
| ||||||||
| ABC/3TC | Total | 12 (22.6) | 18 (25.4) | 28 (31.5) | 40 (34.8) | 42 (32.3) | 50 (32.5) | 55 (34.4) |
| <50 | 8 (15.1) | 11 (15.5) | 16 (18.0) | 22 (19.1) | 22 (16.9) | 28 (18.2) | 33 (20.6) | |
| ≥50 | 4 (7.5) | 7 (9.9) | 12 (13.5) | 18 (15.7) | 20 (15.4) | 22 (14.3) | 22 (13.8) | |
|
| ||||||||
| TDF/FTC | Total | – | 22 (31.0) | 32 (36.0) | 46 (40.0) | 77 (59.2) | 93 (60.4) | 45 (28.1) |
| <50 | – | 10 (14.1) | 16 (18.0) | 24 (20.9) | 39 (30.0) | 52 (33.8) | 27 (16.9) | |
| ≥50 | – | 12 (16.9) | 16 (18.0) | 22 (19.1) | 38 (29.2) | 41 (26.6) | 18 (11.3) | |
|
| ||||||||
| ZDV/3TC | Total | 29 (54.7) | 28 (39.4) | 27 (31.5) | 27 (23.5) | 7 (5.4) | 5 (3.2) | 4 (2.5) |
| <50 | 17 (32.1) | 15 (21.1) | 12 (13.5) | 11 (9.6) | 4 (3.1) | 2 (1.3) | 1 (0.6) | |
| ≥50 | 12 (22.6) | 13 (18.3) | 15 (18.0) | 16 (13.9) | 3 (2.3) | 3 (1.9) | 3 (1.9) | |
|
| ||||||||
| 3TC/d4T | Total | 7 (13.2) | – | – | – | – | – | – |
| <50 | 2 (3.8) | – | – | – | – | – | – | |
| ≥50 | 5 (9.4) | – | – | – | – | – | – | |
|
| ||||||||
| TAF/FTC | Total | – | – | – | – | – | – | 50 (31.3) |
| <50 | – | – | – | – | – | – | 26 (16.3) | |
| ≥50 | – | – | – | – | – | – | 24 (15.0) | |
|
| ||||||||
| 3TC/ddI | Total | 1 (1.9) | – | – | – | – | – | – |
| <50 | 1 (1.9) | – | – | – | – | – | – | |
| ≥50 | – | – | – | – | – | – | – | |
|
| ||||||||
| 3TC | Total | 3 (5.7) | – | – | – | – | ||
| <50 | 2 (3.8) | – | – | – | – | |||
| ≥50 | 1 (1.9) | – | – | – | – | – | – | |
|
| ||||||||
| Not used | Total | 1 (1.9) | 3 (4.2) | 2 (2.2) | 2 (1.7) | 4 (3.1) | 6 (3.9) | 6 (3.8) |
| <50 | 1 (1.9) | 2 (2.8) | 2 (2.2) | 2 (1.7) | 2 (1.5) | 3 (1.9) | 3 (1.9) | |
| ≥50 | – | 1 (1.4) | – | – | 2 (1.5) | 3 (1.9) | 3 (1.9) | |
|
| ||||||||
| NNRTIs | (n=13) | (n=14) | (n=10) | (n=15) | (n=15) | (n=28) | (n=14) | |
|
| ||||||||
| EFV | Total | 9 (69.2) | 11 (78.6) | 8 (88.9) | 13 (86.7) | 10 (66.7) | 2 (7.1) | – |
| <50 | 5 (38.5) | 6 (42.9) | 3 (30.0) | 5 (33.3) | 4 (26.7) | 1 (3.6) | – | |
| ≥50 | 4 (30.8) | 5 (35.7) | 5 (50.0) | 8 (53.3) | 6 (40.0) | 1 (3.6) | – | |
|
| ||||||||
| ETR | Total | 1 (7.7) | 3 (21.4) | 2 (20.0) | 2 (13.3) | 4 (26.7) | 6 (21.4) | 6 (42.9) |
| <50 | 1 (7.7) | 2 (14.3) | – | – | 3 (20.0) | 3 (10.7) | 3 (21.4) | |
| ≥50 | – | 1 (7.1) | – | – | 1 (6.7) | 3 (10.7) | 3 (21.4) | |
|
| ||||||||
| NVP | Total | 3 (23.1) | – | – | – | – | – | – |
| <50 | 2 (15.4) | – | – | – | – | – | – | |
| ≥50 | 1 (7.7) | – | – | – | – | – | – | |
|
| ||||||||
| RPV | Total | – | – | – | – | 1 (6.7) | 20 (71.4) | 8 (57.1) |
| <50 | – | – | – | – | – | 10 (35.7) | 4 (28.6) | |
| ≥50 | – | – | – | – | 1 (6.7) | 10 (35.7) | 4 (28.6) | |
|
| ||||||||
| PIs | (n=44) | (n=53) | (n=64) | (n=79) | (n=74) | (n=72) | (n=45) | |
|
| ||||||||
| ATV | Total | 13 (29.5) | 17 (32.1) | 19 (29.7) | 17 (21.5) | 6 (8.1) | 3 (4.2) | 1 (2.2) |
| <50 | 5 (11.4) | 9 (17.0) | 10 (15.6) | 9 (11.4) | 3 (4.1) | 1 (1.4) | 1 (2.2) | |
| ≥50 | 8 (18.2) | 8 (15.1) | 9 (14.1) | 8 (10.1) | 3 (4.1) | 2 (2.8) | – | |
|
| ||||||||
| ATV/RTV | Total | – | – | – | 7 (8.9) | 6 (8.1) | 4 (5.6) | 1 (2.2) |
| <50 | – | – | – | 4 (5.1) | 5 (6.8) | 4 (5.6) | 1 (2.2) | |
| ≥50 | – | – | – | 3 (3.8) | 1 (1.4) | – | – | |
|
| ||||||||
| ATV/COBI | Total | – | – | – | – | – | – | 2 (4.4) |
| <50 | – | – | – | – | – | – | 1 (2.2) | |
| ≥50 | – | – | – | – | – | – | 1 (2.2) | |
|
| ||||||||
| LPV/RTV | Total | 27 (61.4) | 30 (56.6) | 39 (60.9) | 45 (57.0) | 35 (47.3) | 33 (45.8) | 30 (66.7) |
| <50 | 18 (40.9) | 19 (35.8) | 24 (37.5) | 27 (34.2) | 19 (25.7) | 19 (26.4) | 17 (37.8) | |
| ≥50 | 9 (20.5) | 11 (20.8) | 15 (23.4) | 18 (22.8) | 16 (21.6) | 14 (19.4) | 13 (28.9) | |
|
| ||||||||
| DRV/RTV | Total | 1 (2.3) | 4 (7.5) | 4 (6.3) | 9 (11.4) | 26 (35.1) | 32 (44.4) | 7 (15.6) |
| <50 | 1 (2.3) | 3 (5.7) | 3 (4.7) | 7 (8.9) | 16 (21.6) | 20 (27.8) | 6 (13.3) | |
| ≥50 | – | 1 (1.9) | 1 (1.6) | 2 (2.5) | 10 (13.5) | 12 (16.7) | 1 (2.2) | |
|
| ||||||||
| DRV/COBI | Total | – | – | – | – | – | – | 4 (8.9) |
| <50 | – | – | – | – | – | – | 2 (4.4) | |
| ≥50 | – | – | – | – | – | – | 2 (4.4) | |
| IDV | Total | 3 (6.8) | 2 (3.8) | 2 (3.1) | 1 (1.3) | – | – | – |
| <50 | 2 (4.5) | 1 (1.9) | 1 (1.6) | – | – | – | – | |
| ≥50 | 1 (2.3) | 1 (1.9) | 1 (1.6) | 1 (1.3) | – | – | – | |
|
| ||||||||
| DRV | Total | – | – | – | – | 1 (1.4) | – | – |
| <50 | – | – | – | – | – | – | – | |
| ≥50 | – | – | – | – | 1 (1.4) | – | – | |
|
| ||||||||
| INSTIs | (n=1) | (n=10) | (n=20) | (n=26) | (n=51) | (n=65) | (n=112) | |
|
| ||||||||
| DTG | Total | – | – | – | – | 1 (2.0) | 11 (16.9) | 36 (32.1) |
| <50 | – | – | – | – | 1 (2.0) | 8 (12.3) | 22 (19.6) | |
| ≥50 | – | – | – | – | – | 3 (4.6) | 14 (12.5) | |
|
| ||||||||
| EVG/COBI | Total | – | – | – | – | 20 (39.2) | 30 (46.2) | 64 (57.1) |
| <50 | – | – | – | – | 12 (23.5) | 18 (27.7) | 34 (30.4) | |
| ≥50 | – | – | – | – | 8 (15.7) | 12 (18.5) | 30 (26.8) | |
|
| ||||||||
| RAL | Total | 1 (100.0) | 10 (100.0) | 20 (100.0) | 26 (100.0) | 30 (58.8) | 24 (36.9) | 12 (10.7) |
| <50 | 1 (100.0) | 2 (20.0) | 7 (35.0) | 9 (34.6) | 11 (21.6) | 8 (12.3) | 7 (6.3) | |
| ≥50 | – | 8 (80.0) | 13 (65.0) | 17 (65.4) | 19 (37.3) | 16 (24.6) | 5 (4.5) | |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; COBI, cobicistat; d4T, stavudine; ddI, didanosine; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; ETR, etravirine; EVG, elvitegravir; FTC, emtricitabine; IDV, indinavir; INSTIs, integrase strand transfer inhibitors; LPV, lopinavir; NNRTIs, nonnucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; NVP, nevirapine; PIs, protease inhibitors; RAL, raltegravir; RPV, rilpivirine; RTV, ritonavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
The classes of drugs coadministered with ARVs are presented in an age-wise manner in Table 4. The drug classes that had significantly higher rates of use in patients aged ≥50 years than in those aged <50 years were drugs prescribed for ailments of the alimentary tract and metabolism; dermatologicals; and drugs for diseases of the cardiovascular system, blood and blood forming organs, and the genitourinary system and sex hormones.
Table 4.
Coadministered drug classes with antiretrovirals among the patients included in the study
| Class | All patients, n (%) (n=183) | <50 years, n (%) (n=104) | ≥50 years, n (%) (n=79) | P-value |
|---|---|---|---|---|
| Alimentary tract and metabolism | 105 (57.4) | 48 (46.2) | 57 (72.2) | <0.001 |
| Anti-infectives for systemic use | 77 (42.1) | 45 (43.3) | 32 (40.5) | 0.708 |
| Dermatologicals | 49 (26.8) | 20 (19.2) | 29 (36.7) | 0.008 |
| Musculoskeletal system | 47 (25.7) | 28 (26.9) | 19 (24.1) | 0.660 |
| Nervous system | 47 (25.7) | 23 (22.1) | 24 (30.4) | 0.205 |
| Respiratory system | 44 (24.0) | 23 (22.1) | 21 (26.6) | 0.484 |
| Cardiovascular system | 43 (23.5) | 18 (17.3) | 25 (31.6) | 0.023 |
| Systemic hormonal preparations, excluding sex hormones | 22 (12.0) | 13 (12.5) | 9 (11.4) | 0.819 |
| Blood and blood forming organs | 20 (10.9) | 7 (6.7) | 13 (16.5) | 0.037 |
| Genitourinary system and sex hormones | 18 (9.8) | 5 (4.8) | 13 (16.5) | 0.009 |
| Antineoplastic and immunomodulating agents | 10 (5.5) | 7 (6.7) | 3 (3.8) | 0.518 |
| Sensory organs | 7 (3.2) | 3 (2.9) | 4 (5.1) | 0.467 |
| Antiparasitic products, insecticides, and repellents | 2 (1.1) | 1 (1.0) | 1 (1.3) | 0.845 |
Based on the data obtained by using the Liverpool HIV Drug Interactions website, the potential or contraindicated DDIs are summarized in Table 5. A total of 194 potential or contraindicated DDIs were identified, and among them, 12 (6.2%) contraindicated DDIs were found. Contraindicated DDIs occurred most frequently between boosted PIs with RTV or EVG/COBI and coprescribed drugs such as alfuzosin, clopidogrel, quetiapine, rifampicin, simvastatin, and phenytoin.
Table 5.
Potential or contraindicated drug–drug interactions between antiretrovirals and other drugs coprescribed with antiretrovirals
| Antiretroviral drug | Comedication | Frequency, n (%) (n=194) | Strength of recommendationa | Quality of evidenceb |
|---|---|---|---|---|
|
| ||||
| NRTIs | ||||
|
| ||||
| 3TC | Amphotericin | 1 (0.5) | Potential interaction | Very low |
| TDF | Acyclovir | 5 (2.6) | Potential interaction | Very low |
| Celecoxib | 2 (1.0) | Potential interaction | Very low | |
| Clarithromycin | 1 (0.5) | Potential interaction | Very low | |
| Ganciclovir | 4 (2.1) | Potential interaction | Very low | |
| Naproxen | 3 (1.5) | Potential interaction | Very low | |
| Nimesulide | 6 (3.1) | Potential interaction | Very low | |
| Pentamidine | 1 (0.5) | Potential interaction | Very low | |
| Topiramate | 1 (0.5) | Potential interaction | Very low | |
| Verapamil | 1 (0.5) | Potential interaction | Very low | |
|
| ||||
| ZDV | Fluconazole | 2 (1.0) | Potential interaction | Low |
| Trimethoprim/sulfamethoxazole | 1 (0.5) | Potential interaction | Low | |
|
| ||||
| NNRTIs | ||||
|
| ||||
| EFV | Amlodipine | 1 (0.5) | Potential interaction | Very low |
| Moxifloxacin | 1 (0.5) | Potential interaction | Very low | |
| Nimesulide | 1 (0.5) | Potential interaction | Very low | |
| Zolpidem | 1 (0.5) | Potential interaction | Very low | |
|
| ||||
| ETR | Clarithromycin | 1 (0.5) | Potential interaction | Moderate |
| Fluconazole | 2 (1.0) | Potential interaction | Low | |
| Glimepiride | 2 (1.0) | Potential interaction | Very low | |
| Lercanidipine | 1 (0.5) | Potential interaction | Very low | |
| Naproxen | 1 (0.5) | Potential interaction | Very low | |
| Oxycodone | 1 (0.5) | Potential interaction | Very low | |
| Rifampicin | 1 (0.5) | Do not coadminister | Moderate | |
| Sildenafil | 1 (0.5) | Potential interaction | High | |
| Tamsulosin | 1 (0.5) | Potential interaction | Very low | |
|
| ||||
| RPV | Diltiazem | 1 (0.5) | Potential interaction | Very low |
| Famotidine | 2 (1.0) | Potential interaction | Low | |
| Fluconazole | 1 (0.5) | Potential interaction | Very low | |
| Itraconazole | 1 (0.5) | Potential interaction | Very low | |
|
| ||||
| PIs | ||||
|
| ||||
| ATV | Atovaquone/proguanil | 1 (0.5) | Potential interaction | Moderate |
| Buspirone | 1 (0.5) | Potential interaction | Very low | |
| Clarithromycin | 1 (0.5) | Potential interaction | Low | |
| Escitalopram | 1 (0.5) | Potential interaction | Very low | |
| Famotidine | 2 (1.0) | Potential interaction | Low | |
| Lansoprazole | 1 (0.5) | Do not coadminister | Low | |
| Nortriptyline | 2 (1.0) | Potential interaction | Very low | |
| Prednisolone | 1 (0.5) | Potential interaction | Very low | |
| Rifabutin | 1 (0.5) | Potential interaction | High | |
| Tamsulosin | 1 (0.5) | Potential interaction | Very low | |
| Zolpidem | 1 (0.5) | Potential interaction | Very low | |
|
| ||||
| ATV/COBI | Atorvastatin | 1 (0.5) | Potential interaction | Very low |
|
| ||||
| ATV/RTV | Atorvastatin | 2 (1.0) | Potential interaction | Very low |
| Clopidogrel | 1 (0.5) | Do not coadminister | Low | |
| Methylprednisolone | 1 (0.5) | Potential interaction | Very low | |
|
| ||||
| LPV/RTV | Alfuzosin | 1 (0.5) | Do not coadminister | Moderate |
| Alprazolam | 1 (0.5) | Potential interaction | Very low | |
| Amlodipine | 3 (1.5) | Potential interaction | Very low | |
| Azithromycin | 1 (0.5) | Potential interaction | Very low | |
| Atorvastatin | 8 (4.1) | Potential interaction | High | |
| Clarithromycin | 3 (1.5) | Potential interaction | Very low | |
| Clindamycin | 1 (0.5) | Potential interaction | Very low | |
| Estradiol | 1 (0.5) | Potential interaction | Very low | |
| Fentanyl | 2 (1.0) | Potential interaction | Very low | |
| Gliclazide | 1 (0.5) | Potential interaction | Very low | |
| Glimepiride | 4 (2.1) | Potential interaction | Very low | |
| Hydroxyzine | 3 (1.5) | Potential interaction | Very low | |
| Lacidipine | 1 (0.5) | Potential interaction | Very low | |
| Methylprednisolone | 2 (1.0) | Potential interaction | Very low | |
| Mirtazapine | 2 (1.0) | Potential interaction | Very low | |
| Nortriptyline | 2 (1.0) | Potential interaction | Very low | |
| Nifedipine | 1 (0.5) | Potential interaction | Very low | |
| Oxcarbazepine | 1 (0.5) | Potential interaction | Very low | |
| Quetiapine | 1 (0.5) | Do not coadminister | Very low | |
| Rifabutin | 1 (0.5) | Potential interaction | High | |
| Rifampicin | 1 (0.5) | Do not coadminister | High | |
| Risperidone | 1 (0.5) | Potential interaction | Very low | |
| Sildenafil | 1 (0.5) | Potential interaction | High | |
| Simvastatin | 1 (0.5) | Do not coadminister | Moderate | |
| Tadalafil | 2 (1.0) | Potential interaction | High | |
| Tamsulosin | 1 (0.5) | Potential interaction | Very low | |
| Trazodone | 1 (0.5) | Potential interaction | Moderate | |
| Valproate | 1 (0.5) | Potential interaction | Moderate | |
| Voriconazole | 1 (0.5) | Potential interaction | Moderate | |
| Zolpidem | 6 (3.1) | Potential interaction | Very low | |
|
| ||||
| DRV/RTV | Atorvastatin | 3 (1.5) | Potential interaction | High |
| Atovaquone/proguanil | 1 (0.5) | Potential interaction | Very low | |
| Clarithromycin | 1 (0.5) | Potential interaction | Moderate | |
| Clopidogrel | 1 (0.5) | Do not coadminister | Low | |
| Colchicine | 1 (0.5) | Potential interaction | Very low | |
| Glimepiride | 1 (0.5) | Potential interaction | Very low | |
| Hydrocortisone | 1 (0.5) | Potential interaction | Very low | |
| Hydroxyzine | 2 (1.0) | Potential interaction | Very low | |
| Itraconazole | 1 (0.5) | Potential interaction | Very low | |
| Methylprednisolone | 1 (0.5) | Potential interaction | Very low | |
| Nifedipine | 1 (0.5) | Potential interaction | Very low | |
| Oxycodone | 1 (0.5) | Potential interaction | Very low | |
| Prednisolone | 2 (1.0) | Potential interaction | Very low | |
| Quetiapine | 1 (0.5) | Do not coadminister | Very low | |
| Rifabutin | 1 (0.5) | Potential interaction | Low | |
| Sildenafil | 1 (0.5) | Potential interaction | Very low | |
| Valproate | 1 (0.5) | Potential interaction | Very low | |
| Zolpidem | 3 (1.5) | Potential interaction | Very low | |
|
| ||||
| IDV | Glimepiride | 1 (0.5) | Potential interaction | Very low |
|
| ||||
| INSTIs | ||||
|
| ||||
| DTG | Magnesium | 1 (0.5) | Potential interaction | Very low |
| Metformin | 5 (2.6) | Potential interaction | Low | |
|
| ||||
| EVG/COBI | Alprazolam | 1 (0.5) | Potential interaction | Very low |
| Amlodipine | 2 (1.0) | Potential interaction | Very low | |
| Atorvastatin | 1 (0.5) | Potential interaction | Very low | |
| Clonazepam | 1 (0.5) | Potential interaction | Very low | |
| Dexamethasone | 1 (0.5) | Potential interaction | Very low | |
| Fentanyl | 1 (0.5) | Potential interaction | Very low | |
| Glimepiride | 1 (0.5) | Potential interaction | Very low | |
| Hydroxyzine | 1 (0.5) | Potential interaction | Very low | |
| Iron supplement | 1 (0.5) | Potential interaction | Very low | |
| Itraconazole | 1 (0.5) | Potential interaction | Very low | |
| Magnesium | 1 (0.5) | Potential interaction | Very low | |
| Metformin | 2 (1.0) | Potential interaction | Very low | |
| Midazolam | 1 (0.5) | Potential interaction | Very low | |
| Nifedipine | 1 (0.5) | Potential interaction | Very low | |
| Phenytoin | 1 (0.5) | Do not coadminister | Very low | |
| Quetiapine | 1 (0.5) | Do not coadminister | Very low | |
| Rifabutin | 1 (0.5) | Potential interaction | Low | |
| Rifampicin | 1 (0.5) | Do not coadminister | Moderate | |
| Saxagliptin | 1 (0.5) | Potential interaction | Very low | |
| Sildenafil | 1 (0.5) | Potential interaction | Very low | |
| Tamsulosin | 1 (0.5) | Potential interaction | Very low | |
| Trazodone | 1 (0.5) | Potential interaction | Very low | |
| Valproate | 1 (0.5) | Potential interaction | Very low | |
| Zolpidem | 5 (2.6) | Potential interaction | Very low | |
|
| ||||
| RAL | Calcium supplement | 1 (0.5) | Potential interaction | Very low |
| Iron supplement | 2 (1.0) | Potential interaction | Very low | |
| Magnesium | 6 (3.1) | Potential interaction | Very low | |
| Rifampicin | 2 (1.0) | Potential interaction | Moderate | |
Notes:
Do not coadminister: these drugs should not be coadministered; Potential interaction: potential clinically significantly interaction that is likely to require additional monitoring, alteration of drug dosage or timing of administration.
Find more information on quality of evidence at the following website: https://www.hiv-druginteractions.org/.
Abbreviations: 3TC, lamivudine; ATV, atazanavir; COBI, cobicistat; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; ETR, etravirine; EVG, elvitegravir; IDV, indinavir; INSTIs, integrase strand transfer inhibitors; LPV, lopinavir; NNRTIs, non-nucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors; RAL, raltegravir; RPV, rilpivirine; RTV, ritonavir; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
Discussion
In this study, the usage patterns of ARV regimens in HIV-positive patients were studied in an age-wise manner and potential or contraindicated DDIs between ARV and non-ARV drugs were investigated. Recently, the most frequently prescribed regimen involved treatment with NRTIs/INSTIs followed by therapy with NRTIs/PIs. ABC/3TC, TDF/FTC, and TAF/FTC among the NRTIs, LPV/RTV among the PIs, and EVG/COBI and DTG among the INSTIs were frequently prescribed. In addition, DDIs between boosted PIs with RTV or EVG/COBI and coprescribed drugs occurred most frequently.
Numerous diseases such as osteoporosis, DM, chronic liver disease, chronic kidney disease, and CVDs have higher rates of occurrence in the aging population of PLWH than in their HIV-uninfected counterparts.18–20 Therefore, in order to effectively manage health conditions in the aging population of PLWH, it is not only essential to suppress the viral load of HIV and allow the immune system to recover but also to control age-associated, non-AIDS illnesses. However, the combined use of ARV and non-ARV drugs is likely to cause various drug-related problems such as DDIs.
The coformulation of ZDV and 3TC was a preferred choice for the treatment of HIV infections in 2011, but it was rarely used in 2017. Instead of this NRTI combination, tenofovir-based combinations (TDF/FTC and TAF/FTC) or the ABC/3TC combination was usually administered. This trend may be due to several reasons as stated hereafter. The prolonged use of ZDV can lead to more severe adverse reactions such as hematological toxicities (anemia and/or neutropenia) and symptomatic myopathy.21 ZDV/3TC requires twice-daily dosing for effectively suppressing the levels of HIV RNA, whereas the TDF/FTC, TAF/FTC, and ABC/3TC combinations require once-daily dosing, which may reduce the pill burden for HIV-infected patients and improve their medication adherence rates.21–26 Furthermore, it was reported that the efficacy of the ZDV/3TC combination was less robust than TDF/FTC-based ART in achieving viral suppression.27,28
The most frequently prescribed regimen in 2017 was NRTIs/INSTIs followed by NRTIs/PIs. Specifically, TDF/FTC/COBI/EVG (Stribild®) and TAF/FTC/COBI/EVG (Genvoya®), approved by the US Food and Drug Administration (FDA) in 2012 and 2015, respectively, consisted of two NRTIs and one INSTI with one booster.24,25 The ABC/3TC/DTG (Triumeq®) combination, consisting of two NRTIs and one INSTI, was approved by the FDA in 2014.26 This tendency might have appeared owing to the use of the once-daily, single-tablet regimens as the initial therapy for treatment-naïve patients and switching to simplified, less toxic regimens for treatment-experienced patients.29,30
Until 2016, Stribild had been the only tenofovir-based combination; however, in 2017, Genvoya was introduced in the hospital where this study was conducted. As shown in Table 3, the prescription rate of TDF/FTC, the two NRTIs in Stribild, in 2017 (28.1%) was almost half of that in 2016 (60.4%). However, the prescription rate of TAF/FTC, the two NRTIs in Genvoya, was 31.3% in 2017, which is likely to account for the decrease in the prescription rate of TDF/FTC in 2017. This result may be further explained by the differences in the efficacy and safety of the regimens containing TDF/FTC and TAF/FTC for the management of HIV infection. In comparison to TDF/FTC-based regimens, TAF/FTC-based regimens have similar or better efficacy, and their use improved renal and bone health.31–33 According to the study by Sax et al, which employed treatment-naïve HIV-1-infected patients, high virological success rates (HIV-1 RNA <50 copies/mL) were achieved at week 48 in patients receiving both TAF/FTC/COBI/EVG (92%) and TDF/FTC/COBI/EVG (90%).34 In the study by Mills et al on virologically suppressed HIV-1-infected patients, virological success (HIV-1 RNA <50 copies/mL) at week 48 occurred in 97% of the patients receiving TAF/FTC/COBI/EVG and 93% of those administered with the TDF-containing regimen (P<0.0002).35 In particular, TAF/FTC/COBI/EVG was preferred over TDF/FTC/COBI/EVG for HIV-1-infected patients who were aged ≥50 years.33,34
The intracellular concentration of tenofovir-diphosphate, which is an active metabolite, was approximately four times higher after treatment with TAF than that after treatment with TDF.34 This indicated that compared with TDF, TAF is required at much lower doses, and the systemic exposure of tenofovir was also expected to be much lower in patients under therapy with TAF than in those treated with TDF.34 Consequently, this is likely to improve tenofovir-associated AEs such as renal toxicity and reduced bone mineral density (BMD). Sax et al reported that therapy with TAF/FTC/COBI/EVG induced a significantly smaller increase in mean serum creatinine (0.08 vs 0.12 mg/dL; P<0.0001), significantly lesser proteinuria (median % change, −3 vs 20; P<0.0001), and a significantly smaller decrease in the BMD of the spine (mean % change, −1.30 vs −2.86; P<0.0001) and hip (−0.66 vs −2.95; P<0.0001) at week 48 than those observed after treatment with TDF/FTC/COBI/EVG.34 Mills et al additionally reported that compared with treatment with TDF-containing regimens, therapy with TAF/FTC/COBI/EVG significantly improved the BMD of the spine (mean % change from the baseline, 1.56 vs −0.44; P<0.0001) and hip (1.47 vs −0.34; P<0.0001) and the mean serum creatinine (−0.4 vs 2.9 μmol/L; P<0.0001) at week 48.35 Comprehensively, TAF/FTC/COBI/EVG may be a better choice than TDF/FTC/COBI/EVG for the treatment of HIV-infected patients, especially those aged ≥50 years, and patients who have reduced renal function, medical history of fractures, osteopenia, or osteoporosis.
Exposure to ABC, the NRTI present in Triumeq, may lead to an increase in the risks of CVD events, such as coronary artery disease and myocardial infarction.31,36 According to the study that assessed the risk of CVD events in HIV-infected patients administered with ARV drugs, a higher incidence rate of CVD events was observed in the patients who were currently exposed to ABC than in those who were currently exposed to other ARV drugs (9.74/1,000 person-years vs 5.75/1,000 person-years).36 The HRs of CVD events for patients under current (1.43; P=0.001), recent (1.41; P=0.001), and cumulative (1.18 [per year]; P=0.002) exposure to ABC increased with statistical significance.36 The HR of CVD events for cumulative exposure to ABC also increased for up to 24 months and decreased thereafter.36 Consequently, ABC should be cautiously used in HIV-infected patients, especially those aged ≥50 years, and patients with risk factors (such as HTN, hyperlipidemia, DM, and smoking) for coronary artery disease and myocardial infarction, by appropriately managing those risk factors prior to initiating regimens containing ABC.
The DDIs between ART and non-ART drugs make it difficult to design effective and safe ART regimens, especially in older HIV-infected patients (≥50 years of age), who are more likely to take one or more comedications with ART drugs in order to manage multiple comorbidities than younger HIV-infected patients (<50 years of age) are.4,37 The independently associated variables with potential or contraindicated DDIs include older age, dyslipidemia, higher daily drug burden of non-ARTs, and prescription of PIs.4 In this study, contraindicated drugs, such as alfuzosin, clopidogrel, quetiapine, rifampicin, simvastatin, and pheny-toin, were frequently prescribed along with ARV regimens including pharmacokinetic boosters (ie, RTV and COBI). Potential DDIs mainly occurred between boosted ARV regimens and non-ART drugs, such as drugs prescribed for gastrointestinal, metabolic, cardiovascular, and central nervous system ailments, which was similar to the results obtained from previous studies.4,37 ARV regimens including pharmacokinetic boosters should be cautiously administered to poly-medicated patients. Other regimens including INSTIs (ie, DTG and RAL) are preferable in those cases. Additionally, comprehensive pharmacist-led medication review and intervention in HIV-positive patients, especially those under complex medication regimens, may reduce the incidence rates of AEs and DDIs, as demonstrated in a previous study.7
This study has some limitations which should be borne in mind while interpreting the results. All data pertaining to the prescribed medications including ART agents were retrospectively collected by reviewing the electronic medical charts of the patients. Therefore, it could not be confirmed whether the patients had actually partaken of the prescribed medication and whether the DDIs had actually occurred. This limitation may be solved by providing the patients with self-reporting questionnaires concerning medication adherence and DDIs in the future. It was difficult to determine when the comorbidities had occurred owing to the cross-sectional design of this study. The last limitation was the representativeness of the patients included in this study. Most of the patients were likely to be current residents of North Jeolla Province in South Korea; thus, it may be somewhat difficult to generalize the results of this study and extend them to the residents of other regions of South Korea. In order to overcome this shortcoming, it is necessary to collaborate with other hospitals in the near future. However, since studies on the age-wise usage pattern of ARVs and their DDIs with non-ARV drugs have been rarely conducted in Korea, this study is of significance and could aid the identification of more appropriate ARV drug regimens having few DDIs with non-ARVs in an aging population of PLWH in Korea.
Conclusion
The advent of combination ARTs has enabled PLWH to live up to older ages, causing these individuals to experience age-related illnesses, which necessitates the use of non-ARV medications for managing these illnesses. It is important to use appropriate ART regimens that are less likely to interact with other comedications and affect non-AIDS illnesses. The most frequently prescribed ART regimen involves treatment with NRTIs/INSTIs (ie, ABC/3TC/DTG, TAF/FTC/COBI/EVG, and TDF/FTC/COBI/EVG). TAF/FTC/COBI/EVG may be a better option than TDF/FTC/COBI/EVG and ABC/3TC/DTG for patients, especially those aged ≥50 years, and those having low BMD, reduced kidney function, or cardiovascular diseases. EVG/COBI and boosted PIs with RTV or COBI may not be good options for poly-medicated patients due to the high risks of DDIs, and DTG or RAL regimens may be preferred in this situation. However, EVG/COBI was most frequently prescribed in 2017. Further research should be performed to evaluate the impact of pharmacist-led medication review and intervention on AEs and DDIs in HIV-positive patients in Korea under complex medication regimens.
Acknowledgments
This work was supported by the National Research Foundation of Korea grant funded by the Korea government (NRF-2016R1C1B1015938).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Oguntibeju OO. Quality of life of people living with HIV and AIDS and antiretroviral therapy. HIV AIDS. 2012;4:117–124. doi: 10.2147/HIV.S32321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maggi P, Di Biagio A, Rusconi S, et al. Cardiovascular risk and dyslipidemia among persons living with HIV: a review. BMC Infect Dis. 2017;17(1):551. doi: 10.1186/s12879-017-2626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahy M, Autenrieth CS, Stanecki K, Wynd S. Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS. 2014;28(Suppl 4):S453–S459. doi: 10.1097/QAD.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holtzman C, Armon C, Tedaldi E, et al. Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med. 2013;28(10):1302–1310. doi: 10.1007/s11606-013-2449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin CF, Wang CY, Bai CH. Polypharmacy, aging and potential drug-drug interactions in outpatients in Taiwan: a retrospective computerized screening study. Drugs Aging. 2011;28(3):219–225. doi: 10.2165/11586870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Secoli SR, Figueras A, Lebrão ML, de Lima FD, Santos JL. Risk of potential drug-drug interactions among Brazilian elderly: a population-based, cross-sectional study. Drugs Aging. 2010;27(9):759–770. doi: 10.2165/11538460-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.McNicholl IR, Gandhi M, Hare CB, Greene M, Pierluissi E. A pharmacist-led program to evaluate and reduce polypharmacy and potentially inappropriate prescribing in older HIV-positive patients. Pharmacotherapy. 2017;37(12):1498–1506. doi: 10.1002/phar.2043. [DOI] [PubMed] [Google Scholar]
- 8.Cantudo-Cuenca MR, Jiménez-Galán R, Almeida-Gonzalez CV, Morillo-Verdugo R. Concurrent use of comedications reduces adherence to antiretroviral therapy among HIV-infected patients. J Manag Care Spec Pharm. 2014;20(8):844–850. doi: 10.18553/jmcp.2014.20.8.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baecke C, Gyssens IC, Decoutere L, van der Hilst JCH, Messiaen P. Prevalence of drug-drug interactions in the era of HIV integrase inhibitors: a retrospective clinical study. Neth J Med. 2017;75(6):235–240. [PubMed] [Google Scholar]
- 10.Tseng A, Szadkowski L, Walmsley S, Salit I, Raboud J. Association of age with polypharmacy and risk of drug interactions with antiretroviral medications in HIV-positive patients. Ann Pharmacother. 2013;47(11):1429–1439. doi: 10.1177/1060028013504075. [DOI] [PubMed] [Google Scholar]
- 11.Bastida C, Grau A, Márquez M, et al. Polypharmacy and potential drug-drug interactions in an HIV-infected elderly population. Farm Hosp. 2017;41(5):618–624. doi: 10.7399/fh.10778. [DOI] [PubMed] [Google Scholar]
- 12.Smith JM, Flexner C. The challenge of polypharmacy in an aging population and implications for future antiretroviral therapy development. AIDS. 2017;31(Suppl 2):S173–S184. doi: 10.1097/QAD.0000000000001401. [DOI] [PubMed] [Google Scholar]
- 13.Molas E, Luque S, Retamero A, et al. Frequency and severity of potential drug interactions in a cohort of HIV-infected patients identified through a multidisciplinary team. HIV Clin Trials. 2018;19(1):1–7. doi: 10.1080/15284336.2017.1404690. [DOI] [PubMed] [Google Scholar]
- 14.Ghidei L, Simone MJ, Salow MJ, et al. Aging, antiretrovirals, and adherence: a meta analysis of adherence among older HIV-infected individuals. Drugs Aging. 2013;30(10):809–819. doi: 10.1007/s40266-013-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AIDSinfo Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. [Accessed April 4, 2018]. Available from: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/0.
- 16.World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach – second edition. 2016. [Accessed April 4, 2018]. Available from: http://www.who.int/hiv/pub/arv/arv-2016/en/ [PubMed]
- 17.Liverpool HIV Drug Interactions [homepage on the Internet] [Accessed September 6, 2018]. Available from: https://www.hiv-druginteractions.org/
- 18.Escota GV, O’Halloran JA, Powderly WG, Presti RM. Understanding mechanisms to promote successful aging in persons living with HIV. Int J Infect Dis. 2018;66:56–64. doi: 10.1016/j.ijid.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Güerri-Fernandez R, Vestergaard P, Carbonell C, et al. HIV infection is strongly associated with hip fracture risk, independently of age, gender, and comorbidities: a population-based cohort study. J Bone Miner Res. 2013;28(6):1259–1263. doi: 10.1002/jbmr.1874. [DOI] [PubMed] [Google Scholar]
- 20.Palella FJ, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 21.COMBIVIR® Package Insert. [Accessed April 11, 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/20857s030lbl.pdf.
- 22.Flynn PM, Rodman J, Lindsey JC, et al. Intracellular pharmacokinetics of once versus twice daily zidovudine and lamivudine in adolescents. Antimicrob Agents Chemother. 2007;51(10):3516–3522. doi: 10.1128/AAC.01626-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruane PJ, Richmond GJ, Dejesus E, et al. Pharmacodynamic effects of zidovudine 600 mg once/day versus 300 mg twice/day in therapy-naïve patients infected with human immunodeficiency virus. Pharmacotherapy. 2004;24(3):307–312. doi: 10.1592/phco.24.4.307.33184. [DOI] [PubMed] [Google Scholar]
- 24.STRIBILD® Package Insert. [Accessed April 11, 2018]. Available from: https://www.gilead.com/~/media/files/pdfs/medicines/hiv/stribild/stribild_pi.pdf?la=en.
- 25.GENVOYA® Package Insert. [Accessed April 11, 2018]. Available from: https://www.gilead.com/~/media/files/pdfs/medicines/hiv/genvoya/genvoya_pi.pdf?la=en.
- 26.TRIUMEQ® Package Insert. [Accessed April 11, 2018]. Available from: https://www.viivhealthcare.com/media/80846/Triumeq-PI-MG.pdf.
- 27.Gallant JE, Dejesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354(3):251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 28.Pozniak AL, Gallant JE, Dejesus E, et al. Tenofovir Disoproxil Fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes-a 96-week analysis. J Acquir Immune Defic Syndr. 2006;43(5):535–540. doi: 10.1097/01.qai.0000245886.51262.67. [DOI] [PubMed] [Google Scholar]
- 29.Truong WR, Schafer JJ, Short WR. Once-daily, single-tablet regimens for the treatment of HIV-1 infection. P&T. 2015;40(1):44–55. [PMC free article] [PubMed] [Google Scholar]
- 30.Sebaaly JC, Kelley D. Single-tablet regimens for the treatment of HIV-1 infection. Ann Pharmacother. 2017;51(4):332–344. doi: 10.1177/1060028016682531. [DOI] [PubMed] [Google Scholar]
- 31.Angione SA, Cherian SM, Özdener AE. A review of the efficacy and safety of Genvoya® (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) in the Management of HIV-1 infection. J Pharm Pract. 2018;31(2):216–221. doi: 10.1177/0897190017710519. [DOI] [PubMed] [Google Scholar]
- 32.Raffi F, Orkin C, Clarke A, et al. Brief Report: long-term (96-week) efficacy and safety after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in HIV-infected, virologically suppressed adults. J Acquir Immune Defic Syndr. 2017;75(2):226–231. doi: 10.1097/QAI.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arribas JR, Thompson M, Sax PE, et al. Brief report: randomized, double-blind comparison of tenofovir alafenamide (TAF) vs tenofovir disoproxil fumarate (TDF), each coformulated with elvitegravir, cobicistat, and emtricitabine (E/C/F) for initial HIV-1 treatment: Week 144 results. J Acquir Immune Defic Syndr. 2017;75(2):211–218. doi: 10.1097/QAI.0000000000001350. [DOI] [PubMed] [Google Scholar]
- 34.Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 35.Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16(1):43–52. doi: 10.1016/S1473-3099(15)00348-5. [DOI] [PubMed] [Google Scholar]
- 36.Dorjee K, Baxi SM, Reingold AL, Hubbard A. Risk of cardiovascular events from current, recent, and cumulative exposure to abacavir among persons living with HIV who were receiving antiretroviral therapy in the United States: a cohort study. BMC Infect Dis. 2017;17(1):708. doi: 10.1186/s12879-017-2808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marzolini C, Back D, Weber R, et al. Ageing with HIV: medication use and risk for potential drug-drug interactions. J Antimicrob Chemother. 2011;66(9):2107–2111. doi: 10.1093/jac/dkr248. [DOI] [PubMed] [Google Scholar]