ABSTRACT
Cadmium (Cd) is one of the environmental risk factors for bone loss. The present study included 40 sewage workers occupationally exposed to Cd. Forty nonexposed men were included as a control group. Current smokers represented 65% and 47.5% of the exposed and control groups, respectively.
The study aimed to investigate the hazard of occupational Cd exposure on bone health. This was achieved through measuring serum and urinary Cd, and calcium (Ca), in addition to serum osteoprotegerin (OPG) and estrogen receptor-α gene. Results showed significant elevation in serum Cd, OPG, and urinary Ca levels in the exposed compared to the controls. Bony aches and joint pain were more prevalent among the exposed workers. Serum and urinary Cd increased in exposed smokers relative to control smokers. Also, serum OPG levels showed significant rise among exposed smoker and nonsmoker compared to control smoker and nonsmoker groups. Serum Cd level increased significantly in PP and pp genotypes in exposed workers compared to controls, while elevated levels of serum OPG was observed in PP and Pp genotypes in exposed workers relative to controls.
Urinary Cd exhibited significant rise in both PP and pp genotypes in exposed workers, while Ca excretion was elevated in pp genotype only. The study reflected an association of genetic predisposition and Cd exposure in progression of osteoporosis. Further research is needed to explain the mechanisms of Cd impact on bone. The role of smoking is important and hence smoking cessation programs are essential for sewage workers.
KEYWORDS: Bone, osteoporosis, Cd, osteoprotegerein, estrogen receptor-α, gene, smoking, environment, sewage workers
Introduction
Cadmium (Cd) is definitely an ever-present environmental pollutant [1]. Abdel-Sabour [2] reported that Cd releases are still rising. Strict measures must be taken to reduce Cd emissions. In the Egyptian environment, Cd flow may vary from one compartment to another at different rates, leading to a buildup in compartments as soils and biota. Accumulation is expected to occur with continued emissions. Both natural and anthropogenic sources of Cd need attention, especially in the industrial cities in Egypt [2]. Cd in sewage, industrial effluent (also, other liquid and solid wastes), and sewage sludge will increase soil levels and is expected to raise dietary levels and body burdens. Other study recorded that Cd can enter the body through the food chain or cigarette consumption.
Bone is a target organ for Cd toxicity. Epidemiological studies showed that Cd could induce bone loss, osteoporosis with an increase in the potential of bone fractures risk [3]. Once Cd is absorbed, it can retain in the body where it accumulates during life. Even at environmental level, Cd exposure is associated with reduced bone mineral density which may cause osteoporosis with increased risk of bone fracture [4]. Animal studies revealed that Cd stimulates the development of osteoclasts with modification of its activities and breaking down the bone collagen matrix [5]. Trzcinka-Ochocka et al. [6] established that the main factors that have impact on bone density are body weight, age in the female subjects, and the entire body weight with calcium excretion in males. Long-term exposure to Cd (both environmental and occupational) may cause severe kidney and bone damage. Another effect of Cd toxicity is reduced bone thickness due to prolonged exposure. Three main pathways of Cd effects on bone mass have been supposed: (1) disturbance with the normal activation process of vitamin D following renal tubular injury [7], (2) interference with Ca absorption from the digestive system [8], and (3) direct effect of Cd on bone metabolic process without renal injury [9].
Osteoclasts represent one of the main cells which are responsible for bone resorption [10]. It has been stated that Cd exposure could affect metabolic biomarker of bone resorption [11]. Low level of Cd might promote the formation of osteoclasts in vitro [12].
Osteoprotegerin (OPG) is a secreted glycoprotein that represents one of the members of the tumor necrosis factor family. It prevents receptor activator of the nuclear factor kappa B ligand (RANKL) from binding to receptor activator of the nuclear factor kappa B (RANK), thereby resulting in inhibition of osteoclast differentiation and activation [13]. It is produced by osteoblasts and plays a critical role in numerous physiological processes, especially osteoclastogenesis [14].
Other study concluded that OPG has a critical role in bone resorption regulation by modifying osteoclast differentiation. Consequently, overexpression of OPG in mice has been reported to result in osteoporosis [15].
Accumulating evidence suggested that estrogen seems to play a major role in male bone metabolism [16]. Estrogen effect was mediated by estrogen receptor (estrogen receptor alpha [ER-α]), which represents a member of steroid hormone receptor family. It functions as an important part of the hormone–receptor complex that promotes specific target genes expression [17]. Genetic alterations in multiple genes can account for this genetic component and ER-α gene represents one of the various potential candidates. Kim et al. [17] recorded that ER-α gene polymorphism is associated with osteoporosis development in Korean vegetarian men. Polymorphism of the ER-α gene found on intron 1 may affect gene expression and/or interindividual susceptibility in the estrogen hormone through its regulatory action in mRNA transcription. Other possibility was due to that this polymorphism might be associated with unidentified polymorphisms that is responsible for the quality or function of the protein encoded by the ER-α gene [18].
Therefore, homozygous mutations in estrogen receptor (ER-α) or aromatase [18] genes led to unfused epiphyses, increased bone remodeling, and impairment in bone mass throughout adolescence.
Different interventional studies demonstrated that estrogen performed significant and possibly dominant regulatory role in men during bone resorption process [19], with contribution of estrogen and testosterone in maintaining bone formation [20]. Estrogen effects on skeleton were mediated by ERs which are found in osteoblasts and osteoclasts [16]. Expression of both ER-α and ER-B by bone cells occurs in vitro and in vivo [21]. Experimental studies on mouse knockout models mentioned that while ER-α seems to have the main estrogen receptor mediating action in bone, ER-B (in females primarily) can modify ER-α action [22]. Following binding and activation of the ER, estrogen regulates the production of numerous growth factors in addition to different cytokines. Also, it exerts direct effects on the differentiation of osteoblast (including production of type I collagen) and apoptosis in addition to regulation in osteoclast development, activity, and apoptosis [16].
Recent reports referred that Cd is a new environmental estrogen. It was proven that Cd can mimic estradiol effects in estrogen-responsive breast cancer cell line as MCF-7 [23]. In addition, Cd blocks estradiol binding to ER-α in a noncompetitive manner, suggesting that Cd can interact with receptor hormone-binding domain [22]. In other words, Cd binds with higher affinity to ER-α-binding domain with blocking of estradiol binding [23].
The objective of this study was to investigate the hazard of occupational Cd exposure on bone health. This was achieved through studying its effect on bone turnover and bone formation. Moreover, a direct osteotoxic effect of this metal has been also suggested. The current work may help to support or exclude this hypothesis. It also studied the relationship between Cd and ER-α (osteoporosis candidate gene).
Subjects and methods
The present work was a cross-sectional comparative study. It included 80 men classified into 2 groups, each consisted of 40 subjects. The first group represented the exposed workers who worked in a sewage plant in Helwan City, Cairo, Egypt. Their age ranged between 39 and 59 years (mean age 48.9 years). They have a mean duration of exposure 21.9 ± 6.04 years. The second group, a control group, aged between 33 and 60 years (mean age 50.53 ± 5.98 years). The two groups were matched for age and socioeconomic status. The participants answered a designed questionnaire including detailed occupational history, smoking habit, and medical complaints. Clinical examination was performed. Approval of the Ethical committee of the National Research Centre was taken prior to the study (Registration number 15170). Subjects with past history of rheumatoid or any bone diseases were excluded from the study. Smoking index was based on the result of multiplication of number of cigarettes per day and duration of smoking [24]. In the exposed group, 65% were current smokers, with mean smoking index (554.52 ± 201.72) compared to 47.5% current smokers in the control group with mean smoking index (401 ± 203.56).
A blood sample (7 mL) was collected from each participant and divided into two tubes. The first one was a dry tube which was left at room temperature for 30 min and then centrifuged at 3000 rpm for 5 min to separate serum for the measurement of serum Cd, Ca, and OPG. The second one is an Ethylenediaminetetraacetic acid (EDTA) tube which was used for ERα genotyping. The participants collected urine for the measurement of Cd, Ca, and creatinine (creat).
Biochemical measurements
Serum and urine cadmium
Cd in serum was determined by inductively coupled plasma mass spectrometry (ICP-MS) according to Li et al. [25].
Also, urinary cadmium (U-Cd) was measured by ICP-MS in samples diluted 10 times with an alkaline solution and corrected for molybdenum oxide-based interference [26].
Serum and urine calcium
Serum and urinary calcium (U-Ca) were analyzed, according to Vurek [27] by colorimetric method, using Centronic, GMBH kit. Ca ion forms a blue dye, in the presence of methylthymolblue, which is proportional to Ca concentration, read at 578 nm. Urine samples were diluted 4 times with distilled water and acidified to pH 3–4 with HCl N/10, and the result is multiplied by dilution factor.
Determination of urinary creatinine
To adjust the values of the urinary Cd and Ca, urinary creat was carried out by using the Jaffe method without deproteinization according to Bartels [28].
Measurement of serum OPG
Serum OPG levels were measured using an enzyme-linked immunosorbent assay commercial kit (SunRed Biotechnology). The test is based on the principle of double-antibody sandwich technique. The detection limit of OPG was 2.044 ng/L.
Genomic DNA analysis and ER genotyping
Genomic DNA was extracted from peripheral leucocytes using QIAamP DNA mini kit. The isoform of ER gene was amplified by polymerase chain reaction (PCR) according to Kobayashi et al. [29]. The following primers were used: forward, 5′CTGCCACCCTATCTGTATCTTTTCCTATTCTCC′3; and reverse, 5′TCTTTCTCTGCCACCCTGGCGTCGATTATCTGA′3.
The 50-μL final reaction contained 0.1 μg DNA, 1 unit of Taq polymerase, 10 mM Tris–HCl, pH 8.3, 50 mM KCl, 1 mM MgCl2, 200 μM each of the four deoxyribonucleotides, and 0.4 μM each of the primers. The reaction was run for 30 cycles with denaturation at 94°C for 30 s, annealing at 61°C for 40 s, and extension at 72°C for 90 s. Then, the final (PCR) product was digested with 6 U PvuII restriction endonuclease and incubated in 37°C over night. The digested material was subjected to 2% agarose gel with ethidium bromide staining. The results were read as capital P represents the absence of the restriction site while small p indicates the presence of the restriction site and Pp genotype indicated presence of PvuII restriction sites on one of the two alleles.
Statistical analysis
It was carried out using SPSS program version 17. The data were expressed as mean ± SD. Differences between groups were compared using Student’s t-test for independent groups. Chi-square test was used for frequency tables where odds ratio (OR) calculated the outcome in two groups. P < 0.05 was set as significant. Differences among various ER genotypes were assessed by one-way analysis of variance.
Results
There was a highly significant increase in the mean of serum Cd in exposed compared to controls (1.02 ± 0.6 vs. 0.60 ± 0.2 µg/L, respectively; P= 0.001) and serum OPG (668.9 ± 174.6 vs. 534.4 ± 140.4 ng/L, P = 0.019), and the mean U-Ca level was significantly higher (0.52 ± 0.04 vs. 0.65 ± 0.06 mg/g creat, respectively; P = 0.048) (Table 1).
Table 1.
Biochemical parameters in exposed and control groups.
| Exposed (n = 40) | Controls (n = 40) | t-Test | P-value | |
|---|---|---|---|---|
| Serum Cd (µg/L) | 1.02 ± 0.6 | 0.60 ± 0.2 | 2.47 | 0.006** |
| Serum OPG (ng/L) | 668.9 ± 174.6 | 534.4 ± 140.4 | 3.79 | 0.039** |
| Serum Ca (mg/dL) | 10.3 ± 0.8 | 12.6 ± 1.2 | 0.99 | NS |
| U-Ca (mg/g creat) | 0.52 ± 0.04 | 0.65 ± 0.06 | 1.05 | 0.048* |
| U-Cd (µg/g creat) | 3.28 ± 0.04 | 2.94 ± 0.02 | 0.91 | NS |
*P < 0.05, significant; **P < 0.01, highly significant. NS: Nonsignificant (P ≥ 0.05).
The percentage of bony aches in the exposed workers showed a highly significant increase compared to the controls (95% vs. 37.5%, respectively; P = 0.001). The OR showed marked increase in the risk of bony aches among exposed 31.7 times the risk among controls (95% CI: 6.1–139.14). Also, the percentage of joint pain in the exposed workers showed a highly significant increase compared to the controls (60% vs. 28.9%, respectively; P = 0.007). The OR showed marked increase of the risk of joint pain in the exposed 3.68 times the risk among the controls (95% CI: 1.43–9.47) (Table 2).
Table 2.
Frequency of clinical symptoms among both groups.
| Exposed (n = 40) | Controls (n = 40) | χ2 | P-value | OR | 95% CI |
||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Bony aches | 38 (95.0%) | 15 (37.5%) | 27.6 | 0.001** | 31.7 | 6.7 | 150.6 |
| Joint pain | 24 (60.0%) | 11 (28.9%) | 7.6 | 0.007** | 3.68 | 1.43 | 9.47 |
| Bone fracture | 4 (10.0%) | 3 (7.9%) | 0.11 | 0.5 (NS) | 1.3 | 0.27 | 6.22 |
95% CI: 95% Confidence interval; NS: nonsignificant (P ≥ 0.05).
P < 0.05, significant; **P < 0.01, highly significant.
A significant increase in serum Cd and U-Cd level (Figure 1) was observed among the exposed smoker compared to the control smoker groups.
Figure 1.
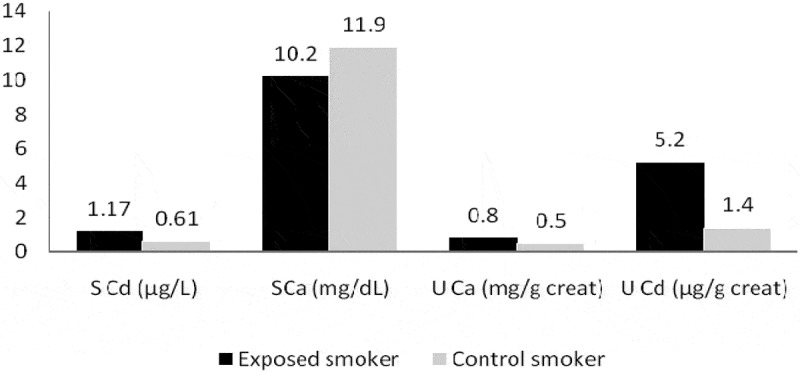
Association of smoking in exposed and control groups with different biochemical parameters.
Figure 2 showed significant rise in serum OPG in the exposed smoker and control smoker groups. In addition, significant elevation in serum OPG among nonsmoker exposed and nonsmoker controls was detected.
Figure 2.
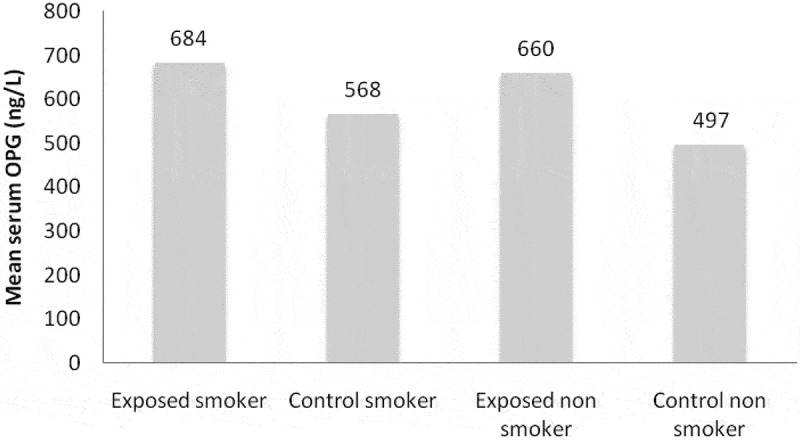
Mean serum OPG in smokers and nonsmokers exposed and control groups.
Significant difference in the percentage distribution of ER gene between the exposed workers and the control group (P = 0.006) was illustrated in Figure 3.
Figure 3.
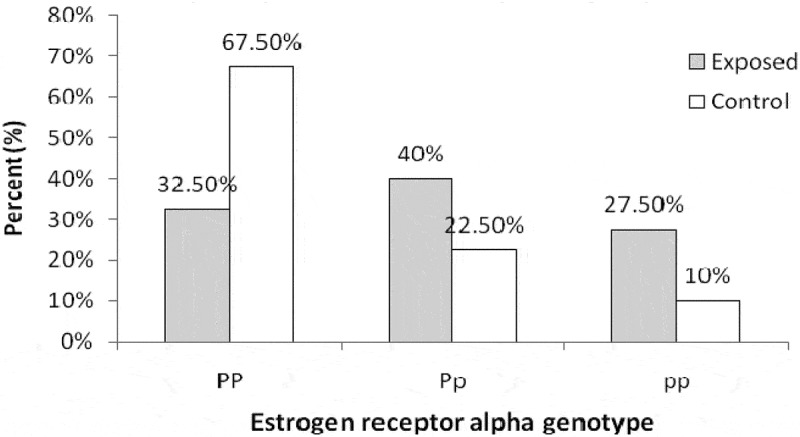
Estrogen receptor alpha genotype frequency in control and exposed groups.
The mean serum Cd level showed significant increase in PP genotype among exposed workers compared to controls (1.2 ± 0.7 vs. 0.6 ± 0.2 µg/L, respectively; P = 0.002) and in pp genotypes (1.04 ± 0.9 vs. 0.33 ± 0.011 µg/L, respectively; P = 0.03), while serum OPG revealed high significant increase in PP and Pp genotypes in the exposed workers compared to the controls (719.54 ± 152.7 vs. 531.56 ± 144.6 ng/L, respectively; P = 0.001; and 651.31 ± 190.8 vs. 519.56 ± 127.4 ng/L; P = 0.025) (Table 3).
Table 3.
Serum Cd, OPG, and Ca in different genotypes of ER-α gene.
| PP |
Pp |
pp |
||||
|---|---|---|---|---|---|---|
| Exposed (N = 13) | Control (N = 27) | Exposed (N = 16) | Control (N = 9) | Exposed (N = 11) | Control (N = 4) | |
| S-Cd (µg/L) | 1.2 ± 0.7 | 0.6 ± 0.2 | 0.87 ± 0.3 | 0.6 ± 0.2 | 1.04 ± 0.9 | 0.33 ± 0.011 |
| P-value | 0.002** | NS | 0.03** | |||
| OPG (ng/L) | 719.54 ± 152.7 | 531.56 ± 144.6 | 651.31 ± 190.8 | 519.56 ± 127.4 | 634.55 ± 176.7 | 587.50 ± 165.2 |
| P-value | 0.001** | 0.025** | 0.65 | |||
| S-Ca (mg/dL) | 10.16 ± 0.9 | 11.18 ± 0.8 | 9.48 ± 2.9 | 10.18 ± 0.8 | 10.15 ± 1.0 | 10.50 ± 0.6 |
| P-value | NS | NS | NS | |||
P < 0.05, significant; **P < 0.01, highly significant. NS: Nonsignificant (P ≥ 0.05).
On the other hand, mean U-Ca exhibited high significant increase in pp genotype in the exposed workers compared to the control group (0.042 ± 0.03 vs. 0.073 ± 0.07 mg/g creat, respectively; P = 0.009). As regards mean U-Cd in both PP and pp genotypes, there was a high significant increase in the exposed group compared to the controls (1.10 ± 4.4 vs. 0.28 ± 0.2 µg/g creat; P = 0.027; and 0.34 ± 0.2 vs. 0.07 ± 0.06 µg/g creat; P = 0.021) (Table 4).
Table 4.
Urinary Cd, Ca, Ca/creat, and Cd/creat ratio in different genotypes of ER-α gene.
| PP |
Pp |
pp |
||||
|---|---|---|---|---|---|---|
| Exposed (N = 13) | Control (N = 27) | Exposed (N = 16) | Control (N = 9) | Exposed (N = 11) | Control (N = 4) | |
| U-Ca (mg/g creat) |
0.058 ± .04 | 0.071 ± 0.07 | 0.054 ± 0.04 | 0.071 ± 0.07 | 0.042 ± 0.03 | 0.073 ± 0.07 |
| P-value | 0.56 | 0.44 | 0.009** | |||
| U-Cd (µg/g creat) |
1.10 ± 4.4 | 0.28 ± 0.2 | 0.31 ± 0.3 | 0.24 ± 0.2 | 0.34 ± 0.2 | 0.07 ± 0.06 |
| P-value | 0.027* | 0.48 | 0.021* | |||
*P < 0.05, significant; **P < 0.01, highly significant. NS: Nonsignificant (P ≥ 0.05).
Discussion
Osteoporosis is a disorder of reduced bone strength that leads to skeletal fragility with increasing fracture risk [30]. Different epidemiological studies recorded that Cd exposure may lead to bone loss, in addition to osteoporosis and increased bone fractures risk [31].
Assessment of Cd body burden is through measuring levels of Cd in blood. It is a good estimate for Cd body burden in exposed populations [32].
Our study revealed significant high increase in serum Cd p<0.006 in exposed compared to controls which was in accordance with Al-Rmalli et al. [32]. They recorded that blood Cd represented a good and valuable biomarker of both recent low level and long-term exposure. Increasing age and smoking were associated with high Cd levels in blood. Cigarette smoking may probably represent the most critical factor or indicator that reflects Cd body burden as cigarettes contain a lot of Cd taken up through tobacco plant [33].
Previous epidemiological data mentioned an association between low level of Cd exposure in environment and increased risk of Cd-inducing bone disturbances [6].
Also, mean U-Ca level was significantly higher in exposed workers compared to control group. That was in agreement with Nawrot et al. [1], who suggested that Cd-induced renal tubular damage leads to reduced Ca reabsorption from the nephron. This results in hypercalciuria and low Body mass index (BMD) and therefore increased risk of fracture, especially in postmenopausal women or elderly men.
Brzóska and Moniuszko-Jakoniuk [33] demonstrated significant increase in U-Ca and unchanged serum Ca levels that is in agreement with our results. They suggested that in case of Ca decreased levels in the extracellular fluid, calcemia is compensated through its release from bone. That explained the unchanged serum Ca levels in experimental rats exposed to Cd. The above mechanisms in addition to direct effect of Cd on bone cells could be considered as possible causes of bone loss induced by Cd [32].
At environmental low levels of Cd exposure, consistent associations were found between bone density and lifetime Cd exposure, which was reflected as U-Cd, independent of tubular dysfunction. Epidemiologic evidence for direct osteotoxic Cd effect was strongly supported by experimental studies. In Cd exposed animals, bone demineralization takes place early following Cd exposure and preceding renal injury [1]. Other explanation is that blood stream Cd was bound to a greater extent to proteins as metallothionein that can be filtered as low molecular weight proteins in the glomeruli and then reabsorbed in the tubules. Thus, a fall in tubular reabsorption could result in an increase in U-Cd excretion [34].
Other study suggested that Cd might increase Ca excretion and consequently lead to reduction in active vitamin D [1,25(OH)2D] generation in kidney. Furthermore, decrease in Ca uptake, as well as absorption in gastrointestinal gut, causes inhibition in bone mineralization and formation [35].
Clinical symptoms among studied subjects showed that the percentage of bony aches and joint pain among exposed workers was significantly increased compared to controls with no increase in risk of bone fracture as shown in Table 2.
The current study recorded significant high increase in mean levels of serum and U-Cd in exposed smoker compared to control smoker group (Figure 1).
As outlined by Wallin et al. [36], nonsmokers who are not occupationally exposed to Cd were subjected mainly through diet. As shown in smokers, inhaled smoke from cigarette represents generally the essential Cd exposure source. Recently, different studies have reported that even low Cd exposure level might increase fractures and osteoporosis risk. Other study mentioned that particular increase in U-Cd level is dependent on the amount of consumed daily cigarettes. Thus, smoking represents a confounder for evaluation of U-Cd among populations with high dietary Cd burden [37].
Szulc et al. [38] revealed that OPG is synthesized by different skeletal and extraskeletal tissues and other cell types and is also regulated by various osteotropic and nonosteotropic hormones and cytokines. They related the rise in circulating OPG levels to age in males. They postulated that this rise might be in part able to counteract the increase in bone resorption rate and bone loss which was found to be associated with age. Additionally, levels of circulating OPG may reflect in part the local milieu inside the bone and bone marrow microenvironment [38]. This comes in agreement with our findings that showed high significant increase in the level of serum OPG in the exposed group.
Furthermore, significant elevation in mean levels of serum OPG was detected in exposed smoker and nonsmoker groups compared with nonsmoker exposed and control groups (Figure 2).
Fusby et al. [39] stated that cigarette smoking had an adverse effect on immune system through bone marrow reduction that represents an important risk factor for osteoporosis development.
Genetic factors assessments might be useful in targeting precautionary measures to subjects with higher risk of osteoporosis development [40]. The present work recorded genotype distributions of the Restriction fragment length polymorphism (RFLP) of PvuII in the exposed and controls where PP genotype represented 32.5% vs. 67.5%, Pp (40% vs. 22.5%), and pp (27.5% vs. 10%), respectively (Figure 3).
Recently, a phenotype report of osteoporosis in men with destructive mutation in ER-α gene strongly supports its involvement in the evolution of osteoporosis [41].
Activation of ER-α was recorded at low concentrations of Cd through interaction with receptor hormone-binding domain. Cd binds with higher affinity and blocks estradiol-binding receptor. Cd interaction with the receptor seems to involve numbers of different amino acids within receptor hormone-binding pocket that suggest formation of a coordination complex with hormone-binding domain (by the metal) which activates the receptor [23].
Current study revealed that mean serum Cd level increased significantly in PP and pp genotypes in the exposed workers compared to the controls. Moreover, serum OPG increased significantly in PP and Pp genotypes in the exposed workers compared to the controls (Table 3).
Cigarette smoking can induce alteration in bone metabolism by indirect way through alteration in calciotropic hormone metabolism in addition to estradiol binding [42]. Another mechanism is through adrenal cortical hormone metabolism alteration. Direct effects on osteogenesis that include RANK–RANKL–OPG system alteration, collagen metabolism [43], and bone angiogenesis are other suggested mechanisms [44,45].
Khosla et al. [16] mentioned that in males aged 60–90 years, correlation was detected between bone loss rates at the midradius with bioavailable E2 levels in subjects with P alleles, however not in individuals with pp genotypes. Alternatively, modulation of bone mass density or bone loss rate by ER genotypes could be present only within certain threshold levels of serum E2. In turn, the hypothesis that explains lack of interaction between free E2 levels and PvuII genotypes may be due to estrogen sufficiency in young males.
Mean U-Ca exhibited significant increase in pp genotype in exposed workers compared to controls and mean U-Cd showed significant elevation in both PP and pp genotypes (Table 4).
Osteoporotic phenotype observation in man with ER-α gene disruptive mutation [46] as well as in mice with functional ER-α loss and decreased bone mass density [47] supported the hypothesis of ER-α as an osteoporosis candidate gene. It can be suggested that common allelic variants of ER-α gene led to different responsiveness to estrogen in different population [45].
A previous study suggested that pp genotype could not be relatively sensitive to estrogen, and in consequence, subjects with P allele will benefit more from the defensive estrogen actions on bone than subjects with all p allele. Moreover, negative or positive associations might be dependent on levels of circulating estrogen [16].
In summary, workplace exposure to Cd remains possible in several societies. Our results supported the hypothesis that Cd along with smoking can be definitely osteotoxic. In addition, Cd is associated with increased Ca excretion which may lead to osteoporosis in sewage workers. Moreover, overexpression of OPG which is associated with elevation in bone resorption may be related to the protective mechanism of the skeleton to compensate the increased rate of bone resorption and bone loss.
Disruptive mutation of the ER-α gene was associated with increased levels in serum Cd and OPG, U-Cd, and U-Ca in several genotypes which strongly supports the involvement of ER-α gene in osteoporosis development. Smoking cessation is recommended and much more research is necessary in order to explain the mechanisms of Cd effects on bone and Ca metabolism.
Funding Statement
This research received fund from National Research Centre, Egypt. The grant number is [p100801].
Disclosure statement
No potential conflict of interest was reported by the author.
References
- [1].Nawrot T, Geusens P, Nulens TS, et al. Occupational cadmium exposure and calcium excretion, bone density, and osteoporosis in men. J Bone Miner Res. 2010;25(6):1441–1445.doi:10.1002/jbmr.22 [DOI] [PubMed] [Google Scholar]
- [2].Abdel-Sabour MF.Cadmium status in Egypt. J Environ Sci. 2001;13(3):351–360. [PubMed] [Google Scholar]
- [3].Tang L, Chen X, Bao Y, et al. CT imaging biomarkers of bone damage induced by environmental level of cadmium exposure in male rats. Biol Trace Elem Res. 2016;170(1):146–151. [DOI] [PubMed] [Google Scholar]
- [4].Chen X, Qin B, Li X, et al. Effects of fluoride and cadmium co-exposure on bone in male rats. Biol Trace Elem Res. 2013a;154(3):396–402. [DOI] [PubMed] [Google Scholar]
- [5].Kazantzis G. Cadmium, osteoporosis and calcium metabolism (review). Biometals. 2004;17(5):493–498. [DOI] [PubMed] [Google Scholar]
- [6].Trzcinka-Ochocka M, Jakubowski M, Szymczak W, et al. The effects of low environmental cadmium exposure on bone density. Environ Res. 2010;110(3):286–293. [DOI] [PubMed] [Google Scholar]
- [7].Jarup L, Berglund M, Elinder CG, et al. Health effects of cadmium exposure a review of the literature and a risk estimate. Scand J Work, Environ Health. 1998;24(Supp1):1–52. [PubMed] [Google Scholar]
- [8].Nordberg GF, Fowler BA, Nordberg M, et al. Handbook on the toxicology of metals. 3rd ed. Oxford (UK): Academic Press, Elsevier; 2007. [Google Scholar]
- [9].Uriu K, Morimoto I, Kai K, et al. Uncoupling between bone formation and resorption in ovariectomized rats with chronic cadmium exposure. Toxicol Appl Pharmacol. 2000;164(3):264–272. [DOI] [PubMed] [Google Scholar]
- [10].Fu YX, Gu JH, Zhang YR, et al. Osteoprotegerin influences the bone resorption activity of osteoclasts. Int J Mol Med. 2013;31(6):1411–1417. [DOI] [PubMed] [Google Scholar]
- [11].Sughis M, Penders J, Haufroid V, et al. Bone resorption and environmental exposure to cadmium in children: a cross-sectional study. Environ Health. 2011;10:104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen X, Wang G, Li X, et al. Environmental level of cadmium exposure stimulates osteoclasts formation in male rats. Food Chem Toxicol. 2013b;60:530–535. [DOI] [PubMed] [Google Scholar]
- [13].Liu W, Xu C, Zhao H, et al. Osteoprotegerin induces apoptosis of osteoclasts and osteoclast precursor cells via the Fas/Fas ligand pathway. PLoS One. 2015;10(11):e0142519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cvijetic S, Grazio S, Kosovic P, et al. Osteoporosis and polymorphisms of osteoprotegerin gene in postmenopausal women - a pilot study. Reumatologia. 2016;54(1):10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ohmori H, Makita Y, Funamizu M, et al. Linkage and association analyses of the osteoprotegerin gene locus with human osteoporosis. J Hum Genet. 2002;47:400–406. [DOI] [PubMed] [Google Scholar]
- [16].Khosla S, Riggs BL, Atkinson EJ, et al. Relationship of estrogen receptor genotypes to bone mineral density and to rates of bone loss in men. J Clin Endocrinol Metab. 2004;89:1808–1816. [DOI] [PubMed] [Google Scholar]
- [17].Kim SY, Kim HH, Nam CM, et al. Association of estrogen receptor-alpha gene polymorphism with pathogenesis of osteoporosis in Korean vegetarian men. Med Princ Pract. 2010;19(3):200–205. [DOI] [PubMed] [Google Scholar]
- [18].Rochira V, Faustini-Fustini M, Balestrieri A, et al. Estrogen replacement therapy in a man with congenital aromatase deficiency: effects of different doses of transdermal estradiol on bone mineral density and hormonal parameters. J Clin Endocrinol Metab. 2000;85:1841–1845. [DOI] [PubMed] [Google Scholar]
- [19].Leder BZ, LeBlanc KM, Schoenfeld DA, et al. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab. 2003;88:204–210. [DOI] [PubMed] [Google Scholar]
- [20].Taxel P, Kennedy DG, Fall PM, et al. The effect of aromatase inhibition on sex steroids, gonadotropins, and markers of bone turnover in older men. J Clin Endocrinol Metab. 2001;86:2869–2874. [DOI] [PubMed] [Google Scholar]
- [21].Bord S, Horner A, Beavan S, et al. Estrogen receptors α and β are differentially expressed in developing human bone. J Clin Endocrinol Metab. 2001;86:2309–2314. [DOI] [PubMed] [Google Scholar]
- [22].Sims NA, Clement-Lacroic P, Minet D, et al. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest. 2003;111:1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stoica A, Katzenellenbogen BS, Martin MB. Activation of estrogen receptor-a by the heavy metal cadmium. Mol Endocrinol. 2000;14:545–553. [DOI] [PubMed] [Google Scholar]
- [24].Bano R, Ahmad N, Mahagaonkar AM, et al. Study of lung functions in smokers and non-smokers in rural India. Indian J Physiol Pharmacol. 2011;55(1):84–88. [PubMed] [Google Scholar]
- [25].Li G, Brockman JD, Lin S-W, et al. Measurement of the trace elements Cu, Zn, Fe, and Mg and the ultratrace elements Cd, Co, Mn, and Pb in limited quantity human plasma and serum samples by inductively coupled plasma-mass spectrometry. Amer J Anal Chem. 2012;3:646–650. [Google Scholar]
- [26].Momen AA, Khalid MAA, Elsheikh MAA, et al. Assessment and modifications of digestion procedures to determine trace elements in urine of hypertensive and diabetes mellitus patients. J Health Specialties. 2013;1(3):122–128. [Google Scholar]
- [27].Vurek GG. Calcium measurement: picomole quantitation by continuous flow colorimetry. Anal Biochem. 1981;114:288–293. [DOI] [PubMed] [Google Scholar]
- [28].Bartels H. Determination of serum and urinary creatinine by Jaffe s method without deproteinsation in a 2-point reaction rate measurement in 2 minutes. Clin Chim Acta. 1971;32:81–84.5096431 [Google Scholar]
- [29].Kobayashi S, Inoue S, Hosoi T, et al. Association of bone mineral density with polymorphism of the oestrogen receptor gene. J Bone Mineral Res. 1996;11:306–311. [DOI] [PubMed] [Google Scholar]
- [30].McClung M. Role of RANKL inhibition in osteoporosis. Arthritis Res Ther. 2007;9(Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Engström A, Michaëlsson K, Vahter M, et al. Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone. 2012;50:1372–1378. [DOI] [PubMed] [Google Scholar]
- [32].Al-Rmalli SW, Belazi AM, Mustafa HA, et al. Blood cadmium concentrations in general population of Tripoli region, Libya. J Appl Chem. 2015;8(7):76–80. [Google Scholar]
- [33].Brzóska MM, Moniuszko-Jakoniuk J. Low-level lifetime exposure to cadmium decreases skeletal mineralization and enhances bone loss in aged rats. Bone. 2004;35(5):1180–1191. [DOI] [PubMed] [Google Scholar]
- [34].Wallin M, Sallsten G, Lundh T, et al. Low-level cadmium exposure and effects on kidney function. Occup Environ Med. 2014;71(12):848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Horiguchi H, Oguma E, Sasaki S, et al. Environmental exposure to cadmium at a level insufficient to induce renal tubular dysfunction does not affect bone density among female Japanese farmers. Environ Res. 2005;97:83–92. [DOI] [PubMed] [Google Scholar]
- [36].Wallin M, Barregard L, Sallsten G, et al. Low-level cadmium exposure is associated with decreased bone mineral density and increased risk of incident fractures in elderly men: the MROS Sweden study. J Bone Miner Res. 2016;31(4):732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ikeda M, Moriguchi J, Ezaki T, et al. Smoking-induced increase in urinary cadmium levels among Japanese women. Int Arch Occup Environ Health. 2005;78(7):533–540. [DOI] [PubMed] [Google Scholar]
- [38].Szulc P, Hofbauer LC, Heufelder AE, et al. Osteoprotegerin serum levels in men: correlation with age, estrogen, and testosterone status. J Clin Endocrinol Metab. 2001;86(7):3162–3165. [DOI] [PubMed] [Google Scholar]
- [39].Fusby JS, Kassmeier MD, Palmer VL, et al. Cigarette smoke-induced effects on bone marrow B-cell subsets and CD4+: CD8+T-cell ratios are reversed by smoking cessation: influence of bone mass on immune cell response to and recovery from smoke exposure. Inhal Toxicol. 2010;22(9):785–796. [DOI] [PubMed] [Google Scholar]
- [40].Masi L, Maddali Bongi S, Angotti C, et al. The role of osteoprotegerin (OPG) and estrogen receptor (ER-α) gene polymorphisms in rheumatoid arthritis. Clin Cases Miner Bone Metab. 2007;4(2):156–160. [PMC free article] [PubMed] [Google Scholar]
- [41].Nam HS, Shin MH, Kweon SS, et al. Association of estrogen receptor-α gene polymorphisms with bone mineral density in postmenopausal Korean women. J Bone Miner Metab. 2005;23:84–89. [DOI] [PubMed] [Google Scholar]
- [42].Rapuri PB, Gallagher JC, Balhorn KE, et al. Smoking and bone metabolism in elderly women. Bone. 2000;27:429–436. [DOI] [PubMed] [Google Scholar]
- [43].Sørensen LT, Toft BG, Rygaard J, et al. Effect of smoking, smoking cessation, and nicotine patch on wound dimension, vitamin C, and systemic markers of collagen metabolism. Surgery. 2010;148:982–990. [DOI] [PubMed] [Google Scholar]
- [44].Ma L, Zheng LW, Sham MH, et al. Uncoupled angiogenesis and osteogenesis in nicotine-compromised bone healing. J Bone Miner Res. 2010;25:1305–1313. [DOI] [PubMed] [Google Scholar]
- [45].Yoon V, Maalouf NM, Sakhaee K. The effects of smoking on bone metabolism. Osteoporos Int. 2012;23(8):2081–2092. [DOI] [PubMed] [Google Scholar]
- [46].Gennari L, Merlotti D, De Paola V, et al. Estrogen receptor gene polymorphisms and the genetics of osteoporosis: a huge review. Am J Epidemiol. 2005; 15;161(4):307–320. [DOI] [PubMed] [Google Scholar]
- [47].Smith EP, Boyod J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen receptor gene in a man. N Engl J Med. 1994;331:1056–1061. [DOI] [PubMed] [Google Scholar]


