Abstract
Objective: The purpose of this study was to assess the effects of the Mulligan Concept (MC) ‘squeeze’ technique compared to a sham technique in participants with a clinically diagnosed meniscal tear.
Methods: A multi-site randomized sham-controlled trial of participants (n = 23), aged 24.91 ± 12.09 years, with a clinically diagnosed meniscal tear were equally and randomly divided into two groups. Groups received a maximum of six treatments over 14 days. Patient outcomes included the numeric pain rating scale (NRS), patient-specific functional scale (PSFS), the disablement in the physically active (DPA) scale and the knee injury osteoarthritis outcome score. Data were analysed using univariate ANOVA, univariate ANCOVA, and descriptive statistics.
Results: All participants in the MC ‘squeeze’ group met the discharge criteria of ≤2 points on the NRS, ≥9 points on the PSFS, and ≤34 points or ≤23 on the DPA Scale for chronic or acute injuries, respectively within the treatment intervention timeframe. A significant difference was found in favor of the MC ‘squeeze’ technique in PSFS scores (F(1, 21) = 4.40, p = .048, partial eta squared = .17, observed power = .52) and in DPA Scale scores (F(1, 21) = 7.46, p = .013, partial eta squared = .27, observed power = .74).
Discussion: The results indicate the MC ‘squeeze’ technique had positive effects on patient function and health-related quality of life over a period of 14 days and was clinically and statistically superior to the sham treatment. Further investigation of the MC ‘squeeze’ technique is warranted.
Keywords: Meniscal tears, manual therapy, knee pain, rehabilitation
Introduction and background
Meniscal tears are suspected to affect critical functions of the meniscus, such as joint congruency, load transmission, and shock absorption [1,2] leading to the classic signs and symptoms of a meniscal tear: catching, locking, or clicking; joint line pain; and a feeling of ‘giving out’ or instability [3]. Despite the importance of the meniscus tissue for function, incidental findings of asymptomatic tears on magnetic resonance imaging (MRI) are relatively common [4–7], suggesting the presence of a meniscal tear does not directly correlate to knee disability. Therefore, the presence of meniscal lesions on MRI findings may not equate to the pathology being the root cause of dysfunction [5,6].
Treatment options are typically categorized as surgical, involving partial meniscectomy or meniscal repair, or non-surgical, which is defined as conservative therapy [8]. Arthroscopic surgery currently remains the proposed gold standard for treatment of meniscal tears [9]. Preservation of the meniscus through arthroscopic surgical repair is considered the most ideal option [10]; however, failure rates have been reported as high as 42% following those procedures [11] and the prevalence of subsequent surgeries is as high as 20% [12]. Arthroscopic partial meniscectomy (APM) is the most common procedure used to treat meniscal tears [11]. While common, the APM procedure has inconsistent results for alleviating the symptoms of meniscal tears [13–17] and 50% of patients who undergo APM develop knee OA symptoms confirmed by radiographic images years after surgery [10,16–20]. Consequently, patients who undergo any type of meniscal surgery are at risk for requiring subsequent surgeries [12], which suggests clinicians should exhaust conservative care options for meniscus tears before pursuing surgical options [21].
Conservative therapy for meniscus tears commonly includes active exercises focused on increasing range of motion (ROM) and muscle strength while improving balance and flexibility [8,21]. Although conservative therapy protocols are recommended as an alternative to surgery [13,21,22], lengthy timelines [8] and poor outcomes [13,21,22] may make those protocols less appealing to patients. Reported outcomes of surgery and conservative care are similar and have inconsistent results [13,21,22]; therefore, there is a need for additional research into non-operative alternative treatment methods for treating the symptoms of meniscal tears; as there is limited support that non-operative alternative treatment are effective [23].
The Mulligan Concept ‘squeeze’ technique is a manual therapy treatment within the Mulligan Concept (MC) paradigm that could be used to rapidly treat range of motion deficits and pain localized to the knee joint line during movement [24]. Such symptoms are often reported in the presence of meniscal tears due to altered joint mechanics and function caused secondarily by the disruption of meniscal tissue [25]. If meniscal tissue is dislodged or subluxed from its normal anatomical position after a tear, the disrupted tissue may cause increased pressure on the highly innervated periphery of the meniscus tissue and result in the commonly reported symptoms [2,6,26,27].
The MC ‘squeeze’ technique has produced favorable patient outcomes for clinically classified meniscal tears in anecdotal reports and published a priori case studies [28,29]. In these reports, patients reported positive changes in pain, function, disability, and psychosocial well-being on patient reported outcome measures; however, the small sample size and lack of comparison groups necessitates the need for further investigation to determine the effectiveness of the MC ‘squeeze’ technique. Therefore, the purpose of this study was to assess the effects of the MC squeeze technique compared to a sham technique in participants presenting with a clinically diagnosed meniscal tear.
Methods
Study design
The study was a multi-site randomized sham-controlled trial, conducted across four clinics with four athletic trainers providing treatments. The study was approved by the Institutional Review Boards at the four clinical sites. Participants signed written informed consent acknowledging possible publication of de-identified outcomes, and consent/assent forms were collected from all minors participating in this study.
Participant selection
Participants were recruited as a sample of convenience of physically active and sedentary participants ranging from 14 to 62 years of age. A priori randomization was designed to ensure equal distribution of participants into either the MC ‘squeeze’ technique treatment group or the sham group. Participants who reported common symptoms of a meniscal tear with various mechanisms of injury or onset of symptoms (i.e. acute and chronic) were considered for participation in this study at each clinical site.
Meniscal tears were identified solely based on a clinical exam. Inclusion criterion were a positive finding in at least three of the following: McMurray’s test, pain with maximal knee flexion, pain with maximal knee extension, joint line tenderness, and a history of clicking and/or popping [3]. The preceding inclusion criteria were formed according to the clinical composite score (CCS) developed by Lowery et al. [3] When three of the signs were present, the CCS had a specificity of 90.2% and a positive prediction value (PPV) of 76.7% [3]; in comparison, an MRI has a specificity of 69–93.3% [9,30] and a PPV of 80.4–83.2% [30] for the detection of meniscal tears. Participants were also required to present with a positive finding in at least one of the following orthopedic tests: Apley’s compression and distraction (specificity = 90%) [31]; and Thessaly’s performed at 20° of knee flexion (specificity = 96–97%) [32]. Exclusion criteria were the presence of knee comorbidities, such as anterior cruciate ligament (ACL) tears, knee contusion, fracture, knee dislocation, other knee ligament instability, non-mechanical causes of pain (e.g. hyperalgesia), and any injury or illness for which manual therapy is contraindicated.
Outcome measures
Patient outcomes included the numeric pain rating scale (NRS), the patient-specific functional scale (PSFS), the disability in the physically active (DPA) scale, and the knee injury and osteoarthritis outcome score (KOOS). Cumulative NRS and PSFS were collected at intake, daily pretreatment, and 24-h after the final treatment. Current NRS and PSFS scores were also collected daily after each treatment intervention. The DPA Scale and KOOS were only collected at intake and 24-h after the final treatment.
Treatment interventions
Treatment and participant position began in the same position that elicited knee symptoms during assessment, which was either supine/non-weight bearing (NWB), partial weight bearing (PWB), or full weight bearing (FWB) [24] for both treatment options.
Mulligan concept ‘squeeze’ intervention
The clinicians placed themselves in a position of biomechanical advantage based on each participant’s individual treatment position. The participant actively placed the involved knee in approximately 90° of flexion (allowing access to the joint line) or to the participant’s pain-free limit of flexion in NWB. The clinician then placed the medial border of one thumb (i.e. the contact thumb) on the site of joint line tenderness and/or joint line edema, while the other thumb (i.e. the mobilizing thumb) was used to apply a force through the first thumb in an overlapping manner (Figure 1(A)). Next, the participant extended their knee through their pain-free range, while the clinician maintained contact force with thumbs, releasing the force as the joint space closed in maximal knee extension (Figure 1(B)). The participant then performed active knee flexion as the clinician continued to apply a ‘squeezing’ force towards the center of the joint until maximal pain-free knee flexion was reached (Figure 1(C)). The clinician held the pressure at the joint line for two seconds as the participant applied pain-free overpressure by pulling their tibia with both hands to their end range of knee flexion (Figure 1(C)). If a participant could not grasp their tibia, they were given a strap to assist them into flexion. The participants returned to their end-range of knee extension, while the clinician released the force as the joint space closed. The participants could experience localized discomfort from the overlap grip, but the localized discomfort was not exacerbated with movement.
Figure 1.
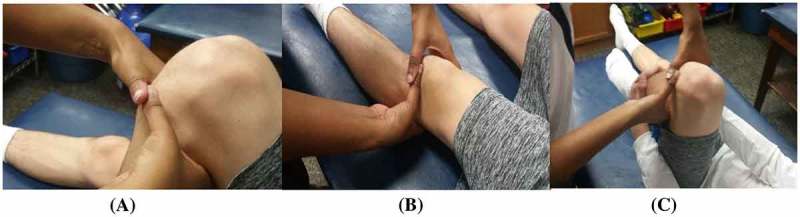
Starting hand placement showing the overlap thumb grip. (A) Clinician hand placement in NWB (supine) for the MC ‘squeeze’ technique treatment in full knee extension. Clinician alleviates pressure on joint line. (B) Clinician hand placement in NWB (supine) for the MC ‘squeeze’ technique treatment in full knee flexion. Pain-free over-pressure is provided by the participant (C).
Participants who were restricted in flexion were asked to perform active knee flexion only (Figure 1(C)). Participants who were restricted in extension were asked to perform active knee extension only (Figure 1(B)). Participants who were restricted in both flexion and extension were first asked to perform knee flexion followed by knee extension. The treatment consisted of three sets of 10 repetitions with a minimum of 30 s of rest between each set. As the participants progressed towards full weight bearing, their position during treatment application also progressed from supine to partial weight bearing (Figure 2) to full weight bearing (Figure 3). Each participant was monitored for any increase in pain throughout the technique in accordance with MC treatment principles.
Figure 2.
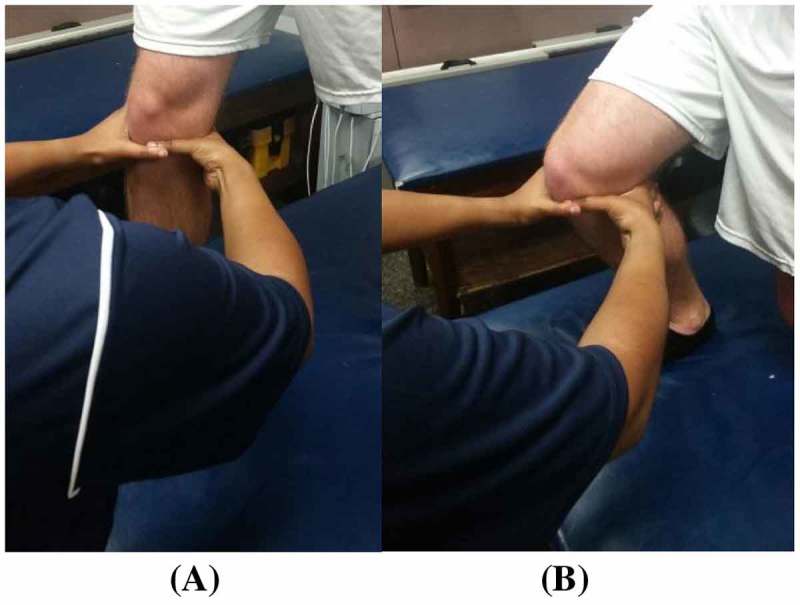
Clinician hand placement in PWB (lunge) starting. (A) and ending position. (B) for the MC ‘squeeze’ technique.
Figure 3.
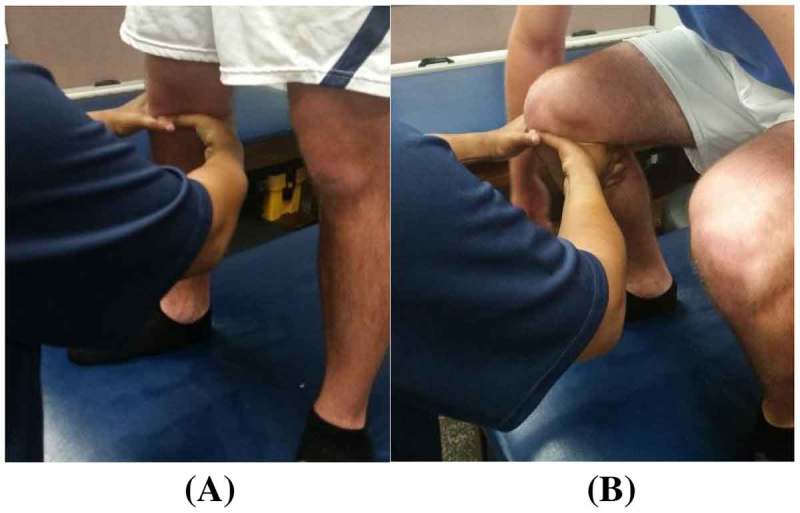
Clinician hand placement in FWB (squat) starting. (A) and ending position. (B) for the MC ‘squeeze’ technique.
Sham intervention
The ‘sham’ treatment followed the same protocol as the MC ‘squeeze’ group (i.e. flexion/extension movement pattern was consistent) except for the hand placement and the force. The hand placement for the sham treatment consisted of the same overlap grip of the thumbs, but the clinician applied the ‘squeeze’ a ½ inch below the point of maximal joint line tenderness. To provide consistent force using the sham treatment across treatment applications and participants, the clinician used only enough force to blanch the nail bed of the reinforcing thumb when applying the ‘sham’ treatment.
Discharge criteria
The discharge criteria for both treatment groups included: a PSFS score of nine or higher for the reported patient-specific activity, a cumulative NRS score of two or less (with no greater than a one on current pain), and a DPA Scale score of 34 or less for persistent/chronic injuries and 23 or less for acute injuries. Participants were discharged from the study once they reached the predetermined criteria and maintained the outcomes a minimum of 24 h post-treatment.
Treatment application protocol
The protocol consisted of a maximum of six treatments within a 14-day period. Treatment applications were separated by a minimum of 24 h and a maximum of 72 h between each treatment session. If participants reached discharge criteria prior to the sixth treatment, they could be discharged successfully from the study prior to completing all six treatments. Participants in the sham group that did not reach discharge criteria by the end of six treatments were automatically placed into a third group which received the MC ‘squeeze’ treatment (Figure 4). A minimum of 24 h was required after the last treatment to assess a participant for discharge. Participants were not restricted from any activities of daily living and could participate as tolerated (based on clinical presentation and clinician assessment) in any specific sport activities throughout the duration of this study.
Figure 4.
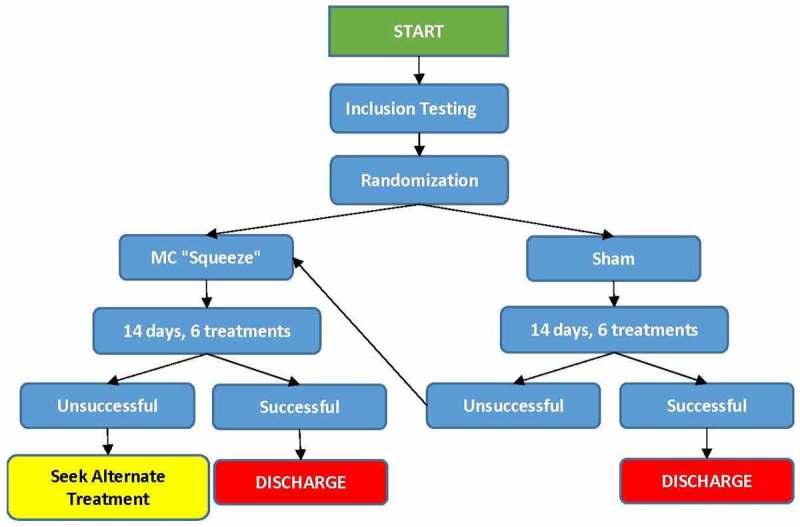
Flow chart of methods and post-study treatment options provided to each participant if assigned treatment was unsuccessful.
Data analysis
Descriptive statistics (mean ± SD) were calculated for all participant demographics. Using NRS, PSFS, DPA, and KOOS scores from a pilot study, an a priori analysis using G power determined a minimum of 16 participants would be required for this study. A series of one-way analyses of variance (ANOVAs) was performed on the NRS and PSFS scores due to the variance in baseline scores between each group (i.e. linearity and homogeneity of regression did not exist). A series of one-way analyses of covariance (ANCOVAs), with baseline scores as the covariate, was performed on DPA Scale and KOOS5 scores. Patient outcomes on NRS and PSFS were used to assess the effect of each intervention after a single treatment, and NRS, PSFS, DPA, and KOOS5 were used to assess the effect of each treatment intervention after final treatment. Mean differences, ± standard deviation (SD), were calculated with statistical significance set at p ≤ .05, confidence intervals (CI) at 95%, and partial eta squared values: small = .02, medium = .13, and large = .26 [33]. All data analyses were performed using Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 23.0.
Results
Participant demographics
Twenty-eight participants (males = 14, females = 14) qualified for this study. Five participants elected to withdraw prior to reaching discharge criteria in the allotted 14-day period. Two participants withdrew due to the time constraints of the study (MC ‘squeeze’ group = 1, sham group = 1), two sustained additional injuries (sham group = 2), and the last did not offer a reason (sham group = 1). The remaining 23 participants (age = 24.91 ± 12.09, males = 11, females = 12) were included in the final data analysis. The MC ‘squeeze’ group was composed of 12 participants (acute = 6, chronic = 6) and the sham group was composed of 11 participants (acute = 3, chronic = 8). Participants were generally healthy (i.e. no general medical or orthopedic comorbidities) with a mean BMI of 28.48 ± 5.35, from both athletic and general populations (MC ‘squeeze’ BMI = 25.98 ± 5.62, Sham BMI = 26.35 ± 5.17; Table 1). The results of each participant’s clinical exam are presented in Table 2.
Table 1. Participant demographic data for the MC ‘squeeze’ and sham group.
| Participant ID # | Gender | Age | Sport/activity | BMI | Onset (duration of symptoms) | Joint line point of treatment | Total treatments |
|---|---|---|---|---|---|---|---|
| 101 | Male | 45 | Football coach | 35.6 BMI | Chronic | Medial | 5 squeeze |
| 102 | Male | 23 | Football | 32.8 BMI | Chronic | Medial | 4 squeeze |
| 103 | Female | 53 | General population | 24.0 BMI | Chronic | Lateral | 6 squeeze |
| 104 | Male | 22 | Soccer | 24.3BMI | Chronic | Medial | 3 squeeze |
| 105 | Male | 20 | Baseball | 32.5 BMI | Acute | Medial | 5 squeeze |
| 106 | Male | 21 | Track & field | 23.6 BMI | Acute | Lateral | 6 squeeze |
| 107 | Male | 14 | Basketball | 18.5 BMI | Acute | Medial | 3 squeeze |
| 108 | Female | 18 | Dance | 29.9 BMI | Chronic | Lateral | 5 squeeze |
| 109 | Female | 21 | ROTC | 24.0 BMI | Acute | Medial | 6 squeeze |
| 110 | Female | 25 | Swim coach | 26.8 BMI | Acute | Medial | 6 squeeze |
| 111 | Female | 20 | Basketball | 21.30BMI | Chronic | Medial | 6 squeeze |
| 112 | Male | 16 | Soccer | 18.5 BMI | Acute | Lateral | 4 squeeze |
| 113a | Male | 33 | Football/track coach | 23.0 BMI | Chronic | Lateral | 6 sham + 4 squeeze |
| 114a | Male | 19 | Baseball | 25.7 BMI | Chronic | Lateral | 4 sham |
| 115a | Female | 20 | Soccer | 24.4 BMI | Chronic | Medial | 5 sham |
| 116a | Female | 19 | Cross country | 20.4 BMI | Acute | Medial | 6 sham + 6 squeeze |
| 117a | Male | 23 | Football | 31.0 BMI | Acute | Medial | 6 sham + 5 squeeze |
| 118a | Female | 19 | ROTC | 24.1 BMI | Acute | Lateral | 6 sham |
| 119a | Female | 18 | Recreational basketball | 21.3 BMI | Chronic | Medial | 6 sham |
| 120a | Female | 21 | General population | 35.2 BMI | Chronic | Medial | 6 sham + 6 squeeze + 1 MWM** |
| 121a | Female | 62 | General population | 30.4 BMI | Chronic | Posterior lateral | 6 sham + 4 squeeze |
| 122a | Male | 23 | General population | 33 BMI | Chronic | Lateral | 5 sham |
| 123a | Female | 18 | Recreational basketball | 21.3 BMI | Chronic | Medial | 6 sham |
Sham treatment group.
Notes alternate treatment used according to the study protocol in Figure 4.
Table 2. Signs and symptoms present among all participants at intake and discharge/after the six treatments.
| Sign/symptoms | MC ‘squeeze’ group (n = 12) | Sham group (n = 11) | Cross-over group - sham to MC ‘squeeze’ (n = 5) | |||
|---|---|---|---|---|---|---|
| Intake | Final treatment | Intake | Final treatment | Pre-MC | Final treatment | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Popping/clicking | 10 (83.33) | 2 (16.67) | 9 (81.82) | 9 (81.81) | 5 (100) | 3 (60) |
| JLT | 12 (100) | 4 (33.33) | 11 (100) | 8 (72.73) | 5 (100) | 3 (60) |
| Pain in TKE | 6 (50) | 0 (0) | 6 (54.55) | 6 (54.55) | 3 (60) | 0 (0) |
| Pain in TKF | 11 (91.17) | 0 (0) | 10 (90.90) | 6 (54.55) | 4 (80) | 0 (0) |
| Positive McMurray’s test | 11 (91.17) | 2 (16.67) | 10 (90.90) | 8 (72.73) | 5 (100) | 1 (20) |
| Positive Thessaly’s test | 10 (83.33) | 0 (0) | 11 (100) | 6 (54.55) | 3 (60) | 0 (0) |
| Positive Apley’s Test | 5 (41.67) | 0 (0) | 2 (18.18) | 2 (18.18) | 2 (40) | 0 (0) |
| Edema | 0 (0) | 0 (0) | 1 (9.09) | 1 (9.09) | 0 (0) | 0 (0) |
| NWB/PWB | 3 (25) | 0 (0) | 1 (9.09) | 0 (0) | 0 (0) | 0 (0) |
MC = Mulligan concept; JLT = joint line tenderness; TKE = terminal knee extension; TKF = terminal knee flexion NWB = non weight-bearing; PWB = Partial weight-bearing.
Numeric rating scale outcomes
A univariate ANOVA was used to assess the change in current pain between the MC ‘squeeze’ and sham groups immediately after the first treatment. No significant difference was found (F(1, 21) = .006, p = .938, partial eta squared = .000, observed power = .051) between the two groups. The MC ‘squeeze’ group reported a mean reduction on current NRS of 1.56 ± 1.01 after a single treatment, while the sham group reported a mean reduction of 1.30 ± 1.51.
A univariate ANOVA revealed no significant difference in cumulative pain scores between the MC ‘squeeze’ and sham groups after the final treatment (F(1,21) = 1.70, p = .21, partial eta squared = .075, observed power = .24) (Table 3). However, the MC ‘squeeze’ group reported a mean reduction on cumulative NRS of 2.19 ± 1.00 effectively meeting the minimal clinically important difference (MCID) of 2 points for NRS [34], while the sham group only reported a mean reduction of 1.24 ± 2.31 (Table 3). All 12 (100%) participants in the MC ‘squeeze’ group met the discharge criteria of ≤ cumulative two points on NRS at the end of the treatment intervention, while only four (36%) of the 11 sham participants met the discharge criteria for NRS.
Table 3. Analysis of variance (ANOVA) in outcome measures from intake to final treatment between groups.
| Outcomes | MC ‘squeeze’ group M (±SD) | Sham group M (±SD) | p | Effect size | Power | ||
|---|---|---|---|---|---|---|---|
| Intake | Final treatment | Intake | Final treatment | ||||
| NRS (Avg) | 2.64 (± .89) | .44 (± .44) | 3.67 (± 2.50) | 2.42 (± 1.96) | .206 | .075 | .238 |
| PSFS | 3.67 (± 1.72) | 9.50 (± 1.85) | 6.45 (± 1.57) | 7.00 (± 2.07) | .000*b | .666*b | 1.00 |
| aDPA | 23.92 (± 10.05) | 9.00 (± 8.12) | 24.91 (± 11.96) | 18.55 (± 14.05) | .013*b | .272*b | .739 |
| aKOOS5 | 65.50 (± 12.26) | 79.32 (± 15.22) | 60.76 ± 18.32) | 69.84 (± 13.69) | .162 | .095 | .282 |
MC = Mulligan concept; NRS = numeric rating scale for pain; Avg = average; PSFS = patient-specific functional scale; DPA = disablement in the physically active scale; KOOS5 = knee injury and osteoarthritis outcome score (composite score).
Notes statistical significance
ANCOVA with baseline scores extracted as covariates.
Notes large effect size.
Patient-specific functional scale outcomes
A univariate ANOVA was used to assess the change in PSFS scores between the MC ‘squeeze’ and the sham groups immediately after the first treatment. A significant difference was found (F(1, 21) = 4.40, p = .048, partial eta squared = .17, observed power = .52) between the two groups. The MC ‘squeeze’ group reported a mean improvement of function on PSFS of 1.58 ± 2.69 after a single treatment application, while the sham group reported a mean reduction of .46 ± 1.86. Four (33%) participants in the MC ‘squeeze’ group reported a minimal detectable change (MDC) on the PSFS after the first treatment while no participants in the sham group reported clinically meaningful improvements in function.
A univariate ANOVA revealed a significant difference in the change in PSFS scores between the MC ‘squeeze’ and the sham groups after the final treatment (F(1, 21) = 41.92, p < .001, partial eta squared = .67, observed power = .10) (Table 3). After the final treatment, the MC ‘squeeze’ group reported a mean change on PSFS of 5.83 ± 1.85, twice the MDC of 2.5 for PSFS [35], while the sham group only reported a mean change of .55 ± 2.07 (Table 3). All 12 (100%) participants in the MC ‘squeeze’ group reported a PSFS score equal or greater than 9 points after final treatment, while only four (36%) of the 11 sham participants reported equivalent PSFS scores, and produced a moderate effect size [33].
Disablement in the physically active scale outcomes
A univariate ANCOVA, with baseline scores set as the covariate (p < .001), revealed a significant difference in DPA Scale scores between the MC ‘squeeze’ and sham groups after the final treatment (F(1, 21) = 7.46, p = .013, partial eta squared = .27, observed power = .74) (Table 3). The mean difference in DPA Scale scores between the two groups was 8.78 (p = .013, 95% CI: −15.48, −2.08). After the final treatment, the MC ‘squeeze’ group reported a mean DPA Scale score of 9.00 ± 8.12, 14 points below the accepted ‘return to play’ score of 23 [36], while the sham group reported a mean score of 18.55 ± 14.05 (Table 3). The mean change for the MC ‘squeeze’ group was 14.92 ± 7.68, more than twice the mean change of the sham group (mean change = 6.36 ± 8.15) (Table 3).
Knee injury osteoarthritis and outcome scores
A univariate ANCOVA, with baseline scores set as the covariate (p < .001), did not reveal a significant difference in KOOS5 scores between the MC ‘squeeze’ and sham groups after the final treatment (F(1, 21) = 2.11, p = .16, partial eta squared = .095, observed power = .28) (Table 3). The mean difference in KOOS5 scores between the two groups was 6.23 (p = .16, 95% CI: −2.73, 15.19). However, after final treatment, the MC ‘squeeze’ group reported a mean KOOS5 score of 79.32 ± 15.23, while the sham group only reported a mean score of 69.84 ± 13.69 (Table 3). The mean change for the MC ‘squeeze’ group was 13.82 ± 10.94, more than the mean change of the sham group (mean change = 9.07 ± 11.13) (Table 3). Five (42%) of the 12 participants in the MC ‘squeeze’ group reported KOOS5 scores of ≥80/100 points by the end of the treatment intervention, while only 2 (18%) of the 11 sham participants reported equivalent scores.
Discussion
Participants among both treatment groups in this randomized sham-controlled study experienced positive effects, but the results suggest the improvements reported by the MC ‘squeeze’ group were superior overall. All 12 participants in the MC group met discharge criteria within the 14-day, six treatment time frame; whereas only four sham participants (n = 11) met discharge criteria within the research time frame. Additionally, five of the seven sham participants who did not meet discharge criteria elected to receive the MC ‘squeeze’ treatment protocol after study conclusion and four of the five participants met discharge criteria within the additional 14-day, six treatment time frame, (the 5th participant required one additional treatment; Table 4). When considering symptom presentation, 42% (n = 5) of the MC ‘squeeze’ participants displayed a full resolution of positive findings on a clinical exam; 58% (n = 7) displayed two or fewer positive findings, despite self-reporting as asymptomatic (Table 2). In comparison, none of the sham participants displayed a full resolution of positive findings on a clinical exam by the end of the study (Table 2). The MC ‘squeeze’ technique was able to alleviate signs and symptoms of the clinical prediction rule for meniscus tears [3], thus lending support for the technique to resolve symptoms while not actually repairing a tear in the tissue. As previously mentioned, incidental findings of meniscal tears on MRI’s are relatively common and do not indicate pathological cause of symptoms [5,6]. As a result, functional patients who have been treated with the MC ‘squeeze’ technique may be able to continue with activity with minimal or no symptoms and without the financial and physiological burden of surgery.
Table 4. Outcomes scores after sham treatment, compared to outcomes scores for the same participants after having received the MC ‘squeeze’ treatment intervention.
| Participant ID # | NRS (Avg) | PSFS | DPA | KOOS5 | Total treatments | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sham | MC ‘squeeze’ | Sham | MC ‘squeeze’ | Sham | MC ‘squeeze’ | Sham | MC ‘squeeze’ | ||
| 113 | 3.33 | .33 | 5 | 9 | 12 | 0 | 63.6 | 96.6 | 6 sham + 4 squeeze |
| 116 | 4.33 | 1 | 7 | 9 | 26 | 6 | 68.8 | 92 | 6 sham + 6 squeeze |
| 117 | 3.33 | 1.33 | 7 | 9 | 29 | 16 | 61.8 | 73.6 | 6 sham + 5 squeeze |
| 120a | 3.33 | .33 | 4 | 9 | 44 | 23 | 48.2 | 79.2 | 6 sham + 6 squeeze + 1 MWMb |
| 121 | 2.67 | .33 | 6 | 9 | 22 | 7 | 71.2 | 78.6 | 6 sham + 4 squeeze |
Participant #120 reported a PSFS score which did not meet discharge criteria after the sixth squeeze treatment, therefore, was placed in the ‘additional treatments’ group, involving the application of a mobilization with movement (3 × 10).
MWM = Mobilization with Movement.
A significant difference was not found between groups on the NRS; both groups reported a decrease in pain immediately after the first treatment and over the course of treatment. However, there is a possibility of a type II error occurring in the interpretation of this analysis. The analysis of change in pain scores yielded a low power (.051) immediately after the first treatment and a low power (.24) from intake to discharge. The lack of significant difference between the groups on the NRS at any point during the study may be attributed to higher intake scores and more variability in pain for the sham group. Lower mean NRS scores at intake for the MC ‘squeeze’ group afforded less room for improvement compared to the sham group during the course of treatment; thus, a ‘floor/ceiling’ effect for the MC group may have limited the ability to detect a statistically significant difference between groups. A notable clinical difference was found between groups; after the first treatment, 50% of participants in the MC ‘squeeze’ group reported an MCID on the NRS, while only 36% of participants in the sham group reported equivalent results. Furthermore, 100% of the MC ‘squeeze’ group reported NRS scores of one or less at the completion of the study, as opposed to only 36% of the sham group.
Analysis of the PSFS scores revealed a statistically significant difference between the two groups, immediately after the first treatment and over the course of treatment, in favor of the MC ‘squeeze’ group. In addition, the MC ‘squeeze’ group experienced clinically significant improvements (i.e. MDC) immediately after the first treatment and over the course of treatment on the PSFS. It is possible the sham group experienced a ‘floor/ceiling’ effect due to a smaller window for improvement with mean PSFS scores at baseline of 6.45 ± 1.57 as compared to the MC ‘squeeze’ group’s mean baseline scores of 3.67 ± 1.72; however, further consideration of the outcomes suggests the MC group experienced superior outcomes to the sham group. For example, none of the sham patients reported an MDC on the PSFS after the first treatment, whereas 33% of the MC ‘squeeze’ group did. Moreover, 100% of the participants in the MC ‘squeeze’ group reported a PSFS score of 9 or better over the course of treatment as compared to just 36% of the sham. Thus, the differences between the MC ‘squeeze’ group and the sham group suggest the MC ‘squeeze’ technique may have had advantageous effects in alleviating the functional activity symptoms associated with clinically diagnosed meniscal tears compared to the sham intervention.
In addition to improving functional activity, the MC ‘squeeze’ treatment also improved the group’s perception of their disability as reported in their DPA Scale scores; similarly to the improvement reported in a previous case study examining the effect of the MC on an apparent meniscal injury (Rhinehart, 2015) A statistically significant difference was found between the MC ‘squeeze’ group and the sham group over the course of treatment. The MC ‘squeeze’ group reported lower scores on the DPA Scale, with 100% of participants reporting scores of less than 23 points by the end of the treatment intervention. In contrast, only 55% of the sham participants reported scores of less than 23 points. A score below 23 is clinically relevant for the participants in this study because it is indicative of normative values reported after discharge from treatment for an acute injury and would also fall within the published normal, healthy range (0–34 points) for uninjured people [36].
A statistically significant difference between groups was not found on the KOOS5. The lack of significant difference between the MC ‘squeeze’ and sham groups could be due to the KOOS5 inquiring about symptoms within the past week or the goal of the outcome scale to measure dysfunction in a group that was excluded from this study, e.g. osteoarthritis patients. The timeframe of this study was two weeks and the KOOS5 was administered within 24 to 72 h of the participants reporting being symptom-free or completing the 6 treatment sessions. Although several participants were asymptomatic (e.g. pain resolved, etc.) at the time of KOOS5 administration, it is a possible that participants may have still been symptomatic within the week the final KOOS questionnaire was completed, which may have led to depressed scores. It is also worth noting that there was a moderate effect size and a low power for the KOOS5 analysis; thus, it is possible a Type II error is being committed by accepting there is not a difference between groups.
One potential reason for the positive effects experienced by the MC ‘squeeze’ group is the treatment’s theorized effect on the meniscal tissue [24,37]. After meniscal injury, meniscal tissue can become dislodged from its normal anatomical position [26,27,38], defined as meniscal derangement [39]. Tissue derangement has been theorized to contribute to approximately 42% of all knee pain [40]. In the presence of tissue derangement at the knee, pressure may be placed on the highly innervated joint line structures [2,26,27,38]. Hypothetically, the MC ‘squeeze’ technique repositions the deranged meniscal tissue into its normal anatomical position and therefore alleviates the symptoms commonly associated with meniscal tears [24,37]. However, these ideas remain purely theoretical, as there is a paucity of research available on the tissue derangement model in the extremities [39].
The positive effects experienced by the sham group also cannot be ignored. Approximately 36% of the sham group experienced symptom improvement that qualified those patients for discharge from the study. Additionally, most the sham group experienced some positive effects on most outcome instruments. The positive effects in the sham group could be attributed to the resemblance of our sham treatment to the repeated directional preference movements in the Mechanical Diagnosis and Therapy (MDT) paradigm. The MDT paradigm involves the classification of patients according to how their symptoms respond to repetitive or sustained unidirectional movements, the most common of which is a ‘derangement syndrome’ [39–44]. Derangement is defined as an anatomical disturbance in the normal resting position of a joint [39,40,43–45]. Patients with a reducible derangement will present a directional preference during the MDT evaluation [39,40,43–45]. While the MDT evaluation method was not followed in this study, it was possible that sham participants experienced improvements, or even complete abolishment of symptoms, due to the ‘sham’ treatment resulting in applied repeated motion in a directional preference. Patients classified with a knee derangement have experienced significantly better outcomes in pain and function when compared to a control group [39].
The positive effects achieved by the sham group could also be attributed to the psychological mechanisms of the placebo effect. The magnitude of the placebo effect depends largely on patient expectation [46–48]. The participants in this case series were blinded to the intervention that they received. As a result, patient outcomes may have improved based on the participant’s expectation of being randomized into the treatment group. The positive effects reported by our sham participants are comparable to other placebo-controlled studies in which participants are told they will either receive a treatment or a placebo and results in small, but significant improvements in pain with small effects sizes [49]. Additionally, the sham participants that reached discharge criteria is not a new phenomenon; the placebo effect has been attributed to up to 50% of patients reaching discharge criteria, particularly in manual therapy [50]. While placebos may not alter the pathophysiology, they can alleviate symptoms (e.g. patient-reported pain) [46].
Different types of manual therapies or therapeutic touch elicit various mechanisms of pain control associated with Central Nervous System (CNS) descending pain modulation including, but not limited to, an increase in β-endorphins, serotonin meditation, increases in dopamine production and oxytocin mediation [51]. Therefore, the placebo effect could explain why some participants experienced improvements in symptoms but most participants did not experience the significant improvements in functional activity and disability reported by the MC ‘squeeze’ group.
Limitations do exist within this study. Such limitations include non-blinded clinicians, lack of long-term outcomes data (i.e. six-months, one-year, and beyond) and a relatively small sample size. A power analysis was calculated based on pilot data of a five-participant sample and, although the minimum sample size (n = 16) was surpassed in this study, a larger sample size including a more diverse patient population would allow for greater generalization to clinical practice. A larger sample size is also likely necessary in this study due to the number of scales used and is evident in the low power, but moderate effect size noted on certain outcomes measures (e.g. KOOS5). Specifically regarding the KOOS, there was a limitation in study design because the final data collection was 24-h post symptom resolution and/or sixth treatment intervention and the scale requires patients to analyze symptoms over the past week when symptoms may have still been present. Therefore, a true analysis of improvement on the KOOS may not have occurred with the study design. Results reported in this study may have been further improved by determining which MC technique was best for each individual participant or through utilizing multiple interventions within the MC.
Future research on the effects of the MC ‘squeeze’ technique should include sub-classification of participants (e.g. acute versus chronic mechanism, etc.) prior to randomization. Because most of the participants included in this study were younger athletic patients with BMIs below the obesity level, additional research assessing older, sedentary individuals with higher BMIs would be advantageous because chronic degenerative meniscus tears are typically observed in populations who are older, sedentary, and overweight [11,52]. Finally, future research should include follow-up data (short term and long term), identifying the time frames improvements are maintained following a return to sport or activities of daily living.
Conclusion
The results in this study indicate the MC ‘squeeze’ technique had a positive effect on patient function over a period of 14 days that was, in general, clinically and statistically superior to the sham treatment. While participants in both groups experienced a decrease in pain, only the MC ‘squeeze’ group reported a significant increase in functional activity and decrease in disability. The results in this study indicate that the MC ‘squeeze’ technique was effective in reducing the symptoms associated with meniscal tears in all participants who met the criteria for a clinical diagnosis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Robinetta Hudson is the head athletic trainer at Concordia Lutheran High School. She is focused on patient-centered care and the continued advancement of her clinical practice through the use of multiple treatment paradigms including but not limited to the Mulligan concept, reactive neuromuscular stabilization and primal reflex release techniques.
Amy Richmond is an athletic trainer at High Point University. Her research interest is in innovative and holistic healthcare and providing the best quality patient care.
Belinda Sanchez is a clinical assistant professor at the University of Idaho. Her research interests include innovative manual therapy techniques and psychosocial interventions for the treatment of chronic musculoskeletal injuries.
Valerie Stevenson is an assistant athletic trainer at Texas Women University. Her primary research interest involve investigating conservative treatment techniques for meniscus pathology as well as determining the probable implications that the nervous systems’ primal reflexes play in pain presentation and function
Russell T. Baker is a clinical assistant professor and the director of the MSAT program at the University of Idaho. His research interests include rehabilitation of acute and chronic musculoskeletal pathologies using manual therapy and patient reported outcome instrument psychometrics.
James May is a clinical assistant professor and DAT clinical education coordinator of athletic training at the University of Idaho.
Alan Nasypany is a clinical associate professor and DAT program director of athletic training at the University of Idaho.
Don Reordan is a physical therapist in Jacksonville, Oregon, an orthopedic clinical specialist, certified by the American Board of Physical Therapy Specialties and an accredited member of the Mulligan Concept Teachers Association.
Supplementary Material
References
- [1].Fox AJS, Bedi A, Rodeo SA. The basic science of human knee menisci: structure, composition, and function. Sports Health. 2012;4(4):340–351. DOI: 10.1177/1941738111429419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lee JM, Fu FH. The meniscus: basic science and clinical applications. Oper Tech Orthop. 2000;10(3):162–168. DOI: 10.1053/otor.2000.5289. [DOI] [Google Scholar]
- [3].Lowery DJ, Farley TD, Wing DW, et al. A clinical composite score accurately detects meniscal pathology. Arthroscopy. 2006;22(11):1174–1179. DOI: 10.1016/j.arthro.2006.06.014. [DOI] [PubMed] [Google Scholar]
- [4].Walczak BE, McCulloch PC, Kang RW, et al. Abnormal findings on knee magnetic resonance imaging in a asymptomatic NBA players. Am J Knee Surg. 2008;21(1):27–33. [DOI] [PubMed] [Google Scholar]
- [5].Shellock FG, Hiller WDB, Ainge GR, et al. Knees of ironman triathletes: magnetic resonance imaging assessment of older (>35 years old) competitors. J Magn Reson Imaging. 2003;17(1):122–130. 10.1002/(ISSN)1522-2586 [DOI] [PubMed] [Google Scholar]
- [6].Zanetti M, Pfirmann CWA, Schmid MR, et al. Patients with suspected meniscus tears: prevalence of abnormalities seen on MRI of 100 symptomatic and contralateral asymptomatic knees. Am J Roentgenol. 2003;181:635–641. [DOI] [PubMed] [Google Scholar]
- [7].Ludman CN, Hough DO, Cooper TG, et al. Silent meniscal abnormalities in athletes: magnetic resonance imaging of asymptomatic competitive gymnasts. Br J Sports Med. 1999;33(6):414–416. 10.1136/bjsm.33.6.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mordecai SC, Al-Hadithy N, Ware HE, et al. Treatment of meniscal tears: an evidence based approach. World J Orthop. 2014;5(3):233–241. DOI: 10.5312/wjo.v5.i3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nikolaou V, Chronopoulos E, Savvidou C, et al. MRI efficacy in diagnosing internal lesions of the knee: a retrospective analysis. J Trauma Manag Outcomes. 2008;2(1):4 DOI: 10.1186/1752-2897-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Englund M, Guermazi A, Lohmander LS. The meniscus in knee osteoarthritis. Rheum Dis Clin North Am. 2009;35(3):579–590. DOI: 10.1016/j.rdc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [11].Getgood A, Robertson A. (v) Meniscal tears, repairs and replacement – a current concepts review. Orthop Trauma. 2010;24(2):121–128. DOI: 10.1016/j.mporth.2010.03.01. [DOI] [Google Scholar]
- [12].Paxton ES, Stock MV, Brophy RH. Meniscal repair versus partial meniscectomy: a systematic review comparing reoperation rates and clinical outcomes. Arthroscopy. 2011;27(9):1275–1288. 10.1016/j.arthro.2011.03.088 [DOI] [PubMed] [Google Scholar]
- [13].Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368(18):1675–1684. DOI: 10.1056/NEJMoa1301408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lyman S, Hidaka C, Valdez AS, et al. Risk factors for meniscectomy after meniscal repair. Am J Sports Med. 2013;41(12):2772–2778. DOI: 10.1177/0363546513503444. [DOI] [PubMed] [Google Scholar]
- [15].Sihvonen R, Paavola M, Malmivaara A, et al. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369(26):2515–2524. 10.1056/NEJMoa1305189 [DOI] [PubMed] [Google Scholar]
- [16].Nepple JJ, Dunn WR, Wright RW. Meniscal repair outcomes at greater than five years. J Bone Joint Surg Am. 2012;94(24):2222–2227. DOI: 10.2106/JBJS.K.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pujol N, Barbier O, Boisrenoult P, et al. Amount of meniscal resection after failed meniscal repair. Am J Sports Med. 2011;39(8):1648–1652. DOI: 10.1177/0363546511402661. [DOI] [PubMed] [Google Scholar]
- [18].Englund M, Paradowski P, Lohmander LS. Radiographic hand osteoarthritis is associated with radiographic knee osteoarthritis after meniscectomy. Arthritis Rheum. 2004;50:469–475. 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- [19].Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a 16-year follow-up of meniscectomy with matched controls. Arthritis Rheum. 2003;48:2178–2187. 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- [20].Englund M, Roos EM, Roos HP, et al. Patient‐relevant outcomes fourteen years after meniscectomy: influence of type of meniscal tear and size of resection. Rheumatology. 2001;40:631–639. 10.1093/rheumatology/40.6.631 [DOI] [PubMed] [Google Scholar]
- [21].Herrlin S, Hållander M, Wange P, et al. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomized trial. Knee Surg Sports Traumatol Arthrosc. 2007;15(4):393–401. DOI: 10.1007/s00167-006-0243-2. [DOI] [PubMed] [Google Scholar]
- [22].Hwang YG, Kwoh CK. The meteor trial: no rush to repair a torn meniscus. Cleve Clin J Med. 2014;81(4):226–232. DOI: 10.3949/ccjm.81a.13075. [DOI] [PubMed] [Google Scholar]
- [23].Rhinehart AJ. Effective treatment of an apparent meniscal injury using the Mulligan Concept. J Sports Med Allied Health Sci. 2015;1(2):1–5. [Google Scholar]
- [24].Mulligan BR. Manual therapy nags, snags, mwms etc. 6th ed Wellington: Plan View Services Ltd; 2010. [Google Scholar]
- [25].Bedi A, Kelly NH, Baad M, et al. Dynamic contact mechanics of the medial meniscus as a function of radial tear, repair, and partial meniscectomy. J Bone Joint Surg Br. 2010;92(6):1398–1408. DOI: 10.2106/JBJS.I.00539. [DOI] [PubMed] [Google Scholar]
- [26].Dye SF, Vaupel G, Dye CC. Conscious Neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773–777. 10.1177/03635465980260060601 [DOI] [PubMed] [Google Scholar]
- [27].Renstrom P, Johnson RJ. Anatomy and biomechanics of the menisci. Clin Sports Med. 1990;9(3):523–538. [PubMed] [Google Scholar]
- [28].Hudson R, Richmond A, Sanchez B, et al. An alternative approach to the treatment of meniscal pathologies: a case report analysis of the Mulligan concept ‘squeeze’ technique. Int J Sports Phys .2016; 11 (4). 1–11. [PMC free article] [PubMed] [Google Scholar]
- [29].Brody K, Baker R, Nasypany A, et al. Meniscal lesions: the physical examination and evidence for conservative treatment. Int J Athl Ther Train. 2015;20(5):35–38. 10.1123/ijatt.2014-0103 [DOI] [Google Scholar]
- [30].Crawford R, Walley G, Bridgman S, et al. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Brit Med J. 2007;84(1):5–23. DOI: 10.1093/bmb/ldm022. [DOI] [PubMed] [Google Scholar]
- [31].Karachalios T, Hantes M, Zibis A, et al. Diagnostic accuracy of a new clinical test (the thessaly test) for early detection of meniscal tears. J Bone Joint Surg Am. 2005;87(5):955–962. 10.2106/JBJS.D.02338 [DOI] [PubMed] [Google Scholar]
- [32].Kurosaka M, Yagi M, Yoshiya S, et al. Efficacy of the axially loaded pivot shift test for the diagnosis of a meniscal tear. Int Orthop. 1999;23(5):271–274. 10.1007/s002640050369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cohen J. Statistical power analysis and research results. Am Educ Res J. 1973;10(3):225 DOI: 10.2307/1161884. [DOI] [Google Scholar]
- [34].Salaffi F, Stancati A, Silvestri CA, et al. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283–291. 10.1016/j.ejpain.2003.09.004 [DOI] [PubMed] [Google Scholar]
- [35].Chatman AB, Hyams SP, Neel JM, et al. The patient-specific functional scale: measurement properties in patients with knee dysfunction. Phys Ther. 1999;77(8):820–829. [DOI] [PubMed] [Google Scholar]
- [36].Vela LI, Denegar CR. The disablement in the physically active scale, Part II: the psychometric properties of an outcomes scale for musculoskeletal injuries. J Athl Train. 2010;45(6):630–641. DOI: 10.4085/1062-6050-45.6.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mulligan B. Manual therapy rounds: mobilisations with movement (MWM’s). J Man Manip Ther. 1993;1(4):154–156. 10.1179/jmt.1993.1.4.154 [DOI] [Google Scholar]
- [38].Wilson AS, Legg PG, McNeur JC. Studies on the innervation of the medial meniscus in the human knee joint. Anat Rec. 1969;165(4):485–491. 10.1002/(ISSN)1097-0185 [DOI] [PubMed] [Google Scholar]
- [39].Rosedale R, Rastogi R, May S, et al. Efficacy of exercise intervention as determined by the McKenzie System of mechanical diagnosis and therapy for knee osteoarthritis: a randomized controlled trial. J Orthop and Sports Phys Ther. 2014;44(3):173–181. DOI: 10.2519/jospt.2014.4791. [DOI] [PubMed] [Google Scholar]
- [40].May S, Rosedale R. A survey of the McKenzie classification system in the extremities: prevalence of mechanical syndromes and preferred loading strategies. Phys Ther. 2012;92(9):1175–1186. DOI: 10.2522/ptj.20110371. [DOI] [PubMed] [Google Scholar]
- [41].Apeldoorn AT, Helvoirt H, Meihuizen H, et al. The influence of centralization and directional preference on spinal control in patients with nonspecific low back pain. J Ortho Sports Phys Ther. 2016;46(4):258–269. DOI: 10.2519/jospt.2016.6158. [DOI] [PubMed] [Google Scholar]
- [42].Dunsford A, Kumar S, Clarke S. Integrating evidence into practice: use of McKenzie-based treatment for mechanical low back pain. J Multidisc Healthc. 2011;4:393–402. DOI: 10.2147/JMDH.S24733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].May S, Ross J. The McKenzie classification system in the extremities: a reliability study using mckenzie assessment forms and experienced clinicians. J Manip Physiol Ther. 2009;32(7):556–563. DOI: 10.1016/j.jmpt.2009.08.007. [DOI] [PubMed] [Google Scholar]
- [44].Hefford C. McKenzie classification of mechanical spinal pain: profile of syndromes and directions of preference. Man Ther. 2008;13:75–81. DOI: 10.1016/j.math.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [45].Aytona MC, Dudley K. Rapid resolution of chronic shoulder pain classified as derangement using the McKenzie method: a case series. J Man Manip Ther. 2013;21(4):207–212. DOI: 10.1179/2042618613Y.0000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kaptchuk T, Miller F. Placebo effects in medicine. N Engl J Med. 2015;373(1):8–9. DOI: 10.1056/nejmp1504023. [DOI] [PubMed] [Google Scholar]
- [47].Vase L, Petersen GL, Riley JL 3rd, et al. Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. Pain. 2009;145:36–44. 10.1016/j.pain.2009.04.008 [DOI] [PubMed] [Google Scholar]
- [48].Ernst E. Does spinal manipulation have specific treatment effects? J Fam Pract. 2000;17:554–556. 10.1093/fampra/17.6.554 [DOI] [PubMed] [Google Scholar]
- [49].Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;44:1594–1602. 10.1056/NEJM200105243442106 [DOI] [PubMed] [Google Scholar]
- [50].Chaibi A, Benth JS, Russell MB. Validation of placebo in a manual therapy randomized controlled trial. Sci Rep. 2015;5(11774):1–8. DOI: 10.1038/srep11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vigotsky AD, Bruhns RP. The role of descending modulation in manual therapy and its analgesic implications: a narrative review. Pain Res Treat. 2015;2015:292805 DOI: 10.1155/2015/292805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yeh PC, Starkey C, Lombardo S, et al. Epidemiology of isolated meniscal injury and its effect on performance in athletes from the national basketball association. Am J Sports Med. 2012;40(3):589–594. DOI: 10.1177/03635465114286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


