ABSTRACT
The recent Colorado Gold King Mine waste-water spill and Michigan’s water supply re-routing program catastrophe, has directed renewed public attention towards resurgent environmental lead contamination threats. Leaded environments present social justice issues for children and mothers possessing blood lead levels (BLLs) > 5 μg/dL. Childhood lead exposure remains a continual U.S. public health problem manifesting in lifelong adverse neuropsychological consequences. The 2007 Inspector General Report demonstrated low BLL screening rates across the U.S. and this study examined the regularity of children’s BLL screening rates. The Centers for Disease Control and Prevention (CDC) Lead Poisoning National Surveillance 2010–2014 children’s BLL screening rates, were examined to assess BLL screening regularity in states traditionally known to have regularly occurring BLL screenings: New York, New Jersey, and Pennsylvania. The results extracted from the CDC data showed that < 50% of children were BLL screened by six-years of age across the states that were sampled. The findings highlight that without a “clear map” of lead exposed areas through accurate and consistent BLL screenings, how the potential for such disparities within – and between-states within the U.S. could arise due to environmental social justice issues in relation to BLL screening barriers. Barriers preventing children’s BLL screenings were considered, and public health interventions recommended to improve screening rates included: routine BLL screening for all pregnant women, lactating mothers, and children; while, removing known lead exposure sources within communities. This study calls for action during a time of renewed public attention to resurgent lead poisoning within the U.S.
KEYWORDS: Blood lead levels, lead poisoning, environmental social justice, lead prevention, children, pregnant women, lactating mothers, public health
Introduction: historical context of childhood lead poisoning in the U.S
In the United States (U.S.), childhood lead poisoning caused by environmental exposures has been well-documented as a continual public health problem since the 20th century. Adverse neuropsychological consequences from lead sequelae begin in early gestational development and persist across the lifespan [1,2]. Over the last 50 years, animal biomedical and human clinical research regarding environmental lead exposures on neurodevelopment have provided substantial and fundamental evidence defining lead as a neurotoxicant [3]. Contemporaneous with the research done on lead poisoning during that half-century in the U.S., critical public health legislation facilitated the removal of large amounts of lead from the environment in the 1970s (i.e. the Lead-Based Paint Poisoning Prevention Act [PL 91–695 and PL 93–151] to address children engaging in Pica and eating paint chips, as well as the Clean Air Act [PL 88–206] that was directed at removing leaded gasoline) [4–8]. Despite these laws reducing high blood lead levels (HBLLs) in children through conscientious and responsible public health efforts, today children in the U.S. remain continually at-risk for low blood lead level (LBLL) exposures that are still deemed to have concerning neurodevelopmental effects as the brain is particularly sensitive to low-levels of lead [3]. Currently, LBLL environmental exposures are acquired from residual industrial byproducts such as but not limited to: lead-painted toys [9,10]; lead-contaminated candies [11–14] and their wrappers; [15,16] unabated/improperly abated housing when renovating pre-1978 homes containing lead paint, lead-soldered and/or plumbing delivering water to pre-1978 homes, schools, and others institutions [17]. More recently, events such as the U.S. Environmental Protection Agency (EPA) Gold King Mine waste-water spill on 5 August 2015 in Silverton, Colorado [18] and Flint, Michigan’s 2014 water supply re-routing program catastrophe [19,20] further demonstrate continued public health threats of environmental lead exposures that exceed the Center for Disease Control and Prevention’s (CDC) actionable levels (i.e. ≥ 5 µg/dL). Thus, warranting further investigation into the causes, sources of exposure(s), the associated public health impacts/concerns, and more importantly the environmental social justice issues faced by surrounding populations of children across the U.S. defined as most-at-risk for resurgent lead poisoning.
In the U.S., certain populations may not have the financial ability to lead-abate their residence nor are they adequately educated in how to deal with or approach a lead-removal resolution to mitigate their family’s potential risks. Further, if they have a child who is lead poisoned they often cannot afford to relocate, regularly visit a physician, or pay for medical treatments/prescription medicine due to a lack of medical insurance coverage and/or insufficient income. Thus, due to a combination of socio-economic-status (SES) factors, some families and their children may be left with no other option, but to unfortunately endure the negative consequences of a lead-contaminated environment where their children will reside and increase their risk for acquiring a neurodevelopmental disorder. This presents with a rather profound environmental social justice situation in which the U.S. government should step in to help these families experiencing such residential predicaments (i.e. in essence, a form of environmental learned helplessness), as the government is capable to remove and further prevent the sources of lead exposures these families continue to experience within the U.S.
Moreover, families residing in these low SES areas may have recently immigrated to the U.S. during the last 50 years and are potentially unaware of the history of lead poisoning issues within the U.S., its evidence regarding neurodevelopmental impacts in children, and whether their immediate environment places them and their children at risk. Further, when families immigrate over to the U.S. they may not be able to translate lead poisoning educational materials provided to them in English and would require it to be translated into their native language to result in a functional educational intervention to reduce their family’s potential risks. Regrettably, HBLLs diagnosed in children residing within these lead contaminated areas have resulted in a renewed public attention to the deleterious effects of this neurotoxicant within their surrounding environments. The greatest blood lead level (BLL) concerns have been raised by pregnant mothers within their first trimester seeking more education on the relationship between maternal lead body burden and fetal lead poisoning within these at-risk communities [21,22]. Thus, environmental lead exposures arising from new, as well as historically unaddressed environmental exposure sources (i.e. lead unabated houses and lead soldered plumbing eroding through its water supply, etc.) within the U.S. remains a major environmental social justice issue and a public health problem for pregnant women, lactating mothers, and children with adverse consequences impacting their quality of life outcomes across their lifespans.
The first aim of this study was to examine the current rates of children in the U.S. that have been screened for BLLs within three states (i.e. New York, New Jersey, and Pennsylvania) that are traditionally known to have a broad range of BLL screening rates using publicly available data from the CDC, and to compare those datasets with two states with resurgent lead exposures (i.e. Colorado and Michigan) to assess the public health risks therein. The public health risks associated with lead poisoning considered the potential barriers to both primary (e.g. lead removal) and secondary (e.g. BLL screening) efforts within the states sampled. From these findings, a proposal for renewed public health interventions addressing these environmental social justice issues were discussed in the context of directing lead poisoned pregnant women, lactating mothers, and children to seek the appropriate medical professionals to receive individualized treatment. Additionally, findings from these families lead exposure sources can help local government officials to identify ways in which to prevent the recurrence and resurgence of lead contamination for future generations; similar to what was accomplished with the removal of leaded paint and gasoline within the U.S. The concern that areas in which BLL screenings remain ill-defined, creates a real problem in identifying a clear map of disparities to inform the public of where BLL screenings are lacking and subsequently where interventions may need to take place in the U.S. Notably, the issue of addressing lead exposures is also an international public health concern, as argued by Silbergeld [23]; however, the present recommendations are specific to the structure of the U.S. housing, environment, and health care systems response to these resurgent sources of lead contamination. The second aim of this study also sought to renew a call for research and action around lead poisoning prevention as an environmental social justice issue.
Background: neuropsychopathological costs of lead poisoning and historical efforts to monitor and remove lead threats
It is necessary to briefly review the neuropsychopathological effects of lead poisoning to demonstrate the importance of addressing this environmental contaminant and central nervous system neurotoxicant. One mechanism by which lead ions (Pb2+) enter the brain is through their ability to penetrate the blood brain barrier (BBB) as a calcium ion (Ca2+) substitute. Further, it has been shown that lead exposure in vitro can be actively transported into the brain via the Ca-ATPase pumps through endothelial capillary cells [24]. The Agency for Toxic Substances and Disease Registry (ATSDR) [25] reported that lead is not uniformly distributed between blood, soft, and mineralizing tissues, and as a result requires careful medical management in children [26]. Lead is filtered from the child’s circulating blood in approximately 25 days, whereas when it is mobilized into the child’s bone tissues as a calcium substitute, lead has been estimated to remain for up to 30 years in cortical bone [27–29]; thereby, increasing the child’s risk for disrupted or altered neural development. Notably, due to technological and practical issues, it is not possible to accurately measure lead accumulation and deposition within the brains of children (arguably the most important organ of interest), unless a post-mortem analysis is conducted [3,30].
Lead-exposed children when clinically assessed as adults, evidence that 70% of their lead accumulation may remain in their bones as a historical record of their developmental exposures [25,26] and present with an internal ongoing source of potential re-exposure as they age. During these formative years in which a developing child may acquire a lead body burden, children are continually at-risk for recurrent lead recirculation leaching from their bone stores into their bloodstream and subsequent lead (re)deposition into their brain, making children remarkably vulnerable to lead poisoning across their lifespan [24–29]. Thus, the longer lead remains within a child’s body, it increases the probability for their brain to become susceptible to and damaged by this neurotoxicant. The prognosis of chronic lead exposures contributes to cumulative and compounded neuropsychological deficits that will ensue across their lifespan [3,29,31]. This lead-brain burden will severely influence the child’s trajectory of normal cognitive, emotional, and behavioral functions [29–32], and reliably predicts for the child to experience a lifetime of social-emotional, adaptive behavioral and educational advancement problems [32–36]. Further, individuals from low SES environments often experience more life stressors, and are often times further compounded by psychological stress, which has been shown to increase lead mobilization form their bodily stores; this is especially demonstrated among pregnant women, lactating mothers, and developing children [21,22,29]. Therefore, it can be argued that a lead poisoned child residing in a more stressful environment (i.e. not restricted to impoverished environments) may have a worse prognosis than a lead poisoned child residing in a less stressful environment; thus, establishing an epigenetic susceptibility profile for lead developmental neurotoxicity. Interestingly, most cases of lead poisoned families are identified as residing in low SES environments with significant stressors [21,22,29,33,36].
Bellinger [30] reported that children identified as having a BLL of 10 μg/dL correlated with up to a 5-point loss in IQ, and Lanphear et al. [37] reported that children with LBLLs < 10 μg/dL showed cognitive deficits at BLLs previously considered by the CDC to be safe. Further, Chiodo et al. [38] examined LBLLs in an underrepresented minority (URM) population of inner city children at approximately seven-years of age, and found that what was previously determined as “safe lead levels” produced deficits in intelligence, reaction time, visual-motor integration, fine motor skills, attention, executive functions, and an increase in off-task non-educational behaviors. Moreover, the teachers of the examined children reported withdrawal and related classroom educational avoidant behaviors. Altogether, given the history of reports on these issues it is now argued that since lead is an established neurotoxicant, no BLL should be deemed safe [30,31,39]. The Advisory Committee on Childhood Lead Poisoning Prevention (ACCLPP), developed by the CDC to provide guidance regarding childhood lead poisoning prevention, published a report in 2012, which changed several previous recommendations based on the more recent LBLL datasets. The previous “level of concern” language, which often prompted inaction within communities that endured low-levels of lead toxicity, are now shown to cause similar neuropsychological impacts as levels already deemed unsafe [40]. The committee stated that “it is not possible to determine a threshold below which BLLs are not inversely related to IQ”[40]. Due to the evident cognitive, behavioral, and educational problems resulting from environmental lead exposure, childhood and maternal lead poisoning has deleterious social and economic associated costs that governments should carefully approach and seriously consider [34].
In particular, prenatal lead exposure is also a major concern as reports have shown increased miscarriages and fetal deaths to be associated with pregnant women residing in areas containing lead-contaminated drinking water [21,22]. Notably, lead pills were used as an abortifacient in the early 20th century [21]. Consistent with these reports, the risk of birth defects or stillbirths are elevated for paternal HBLL exposure occupations [41]. Further, prenatal lead exposure is associated with decreased intellectual development in the resulting child when carried to term [22,41]. However, given these findings there is no clear indication for a threshold BLL for this precise relationship, which suggests lead exposure may be one contributing factor, amongst others, that may further increase the risk of miscarriage or fetal neurodevelopmental or neuropathological problems. In the case of healthy pregnancy outcomes, it should also be noted that no amount of lead exposure should be considered safe for pregnant women. Moreover, data on populations of lead-exposed pregnant women are lacking and future studies should be directed towards improving the public’s understanding regarding the individual medical health needs of lead-poisoned pregnant women and lactating mothers’ in relation to their residential environments.
Secondary prevention through blood lead level (BLL) monitoring
Historically, one of the main forms of prevention against lead poisoning has been BLL screening. Although, BLL screening is used to diagnose a child or family member with lead poisoning, it alone will not prevent the consequences associated with lead exposures. However, BLL screening, if conducted by a fertility/obstetrics and gynecology physician (i.e. for both low- and high-risk pregnancies) or a midwife when the mother is in the first trimester of her pregnancy can inform the mother and/or family of any potential lead-associated medical health risks during fetal development. Moreover, if the mother is unremarkable for a BLL, there is still the potential for the child to acquire lead poisoning from its environment during postnatal life. Even if the child was absent of lead exposure during pregnancy, the developing brain remains vulnerable to environmental lead exposures and such risks should not be dismissed. Therefore, it is also imperative for the child to be tested for a BLL at birth, within the first and second year of life, and followed up annually thereafter until the age of six. If the child is found to be positive for a BLL, then they should be followed up every six-months or sooner and evaluated for potential chelation, calcium-dietary supplementation, and/or blood transfusion therapies where applicable and as symptoms dictate the need for such treatment interventions.
Through such a proactive approach, BLL screenings can provide the family and physicians with a more informed timeline in isolating when the lead exposure occurred and subsequently can begin to hone in on identifying the possible source(s) of exposure(s) within and across the environments, they traverse daily. In this way, lead screening can inform families to investigate environmental sources of lead exposure and prevent further exposing themselves or their children to these areas. This can help to increase the dialogue amongst residents and property owners (i.e. both private apartments and residential homes), government owned properties (i.e. public housing developments and schools), school officials, day cay centers, and other public areas to investigate continual sources of lead contamination. It is when this “reverse detective work” is done and followed up correctly that the government or other responsible parties can be contacted to facilitate the removal and eliminate the source of lead exposure; thereby, preventing others from becoming lead poisoned in the future. Through such “reverse detective work” a map of local environments and the degree to which lead exposure risks occur can be documented accurately. Notably, this “reverse detective work” in producing a map identifying the source(s) of lead exposure(s) to prevent recurrences, arguably begins with BLL screening coupled with appropriate lead poisoning education. Thus, without such regularly conducted BLL screenings, society may be unaware of or unable to prevent such sources of continual or new lead exposures if such a map fails to be generated. Ultimately, if BLL screenings are not done consistently during the first trimester of pregnancy or followed over the first few years of the child’s life, a lack of BLL screening can evolve into a social misattribution of lead poisoning risk. This predicament may foster perhaps a greater danger of instilling the psycho-social idea unto others that, “there is no such risk for lead poisoning as it was addressed when lead was banned from paint and gasoline.”
Thoughtful and well-monitored BLL screenings offer families and physicians with a series of opportunities for monitoring lead-related health effects, beginning discussions regarding early intervention(s), and potentially proactively planning other educational related service outcomes for their school-aged years. Given the aforementioned relationship between BLL screening and lead prevention, it is striking to learn that in 2018 within the U.S., only Medicaid-enrolled children are mandated to have BLL screenings at 12- and 24-months of age. This results in a large proportion of privately-insured and uninsured children, pregnant women, and lactating mothers that remain unscreened for lead exposures (i.e. especially, low SES families). Further, even among the few mandated populations for BLL screening (i.e. Medicaid recipients), a study conducted by the U.S. Inspector General examined data from 2007, and at that time found that over 50% of children did not receive mandatory BLL screenings [42]. Similarly, Kemper & Clark [43] found that 68% of pediatricians reported that they screened one-year old Medicaid-enrolled children for lead exposures, but only 42% of these children previously tested at one-years of age were routinely screened for follow-up lead exposures at two-years of age (i.e. ~ 34% of the original census sample). This suggests that the U.S. BLL screening rates, defined in this context as the population of children identified within a given state’s census period that were actually screened for BLLs during that same census period without duplication/replication, and were reported to the CDC, are presently not being regularly nor adequately screened for lead poisoning. Other studies have reported that routine BLL screening rates comprise as low as 27% of children under six-years of age [44,45]. Currently, data representing the BLLs of immigrant children (i.e. both those documented and undocumented) who do not qualify for Medicaid or have private insurance, remain to be elucidated as their risks for lead poisoning remain high and their BLLs unknown. However, it is well documented that vulnerable migrant families within the U.S. often find residence in lower income neighborhoods – where these risks are the highest – thus, further increasing migrant families’ risks of having lead-poisoned children needing equal access to such medical care services and treatment [46].
Research has also found both patients and physicians presenting with or experiencing barriers from receiving childhood BLL screenings, which may account for these disturbingly low screening rates in the U.S. Of the physicians who do not screen their Medicaid patients for lead exposures, they reported it was because they perceived their patients did not reside in a high-risk lead-containing environment, and as such, their circumstances did not warrant BLL screening [43–45]. Another barrier to BLL screenings for physicians was their patients’ ability to access or afford testing off-site [43]. Finally, low reimbursement rates for physicians and health care professionals were blamed for the lack of regularly conducted on-site BLL screenings [43]. Essentially, the clinics kept diagnostic materials for select tests in which reimbursement rates were reasonable or which the physicians perceived to be more important or vital. This presents with a serious ethical concern for physicians within the U.S. as their perceptions of medical interventions are inconsistent with the CDCs campaign to eliminate HBLLs through the Childhood Lead Poisoning Prevention Program. The CDC is committed to the Healthy People by 2020 campaign, which seeks to eliminate lead screening biases related to race, SES, and associated public health concerns [47], yet physicians are under screening children for BLLs each year nationally. More perturbing is how the CDC will manage and interpret the data physicians provide regarding lead exposed children as they are currently being under and selectively reported from only low SES populations. This situation suggests that the reduction in lead-positive children (i.e. arguably a false-negative testing result) is being obscured by the scarce and inadequate number of U.S. children actually being BLL screened, rather than all children in the U.S. being BLL screened and diagnosed as lead-negative. This later point is a critical principle for the Healthy People 2020 initiative, as it is an inconceivable environmental social injustice that continues to occur in a modern age of medical technological advancements and increasing social reform. Yet, a significant number of children within the U.S. are not being granted, recommended, afforded, and even are at times prevented from being screened for a BLL at ages when their brains are most vulnerable to leads neurotoxicant impacts.
Potential barriers to BLL screenings faced by parents and guardians have been examined in depth as well. Boreland & Lyle [48] conducted a literature review of studies that specifically examined parent attitudes towards BLL screenings. Several studies within their review found that parents are often unfamiliar with the effects of lead poisoning and the sources of lead exposure (i.e. an educational intervention deficit). If families are unaware of lead sources of exposure, they will be incapable of preventing their children from lead toxicity. Further, their lead poisoning educational intervention should take place during routine visits with their physician during the course of their pregnancy. Accessibility of services was another important, yet separate issue. Sometimes BLL screenings were not offered, and when they were, they were not always performed onsite, presenting logistical barriers for parents [48]. Finally, concerns with the discomfort children experienced during invasive blood draws for BLL screening were noted as additional barriers for parents and child caregivers (See also Polivka & Gottesman [49]).
Since 1995, the CDC has collected childhood BLL surveillance data from U.S. state and local health departments that were generated by physicians and pediatricians. States are responsible to maintain their own databases to prevent erroneous duplication and/or sequential replication of a single child once identified as lead-positive, and are further expected to report these data back to the CDC. Historically, these databases were intended to monitor and support public health action and prevention regarding medical treatment interventions, environmental investigations, assessment/reassessment, abatement, and identifying new-sources of lead exposure that continually place children at-risk within the U.S. Despite this being a state requirement, some states have more consistently reported their children’s BLLs than others have. Using the recently available CDC data compiled from states across the U.S., the following questions were investigated: What are the current BLL screening rates for states in which lead disasters have occurred? Do the children’s BLL screening data reflect differences when compared to states known as traditionally having higher than average BLL screening rates from the CDC? Additionally, the investigation sought to determine whether children’s BLL screening rates were decreasing or increasing since 2010 to 2014. These data were compared to previous studies of barriers to children’s BLL screenings within the U.S., in order to consider recommendations for improved primary (e.g. lead removal) and secondary (e.g. BLL screening) prevention efforts.
Methods: CDC lead poisoning national surveillance data (2010–2014)
In order to assess the current situation of childhood lead screenings in five states, publicly available data from the CDC Lead Poisoning National Surveillance Database (LPNSD) 2010–2014 [50] on lead poisoned children were extracted and compared to the 2010 U.S. Inspector General report [42]. The BLL data starting from 2010, were selected since it was the first year in which the LPNSD included a sub-classification for children with BLLs ranging from 5–9 µg/dL (with all prior years listing the lowest BLLs measured by the CDC as 10–14 µg/dL (For Review See CDC [50]). The datasets included children six-years of age or younger (i.e. < 72 months old) and were sub-classified by: state; year; population of children identified within each census; rates of children that were reported to have been BLL screened (i.e. irrespective of Medicaid-enrollment or lack of insurance); confirmed rates of children who were lead-positive from the annual BLL screenings with BLLs ranging from: 5–9 μg/dL, 10–14 μg/dL, 15–19 μg/dL, 20–24 μg/dL, 25–44 μg/dL, 45–69 μg/dL, ≥ 70 μg/dL; and the summative rates of children screened for BLLs exceeding 5 μg/dL.
Given the perspicacious concerns regarding children’s developmental and neuropsychopathological impacts associated with BLLs < 10 μg/dL, these CDC datasets offer timely insights towards elucidating the number of children who remain affected by or are at-risk for environmental lead poisoning 50-years following the removal of leaded paint and gasoline. Since children in the U.S. remain lead poisoned following the removal of leaded paint and gasoline from the environment, this suggests that other sources of lead exposure may have been less obvious and not addressed over the last half-century or alternatively new sources of lead exposures are being observed in the environment. Consistent with the latter point regarding new sources of environmental water-based lead exposures, the states of Colorado (i.e. exploratory excavation work in the Gold King Mine contaminated nearby inter-state rivers) and Michigan (i.e. city water re-routing program contaminated Flint’s residential urban water supply) were examined. Contrastingly, the states of New York (separated into two sub-populations: New York City [NYC] vs. New York State excluding NYC), New Jersey, and Pennsylvania were used as comparative reference populations, since a consistent BLL screening dataset was obtained from the CDC (2014) surveillance from 1997–2014 [50]. These states were also chosen since New York has historically remained active in promoting lead conscious policies such as educating its public [51], in addition to its intent to separate the urban metropolitan city data from more suburban and rural areas as a within-state comparison. Compared to New York, New Jersey has been viewed as less proactive in educating its public, as it views the BLL actionable threshold of concern to be 10 μg/dL [52] and Pennsylvania [53] is a state in which its lead educational efforts fall between New York and New Jersey [50].
The raw data from the CDC publicly available 2010 to 2014 [50] children’s BLL annual screening rates were extracted for the states of NYC, New York excluding NYC, New Jersey, Pennsylvania, Michigan, and Colorado. The population of children annually screened for BLLs were used to determine trends for improved lead screening and prevention for children with > 5 µg/dL (Table 1) and < 5 µg/dL (Table 2). The extracted datasets were processed using SPSS V24 that employed a multivariate ANOVA using State and Year as independent factors and either the population of children (Figure 1) or the percentage of children (Figure 2) screened for BLLs as the dependent variables. The percentage of children annually screened for lead poisoning was calculated by dividing the number of children lead screened annually by the U.S. children census data for that state and multiplied by 100. A Wilks Lambda Ʌ test was conducted to determine differences between State means. A Dunnett’s test was conducted using NYC as the control group and subsequent post hoc pairwise comparisons were used to reveal differences between States through multiple and one-to-one comparisons. The criterion for significance was set at α = 0.05 and a confidence interval of 95%. Notably, the CDC does not require BLL screening rates for pregnant and lactating women; thus, the present data analysis is limited to only children’s BLL screening rates [54,55]. However, lead risk determination is recommended for pregnant women prior to considering a BLL screening and this recommendation is supported by both the American College of Obstetricians and Gynecologists [54], as well as the CDC [55].
Table 1.
The population of children with BLL’s > 5 μg/dL extracted from the CDC National surveillance data [50].
| State | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|
| New York City | 14,400 | 12,009 | 8,6884 | 7,689 | 6,997 |
| New York (Excluding NYC) | 15,621 | 13,786 | 3,383 | No Data | 2,497 |
| New Jersey | No Data | 8,063 | 6,604 | 6,424 | No Data |
| Pennsylvania | 20,955 | 17,252 | 14,480 | 12,906 | 11,918 |
| Michigan | 18,289 | 14,737 | 12,622 | 3,824 | 4,997 |
Table 2.
The population of children with BLL’s < 5 μg/dL extracted from the CDC National surveillance data [50].
| State | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|
| New York City | 12,895 | 10,734 | 7,668 | 6,813 | 6,059 |
| New York (Excluding NYC) | 13,091 | 11,649 | 2,721 | No Data | 2,721 |
| New Jersey | No Data | 6,816 | 5,639 | 5,583 | No Data |
| Pennsylvania | 17,804 | 14,392 | 12,036 | 10,969 | 10,125 |
| Michigan | 15,939 | 12,869 | 11,148 | 3,381 | 4,362 |
Figure 1.
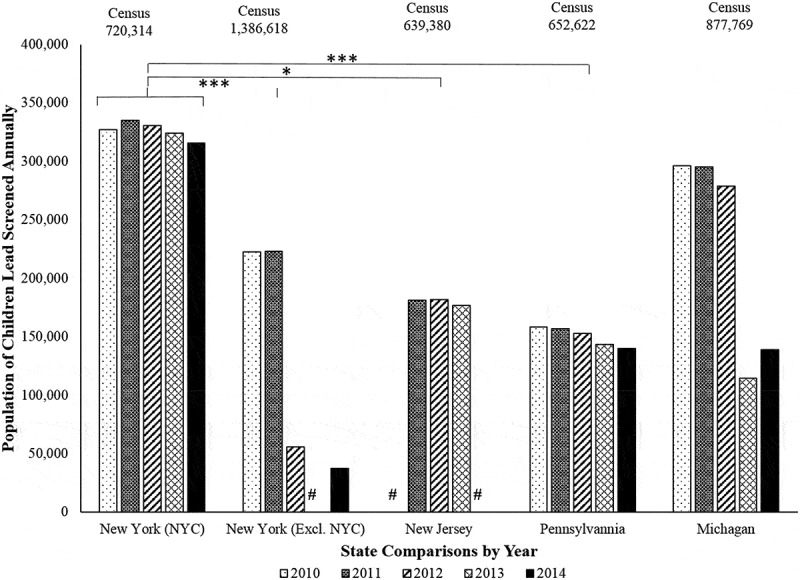
Population of children by state annually screened for lead poisoning in 2010–2014. Using NYC as a control group, the population of children annually screened for lead poisoning from the 2010–2014 CDC data [50] showed that NYC was rather stable in screening children from 2010–2014. In contrast, when compared to NYC, New York excluding NYC (p < 0.001***), New Jersey (p < 0.016*), and Pennsylvania (p < 0.001***) showed significantly less of the population of children annually screened for lead poisoning. The state of Michigan (p = 0.060 n/s) showed stable, but not significantly lower screening rates of children when compared to NYC. The (#) indicates that no data were available for that state in that year. No records for the years examined were available from the CDC data [50] for Colorado.
Figure 2.
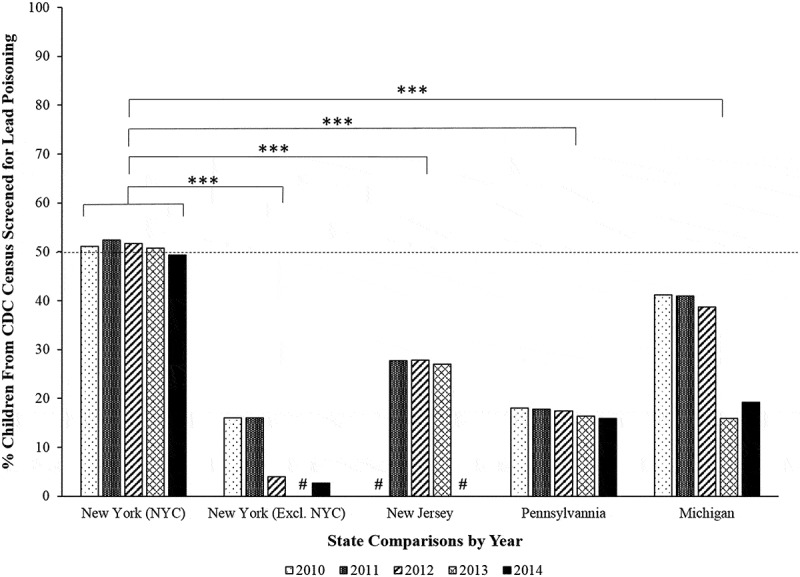
Percent of children by state annually screened for lead poisoning in 2010 to 2014. Using NYC as a control group, the percent of children annually screened for lead poisoning from the 2010–2014 CDC data [50] showed that approximately 50% of children were lead screened in NYC. In contrast, New York excluding NYC (p < 0.001***), New Jersey (p < 0.001***), Pennsylvania (p < 0.001***), and Michigan (p < 0.001***) annually screened significantly fewer children for lead poisoning than NYC based on the percentage of children reported from each states Census data. The (#) indicates that no data were available for that state in that year. No records for the years examined were available from the CDC data [50] for Colorado.
Results: trends in childhood BLL screening rates
The results obtained indicate that, despite modern advancements in medical technology, there still appears to be psychosocial and public health concerns regarding the rates of LBLL screening and the amount of unscreened children within the U.S. Table 1. lists the LPNSD rates of children screened from the sampled states possessing BLLs > 5 µg/dL. The data revealed that from 2010 to 2014 the screening rates of children with BLLs > 5 µg/dL reduced from: 14,400 to 6,997 in New York City (51.41%); 15,621 to 2,497 in New York excluding NYC (84.02%); 20,955 to 11,918 in Pennsylvania (43.13%); and 18,289 to 4,997 in Michigan (72.68%), respectively. New Jersey reported no children’s BLL screening data during the years of 2010 and 2014, yet data from 2011 to 2013 revealed BLL screening rates reduced from 8,063 to 6,424 (20.33%). Lastly, Colorado had no data reported during the 2010–2014 period that was examined.
These data suggest that state efforts have been ongoing and continue to reduce BLLs exceeding 5 µg/dL, but as a natural consequence to what each state defines as an actionable concern for BLLs, there may exist the potential for certain states to be more tolerant to accept children with BLL < 5 µg/dL without further seeking to remove and eliminate more sources of lead exposure (i.e. potentially sensitizing inaction in certain states). In order to evaluate the potential for this concern, Table 2, lists the LPNSD rates of children screened from the sampled states possessing BLLs < 5 µg/dL. The data revealed that from 2010 to 2014 the screening rates of children with BLLs < 5 µg/dL reduced from: 12,895 to 6,059 in New York City (53.01%); 13,091 to 1,951 in New York excluding NYC (85.01%); 17,804 to 10,125 in Pennsylvania (43.13%); and 15,939 to 4,362 in Michigan (72.63%), respectively. New Jersey reported no children’s BLL screening data during the years of 2010 and 2014, yet data from 2011 to 2013 revealed BLL screening rates reduced from 6,816 to 5,583 (18.09%). Lastly, Colorado had no data reported during the 2010–2014 period that was examined. Taken together, these data suggest that Michigan, New York excluding NYC, NYC, and Pennsylvania have been very active in their childhood lead prevention efforts. In contrast, New Jersey has made efforts in reducing lead exposures that could negatively impact children, but compared to the other states examined, New Jersey’s efforts met far less of the public’s needs.
In order to better understand the LPNSD data and its relationship with testing all children within each state, these values were compared to the population of children identified by each state’s 2010 census to the LPNSD annual childhood BLL screening rates. In every state examined, the number of children with BLLs > 5 μg/dL were purported to have decreased over time (See Tables 1 & 2) and were confirmed by the data examined. However, one must be cautious in interpreting this dataset as it may not reflect clear evidence of decreased environmental lead exposure threats to children or prevention. Since Medicaid children are mandated for BLL screenings and children often associated with lower SES are recommended or perceived by physicians to warrant BLL screenings, children who don’t match or meet these criteria would remain unscreened. This presents with a unique problem in that through such Medicaid mandated testing program, other sources of environmental lead exposure risks away from where these families reside would not be considered. In addition, children from these demographic backgrounds have been reported to lack access to such off-site BLL screening, which also suggests that the rates of these specific populations may be underreported within the LPNSD datasets as well. In order to address this concern, first the annual children’s BLL screening rates were evaluated. Second, the annual LPNSD data from 2010 to 2014 were divided by the 2010 children’s census data and converted to a percentage of children within each state that were BLL screened and the differences revealed the remainder of children left unscreened within each state.
Figure 1. demonstrates that in the U.S. a decreasing trend in the population of children six-years of age or younger who were annually BLL screened from 2010 to 2014 in the states of Michigan, New York (excluding NYC), and Pennsylvania; whereas New York City and the state of New Jersey screening rates have remained relatively stable. The (#) indicates that no data were available for that state in that year. Colorado data were intended to be included, but no record for the years examined were available from the LPNSD. The results show that compared to NYC, New York State (excluding NYC), New Jersey, Pennsylvania, and Michigan have reported a reduction in BLL annual screening rates (i.e. this suggests that many children may remain unscreened). A multivariate ANOVA using the factors, Year and State, revealed a significant effect of State using a Wilks’ Lambda Ʌ F (4,17) = 4.102, p < 0.001***, = 0.506 and a MANOVA F (4,17) = 7.416, p < 0.001***, = 0.636, yet Year was not significant F (4) = 0.021, p = 0.999. A Dunnet’s post hoc test, using NYC as the control group [50], revealed significant differences when comparing New York excluding NYC (p < 0.001***), New Jersey (p < 0.016*), and Pennsylvania (p < 0.001***), but Michigan was not significant (p = 0.060).
To address the second query, Figure 2. illustrates the percent of children BLL screened relative to the census for each state. The data showed that with the exception of NYC, less than 50% of children were actually BLL screened by six-years of age. The data revealed that approximately 50% or less of all children (horizontal dotted line) identified by the census were BLL screened across all the states examined (Figure 2). This suggests that more children may be affected by or at risk of environmental lead poisoning, but are not accounted for based on a combination of factors and barriers that may prevent them from being BLL screened annually. A multivariate ANOVA using the factors Year and State revealed a significant effect of State determined by Wilks’ Lambda Ʌ F (4,17) = 9.181, p < 0.001***, = 0.697 and a MANOVA F (4,17) = 24.675, p < 0.001***, = 0.853, yet Year was not significant MANOVA F (4,17) = 0.21, p = 9.99, = 0.005 (Figure 2). A Dunnet’s post hoc test for pairwise comparisons, using NYC as the control group, revealed significant differences in the percent of children BLL screened when compared with New York excluding NYC (p < 0.001***), New Jersey (p < 0.001***), Pennsylvania (p < 0.001***), and Michigan (p < 0.001***) (Figure 2). Taken together, these findings demonstrate a decline in the number of children confirmed with lead poisoning both > 5 µg/dL and < 5 µg/dL; however, they do not adequately reflect all children within each state’s census as there is approximately 50% or more children within the states examined in this study unscreened for BLLs each year in the U.S.
Discussion: BLL screening inconsistent despite major concerns about lead exposure
Consistent with this study’s findings, the nine state study conducted by the U.S. Inspector General found that 57.4% of children did not receive mandatory (i.e. Medicaid-sponsored) BLL screenings [42]. In fact, the findings reported herein evidenced even lower rates of childhood BLL screenings for the five-states examined. This is likely due to the inclusion of children within the census who were not mandated to be lead screened (i.e. uninsured and privately insured children). The data obtained indicate that in the cases of the states examined that they have continued to report reductions in children with BLLs annually. However, given the history of childhood lead poisoning, the conversation in the U.S. should now begin to shift towards ensuring all children are annually BLL screened to confirm these reductions for all children and not a select-subset of the population of children within a given state (i.e. BLL screening across the board). Since this study examined only five-states, of which four-states’ data could be examined, it may serve prudent for the CDC and LPNSD to evaluate the potential information gained from a pilot study mandating all children to receive annual BLL screenings in New York, New Jersey, Pennsylvania, and Michigan. This data could then be compared to the report compiled herein to resolve the gaps within the data, initiate a map of environmental exposure risks, further elucidate and address remaining barriers to BLL screenings, as well as associated public health concerns as a means to address this environmental social justice issue. This information could be rather informative as the CDC data examined herein is based upon Michigan’s data prior to the Flint water re-routing catastrophe and can serve as an index of increased environmental and public health improvements or deficits for the Michigan populations that were directly affected in the coming years.
Based on the BLL screening data examined, it is unclear why some states are collecting data annually and not others (i.e. Colorado), or intermittently collecting data (i.e. New York excluding NYC and New Jersey), and why some states’ BLL screening rates are stable and others unstable (i.e. New York excluding NYC and Michigan). Although in each of the states the rates of children with BLLs exceeding 5 μg/dL have been declining, these statistics only account for less than 50% of all children screened. Thus, failing to provide an adequate depiction of how many children are affected and remain in need of critical health care interventions. For example, although NYC and Pennsylvania have been stable in their annual children’s BLL screenings, as determined by the census, NYC screens more (i.e. 49.39% in 2014) and has less children with > 5 μg/dL lead body burdens (i.e. 6,977 in 2014), whereas Pennsylvania screens less (i.e. 15.92% in 2014) and has more children with > 5 μg/dL lead body burdens (i.e. 11,918 in 2014). Moreover, from the 2014 census data, it is unclear as to whether the unscreened 51.61% of children in NYC and 85.08% of children in Pennsylvania are lead poisoned. The lack of data in Colorado and the recent low-rates in Michigan are more concerning; especially, given the resurgent lead exposures of late. In fact, the CDC data examined herein only covers 38 out of the 50 states [50]. The CDC dataset [50] that was examined excluded children’s BLL screening data from: Alaska, Colorado, Idaho, Montana, Nebraska, New Mexico, North Dakota, South Carolina, South Dakota, Utah, and Wisconsin.
The summary of this study’s findings suggest that the CDC should mandate each state across the U.S. to report children’s BLL screening data annually to proactively evaluate the potential for addressing public health concerns of lead poisoning early and to identify sources within and between states that may cause concerns. One such example is the Gold King mine excavation that has affected the rivers between Colorado, New Mexico, and Utah. However, given the current CDC dataset, neither one of these states’ children’s BLL screening data were reported. This presents with a peculiar situation where the lead source of contamination from Colorado may cause new and/or additional lead contamination sources to its neighboring states. If all states had previously reported children’s BLL screening data to the CDC regularly, the CDC would have a current “baseline” of BLLs for children in each of these states and could effectively monitor for any increases in BLLs annually, due to such environmental misfortunes. The real problem is that situation creates an environmental social justice issue that is perhaps best explained in a quote from a world-renowned epidemiologist Bruce Lampher [56], “children, in essence, are being used as biological indicators of lead-contaminated housing because society has not been willing to invest in efforts to remove the lead from children’s environments.” If local state and Department of Health (DOH) officials could work together in addressing, diminishing, and ultimately removing lead contamination within the areas they govern, the quality of life for future generations of children in the U.S. could improve.
The following considerations have been posited based on this study’s findings: is it possible that lead disasters are more likely in states that demonstrate a reduction in or lack of public education and/or lead conscious practices? Perhaps recognition of lead toxicity as demonstrated by children’s BLL screening rates also reflects recognition (or lack thereof) or potential environmental hazards such as: irresponsibly changing the water sources in Flint [19,20], drilling to extract wastes in the Gold King Mine mishap [18], the potential for similar outcomes to occur due to oil fracking conditions in Pennsylvania [57], as well as the ever-increasing need to replace outdated state-wide sewer and plumbing systems in local neighborhoods, schools, pre-1970s houses, public housing, and apartment buildings [26,29]. The efforts made by each states’ DOH as in the case of the states examined herein [51–53] show New York and Pennsylvania to be more actively involved in lead poisoning public education, but still may fail short in addressing the issue. However, across all the states examined in this study, childhood BLL screening rates appear to be low, as well as both under and inconsistently reported.
Recommendations: a public health perspective
The present study’s findings reveal the importance of addressing lead poisoning prevention in every state, including those traditionally known as being lead conscious. Medicaid-enrolled children are mandated to have BLL screenings at 12–24 months of age, although as this and former studies have demonstrated, actual BLL screening rates remain inconsistent. The CDC task force recommends [40] that, “all children between the ages of 12–24 months, regardless of insurance status” be lead screened to obtain an accurate measure of children’s BLLs across the nation. It is further recommended that legislation require private insurance companies to reimburse physicians and other health care professionals to conduct BLL screenings for all pregnant women, lactating mothers, in addition to children 12–24 months of age; which are contemporaneous with the most critical time-periods for a child’s developing brain and is equivalent to other early neuropsychological screening measures for pica risk factors (i.e. soil, dirt, paint chips, etc.), as well as Autism and its associated spectrum disorders [58,59].
The CDC task force also reiterates the important role of physicians, pediatricians, and other health care professionals (i.e. midwives, fertility, obstetrics and gynecology doctors) in not only proactively preventing and detecting lead exposure, but also reactively managing the negative consequences of lead in exposed children and mothers. If a child were determined to be lead poisoned, clear policies and procedures should be mandated for physicians and other health care professionals to track a child from 12 to 24 months of age up until their BLLs have been eliminated. Additionally, physicians and other health care professionals should be tracking BLLs against anthropometric measures such as the CDC clinical growth charts [60] for child head circumference and other relevant nutritional and body weight factors. If a child is identified as being lead poisoned and the source of lead exposure is ill- or undefined, it is imperative to continue providing BLL screenings to children beyond 36 months of age. Notably, if a child’s BLL screening results come back negative at 12 months of age, it does not mean they should be exempt from being re-evaluated at 24–36 months of age as they can still be at risk for contracting lead poisoning from a range of environmental sources known to produce LBLLs (i.e. contracting lead exposure from public schools, water supplies, candies, toys, electronics, etc.).
Further, simply monitoring children’s BLLs and anthropometric measures at 12–24 months is insufficient and arguably unethical given what is known about the neurotoxic effects of lead on brain development, intellectual ability, and quality of life outcomes. In particular, health care provider assistance through patient education regarding nutritional supplement and replacement therapy approaches that can potentially minimize lead absorption remains promising. Additional recommendations would encourage programs such as the Supplemental Nutrition Assistance Program (SNAP), the Women, Infants, and Children program (WIC) to ensure they include foods and supplements containing such replacement vitamins and minerals (i.e. calcium and vitamin E) to reduce and/or mitigate lead absorption within pregnant women, lactating mothers, and children [46,60–62]. Education regarding nutritional options are only useful if the resources are readily and consistently available for these often-vulnerable families to access the necessary food and nutritional supplements. However, it should be noted here that more research is needed to understand whether such evidence-based therapies not only reduce circulating BLLs, but also more importantly, ameliorate brain lead deposition [46,60–62]. Consistently, new cost-effective therapies as dietary taurine intake in addressing lead induced neurodevelopmental problems across the lifespan are currently being explored in animal models [63–65], but require a concerted effort in confirming its efficacy through clinical studies.
The U.S. would be best served if each state were to develop a new program for increasing the public’s knowledge of the sources of environmental lead exposures and its negative neurodevelopmental and neuropsychopathological consequences that may result from childhood lead poisoning. The first program would include utilizing the mobile medical services model in which mobile lead poisoning education for both mother’s and children’s BLL screening services could be dispatched into communities. These mobile medical services could team up with other prevention efforts, or react in the event of a known, suggested, or presumed environmental lead exposure source such as those in the Gold King mine of Silverton, Colorado [18] and water supply issues in Flint, Michigan [19,20,66]. These mobile medical teams would be comprised of nurses and health care professionals who would both educate the community about lead exposures and screen mothers and children for BLLs directly in the field. When BLL screenings are unavailable due to the aforementioned barriers, these mobile medical units may prove as a more effective intervention to mitigate accessibility issues, and vouchers can be distributed to alleviate the financial barrier to families wanting to screen their children for lead exposures. Considering the lack of BLL screenings in all communities, this will likely serve the public well in assessing those environments housing children with no insurance – often children without documents or above the Medicaid threshold for their state – or those with private insurance.
In addition to programming, it is also recommend for future research to examine BLL screening barriers to determine ways in which to maintain adequate BLL screening in urban communities, who are most often at-risk for environmental lead exposures [38]. Notably, this recommendation may only address urban areas within the U.S. medical structure; however, as noted by Silbergeld [23], this is an international problem that cannot be solved alone and requires international policy discussions. Data drawn from physicians, other health care professional offices, and chart reviews leaves many families out of the current understanding regarding the barriers they experience in obtaining BLL screenings for their children. Thus, additional studies are needed to fully understand the extent of missing children from the CDC data currently available, and data collection needs to begin for pregnant and lactating women. The former requires an active dialogue and ongoing civic engagement; especially, as lead contamination may arise from trading goods with foreign countries, electronic recycling or E-waste, and other forms of manufacturing of products offshore that may indirectly effect the U.S. as new lead source exposures [67–69]. Through discussions that are more pointed and education inclusive of local communities, the government, and health care providers, the U.S. population can learn how to effectively address and manage the barriers most likely inhibiting annual BLL screenings of pregnant women, lactating mothers, and children today.
Finally, while the aforementioned demonstrates an abundance of research that has reviewed and examined the effects of lead poisoning in children and its associated barriers to secondary prevention – such as BLL screenings – limited research has focused on primary prevention. The CDC’s 2012 task force [40] reported a renewed call for primary prevention by proactively identifying and removing lead exposure sources within the environment, such as within housing, water, and/or consumer products, prior to detecting elevated BLLs in children post-screening.
Primary prevention is particularly important, especially in vulnerable communities in which pre-1978 public and private housing, as well as water sources are more likely to be lead contaminated, as in the case of Silverton, Colorado [18] and Flint, Michigan [19,20,66]. Low-income communities already face structural inequalities that negatively influence their access to education, academic achievement, health care, and economic opportunity, whereas environmental lead poisoning further inhibits access by causing developmental and intellectual disabilities in children that persist across their lifespans. Thoroughly analyzing the National Health and Nutrition Examination Surveys (NHANES), Jones et al. [61] found that risk factors for HBLLs continue to negatively affect vulnerable communities disproportionately. In their study, HBLLs were associated with populations residing in older pre-1978 housing, poverty, and coming from underrepresented minority backgrounds.
It is the public health perspective that all communities – regardless of income level, ethnicity or documentation status – deserve safe housing and uncontaminated drinking water, and this should be the primary prevention strategy for effectively eliminating environmental lead exposures. The second form of prevention recommended by the CDC task force [55] relates to physicians educating their patients on the sources and dangers of lead exposure. Such an environmental social justice framed educational strategy would help increase parents’ abilities to make informed decisions about living spaces, drinking water sources, and any potentially unsafe home renovation projects. This recommendation, however, does not account for the limited choices low-income families may face when deciding where to live in many U.S. communities. It is further recommend that the burden not only fall on the physicians and medical health professionals to educate their patients, but rather equal responsibility should fall on the housing and urban development authorities to remove known environmental lead threats from all low-income housing across the U.S. Moreover, state systems should require lead neurotoxicity education and BLL screenings in order to approve permits for pre-1978 home renovation projects and/or renting apartments in pre-1978 buildings, as this continues to present yet another major source of lead exposure for pregnant women, lactating mothers, and children.
Conclusion
Despite the many historically significant improvements in removing lead from paint and gasoline in the U.S., alternative lead sources remain within the environment, posing continual increased risks for children to be exposed to lead with the potential for developmental disabilities and associated neuropsychological disorders [3,29–31]. Yet, childhood BLL screenings and subsequent medical treatments in the U.S. remains unsatisfactory. The findings from this study reveal that approximately 50% or less children in New York, New Jersey, Pennsylvania, and Michigan from the CDC data that were analyzed are regularly screened for BLLs annually. Further, some states, like Colorado, are not reporting data, leaving the actual picture of BLL screening rates and levels of lead toxicity in children for this state to remain unknown. Another point of interest is that no other state across the U.S. besides New York separates their annual children’s BLL screening data between urban and suburban/rural populations (i.e. NYC vs. New York excluding NYC). This may inadvertently result in New York having more sensitive datasets when compared to other states; thus, justifying them as an adequate reference state in which to draw comparisons. Further, within states that may have more suburban and rural areas, this may obscure the ability for the CDC and local governments to localize lead sources of exposure as the percent of children affected may be negatively skewed by these differences in areas (i.e. metropolitan vs. non-metropolitan) creating a false-negative of lead exposure concerns for children. The alternate situation should also be considered as states with more metropolitan areas and less rural areas may present with a false-positive suggesting greater lead exposure concerns for children. In order to prevent the potential for such skewed data reporting issues, the CDC should refine their data requirements from local state and government officials to parse each states’ children’s BLL screening populations by urban vs. suburban and rural areas.
Additionally, regardless of the state, certain communities within each state across the U.S. still remain at higher risk than others to lead exposure sources and no current map of lead exposure risk accurately reflects these issues. Finally, despite recognizing the negative effects of lead on children, there lacks a universal requirement or expectation to screen pregnant and lactating women for lead poisoning during their first, second, and third trimester as a proactive strategy well in advance of their child’s birth. Also, there lacks a universal requirement for children once born to be screened for lead poisoning and followed up at 12 and 24 months of age. This presents with a psychosocial diminishment of lead poisoning education and consultation between families and physicians, due to a misattribution that lead poisoning either no longer is a concern or no longer exists in the current environment (i.e. an idea that was once socialized as a medical concern 50 years ago).
Resurgent lead exposures have renewed public interest regarding this important and continual public health issue as recent research has suggested that environmental neurotoxicants such as lead can cause epigenetic modifications that may negatively impact a child’s neurodevelopment across their lifespan with transgenerational vulnerabilities [70] and may further increase a populations public health risks as those observed in Flint, Michigan [71]. With this renewed interest, the present day should be considered an optimal time to call action for removing environmental lead contaminants in the U.S. Primary prevention of lead removal in pre-1978 home, public work and school spaces; water sources; and consumer products must take precedence, but so should consistent and reliable BLL screening methods during every child’s annual health checkup. Having all lead exposure sources removed and children BLL screenings conducted annually will facilitate the removal of racial, ethnic, financial, and economic barriers long associated with select populations most vulnerable to environmental lead exposures. This will shift society’s historic perspective of lead poisoning being a disease often associated with poverty (i.e. which is both socially inappropriate and outdated), to a more timely and well-suited philosophy of being a disease that impacts the lives of all children irrespective of race, ethnicity, immigrant status, SES, and/or social class.
It is argued that universal screening can only be achieved if insurance companies are mandated to cover childhood BLL screenings, and reimbursement rates for publicly funded insurance companies are increased. Further, by developing new mobile education and BLL screening clinics to provide improved access across the U.S., in urban, suburban, and more rural communities barriers to accessibility, on- and off-site BLL screenings can be circumvented and more sensitive data collected for each state. At present, given the aforementioned it is difficult to accurately estimate the number of cases of children with > 5 µg/dL occur within the U.S. population. However, since the children in families that receive Medicaid, had their mandated BLL screening, and were identified as lead-positive, only 43.5% were screened at one year of age. Moreover, 42% of the original sample were followed up with, indicating a 58% medical drop out perhaps due to barriers to continued BLL screenings. A fair estimate would suggest that 54.7% of children receiving Medicaid in each state are not BLL screened and could be lead poisoned. As for non-Medicaid recipient families, it remains to be elucidated what lead exposures these children may face due to the way in which individual states screen for BLLs; thereby, making national comparisons less comparable than one would hope given the longstanding history of childhood lead poisoning. Current lead exposure disasters, such as those in Silverton, Colorado [18], and Flint Michigan [19,20,66], require ongoing conscientious lead assessment, consistent BLL screening methods for mothers and children, as well as timely, reliable pediatric follow up of lead-positive children. This study serves as a call to reactivate the removal of lead sources in the environment and to encourage local governments to mandate state BLL screenings for all pregnant women, lactating mothers, as well as children across the U.S.
Funding Statement
This work was supported by SUNY Old Westbury’s Faculty Development Grant awarded to LSN.
Acknowledgments
The author would like to thank Dr. Sarah A. Smith from SUNY Old Westbury’s Department of Public Health and Health Disparities Institute for their helpful comments, insights, and suggestions to earlier versions of this manuscript, as without their input this publication would not have been possible. The author would also like to thank our anonymous reviewers for their instructive comments and feedback on this publication. A final acknowledgement is most deserved to highlight the many seminal papers and scientific contributions to the public’s understanding of childhood lead poisoning by Claire C. Patterson, Herbert L. Needleman, and the late John F. Rosen.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- [1]. Needleman HL, Landrigan PJ.. Raising children toxic free: how to keep your child safe from lead, asbestos, pesticides, and other environmental hazards. New York, NY: Avon Books; 1994. [Google Scholar]
- [2]. Pueschel SM, Linakis JG, Anderson AC.. Lead poisoning in childhood. Baltimore, MD: Paul H. Brookes Publishing Co; 1996. [Google Scholar]
- [3]. Lidsky TI, Schneider JS.. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126:5–19. [DOI] [PubMed] [Google Scholar]
- [4]. Lead-Based Paint Poisoning Prevention Act, PL 91-695 Title 42, U.S.C. §§ 4801 et seq. U.S. Statutes at Large. 1971. January 13;84:2078–2080. [Google Scholar]
- [5]. Lead-Based Paint Poisoning Prevention Act Amendments, PL 93-151 Title 42, U.S.C. §§ 4821-4822. U.S. Statutes at Large. 1973. November 9;87:560–568. [Google Scholar]
- [6]. Baltimore Health Department Chronology of lead poisoning control: baltimore 1931-71. Baltimore Health News. 1971. December;34–40. [Google Scholar]
- [7]. Clean Air Act, PL 88-206 Title 42, U.S.C. §§7401 et seq. U.S. Statues at Large. 1963. December 17;77:392–401. [Google Scholar]
- [8]. Clean Air Act Amendments of PL 93-15. (April 9, 1973). Title 42, U.S.C. § 7545. U.S. Statutes at Large. 1973;86:11. [Google Scholar]
- [9]. Feng T, Keller LR, Wang L, et al. Product quality risk perceptions and decisions: contaminated pet food and lead-painted toys. Risk Anal. 2010;30(10):1572–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Kumar A, Pastore P. Lead and cadmium in soft plastic toys. Curr Sci. 2007;93(6):818–822. [Google Scholar]
- [11]. Gerstenberger S, Savage G, Sellers C, et al. Lead-contaminated candies in southern nevada. Public Health Rep. 2007;122(5):572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Rankin CW, Nriagu JO, Aggarwal JK, et al. Lead contamination in cocoa and cocoa products: isotopic evidence of global contamination. Environ Health Perspect. 2005;113(10):1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Medlin J. Lead: sweet candy, bitter poison. Environ Health Perspect. 2004;112(14):A803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Lynch RA, Boatright DT, Moss SK. Lead-contaminated imported tamarind candy and children’s blood lead levels. Public Health Rep. 2000;115(6):537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Kim KC, Park YB, Lee MJ, et al. Levels of heavy metals in candy packages and candies likely to be consumed by small children. Food Res Int. 2008;41(4):411–418. [Google Scholar]
- [16]. Fuortes L, Bauer E. Lead contamination of imported candy wrappers. Vet Hum Toxicol. 2000;42(1):41–42. [PubMed] [Google Scholar]
- [17]. Levin R, Brown MJ, Kashtock ME, et al. Lead exposure in U.S. children, 2008: implications for prevention. Environ Health Perspect. 2008;116(10):1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Fiscor S. Gold King spill daylights EPAs poor remediation practices. Eng Mining J. 2015;216(9):66–68. [Google Scholar]
- [19]. Wilkinson M. (2016). Kid’s lead levels high in many michigan cities. Bridge Magazine, The Detroit News, (cited 2018 March18 Available from: http://www.detroitnews.com/story/news/michigan/flint-water-crisis/2016/01/27/many-michigan-cities-higher-lead-levels-flint/79438144/
- [20]. Botelho G. (2016). Flint water crisis: city gets $28 million in state aid. CNN Breaking News, (cited 2018 March18 Available from: http://www.cnn.com/2016/01/29/us/flint-michigan-water-crisis/
- [21]. Edwards M. Fetal death and reduced birth rates associated with exposure to lead-contaminated drinking water. Environ Sci Technol. 2013;48(1):739–746. [DOI] [PubMed] [Google Scholar]
- [22]. Schnaas L, Rothenberg SJ, Flores MF, et al. Reduced intellectual development in children with prenatal lead exposure. Environ Health Perspect. 2006;114(5):791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Silbergeld EK. The international dimensions of lead exposure. Int J Occup Environ Health. 1995;1(4):336–348. [DOI] [PubMed] [Google Scholar]
- [24]. Bradbury MW, Deane R. Permeability of the blood-brain barrier to lead. Neurotoxicol. 1993;14:131–136. [PubMed] [Google Scholar]
- [25]. U.S. Department of Health and Human Services (2007). Public health service agency for toxic substances and disease registry. (cited 2018 March17). Available from: http://www.atsdr.cdc.gov/toxprofiles/tp13.pdf
- [26]. Chisolm Jr JJ. Medical management In: Pueschel SM, Linakis JG, Anderson AC, editors. Lead poisoning in children. Baltimore, MD: Paul H. Brookes Publishing Co; 1996. p. 141–162. [Google Scholar]
- [27]. Goldman RH, White R, Kales SN, et al. Lead poisoning from mobilization of bone stores during thyrotoxicosis. Am J Ind Med. 1994;25:417–424. [DOI] [PubMed] [Google Scholar]
- [28]. Markowitz M, Weinberger HL. Immobilization-related lead toxicity in previously lead-poisoned children. Pediatrics. 1990;86:455–457. [PubMed] [Google Scholar]
- [29]. Shannon MW. Etiology of childhood lead poisoning In: Pueschel SM, Linakis JG, Anderson AC, editors. Lead poisoning in children. Baltimore, MD: Paul H. Brookes Publishing Co; 1996. p. 37–57. [Google Scholar]
- [30]. Bellinger DC, Dietrich KN. Low-level lead exposure and cognitive function in children. Pediatr Ann. 1994;23:600–605. [DOI] [PubMed] [Google Scholar]
- [31]. Lidsky TI, Schneider JS. Adverse effects of childhood lead poisoning: the clinical neuropsychological perspective. Environ Res. 2006;100:284–293. [DOI] [PubMed] [Google Scholar]
- [32]. Canfield RL, Henderson Jr CR, Cory-Slechta DA, et al. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348(16):1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Landrigan PJ, Schechter CB, Lipton JM, et al. Environmental pollutants and disease in American children: estimates of morbidity, mortality, and costs for lead poisoning, asthma, cancer, and developmental disabilities. Environ Health Perspect. 2002;119(7):721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Levin R. The attributable annual health costs of U.S. occupational lead poisoning. Int J Occup Environ Health. 2016;22(2):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Wasserman GA, Liu X, Lolacono NJ, et al. Lead exposure and intelligence in 7-year old children: the yugoslavie prospective study. Environ Health Perspect. 1997;105:956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Gould E. Childhood lead poisoning: conservative estimates of the social and economic benefits of lead hazard control. Environ Health Perspect. 2009;117(7):1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Lamphear BP, Dietrich K, Auinger P, et al. Cognitive deficits associated with blood lead concentrations <10µg/dl in US children and adolescents. Public Health Perspect. 2000;115:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Chiodo LM, Jacobson SW, Jacobson JL. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol. 2004;26(3):359–371. [DOI] [PubMed] [Google Scholar]
- [39]. Barbosa Jr F, Tanus-Santos JE, Gerlach RF, et al. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect. 2005;113(12):1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Centers for Disease Control and Prevention (CDC) (2012). Low level lead exposure harms children. A renewed call for primary prevention. Report of the Advisory Committee on Childhood Lead Poisoning Prevention. (cited 2018 March17). Available from: https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf
- [41]. Alexander BH, Checkoway H, Van Netten C, et al. Paternal occupational lead exposure and pregnancy outcome. Int J Occup Environ Health. 1996;2(4):280–285. [DOI] [PubMed] [Google Scholar]
- [42]. Office of Inspector General (2010). Most medicaid children in nine states are not receiving all required preventive screening services. (cited 2018 March17). Available from: http://oig.hhs.gov/oei/reports/oei-05-08-00520.pdf
- [43]. Kemper AR, Clark SJ. Physician barriers to lead testing of Medicaid-enrolled children. Ambul Pediatrics. 2005;5(5):290–293. [DOI] [PubMed] [Google Scholar]
- [44]. Feinberg AN, Cummings CK. Blood lead screening. Clin Pediatr (Phila). 2005;44(7):569–574. [DOI] [PubMed] [Google Scholar]
- [45]. Ferguson SC, Lieu T. Blood lead testing by pediatricians: practice, attitudes, and demographics. Am J Public Health. 1997;87(8):1349–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Cleveland LM, Minter ML, Cobb KA, et al. Lead hazards for pregnant women and children: part1: immigrants and the poor shoulder most of the burden of lead exposure in this country. Part 1 of a two-part article details how exposure happens, whom it affects, and the harm it can do. Am J Nurs. 2008;108(10):40–49. [DOI] [PubMed] [Google Scholar]
- [47]. Centers for Disease Control and Prevention (CDC) (2017). Lead. Healthy People 2020. (cited 2018 March17 Available from: https://www.cdc.gov/nceh/lead/
- [48]. Boreland F, Lyle D. Screening children for elevated blood lead—learnings from the literature. Sci Total Environ. 2008;390(1):13–22. [DOI] [PubMed] [Google Scholar]
- [49]. Polivka BJ, Gottesman MM. Parental perceptions of barriers to blood lead testing. J Pediatr Health Care. 2005;19(5):276–284. [DOI] [PubMed] [Google Scholar]
- [50]. Centers for Disease Control and Prevention (CDC) (2014). National surveillance data (1997-2014)—united States [Data file]. (cited 2018 March18). Available from: http://www.cdc.gov/nceh/lead/data/national.htm
- [51]. New York State Department of Health (2016). Educational materials for lead poisoning prevention. (cited 2018 March18). Available from: https://www.health.ny.gov/environmental/lead/education_materials/index.htm
- [52]. New Jersey State Department of Health (2017). Childhood lead. (cited 2018 March18). Available from: http://www.state.nj.us/health/childhoodlead/
- [53]. Pennsylvania State Department of Health (n.d.). Lead (Pb) fact sheet (cited 2018 March18 Available from: http://www.health.pa.gov/My%20Health/Environmental%20Health/Environmental%20Fact%20Sheets/Pages/Lead.aspx#.WZRPLsfD_Io
- [54]. The American Congress of Obstetricians and Gynecologists (2016). Lead screening during pregnancy and lactation. (cited 2018 March17).Available from: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Lead-Screening-During-Pregnancy-and-Lactation
- [55]. Centers for Disease Control and Prevention (2010). Guidelines for the identification and management of lead exposure in pregnant and lactating women. (cited 2018 March17). Available from: https://www.cdc.gov/nceh/lead/publications/leadandpregnancy2010.pdf
- [56]. Cabrera Y. (2017). Urban children are playing in toxic dirt: decades of build-up have left invisible mountains of lead in America’s urban centers, including Santa Ana, California. A ThinkProgress investigation. (cited 2018 March18). Available from: https://thinkprogress.org/urban-children-are-playing-in-toxic-dirt-41961957ff23/
- [57]. Rabe BG, Borick C. Conventional politics for unconventional drilling? Lessons from pennsylvania’s early move into fracking policy development. Int Rev Poult Res Politics Policy Sci Technol. 2013;30(3):321–340. [Google Scholar]
- [58]. Lidsky TI, Schneider JS. Autism and autistic symptoms associated with childhood lead poisoning. J Appl Res. 2005;5(1):80–87. [Google Scholar]
- [59]. Corsello CM. Early intervention in autism. Infants & Young Children. 2005;18(2):74–85. [Google Scholar]
- [60]. Centers for Disease Control and Prevention (CDC) (2009). Clinical growth charts. (cited 2018 March17). Available from: http://www.cdc.gov/growthcharts/clinical_charts.htm
- [61]. Jones RL, Homa DM, Meyer PA, et al. Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988–2004. Pediatrics. 2009;123(3):e376–e385. [DOI] [PubMed] [Google Scholar]
- [62]. Anderson AC, Pueschel SM, Linakis JG. Pathophysiology of lead poisoning In: Pueschel SM, Linakis JG, Anderson AC, editors. Lead poisoning in children. Baltimore, MD: PH Brookes; 1996. p. 75–96. [Google Scholar]
- [63]. Neuwirth LS. (2014). The characterization of Pb2+ toxicity in rat neural development: An assessment of Pb2+ effects on the GABA shift in neural networks and implications for learning and memory disruption. UMI Proquest Dissertations & Theses 3612469. DAI/B 75-06(E), cited 2014 April. [Google Scholar]
- [64]. Neuwirth LS, Volpe NP, Corwin C, et al. Taurine recovery of learning deficits induced by developmental Pb2+ exposure In: Lee DH, Shaffer S, Park E, et al, editors. Taurine 10. Vol. 975, 2017; New York, NY: Springer Press. p. 39–55. [DOI] [PubMed] [Google Scholar]
- [65]. Neuwirth LS, Phillips GR, El Idrissi A. Perinatal Pb2+ exposure alters the expression of genes related to the neurodevelopmental GABA-shift in postnatal rats. J Biomed Sci. 2018;25(45):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Hanna-Attisha M, LaChance J, Sadler RC, et al. Elevated blood lead levels in children associated with flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Pascale A, Sosa A, Bares C, et al. E-waste informal recycling: an emerging source of lead exposure in South America. Ann Glob Health. 2016;82(1):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Sepúlveda A, Schluep M, Renaud FG, et al. A review of the environmental fate and effects of hazardous substances released from electrical and electronic equipments during recycling: examples from China and India. Environ Impact Assess Rev. 2010;30(1):28–41. [Google Scholar]
- [69]. Huo X, Xu X, Zheng L, et al. Elevated blood lead levels of Children in Guiyu, an electronic waste recycling town in China. Environ Health Perspect. 2007;115(7):1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Rothstein MA, Harrell HL, Marchant GE. Transgenerational epigenetics and environmental justice. Environ Epigenetics. 2017;3(3):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Hanna-Attisha M, LaChance J, Sadler RC, et al. Elevated blood lead levels in children associated with the flint driking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]


