Abstract
Objective:
To compare non-Hodgkin lymphoma (NHL) incidence rates in adults who started antiretroviral therapy (ART) across the Asia-Pacific, South Africa, Europe, Latin, and North America.
Methods:
We included cohort data of adults living with HIV who started ART after 1995 within the framework of the International epidemiology Databases to Evaluate AIDS (IeDEA) and the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE). We used flexible parametric survival models to compare regional NHL rates at 2 years after ART start and to identify risk factors for NHL.
Results:
We included 210,898 adults with 1.1 million person-years (pys) of follow-up and 1,552 incident NHL cases (raw overall incidence rate 142/100,000 pys). After adjusting for age at ART start, first-line ART regimen, calendar period of ART start, and especially current CD4 cell count, NHL rates were similar across regions for most population groups. However, South African women remained at increased risk of developing NHL compared with their European counterparts (adjusted hazard ratio [aHR] 1.79, 95% CI 1.19–2.70). In Europe, Latin and North America, NHL risk was highest in men who have sex with men (MSM, aHR 1.30, 95% CI 1.14–1.48), followed by heterosexual men (referent), and women (aHR 0.66, 95% CI 0.57–0.78).
Conclusions:
The risk of developing NHL is higher in women in South Africa than in Europe and higher in MSM compared with heterosexual men and women. Reasons for these differences remain unclear. Early ART access and regular patient monitoring to avert low CD4 cell counts remain key for NHL prevention.
Keywords: Non-Hodgkin lymphoma, HIV, antiretroviral therapy, cohort study, incidence rates
Introduction
With the introduction of combination antiretroviral therapy (ART) the risk of developing human immunodeficiency virus (HIV)-related non-Hodgkin lymphoma (NHL) has substantially decreased [1–6]. However, NHL is still one of the most common cancers and a frequent cause of death among adults living with HIV [7–10]. The pathogenesis of HIV-related NHL is not well understood, but HIV-induced immune dysregulation and impaired control of oncogenic viruses seem to play an important role [11]. Epstein Barr virus (EBV), human herpesvirus 8 (HHV-8), and hepatitis C virus (HCV) have been recognized as causative agents of NHL, whereas the role of hepatitis B virus (HBV) in lymphomagenesis is being evaluated [12,13]. Prevalence and average age at acquisition of these viruses differ by population and geographic area. For example, EBV infection is typically delayed in high-income countries compared with low- and middle-income countries [12]. HHV-8 is highly prevalent in HIV-positive men who have sex with men (MSM) in most regions but is also common in heterosexual men and women in sub-Saharan Africa [14]. HCV prevalence is high in persons who inject drugs (PWID) [15], and it is increasing in MSM [16]. Furthermore, access to effective ART also varies across geographic regions. Median CD4 cell count at ART start is considerably lower in sub-Saharan Africa and Latin America than in Central Europe and North America [17]. Nevertheless, studies comparing the risk of developing NHL among adults living with HIV across different geographic regions are not available. We compared NHL incidence rates in adults who had started ART in the Asia-Pacific, South Africa, Europe, Latin America, or North America, and assessed factors associated with regional differences in NHL rates.
Methods
We analyzed cohort data from the International epidemiology Databases to Evaluate AIDS (IeDEA) and the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE) in EuroCoord. For further information on data collection and merging, see Supplementary Box S1. We included adults (≥16 years old) living with HIV who started ART after cohort enrollment from 1996 onwards. Incident NHL was defined as NHL diagnosed after ART start. Person-years were measured from ART start to the first occurrence of NHL, last visit, death, or database closure. In a sensitivity analysis, we excluded NHL cases diagnosed within the first six months after ART start. We assumed that adults remained on ART throughout follow-up and did not consider treatment interruptions and terminations. We estimated NHL incidence rates in two different ways: i) by dividing the number of incident NHL cases by person-years (pys) at risk (referred to as raw incidence rates), and ii) using proportional hazard flexible parametric survival models [18]. Flexible parametric survival models allow estimating instantaneous NHL incidence rates at given time points and displaying the change in incidence rates over time graphically. Using these models, we estimated regional crude and adjusted NHL incidence rates over time after ART start and identified risk factors for NHL. We modeled the baseline hazard using restricted cubic splines with four degrees of freedom and allowed for time-dependent region effects with two degrees of freedom. We compared crude and adjusted NHL rates at 2 years after ART start across geographic regions and in a sensitivity analysis, we compared regional NHL rates at 5 years after ART start. We used likelihood ratio tests to test whether the effect of a risk factor on NHL risk differed across regions. We assessed sex, exposure group (MSM, heterosexual men, women), injection drug use (yes, no), age at ART start (continuous variable), first-line ART regimen (non-nucleoside reverse transcriptase inhibitor [NNRTI]-based, protease inhibitor [PI]-based, other), calendar period of ART start (1996–1998, 1999–2003, 2004–2007, 2008–2014), and current (time-varying) CD4 cell count (continuous variable) in regression analyses. We used the last observation carried forward method for current CD4 cell count, i.e. we assumed that CD4 cell counts remained stable until a new measurement became available. CD4 cell count at ART start and HIV RNA load at ART start were assessed in descriptive analyses.
We used models including region and only one additional covariate with or without its interaction with region (referred to as crude models) to compare the burden of incident NHL across regions. Adjusted models with relevant risk factors and their interaction with region (if necessary) were fit to assess remaining differences in regional NHL rates. Variable selection was not automated, but rather based on clinical and epidemiological reasoning as well as data availability and quality. We derived three adjusted models: model 1 included region, sex and its interaction with region, current CD4 cell count and its interaction with region, age at ART start, first-line ART regimen, and calendar period of ART start. Model 2 was restricted to regions with data on sexual orientation and PWID status (i.e. Europe, Latin and North America), and included region, age at ART start, current CD4 cell count, first-line ART regimen, calendar period of ART start, PWID status, and exposure group. To compare exposure group specific NHL rates across regions, we created model 3 by adding an interaction term between exposure group and region to model 2. To account for differences in HIV-related risk factors across regions, we used the fitted models to predict NHL incidence rates for a chosen set of covariates (including region) and different time points. For men and women (model 1), we used the following covariate profile: start of an NNRTI-based regimen between 2008–2014 at age 40 and current CD4 cell count of 450 cells/μl. For MSM, heterosexual men and women (model 3), we chose the following covariate profile: start of an NNRTI-based regimen between 2008–2014 at age 40, no injection drug use, and current CD4 cell count of 450 cells/μl. We present number and percentages of adults, medians with interquartile ranges (IQR), incidence rates per 100,000 pys, and hazard ratios (HRs) with 95% confidence intervals (CI). All analyses were done in STATA 14 (Stata Corporation, USA), and R (R Foundation, Austria).
Results
Descriptive analyses
We received data for 408,395 adults living with HIV enrolled in 49 eligible cohorts in the Asia-Pacific, Australia, Europe, South Africa, Latin and North America. We excluded 133,420 adults who did not start ART and another 64,077 adults for reasons detailed in Supplement Figure S1. We excluded five cohorts with <100 eligible adults and one region with <500 eligible adults (Australia). In a last step, we excluded 1,792 adults who did not have any CD4 cell count measurement. The Asia-Pacific region with 2,638 adults and 9 incident NHL cases was included for descriptive analyses but excluded from regression models.
The descriptive dataset included 210,898 adults from the Asia-Pacific (n=2,638), South Africa (n=21,656), Latin America (n=8,569), North America (n=16,986) and Europe (n=161,049), see Table 1. Total follow-up was 1.1 million pys with a median of 4.1 years per person (IQR 1.7–7.9); 1,552 adults developed NHL after starting ART (Europe 1,225, North America 204, South Africa 63, Latin America 51, Asia-Pacific 9). Age at ART start was similar across regions with a median of 37.3 years (IQR 31.4–44.4). In the Asia-Pacific, Europe, North, and Latin America about 70% of adults were male, whereas in South Africa 63% were female. In Europe, Latin America, and North America, ≥40% of adults were MSM in contrast to 23% in the Asia-Pacific. Data on MSM and PWID were not available for South Africa. First-line ART regimens differed by region, with NNRTI used by ≥90% of adults in South Africa and the Asia-Pacific, and about 40% in Europe and North America. Median CD4 cell count at ART start was highest in Europe (250, IQR 126–368) and lowest in South Africa (107 cells/μL, IQR 43–176). While in South Africa and the Asia-Pacific >95% of adults started ART after 2003, 39% of the European and 63% of the North American study population had started ART before 2004 (Table 1).
Table 1:
Patient characteristics at start of ART, stratified by region.
| Asia-Pacific | South Africa | Latin America | North America | Europe | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| All adults | 2,638 (100%) | 21,656 (100%) | 8,569 (100%) | 16,986 (100%) | 161,049 (100%) |
| Median follow-up time (IQR) [years] | 2.7 (1.7–3.9) | 2.0 (0.8–4.0) | 4.7 (2.0–8.2) | 4.3 (1.7–8.5) | 4.5 (1.9–8.5) |
| Sex / exposure group | |||||
| Women | 814 (31%) | 13,667 (63%) | 2,267 (26%) | 4,203 (25%) | 44,180 (27%) |
| Men | 1,824 (69%) | 7,989 (37%) | 6,302 (74%) | 12,783 (75%) | 116,869 (73%) |
| Heterosexual | 1,200 (45%) | 6,776 (31%) | 1,927 (22%) | 3,285 (19%) | 45,110 (28%) |
| MSM | 616 (23%) | NR | 3,637 (42%) | 8,692 (51%) | 64,232 (40%) |
| Missing | 8 (<1%) | 1,213 (6%) | 738 (9%) | 806 (5%) | 7,527 (5%) |
| PWID | |||||
| Yes | 172 (7%) | NR | 170 (2%) | 2,174 (13%) | 17,755 (11%) |
| No | 2,458 (93%) | NR | 8,348 (97%) | 14,812 (87%) | 143,294 (89%) |
| Missing | 8 (<1%) | 21,656 (100%) | 51 (1%) | 0 (0%) | 0 (0%) |
| Median age at ART start (IQR) [years] | 36.3 (30.5–43.0) | 36.4 (31.0–42.7) | 35.6 (29.7–42.9) | 39.6 (33.8–46.1) | 37.3 (31.4–44.5) |
| Age at ART start [years] | |||||
| 16–25 | 232 (9%) | 1,737 (8%) | 1,008 (12%) | 927 (5%) | 13,262 (8%) |
| 26–35 | 1,055 (40%) | 8,643 (40%) | 3,426 (40%) | 4,838 (28%) | 57,975 (36%) |
| 36–45 | 867 (33%) | 7,777 (36%) | 2,609 (30%) | 6,945 (41%) | 55,577 (35%) |
| 46–55 | 354 (13%) | 2,883 (13%) | 1,114 (13%) | 3,345 (20%) | 23,879 (15%) |
| ≥56 | 130 (5%) | 616 (3%) | 412 (5%) | 931 (5%) | 10,356 (6%) |
| First line treatment | |||||
| NNRTI-based | 2,366 (90%) | 20,492 (95%) | 6,407 (75%) | 6,200 (37%) | 67,059 (42%) |
| PI-based | 235 (9%) | 1,117 (5%) | 1,959 (23%) | 9,231 (54%) | 82,614 (51%) |
| Other ART | 37 (1%) | 47 (<1%) | 203 (2%) | 1,555 (9%) | 11,376 (7%) |
| Calendar year of ART start | |||||
| 1996–1998 | 0 (0%) | 0 (0%) | 109 (1%) | 5,466 (32%) | 19,216 (12%) |
| 1999–2003 | 100 (4%) | 99 (<1%) | 1,969 (23%) | 5,228 (31%) | 43,675 (27%) |
| 2004–2007 | 513 (19%) | 10,479 (48%) | 2,811 (33%) | 4,091 (24%) | 39,881 (25%) |
| 2008–2014 | 2,025 (77%) | 11,078 (51%) | 3,680 (43%) | 2,201 (13%) | 58,277 (36%) |
| Median CD4 cell count at ART start (IQR) [cells/μl] | 138 (43–234) | 107 (43–176) | 165 (61–273) | 233 (92–378) | 250 (126–368) |
| CD4 cell count at ART start (IQR) [cells/μl] | |||||
| <50 | 677 (26%) | 5,507 (25%) | 1,527 (18%) | 2,652 (16%) | 18,051 (11%) |
| 50–99 | 327 (12%) | 3,940 (18%) | 1,035 (12%) | 1,319 (8%) | 11,903 (7%) |
| 100–199 | 603 (23%) | 7,242 (33%) | 1,658 (19%) | 2,631 (15%) | 26,061 (16%) |
| 200–349 | 735 (28%) | 2,626 (12%) | 2,151 (25%) | 4,205 (25%) | 48,356 (30%) |
| 350–499 | 96 (4%) | 398 (2%) | 589 (7%) | 2,348 (14%) | 23,977 (15%) |
| 500–699 | 16 (1%) | 185 (1%) | 205 (2%) | 1,344 (8%) | 11,253 (7%) |
| ≥700 | 6 (<1%) | 82 (<1%) | 81 (1%) | 694 (4%) | 5,510 (3%) |
| Missing | 178 (7%) | 1,676 (8%) | 1,323 (15%) | 1,793 (11%) | 15,938 (10%) |
| Median HIV RNA at ART start (IQR) [log10 copies/ml] | 5.0 (4.5–5.4) | 4.5 (2.7–5.3) | 4.9 (4.3–5.4) | 4.5 (3.5–5.2) | 4.8 (4.1–5.3) |
| HIV RNA at ART start (IQR) [log10 copies/ml] | |||||
| <2.7 | 44 (2%) | 1,202 (6%) | 285 (3%) | 2,537 (15%) | 13,187 (8%) |
| 2.7–3.9 | 200 (8%) | 596 (3%) | 704 (8%) | 2,417 (14%) | 18,431 (11%) |
| 4.0–4.9 | 760 (29%) | 1,319 (6%) | 2,293 (27%) | 5,183 (31%) | 51,333 (32%) |
| 5.0–5.9 | 961 (36%) | 1,379 (6%) | 2,484 (29%) | 4,442 (26%) | 50,451 (31%) |
| ≥6.0 | 78 (3%) | 278 (1%) | 239 (3%) | 122 (1%) | 5,078 (3%) |
| Missing | 595 (23%) | 16,882 (78%) | 2,564 (30%) | 2,285 (13%) | 22,569 (14%) |
ART, antiretroviral therapy; IQR, interquartile range; MSM, men who have sex with men; NNRTI, non-nucleoside reverse-transcriptase inhibitor; NR, not reported; PI, protease-inhibitor; PWID, people who inject drugs; RNA, ribonucleic acid.
In adults who developed NHL, median time from ART start to NHL diagnosis was 1.1 years (IQR 0.3–3.6), see Supplementary Table S1. Fifty-seven (4%) adults diagnosed with incident NHL had a history of Kaposi sarcoma (KS); 39 of them (68%) were MSM. Median CD4 cell count at NHL diagnosis was 220 cells/μL (IQR 96–379); median age at NHL diagnosis was 42.8 years (IQR 36.6–50.6). The raw overall NHL incidence rate was 142/100,000 pys (95% CI 135–150). Raw NHL incidence rates per 100,000 pys were similar in Europe (137, 95% CI 130–145), South Africa (116, 95% CI 91–149), Asia-Pacific (116, 95% CI 60–223), and Latin America (112, 95% CI 85–147), but higher in North America (225, 95% 196–258), see Supplementary Table S2. Incidence rates were particularly high (>1,000/100,000 pys) in adults with current CD4 cell counts <50 cells/μL in North America and Europe (Supplementary Table S3). In contrast, in South Africa, NHL incidence rates were relatively low (150/100,000 pys) in adults with CD4 cell counts <50 cells/μL.
NHL risk factors
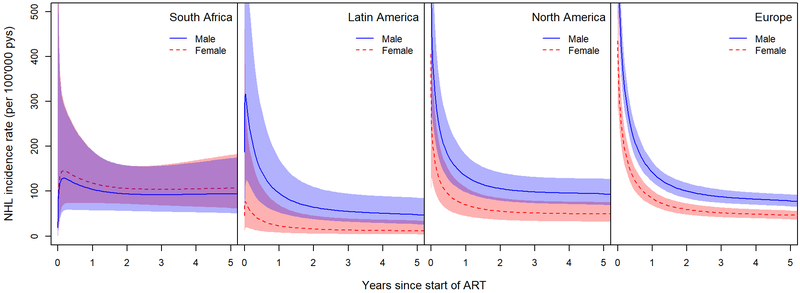
In all regions (model 1), NHL incidence rates were highest immediately after starting ART and decreased thereafter (Figure 1), but the decrease was less pronounced in South Africa. The effect of sex on the risk of developing NHL differed across regions (p-value for interaction=0.016). Based on both crude and adjusted analyses, NHL rates were higher among men than among women in Europe, Latin, and North America (Table 2). In South Africa, the risk of developing NHL was similar in women and men (aHR 1.13, 95% CI 0.67–1.91, referent: men).
Figure 1: NHL incidence rates by time since ART start in men and women across regions.
Incidence rates are predicted for adults with a current CD4 cell count of 450 cells/μl who started an NNRTI-based first-line ART regimen between 2008–2014 at the age of 40 years (model 1). ART, antiretroviral therapy; NHL, non-Hodgkin Lymphoma; NNRTI, non-nucleoside reverse-transcriptase inhibitor; pys, person-years.
Table 2:
Crude and adjusted hazard ratios for the regional effect of sex and current CD4 cell count on the risk of developing NHL in adults who started ART.
| South Africa | Latin America | North America | Europe | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Sex | ||||
| Men | 1.00 | 1.00 | 1.00 | 1.00 |
| Women (crude) | 1.00 (0.60 – 1.69) | 0.23 (0.08 – 0.64) | 0.52 (0.36 – 0.75) | 0.54 (0.47 – 0.63) |
| Women (adjusted*) | 1.13 (0.67 – 1.91) | 0.24 (0.09 – 0.67) | 0.52 (0.36 – 0.76) | 0.59 (0.51 – 0.69) |
| Current CD4 cell count | ||||
| Per 100 cells/μl increase (crude) | 0.91 (0.77 – 1.07) | 0.66 (0.54 – 0.80) | 0.66 (0.61 – 0.71) | 0.71 (0.69 – 0.73) |
| Per 100 cells/μl increase (adjusted**) | 0.92 (0.78 – 1.08) | 0.66 (0.55 – 0.80) | 0.65 (0.60 – 0.71) | 0.72 (0.69 – 0.74) |
Adjusted for age at ART start, calendar year of ART start, first-line ART regimen, current CD4 cell count and its interaction with region (model 1).
Adjusted for age at ART start, calendar year of ART start, first-line ART regimen, sex and its interaction with region (model 1).
ART, antiretroviral therapy; CI, confidence interval; HR, hazard ratio; NHL, non-Hodgkin lymphoma.
The effect of current CD4 cell counts on the risk of developing NHL also varied by region (model 1, p=0.004). In Europe, Latin and North America, NHL rates decreased with increasing current CD4 cell counts (Table 2). Per 100 cells/μl increase, NHL rates declined by about one-third in Latin America (aHR 0.66, 95% CI 0.55–0.80), North America (aHR 0.65, 95% CI 0.60–0.71) and Europe (aHR 0.72, 95% CI 0.69–0.74). In contrast, no clear association between current CD4 cell count and the risk of developing NHL was found in South Africa (aHR 0.92, 95% CI 0.78–1.08). The effect of age at ART start, calendar period of ART start, and first-line regimen did not differ across regions (model 1, Supplementary Table S4). In all regions, NHL rates increased with age (per 10-year increase, aHR 1.29, 95% CI 1.23–1.35). NHL rates decreased from the period 1996–1998 to 1999–2003 and remained stable thereafter. There was no evidence for an association between first-line ART regimen and the risk of developing NHL.
In model 2 without South Africa, we explored the effect of exposure group and injection drug use on NHL risk (Table 3, Supplementary Figure S2). In adjusted analyses, MSM had the highest NHL rates, followed by heterosexual men and women. Compared with heterosexual men, the risk of developing NHL was 30% higher among MSM (aHR 1.30, 95% CI 1.14–1.48), and it was 34% lower in women (aHR 0.66, 95% CI 0.57–0.78). The effect of exposure group on the risk of developing NHL did not differ across Europe, North and Latin America (p=0.330). NHL rates were similar in PWID compared with persons who did not inject drugs in crude (HR 0.98; 95% CI 0.84–1.16) and adjusted analyses (aHR 0.94, 95% CI 0.79–1.12). The effect of injection drug use on NHL rates did not vary by region (p=0.121).
Table 3:
Crude and adjusted hazard ratios for the effect of exposure group and drug use on the risk of developing NHL in adults who started ART, restricted to North America, Latin America, and Europe.
| Crude | Adjusted* | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Exposure group | ||
| Women | 0.52 (0.44 – 0.61) | 0.66 (0.57 – 0.78) |
| Heterosexual men | 1.00 | 1.00 |
| MSM | 0.95 (0.84 – 1.07) | 1.30 (1.14 – 1.48) |
| PWID | ||
| No | 1.00 | 1.00 |
| Yes | 0.98 (0.84 – 1.16) | 0.94 (0.79 – 1.12) |
Adjusted for region, age, calendar year of ART start, first-line ART regimen, current CD4 cell count, and exposure group or PWID, respectively (model 2)
ART, antiretroviral therapy; CI, confidence interval; HR, hazard ratio; MSM, men who have sex with men; NHL, non-Hodgkin lymphoma; PWID, people who inject drugs.
Comparison of NHL rates across geographic regions
The risk of developing NHL was higher in South African women than in European women (aHR 1.79, 95% CI 1.19–2.70, at 2 years after starting ART). In male adults, NHL rates were similar in South Africa and Europe. In crude analyses excluding South Africa, NHL rates at 2 years after ART start were higher in North America than in Europe across all exposure groups, see Table 4. However, after adjustment for HIV-related risk factors – in particular current CD4 cell count – NHL rates among North American women (aHR 0.97, 95% CI 0.64–1.49), heterosexual men (aHR 1.26, 95% CI 0.92–1.74) and MSM (aHR 1.20, 95% CI 0.96–1.49) became more comparable to their European counterparts’ rates. In Latin American women, NHL rates at 2 years after starting ART were lower than in European women (aHR 0.26, 95% CI 0.09–0.77). Of note, this estimate was based on only four incident NHL cases in Latin American women. Among heterosexual men (aHR 0.72, 95% CI 0.41–1.27) and MSM (aHR 0.84, 95% CI 0.55–1.27) in Latin America, NHL rates were similar to their European counterparts’ rates.
Table 4: Comparison of NHL rates between different regions and Europe:
Crude and adjusted HRs for being diagnosed with NHL at 2 years after ART start in different population groups.
| Women1 | All men1 | Heterosexual men2 | MSM2 | |||||
|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | Adjusted HR* (95% CI) | Crude HR (95% CI) | Adjusted HR* (95% CI) | Crude HR (95% CI) | Adjusted HR** (95% CI) | Crude HR (95% CI) | Adjusted HR** (95% CI) | |
| Region | ||||||||
| Europe | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| North America | 1.66 (1.14 – 2.41) | 0.97 (0.64 – 1.49) | 1.74 (1.45 – 2.08) | 1.10 (0.85–1.43) | 1.74 (1.26–2.40) | 1.26 (0.92–1.74) | 1.72 (1.38–2.14) | 1.20 (0.96–1.49) |
| Latin America | 0.36 (0.13 – 0.98) | 0.26 (0.09 – 0.77) | 0.84 (0.59 – 1.22) | 0.64 (0.38 – 1.09) | 0.80 (0.45–1.43) | 0.72 (0.41–1.27) | 1.03 (0.68–1.57) | 0.84 (0.55–1.27) |
| South Africa | 1.48 (1.03 – 2.14) | 1.79 (1.19 – 2.70) | 0.80 (0.51 – 1.25) | 0.94 (0.56 – 1.58) | − | − | − | − |
From models including the variable “sex” and its interaction with region.
From models including the variable “exposure group” and its interaction with region.
Adjusted for age at ART start, calendar period of ART start, first-line ART regimen, current CD4 cell count and its interaction with region, and sex and its interaction with region (model 1).
Adjusted for age at ART start, calendar period of ART start, first-line ART regimen, current CD4 cell count, drug use, and exposure group and its interaction with region (model 3).
ART, antiretroviral therapy; CI, confidence interval; HR, hazard ratio; NHL, non-Hodgkin lymphoma.
Sensitivity analyses
When we excluded NHL cases diagnosed within the first six months after ART start in sensitivity analyses, raw NHL incidence rates decreased (raw overall NHL incidence rate: 100/100,000 pys, 95% CI 94–106, Supplementary Table S5), but the risk factor analyses (Supplementary Tables S6 and S7) and the regional comparison of NHL rates (Supplementary Table S8) remained similar. When comparing NHL rates at 5 years (instead of 2 years) after ART start, the regional NHL incidence pattern did not change much. Women in South Africa still had considerably higher NHL rates than European women (aHR 2.30, 95% CI 1.34–3.95). After adjustment for HIV-related risk factors, NHL rates in all population groups in North America became more similar to their European counterparts’ rates, but they remained elevated (Supplementary Table S9).
Discussion
The overall NHL incidence rate in adults who had started ART was 142/100,000 pys. This exceeds the NHL incidence rates of up to 10/100,000 pys reported for a comparable age group (40–44 years) from the general population in the regions included in our analysis [19]. After adjustment for HIV-related risk factors, NHL rates after starting ART were similar among men in all regions. In contrast, NHL rates were higher in South African than in European women. Across Europe, Latin and North America, the risk of developing NHL was highest in MSM, followed by heterosexual men, and women. In South Africa, NHL rates were similar in men and women. With increasing current CD4 cell counts, the risk of developing NHL decreased among adults in Europe, Latin and North America. However, we did not find an association between current CD4 cell count and the risk of developing NHL in South African adults.
Our analysis is based on a large dataset of adults living with HIV in five continents who had started ART, and it is the first analysis providing a direct comparison of NHL rates in adults living with HIV across geographic regions. We restricted our analysis to adults who had started ART without prior NHL, and we adjusted for current CD4 cell count to account for regional differences in ART provision. The age structures of the included adult populations were similar across regions. We accounted for remaining differences by including age at ART start in the adjusted models. However, NHL ascertainment was not standardized across regions with some cohorts using record linkages with cancer registries and others reviewing medical charts and pathology reports to complement their routine data collection. Variation in the completeness of NHL ascertainment across regions might, therefore, have contributed to regional differences in NHL rates. Information on NHL subtypes was unavailable for most regions and subtype-specific analyses were not possible. However, without standardized protocols for histopathological assessments of NHL across regions, subtype-specific analyses might also be of limited value. For South Africa, reliable cancer data were only available for two urban cohorts that had improved their cancer case recording through record linkages with the National Cancer Registry [20]. These data might not be representative for rural areas of South Africa and for other countries in the Southern African region. We did not take into account ART interruptions and terminations, and not all included adults who had started ART will have remained on ART. Therefore, our NHL incidence rate estimates are not necessarily representative for adults who stayed on ART continuously. HIV RNA data were largely missing in some regions and could not be used to assess ART effectiveness. Data on MSM and PWID were not available for South Africa, but in this region, HIV is mainly spread through heterosexual and vertical transmission. Comprehensive data on ethnicity or region of origin were only available for North America or Europe, respectively, and could not be included in our multiregional analyses. Information on other NHL risk factors including time from HIV infection to ART start and sero-status for potentially relevant co-infections such as EBV, HBV, HCV, and HHV-8 were also mostly missing.
Our study confirms that adults who have started ART remain at considerable risk of developing NHL. The overall incidence rate was 142/100,000 pys which is comparable to incidence rates reported from participating [1,2,4,20–22] and other study groups [5,23–25]. We and others have shown that NHL incidence rates are highest within the first six months after starting ART [1], which might be explained by more frequent NHL diagnoses in this time window due to a closer examination of adults at ART start. Other studies have suggested that immune reconstitution inflammatory syndrome may contribute to the increased number of NHL cases in the first months of ART [26,27]. Our study showed that in most regions men had higher NHL rates than women, which is line with findings from the general population [19] and previous studies in HIV-positive populations [1,2,22,28]. In our study, NHL rates in South Africa were about the same in men and women. Previous studies in this region had not assessed the effect of sex on the risk of developing NHL [20,29]. Across all regions where data on exposure group were available, MSM were at highest risk of developing NHL followed by heterosexual men and women. Similar differences in NHL rates by exposure group have been described before in the Swiss HIV Cohort Study [30], the French Hospital Database on HIV [22] and the COHERE in EuroCoord collaboration [2] – data sources also included in the current analysis. We and others [1,2,22,30] did not observe increased NHL rates in PWID. In line with previous studies, we found that across all regions NHL rates increased with age [1,2,22,28] and declined over calendar periods [1,3–6,31]. As previously reported [2,22,26,28,30,32,33,34], we also observed that in most regions the risk of developing NHL decreased with increasing CD4 cell counts. The lack of an association between current CD4 cell count and NHL rates in South Africa was mainly driven by a small number of NHL cases among adults with low CD4 cell counts in that region. This might be due to selection and detection biases. In settings with limited resources, adults with very low CD4 cell counts might die before an NHL diagnosis can be made. In addition, NHL often presents with non-specific symptoms such as weight loss and night sweats, which might be misdiagnosed as tuberculosis, especially in regions where tuberculosis prevalence is high [35,36].
HIV-associated immunosuppression and co-infection with oncogenic viruses play an important role in the pathogenesis of HIV-related NHL [11]. Early access to HIV care and maintenance of high CD4 cell counts are, therefore, key measures to lower the risk of developing NHL [22,23,37]. In our study, population group specific NHL rates were mostly similar across geographic regions when taking into account differences in HIV-related risk factors in these populations. For example, in crude analyses, NHL rates in all population groups were higher in North America than in Europe, but the rates became similar when we adjusted for age at ART start, first-line regimen, calendar period of ART start, and in particular current CD4 cell counts. However, also in adjusted analyses, MSM had higher NHL rates than heterosexual men and women living with HIV. Furthermore, South African women had a higher risk of developing NHL than European women. It remains unclear why the risk of developing NHL is elevated among those population groups. More frequent clinical assessment in MSM compared with other population groups could lead to a detection bias of NHL in MSM. However, a previous study observed the association between MSM and higher cancer risk specifically for NHL, KS, and anal cancer, but not for other cancers [22]. Co-infection patterns might also contribute to the increased NHL rates in MSM and South African women. Both South African women and MSM are at high risk of co-infection with HHV-8 [14], which has been associated with an increased NHL risk. Yet, HHV-8 has only been associated with two NHL subtypes, i.e. primary effusion lymphoma (PEL) and HHV-8-associated diffuse large B-cell lymphoma, a form of multicentric Castleman’s disease [38], and these NHL subtypes are rare entities. For example, PEL represents <5% of all NHLs in HIV-positive populations [39,40]. Therefore, these HHV-8-related NHL subtypes are unlikely to fully explain the increased NHL risk observed among MSM and South African women. HCV has been associated with NHL development [12,13], and its prevalence is increasing in MSM [16], but we did not find increased NHL rates among PWID, the population group at highest risk for HCV co-infection. The role of co-infections in lymphomagenesis is complex and incompletely understood. Dedicated studies including information on co-infection status of adults living with HIV are needed to clarify whether the increased NHL risk we observed among MSM and South African women can be explained by underlying co-infection patterns.
Conclusion
A better understanding of lymphomagenesis and associated etiologic factors is needed to eventually be able to develop specific preventive measures against NHL in adults living with HIV. In the meantime, early access to ART and regular patient monitoring to avert low current CD4 cell counts remain key for NHL prevention.
Supplementary Material
Acknowledgements
We thank all patients, care providers and data managers in the different IeDEA regions and COHERE in EuroCoord. More detailed acknowledgements concerning the participating consortia can be found in the supplementary material.
Funding
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), and the National Cancer Institute (NCI) of the U.S. National Institutes of Health (NIH) under Award Number U01AI069924 (Southern Africa), U01AI069907 (Asia-Pacific), U01AI069923 (Caribbean, Central, and South America), U01-AI069918 (North America), and U01A1096186 (the IeDEA Network Coordinating Center at Vanderbilt). The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) was also supported by NIH grants F31DA037788, G12MD007583, K01AI093197, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, M01RR000052, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01CA165937, R01DA011602, R01DA012568, R24AI067039, U01AA013566, U01AA020790, U01AI031834, U01AI034989, U01AI034993, U01AI034994, U01AI035004, U01AI035039, U01AI035040, U01AI035041, U01AI035042, U01AI037613, U01AI037984, U01AI038855, U01AI038858, U01AI042590, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01AI103390, U01AI103397, U01AI103401, U01AI103408, U01DA03629, U01DA036935, U01HD032632, U10EY008057, U10EY008052, U10EY008067, U24AA020794,U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR000454, UM1AI035043, Z01CP010214, and Z01CP010176; contracts CDC-200-2006-18797 and CDC-200-2015-63931 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; grants CBR-86906, CBR-94036, HCP-97105, and TGF-96118 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. Additional support was provided by the National Cancer Institute, National Institute for Mental Health and National Institute on Drug Abuse. The TREAT Asia HIV Observational Database (TAHOD) and the Australian HIV Observational Database (AHOD) are initiatives of TREAT Asia, a program of amfAR, The Foundation for AIDS Research. The AHOD is also funded by unconditional grants from Merck Sharp & Dohme, Gilead Sciences, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen-Cilag, ViiV Healthcare. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia. The COHERE study group has received unrestricted funding from: Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS), France; HIV Monitoring Foundation, The Netherlands; and the Augustinus Foundation, Denmark. The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under EuroCoord grant agreement no. 260694. A list of the funders of the participating cohorts can be found at www.COHERE.org. JMM received a personal 80:20 research grant from the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain during 2017–19. This study was also made possible by the generous support of the American people through the United States Agency for International Development (INROADS USAID-674-A-12–00029), and by a grant from the Swiss National Science Foundation (Ambizione-PROSPER PZ00P3_160407 to JB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Writing group: Eliane Rohner1, Lukas Bütikofer2, Kurt Schmidlin1, Mazvita Sengayi3, Mhairi Maskew4, Janet Giddy5, Richard D. Moore6, James J. Goedert7, M. John Gill8, Michael J. Silverberg9, Pragna Patel10, Jessica Castilho11, Jennifer Hoy12, Annette Sohn13, Firouze Bani-Sadr14, Ninon Taylor15, Vassilios Paparizos16, Vincent Le Moing17, Fabrice Bonnet18,19, Annelies Verbon20, Jörg Janne Vehreschild21,22, Frank A. Post23, Caroline Sabin24, Amanda Mocroft24, Fernando Dronda25, Niels Obel26, Sophie Grabar27,28, Vincenzo Spagnuolo29, Andrea Antinori30, Eugenia Quiros-Roldan31, Cristina Mussini32, José M. Miro33, Laurence Meyer34,35, Barbara Hasse36, Deborah Konopnicki37, Bernardino Roca38, Francois Boué39,40, Diana Barger19,41, Dorthe Raben42, Gary M. Clifford43, Silvia Franceschi44, Norbert Brockmeyer45, Matthias Egger1,46, Julia Bohlius1
Affiliations: 1IInstitute of Social and Preventive Medicine, University of Bern, Switzerland; 2CTU Bern, University of Bern, Switzerland; 3National Cancer Registry, National Health Laboratory Service, Johannesburg, South Africa; 4Health Economics and Epidemiology Research Office, Department of Internal Medicine, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; 5Department of Medicine, McCord Hospital, Durban, South Africa; 6Johns Hopkins University, School of Medicine, Baltimore, Maryland; 7Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland; 8University of Calgary, Alberta, Canada; 9Division of Research, Kaiser Permanente Northern California, Oakland, USA; 10Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; 11Vanderbilt University Medical Center, Nashville, TN, USA; 12Department of Infectious Diseases, Alfred Hospital and Monash University, Melbourne, Victoria, Australia; 13 TREAT Asia/amfAR - The Foundation for AIDS Research, Bangkok, Thailand; 14Reims Champagne-Ardenne University, Faculté de médecine, CHU Reims, Hôpital Robert Debré, Tropical and Infectious Diseases, Reims, France; 15IIIrd Medical Department with Haematology, Medical Oncology, Haemostaseology, Infectious Diseases and Rheumathology, Oncologic Center, Paracelsus Medical University, Salzburg, Austria, Present address: Department of Dermatology, University Hospital Salzburg, Paracelsus Medical University, Salzburg, Austria; 16AIDS Unit, Clinic of Venereologic and Dermatologic Diseases, Athens Medical School, “Syngros” Hospital, Athens, Greece; 17Montpellier University, Montpellier, France; 18CHU de Bordeaux, Service de Médecine Interne et Maladies Infectieuses, Hôpital Saint-André, Bordeaux, France; 19INSERM, ISPED, Centre INSERM U1219-Bordeaux Population Health, F-33000 Bordeaux, France; 20Department Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands; 21Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany; 22German Centre for Infection Research, partner site Bonn-Cologne, Cologne, Germany; 23King's College Hospital NHS Foundation Trust, London, UK; 24Institute for Global Health, UCL, London, United Kingdom; 25Department of Infectious Diseases, Hospital Ramón y Cajal, Madrid, Spain; 26Department of Infectious Diseases, Copenhagen University Hospital, Copenhagen, Denmark; 27Sorbonne Université, INSERM, UMR_S 1136, Institut Pierre Louis d’Epidémiologie et de Santé Publique (IPLESP) Paris, France; 28Université Paris Descartes, Sorbonne Paris Cité, Assistance Publique Hôpitaux de Paris (AP-HP), Hôpitaux Universitaire Paris Centre, Unité de Biostatistique et d’Epidémiologie, Paris, France; 29Department of Infectious Diseases, San Raffaele Scientific Institute, Milan, Italy; 30INMI ‘L. Spallanzani’, Rome, Italy; 31Infectious and Tropical Diseases Institute, University of Brescia, Brescia, Italy; 32Infectious Diseases Clinics, University Hospital, Modena, Italy; 33Infectious Diseases Service, Hospital Clinic – IDIBAPS, University of Barcelona, Barcelona, Spain; 34INSERM, U1018, Epidemiology of HIV, Reproduction, Paediatrics, CESP, University Paris-Sud, Paris, France; 35Department of Public Health and Epidemiology, Bicêtre Hospital, AP-HP, Le Kremlin Bicêtre, Paris, France; 36Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Switzerland; 37Department of Infectious Diseases, St Pierre University Hospital, Université Libre de Bruxelles, Brussels, Belgium; 38Hospital General Universitario, Castellón, Spain; 39Université Paris Sud, Paris, France; 40Service Médecine interne et immunologie, AP-HP, Groupe Hospitalier Paris Sud, Hôpital Antoine-Béclère, Clamart, France; 41Univ. Bordeaux, ISPED, Centre INSERM U1219-Bordeaux Population Health, F-33000 Bordeaux, France; 42CHIP, Department of Infectious Diseases, Rigshospitalet, Copenhagen, Denmark; 43International Agency for Research on Cancer, Lyon, France; 44IRCCS CRO Centro di Riferimento Oncologico, Aviano, Italy; 45Department of Dermatology, Venerology and Allergology, Center for Sexual Health and Medicine, St. Josef Hospital, Ruhr-Universität Bochum, Bochum, Germany; 46Centre for Infectious Disease Epidemiology and Research, University of Cape Town, Cape Town, South Africa.
References
- 1.Polesel J, Clifford GM, Rickenbach M, Dal Maso L, Battegay M, Bouchardy C, et al. Non-Hodgkin lymphoma incidence in the Swiss HIV Cohort Study before and after highly active antiretroviral therapy. AIDS 2008; 22:301–306. [DOI] [PubMed] [Google Scholar]
- 2.Bohlius J, Schmidlin K, Costagliola D, Fätkenheuer G, May M, Caro-Murillo A, et al. Incidence and risk factors of HIV-related non-Hodgkin’s lymphoma in the era of combination antiretroviral therapy: a European multicohort study. Antivir Ther 2009; 14:1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschi S, Lise M, Clifford GM, Rickenbach M, Levi F, Maspoli M, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer 2010; 103:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverberg MJ, Lau B, Achenbach CJ, Jing Y, Althoff KN, D’Souza G, et al. Cumulative Incidence of Cancer Among Persons With HIV in North America. Ann Intern Med 2015; 163:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park LS, Tate JP, Sigel K, Rimland D, Crothers K, Gibert C, et al. Time trends in cancer incidence in persons living with HIV/AIDS in the antiretroviral therapy era. AIDS 2016; 30:1795–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS 2014; 28:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst 2015; 107. doi: 10.1093/jnci/dju503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet F, Burty C, Lewden C, Costagliola D, May T, Bouteloup V, et al. Changes in cancer mortality among HIV-infected patients: the Mortalité 2005 Survey. Clin Infect Dis 2009; 48:633–9. [DOI] [PubMed] [Google Scholar]
- 9.Engels EA, Yanik EL, Wheeler W, Gill MJ, Shiels MS, Dubrow R, et al. Cancer-Attributable Mortality Among People With Treated Human Immunodeficiency Virus Infection in North America. Clin Infect Dis 2017; 65:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morlat P, Roussillon C, Henard S, Salmon D, Bonnet F, Cacoub P, et al. Causes of death among HIV-infected patients in France in 2010 (national survey). AIDS 2014; 28:1181–1191. [DOI] [PubMed] [Google Scholar]
- 11.Rubinstein PG, Aboulafia DM, Zloza A. Malignancies in HIV/AIDS. AIDS 2014; 28:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100B. International Agency for Research on Cancer; 2012. [Google Scholar]
- 13.Wang Q, De Luca A, Smith C, Zangerle R, Sambatakou H, Bonnet F, et al. Chronic Hepatitis B and C Virus Infection and Risk for Non-Hodgkin Lymphoma in HIV-Infected Patients: A Cohort Study. Ann Intern Med 2017; 166:9–17. [DOI] [PubMed] [Google Scholar]
- 14.Rohner E, Wyss N, Heg Z, Faralli Z, Mbulaiteye SM, Novak U, et al. HIV and human herpesvirus 8 co-infection across the globe: Systematic review and meta-analysis. Int J Cancer 2016; 138:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 16.Taylor LE, Swan T, Mayer KH. HIV Coinfection With Hepatitis C Virus: Evolving Epidemiology and Treatment Paradigms. Clin Infect Dis 2012; 55:S33–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderegg N, Panayidou K, Abo Y, Alejos B, Althoff KN, Anastos K, et al. Global Trends in CD4 Cell Count at the Start of Antiretroviral Therapy: Collaborative Study of Treatment Programs. Clin Infect Dis 2018; 66:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Royston P, Lambert P. Flexible parametric survival analysis using Stata: beyond the Cox model (ed 1). College Station, TX: StataCorp, 2011. [Google Scholar]
- 19.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on 24/05/2018. [Google Scholar]
- 20.Sengayi M, Spoerri A, Egger M, Kielkowski D, Crankshaw T, Cloete C, et al. Record linkage to correct under-ascertainment of cancers in HIV cohorts: The Sinikithemba HIV clinic linkage project. Int J Cancer 2016; 139:1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink VI, Shepherd BE, Cesar C, Krolewiecki A, Wehbe F, Cortés CP, et al. Cancer in HIV-infected persons from the Caribbean, Central and South America. J Acquir Immune Defic Syndr 2011; 56:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol 2009; 10:1152–1159. [DOI] [PubMed] [Google Scholar]
- 23.Achenbach CJ, Buchanan AL, Cole SR, Hou L, Mugavero MJ, Crane HM, et al. HIV Viremia and Incidence of Non-Hodgkin Lymphoma in Patients Successfully Treated With Antiretroviral Therapy. Clin Infect Dis 2014; 58:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson TM, Morton LM, Shiels MS, Clarke CA, Engels EA. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. AIDS 2014; 28:2313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanik EL, Achenbach CJ, Gopal S, Coghill AE, Cole SR, Eron JJ, et al. Changes in Clinical Context for Kaposi’s Sarcoma and Non-Hodgkin Lymphoma Among People With HIV Infection in the United States. J Clin Oncol 2016; 34:3276–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gopal S, Patel MR, Achenbach CJ, Yanik EL, Cole SR, Napravnik S, et al. Lymphoma immune reconstitution inflammatory syndrome in the center for AIDS research network of integrated clinical systems cohort. Clin Infect Dis 2014; 59:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaffe HW, De Stavola BL, Carpenter LM, Porter K, Cox DR, CASCADE Collaboration. Immune reconstitution and risk of Kaposi sarcoma and non-Hodgkin lymphoma in HIV-infected adults. AIDS 2011; 25:1395–403. [DOI] [PubMed] [Google Scholar]
- 28.Zoufaly A, Stellbrink HJ, an der Heiden M, Kollan C, Hoffmann C, Van Lunzen J, ClinSurv Study Group. Cumulative HIV Viremia during Highly Active Antiretroviral Therapy Is a Strong Predictor of AIDS-Related Lymphoma. J Infect Dis 2009; 200:79–87. [DOI] [PubMed] [Google Scholar]
- 29.Mbulaiteye SM, Katabira ET, Wabinga H, Parkin DM, Virgo P, Ochai R, et al. Spectrum of cancers among HIV-infected persons in Africa: the Uganda AIDS-Cancer Registry Match Study. Int J Cancer 2006; 118:985–90. [DOI] [PubMed] [Google Scholar]
- 30.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst 2005; 97:425–32. [DOI] [PubMed] [Google Scholar]
- 31.Hleyhel M, Belot A, Bouvier AM, Tattevin P, Pacanowski J, Genet P, et al. Risk of AIDS-defining cancers among HIV-1-infected patients in France between 1992 and 2009: Results from the FHDH-ANRS CO4 cohort. Clin Infect Dis 2013; 57:1638–1647. [DOI] [PubMed] [Google Scholar]
- 32.Silverberg MJ, Chao C, Leyden WA, Xu L, Horberg MA, Klein D, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev 2011; 20:2551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petoumenos K, van Leuwen M, Vajdic C, Woolley I, Chuah J, Templeton D, et al. Cancer, immunodeficiency and antiretroviral treatment: results from the Australian HIV Observational Database (AHOD). HIV Med 2013; 14:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shepherd L, Ryom L, Law M, Hatleberg CI, de Wit S, Monforte AD, et al. Differences in Virological and Immunological Risk Factors for Non-Hodgkin and Hodgkin Lymphoma. J Natl Cancer Inst. 2018; 110:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buyego P, Nakiyingi L, Ddungu H, Walimbwa S, Nalwanga D, Reynolds SJ, et al. Possible misdiagnosis of HIV associated lymphoma as tuberculosis among patients attending Uganda Cancer Institute. AIDS Res Ther 2017; 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 2015; 29:1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanik EL, Napravnik S, Cole SR, Achenbach CJ, Gopal S, Olshan A, et al. Incidence and timing of cancer in HIV-infected individuals following initiation of combination antiretroviral therapy. Clin Infect Dis 2013; 57:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonçalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS 2017; 31:1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olszewski AJ, Fallah J, Castillo JJ. Human immunodeficiency virus-associated lymphomas in the antiretroviral therapy era: Analysis of the National Cancer Data Base. Cancer 2016; 122:2689–2697. [DOI] [PubMed] [Google Scholar]
- 40.Riedel DJ, Rositch AF, Redfield RR, Blattner WA. HIV-associated lymphoma sub-type distribution, immunophenotypes and survival in an urban clinic population. Leuk Lymphoma 2016; 57:306–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.