Abstract
The C11–N26 fragment of griseoviridin has been prepared enantioselectively. The 1,3-syn diol synthon was derived from lipase-catalyzed selective acylation of(±)-1-(benzyloxy)pent-4-en-2-ol. The conjugated (E,E)-dienylmethyl azide functionality was installed by a Pd-catalyzed allylic azidation reaction.
Keywords: alkenes, azides, antibiotics, catalysis, palladium
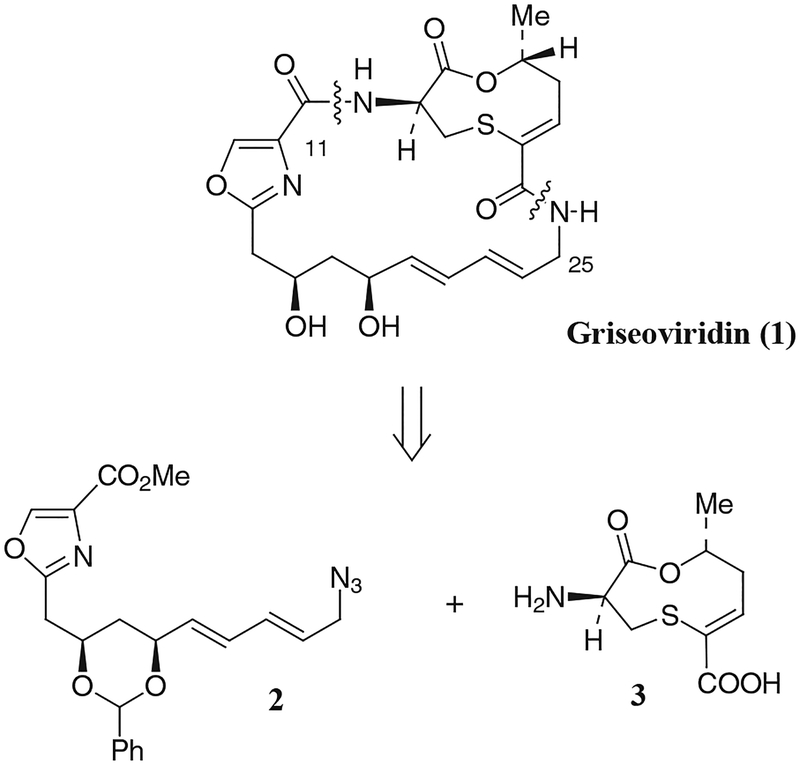
Griseoviridin (1), a member of the streptogramin family of antibiotics, is a broad-spectrum antibiotic produced by a strain of Streptomyces griseus.1 It has exhibited in vitro inhibitory properties against various pathogenic bacteria and fungi. Griseoviridin (1) inhibits protein synthesis by interfering with bacterial ribosomal function.2 The structure of 1 and its absolute configuration have been determined by X-ray crystallography.3 The unique structural features of griseoviridin (1) and its biological properties have attracted much synthetic attention. Retrosynthetically, griseoviridin can be assembled in a convergent manner from two appropriately protected fragments: a C11–N26 dienyl azide fragment 2 and a nine-membered macrocycle 3 (Figure). To date, the only total synthesis of griseoviridin (1) has been recently achieved by Meyers.4 Several approaches for the synthesis of the oxazole or macrocyclic fragments of 1 have also been published over the years.5 Herein we report an enantioselective synthesis of the protected C11–N26 fragment 2 of griseoviridin (1) utilizing an optically active 1,3-syn diol synthon derived from lipase-catalyzed selective acylation of (±)-1-benzyloxypent-4-en-2-ol. A Pd-catalyzed allylic azidation has been utilized to install the conjugated (E,E)-dienylmethyl azide functionality selectively.
Figure.
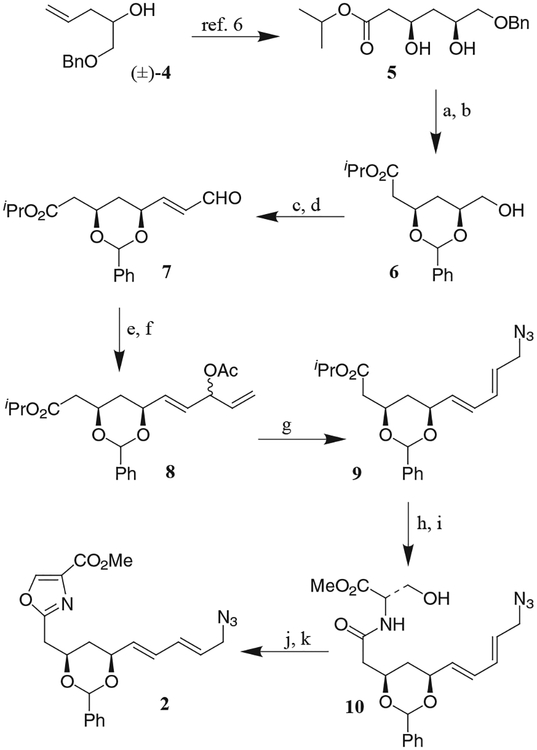
The synthesis of C11–N26 fragment 2 is illustrated in the Scheme. The known optically active diol 5 was prepared in multigram quantities by enzymatic acylation of (±)-1-benzyloxypent-4-en-2-ol as described previously.6 Removal of the benzyl group by catalytic hydrogenation of 5 over Pearlman’s catalyst provided a triol which was reacted with dimethylbenzylidene acetal in CH2Cl2 in the presence of p-TsOH at 23 °C to afford benzylidene derivative 6 in 83% yield over 2 steps. Swern oxidation of 6 followed by Wittig olefination of the resulting aldehyde with (triphenylphosphoranylidene)acetaldehyde in benzene at reflux provided a mixture of α, β-unsaturated aldehyde 7 being the major isomer (E/Z ratio of 12:1 by 1H and 13C NMR analysis). To install the conjugated primary (E,E)-dienylmethyl azide we have elected to carry out a palladium-catalyzed azidation of the corresponding allyl ester. While Murahashi and co-workers have extensively investigated Pd(0)-catalyzed azidation of allyl esters to primary allyl azides, selective formation of dienylmethyl azides from the corresponding diene acetates has not been examined.7 Such conjugated allylamines are structural features of many biologically important natural products.8 Thus, treatment of 7 with vinylmagnesium bromide in diethyl ether at 0 °C provided the allylic alcohol which was acetylated with acetic anhydride and triethylamine in the presence of a catalytic amount of DMAP to furnish acetate derivative 8 as a 1:1 mixture in 74% yield for two steps. Exposure of 8 to NaN3 in a mixture (1:1) of THF and water at 65 °C in the presence of a catalytic amount (5 mol%) of Pd(PPh3)4 afforded (E,E)-dienylmethyl azide 9 exclusively in 81% isolated yield. The corresponding reaction with Pd(OAc)2 and PBu3-derived catalyst was less effective forming azide 9 in only 71% yield. The configuration of the newly formed double bond was confirmed to be E by a proton decoupling experiment which showed the coupling constant of the olefinic protons to be 14.7 Hz. To elaborate the oxazole functionality, the isopropyl ester (9) was saponified by treatment with aqueous sodium hydroxide in methanol at 23 °C. The resulting acid was coupled with L-serine methyl ester in the presence of isobutyl chloroformate and N-methylmorpholine to afford amide 10 in 80% yield for two steps. The serine amide 10 was converted to the corresponding oxazoline by treatment with Burgess’ salt in THF at reflux for 6 hours.9 Subsequent oxidation of the oxazoline with bromotrichloromethane and 1,8-diazabicyclo[5.4.0]undec-7-ene as described by Williams furnished 2 in 62% yield for two steps.10
Scheme.
(a) H2, Pd(OH)2, EtOAc, 91%; (b) PhCH(OMe)2, p-TsOH, CH2Cl2, 91%; (c) (COCl)2, DMSO, Et3N, CH2Cl2, −78 °C; (d) Ph3P=CHCHO, benzene, reflux, 70% for 2 steps; (e) CH2=CHMgBr, Et2O, 0 °C; (f) Ac2O, Et3N, DMAP, CH2Cl2, 74% for 2 steps; (g) Pd(PPh)4, NaN3, THF–H2O, 65 °C, 81%; (h) NaOH, MeOH–H2O; (i) N-methylmorpholine, isobutyl chloroformate, serine methyl ester · HCl, THF, −30 to +23 °C, 80% 2 for steps; (j) Burgess salt, THF, reflux; (k) BrCCl3, DBU, CH2Cl2, 0 to 23 °C, 62% for 2 steps
In conclusion, an enantioselective synthesis of the C11–N26 fragment of griseoviridin (1) has been achieved. Efficient installation of the conjugated (E,E)-dienylmethyl azide functionality by a Pd-catalyzed allylic azidation is noteworthy.
All moisture sensitive reactions were carried out under N2. Anhyd solvents were obtained as follows: THF, distilled from sodium and benzophenone; CH2Cl2, distilled from P2O5; Et3N, distilled from CaH2. All other solvents were HPLC grade. Column chromatography was performed with Whatman 240–400 mesh silica gel under low pressure of 5–10 psi. TLC was carried out with E.Merck silica gel 60-F254 plates. 1H and 13C NMR spectra were recorded on Bruker AM 400 (400 MHz), Avance 400 (400 MHz) and Avance 500 (500 MHz) spectrometers.
(4S,6R)-6-Isopropyloxycarbonylmethyl-4-hydroxymethyl-2-phenyl-1,3-dioxane (6)
To a stirred solution of 5 (804 mg, 2.7 mmol) in EtOAc (30 mL) and MeOH (15 mL) was added Pd(OH)2 (161 mg). The resulting solution was placed under a H2 balloon and stirred at 23 °C for 2 h. The mixture was filtered and the filtrate was evaporated under reduced pressure to provide the crude triol (519 mg, 91%), which was used directly for the next step without further purification.
1H NMR (CDCl3): δ = 5.01–5.07 (m, 1 H), 4.29 (m, 1 H), 3.98 (m, 1 H), 3.63 (dd, 1 H, J = 3.5, 11.2 Hz), 3.49 (dd, 1 H, J = 6, 11.2 Hz), 3.30 (br, 3 H), 2.45 (d, 2 H, J =6 Hz), 1.55–1.72 (m, 2 H), 1.24 (d, 6 H, J = 6 Hz).
HRMS (EI): m/z calcd for C9H19O5 (M + H+) 207.1232, found (M + H+) 207.1236.
To a stirred solution of the above triol (992 mg, 4.81 mmol) and benzaldehyde dimethyl acetal (1.8 mL, 12 mmol) in CH2Cl2 (40 mL) was added p-TsOH (46 mg, 0.24 mmol). The resulting mixture was stirred at r.t. for 1 h and was quenched by sat. aq NaHCO3. The layers were separated and the aqueous layer was extracted with CH2Cl2 (2×30 mL). The combined organic layers were washed with brine and dried (Na2SO4). Evaporation of the solvent under reduced pressure provided a residue which was purified by column chromatography on silica gel (30% EtOAc in hexanes) to afford the alcohol 6 (1.28 g, 91%); [α]D23−3.64 (c = 2.75, CHCl3).
IR (film): 3450, 2979, 2924, 1729, 1106, 1023 cm−1.
1H NMR (400 MHz, CDCl3):δ = 7.47–7.50 (m, 2 H), 7.34–7.36 (m, 3 H), 5.59 (s, 1 H), 5.04 (m, 1 H), 4.35 (m, 1 H), 4.03 (m, 1 H), 3.60–3.74 (m, 2 H), 2.70 (dd, 1 H, J = 7, 15.6 Hz), 2.50 (dd, 1 H, J = 6, 15.6 Hz), 2.11 (br, 1 H), 1.51–1.66 (m, 2 H), 1.23 (d, 6 H, J = 6.6 Hz).
13C NMR (100 MHz, CDCl3): δ = 170.1, 138.0, 128.8, 128.1, 126.1, 100.5, 77.4, 72.9, 68.0, 65.3, 41.2, 31.9, 21.7.
HRMS (EI): m/z calcd for C16H22 O5Na (M + Na+) 317.1365, found (M + Na+) 317.1382.
(4S,6R)-6-Isopropyloxycarbonylmethyl-2-phenyl-4-[(E)-3-oxoprop-1-enyl]-1,3-dioxane (7)
Oxalyl chloride (0.2 mL, 2.2 mmol) was added to CH2Cl2 (30 mL) with stirring at −78 °C. The resulting mixture was stirred for 5 min and DMSO (0.3 mL, 4.28 mmol) was added. After 15 min, alcohol 6 (210 mg, 0.71 mmol) in CH2Cl2 (5 mL) was added dropwise. The resulting mixture was stirred at −78 °C for 30 min and Et3N (1 mL, 7.1 mmol) was added. The solution was warmed to 23 °C and quenched with aq sat. NH4Cl solution. The layers were separated and the aqueous layer was extracted with CH2Cl2 (2 × 15 mL). The combined organic layers were washed with brine, dried (Na2SO4) and evaporated under reduced pressure to provide the crude aldehyde, which was used directly for next step without further purification. The crude aldehyde and (triphenylphosphoranylidene)-acetaldehyde (216.1 mg, 0.71 mmol) were dissolved in CHCl3 and heated to reflux for 15 h. The resulting mixture was cooled to r.t. and the solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel (30% EtOAc in hexanes) to provide aldehyde 7 (156 mg, 70%); [α]D23−13.2 (c = 1.0, CHCl3).
IR (film): 2980, 1728, 1691, 1375, 1147, 1109, 1017 cm−1.
1H NMR (400 MHz, CDCl3): δ = 9.58, (d, 1 H, J = 8 Hz), 7.49–7.51 (m, 2 H), 7.34–7.38 (m, 3 H), 6.81 (dd, 1 H, J = 4.1, 16 Hz), 6.40 (ddd, 1 H, J = 1.6, 7.9, 16 Hz), 5.67 (s, 1 H), 5.04 (m, 1 H), 4.70 (m, 1 H), 4.40 (m, 1 H), 2.72 (dd, 1 H, J = 7, 15.7 Hz), 2.52 (dd, 1 H, J = 6.4, 15.7 Hz), 1.93 (m, 1 H), 1.60 (m, 1 H), 1.24 (d, 6 H, J = 6.3 Hz).
13C NMR (100 MHz, CDCl3): δ= 193.3, 169.9, 153.9, 137.6, 131.1, 129.0, 128.3, 120.0, 100.6, 74.8, 73.2, 68.2, 40.9, 35.4, 21.8
HRMS (EI): m/z calcd for C18H22O5Na (M + Na+) 341.1365, found (M + Na+) 341.1357.
(4S,6R)-4-[(1E)-3-Acetyloxypenta-1,4-dienyl]-6-isopropoxycarbonylmethyl-2-phenyl-1,3-dioxane (8)
To a stirred solution of aldehyde 7 (156.1 mg, 0.49 mmol) in Et2O (10 mL) cooled at 0 °C was added a 1 M solution of vinylmagnesium bromide in THF (1 mL, 1 mmol) dropwise. The resulting mixture was stirred at 0 °C for 30 min and was quenched by sat. aq NH4Cl solution. The layers were separated and the aqueous layer was extract with EtOAc (2 × 10 mL). The combined organic layers were washed with brine, dried (Na2SO4) and evaporated under reduced pressure to provide the crude alcohol, which was used directly for next step without further purification. To a stirred solution of the above alcohol in CH2Cl2 (10 mL) at 0 °C was added Ac2O (92 μL, 1 mmol), Et3N (140 μL, 1 mmol) and DMAP (6 mg, 0.05 mmol). The resulting mixture was stirred for 1 h and was quenched by sat. aq NH4Cl solution. The layers were separated and the aqueous layer was extracted with CH2Cl2 (2 × 10 mL). The combined organic layers were washed with brine, dried (Na2SO4) and evaporated and the residue was purified by column chromatography on silica gel (10% EtOAc in hexanes) to provide a mixture of acetates 8 as a colorless oil (146 mg, 74% 2 steps).
IR (film): 2961, 1734, 1371, 1234, 1105, 1019 cm−1.
1H NMR (400 MHz, CDCl3: δ = 7.48–7.51(m, 2 H), 7.33–7.35 (m, 3 H), 5.77–5.87 (m, 3 H), 5.73 (m, 1 H), 5.61 (s, 1 H), 5.28 (m, 2 H), 5.04 (m, 1 H), 4.43 (m, 1 H), 4.34 (m, 1 H), 2.69 (dd, 1 H, J = 7, 15.6 Hz), 2.49 (dd, 1 H, J = 6.3, 15.6 Hz), 2.08 (s, 3 H), 1.78–1.83 (m, 1 H), 1.53–1.60 (m, 1 H), 1.24 (d, 6 H, J = 6.3 Hz).
13C NMR (100 MHz, CDCl3): δ = 170.1, 169.9, 138.2, 134.9, 132.6, 132.5, 128.8, 128.2, 128.0, 127.9, 126.1, 117.6, 100.6, 75.9, 75.8, 74.2, 74.1, 73.2, 68.1, 41.2, 36.4, 21.8, 21.2.
HRMS (EI): m/z calcd for C22H32NO6 (M + NH4+) 406.2230, found (M + NH4+) 406.2235.
(4S,6R)-4-[(1E,3E)-5-Azidopenta-1,3-dienyl)-6-isopropoxycarbonylmethyl-2-phenyl-1,3-dioxane (9)
To a stirred solution of acetate 8 (129 mg, 0.33 mmol) in THF (10 mL) and H2O (4 mL) was added NaN3 (43 mg, 0.7 mmol) and Pd(PPh3)4 (19 mg, 0.016 mmol). The resulting mixture was heated to reflux for 1 h and cooled to r.t. The layers were seperated and the aqueous layer was extracted with EtOAc (3 × 5 mL). The combined organic layers were washed with brine, dried (Na2SO4) and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel (5% EtOAc in hexanes) to provide azide 9 (99 mg, 81%) as a colorless oil; [α]D23+0.76 (c = 1.3, CHCl3).
1H NMR (400 MHz, CDCl3): δ= 7.49–7.51 (m, 2 H), 7.34–7.36 (m, 3 H), 6.24–6.38 (m, 2 H), 5.73–5.86 (m, 2 H), 5.63 (s, 1 H), 5.05 (m, 1 H), 4.46 (m, 1 H), 4.35 (m, 1 H), 3.81 (d, 2 H, J = 6.7 Hz), 2.71 (dd, 1 H, J = 7, 15.6 Hz), 2.50 (dd, 1 H, J = 6.3, 15.6 Hz), 1.80 (m, 1 H), 1.60 (m, 1 H), 1.24 (d, 6 H, J = 6.2 Hz).
13C NMR (100 MHz, CDCl3): δ = 170.1, 138.1, 133.8, 133.7, 129.4, 128.7, 128.1, 126.5, 126.1, 100.6, 76.2, 73.1, 67.9, 52.4, 41.1, 36.3, 21.7.
HRMS (EI): m/z calcd for C20H25N3O4Na (M + Na+) 394.1743, found (M + Na+) 394.1732.
(1S,4S,6R)-4-[(1E,3E)-5-Azidopenta-1,3-dienyl)-6-[N-(1-hydroxylmethyl-2-methoxycarbonyl)acetylamino]-2-phenyl-1,3-dioxane (10)
Azide 9 (18 mg, 0.05 mmol) in a mixture of MeOH (3 mL) and 4 N aq NaOH (3 mL) was stirred at r.t. for 12 h. After this period, the mixture was concentrated under reduced pressure. The resulting residue was cooled to 0 °C and acidified to pH 3 with 25% citric acid. The mixture was extracted with EtOAc (3 × 5 mL). The combined organic layers were dried (Na2SO4) and evaporated under reduced pressure to provide the crude acid, which was used directly without further purification. To a solution of the above acid in THF (5 mL) cooled at −30 °C was added N-methylmorpholine (17μL, 0.16 mmol) and isobutyl chloroformate (7μL, 0.05 mmol). The resulting mixture was stirred at −30 °C for 30 min. L-Serine methyl ester (8 mg, 0.05 mmol) was added and the reaction mixture was warmed to 23 °C and stirred for 15 h. After this period, the mixture was evaporated under reduced pressure and purified by column chromatography on silica gel (80% EtOAc in hexanes) to provide amide 10 (17 mg, 80% 2 steps); [α]D23+11.5 (c = 2.26, CHCl3).
IR (film): 3367, 2952, 2100, 1744, 1653, 1533, 1217 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.49 7.52 (m, 2 H), 7.35–7.39 (m, 3 H), 6.95 (d, 1 H, J = 7.5 Hz), 6.23–6.37 (m, 2 H), 5.71–5.83 (m, 2 H), 5.63 (s, 1 H), 4.65 (m, 1 H), 4.44 (m, 1 H), 4.34 (m, 1 H), 3.79–3.91 (m, 4 H), 3.73 (s, 3 H), 2.51–2.62 (m, 2 H), 1.60–1.80 (m, 2 H).
13C NMR (100 MHz, CDCl3): δ = 170.7, 170.4, 137.9, 133.7, 133.4, 129.7, 129.1, 128.3, 126.8, 126.2, 100.9, 76.3, 73.6, 63.1, 54.7, 52.7, 52.5, 42.8, 36.2.
HRMS (EI): m/z calcd for C21H26N4O6Na (M + Na+) 453.1750, found (M + Na+) 453.1755.
(4S,6R)-4-[(1E,3E)-5-Azidopenta-1,3-dienyl)-6-[(4-(methoxycarbonyl)-2-oxazolyl)methyl]-2-phenyl-1,3-dioxane (2)
To a stirred solution of the amide (6 mg, 0.01 mmol) in THF (5 mL) was added methyl-N-(triethylammoniosulfonyl)carbamate (Burgess’ reagent) (6 mg, 0.03 mmol). The resulting mixture was heated to 60 °C for 2 h. After this period, the mixture was cooled to r.t. and passed through a short silica gel column. Evaporation of the solvent under reduced pressure provided the crude oxazoline, which was used directly without further purification. To a stirred solution of the above crude oxazoline in CH2Cl2 (3 mL) cooled at 0 °C was added DBU (6 μL, 0.04 mmol) and BrCCl3 (4 μL, 0.04 mmol). The mixture was warmed to 23 °C and stirred for 15 h. After this period, the mixture was quenched with sat. aq NH4Cl. The layers were separated and the aqueous layer was extracted with CH2Cl2. (3 × 3 mL). The combined organic layers were washed with brine, dried (Na2SO4) and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel (50% EtOAc in hexanes) to provide oxazole 2 (3.4 mg, 62% 2 steps); [α]D23−6.25 (c = 0.48, CHCl3).
IR (film): 2923, 2102, 1732, 1112 cm−1.
1H NMR (400 MHz, CDCl3): δ = 8.19, (s, 1 H), 7.46–7.52 (m, 2 H), 7.31–7.36 (m, 3 H), 6.23–6.37 (m, 2 H), 5.71–5.85 (m, 2 H), 5.62 (s, 1 H), 4.40–4.48 (m, 2 H), 3.92 (s, 3 H), 3.80 (d, 2 H, J = 6.4 Hz), 3.24 (dd, 1 H, J = 6.5, 15 Hz), 3.08 (dd, 1 H, J = 6.5, 15 Hz), 1.78–1.95 (m, 2 H).
13C NMR (100 MHz, CDCl3): δ = 162.1, 161.5, 144.0, 137.9, 133.6, 133.3, 129.6, 128.8, 128.1, 126.7, 126.1, 125.9, 100.7, 73.9, 52.4, 52.1, 36.2, 34.6, 29.6.
HRMS (EI): m/z calcd for C21H22N4O5K (M + K+) 449.1227, found (M + K+) 449.1240.
Acknowledgment
Financial support of this work by the National Institutes of Health (GM 55600) is gratefully acknowledged.
References
- (1).Ehrlich J; Coffey GL; Fisher MW; Galbraith MM; Knudsen MP; Sarber RW; Schlingman AS; Smith RM; Weston JK Antibiot. Annu 1955, 790. [Google Scholar]
- (2).Vazquez D Antibiotics, Vol. III; Corcoran JW; Hahn FE, Eds.; Springer Verlag: New York, 1975. [Google Scholar]
- (3).Birubaum GI; Hall SR J. Am. Chem. Soc 1976, 98, 1926. [DOI] [PubMed] [Google Scholar]
- (4).Dvorak CA; Schmitz WD; Poon DJ; Pryde DC; Lawson JP; Amos RA; Meyers AI Angew. Chem., Int. Ed 2000, 39, 1664. [DOI] [PubMed] [Google Scholar]
- (5).(a) Meyers AI; Amos RA J. Am. Chem. Soc 1980, 102, 870. [Google Scholar]; (b) Butera J; Rini J; Helquist PJ Org. Chem 1985, 50, 3676. [Google Scholar]; (c) Liu L; Tanke RS; Miller MJ J. Org. Chem 1986, 51, 5332. [Google Scholar]; (d) Marcantoni E; Massaccesi M; Petrini MJ Org. Chem 2000, 65, 4553. [DOI] [PubMed] [Google Scholar]
- (6).Ghosh AK; Lei HJ Org. Chem 2000, 65, 4779. [DOI] [PubMed] [Google Scholar]
- (7).Murahashi S; Taniguchi Y; Imada Y; Tanigawa YJ Org. Chem 1989, 54, 3292. [Google Scholar]
- (8).(a) Cocito C Microbiol. Rev 1979, 43, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Service RF Science 1995, 270, 724. [DOI] [PubMed] [Google Scholar]
- (9).Burgess EM; Penton HR; Taylor EA; Williams WM Org. Synth. Coll. Vol. VI; Wiley: New York, 1988, 788. [Google Scholar]
- (10).Williams DR; Lowder PD; Gu YG; Brooks DA Tetrahedron Lett. 1997, 38, 331. [Google Scholar]