Abstract
Background:
Major depressive disorder (MDD) and anxiety disorders are highly comorbid, sharing many similar symptoms, including impairments in cognitive control. Deficits in cognitive control could be a potential mechanism underlying impaired emotion regulation in mood disorders.
Methods:
Participants were 44 individuals with no history of mental illness (healthy controls, HC), 31 individuals in the remitted state of MDD (rMDD), and 18 individuals who met lifetime DSM-IV-TR criteria for rMDD and an anxiety disorder in remission (Comorbid). Participants completed a Parametric Go/No-Go (PGNG) test during fMRI. Event-related analyses modeled activity for cognitive control successes (Hits for Targets, Rejections for Lures) and failures (Commissions on Lures) on the PGNG task.
Results:
The rMDD group showed significantly reduced activity within the cognitive control network (CCN) during Commission errors, including the middle frontal gyrus and inferior parietal lobule (IPL). The Comorbid group showed significantly reduced activity in several clusters within the CCN during correct Rejections, including the left IPL and right inferior frontal gyrus and greater subgenual cingulate. Notably, during correct Rejections, 60% of activation for the Comorbid group was within the Salience and Emotion Network (SEN), with 0% within the CCN.
Limitations:
The size of the Comorbid subgroup was modest, preventing subanalysis of the different AD subtypes.
Conclusions:
There is evidence that CCN activity declines in rMDD and that there may be compensatory SEN activity in individuals with Comorbid rMDD and anxiety. Our findings support the identification of comorbid anxiety as a meaningful subtype of MDD that may obscure group differences between rMDD and HCs.
Keywords: cognitive control network, remitted depression, executive control, fMRI, salience network, compensatory error monitoring
Introduction
Deficits in cognitive control are a potential mechanism underlying impaired emotion regulation in mood disorders (S. A. Langenecker, Jacobs, & Passarotti, 2014; S. A. Langenecker et al., under review; Phillips, Drevets, Rauch, & Lane, 2003). Major Depressive Disorder (MDD) is consistently associated with cognitive deficits, including in executive function, working memory and attention (Bora, Harrison, Yucel, & Pantelis, 2013; Jenkins, Peters, Jacobs, & Langenecker, 2016; McIntyre et al., 2013; H. R. Snyder, 2013). Anxiety Disorders (AD) are also associated with cognitive deficits, although to a lesser extent (Castaneda, Tuuio-Henriksson, Marttunen, Suvisaari, & Lonnqvist, 2008; S. A. Langenecker, Caveney, et al., 2007). MDD and AD are highly comorbid, sharing many symptoms (Kessler et al., 1996; Lamers et al., 2011; McIntyre et al., 2016). Around two thirds of individuals with MDD have a comorbid AD (Gorman, 1997), with common comorbid ADs including panic disorder, generalized anxiety disorder, obsessive-compulsive disorder and social phobia (Kessler et al., 1996). The present study investigated whether individuals with comorbid anxiety perform differently or activate different neural regions during successful and unsuccessful cognitive control compared to individuals with MDD only, to achieve a better understanding of different underlying pathophysiologies and possibly identify different treatment options for these subgroups.
Anxiety and depression are both associated with abnormal response monitoring (Holmes & Pizzagalli, 2008; Moser, Moran, Schroder, Donnellan, & Yeung, 2013; Olvet & Hajcak, 2008), or continuous, real-time self-assessment of performance capabilities, an essential cognitive control function. Yet our current understanding of anxiety-related modulation of attentional and cognitive control still relies on a diffuse and not easily reconciled set of theories and results. Theories suggest two components of cognitive control, and neuroimaging research supports this functional dissociation. First, implementation of cognitive control via provision of resources for top-down control is supported by the dorsolateral prefrontal cortex (DLPFC) and inferior parietal lobule (IPL). Second, response appraisal and monitoring, necessary for determining whether cognitive control is being allocated effectively, is supported in part by the medial prefrontal cortex (MacDonald, Cohen, Stenger, & Carter, 2000). With increased processing demands (e.g. task difficulty), there are greater demands for both of these functions, and it becomes difficult to dissociate the contribution of the two sets of regions to either process (MacDonald et al., 2000).
Theories of cognitive control related to provision and implementation, monitoring and adjustment are relatively well-developed within anxiety disorders. Convergence for cognitive control monitoring as a core feature of disruption in anxiety disorders is illustrated best by electroencephalography (EEG) studies (Carrasco et al., 2013; Moser et al., 2013), whereby there is exaggerated response monitoring, as demonstrated by a heightened error-related negativity (ERN) of the human event-related potential in the medial prefrontal cortex. However, this exaggerated response does not appear to translate into increased cognitive control performance, as post-error behaviour does not improve (Hajcak, McDonald, & Simons, 2003; Moran, Bernat, Aviyente, Schroder, & Moser, 2015). The ERN is, however, sensitive to the perceived consequences of errors (reactions), which, among other findings, has lead to some researchers suggesting that the ERN is related to performance motivation (Hajcak, 2012).
A similar, more cognitive-based theory of enhanced ERN in anxiety is the Compensatory error-monitoring hypothesis (Moser et al., 2013), whereby anxious individuals utilize reactive control (appraisal and monitoring) which is posited to be transient and stimulus-driven, rather than proactive control (provision and implementation) which is anticipatory and more effortful, requiring active maintenance of task goals (Braver, 2012). In this context, the error-monitoring signal is not properly conveyed to cognitive control regions, leading to inefficient corrective behaviours (2015).
Another dual model of anxiety (Heller, Nitschke, Etienne, & Miller, 1997) is centered on the notion that panic and worry have distinct psychological and physiological characteristics that can also change motivational and attentional processes. The anxious arousal/apprehension model distinguishes between apprehension, a cognitive, verbal, ruminative form of anxiety involving worry and primarily concerns for the future (which may drive proactive control), and anxious arousal, characterized by current somatic symptoms of physiological hyperarousal, predominant in panic (perhaps related to reactive control).
The salience and emotion network (SEN) is a large-scale network that functions to integrate sensory, emotional and cognitive information to detect and signal the salience of stimuli to the organism to enable a rapid behavioural response (Seeley et al., 2007, Menon, 2011). It’s numerous subregions, which include the amygdala, dorsal anterior cingulate, anterior insula, ventral striatum and the ventromedial prefrontal cortex (including the subgenual anterior cingulate cortex, sgACC) have been shown to have related but distinct specializations. For example, one study found that the amygdala is specialized for representing intrinsic value whereas the sgACC was more important for indicating personal relevance (Phan et al., 2004). The similarities for these theories of reactive or stimulus-driven attention/control in ADs (relative to goal-driven, directed attention) comes from functional neuroimaging studies of individuals with ADs that implicate regions of the SEN, including the amygdala, anterior insula and anterior cingulate cortex (Damsa, Kosel, & Moussally, 2009; Etkin & Wager, 2007), regions that might be considered as more relevant for reactive control, vis-a-vis stimulus-driven attention. For example, individuals with OCD show hyperactivation of the SEN, including ventromedial PFC and anterior insula, during an interference task (Stern et al., 2011), although these regions may extend beyond SEN and into error-monitoring regions. Alternatively, those with greater arousal/apprehension might engage in greater proactive control.
Surprisingly few studies have examined the neural correlates of cognitive control in comorbid anxiety and depression using fMRI. One fMRI study of comorbid MDD and anxiety reported trend level increases in left DLPFC with planning load relative to MDD only (van Tol et al., 2012). Another study with an emotional stroop task found that anhedonic depression was associated with increased right DLPFC and reduced right IFG activation, when anxious arousal was elevated and anxious apprehension was low. These researchers suggested that anxious arousal with depression results in a failure to implement top down CCN processing due to increased SEN activity (Engels et al., 2010). Recently, McTeague et al. (2017) conducted an important transdiagnostic meta-analysis of cognitive control during fMRI studies, with a subanalysis of non-psychotic disorders, including unipolar and bipolar depressive disorders, and anxiety disorders (e.g., generalized, social, specific, obsessive-compulsive and posttraumatic stress disorders). They reported that patients with non-psychotic disorders showed aberrant activation (increases and decreases) in regions including the right ventrolateral prefrontal cortex/insula and intraparietal sulcus. They conclude that their results reflect aberrant activation of the multiple demand network, which incorporates regions of the cognitive control and salience networks (Camilleri et al., 2017). Relevant to our hypotheses below, McTeague et al. (2017) interpreted increases in activation in patients compared to controls as reflecting compensation (Nielson, Langenecker, & Garavan, 2002). Furthermore, they attribute discordant hypo- and hyperactivations as potentially related to closer coupling of regions with cognitive regions compared to salience regions.
Recently, we compared individuals with active (a) MDD to those with aMDD and comorbid anxiety using a Parametric Go/No-Go task (PGNG) (S. A. Langenecker, Caveney, et al., 2007; Votruba & Langenecker, 2013) during fMRI (Crane et al., 2016). Individuals with comorbid anxiety showed increased activity in IPL during successful sustained attention (possibly greater proactive control) and reduced activity in the posterior cingulate and hippocampus during correct Rejections compared to HCs. Furthermore, during Commission errors, the comorbid group showed reduced activity in the right inferior frontal gyrus (IFG) relative to HCs, and in the mid-cingulate, IPL, and superior temporal gyrus relative to individuals with aMDD only. These results supported the hypothesis of hypervigilance (proactive control) within the CCN in comorbid MDD and anxiety, which could be interpreted as compensatory activity to achieve adequate performance (Eysenck, Derakshan, Santos, & Calvo, 2007). However, results did not support the hypothesis of greater SEN activity in the comorbid group (e.g., greater reactive control). Our results in aMDD are counterintuitive in light of theories of enhanced bottom-up activity (reactive control) for comorbid anxiety and MDD. This is potentially because patterns are obscured by active symptoms, and/or that cognitive control impairments may be a trait marker for MDD (Airaksinen, Wahlin, Forsell, & Larsson, 2007; S. A. Langenecker, Caveney, et al., 2007; S. A. Langenecker, Jacobs, R.H. & Passarotti, A.M. , 2014; McInerney, Gorwood, & Kennedy, 2016; A. T. Peters et al., 2017; H.R. Snyder, 2013). Therefore, we wanted to clarify the neural underpinnings of comorbid AD and MDD by examining the effect of comorbid anxiety on the neural correlates of cognitive control in young adults in the remitted state of MDD (rMDD), with few episodes of illness, and in a more constrained age window. The following hypotheses were proposed:
CCN hypotheses.
First, based on Crane et al. (Crane et al., 2016) we hypothesized that the rMDD and Comorbid groups would show reduced activity in CCN regions compared to HCs during Commission errors (failed engagement of proactive control in depression and anxiety hypothesis). We expected this to be relatively more evident in the rMDD than comorbid group. Second, also based on Crane et al., we hypothesized that to achieve correct Rejections, the Comorbid group would show greater CCN activity than HCs (compensation by proactive control in anxiety hypothesis), who would show greater activity than the rMDD only group.
SEN hypotheses.
Third, based on theories of reactive control in anxiety (Heller et al., 1997; Moser et al., 2013), we hypothesized that during Commission errors, the Comorbid group would show increased activity in SEN regions (failed engagement of reactive control in anxiety hypothesis). Fourth, again based on theories of reactive control leading to compensatory performance in anxiety (Eysenck et al., 2007), we hypothesized that during correct Rejections, the Comorbid group would show increased activity in SEN regions compared to the rMDD only and HC groups (compensation by reactive control in anxiety hypothesis). Unfortunately, we note that fMRI time course is insufficient to distinguish between SEN activation that is related to an alerting function (attend now for Rejections) and a correction function (error, needs adjustment, for Commissions). Both functions would be encompassed within a framework of reactive control.
Methods
Participants
Participants were 93 individuals (63 female) aged 18-23 years, comprising 44 healthy individuals and 49 with a history of MDD. The HC group (29 female) had no personal history of psychiatric disorder. Individuals in the rMDD group (34 female) were early in the course of MDD (1-6 episodes, median of 1), but in remission at the time of the study as defined by DSM-IV-TR criteria. Of the individuals with rMDD, a subset of 18 individuals (11 female) also met full criteria (lifetime) for a DSM-IV-TR AD (Comorbid group) for which they were also in remission (Hamilton Anxiety Scale score 0-17, Hamilton, 1969), and the remaining 31 individuals (23 female) did not have any lifetime AD (rMDD only). ADs included generalized AD, specific/simple phobia, agoraphobia, social AD, OCD, posttraumatic stress disorder, and panic disorder. Although the ERN is most consistently aligned with anxious apprehension (Vaidyanathan et al., 2012; Weinberg et al., 2012), and the theory may be more relevant to these individuals, studies such as Etkin and Wagner’s 2007 meta-analysis of PTSD, SAD and specific phobia have found that specific phobia also has increased activation of SEN regions, therefore we chose to included individuals with specific phobias in the comorbid group.
Table 1 reports demographic and clinical comparisons between groups.Participants were recruited from two sites: University of Michigan (UM, n=32, 16 HC) and University of Illinois at Chicago (UIC, n=61, 28 HC). Exclusion criteria were substance abuse or dependence within previous year, psychostimulant use within the past 2 days, regular smoking, hospitalization for suicidal intent within the past three months or suicide attempt within the past six months, neurological condition, personal or family history of psychosis or bipolar disorder, or contraindications for MRI. Participants were required to be free from psychoactive medication use within the past 30 days before enrollment, however they could have used medication previously. Previous and current psychotherapy were not exclusion criteria. No individual was asked to stop using existing treatments.
Table 1.
Demographic and Clinical Characteristics
| HC | rMDD only | Comorbid | posthoc | |
|---|---|---|---|---|
| N (male) | 44 (15) | 31 (8) | 18 (7) | |
| Age | 20.8 (1.6) | 21.1 (1.6) | 21.5 (1.4) | |
| Education | 14.6 (1.4) | 14.5 (1.3) | 14.4 (1.6) | |
| Years well | 2.7 (1.9) | 2.9 (1.7) | ||
| VIQ | 106.1 (9.2) | 109.6 (7.8) | 102.1 (9.3) | rMDD > Comorbid*1 |
| HDRS | 0.4 (1.0) | 1.5 (1.6) | 1.8 (2.3) | HC < rMDD, Comorbid** |
| HARS | 0.5 (1.3) | 2.5 (3.1) | 2.8 (4.2) | HC < rMDD, Comorbid** |
| BSPS Total | 2.6 (3.5) | 5.0 (8.6) | 7.4 (7.7) | HC < Comorbid* |
| BAI | 1.5 (2.3) | 4.5 (4.7) | 4.7 (6.3) | HC < rMDD, Comorbid* |
| MAQ | 59.4 (4.7) | 62.6 (7.7) | 62.9 (10.2) |
Note.
p< .05
p< .01.
HC = healthy control, rMDD = remitted Major Depressive Disorder, VIQ = verbal intelligence quotient, HDRS = Hamilton Depression Rating Scale, HARS = Hamilton Anxiety Rating Scale, BSPS = Brief Social Phobia Scale, BAI = Beck Anxiety Inventory, MAQ = Multidimensional Anxiety Questionnaire. Values in parentheses are standard deviations (except for N male).
This significant difference was driven by two individuals with rMDD with VIQs of 126 and one individual in the Comorbid group with a VIQ of 78. When these three individuals were excluded, there was no significant difference in VIQ (p=.15). Repeating the main analyses, there was still no significant group difference in behavioural performance between groups (p=.33). A MANCOVA of the extracted beta weights (shown in Figure 2) for N=90, with PCTT, PCIT and site as covariates, excluding these three individuals, there was still a significant main effect of diagnosis across conditions (p<.001).
Measures
The Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994) ascertained diagnosis and current remitted state. No participants had any active disorder at the time of study. Diagnostic history was confirmed with parent/guardian interview using a modified Family Interview for Genetic Studies (Maxwell, 1992) or treatment records. The Hamilton Depression Rating Scale-17 (HDRS) (Hamilton, 1960) assessed current depression symptom severity (Zimmerman, Martinez, Young, Chelminski, & Dalrymple, 2013). The Hamilton Anxiety Rating Scale (HARS) (Hamilton, 1959) and the Beck Anxiety Inventory (BAI) (Beck & Steer, 1993) assessed current severity of anxiety symptoms. The Brief Social Phobia Scale (BSPS) (Davidson et al., 1997) measured symptoms of social phobia using three subscales: Fear, Avoidance and Physiological Arousal. The Multidimensional Anxiety Questionnaire (MAQ) (Reynolds, 1999) assessed four symptom domains: Physiological Panic, Social Phobia, Worry-Fears and Negative Affectivity. The BSPS and the MAQ are in supplemental analyses.
The Parametric Go/No-Go test (PGNG) (S. A. Langenecker et al., 2005; S. A. Langenecker, Zubieta, Young, Akil, & Nielson, 2007; Votruba & Langenecker, 2013) measured cognitive control, including proactive (correct Target hits and correct Rejections) and reactive control (incorrect rejections, i.e. Commissions). The PGNG presents participants with a rapid presentation of a continuous stream of letters, with 500 ms presentation and 0 ms ITI. Go and no-Go events are presented in a fixed, jittered order such that there are variable delays from Go target to Go target and N0-go Rejection to No-go Rejection, to optimize the hemodynamic modeling (jittered, fast event-related design). Participants respond or inhibit responses according to increasing difficulty levels (2 vs 3 target rotations of Go and No-go stimuli). The correct response (Go vs No-go) is contingent upon the last response, a contextual non-repeating rule. The task measures attention, set-shifting (Targets), processing speed, and inhibitory control via correct (Rejections) and incorrect responses (Commissions) to lure (No-go stimuli) items. See Supplement for test diagram (Figure S0). Performance measures are Percent Correct Target Trials (PCTT, Go), Percent Correct Inhibitory Trials (PCIT, No-go).
Procedures
Written informed consent was obtained according to the guidelines of the Institutional Review Boards of UM and UIC and consistent with the Declaration of Helsinki (BMJ 1991; 302: 1194). Participants were compensated for their involvement.
Participants completed a practice trial of the PGNG prior to fMRI scanning to increase familiarity and chances for successful understanding and engagement during fMRI (A. Peters et al., 2015). The PGNG takes 24 minutes and was completed at the beginning of a 90-minute fMRI session. Other tasks, completed later in the scanning session, are reported in separate publications.
MRI acquisition
Whole-brain imaging was performed at two sites, both using a 3T GE scanner. Site was included as a covariate in all analyses. At UM a Signa (release VH3) scanner used a forward/reverse spiral sequence to acquire 36 slices, 4 mm thick. The image matrix was 64 × 64 over a 20cm field of view (FOV) for a 3.44 × 3.44 voxel. TR=2000 ms, TE=30 ms. Additionally, 116 high-resolution T1 spoiled gradient echo (SPGR) slices were collected for co-registration purposes, with slice thickness= 1.2mm, FOV=26cm, matrix size=256 × 256. At UIC a Discovery scanner acquired images with a gradient-echo axial echo-planar imaging sequence. The image matrix was 64 × 64 over a 22 cm FOV with 3mm slice thickness (0 gap) for a 3.44 × 3.44 voxel. TR=2000ms, TE=minFul (22.2ms), 90° flip, 44 slices (ascending, interleaved). The anatomical scan was a T1SPGR echo, FOV=22cm, matrix size=256 × 256, 1mm slice thickness, voxel size=0.86 × 0.86, including 182 slices during a scan time of around 4 minutes.
MRI preprocessing
fMRI data were despiked, slice-time corrected, then realigned to the 10th volume in FSL using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002). Brain extraction of anatomical images was performed with FSL’s Brain Extraction Tool (Smith, 2002), co-registered to functional images and spatially normalized to Montreal Neurological Institute (MNI) space, with a final reconstructed spatial resolution of 2×2×2mm. Smoothing used a full-width-half-maximum filter of 5mm. Movement parameters for x, y, and z translation were included in first and second level models as covariates of no interest.
Statistical analysis
The three neuroimaging contrasts of correct Rejections, Commissions and Targets were analysed using a MANCOVA factorial model. For an event-related design, stimuli were modeled in relation to the onset of the event (time locked to onset), and deconvolved with the hemodynamic response function in relation to the implicit baseline. Covariates included in the model were sex, site, behavioural variables of Percent Correct Target Trials (PCTT) and Percent Correct Inhibitory Trials (PCIT) and the mean values (across all 4 runs of PGNG task) of the standard deviations of translation in x, y and z planes after realignment. The threshold of significance for the fMRI analysis was p < .005 and mm3= 328 (p < .05 whole brain, after applying fwhm adjustments for multiple testing based upon 10,000 Monte Carlo simulations in 3dClustSim, December 2015).
To test hypotheses related to the CCN and SEN, masks were created of these two networks, as per (S. A. Langenecker et al., under review). These were defined based on Yeo et al. (2011), who identified and replicated a 7-network cortical parcellation using intrinsic functional connectivity of 1000 people. The "7 Networks MNI 152 Freesurfer conformed 1mm liberal parcellations" were used to create masks for subsequent SPM8 analyses. To simplify analysis, we combined the ventral attention and limbic parcellations to create a SEN mask, and the dorsal attention and fronto-parietal parcellations to create a CCN mask, in line with network models (Seeley et al., 2007) (Menon, 2011). Further, we note that the Yeo et al.’s (2011) parcellations do not include medial temporal structures (e.g. amygdalae) and have poor coverage of the subgenual anterior cingulate (sgACC, see Supplement, Figure S2). Due to the prominent role that amygdala and sgACC have demonstrated in anxiety and depression, ROIs were built of these structures. WFU Pickatlas was used to create a mask of a) the bilateral amygdalae and b) the sgACC, incorporating bilateral Brodmann areas (BA) 25, and the caudal portions of BAs 24 and 32 (Johansen-Berg et al., 2008; Lancaster et al., 1997; Lancaster et al., 2000; Maldjian, Laurienti, & Burdette, 2004; Maldjian, Laurienti, Kraft, & Burdette, 2003) with a dilation of 2 for both ROIs to account for individual differences in anatomy. This allowed us to conduct specific tests of the SEN hypothesis beyond the SEN network mask in these ROIs. For these ROI analyses, an unadjusted p value of < .05 was used.
Results
Behavioural Performance Results
The behavioural data from the trials of interest Percentage Correct Inhibitory Trials (PCIT) were normally distributed, with no outliers. The Percentage Correct Target Trials (PCTT) data were negatively skewed with one HC scoring > 3 standard deviations below the mean of the HC group. Since this person had normal PCIT data, we did not exclude this person.
Univariate Analysis of CoVariance (ANCOVAs), including site as a covariate, found no significant group difference in PCTT, F(2, 89)= 0.73, p= .49, or PCIT, F(2, 89)= 1.75, p= .18 (Figure 1).
Figure 1.
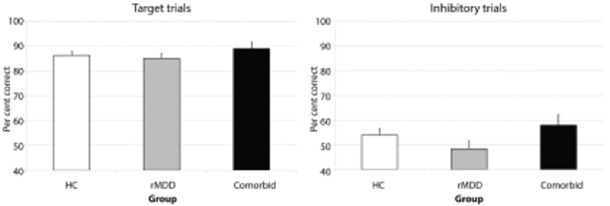
Estimated marginal means for diagnostic subgroups in behavioural performance (per cent correct) for Target trials (left, PCTT) and Inhibitory trials (right, PCIT).
fMRI Results
Whole-brain analysis
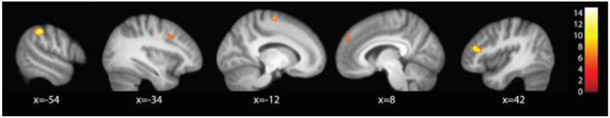
Across all task conditions, there was a significant main effect of Diagnosis in five clusters (Figure 2). These were in the right inferior frontal gyrus, left inferior parietal lobule, bilateral superior frontal gyri and the left middle frontal gyrus. Four of these clusters, the left IPL, SFG and MFG and the right IFG, overlapped with the CCN mask. Peaks of activity are reported in the Supplement.
Figure 2.
Main effect of Diagnosis across task types (Targets, Rejections, and Commissions).Activation differences were observed in left IPL (x = −54), left MFG (x = −34), left SFG (x = −12), right SFG (x = 8), and right IFG (x = 42). (p < .05, whole brain-corrected.)
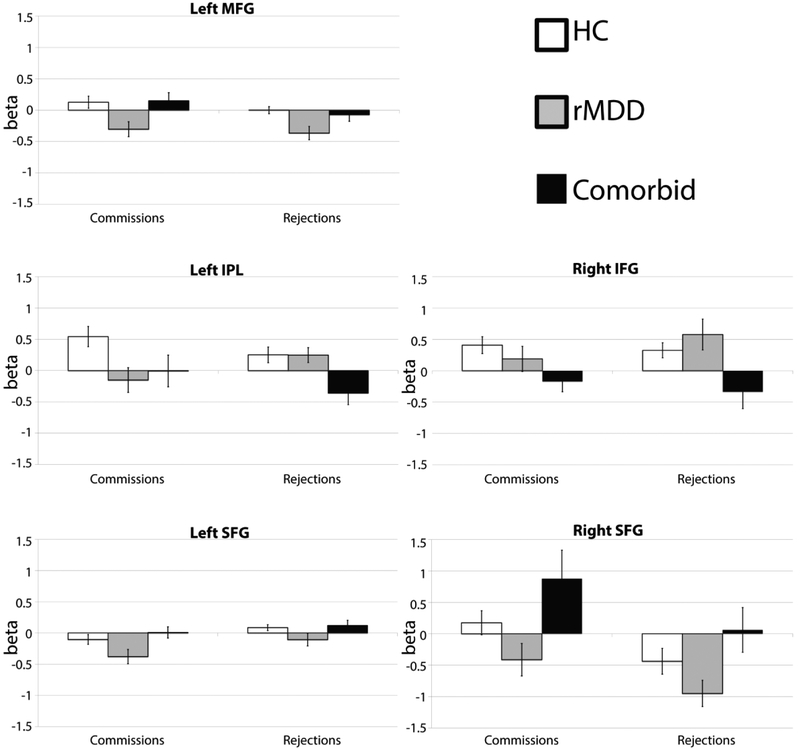
As this whole brain analysis of the main effect of Diagnosis was across all task conditions, we calculated post hoc ANOVAs (5 ROIs × 2 conditions) of the significant main effect of Diagnosis reported above, solely for the purpose of determining the direction of effects for the correct Rejections and Commission errors task conditions. These results are displayed in Figure 3. None of these regions were correlated with any movement parameter for x, y, and z translations for Commissions (rs < ∣.20∣, ps > .07) or Rejections (rs < ∣.17∣, ps > .10). Our hypotheses did not concern the Targets condition, thus these results are included in the Supplement.
Figure 3.
Mean beta values (SE bars) from each Diagnosis in the five significant clusters from the main effect of Diagnosis (across conditions), for Commissions and Rejections. (p < .05, whole brain-corrected.)
Commissions
Tukey post-hoc tests found that the rMDD group had significantly less activation than the Comorbid group for the left SFG (p= .04) and the right SFG (p= .01), than the HC group (p= .01) and the Comorbid group (p= .04) for the left MFG, and than the HC group (p= .02) for the left IPL (Figure 3).
Rejections
Tukey post-hoc tests revealed that the HC group had more activation than the rMDD group (p= .002) for the left MFG. The HC had more activation than the Comorbid group (p= .02) and the rMDD group had more activation than the comorbid group (p= .03) for the left IPL. The Comorbid group had significantly less activity than the rMDD group for the right IFG (p= .02), and significantly greater activity than the rMDD group (p= .03) for the right SFG (Figure 3). As an alternative, exploratory analysis strategy, for each Diagnosis, we calculated the percentage of significant activity that was located within the CCN and SEN network masks for each contrast (see also Table S1 for per cent of each network active by Diagnosis and Condition).
ROI analyses
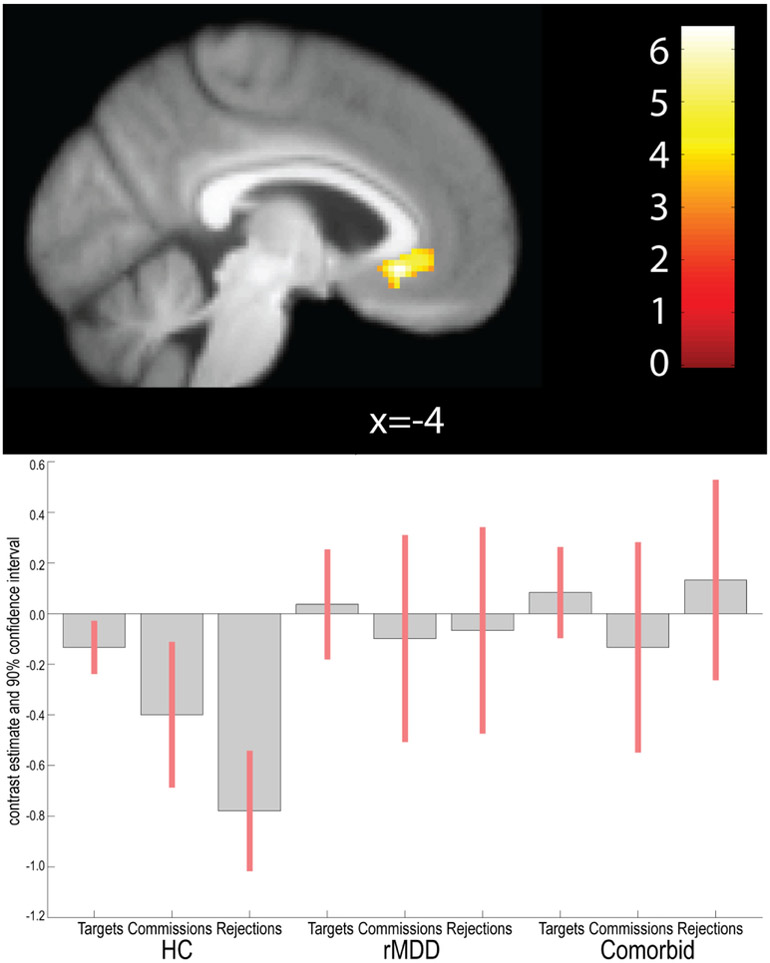
sgACC
Across all conditions, we found a significant main effect of Diagnosis in the bilateral sgACC in a cluster of 166 contiguous voxels (1328mm3), peaking at −4, 26, −8 (Figure 5). There was no significant interaction between Diagnosis and Condition in sgACC. There were no differences between groups in Tukey posthoc tests for diagnosis.
Figure 5.
Main effect of Diagnosis across all conditions in the sgACC ROI. (p< .05, uncorrected).
Amygdala
Across all conditions, there was no significant effect of Diagnosis or interaction between Diagnosis and Condition in bilateral amygdala ROIs.
Discussion
We tested hypotheses of the influence of comorbid anxiety, as a source of heterogeneity in MDD, on proactive and reactive control in rMDD during a cognitive control fMRI task. We found some support for the hypothesis that rMDD and Comorbid (i.e. rMDD + remitted anxiety) groups would show reduced CCN activity compared to HCs during Commissions, in line with models of emotion regulation in mood disorders (S. A. Langenecker et al., 2014; Phillips et al., 2003). We also found some evidence for the hypothesis that during correct Rejections, the Comorbid group would show increased activity in the SEN, possibly a reflection of increased engagement through reactive control.
Based on models of emotion regulation (S. A. Langenecker et al., 2014; Phillips et al., 2003), hypothesis one predicted that rMDD and Comorbid groups would show reduced CCN activity compared to HCs during Commissions. This was partly supported as the rMDD group exhibited less activation in the left MFG and IPL compared to HCs, important regions of the CCN (Menon, 2011; Seeley et al., 2007). These results were corroborated by inverse relations with the MAQ Social Phobia scale reported in the Supplement. However hypothesis one was not supported for the Comorbid group. Reduced CCN activity for Commissions in rMDD only perhaps represents decreased ability to recruit cognitive resources, and some theoretical models posit impaired cognitive regulation in MDD (S. A. Langenecker et al., 2014; Phillips et al., 2003). Although brain regions that are more efficient at a particular task may display less rather than more activity, since Commissions are errors of cognitive control, we interpret this hypoactivation as evidence of failed engagement of the CCN in rMDD.
The main analyses found no evidence for the second hypothesis, compensation by proactive control, as the Comorbid group did not demonstrate greater activity in the CCN during Rejections. In fact, we observed a result in the opposite direction, as the Comorbid group showed significantly reduced activity compared to HCs in the left IPL and right IFG within the CCN. There was a dissociation of rMDD and Comorbid group in these two regions, with the Comorbid group unexpectedly and markedly lower than the rMDD and HC groups, particularly for Rejections. For the right IFG, this was also true for Commissions. During Rejections, the Comorbid group had a marked absence of CCN engagement (Figure 4), whereas the rMDD group showed a similar spatial extent of CCN engagement as the HCs. The cross-condition hypoactivation of right IFG in the comorbid group suggests that there may be compensation in other regions (see SFG discussion below for SEN) to attain equivalent performance for Rejections. This equivalent performance-compensation via SEN may be a vulnerability or protective adjustment. In contrast, illustrated within the Supplement, higher MAQ social phobia was related to greater right inferior parietal and middle frontal activation during correct Rejections (Figure S3, Panel C).
Figure 4.
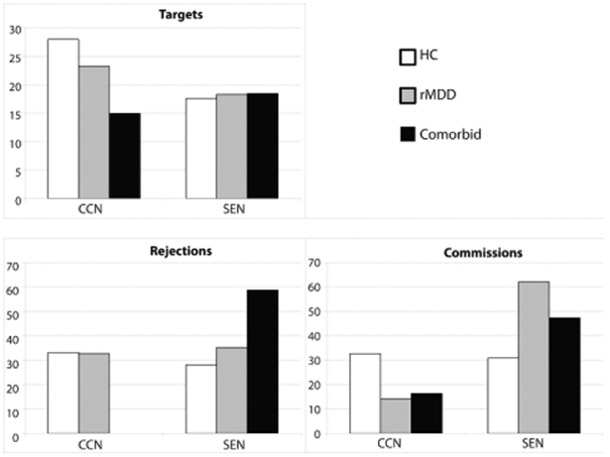
Percentage of active voxels for each condition located within each network for each group. Unreported percentage of active voxels were located outside of CCN and SEN masks, and may include adjoining regions, other networks or smoothing effects/misregistration with the mask/white matter.
The right SFG showed higher activation in the Comorbid group than the rMDD group for Commissions. The pattern of differential network activation by diagnosis was most apparent in Figure 4, wherein the Comorbid group engaged the SEN to a greater degree than the other two groups, and only for correct Rejections. Although speculative, it is possible that compensatory activity within the SEN in the Comorbid group for Rejections was sufficient to achieve similar performance as HCs, such that CCN hyperactivation was not necessary. Alternatively, it may be that compensatory CCN activity is more likely to occur among individuals with symptoms that are active, rather than remitted. A previous study of individuals with rMDD also found hypoactivity of the right dorsomedial prefrontal cortex during error commissions on a go/no-go task (Nixon et al., 2013). Unfortunately, our results are not directly comparable as this prior study did not conduct a whole-brain analysis or include a comorbid anxiety subgroup, which are advantages of the present study.
The hypotheses of increased SEN activity for the Comorbid group during Commissions (failed engagement of reactive control, hypothesis three) and correct Rejections (compensatory reactive control, hypothesis four), were based upon the Compensatory error-monitoring hypothesis (Moser et al., 2013). This posits that anxious individuals utilize stimulus-driven, reactive control, which we argued occurs in the SEN, given studies that have found increased SEN activity in ADs (Damsa et al., 2009; Etkin & Wager, 2007). Although not significant, potentially due to the small size of the Comorbid group, the behavioural results were in the direction hypothesized, with higher percentages correct for both target and inhibitory trials, a hypothesized byproduct of hypervigilance and proactive control. For the fMRI results, our main effect of Diagnosis did not support these hypotheses, as none of the five clusters were located within the SEN. However, dorsomedial activation was higher for the Comorbid group in both right and left SFG regions for Commissions, suggesting that error-monitoring regions outside of SEN are engaged and may lead to greater interference. These regions are anterior to SEN derived by Yeo and colleagues, but have previously been considered as functionally equivalent to SEN (Capuron et al., 2005). Moreover, Figure 4, the proportion of each groups significant activity that overlapped with network masks, is consistent with hypothesis four, as approximately two thirds of active voxels for the Comorbid group were located within the SEN, compared to around one third each for the HC and rMDD groups during Rejections. This increased SEN activity is possibly a reflection of increased engagement through reactive control. Combined with the finding of no significant activity in the CCN during Rejections in the Comorbid group, this tentatively suggests that correct inhibitory control performance in Comorbid anxiety and rMDD is characterized by inefficient utilization of cognitive resources, including failed CCN activity and compensatory SEN activity. Future research with a larger Comorbid sample is required to investigate this further.
There was a significant effect of Diagnosis in the sgACC, which is an important part of the SEN. However, posthoc tests did not reveal significant effects of diagnosis for Commissions or Rejections, potentially due to the modest sample size of the Comorbid group. The sgACC is critical for modulating negative mood states (Mayberg et al., 1999), and has been the target of deep brain stimulation for treatment resistant MDD (Mayberg et al., 2005), based on neuroimaging findings of hyperactivity of this region. This hyperactivity was inversely correlated with activity in dorsolateral prefrontal cortical regions involved in cognitive control (Mayberg et al., 1999), and has been reported to resolve following treatment of MDD (Mayberg et al., 2005). Our results indicate that during cognitive control, hyperactivity of this region may be an intermediate phenotype for MDD or a trait risk factor.
The presence of a comorbid disorder may obscure the typical response of the rMDD group (Kentgen et al., 2000; Weinberg, Klein, & Hajcak, 2012) as there were a number of differences between rMDD and comorbid groups relative to HC. There were also a number of regions related to dimensional measures of anxiety in the supplemental analyses, emphasizing heterogeneity in MDD. However, it is important to note that there are some counterintuitive results in the literature, perhaps in part because most prior studies of comorbid depression and anxiety have relied upon actively depressed individuals, e.g., (Crane et al., 2016). The present study’s results among individuals with rMDD are only partly in line with existing theoretical models (S. A. Langenecker et al., 2014; Phillips et al., 2003) suggesting that diminished regulatory capacity in MDD may be more relevant among individuals with no anxiety comorbidity, whereas a compensation or stimulus-driven attention model may be more relevant in the context of comorbid anxiety phenotypes. Our results indicate potential neural bases for cognitive control deficits in these MDD subtypes, and suggest that efficacious treatments, whether of a preventative or a reactive type, may diverge, based on the presence of comorbid anxiety.
Limitations
There were several limitations of the present study. Although the sample of individuals with rMDD was relatively large (n = 49), the Comorbid subgroup was modest (n = 18), preventing subanalysis of the role of different AD subtypes and potentially failing to reject the null hypothesis due to lack of power (e.g,. type II error). Moreover, some of the AD subtypes included OCD and PTSD, and these have recently been conceptualized as somewhat distinct from other ADs. It is possible that the modest sample size of the Comorbid group precluded the detection of true differences in performance. There were fewer males than females, so there is a risk for type II error in sex differences. There is continued debate about appropriate balance between type I and type II error in the field, which regions to search, and other adjustments for multiple comparisons. As a result, the current thresholding based upon joint height and extent probabilities may be too lenient (type I) or too conservative (type II error) and future meta-analytic work can at least address concerns about type I error. Finally, despite the event-related design, reliance on the sluggish BOLD signal precluded the ability to evaluate hypotheses about the precise time-course of CCN and SEN activity following successful and unsuccessful cognitive control; future studies could use similar paradigms with event-related potentials. Future studies should also examine possible differences among anxiety disorders, and in combination with active and remitted MDD.
Conclusions
In conclusion, our results support the consideration of anxiety as a meaningful subtype of MDD that may obscure group differences between rMDD and HCs when not evaluated. Although cognitive control is often emphasized within emotional challenge and regulation paradigms, the present study is one of the first to evaluate cognitive control in comorbid anxiety in rMDD in a non-emotional paradigm. The use of a remitted group and a non-emotional paradigm may provide an important context for pursuing cognitive control dimensions within the RDoC initiative, and in probing top-down models of disruption in MDD. Identifying comorbid anxiety as one subtype or intermediate phenotype could help to clarify heterogeneity in MDD, with the aim of improving our understanding of networks and behaviours that in future might be specific targets for personalized medicine (Lui, Zhou, Sweeney, & Gong, 2016).
Supplementary Material
Highlights.
Cognitive control deficits may cause impaired emotion regulation in mood disorders
rMDD group showed less cognitive control network (CCN) activity during commissions
Comorbid rMDD and anxiety showed increased salience and emotion network activity
increased salience and emotion network activity may be compensatory in anxiety
Comorbid anxiety is a meaningful subtype of MDD
Acknowledgements:
Supported by a NIMH Biobehavioral Research Award for Innovative New Scientists (BRAINS MH091811, SAL). We thank the UM Prechter Bipolar research team (Kelly A. Ryan, Melvin G. McInnis, Gloria Harrington), the Multifaceted Explorations of the Neurobiology of Depressive Disorders laboratory (MEND2, Sara L. Weisenbach, Kelly A. Ryan, Laura B. Gabriel, Anne L. Weldon, Michelle T. Kassel, Kortni K. Meyers, Erica Hymen, Bethany Pester) for assistance in data collection, diagnostic interviews, and longitudinal follow-up evaluations.
Role of the funding source: The funding source had no role in the design, execution, analysis, interpretation of the data or decision to submit results.
Financial disclosures:
This work was supported by a NIMH grant to S.A.L. (MH091811).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airaksinen E, Wahlin A, Forsell Y, & Larsson M (2007). Low episodic memory performance as a premorbid marker of depression: Evidence from a 3-year follow-up. Acta Psychiatrica Scandinavica, 115(6), 458–465. doi: 10.1111/j.1600-0447.2006.00932.x [DOI] [PubMed] [Google Scholar]
- Beck AT, & Steer RA (1993). Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bora E, Harrison BJ, Yucel M, & Pantelis C (2013). Cognitive impairment in euthymic major depressive disorder: A meta-analysis. Psychological Medicine, 43(10), 2017–2026. doi: 10.1017/s0033291712002085 [DOI] [PubMed] [Google Scholar]
- Braver TS (2012). The variable nature of cognitive control: A dual mechanisms framework. Trends in Cognitive Sciences, 16(2), 106–113. doi: 10.1016/j.tics.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri JA, Muller VI, Fox P, Laird AR, Hoffstaedter F, Kalenscher T, & Eickhoff SB (2017). Definition and characterization of an extended multiple-demand network. Neuroimage, 165, 138–147. doi: 10.1016/j.neuroimage.2017.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, & Miller AH (2005). Anterior cingulate activation and error processing during interferon-alpha treatment. Biol.Psychiatry, 58(3), 190–196. doi:S0006-3223(05)00378-1 [pii]; 10.1016/j.biopsych.2005.03.033 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Hong C, Nienhuis JK, Harbin SM, Fitzgerald KD, Gehring WJ, & Hanna GL (2013). Increased error-related brain activity in youth with obsessive-compulsive disorder and other anxiety disorders. Neuroscience Letters, 541, 214–218. doi: 10.1016/j.neulet.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda AE, Tuuio-Henriksson A, Marttunen M, Suvisaari J, & Lonnqvist J (2008). A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. Journal of affective disorders, 106(1–2), 1–27. doi: 10.1016/j.jad.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Crane NA, Jenkins LM, Dion C, Meyers KK, Weldon AL, Gabriel LB, … Langenecker SA (2016). Comorbid anxiety increases cognitive control activation in Major Depressive Disorder. Depression and Anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsa C, Kosel M, & Moussally J (2009). Current status of brain imaging in anxiety disorders. Current Opinion in Psychiatry, 22(1), 96–110. doi: 10.1097/YCO.0b013e328319bd10 [DOI] [PubMed] [Google Scholar]
- Davidson JRT, Miner CM, DeVeaughGeiss J, Tupler LA, Colket JT, & Potts NLS (1997). The Brief Social Phobia Scale: A psychometric evaluation. Psychological Medicine, 27(1), 161–166. doi: 10.1017/s0033291796004217 [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Spielberg JM, Warren SL, Sutton BP, & Banich MT (2010). Co-occurring anxiety influences patterns of brain activity in depression. Cognitive Affective & Behavioral Neuroscience, 10(1), 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–1488. doi: 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, & Calvo MG (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7(2), 336–353. doi: 10.1037/1528-3542.7.2.336 [DOI] [PubMed] [Google Scholar]
- Gorman JM (1997). Comorbid depression and anxiety spectrum disorders. Depression and Anxiety, 4, 160–168. [DOI] [PubMed] [Google Scholar]
- Hajcak G (2012). What we've learned from mistakes: Insights from error-related brain activity. Current Directions in Psychological Science, 21(2), 101–106. doi: 10.1177/0963721412436809 [DOI] [Google Scholar]
- Hajcak G, McDonald N, & Simons RF (2003). To err is autonomic: Error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology, 40(6), 895–903. doi: 10.1111/1469-8986.00107 [DOI] [PubMed] [Google Scholar]
- Hamilton M (1959). The assessment of anxiety states by rating. British Journal of Medical Psychology, 32(1), 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry, 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, & Miller GA (1997). Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology, 106(3), 376–385. doi: 10.1037//0021-843x.106.3.376 [DOI] [PubMed] [Google Scholar]
- Holmes AJ, & Pizzagalli DA (2008). Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives of General Psychiatry, 65(2), 179–188. doi: 10.1001/archgenpsychiatry.2007.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LM, Peters A, Jacobs RH, & Langenecker SA (2016). Neuroscience of functional outcomes and treatment targets in major depressive disorder In McIntyre RS & Cha DS (Eds.), Cognitive impairment in major depressive disorder: Clinical relevance, biological substrates and treatment opportunities (pp. 257–273). Cambridge: Cambridge University Press. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17(2), 825–841. doi: 10.1006/nimg.2002.1132 [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, … Mayberg HS (2008). Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cerebral Cortex, 18(6), 1374–1383. doi: 10.1093/cercor/bhm167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, & Bruder GE (2000).Electroencephalographic asymmetries in adolescents with major depression: Influence of comorbidity with anxiety disorders. Journal of Abnormal Psychology, 109(4), 797–802. doi: 10.1037/0021-843x.109.4.797 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, & Blazer DG (1996). Comorbidity of DSM-III-R major depressive disorder in the general population: Results from the US National Comorbidity Survey. British Journal of Psychiatry, 168, 17–30. [PubMed] [Google Scholar]
- Lamers F, van Oppen P, Comijs HC, Smit JH, Spinhoven P, van Balkom AJ, … Pennix BW (2011). Comorbidity patterns of anxiety and depressive disorders in a large cohort study: The Netherlands study of Depression and Anxiety (NESDA). Journal of Clinical Psychiatry, 72(3), 341–348. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, … Mazziotta JC (1997). Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping, 5(4), 238–242. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas ES, Rainey L, … Fox PT (2000). Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping, 10(3), 120–131. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, & Berent S (2005). Face emotion perception and executive functioning deficits in depression. Journal of Clinical and Experimental Neuropsychology, 27(3), 320–333. doi: 10.1080/13803390490490515720 [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Caveney AF, Giordani B, Young EA, Nielson KA, Rapport LJ, … Zubieta JK (2007). The sensitivity and psychometric properties of a brief computer-based cognitive screening battery in a depression clinic. Psychiatry Research, 152(2-3), 143–154. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Jacobs RH, & Passarotti AM (2014). Current neural and behavioral dimensional constructs across mood disorders. Current Behavioral Neuroscience Reports, 1, 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Jacobs RH & Passarotti AM (2014). Current Neural and Behavioral Dimensional Constructs across Mood Disorders. Current Behavioral Neuroscience Reports, 1, 114–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Jenkins LM, Stange JP, Chang Y-S, DelDonno SR, Bessette KL, … Jacobs RH (under review). Cognitive control inefficiencies predict recurrence of depressive episodes. [Google Scholar]
- Langenecker SA, Zubieta JK, Young EA, Akil H, & Nielson KA (2007). A task to manipulate attentional load, set-shifting, and inhibitory control: Convergent validity and test-retest reliability of the Parametric Go/No-Go Test. Journal of Clinical and Experimental Neuropsychology, 29(8), 842–853. doi: 10.1080/13803390601147611 [DOI] [PubMed] [Google Scholar]
- Lui S, Zhou XJ, Sweeney JA, & Gong Q (2016). Psychoradiology: The Frontier of Neuroimaging in Psychiatry. Radiology, 281(2), 357–372. doi: 10.1148/radiol.2016152149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, & Carter CS (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–1838. doi: 10.1126/science.288.5472.1835 [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, & Burdette JH (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage, 21(1), 450–455. doi: 10.1016/j.neuroimage.2003.09.032 [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–1239. doi: 10.1016/s1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- Maxwell E (1992). Family Interview for Genetic Studies (FIGS): A Manual for FIGS. Clinical Neurogenetics Branch, Intramural Research Program: National Institute of Mental Health. [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, … Fox PT (1999). Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry, 156(5), 675–682. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, … Kennedy SH (2005). Deep brain stimulation for treatment-resistant depression. Neuron, 45(5), 651–660. doi: 10.1016/j.neuron.2005.02.014 [DOI] [PubMed] [Google Scholar]
- McInerney SJ, Gorwood P, & Kennedy SH (2016). Cognition and biomarkers in major depressive disorder: Endophenotype or epiphenomenon? In McIntyre RS & Cha DS (Eds.), Cognitive impairment in major depressive disorder: Clinical relevance, biological substrates and treatment opportunities (pp. 145–159). Cambridge: Cambridge University Press. [Google Scholar]
- McIntyre RS, Cha DS, Soczynska JK, Woldeyohannes HO, Gallaugher LA, Kudlow P, … Baskaran A (2013). Cognitive deficits and functional outcomes in major depressive disorder: Determinants, substrates, and treatment interventions. Depression and Anxiety, 30(6), 515–527. doi: 10.1002/da.22063 [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Woldeyohannes HO, Soczynska JK, Vinberg M, Cha DS, Lee Y, … Kennedy S (2016). The prevalence and clinical characteristics associated with Diagnostic and Statistical Manual Version-5-defined anxious distress specifier in adults with major depressive disorder: results from the International Mood Disorders Collaborative Project. Therapeutic Advances in Chronic Disease, 7(3), 153–159. doi: 10.1177/2040622315627805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, & Etkin A (2017). Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. The American journal of psychiatry, 174(7), 676–685. doi: 10.1176/appi.ajp.2017.16040400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. doi: 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Moran TP, Bernat EM, Aviyente S, Schroder HS, & Moser JS (2015). Sending mixed signals: Worry is associated with enhanced initial error processing but reduced call for subsequent cognitive control. Social Cognitive and Affective Neuroscience, 10(11), 1548–1556. doi: 10.1093/scan/nsv046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB, & Yeung N (2013). On the relationship between anxiety and error monitoring: A meta-analysis and conceptual framework. Frontiers in Human Neuroscience, 7, 19. doi: 10.3389/fnhum.2013.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, & Garavan H (2002). Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychology and Aging, 17(1), 56–71. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, Yorkcooler C, Simpson SG, Harkavyfriedman J, … Ritz AL (1994). Diagnostic Interview for Genetic Studies: Rationale, unique features, and training. Archives of General Psychiatry, 51(11), 849–859. [DOI] [PubMed] [Google Scholar]
- Olvet DM, & Hajcak G (2008). The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review, 28(8), 1343–1354. doi: 10.1016/j.cpr.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Jacobs RH, Crane NA, Ryan KA, Weisenbach SL, Ajilore O, … Langenecker SA (2015). Domain-specific impairment in cognitive control among remitted youth with a history of major depression. Early Intervention in Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AT, Jacobs RH, Crane NA, Ryan KA, Weisenbach SL, Ajilore O, … Langenecker SA (2017). Domain-Specific Impairment in Cognitive Control among Remitted Youth with a History of Major Depression. . Early Intervention in Psychiatry, 11(5), 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, & Lane R (2003). Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry, 54(5), 515–528. doi: 10.1016/s0006-3223(03)00171-9 [DOI] [PubMed] [Google Scholar]
- Reynolds WM (1999). MAQ: Multidimensional Anxiety Questionnaire: Professional manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. doi: 10.1523/jneurosci.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. doi: 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological bulletin, 139(1), 81–132. doi: 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR (2013). Major Depressive Disorder Is Associated With Broad Impairments on Neuropsychological Measures of Executive Function: A Meta-Analysis and Review. Psychological Bulletin, 139, 81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ER, Welsh RC, Fitzgerald KD, Gehring WJ, Lister JJ, Himle JA, … Taylor SF (2011). Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive-compulsive disorder. Biological Psychiatry, 69(6), 583–591. doi: 10.1016/j.biopsych.2010.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol MJ, Demenescu LR, van der Wee NJA, Kortekaas R, Nielen MMA, Den Boer JA, … Veltman DJ (2012). Functional magnetic resonance imaging correlates of emotional word encoding and recognition in depression and anxiety disorders. Biological Psychiatry, 71(7), 593–602. doi: 10.1016/j.biopsych.2011.11.016 [DOI] [PubMed] [Google Scholar]
- Votruba KL, & Langenecker SA (2013). Factor structure, construct validity, and age- and education-based normative data for the Parametric Go/No-Go Test. Journal of Clinical and Experimental Neuropsychology, 35(2), 132–146. doi: 10.1080/13803395.2012.758239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, & Hajcak G (2012). Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. Journal of Abnormal Psychology, 121(4), 885–896. doi: 10.1037/a0028270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology, 106(3), 1125–1165. doi: 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Martinez JH, Young D, Chelminski I, & Dalrymple K (2013). Severity classification on the Hamilton Depression Rating Scale. Journal of affective disorders, 150(2), 384–388. doi: 10.1016/j.jad.2013.04.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.