Abstract
We describe the design, synthesis, and biological evaluation of a series of novel HIV-1 protease inhibitors with carboxamide derivatives as the P2 ligands. We have specifically designed aminothiochromane and aminotetrahydronaphthalene-based carboxamide ligands to promote hydrogen bonding and van der Waals interactions in the active site of HIV-1 protease. Inhibitors 4e and 4j have shown potent enzyme inhibitory and antiviral activity. High resolution X-ray crystal structures of 4d- and 4k-bound HIV-1 protease revealed molecular insights into the ligand-binding site interactions.
Keywords: HIV-1 protease inhibitors, P2 ligand, Drug resistance, Design and synthesis, X-ray crystal structure
Graphical Abstract

1. Introduction
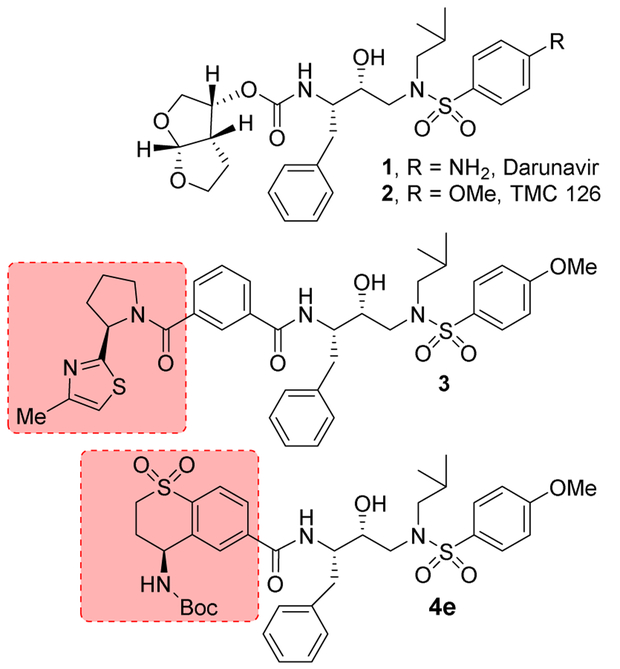
Design and development of HIV-1 protease inhibitors and their introduction in combination therapy represent a major innovation for the treatment of HIV-1 infection and AIDS.1,2 These combination antiretroviral therapies (ART) significantly improve the life expectancy of HIV-1 infected patients.3,4 The mortality rates for HIV/AIDS patients who are treated with ART, have become close to those of the general population.5,6 However, the majority of approved protease inhibitors in ART have limitations due to rapid occurrence of resistant strains, high pill burdens and other side effects.7,8 The last FDA approved protease inhibitor, darunavir (1, Figure 1), has significantly improved properties.9,10 Darunavir has been shown to maintain excellent potency against a broad-spectrum of highly multidrug-resistant HIV-1 variants.11,12 Darunavir and related derivative TMC126 (2) were specifically designed to promote extensive hydrogen-bonding interactions with HIV-1 protease backbone atoms.13,14 Indeed, the X-ray structure of darunavir-bound HIV-1 protease revealed these critical ligand-binding site interactions which are now further utilized in our molecular design.15,16 The design of protease inhibitors continue to be an important area of research. There are recent reports of novel PIs incorporating P2/P2' ligands.17-20 PIs were also designed with new P1 and P2' ligands.21,22 Recently, a new class of piperazine derived non-peptide inhibitors have been reported.23,24
Figure 1.
Structure of protease inhibitors 1-3, 4e.
In our continuing studies towards the design and synthesis of new class of PIs, we have structure-based designed a range of exceptionally potent PIs with intriguing molecular features including, GRL-6579, GRL-02031, GRL-0519, and more recently GRL-0142.14,25-28 These inhibitors exhibit broad-spectrum antiviral activity against highly multidrug-resistant HIV-1 mutant strains and also show high genetic barrier to resistance.27,29 In these inhibitors, we incorporated a variety of cyclic ether-derived templates as the P2-ligand attached to the hydroxyethylamine sulfonamide isostere with a urethane functionality.30 The major design concept behind these PIs is to promote extensive hydrogen bonding with the HIV-1 protease active site backbone atoms like a molecular crab.13,14 Recently, we and others reported a variety of protease inhibitors with benzoic acid amide derivatives as the P2-ligands.31-34 These inhibitors were designed based upon the X-ray structures of FDA approved inhibitors darunavir and nelfinavir-bound to HIV-1 protease.15,16,35 Our efforts led to very potent inhibitors with picomolar enzyme inhibitory activity and low nanomolar antiviral activity. The X-ray structural studies of a number of these inhibitors provided structural insights into the ligand and HIV-1 protease interactions, particularly in the active site of the enzyme.15,35 We have now further explored these molecular insights and we report the design, synthesis, biological evaluation, and X-ray structural studies of a new series of protease inhibitors incorporating stereochemically defined aminothiochromane and aminotetrahydronaphthalene carboxamide derivatives as the P2 ligands.
2. Results and discussion
2.1. Chemistry
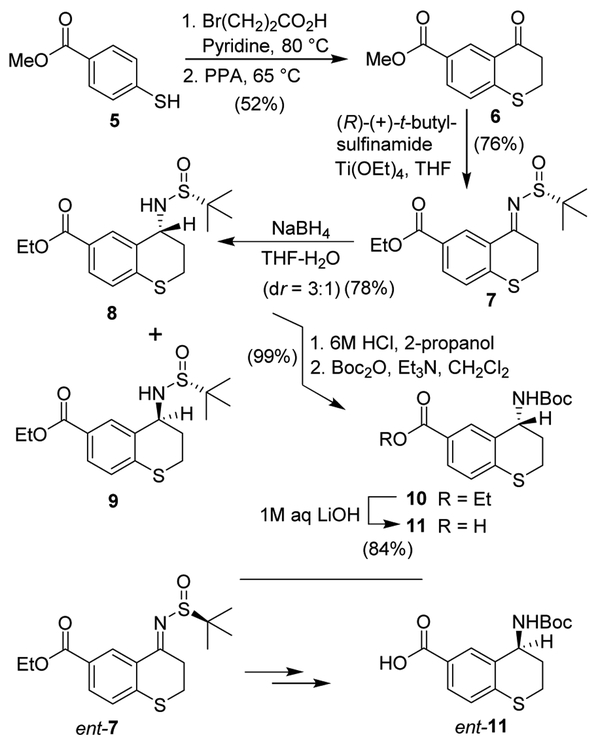
Based upon the X-ray structures of darunavir-bound HIV-1 protease and nelfinavir-bound HIV-1 protease, we designed 4-aminothiochromane and 4-aminotetrahydronaphthalene carboxamide derivatives as the P2 ligand. The synthesis of 4-aminothiochromane-6-carboxylic acid in optically active form is shown in Scheme 1. Reaction of 4-mercaptobenzoic acid methyl ester 5 with 3-bromopropionic acid in the presence of pyridine at 80 °C for 1 h provided the corresponding alkylated product. The resulting acid was reacted with polyphosphoric acid (PPA) at 65 °C for 6 h to afford 4-oxothiochromane derivative 6.36 Reaction of ketone 6 with commercially available (R)-(+)-2-methyl-2-propane sulfinamide in the presence of Ti(OEt)4 in THF at 70 °C furnished sulfinyl imins 7.37,38 Imine derivative 7 was reduced with NaBH4 in a mixture of THF and water at −50 °C to afford a mixture (3:1) of diastereomeric sulfonamides 8 and 9 in 78% combined yield. The diastereomers were separated by silica gel chromatography to provide R-sulfinyl inamide 8 as the major product. Sulfinamide 8 was treated with HCl (6 M solution in isopropanol) in methanol at 23 °C for 1 h to provide the corresponding amine hydrochloride salt. Reaction of this amine salt in CH2Cl2 with Boc2O in the presence of triethylamine afforded Boc- protected amine derivative 10. Saponification of the ethyl ester with aqueous LiOH provided carboxylic acid 11. For the synthesis of the enantiomeric amine derivative ent-11 (enantiomer of compound 11), 4-oxochromane derivative 6 was reacted with commercially available (S)-(−)-2-methyl-2-propane sulfinamide to obtain the corresponding imine which was reduced with NaBH4 to provide mixture of diastereomers. Separation of diastereomers followed by reaction of the major isomers with 6 M HCl, and protection of amine as a Boc-derivative, and aqueous LiOH hydrolysis as described above resulted in enantiomeric acid ent-11.
Scheme 1.
4-Aminothiochromane-6-carboxylic acid.
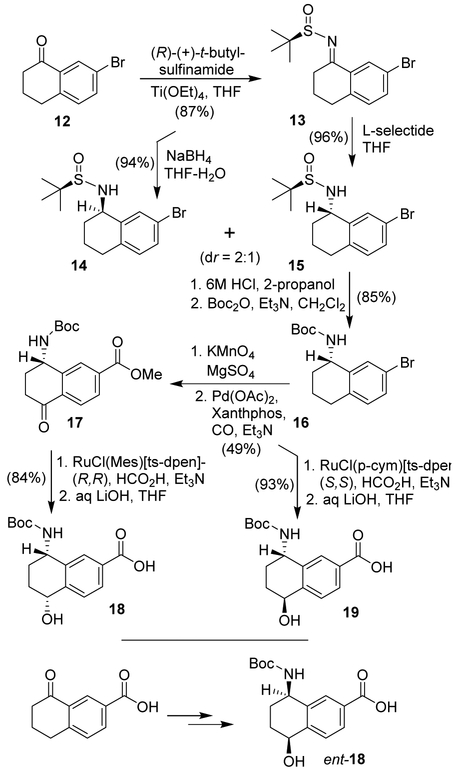
The synthesis of optically active amino-hydroxytetrahydronaphthalene carboxylic acids is shown in Scheme 2. Commercially available bromotetralone 12 was reacted with (R)-(+)-2-methyl-2-propane sulfinamide in the presence of Ti(OEt)4 in THF at 66 °C for 10 h to provide sulfinyl imine 13 in 87% yield. Reduction of 13 with NaBH4 in a mixture of (98:2) THF and water at −50 °C to 23 °C for 3 h provided 14 and 15 in 2:1 ratio in 94% combined yield. The diastereomeric sulfinamides were separated by silica gel chromatography using 30% ethyl acetate in hexanes as the eluent. Reduction of 13 with L-selectride at 0 °C to 23 °C for 3 h afforded only disatereomer 15 in 96% yield.
Scheme 2.
Synthesis of 8-amino-5-hydroxy-tetrahydronaphthalene-2-carboxylic acid.
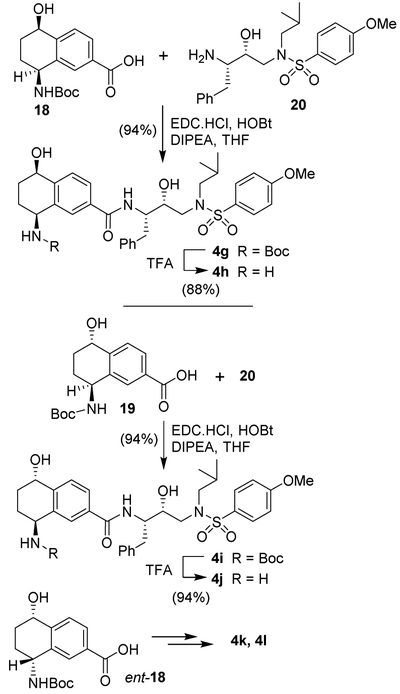
Treatment of sulfinamide 15 with HCl (6M solution in isopropanol) in methanol at 23 °C for 2.5 h afforded the corresponding amine which was reacted with Boc2O in the presence of Et3N in CH2Cl2 at 0 °C to 23 °C for 12 h to provide optically active Boc-derivative 16 in 85% yield over two-steps. For introduction of the 5-hydroxyl group, Boc-amine derivative 16 was oxidized using KMnO4 in the presence of MgSO4 in acetone at 0 °C to 23 °C for 8 h to furnish the corresponding bromoketone derivative in 61% yield. Treatment of the resulting bromoketone in methanol in the presence of catalytic amount Pd(OAc)2, (2 mol%) xantphos (4 mol%) and excess of Et3N under a CO-filled balloon at 70 °C for 3.5 h provided methyl ester derivative 17 in 80% yield.39 Ketoester 17 was converted to the corresponding Boc-aminoalcohol derivative by a catalytic transfer hydrogenation reaction using the Noyori catalyst RuCl(Mes)[R,R-ts-dpen] in DMF in the presence of formic acid and Et3N at 60 °C for 12 h to afford the corresponding alcohol as a single diastereomer in 94% yield.40,41 Saponification of the resulting methyl ester with 1N aqueous LiOH in THF in the presence of a few drops of MeOH at 23 °C for 12 h afforded carboxylic acid 18 in 89% yield. Furthermore, reduction of ketoester 17 with RuCl(p-Cym)[S,S-ts-dpen] under the same reaction conditions mentioned above, provided the diastereomeric alcohol as a single diastereomer in 93% yield. Basic ester hydrolysis furnished ligand acid 19. The synthesis of enantiomeric ligand carboxylic acid ent-18 was carried out from commercially available methyl 8-oxo-5,6,7,8-tetrahydronaphthalene-2-carboxylate and (S)-t-butyl sulfinamide to provide imine which was reduced with NaBH4 to obtain a mixture (2:1) of diastereomers. The major diastereomer was was treated with 6 M HCl to provide the corresponding amine. Protection of the resulting amine as Boc-derivative. This was converted to carboxylic acid ent-18 by following the same sequence of reactions as described above.
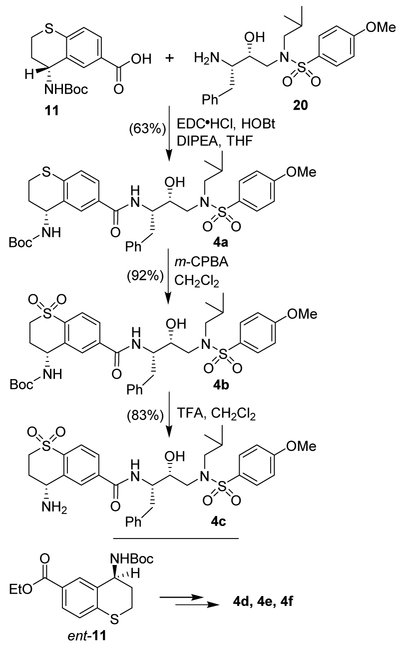
The synthesis of various inhibitors containing the (R)-hydroxyethylaminesulfonamide isostere and various thiochromane derivatives as the P2-ligand is shown in Scheme 3. Optically active thiochromane carboxylic acid 11 with the known aminoalcohol 2025,26 was reacted with EDC and HOBt in the presence of diisopropyl-ethylamine (DIPEA) in THF at 23 °C for 8 h to furnish inhibitor 4a in 63% yield. Oxidation of thiochromane derivative 4a with mCPBA in CH2Cl2 at 0 °C to 23 °C for 6 h afforded sulfone derivative 4b in 92% yield. Treatment of 4b with trifluoroacetic acid (TFA) in CH2Cl2 at 23 °C for 3 h furnished the amine derivative 4c in 83% yield. Enantiomeric ligand acid ent-11 was converted to inhibitors 4d-f as described above. The full structures of these inhibitors are shown in Table 1.
Scheme 3.
Synthesis of protease inhibitors 4a-f.
Table 1.
| Entry | Inhibitor Structure |
Ki
(nM) |
IC50
(nM) |
|---|---|---|---|
| 1 |  |
26.7 | >1000 |
| 2 |  |
0.10 | 476 |
| 3 |  |
18 | >1000 |
| 4 |  |
0.38 | >1000 |
| 5 |  |
0.008 | 47 |
| 6 |  |
15.8 | >1000 |
All antiviral assays were performed using MT-4 cells and HIVNL4-3 (subtype B). Values are the mean value of at least two experiments.
The IC50 values of amprenavir (APV), saquinavir (SQV), indinavir (IDV), and darunavir (DRV) were 0.03, 0.015, 0.03, and 0.003 μM, respectively.
The synthesis of various aminotetrahydronapthalene derivatives as the P2 ligands is shown in Scheme 4. Coupling of acid containing Boc-aminoalcohol derivative 18 with amine 20 using EDCI and HOBt in the presence of DIPEA in THF to provide inhibitor 4g. Removal of Boc-group by exposure to TFA in CH2Cl2 at 23 °C for 3 h provided inhibitor 4h with aminoalcohol functionalities. Coupling of diastereomeric ligand acid 19 with amine 20 under similar coupling conditions afforded inhibitor 4i. Removal of the Boc group with TFA provided inhibitor 4j. The corresponding enantiomeric ligand ent-19 was then converted to inhibitors 4k and 4l as described above. The full structures of these inhibitors are shown in Table 2.
Scheme 4.
Synthesis of protease inhibitors 4g-f.
Table 2.
| Entry | Inhibitor Structure |
Ki
(nM) |
IC50
(nM) |
|---|---|---|---|
| 1 |  |
0.3 | >1000 |
| 2 |  |
17 | >1000 |
| 3 |  |
0.14 | 254 |
| 4 |  |
0.06 | 232 |
| 5 |  |
1.63 | >1000 |
| 6 |  |
1.10 | >1000 |
All antiviral assays were performed using MT-4 cells and HIVNL4-3 (subtype B).
The IC50 values of amprenavir (APV), saquinavir (SQV), indinavir (IDV), and darunavir (DRV) were 0.03, 0.015, 0.03, and 0.003 μM, respectively.
2.2. HIV-1 Protease inhibitory and antiviral activity
Our preliminary model of inhibitor 4a that we created in the Nelfinavir-bound HIV-1 protease active site,35 indicated that the thiochroman heterocycle with (S)-Boc-amine functionality can interact with Asp29 and Asp30 backbone NHs in the S2 subsite, while the thiochroman moiety would fill the hydrophobic pocket. The results of HIV-1 protease inhibitory Ki, and antiviral IC50 values are shown in Table 1. The assay protocol for HIV-1 protease activity is similar to the report of Toth and Marshall.42 Antiviral activity was determined in MT-4 human T-lymphoid cells exposed to HIV-1NL4-3 (subtype B) as described by us previously.11 We chose to utilize a hydroxyethylaminesulfonamide isostere with 4-methoxybenzene sulfonamide as the P2' ligand as in inhibitor 2. As can be seen, inhibitor 4a with 4-(R)-aminothiochroman carboxamide as the P2 ligand, showed a HIV-1 protease inhibitory Ki of 26.7 nM, but did not show any appreciable antiviral activity (IC50 > 1 μM). Since sulfone oxygens are known43,44 to form strong bonding interactions, we oxidized the ring sulfur to its sulfone derivative. The resulting inhibitor 4b showed improvement of potency, exhibiting enzyme Ki, of 0.1 nM. It also exhibited improvement of antiviral activity with an IC50 value of 476 nM (entry 2). The removal of the Boc-group provided 4-amine derivative 4c which displayed significant loss of activity (entry 3). We then examined stereochemical effect and inhibitor 4d with a 4-(S)-aminothiochroman carboxamide as the P2 ligand showed potent enzyme inhibitory activity with a Ki, of 0.38 nM. However, inhibitor 4d did not exhibit appreciable antiviral activity (IC50 > 1 μM). We oxidized the ring sulfur to its sulfone derivative 4e. This led to significant improvement of enzyme inhibitory activity with a Ki of 8 pM (entry 5). Inhibitor 4e also showed very good antiviral activity (IC50 = 47 nM). We presume that the improvement of activity is due to formation of a hydrogen bond through one of the sulfone oxygens of the P2 ligand. Removal of the Boc-group provided inhibitor 4f, which showed substantial loss of enzyme inhibitory and antiviral activity similar to inhibitor 4c (entries 3 and 6). In general, this series of inhibitors showed low cytotoxicity (CC50) values in MT4 cells. The selectivity index of selected inhibitors are shown in Table 3.
Table 3.
Selectivity Index for selected inhibitors.a
| Inhibitor | CC50 (μM) | Selectivity Indexa |
|---|---|---|
| 4b | >100 | >210 |
| 4e | >100 | >2128 |
| 4i | 33.3 | 131 |
| 4j | 35.1 | 151 |
Each selectivity index denotes a ratio of CC50 to IC50.
Since both sulfone derivatives 4b and 4e are significantly more potent than the corresponding sulfides 4a and 4d, we speculated that one of the sulfone oxygens may have formed hydrogen bonding interactions with a residue in the active site. We, therefore, designed tetrahydronapthalene carboxamide derivatives with aminoalcohol substitution on the ring to mimic the interactions of Boc-amine and sulfone functionalities of inhibitor 4e. The results are shown in Table 2. Inhibitor 4g with a (R)-hydroxy derivative as the P2 ligand showed good enzyme activity, but antiviral activity was > 1 μM. The corresponding amine derivative 4h is significantly less potent (entry 2). The 4(S)-hydroxy derivative 4i showed improvement of both enzyme inhibitory and antiviral activity with an IC50 value of 254 nM. The corresponding aminoalcohol derivative 4j exhibited 1-fold improvement in enzyme activity, but showed comparable antiviral activity to its Boc-derivative 4i. We also examined the stereochemical effect of the Boc derivative 4k and the corresponding amine derivative 4l. Both compounds were less potent.
2.3. X-Ray Crystal structure of inhibitor-bound HIV-1 protease
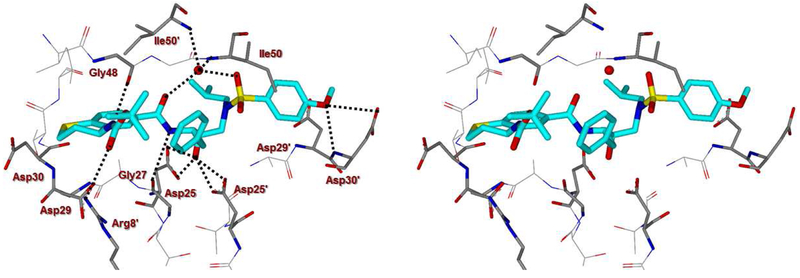
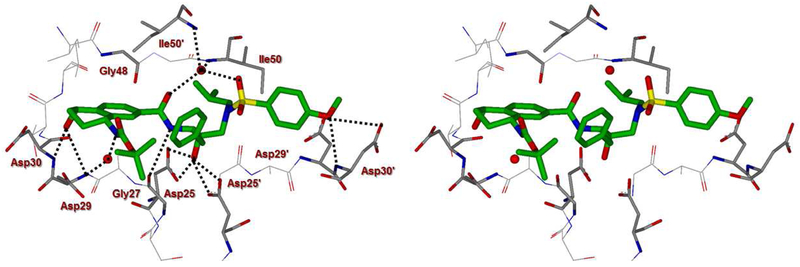
To obtain molecular insight into the ligand-binding site interactions, we set up co-crystallization experiments with several inhibitors and HIV-1 protease.45 The X-ray structures were obtained for the wild-type HIV-1 protease co-crystallized independently with inhibitors 4d (GRL-02815A) and 4k (GRL-04315A) and were refined to a resolution of 1.20 Å and 1.14 Å, respectively. The protease dimer structures were very similar to the darunavir-bound HIV-1 protease complex15 with a RMSD of 0.15 Å for 198 equivalent Cα atoms, and the largest disparity of around 0.4-0.6 Å. In both structures, the active site of the protease dimer was occupied by two alternate conformations of inhibitor related by 180° rotation with a relative occupancy of 0.60/0.40. The two inhibitor conformations show similar interactions with the protease, hence details are given for the major conformation. With the exception of the P2 ligand, both inhibitors retain the hydrogen bonds observed between darunavir and the main chain atoms of protease. These inhibitors have distinctly different P2 ligands from the bis-tetrahydrofuran in darunavir. Inhibitor 4d contains a thiochroman heterocycle with (S)-Boc-amine functionality as the P2 ligand and a stereoview of the active site interactions is shown in Figure 2. Inhibitor 4k, on the other hand, contains a tetrahydronaphthalene carboxamide with (R)-Boc-amine and (S)-hydroxyl functionalities as the P2 ligand and a stereoview of the active site interactions is shown in Figure 3.
Figure 2.
Stereoview of the X-ray structure of inhibitor 4d (turquoise)-bound HIV-1 protease (PDB code: 6DV0). All strong active site hydrogen bonding interactions of inhibitor 4d with HIV-1 protease are shown as dotted lines.
Figure 3.
Stereoview of the X-ray structure of inhibitor 4k (green)-bound HIV-1 protease (PDB code: 6DV4). All strong active site hydrogen bonding interactions of inhibitor 4k with HIV-1 protease are shown as dotted lines.
In inhibitor 4d-bound HIV-1 protease structure, the bulky sulfur atom in the thiochroman group provides hydrophobic interactions with the side chains of Asp29, Asp30 and Ile47. The amide of the carbamate group forms a hydrogen bond of 2.6 Å length with the carbonyl oxygen atom of Gly48 in the flap, and carbonyl oxygen forms a hydrogen bond of 3.4 Å with NH2 moiety of guanidinium side chain of Arg8’. As can be seen from the X-ray structure of 4d, the significant improvement of enzyme inhibitory and antiviral activity of the corresponding sulfone derivative 4e could be due to formation of hydrogen bonding interactions of the sulfone oxygens with the backbone NH’s of Asp29 and Asp30 located in the S2 subsite. The t-butyl group forms van der Waals interactions with the hydrophobic side chains of Pro81, Val82 and Phe53. In the inhibitor 4k-HIV-1 protease complex, the hydroxyl oxygen on the cyclohexane ring is in an equivalent location to the methoxy oxygen of the P2′ ligand and forms similar hydrogen bonds with the main chain amides of Asp29 and Asp30 with bond lengths of 3.5 Å and 3.1 Å, respectively, and a 2.8 Å-long hydrogen bond with the carboxylate oxygen of the Asp30 side chain. The different chiral orientation relative to inhibitor 4d shifts the carbamate away from the flap residue Gly48. The carbamate amide and carbonyl oxygen can only form hydrogen bonds via one or two water intermediates to the amide atom of Asp29 and main chain oxygen and amide of Gly49, respectively. The terminal t-butyl group embeds between the side chains of Arg8’, Pro81 and Val82, forming a C-H…π interaction with the guanidinium group of Arg8’ and van der Waals interactions with Pro81 and Val82. These differences in the P2 group may contribute to the inhibitor potency against HIV-1 protease.
3. Conclusion
In summary, we have reported the structure-based design and synthesis of a series of HIV-1 protease inhibitors incorporating stereochemically defined amino-thiochroman and aminotetrahydronaphthalene carboxamide derivatives as the P2 ligands. We have investigated various stereoisomers in order to promote effective hydrogen bonding interactions with backbone atoms in the S2 subsite. These functionalized ligands were synthesized stereoselectively in optically active form by reduction of chiral sulfinamide derivatives. Also, Noyori transfer hydrogenation using chiral ruthenium catalyst provided selective reduction of the 8-amino-5-tetralone derivatives to the corresponding 5-hydroxy-naphthalene derivatives. Amide derivatives of these ligands on a hydroxyethylamine sulfonamide isostere provided potent inhibitors. Inhibitors 4e and 4j exhibited very potent enzyme inhibitory activity in the picomolar range. These inhibitors have also shown very good antiviral activity. To obtain molecular insights into the ligand-binding site interactions, we determined high resolution X-ray crystal structures of related inhibitors 4d and 4k-bound HIV-1 protease. The structures show key interactions of amino-thiochroman and amino-tetrahydronaphthalene ligands in the S2 subsite. Both amine functionalities formed strong hydrogen bonds with the Asp30 backbone NH. This may explain the high enzyme inhibitory activity of these inhibitors. Further design and ligand optimization using X-ray structural insights are currently underway in our laboratories.
4. Experimental Section
4.1. General experimental conditions
All moisture-sensitive reactions were carried out in oven-dried glassware under an argon atmosphere unless otherwise stated. Anhydrous solvents were obtained as follows: Diethyl ether and tetrahydrofuran were distilled from sodium metal/benzophenone under argon. Toluene and dichloromethane were distilled from calcium hydride under argon. All other solvents were reagent grade. Column chromatography was performed using Silicycle SiliaFlash F60 230-400 mesh silica gel. Thin-layer chromatography was carried out using EMD Millipore TLC silica gel 60 F254 plates. 1H NMR and 13C NMR spectra were recorded on a Varian INOVA300, Bruker ARX400, Bruker DRX500, or Bruker AV-III-500-HD. Low-resolution mass spectra were collected on a Waters 600 LCMS or by the Purdue University Campus-Wide Mass Spectrometry Center. High-resolution mass spectra were collected by the Purdue University Campus-Wide Mass Spectrometry Center. HPLC analysis and purification was done an on Agilent 1100 series instrument using a YMC Pack ODS-A column of 4.6 mm ID for analysis and either 10 mm ID or 20 mm ID for purification. The purity of all test compounds was determined by HPLC analysis to be ≥95% pure.
4.2. Synthesis of inhibitors
Methyl 4-oxothiochromane-6-carboxylate (6)
A mixture of 3-bromopropionic acid (0.91 g, 5.95 mmol) and p-(carbomethoxy)thio-phenol (1 g, 5.95 mmol) was placed in a round-bottom flask. The flask was heated slowly with the aid of an oil bath. When the mixture melted, giving a homogeneous solution, pyridine (0.96 mL, 11.90 mmol) was added and the reaction was allowed to proceed under an atmosphere of nitrogen at 80 °C for 1 h. After this period, the product was dissolved in ethyl acetate and extracted repeatedly with aqueous bicarbonate. Acidification of the bicarbonate layer afforded 3-((4-(methoxycarbonyl)phenyl)thio)propanoic acid (982 mg, 69%) as an amorphous crystals. LRMS-ESI (m/z): 241 [M+H]+.
To the above 3-((4-(methoxycarbonyl)phenyl)-thio)propanoic acid (200 mg, 0.832 mmol), 1.5 g of polyphosphoric acid was added and the resulting mixture was stirred at 65 °C for 6 h. After this period, the reaction mixture was allowed to cool to room temperature and quenched by the addition of cold water and extracted with ethyl acetate (3×25 mL). The organic layer was washed with saturated aq. NaHCO3 solution, water, saturated aq. NaCl solution, and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give 6 (141 mg, 76 %) as a yellow solid. Rf = 0.4 (30% EtOAc/hexanes). LRMS-ESI (m/z): 223 [M+H]+.
Ethyl (S,E)-4-((tert-butylsulfinyl)imino)thiochromane-6-carboxylate (7)
To a stirred solution of 6 (435 mg, 1.92 mmol) in THF (18 mL) was added Ti(OEt)4 (1.0 mL, 4.805 mmol). The solution was stirred at ambient temperature for 5 min before addition of (R)-(+)-2-methyl-2-propanesulfinamide (291 mg, 2.4 mmol). Then reaction mixture refluxed for 12 h. The reaction mixture was cooled to room temperature and concentrated in vacuo, diluted with EtOAc (35 mL). Saturated NaHCO3 (17 mL) was added under vigourous stirring and the slurry was filtered through a pad of celite. The organic phase was separated, dried over Na2SO4 and concentrated in vacuo. The crude residue was purified by silica gel column chromatography (30% EtOAc/hexanes) to furnish 7 (500 mg, 76%). Rf = 0.3 (30% EtOAc/hexanes); 1H NMR (400 MHz, CDCl3) δ 8.81 (d, J = 2.0 Hz, 1H), 7.91 (dt, J = 8.3, 2.0 Hz, 1H), 7.28 (s, 1H), (q, J = 7.1 Hz, 2H), 3.77 – 3.61 (m, 1H), 3.50 (ddd, J = 16.9, 6.7, 5.5 Hz, 1H), 3.14 (dd, J = 7.1, 5.6 Hz, 2H), 1.38 (t, J = 7.3 Hz, 3H), 1.35 (s, 9H); LRMS-ESI (m/z): 340 [M+H]+.
Ethyl (R)-4-(((R)-tert-butylsulfinyl)amino)thiochromane-6-carboxylate (8) and Ethyl (S)-4-(((R)-tert-butylsulfinyl)amino)thiochromane-6-carboxylate (9)
To a srirred solution of 7 (400 mg, 1.22 mmol) in THF/H2O (4 mL, 98:2) was added NaBH4 (139 mg, 3.68 mmol) at −50 °C. The resulting solution was warmed to room temperature over 3 h. The solvent was then removed in vacuo and the resulting residue was triturated with CH2Cl2. The solution was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to furnish a crude product. The crude product was purified by flash column chromatography over silica gel (30% ethyl acetate/hexanes) to furnish 8 (250 mg, 60%) and 9 (76 mg, 18%).
Compound 8
Rf = 0.4 (50% EtOAc/hexanes); [α]D23 = −14.4 (c 0.83, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 1.9 Hz, 1H), 7.77 (dd, J = 2.0 Hz, 1H), 7.16 (d, J = 8.3 Hz, 1H), 4.56 (dt, J = 10.2, 5.5 Hz, 1H), 4.34 (q, J = 7.1 Hz, 2H), 3.49 (d, J = 9.2 Hz, 1H), 3.21 – (m, 2H), 2.43 (qd, J = 6.2, 5.4, 2.7 Hz, 2H), 1.37 (t, J = 7.1 Hz, 3H), 1.27 (s, 9H); LRMS-ESI (m/z): 342 [M+H]+.
Compound 9
Rf = 0.3 (50% EtOAc/hexanes); [α]D23 = +32.2 (c 0.75, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 1.9 Hz, 1H), 7.81 (dd, J = 1.9 Hz, 1H), 7.19 (d, J = 8.3 Hz, 1H), 4.69 (d, J = 4.7 Hz, 1H), 4.35 (q, J = 7.1 Hz, 2H), 3.34 (td, J = 12.7, 3.0 Hz, 1H), 3.17 (s, 1H), 2.85 (dt, J = 12.4, 4.0 Hz, 1H), 2.58 (dq, J = 14.5, 3.9 Hz, 1H), 2.07 – 1.93 (m, 2H), 1.38 (t, J = 7.1 Hz, 3H), 1.23 (s, 9H). LRMS- ESI (m/z): 342 [M+H]+.
Ethyl (R)-4-((tert-butoxycarbonyl)amino)thiochromane-6-carboxylate (10)
To a solution of 8 (350 mg, 1.02 mmol) in MeOH (10 mL) was added 6 M HCl in Isopropanol (4mL) at 23 °C under argon atmosphere. The reaction mixture was stirred at 23 °C for 1 h. After this period, the solvent was removed under reduced pressure to afford the desired amine salt. Thus obtained amine salt and Et3N (0.46 mL, 3.20 mmol) were dissolved in CH2Cl2 (5 mL), cooled to 0 °C and di-tert-butyldicarbonate (349 mg, 1.60 mmol) was added and allowed to warm to 23 °C. After stirring for 12 h, the reaction was diluted with CH2Cl2 and washed with water, brine solution, dried over Na2SO4 and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (10% EtOAc/hexanes) to afford 10 (347 mg, 99% over two steps). Rf = 0.3 (10% EtOAc/hexanes); [α]D23 = +36.5 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.96 (d, J = 1.9 Hz, 1H), 7.77 (dd, J = 2.0 Hz, 1H), 7.15 (d, J = 8.3 Hz, 1H), 4.89 (s, 1H), 4.75 (s, 1H), 4.35 (qd, J = 7.1, 1.4 Hz, 2H), 3.13 (td, J = 11.8, 10.6, 3.1 Hz, 1H), 3.07 – 2.98 (m, 1H), 2.39 (s, 1H), 2.09 (td, J = 10.5, 3.5 Hz, 1H), 1.49 (s, 10H), 1.38 (t, J = 7.1 Hz, 3H). LRMS-ESI (m/z): 355 [M+NH4]+.
(R)-4-((tert-Butoxycarbonyl)amino)thiochromane-6-carboxylic acid (11)
A solution of 10 (364 mg, 1.08 mmol) in THF: MeOH (6 mL, 2:1) was treated with 1N LiOH solution (1.62 mL, 1.62 mmol). The resulting mixture was stirred for 12 h, and then concentrated under reduced pressure. The residue was dissolved in water and acidified with citric acid then extracted with ethyl acetate (3×20 mL). The combined ethyl acetate layer was dried over Na2SO4, filtered, and concentrated to give carboxylic acid 11 (280 mg, 84%). Rf = 0.5 (10% MeOH/CH2Cl2); [α]D23 = +33.2 (c 0.8, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.03 (s, 1H), 7.82 (dd, J = 8.3, 1.9 Hz, 1H), 7.19 (d, J = 8.3 Hz, 1H), 4.91 (s, 1H), 4.78 (s, 1H), 3.14 (t, J = 11.5 Hz, 1H), 3.04 (ddd, J = 12.7, 6.5, 3.6 Hz, 1H), 2.39 (s, 1H), 2.18 – 2.06 (m, 1H), 1.49 (s, 9H). LRMS-ESI (m/z): 332 [M+Na]+.
Ethyl (S)-4-((tert-butoxycarbonyl)amino)thiochromane-6-carboxylate (ent-10)
Compound ent-10 (50 mg, 68%) was synthesized from ent-8 (75 mg, 0.21 mmol) by following the procedure outlined for compound 10. Rf = 0.2 (10% EtOAc/hexanes); [α]D23 = −37.5 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.96 (d, J = 1.9 Hz, 1H), 7.77 (dd, J = 8.3, 2.0 Hz, 1H), 7.15 (d, J = 8.3 Hz, 1H), 4.89 (s, 1H), 4.75 (s, 1H), 4.35 (q, J = 7.1, 1.4 Hz, 2H), 3.12 (td, J = 11.8, 10.6, 3.2 Hz, 1H), 3.06 – 2.97 (m, 1H), 2.38 (s, 1H), 2.18 – 2.03 (m, 1H), 1.48 (s, 9H), 1.38 (t, J = 7.1 Hz, 3H). LRMS-ESI (m/z): 360 [M+Na]+.
(S)-4-((tert-Butoxycarbonyl)amino)thiochromane-6-carboxylic acid (ent-11)
Compound ent-10 (120 mg, 0.37 mmol) was treated with 1N LiOH (0.55 mL, 0.55 mmol) by following the procedure outlined for compound 11 to give compound ent-11 (75 mg, 66%). Rf = 0.5 (10% MeOH/CH2Cl2); [α]D23 = −36.6 (c 0.12, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.03 (s, 1H), 7.81 (dd, J = 8.3, 1.9 Hz, 1H), 7.19 (d, J = 8.3 Hz, 1H), 4.91 (s, 1H), 4.77 (s, 1H), 3.12 (d, J = 11.2 Hz, 1H), 3.07 – 2.98 (m, 1H), 2.39 (s, 1H), 2.18 – 2.06 (m, 1H), 1.49 (s, 9H); LRMS-ESI (m/z): 332 [M+Na]+.
tert-Butyl ((R)-6-(((2S,3R)-3-hydroxy-4-((N-isobutyl-4-methoxyphenyl)sulfonamido)-1-phenylbutan-2-yl)carbamoyl)thiochroman-4-yl)carbamate (4a)
To a solution of 11 (85 mg, 0.27 mmol) and HOBt (56 mg, 0.41 mmol) in anhydrous THF (5 mL) at 0 °C was added EDC.HCl (58 mg, 0.30 mmol) and stirred at 23 °C for 1 h. An isostere amine 20 (112 mg, 0.27 mmol) and DIPEA (0.1 mL, 0.54 mmol) in THF (3 mL) was added and resulting mixture was stirred for 8 h at 23 °C. The reaction mixture was extracted with ethyl acetate and successively washed with 5% citric acid, sat NaHCO3, brine solution, dried over Na2SO4 and concentrated. The crude product was purified by column chromatography over silica gel (30% EtOAc/hexanes) to afford inhibitor 4a (120 mg, 63%). Rf = 0.3 (30% EtOAc/hexanes); [α]D23 = +22.0 (c 0.85, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.66 (t, J = 6.7 Hz, 2H), 7.57 (d, J = 5.4 Hz, 1H), 7.29 (m, 4H), 7.21 (m, 1H), 7.10 – 7.05 (m, 1H), 6.93 (t, J = 7.5 Hz, 2H), 6.48 (d, J = 8.6 Hz, 1H), 4.79 (m, 2H), 4.40 – 4.17 (m, 2H), 3.98 (s, 1H), 3.85 (s, 3H), 3.24 – 2.90 (m, 6H), 2.89 – 2.73 (m, 2H), 2.35 (d, J = 9.5 Hz, 1H), 2.04 (d, J = 12.3 Hz, 1H), 1.91 – 1.67 (m, 2H), 1.51 – 1.34 (m, 9H), 0.85 (t, J = 6.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 167.52, 163.14, 154.95, 138.99, 137.99, 133.15, 129.92, 129.52, 129.00, 128.79, 126.88, 126.79, 126.08, 114.46, 80.15, 73.00, 58.93, 55.75, 54.86, 53.60, 48.10, 35.15, 28.52, 28.25, 27.34, 22.99, 20.24, 20.14; HRMS-ESI (m/z): [M+H]+ calcd for C36H48N3O7S2, 698.2928; found 698.2925.
tert-Butyl ((R)-6-(((2S,3R)-3-hydroxy-4-((N-isobutyl-4-methoxyphenyl)sulfonamido)-1-phenylbutan-2-yl)carbamoyl)-1,1-dioxidothiochroman-4-yl)carbamate (4b)
To a solution of inhibitor 4a (50 mg, 0.07 mmol) in dichloromethane (1 mL), 3- chloroperbenzoic acid (26 mg, 0.15 mmol) was added slowly at 0 °C under argon atmosphere. The reaction was stirred for 6 h at 23 °C. The reaction mixture was diluted with dichloromethane and washed with sat. Na2CO3 solution and brine. The organic layers were dried over Na2SO4, filtered and concentrated in vacuo. The crude residue was purified by flash column chromatography over silica gel to afford inhibitor 4b (48 mg, 92%). Rf = 0.3 (60% EtOAc/hexanes); [α]D23 = −11.0 (c 0.2, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.78 – 7.70 (m, 2H), 7.70 – 7.62 (m, 2H), 7.54 (d, J = 8.1 Hz, 1H), 7.29– 7.26 (m, 4H), 7.20 (m, 1H), 7.00 – 6.91 (m, 3H), 5.20 (d, J = 8.5 Hz, 1H), 4.98 (s, 1H), 4.42 (s, 1H), 4.06 (dt, J = 12.0, 4.8 Hz, 1H), 3.86 (s, 3H), 3.50 (t, J = 11.5 Hz, 1H), 3.37 (t, J = 10.9 Hz, 1H), 3.11 (dt, J = 21.0, 7.4 Hz, 4H), 2.87 (dt, J = 10.1, 5.2 Hz, 2H), 2.70 – 2.47 (m, 2H), 1.87 (m, 1H), 1.47 (s, 9H), 0.86 (dd, J = 6.7, 3.5 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 166.26, 163.24, 155.10, 138.40, 137.88, 137.05, 130.25, 129.84, 129.54, 129.42, 128.79, 128.27, 127.44, 126.86, 124.18, 114.54, 80.64, 72.86, 60.54, 58.92, 55.79, 54.92, 53.42, 48.15, 47.72, 35.03, 28.46, 27.84, 27.33, 21.19, 20.24, 20.13, 14.32; HRMS-ESI (m/z): [M+H]+ calcd for C36H48N3O9S2, 730.2827; found 730.2824.
(S)-4-Amino-N-((2S,3R)-3-hydroxy-4-((N-isobutyl-4-methoxyphenyl)sulfonamido)-1-phenylbutan-2-yl)thiochromane-6-carboxamide 1,1-dioxide (4c)
To a stirred solution of inhibitor 4b (25 mg, 0.034 mmol) in dichloromethane (1.0 mL) was added TFA (0.1 mL) at 0 °C under argon atmosphere. The reaction mixture was warmed to 23 °C and stirred at for 3 h. Upon completion, solvent was removed under reduced pressure. The residue was extracted with dichloromethane and washed with sat. NaHCO3 solution, brine, dried over Na2SO4 and concentrated in vacuo. The crude residue was purified by column chromatography over silica gel to give inhibitor 4c (18 mg, 83%) as an amorphous solid. Rf = 0.2 (5% MeOH/CH2Cl2); [α]D23 = −13.6 (c 0.6, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.82 – 7.74 (m, 2H), 7.69 – 7.63 (m, 2H), 7.56 (dd, J = 8.2, 1.7 Hz, 1H), 7.29 – 7.23 (m, 4H), 7.19 (m, 1H), 6.95 (dd, J = 8.4, 5.4 Hz, 3H), 4.40 (tt, J = 9.4, 5.0 Hz, 1H), 4.15 (dd, J = 7.1, 4.3 Hz, 1H), 4.05 (dt, J = 4.3 Hz, 1H), 3.85 (s, 3H), 3.67 (ddd, J = 13.4, 9.8, 3.0 Hz, 1H), 3.37 – 3.24 (m, 1H), 3.22 – 2.99 (m, 4H), 2.87 (d, J = 7.5 Hz, 2H), 2.65 (dt, J = 20.3, 6.8 Hz, 1H), 2.38 – 2.26 (m, 2H), 1.87 (m, 1H), 0.85 (d, J = 6.6 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 166.71, 163.13, 142.56, 138.11, 137.26, 130.04, 129.54, 129.47, 128.68, 126.71, 114.46, 72.90, 60.51, 58.73, 55.75, 54.95, 53.48, 48.12, 44.11, 34.91, 28.56, 28.47, 27.28, 22.25, 21.73, 20.24, 20.13; HRMS-ESI (m/z): [M+H]+ calcd for C31H40N3O7S2, 630.2302; found 630.2298.
tert-Butyl ((S)-6-(((2S,3R)-3-hydroxy-4-((N-isobutyl-4-methoxyphenyl)sulfonamido)-1-phenylbutan-2-yl)carbamoyl)thiochroman-4-yl)carbamate (4d)
Compound ent-11 (65 mg, 0.21 mmol) was treated with isostere amine 20 (85 mg, 0.21 mmol) by following the procedure outlined for inhibitor 4a to give inhibitor 4d (117 mg, 80%) as an amorphous solid. Rf = 0.2 (30% EtOAc/hexanes); [α]D23 = −6.5 (c 0.49, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J = 8.5 Hz, 2H), 7.54 (s, 1H), 7.27 (d, J = 4.6 Hz, 3H), 7.24 (s, 1H), 7.19 (m, 1H), 7.06 (d, J = 8.3 Hz, 1H), 6.92 (d, J = 8.6 Hz, 2H), 6.43 (d, J = Hz, 1H), 4.78 (s, 2H), 4.32 (m, 1H), 3.96 (s, 1H), 3.84 (s, 3H), 3.22 – 2.91 (m, 6H), 2.85 (d, J = 7.5 Hz, 1H), 2.33 (s, 1H), 2.03 (d, J = 9.7 Hz, 1H), 1.83 (m, 1H), 1.72 (m, 1H), 1.45 (s, 9H), 0.84 (t, J = 5.7 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 167.80, 163.44, 155.25, 139.27, 138.28, 133.42, 130.32, 129.88, 129.10, 127.21, 127.13, 126.50, 114.78, 80.51, 73.21, 59.24, 56.08, 55.04, 53.96, 48.40, 35.43, 28.87, 28.53, 27.67, 23.27, 20.56, 20.44; HRMS-ESI (m/z): [M+H]+ calcd for C36H48N3O7S2, 698.2928; found 698.2920.
tert-Butyl ((S)-6-(((2S,3R)-3-hydroxy-4-((N-isobutyl-4-methoxyphenyl)sulfonamido)-1-phenylbutan-2-yl)carbamoyl)-1,1-dioxidothiochroman-4-yl)carbamate (4e)
Inhibitor 4d (25 mg, 0.035 mmol) was treated with mCPBA (13 mg, 0.075 mmol) by following the procedure outlined for compound 4b to give compound inhibitor 4e (26 mg, 96%) as an amorphous solid. Rf = 0.2 (60% EtOAc/hexanes); [α]D23 = −8.0 (c 0.1, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.70 (s, 1H), 7.67 (d, J = 8.8 Hz, 2H), 7.58 (dd, J = 15.8, 8.3 Hz, 1H), 7.44 – 7.36 (m, 1H), 7.30 – 7.26 (m, 4H), 7.19 (m, 1H), 7.07 (d, J = 8.6 Hz, 1H), 6.94 (d, J = 8.8 Hz, 2H), 5.34 (s, 1H), 4.97 (s, 1H), 4.40 (dq, J = 9.6, 4.8 Hz, 1H), 4.06 (dt, J = 8.3, 4.6 Hz, 1H), 3.85 (s, 3H), 3.53 (ddd, J = 8.8, 3.2 Hz, 1H), 3.36 (dd, J = 13.3, 8.0 Hz, 1H), 3.18 – 3.01 (m, 4H), 2.89 (dd, J = 7.7, 2.1 Hz, 2H), 2.70 – 2.49 (m, 3H), 1.88 (m, 1H), 1.49 (d, J = 5.0 Hz, 9H), 0.86 (d, J = 6.6 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 166.24, 163.21, 155.40, 141.03, 138.30, 137.96, 137.02, 133.68, 129.95, 129.56, 129.50, 128.75, 128.29, 127.45, 126.90, 124.00, 114.52, 80.96, 77.43 72.86, 58.84, 55.79, 54.85, 53.49, 48.04, 47.58, 34.94, 28.51, 27.32, 20.26, 20.11; HRMS-ESI (m/z): [M+H]+ calcd for C36H48N3O9S2, 730.2827; found 730.2822.
(S)-4-Amino-N-((2S,3R)-3-hydroxy-4-((N-isobutyl-4-methoxyphenyl)sulfonamido)-1-phenylbutan-2-yl)thiochromane-6-carboxamide 1,1-dioxide (4f)
Inhibitor 4f (20 mg, 0.027 mmol) was treated with TFA (0.1 mL) by following the procedure outlined for inbitor 4c to give inhibitor 4f (16 mg, 93%) as an amorphous solid; Rf = 0.1 (5% MeOH/CH2Cl2); [α]D23 = +13.7 (c 0.35, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 8.1 Hz, 2H), 7.71 – 7.63 (m, 2H), 7.56 (d, J = 8.2 Hz, 1H), 7.27 (d, J = 4.4 Hz, 4H), 7.20 (m, 1H), 6.99 – 6.91 (m, 2H), 6.88 (d, J = 8.5 Hz, 1H), 4.46 – 4.33 (m, 1H), 4.16 (s, 1H), 4.05 (dt, J = 8.4, 4.2 Hz, 1H), 3.86 (s, 3H), 3.68 (ddd, J = 9.6, 3.0 Hz, 1H), 3.38 – 3.24 (m, 1H), 3.13 (m, 4H), 2.88 (d, J = 7.5 Hz, 2H), 2.65 (t, J = 12.8 Hz, 1H), 2.41 – 2.28 (m, 1H), 1.87 (m, 2H), 0.86 (d, J = 6.6 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 166.45, 163.24, 140.49, 138.15, 137.92, 129.84, 129.56, 129.49, 128.79, 128.09, 126.93, 126.85, 124.19, 114.54, 77.41, 72.81, 58.89, 55.80, 54.84, 53.51, 48.12, 47.53, 35.00, 30.58, 27.31, 20.24, 20.15; HRMS-ESI (m/z): [M+H]+ calcd for C31H40N3O7S2, 630.2302; found 630.2294.
(R,E)-N-(7-Bromo-3,4-dihydronaphthalen-1(2H)-ylidene)-2-methylpropane-2-sulfinamide (13)
A mixture of 5-bromo-1-tetralone 12 (1.5 g, 6.66 mmol), (R)-(+)-2-methyl-2 propanesulfinamide (1.21 g, 9.99 mmol) and titanium (IV) ethoxide (3.01 g, 13.32 mmol) were dissolved in anhydrous THF (15 mL) and stirred at 66 °C for 10 h under argon atmosphere. The reaction mixture was cooled to 23 °C and ethyl acetate and aq sodium bicarbonate was added. The mixture was filtered through a pad of celite and the aqueous layer was extracted with ethyl acetate. The combined organic phase were dried over Na2SO4 and concentrated under reduced pressure. The crude residue was purified by column chromatography over silica gel (5% EtOAc/hexanes) to afford 13 (1.9 g, 87%). Rf = 0.5 (40% EtOAc/hexanes); 1H NMR (400 MHz, CDCl3) δ 8.24 (d, J = 2.2 Hz, 1H), 7.48 (dd, J = 8.2, 2.2 Hz, 1H), 7.07 (d, J = 8.2 Hz, 1H), 3.27 (ddd, J = 17.6, 9.3, 4.8 Hz, 1H), 3.05 (ddd, J = 17.6, 7.5, 4.5 Hz, 1H), 2.81 (t, J = 6.2 Hz, 2H), 1.99 – 1.87 (m, 2H), 1.33 (d, J = 2.3 Hz, 9H).
(R)-N-((R)-7-Bromo-1,2,3,4-tetrahydronaphthalen-1-yl)-2-methylpropane-2-sulfinamide (14)
Compound 13 (175 mg, 0.53 mmol) was dissolved in THF/H2O (2 mL, 98:2) and cooled to −50 °C. To the mixture was then added NaBH4 (61 mg, 1.59 mmol), and the resulting solution was warmed to 23 °C over a period of 3 h. The solvent was then removed in vacuo, and the resulting residue was triturated with dichloromethane, dried over Na2SO4, filtered, and concentrated under reduced pressure.The crude product was purified by column chromatography over silica gel (30% EtOAc/hexanes) to afford 14 (110 mg, 63%) and 15 (55 mg, 31%). Rf = 0.4 (30% EtOAc/hexanes); 1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 2.1 Hz, 1H), 7.30 (dd, J = 8.2, 2.1 Hz, 1H), 6.98 (d, J = 8.2 Hz, 1H), 4.52 (q, J = 4.2 Hz, 1H), 3.21 (d, J = 3.7 Hz, 1H), 2.76 (dt, J = 17.0, 5.2 Hz, 1H), 2.70 – 2.57 (m, 1H), 2.08 – 1.97 (m, 1H), 1.96 – 1.81 (m, 2H), 1.79 – 1.70 (m, 1H), 1.22 (s, 9H).
(R)-N-((S)-7-Bromo-1,2,3,4-tetrahydronaphthalen-1-yl)-2-methylpropane-2-sulfinamide (15)
Compound 13 (275 mg, 0.83 mmol) was dissolved in anhydrous THF (3 mL) and cooled to 0 °C. To this solution was then added L-Selectride (2.5 mL, 1.0 M in THF, 2.51 mmol) and the resulting solution was allowed to warm to 23 °C over period of 3 h. The solution was then concentrated under vacuo to furnish crude product. The crude residue was purified by column chromatography over silica gel (30% EtOAc/hexanes) to give 15 (265 mg, 96%). Rf = 0.3 (30% EtOAc/hexanes); 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 2.1 Hz, 1H), 7.28 (dd, J = 8.1, 2.1 Hz, 1H), 6.96 (d, J = 8.2 Hz, 1H), 4.42 (q, J = 8.0, 7.4 Hz, 1H), 3.36 (d, J = 10.1 Hz, 1H), 2.80 – 2.56 (m, 2H), 2.40 – 2.24 (m, 1H), 1.96 – 1.73 (m, 3H), 1.28 (s, 9H).
tert-Butyl (S)-(7-bromo-1,2,3,4-tetrahydronaphthalen-1-yl)carbamate (16)
A solution of 15 (1.2 g, 3.63 mmol) in MeOH (20 mL) was treated with 6 N HCl in isopropanol (5 mL). After 2.5 h, solvent was removed in vacuo, and the residue was co-evaporated with ethyl acetate. The residue obtained was dried under high vacuum to provide (S)-7-bromo-1,2,3,4-tetrahydronaphthalen-1-amine hydrochloride (0.94 g).
To a stirred solution of above amine hydrochloride (0.94 g, 3.6 mmol) in CH2Cl2 (30 mL) was consecutively added triethylamine (1.5 mL, 10.89 mmol) and di-tert-butyl-dicarbonate (1.17 g, 5.44 mmol) at 0 °C. The reaction mixture was stirred at 23 °C for 12 h. The reaction mixture was then diluted with CH2Cl2 and washed with water and brine solution, dried over Na2SO4 and concentrated. The crude product was purified by column chromatography over silica gel (20% EtOAc/hexanes) to give 16 (1.0 g, 85% over two steps) as an amorphous solid. Rf = 0.7 (20% EtOAc/hexanes); [α]D23 = −9.32 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.54 – 7.39 (m, 1H), 7.33 – 7.09 (m, 1H), 6.94 (d, J = 8.1 Hz, 1H), 4.78 (q, J = 10.6, 8.8 Hz, 2H), 2.88 – 2.50 (m, 2H), 2.02 (td, J = 10.9, 9.9, 4.9 Hz, 1H), 1.78 (ddp, J = 23.3, 11.4, 4.2 Hz, 3H), 1.49 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 155.55, 139.66, 136.43, 131.38, 130.84, 130.31, 119.73, 79.77, 48.67, 30.45, 28.89, 28.57, 20.05.
Methyl (S)-8-((tert-butoxycarbonyl)amino)-5-oxo-5,6,7,8-tetrahydronaphthalene-2-carboxylate (17)
Boc-amine derivative 16 (950 mg, 2.91 mmol) was dissolved in acetone (55 mL) and cooled to 0 °C. MgSO4 (837 mg, 6.99 mmol) and water (23 mL) were added to the solution. KMnO4 (2.38 g, 15.15 mmol) was added to this mixture in small portions over 1 h and stirred for 8 h at 23 °C. The solid was filtered off and the filtrate was treated with a saturated solution of sodium sulfite. The resulting mixture was filtered and the acetone was removed from the filtrate in vacuo. The remaining aqueous residue was extracted with dichloromethane. The combined organic phases were washed with water, brine, dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude residue was purified by column chromatography over silica gel (20% EtOAc/hexanes) to afford bromo ketone derivative (600 mg, 61%) as an amorphous solid. 1H NMR (400 MHz, CDCl3) δ 7.88 (d, J = 8.4 Hz, 1H), 7.62 (s, 1H), 7.53 (dd, J = 8.4, 2.0 Hz, 1H), 5.03 (s, 1H), 4.82 (d, J = 9.0 Hz, 1H), 2.81 (dt, J = 17.4, 5.3 Hz, 1H), 2.66 (ddd, J = 17.1, 11.5, 4.7 Hz, 1H), 2.49 – 2.32 (m, 1H), 2.18 – 1.99 (m, 2H), 1.51 (s, 9H).
A solution of above bromo ketone (600 mg, 1.76 mmol) in triethylamine (5.3 mL) and methanol (1 mL) was degassed with argon and palladium (II) acetate (8.0 mg, 0.035 mmol) and Xantphos (41 mg, 0.070 mmol) were added. The solution was degassed again and CO gas was bubbled through the solution for approximately 2 min. The reaction flask was fitted with a condenser and a CO balloon and the reaction mixture was heated at 70 °C for 3.5 h. The reaction mixture was cooled to 23 °C, diluted with EtOAc and filtered through a pad of celite. The filtrate was evaporated and the residue was purified by silica gel column chromatography (20% EtOAc/hexanes) to afford titled compound 17 (450 mg, 80 %). Rf = 0.3 (20% EtOAc/hexanes); [α]D23 = −10.7 (c 1.28, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.15 (s, 1H), 8.09 (d, J = 8.1 Hz, 1H), 8.03 (d, J = 8.1 Hz, 1H), 5.09 (s, 1H), 4.83 (s, 1H), 3.95 (s, 3H), 2.87 (dt, J = 17.4, 5.0 Hz, 1H), 2.71 (ddd, J = 16.9, 11.4, 4.7 Hz, 1H), 2.50 – 2.32 (m, 1H), 2.22 – 2.03 (m, 1H), 1.52 (s, 9H); LRMS-ESI (m/z): 342 [M+Na]+.
(5R,8S)-8-((tert-Butoxycarbonyl)amino)-5-hydroxy-5,6,7,8-tetrahydronaphthalene-2-carboxylic acid (18)
Argon was bubbled through a solution of 17 (150 mg, 0.46 mmol) and RuCl[(R,R)-TsDPEN](mesitylene) (9.0 mg, 0.014 mmol) in dry DMF (2 mL) for 10 min. A premixed combination of formic acid (35 μL, 0.938 mmol) and Et3N (135 μL, 0.938 mmol) was added and the mixture stirred at 60 °C for 12 h. The mixture was cooled to 23 °C and diluted with CH2Cl2 and successively washed with water, brine and dried over anhydrous Na2SO4. The solvent was removed in vacuo to give the crude product. The crude residue was purified by column chromatography over silica gel (30% EtOAc/hexanes) to afford the desired alcohol (142 mg, 94%) as an amorphous white solid. Rf = 0.3 (30% EtOAc/hexanes); 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 1H), 7.92 (d, J = 7.9 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 4.85 (s, 1H), 4.77 (m, 2H), 3.91 (s, 3H), 2.09 – 1.93 (m, 5H), 1.49 (s, 9H); LRMS-ESI (m/z): 643 [2M+H]+.
To a solution of above methyl ester (80 mg, 0.248 mmol) in THF: MeOH (1.5 mL, (2:1) was added 1N LiOH (0.37 mL, 0.373 mmol) at 23 °C. The reaction mixture was stirred at 23 °C for 12 h. Solvent was removed under reduced pressure, acidified with aq. saturated citric acid to pH 3-4 and the product was extracted with ethyl acetate, dried over Na2SO4 and concentrated to afford 18 (68 mg, 89 %) as an amorphous solid. Rf = 0.3 (10% MeOH/CH2Cl2); [α]D23 = −38.6 (c 0.29, CH3OH); 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H), 7.89 (dd, J = 8.0, 1.9 Hz, 1H), 7.52 (d, J = 8.1 Hz, 1H), 7.08 (d, J = 9.1 Hz, 1H), 4.71 (s, 2H), 2.05 – 1.89 (m, 4H), 1.50 (d, J = 4.7 Hz, 9H); LRMS-ESI (m/z): 615 [2M+H]+.
(5S,8S)-8-((tert-Butoxycarbonyl)amino)-5-hydroxy-5,6,7,8-tetrahydronaphthalene-2-carboxylic acid (19)
Argon was bubbled through a solution of 17 (150 mg, 0.469 mmol) and RuCl(p-cymene)[(S,S)-Ts-DPEN] (9.0 mg, 0.014 mmol) in dry DMF (1.5 mL) for 10 min. A premixed combination of formic acid (35 μL, 0.938 mmol) and Et3N (135 μL, 0.938 mmol) was added and the mixture was stirred at 60 °C for 12 h. The mixture was cooled to 23 °C and diluted with CH2Cl2 and successively washed with water, brine and dried over anhydrous Na2SO4. The solvent was removed in vacuo to give the crude product. The crude product was purified by column chromatography over silica gel (30% EtOAc/ hexanes) to give the desired alcohol (140 mg, 93%) as an amorphous white solid. Rf = 0.3 (30% EtOAc/hexanes); 1H NMR (400 MHz, CDCl3) δ 8.01 (s, 1H), 7.94 – 7.88 (m, 1H), 7.57 (d, J = 8.1 Hz, 1H), 4.88 (s, 1H), 4.83 – 4.59 (m, 2H), 3.90 (s, 3H), 2.38 – 2.16 (m, 2H), 2.07 – 1.94 (m, 1H), 1.89 – 1.66 (m, 2H), 1.49 (s, 9H); LRMS-ESI (m/z): 643 [2M+H]+.
Above methyl ester (75 mg, 0.233 mmol) was treated with 1N LiOH (0.46 mL, 0.466 mmol) by following the procedure outlined for 18 to give the titled compound 19 (60 mg, 85%) as an amorphous solid; Rf = 0.3 (10% MeOH/CH2Cl2); [α]D23 = −6.0 (c 0.5, CH3OH); 1H NMR (400 MHz, CDCl3) δ 7.94 (s, 1H), 7.85 (dd, J = 8.2, 1.8 Hz, 1H), 7.56 (d, J = 8.1 Hz, 1H), 4.79 – 4.67 (m, 2H), 2.24 – 2.11 (m, 3H), 1.74 (td, J = 12.1, 8.8 Hz, 2H), 1.46 (s, 9H); LRMS-ESI (m/z): 615 [2M+H]+.
(5S,8R)-8-((tert-Butoxycarbonyl)amino)-5-hydroxy-5,6,7,8-tetrahydronaphthalene-2-carboxylic acid (ent-18)
Argon was bubbled through a solution of ethyl (R)-8-((tert-butoxycarbonyl)amino)-5-oxo-5,6,7,8-tetrahydronaphthalene-2-carboxylate (100 mg, 0.299 mmol) and RuCl(p-cymene)[(S,S)-Ts-DPEN] (6.0 mg, 0.0089 mmol) in dry DMF (1.0 mL) for 10 min. A premixed combination of formic acid (22 μL, 0.598 mmol) and Et3N (83 μL, 0.598 mmol) was added and the mixture stirred at 60 °C for 12 h. The mixture was cooled to room temperature and diluted with CH2Cl2 and successively washed with water, brine and dried over anhydrous Na2SO4. The solvent was removed in vacuo to give the crude product. The crude product was purified by column chromatography over silica gel (30% EtOAc/ hexanes) to give desired alcohol (87 mg, 86%) as an amorphous white solid. Rf = 0.3 (30% EtOAc/hexanes); 1H NMR (400 MHz, CDCl3) δ 8.03 (s, 1H), 7.94 (d, J = 7.9 Hz, 1H), 7.57 (d, J = 8.1 Hz, 1H), 4.90 (s, 1H), 4.84 – 4.67 (m, 2H), 4.37 (q, J = 7.1 Hz, 2H), 2.46 – 2.12 (m, 2H), 1.90 – 1.67 (m, 3H), 1.50 (s, 9H), 1.39 (t, J = 7.2 Hz, 3H). LRMS-ESI (m/z): 353 [M+NH4]+.
Above ethyl ester (47 mg, 0.11 mmol) was treated with 1N LiOH (0.2 mL, 0.21 mmol) by following the procedure outlined for 18 to give the titled compound ent-18 (40 mg, 89%) as an amorphous solid. Rf = 0.3 (10% MeOH/CH2Cl2); LRMS-ESI (m/z): 615 [2M+H]+.
tert-Butyl ((1S,4R)-4-hydroxy-7-(((2S,3R)-3-hydroxy-4-((N-isobutyl-4-methoxyphenyl)sulfonamido)-1-phenylbutan-2-yl)carbamoyl)-1,2,3,4-tetrahydronaphthalen-1-yl)carbamate (4g)
Carboxylic acid 18 (40 mg, 0.130 mmol) was treated with isostere amine 20 (68 mg, 0.130 mmol) by following the procedure outlined for inhibitor 4a to give inhibitor 4g (85 mg, 94%) as an amorphous white solid. Rf = 0.5 (80% EtOAc/hexanes); [α]D23 = −3.2 (c 0.62, CHCl3); 1H NMR (400 MHz, CDCy δ 7.65 (d, J = 8.5 Hz, 2H), 7.57 (d, J = 17.2 Hz, 1H), 7.40 (m, 1H), 7.31 – 7.18 (m, 4H), 7.18 – 7.07 (m, 2H), 6.952 (d, J = 8.8 Hz, 2H), 6.86 (d, J = 8.5 Hz, 1H), 5.35 – 5.24 (m, 1H), 4.66 (m, 1H), 4.56 (m, 1H), 4.35 (m, 2H), 4.10 (q, J = 7.1 Hz, 1H), 3.97 (s, 1H), 3.83 (s, 3H), 3.27 – 2.93 (m, 5H), 2.85 (d, J = 7.6 Hz, 2H), 1.89 (m, 2H), 1.46 (s, 9H), 0.84 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 167.75, 163.09, 155.79, 142.78, 138.07, 137.74, 133.70, 129.94, 129.52, 128.86, 128.65, 126.90, 126.65, 126.10, 114.44, 79.83, 73.00, 67.38, 58.70, 55.73, 54.57, 53.53, 48.80, 35.08, 29.79, 29.20, 28.56, 27.24, 25.96, 20.21, 20.06; HRMS-ESI (m/z): [M+Na]+ calcd for C37H49N3O8SNa, 718.3133; found 718.3124.
(5R,8S)-8-Amino-5-hydroxy-N-((2S,3R)-3-hydroxy-4-((N-isobutyl-4-methoxyphenyl)sulfonamido)-1-phenylbutan-2-yl)-5,6,7,8-tetrahydronaphthalene-2-carboxamide (4h)
To a solution of 4g (80 mg, 0.114 mmol) in CH2Cl2 (1 mL) was added TFA (0.3 mL) and resulting solution was stirred at 23 °C for 3 h. Then reaction mixture concentrated under vacuo and extracted with ethyl acetate and washed with sat. NaHCO3, water, brine, dried over Na2SO4, filtered and evaporated under reduced pressure. The crude residue was purified by column chromatography over silica gel (10% MeOH/NH3 in CH2Cl2) to give inhibitor 4h (60 mg, 88%) as an amorphous white solid. Rf = 0.15 (10% MeOH/CH2Cl2); [α]D23 = +12.2 (c 0.63, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.66 (dd, J = 9.1, 2.8 Hz, 2H), 7.58 – 7.53 (m, 1H), 7.36 – 7.29 (m, 1H), 7.29 – 7.24 (m, 3H), 7.21 (t, J = 7.4 Hz, 2H), 7.17 – 7.07 (m, 2H), 6.91 (dd, J = 9.5, 2.6 Hz, 2H), 4.54 (d, J = 5.3 Hz, 1H), 4.38 (dp, J = 9.2, 4.8 Hz, 2H), 4.03 (dt, J = 8.4, 4.2 Hz, 2H), 3.86 (d, J = 6.0 Hz, 1H), 3.82 (s, 3H), 3.23 (dd, J = 15.1, 4.0 Hz, 1H), 3.15 – 2.96 (m, 4H), 2.88 (td, J = 13.9, 6.9 Hz, 3H), 1.97 – 1.61 (m, 6H), 0.83 (t, J = 5.6 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 168.11, 163.05, 143.02, 140.41, 138.27, 133.62, 130.06, 129.51, 128.60, 128.46, 126.94, 126.56, 125.91, 114.41, 77.48, 72.92, 67.88, 58.60, 55.71, 54.61, 53.42, 49.18, 34.97, 28.64, 28.50, 27.18, 20.20, 20.11; HRMS-ESI (m/z): [M+H]+ calcd for C32H42N3O6S, 596.2789; found 596.2785.
tert-Butyl ((1S,4S)-4-hydroxy-7-(((2S,3R)-3-hydroxy-4-((N-isobutyl-4-methoxyphenyl)sulfonamido)-1-phenylbutan-2-yl)carbamoyl)-1,2,3,4-tetrahydronaphthalen-1-yl)carbamate (4i)
Carboxylic acid 19 (40 mg, 0.13 mmol) was treated with isostere amine 20 (68 mg, 0.13 mmol) by following the procedure outlined for inhibitor 4a to give inhibitor 4i (85mg, 94%) as an amorphous solid. Rf = 0.5 (80% EtOAc/hexanes); [α]D23 = +15.0 (c 0.22, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.64 (d, J = 8.9 Hz, 2H), 7.53 (s, 1H), 7.46 – 7.43 (m, 1H), 7.41 (d, J = 8.1 Hz, 1H), 7.25 – 7.20 (m, 4H), 7.15 (td, J = 6.2, 2.9 Hz, 2H), 6.90 (d, J = 8.9 Hz, 2H), 5.03 (d, J = 8.9 Hz, 1H), 4.68 (dd, J = 16.6, 10.3 Hz, 2H), 4.28 (d, J = 20.1 Hz, 1H), 3.96 (dt, J = 8.6, 4.3 Hz, 1H), 3.82 (s, 3H), 3.22 – 2.92 (m, 4H), 2.84 (h, J = 7.6, 6.8 Hz, 2H), 2.22 (m, 6H), 1.86 (dt, J = 13.6, 6.7 Hz, 1H), 1.77 – 1.55 (m, 2H), 1.46 (s, 9H), 0.83 (d, J = 6.6 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 168.13, 163.05, 156.10, 143.27, 138.26, 137.31, 133.64, 129.93, 129.49, 128.58, 128.46, 126.57, 114.40, 80.15, 72.86, 67.63, 58.61, 55.70, 54.74, 53.42, 49.80, 49.58, 49.37, 49.03, 34.94, 30.22, 28.49, 27.73, 27.18, 20.11, 20.03; HRMS-ESI (m/z): [M+H]+ calcd for C37H50F2N3O8S, 696.3313; found 696.3305.
(5S,8S)-8-Amino-5-hydroxy-N-((2S,3R)-3-hydroxy-4-((N-isobutyl-4-methoxyphnyl)sulfonamido)-1-phenylbutan-2-yl)-5,6,7,8-tetrahydronaphthalene-2-carboxamide (4j)
Inhibitor 4i (50 mg, 0.071 mmol) was treated with TFA (0.2 mL) by following the procedure outlined for inbitor 4h to give compound inhibitor 4j (40 mg, 94%) as an amorphous solid. Rf = 0.15 (10% MeOH/CH2Cl2); [α]D23 = +35.6 (c 0.8, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.67 (d, J = 8.8 Hz, 2H), 7.62 (s, 1H), 7.38 – 7.31 (m, 2H), 7.30 – 7.24 (m, 4H), 7.22 – 7.14 (m, 1H), 6.98 – 6.86 (m, 2H), 6.83 (d, J = 8.3 Hz, 1H), 4.68 (dd, J = 7.6, 4.3 Hz, 1H), 4.37 (dd, J = 9.4, 4.9 Hz, 1H), 4.04 (m, 1H), 3.84 (s, 3H), 3.23 (dd, J = 15.0, 4.2 Hz, 1H), 3.16 – 2.97 (m, 4H), 2.87 (m 3H), 2.26 – 2.10 (m, 2H), 1.87 (dq, J = 13.8, 6.8 Hz, 2H), 1.69 (q, J = 9.6, 8.2 Hz, 2H), 1.52 (q, J = 11.3, 9.9 Hz, 1H), 0.84 (dd, J = 6.6, 2.5 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 168.10, 163.09, 142.51, 140.87, 138.30, 133.51, 130.08, 129.55, 128.68, 128.33, 126.68, 125.50, 114.44, 72.92, 68.03, 58.72, 55.74, 54.77, 53.50, 49.34, 35.00, 31.05, 30.20, 29.89, 27.22, 20.22, 20.14; HRMS-ESI (m/z): [M+H]+ calcd for C32H42N3O6S, 596.2789; found 596.2784.
tert-Butyl ((1R,4S)-4-hydroxy-7-(((2S,3R)-3-hydroxy-4-((N-isobutyl-4-methoxyphenyl)sulfonamido)-1-phenylbutan-2-yl)carbamoyl)-1,2,3,4-tetrahydronaphthalen-1-yl)carbamate (4k)
Carboxylic acid ent-18 (41 mg, 0.10 mmol) was treated with isostere amine 20 (52 mg, 0.10 mmol) by following the procedure outlined for compound 4a to give compound inhibitor 4k (75 mg, 94%) as an amorphous solid. Rf = 0.5 (80% EtOAc/hexanes); [α]D23 = +5.9 (c 0.57, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.65 (dd, J = 8.5, 6.4 Hz, 3H), 7.34 (d, J = 7.7 Hz, 1H), 7.28 (m, 4H), 7.23 – 7.15 (m, 1H), 6.99 – 6.87 (m, 2H), 6.70 (d, J = 8.7 Hz, 1H), 5.06 (d, J = 8.9 Hz, 1H), 4.71 (m, 1H), 4.64 (m, 1H), 4.47 – 4.27 (m, 2H), 3.99 (s, 1H), 3.85 (s, 3H), 3.21 – 2.99 (m, 4H), 2.85 (d, J = 7.5 Hz, 2H), 2.62 (s, 1H), 1.97 (m, 3H), 1.86 (m, 3H), 1.47 (s, 9H), 0.85 (dd, J = 6.6, 3.3 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 167.89, 163.15, 155.72, 142.88, 138.06, 137.88, 133.87, 129.95, 129.55, 128.96, 128.76, 127.01, 126.75, 126.08, 114.47, 79.93, 73.04, 67.50, 58.87, 55.76, 54.87, 53.62, 48.89, 35.02, 29.83, 29.33, 28.57, 27.34, 26.07, 20.24, 20.13; HRMS-ESI (m/z): [M+H]+ calcd for C37H50N3O8S, 696.3313; found 696.3310.
(5S,8R)-8-Amino-5-hydroxy-N-((2S,3R)-3-hydroxy-4-((N-isobutyl-4-methoxyphenyl)sulfonamido)-1-phenylbutan-2-yl)-5,6,7,8-tetrahydronaphthalene-2-carboxamide (4l)
Inhibitor 4k (30 mg, 0.043 mmol) was treated with TFA (0.1 mL) by following the procedure outlined for inbitor 4h to give inhibitor 4l (23 mg, 89%) as an amorphous solid. Rf = 0.15 (10% MeOH/CH2Cl2); [α]D23 = −9.5 (c 0.2, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.73 – 7.59 (m, 3H), 7.48 – 7.40 (m, 1H), 7.38 – 7.25 (m, 4H), 7.19 (t, J = 7.0 Hz, 1H), 6.93 (d, J = 8.6 Hz, 2H), 6.81 (d, J = Hz, 1H), 4.66 (t, J = 4.7 Hz, 1H), 4.38 (d, J = 8.7 Hz, 1H), 4.01 (dt, J = 10.4, 4.5 Hz, 2H), 3.85 (s, 3H), 3.22 (dd, J = 15.0, 4.3 Hz, 1H), 3.14 – 3.01 (m, 4H), 2.85 (dt, J = 13.5, 7.4 Hz, 2H), 1.96 – 1.76 (m, 6H), 0.85 (d, J = 6.7 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 168.03, 163.12, 143.32, 140.01, 138.25, 133.71, 130.09, 130.08, 129.56, 128.69, 126.82, 126.68, 126.30, 114.45, 73.02, 68.02, 58.67, 55.75, 54.85, 53.40, 49.38, 35.05, 29.83, 28.66, 28.09, 27.24, 20.23, 20.15; HRMS-ESI (m/z): [M+H]+ calcd for C32H42N3O6S, 596.2789; found 596.2786.
4.3. Determination of X-ray structure of HIV-1 protease inhibitor complexes.
For X-ray crystallographic studies, HIV-1 protease was expressed and purified as described.45 The protease-inhibitor complex was crystallized by the hanging drop vapor diffusion method with well solutions of 0.9M NaCl, 0.1M Sodium Cacodylate, pH 6.4 for PR/GRL-02815A (4d) complex, and 0.95M NaCl, 0.1M Sodium Acetate, pH 5.5 for PR/GRL-043-15A (4k) complex. Diffraction data were collected on a single crystal cooled to 90 K at SER-CAT (22-ID beamline), Advanced Photon Source, Argonne National Lab (Chicago, USA) with X-ray wavelength of 1.0 Å. X-ray data were processed by HKL-200046 to give Rmerge values of 8.5% and 7.8% for inhibitors 4d- and 4k-bound HIV-1 protease complexes, respectively. The crystal structures were solved by PHASER47 in CCP4i Suite48-50 using one of the previously reported isomorphous structures51 as the initial model, and refined by SHELX-201452,53 with X-ray data at 1.20 Å resolution for inhibitor 4d and HIV-1 protease complex and 1.14 Å for inhibitor 4k and HIV-1 protease complex. PRODRG-254 was used to construct the inhibitor and geometric restraints for refinement. COOT55,56 was used for modification of the model. Alternative conformations were modeled, and isotropic atomic displacement parameters (B factors) were applied for all atoms including solvent molecules. The final refined solvent structure comprised one Na+ ion, two Cl− ions, one glycerol molecules and 209 water molecules for inhibitor 4d and HIV-1 protease complex and Na+ ion, two Cl− ions, one acetate ion, one glycerol molecules and 142 water molecules for for inhibitor 4k and HIV-1 protease complex. The crystallographic statistics are listed in Table 1 (Please see, supporting information). The coordinates and structure factors of the protease complexes with inhibitors 4d and 4k have been deposited in the Protein Data Bank57 with accession codes of 6DV0 and 6DV4, respectively.
Supplementary Material
Highlights.
Design and synthesis of novel HIV-1 protease inhibitors are reported
Aminothiochromane and aminotetrahydronaphthalene derivatives were synthesized
The synthesis of ligands utilized reduction of chiral sulfinamide derivatives
The X-ray crystal structures of inhibitor-bound HIV-1 protease were determined
Hydrogen bonding and van der Waals interactions were observed in the active site
Acknowledgements
This research was supported by the National Institutes of Health (Grant GM53386 to A.K.G. and Grant GM62920 to I.T.W.). This work was also supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, and in part by a Grant-in-Aid for Scientific Research (Priority Areas) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Monbu Kagakusho), a Grant for Promotion of AIDS Research from the Ministry of Health, Welfare, and Labor of Japan, and the Grant to the Cooperative Research Project on Clinical and Epidemiological Studies of Emerging and Reemerging Infectious Diseases (Renkei Jigyo) of Monbu-Kagakusho.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References and notes
- 1.Ghosh AK, Osswald HL, Prato G, Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS, J. Med. Chem 59 (2016) 5172–5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hue S, Gifford RJ, Dunn D, Fernhill E, Pillay D, Demonstration of Sustained Drug-Resistant Human Immunodeficiency Virus Type 1 Lineages Circulating among Treatment-Naive Individuals, J. Virol, 83 (2009) 2645–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diffenbach CW, Fauci AS, Thirty Years of HIV and AIDS: Future Challenges and Opportunities, Ann. Intern. Med, 154 (2011) 766–771. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley MN, Prevention of HIV-1 Infection with Early Antiretroviral Therapy, Engl. J. Med, 365 (2011) 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohse N, Hansen AB, Gerstoft J, Obel N, Improved survival in HIV-infected persons: consequences and perspectives, J. Antimicrob. Chemother, 60 (2007) 461–463. [DOI] [PubMed] [Google Scholar]
- 6.Montaner JSG, Lima VD, Barrios R, Yip B, Wood E, Kerr T, Shannon K, Harrigan PR, Hogg RS, Daly P, Kendall P, Association of Highly Active Antiretroviral Therapy Coverage, Population Viral Load, and Yearly New HIV Diagnoses in British Columbia, Canada: a Population-Based Study, Lancet, 376 (2010) 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf, accessed on March 31, 2018.
- 8.Hue S, Gifford RJ, Dunn D, Fernhill E, Pillay D, Demonstration of Sustained Drug-Resistant Human Immunodeficiency Virus Type 1 Lineages Circulating among Treatment-Naive Individuals, J. Virol, 83 (2009) 2645–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh AK, Dawson ZL, Mitsuya H, Darunavir, a conceptually new HIV-1 protease inhibitor for the treatment of drug-resistant HIV, Bioorg. Med. Chem, 15 (2007) 7576–7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Béthune MP, Sekar V, Spinosa-Guzman S, Vanstockem M, De Meyer S, Wigerinck P, Lefebvre E, Darunavir (Prezista, TMC114): From Bench to Clinic, Improving Treatment Options for HIV-Infected Patients in Antiviral Drugs: From Basic Discovery Through Clinical Trials. John Wiley & Sons, Inc.; New Jersey, 2011, 31–45. [Google Scholar]
- 11.Koh Y, Nakata H, Maeda K, Ogata H, Bilcer G, Devasamudram T, Kincaid JF, Boross P, Wang Y-F, Tie Y, Volarath P, Gaddis L, Harrison RW, Weber IT, Ghosh AK, Mitsuya H, Novel bis-Tetrahydrofuranylurethane-Containing Nonpeptidic Protease Inhibitor (PI) UIC-94017 (TMC114) with Potent Activity against Multi-PI-Resistant Human Immunodeficiency Virus In Vitro, Antimicrob. Agent Chemother, 47 (2003) 3123–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Meyer S, Azijn H, Surleraux D, Jochmans D, Tahri A, Pauwels R, Wigerinck P, de Béthune MP, TMC114, a Novel Human Immunodeficiency Virus Type 1 Protease Inhibitor Active against Protease Inhibitor-Resistant Viruses, Including a Broad Range of Clinical Isolates, Antimicrob. Agents Chemother, 49 (2005) 2314–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh AK, Chapsal B, Weber IT, Mitsuya H, Design of HIV Protease Inhibitors Targeting Protein Backbone: An Effective Strategy for Combating Drug Resistance, Acc. Chem. Res, 41 (2008) 78–86. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh AK, Anderson DD, Weber IT, Mitsuya H, Enhancing Protein Backbone Binding – A Fruitful Concept for Combating Drug-Resistant HIV, Angew. Chem. Int. Ed, 51 (2012) 1778–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tie Y, Boross PI, Wang Y-F, Gaddis L, Hussain AK, Leshchenko S, Ghosh AK, Louis JM, Harrison RW, Weber IT, High Resolution Crystal Structures of HIV-1 Protease with a Potent Non-peptide Inhibitor (UIC-94017) Active Against Multi-drug-resistant Clinical Strains, J. Mol. Biol, 338 (2004) 341–352. [DOI] [PubMed] [Google Scholar]
- 16.Kovalevsky AY, Liu F, Leshchenko S, Ghosh AK, Louis JM, Harrison RW, Weber IT, Ultra-high resolution crystal structure of HIV-1 protease mutant reveals two binding sites for clinical inhibitor TMC114, J. Mol. Biol, 363 (2006) 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai X, Yang Z, Zhu M, Dong B, Zhou L, Zhang G, Wang J, Wang Y Design and Synthesis of Potent HIV-1 Protease Inhibitors with (S)-Tetrahydrofuran-Tertiary Amine-Acetamide as P2–Ligand: Structure–Activity Studies and Biological Evaluation. Eur. J. Med. Chem 137 (2017) 30–44. [DOI] [PubMed] [Google Scholar]
- 18.Hohlfeld K, Wegner JK, Kesteleyn B, Linclau B, Unge J Disubstituted Bis-THF Moieties as New P2 Ligands in Nonpeptidal HIV-1 Protease Inhibitors (II). J. Med. Chem 58 (2015) 4029–4038. [DOI] [PubMed] [Google Scholar]
- 19.Öhrngren P, Wu X, Persson M, Ekegren JK, Wallberg H, Vrang L, Rosenquist A, Samuelsson B, Unge T, Larhed M HIV-1 protease inhibitors with a tertiary alcohol containing transition-state mimic and various P2 and P1' substituents. MedChemComm 2 (2011) 701–709. [Google Scholar]
- 20.Ekegren JK, Gising J, Wallberg H, Larhed M, Samuelsson B, Hallberg A Variations of the P2 group in HIV-1 protease inhibitors containing a tertiary alcohol in the transition-state mimicking scaffold. Org. & Biomol. Chem 4 (2006) 3040–3043. [DOI] [PubMed] [Google Scholar]
- 21.Yan J, Huang N, Li S, Yang LM, Xing W, Zheng YT, Hu Y Synthesis and Biological Evaluation of Novel Amprenavir-Based P1-Substituted Bi-Aryl Derivatives as Ultra-Potent HIV-1 Protease Inhibitors. Bioorg. Med. Chem. Lett 22 (2012) 1976–1979. [DOI] [PubMed] [Google Scholar]
- 22.Yang ZH, Bai XG, Zhou L, Wang JX, Liu HT, Wang YC Synthesis and Biological Evaluation of Novel HIV-1 Protease Inhibitors Using Tertiary Amine as P2-Ligands. Bioorg. Med. Chem. Lett 25 (2015) 1880–1883. [DOI] [PubMed] [Google Scholar]
- 23.Bungard CJ, Williams PD, Ballard JE, Bennett DJ, Beaulieu C, Bahnck-Teets C, Carroll SS, Chang RK, Dubost DC, Fay JF, Diamond TL, Greshock TJ, Hao L, Holloway MK, Felock PJ, Gesell JJ, Su HP, Manikowski JJ, McKay DJ, Miller M, Min X, Molinaro C, Moradei OM, Nantermet PG, Nadeau C, Sanchez RI, Satyanarayana T, Shipe WD, Singh SK, Truong VL, Vijayasaradhi S, Wiscount CM, Vacca JP, Crane SN, McCauley JA Discovery of MK-8718, an HIV Protease Inhibitor Containing a Novel Morpholine Aspartate Binding Group. ACS Med. Chem. Lett 7 (2016) 702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bungard CJ, Williams PD, Schulz J, Wiscount CM, Holloway MK, Loughran HM, Manikowski JJ, Su HP, Bennett DJ, Chang L, Chu XJ, Crespo A, Dwyer MP, Keertikar K, Morriello GJ, Stamford AW, Waddell ST, Zhong B, Hu B, Ji T, Diamond TL, Bahnck-Teets C, Carroll SS, Fay JF, Min X, Morris W, Ballard JE, Miller MD, McCauley JA Design and Synthesis of Piperazine Sulfonamide Cores Leading to Highly Potent HIV-1 Protease Inhibitors. ACS Med. Chem. Lett 8 (2017) 1292–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh AK, Sridhar PR, Leshchenko S, Hussain AK, Li J, Kovalevsky AY, Walters DE, Wedekind JE, Grum-Tokars V, Das D, Koh Y, Maeda K, Gatanaga H, Weber IT, Mitsuya H, Structure-Based Design of Novel HIV-1 Protease Inhibitors To Combat Drug Resistance, J. Med. Chem, 49 (2006) 5252–5261. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh AK, Xu C-X, Rao KV, Baldridge A, Agniswamy J, Wang Y-F, Weber IT, Aoki M, Miguel SGP, Amano M, Mitsuya H, Probing Multidrug-Resistance and Protein–Ligand Interactions with Oxatricyclic Designed Ligands in HIV-1 Protease Inhibitors, ChemMedChem, 5 (2010) 1850–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh AK, Chapsal BD, Design of the anti-HIV protease inhibitor darunavir In ‘From Introduction to Biological and Small Molecule Drug Research and Development’ Ed. Ganellin CR; Roberts SM; Jefferis R 2013, 355–384. [Google Scholar]
- 28.Ghosh AK, Rao KV, Nyalapatla PR, Kovela S, Brindisi M, Osswald HL, Reddy BS, Agniswamy J, Wang Y-F, Aoki M, Hattori S.-i., Weber IT, Mitsuya H, Design of Highly Potent, Dual Acting and Central Nervous System-Penetrating HIV-1 Protease Inhibitors with Excellent Potency against Multidrug-Resistant HIV-1 Variants ChemMedChem, 13 (2018) 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoki M, Hayashi H, Rao KV, Das D, Higashi-Kuwata N, Bulut H, Aoki-Ogata H, Takamatsu Y, Yedidi RS, Davis DA, Hattori S.-i., Nishida N, Hasegawa K, Takamune N, Nyalapatla PR, Osswald HL, Jono H, Saito H, Yarchoan R, Misumi S, Ghosh AK, Mitsuya H, A novel central nervous system-penetrating protease inhibitor overcomes human immunodeficiency virus 1 resistance with unprecedented aM to pM potency, eLife, 6 (2017) e28020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh AK, Sridhar PR, Kumaragurubaran N, Koh Y, Weber IT, Mitsuya H, Bis-Tetrahydrofuran: a Privileged Ligand for Darunavir and a New Generation of HIV Protease Inhibitors That Combat Drug Resistance, ChemMedChem, 1 (2006) 939–950. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh AK, Schiltz GE, Rusere LN, Osswald HL, Walters DE, Amano M, Mitsuya H, Design and synthesis of potent macrocyclic HIV-1 protease inhibitors involving P1–P2 ligands, Org. Biomol. Chem, 12 (2014) 6842–6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh AK, Swanson LM, Cho H, Hussain KA, Leschenko S, Kay S, Walters DE, Mitsuya H, Structure-Based Design: Synthesis and Biological Evaluation of a Series of Novel Cycloamide-Derived HIV-1 Protease Inhibitors, J. Med. Chem, 48 (2005) 3576–3585. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh AK, Brindisi M, Nyalapatla PR, Takayama J, Ella-Menye J-R, Yashchuk S, Agniswamy J, Wang Y-F, Aoki M, Amano M, Weber IT, Mitsuya H, Design of novel HIV-1 protease inhibitors incorporating isophthalamide-derived P2-P3 ligands: Synthesis, biological evaluation and X-ray structural studies of inhibitor-HIV-1 protease complex, Bioorg. & Med. Chem, 25 (2017) 5114–5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid RC, Pattenden LK, Tyndall JDA, Martin JL, Walsh T, Fairlie DP, Countering Cooperative Effects in Protease Inhibitors Using Constrained β-Strand-Mimicking Templates in Focused Combinatorial Libraries, J. Med. Chem, 47 (2004) 1641–1651. [DOI] [PubMed] [Google Scholar]
- 35.Kaldor SW, Kalish VJ, Davies JF, Shetty BV, Fritz JE, Appelt K, Burgess JA, Campanale KM, Chirgadze NY, Clawson DK, Dressman BA, Hatch SD, Khalil DA, Kosa MB, Lubbehusen PP, Muesing MA, Patick AK, Reich SH, Su KS, Tatlock JH, Viracept (Nelfinavir Mesylate, AG1343): A Potent, Orally Bioavailable Inhibitor of HIV-1 Protease, J. Med. Chem, 40 (1997) 3979–3985. [DOI] [PubMed] [Google Scholar]
- 36.Moriwake T, Syntheses of 3-Cyano-3-methyl-4-thiochromanone and 3-Carbomethoxy-3-methyl-4-thiochromanone, J. Med. Chem, 9 (1966) 163–164. [DOI] [PubMed] [Google Scholar]
- 37.Tanuwidjaja J, Peltier HM, Ellman JA, One-Pot Asymmetric Synthesis of Either Diastereomer of tert-Butanesulfinyl-protected Amines from Ketones, J. Org. Chem, 72 (2007) 626–629. [DOI] [PubMed] [Google Scholar]
- 38.Colyer JT, Andersen NG, Tedrow JS, Soukup TS, Faul MM, Reversal of Diastereofacial Selectivity in Hydride Reductions of N-tert-Butanesulfinyl Imines, J. Org. Chem, 71 (2006) 6859–6862. [DOI] [PubMed] [Google Scholar]
- 39.Martinelli JR, Watson DA, Freckmann DMM, Barder TE, Buchwald SL, Palladium-Catalyzed Carbonylation Reactions of Aryl Bromides at Atmospheric Pressure: A General System Based on Xantphos, J. Org. Chem, 73 (2008) 7102–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noyori R, Ohkuma T, Asymmetric Catalysis by Architectural and Functional Molecular Engineering: Practical Chemo- and Stereoselective Hydrogenation of Ketones, Angew. Chem. Int. Ed, 40 (2001) 40–73. [PubMed] [Google Scholar]
- 41.Ohkuma T, Tsutsumi K, Utsumi N, Arai N, Noyori R, Murata K, Asymmetric Hydrogenation of α-Chloro Aromatic Ketones Catalyzed by η6-Arene/TsDPEN–Ruthenium(II) Complexes, Org. Lett, 9 (2007) 255–257. [DOI] [PubMed] [Google Scholar]
- 42.Toth MV, Marshall GR, A Simple Continuous Fluorometric Assay for HIV Protease, Int. J. Pept. Protein Res, 36 (1990) 544–550. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh AK, Thompson WJ, McKee SP, Duong TT, Lyle TA, Chen JC, Darke PL, Zugay J, Emini EA, Schleif WA, Huff JR, Anderson PS, Cyclic sulfolanes as novel and high-affinity P2 ligands for HIV-1 protease inhibitors, J. Med. Chem, 36 (1993) 924–927. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh AK, Thompson WJ, Culberson C, Holloway MK, McKee SP, Duong TT, Munson PM, Darke PL, Zugay J, Emini EA, Schleif WA, Huff JR, Anderson PS, The Development of Cyclic Sulfolanes as Novel and High-Affinity P2 Ligands for HIV-1 Protease Inhibitors, J. Med. Chem, 37 (1994) 1177–1188. [DOI] [PubMed] [Google Scholar]
- 45.Mahalingam B, Louis JM, Hung J, Harrison RW, Weber IT, Structural implications of drug-resistant mutants of HIV-1 protease: high-resolution crystal structures of the mutant protease/substrate analogue complexes, Proteins, 43 (2001) 455–464. [DOI] [PubMed] [Google Scholar]
- 46.Otwinowski Z, Minor W, Processing of X-ray Diffraction Data Collected in Oscillation Mode Methods in Enzymology, 276: Macromolecular Crystallography, Part A; Carter CW Jr., Sweet RM, Eds.; Academic Press: New York, 1997; pp 307–326. [DOI] [PubMed] [Google Scholar]
- 47.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ, Phaser crystallographic software, J. Appl. Crystallogr, 40 (2007) 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AGW, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A, Wilson KS, Overview of the CCP4 suite and current developments, Acta Crystallogr., Sect. D: Biol. Crystallogr, 67 (2011) 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collaborative Computational Project, Number 4, The CCP4 suite: programs for protein crystallography, Acta Crystallogr., Sect. D: Biol. Crystallogr, 50 (1994) 760–763. [DOI] [PubMed] [Google Scholar]
- 50.Potterton E, Briggs P, Turkenburg M, Dodson E, A graphical user interface to the CCP4 program suite, Acta Crystallogr., Sect. D: Biol. Crystallogr, 59 (2003) 1131–1137. [DOI] [PubMed] [Google Scholar]
- 51.Shen C-H, Wang Y-F, Kovalevsky AY, Harrison RW, Weber IT, Amprenavir complexes with HIV 1 protease and its drug resistant mutants altering hydrophobic clusters, FEBS J, 277 (2010) 3699–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheldrick GM, A short history of SHELX, Acta Crystallogr., Sect. A: Found. Crystallogr, 64 (2008) 112–122. [DOI] [PubMed] [Google Scholar]
- 53.Sheldrick GM, Schneider TR, SHELXL: high-resolution refinement, Meth. Enzymol, 277 (1997) 319–343. [PubMed] [Google Scholar]
- 54.Schuettelkopf AW, van Aalten DMF, PRODRG: a tool for high-throughput crystallography of protein-ligand complexes, Acta Crystallogr., Sect. D: Biol. Crystallogr, 60 (2004) 1355–1363. [DOI] [PubMed] [Google Scholar]
- 55.Emsley P, Lohkamp B, Scott WG, Cowtan K, Features and development of Coot, Acta Crystallogr., Sect. D: Biol. Crystallogr, 66 (2010) 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Emsley P, Cowtan K, Coot: model-building tools for molecular graphics, Acta Crystallogr., Sect. D: Biol. Crystallogr, 60 (2004) 2126–2132. [DOI] [PubMed] [Google Scholar]
- 57.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE, The Protein Data Bank, Nucleic Acids Res, 28 (2000) 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.