Summary
Background
Elevated d-dimer levels are associated with poor clinical outcomes in subjects with treated HIV infection. Protease Activated Receptor-1 (PAR-1) is activated by thrombin and overexpressed on immune cells from HIV-infected subjects. We studied a licensed inhibitor of PAR-1, vorapaxar, to reduce HIV associated hypercoagulation and inflammation.
Methods
We performed a multicentre, double-blind, randomised, placebo-controlled trial involving HIV infected, aviremic participants on stable ART with d-dimer levels >200ng/mL. Outpatients in Australia and the USA were randomly assigned through computer generated block lists to receive vorapaxar (2·5mg orally daily), or matched placebo for 12 weeks. The primary endpoint was treatment group difference in changes from baseline d-dimer levels after 8–12 weeks of treatment in a modified intention-to-treat group. This trial is registered with Clinicaltrials.gov, number NCT02394730, and closed to new participants.
Findings
Between October 21 2015 and July 14 2017, 65 eligible subjects were randomly assigned to the placebo (n=31) or vorapaxar group (n=34). The modified intention to treat population comprised participants with at least one dose of study drug and/or one follow up visit (31 placebo, 33 vorapaxar). D-dimer levels after 8–12 weeks treatment were not different in vorapaxar compared to placebo treated groups (difference −0·02 log10ng/mL, 95% CI of −0·10 to 0·05, p = 0·56). Vorapaxar treatment was safe and well tolerated in this subject cohort, with suppression of HIV replication maintained.
Interpretation
Vorapaxar had no impact on d-dimer levels or inflammatory markers in HIV infected subjects on stable ART but at risk for poor outcomes. Alternative approaches are needed to reduce hypercoagulation, inflammation and adverse long-term outcomes in subjects with treated HIV infection.
Introduction
Elevated expression of d-dimer (a marker of coagulopathy) and elevated hs-CRP and IL-6 (markers of immune activation/inflammation) are associated with increased risk of death and serious end-organ diseases among people with HIV infection.1–3 These markers are increased in untreated HIV replication, but even among people with well controlled HIV on combination antiretroviral therapy (ART) there is a consistent relationship between higher d-dimer levels and poorer clinical outcome.4–7 While ART reduces levels of d-dimer, it does not result in normalisation.6,8,9 Among subjects with suppressed plasma HIV RNA levels, expression of d-dimer and inflammation markers is higher than in age matched populations without HIV infection.10 Interventions to reduce either hypercoagulation and/or immune activation may both permit a clearer understanding of the underlying pathogenesis and be of therapeutic benefit.
The relationship between coagulopathic disorder and immune activation is an evolving area of research interest.11–13 Tissue injury results in the release of tissue factor that promotes the coagulation cascade resulting in thrombus formation. T-cells differentially express receptors linked to this cascade and are activated at times when tissue injury has occurred. A novel observation suggests that CD8+ T lymphocytes from HIV infected persons over-express Protease Activated Receptor-1 (PAR-1).14 PAR-1 is activated by thrombin and CD8+ cells expressing PAR-1 become activated (express cytokines and chemokines) in a dose dependent fashion to exogenous thrombin.
The sources of tissue injury, immune activation and hypercoagulopathy in people with well controlled HIV replication are not known. Increased levels of tissue factor expression are present in monocytes from people with HIV-1 infection.15 Analysis of thrombin generation suggests the net effect of HIV replication is pro-coagulant, although the degree to which this persists after suppression of HIV replication is uncertain.16 It is plausible that tissue injury in the setting of HIV replication promotes thrombin formation and PAR-1 dependent signalling that in turn supports immune activation and inflammation.17 PAR-1 may therefore be a potential target for therapeutic manipulation in the setting of well controlled HIV infection.
Vorapaxar is an oral competitive PAR-1 antagonist that mediates anticoagulation through inhibiting thrombin-induced platelet aggregation. Vorapaxar has been studied in large clinical endpoint trials in cardiovascular disease and is licensed as secondary prophylaxis for subjects with a history of myocardial infarction or peripheral arterial disease.18,19 We hypothesised that vorapaxar could reduce markers of hyper-coagulation and inflammation in subjects with well treated HIV at risk for adverse clinical outcomes.
Methods
Study design and participants
The ADVICE study (Attenuation of D-dimer using Vorapaxar to target Inflammatory and Coagulation Endpoints) was a double-blind, randomised, placebo-controlled trial at seven health centres in 5 hospital clinic or general practice sites in Australia (Melbourne and Sydney) and 2 hospital clinic sites in the USA (Minneapolis and Washington DC). HIV infected people over the age of 40 with suppressed HIV viremia (plasma HIV RNA <50 copies per mL) for at least 24 weeks and a d-dimer level of >200ng/mL were eligible. Antiretroviral regimens excluded HIV protease and non-nucleoside reverse transcriptase inhibitors (except rilpivirine) because of potential drug-drug interactions with vorapaxar. Subjects taking other anti-coagulants or a history of cardiovascular disease were also excluded. A complete list of inclusion and exclusion criteria is provided in the trial protocol (Appendix page 12–111).
The trial was approved by the research ethics board for each trial centre and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All subjects provided written informed consent. The trial was monitored by an independent Data and Safety Monitoring Board (DSMB). The results were collected and analysed by the writing committee. No interim analysis was specified in the protocol nor recommended by the DSMB which reviewed safety data at predefined intervals or in response to reported serious adverse events.
Randomisation and masking
Randomisation was through computer generated block lists of size 2, stratified by site, and allocated double-blind through a web-based database system.
Procedures
Consenting participants were screened and within 14 days randomly allocated to receive either vorapaxar sulphate (2·5mg daily) or matched placebo for 12 weeks. Participants were reviewed and had a blood sample taken at weeks 1, 4, 8 and 12 during treatment. At the week 12 visit, the study treatment was stopped and subjects reviewed and had a blood sample taken at the week 18 final visit.
Cryopreserved plasma samples were retrospectively batch-analysed at Leidos Biomedical Research Inc. (Maryland, USA) D-dimer was measured using an Enzyme Linked Flourescent Assay on a VIDAS instrument (bioMerieux, Marcy l’Etoile, France). Traditional ELISAs were used to measure sCD14 (R&D Systems, Minneapolis, MN, USA) and sCD163 (Aviscera Bioscience Inc., Santa Clara, CA, USA). Hs-CRP and IL-6 were measured by electrochemiluminescense (Meso Scale Discovery, Rockville, MD, USA).
To measure PAR-1 levels, we used flow cytometry for PAR-1 expression on gated CD4 and CD8 T cells. This was performed on fresh blood (<4hr from venepuncture) in the subset of enrolled subjects in Australia (n=39) with access to validated flow cytometry assays. Detailed methods and gating strategies of the flow cytometry assays are shown in the Appendix page 5.
All adverse events (see protocol in the Appendix page 12–111) were collected and summarised by randomised treatment group, severity and relation to study drug. Serious adverse events were summarised for all enrolled participants. A particular focus of safety analyses was bleeding events given the anti-coagulant nature of vorapaxar and were classified according to the Bleeding Academic Research Consortium (BARC) criteria (Appendix page 12–111).
Outcomes
The primary endpoint was the difference between treatment groups in changes in d-dimer from baseline to the average of weeks 8 and week 12. Secondary endpoints included change in d-dimer between week 12 and week 18 (after cessation of vorapaxar/placebo), changesin HIV RNA, changes in CD4 and CD8 T cell counts, and changes in inflammatory markers hs-CRP and IL-6. Pre-specified exploratory endpoints included changes in activation markers of monocytes (soluble CD14 and CD163) and PAR-1 expression on CD4 and CD8 T cells. Full details of all endpoints are provided in the protocol (Appendix page 12–111). Additional exploratory endpoints listed in the protocol of cell-associated HIV levels, ultrasensitive viral load, T cell activation markers and Natural Killer cell functions were not pursued.
Statistical Analysis
A linear regression for change (log10) of d-dimer from baseline to an average of weeks 8 and 12 modelled against treatment and baseline outcome variable was used for the primary endpoint. The standard deviation of d-dimer levels in a previous study of ART-treated subjects with d-dimer >200ng/mL was 0·36 log10 ng/mL at week 12 and 0·41 log10 ng/mL at week 24.20 Using a repeated measures regression analysis at week 8 and 12, assuming variability in change in log10 d-dimer=0·4, and correlation between these two time points as 0·57, then a total sample size of 56 subjects (28 in each arm) gives 80% power to detect a mean difference of 0·26 logs. Recruitment of 60 subjects was planned, allowing for some non-completion. To give an idea of the absolute magnitude of differences we would be powered to detect, assuming no change in log10 d-dimer in the placebo group, this mean difference of 0·26 logs corresponds to a 45% decrease in d-dimer from baseline in the vorapaxar group. This would move most subjects to at least one lower quartile of d-dimer levels. It has previously been calculated that a one quartile change in d-dimer levels is associated with an adjusted odds ratio of 5.3 for the risk of serious non-AIDS events (including cardiovascular events) or death.4 We reasoned that such a change in d-dimer, as a marker, would be required to justify pursuing vorapaxar in larger clinical studies.
A detailed statistical analysis plan was prepared and finalised before the database was finalised (Appendix pages 112–117). A modified intention to treat approach was taken for primary analyses, including all randomised participants who received study drug and had any follow-up data. All available follow-up data were included regardless of whether participants ceased study drug. Changes in continuous endpoints were analysed using regression models adjusted for baseline values. Binary endpoints were analysed using logistic regression. All analyses were done with Stata (version 14.2). The study protocol was registered at clinicaltrials.gov (#NCT02394730).
Role of the funding source
The trial was designed by the authors and supported by funding from the Australian National Health and Medical Research Council and U.S. National Cancer Institute, National Institutes of Health, which were not involved in the trial design, conduct, or analyses. The pharmaceutical company Merck provided vorapaxar and matched placebo but there was no other industry support or funding. Merck was not otherwise involved in the trial design, conduct, or analyses. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
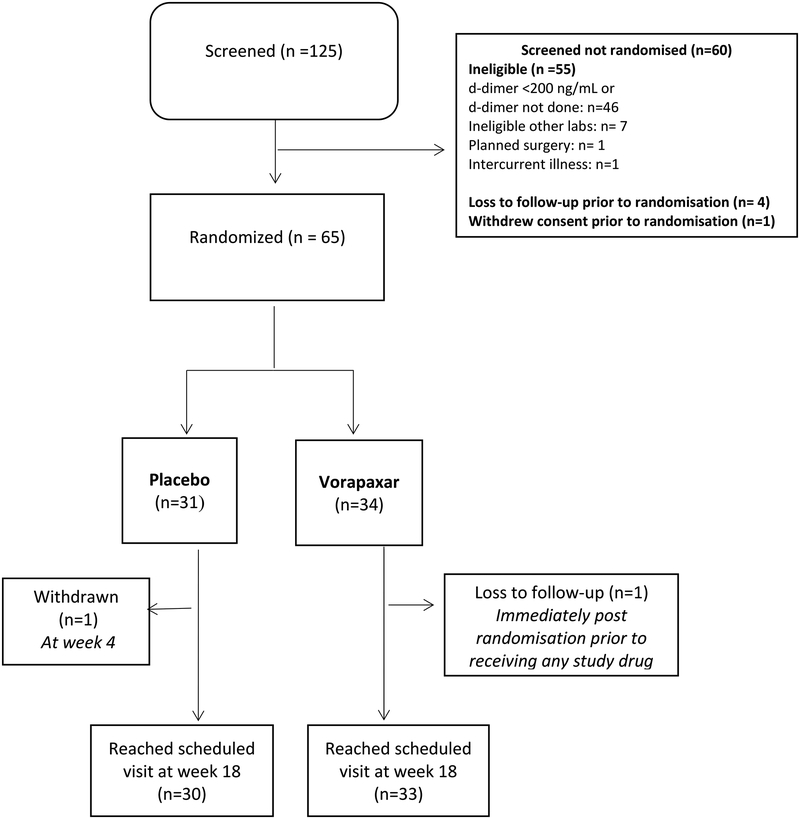
A total of 125 participants underwent screening; 65 were eligible and underwent randomisation from October 14 2015 through July 14 2017 (34 to the vorapaxar group and 31 to the placebo group) at five centres in Australia and two in the United States. Reasons for screening failure were primarily related to d-dimer levels of <200ng/ml (Fig 1). One participant assigned to vorapaxar was lost to follow up immediately after randomisation prior to receiving any study drug, leaving modified intentional to treat (mITT) groups of 31 in the placebo arm and 33 in the vorapaxar arm. One participant in the placebo group withdrew consent at week 4. Thirty participants in the placebo arm and 33 in the vorapaxar arm reached the end of the study at week 18.
Figure 1. Subject disposition.
Diagram illustrating subject disposition. The modified intention to treat group (placebo 31, vorapaxar 34) excluded the one subject randomised to vorapaxar who was lost to follow up before receiving any study drug.
Baseline demographic and clinical characteristics of the two trial groups were balanced (Table 1). Participants were primarily (59 of 64, 91%) male, had a median age of 52 years, and had a baseline risk of CVD within 10 years of 11·4% using a Framingham Heart Study calculator. Participants had been diagnosed with HIV infection for a median of 12·5 years. Participants had controlled HIV RNA and an average CD4+ T cell count of 643 cells per μL. The most common current ART regimen was two N(t)RTI in combination with either dolutegravir or raltegravir (55 of 64, 86%), with the remaining participants on two NRTIs in combination with rilpivirine. The trial successfully recruited participants at risk of future adverse outcomes based on the median d-dimer concentration (ng/mL) at baseline of 421·9ng/mL.
Table 1:
Baseline characteristics
| placebo (n=31) | vorapaxar (n=33) | total (n=64) | |
|---|---|---|---|
| Age (years) | 52 (48–60)* | 52·5 (48–58) | 52 (48–60) |
| Sex (male) | 28 (90·3%) | 31 (91·2%) | 59 (90·8%) |
| Total cholesterol (mmol/L) | 4·7 (3·9–5·4) | 4·6 (4–5·5) | 4·7 (4–5·4) |
| HDL (mmol/L) | 1·3 (0·9–1·6) | 1·2 (1–1·4) | 1·2 (1–1·5) |
| Systolic blood pressure | 127 (120–136) | 125 (115–133) | 126·5 (117·5–135) |
| Diastolic blood pressure | 80 (70–86) | 77 (70–85) | 78·5 (70–85·5) |
| Current smoker (n[%]) | 9 (29%) | 9 (27·3%) | 18 (28·1%) |
| Framingham heart score: 10 year CVD risk %† | 12·1 (8·3–19·4) | 10·6 (7·3–21·1) | 11·4 (7·9–19·8) |
| d-dimer (ng/mL) | 391·6 (302·3–813·7) | 432·5 (298·0–531·1) | 421·9 (299·0–687·6) |
| hs-CRP (μg/mL) | 1·97 (0·61, 4·81) | 1·53 (0·50, 3·01) | 1·58 (0·50, 3·86) |
| IL-6 (pg/mL) | 0·99 (0·69, 1·54) | 0·93 (0·61, 1·39) | 0·94 (0·62, 1·52) |
| Estimated duration of HIV infection (years) | 12·2 (8·6, 22·4) | 12·8 (9·2, 24·3) | 12·5 (8·8, 23·2) |
| Plasma HIV RNA (copies/mL) | 20 (20–48) | 20 (20–48) | 20 (20–48) |
| CD4+ T-cells/mm3 | 698 (490–869) | 639 (504–768) | 642·5 (497–828·5) |
| Time on current ART (years) | 2 (0·7–4) | 1·3 (0·7–3) | 1·5 (0·7–3·9) |
| ART regimens: | |||
| 2 × N(t)RTI + dolutegravir or raltegravir (n[%]) | 28 (90%) | 27 (82%) | 55 (86%) |
| 2 × N(t)RTI + rilvipirine (n[%]) | 3 (10%) | 6 (18%) | 9 (14%) |
| HCV RNA positive/HCV seropositive (n[%]) | 0/3 (10%) | 0/5 (15%) | 0/8 (12%) |
All values are median (IQR) based on modified intention to treat group unless otherwise noted.
As calculated by reference: D’Agnostino RB et al, General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–53
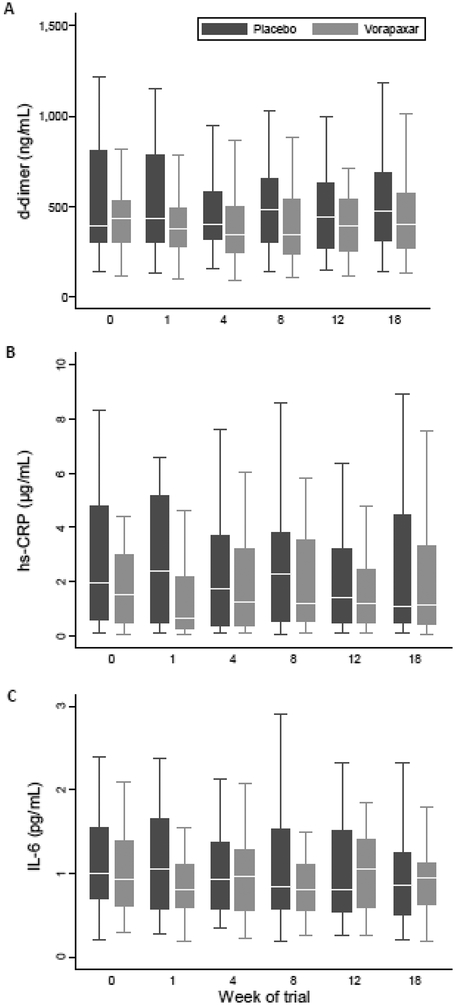
Twelve weeks treatment with vorapaxar did not reduce levels of d-dimer, which were essentially unchanged throughout the trial (Fig 2A). For the primary endpoint calculation, there was no significant change in log10 d-dimer from baseline to the average level at 8 and 12 weeks after treatment with vorapaxar compared to placebo (−0·02 log10 ng/mL, 95% CI −0·10 to 0·05, p = 0·56 using a regression model adjusted for baseline d-dimer level, Table 2). The mean % change in d-dimer from baseline to the average of weeks 8 and 12 was −10·8% and −8·5% for vorapaxar and placebo respectively. There was no difference in d-dimer levels after either 8 weeks or 12 weeks when analysed separately and there was no rise in d-dimer between 12 and 18 weeks after vorapaxar was ceased (Table 2). There was no significant difference in the proportion of subjects in each group that achieved a low d-dimer level of <165ng/mL at week 12 (2/30 in placebo group vs 1/33 of vorapaxar group, p = 0·60).
Figure 2. d-dimer, hs-CRP and IL-6 levels during the trial.
Box and whiskers plots show median (line), interquartile range (box) and whiskers (defined as UQ+1·5xIQR and LQ-1·5xIQR) for the two groups (vorapaxar grey, placebo black boxes) during the trial. Potential outliers are not shown. Vorapaxar or placebo was given for weeks 0–12 and then stopped. A. Plasma d-dimer (ng/mL), B. Plasma hs-CRP (μg/mL), C. plasma IL-6 (pg/mL).
Table 2.
Mean change of d-dimer, IL-6 and hs-CRP
| Endpoint | Mean % [95%CI] change | Log10 transformed data | ||
|---|---|---|---|---|
| placebo | vorapaxar | Difference between treatment groups† | p-value | |
| Baseline to average of week 8·12 | ||||
| *d-dimer (ng/mL) | −8·5 [−18·4, 2·5] | −10·8 [−23·1, 3·4] | −0·02 [−0·10, 0·05] | 0·56 |
| IL-6 (pg/mL) | −11·6 [−29·1, 10·3] | 12·6 [−15·6, 50·4] | 0·08 [−0·06, 0·22] | 0·25 |
| hs-CRP (μg/mL) | −15·7 [−40·9, 20·2] | −0·02 [−41·3, 70·2] | 0·02 [−0·20, 0·24] | 0·84 |
| Baseline to week 8 | ||||
| d-dimer (ng/mL) | −10·7 [−19·5, 2·0] | −12·5 [−25·0, 2·1] | −0·03 [−0·10, 0·05] | 0·52 |
| IL-6 (pg/mL) | −14·3 [−33·4, 10·2] | −1·1 [−27·2, 34·4] | 0·04 [−0·11, 0·19] | 0·63 |
| hs-CRP (μg/m/L) | −14·2 [−41·9, 26·9] | −0·5 [−44·8, 79·4] | 0·02 [−0·24, 0·27] | 0·91 |
| Baseline to week 12 | ||||
| d-dimer (ng/mL) | −8·8 [−19·4, 3·2] | −10·2 [−22·9, 4·5] | −0·02 [−0·09, 0·06] | 0·64 |
| IL-6 (pg/mL) | −13·3 [−29·6, 6·9] | 10·4 [−16·0, 45·1] | 0·08 [−0·05, 0·21] | 0·22 |
| hs-CRP (μg/mL) | −28·2 [−50·5, 4·2] | −24·3 [−50·9, 16·5] | −0·02 [−0·21, 0·16] | 0·79 |
| Week 12 to week 18 | ||||
| d-dimer (ng/mL) | 8·0 [−6·9, 25·2] | 8·9 [−5·0, 24·9] | 0·003 [−0·08, 0·09] | 0·95 |
| IL-6 (pg/mL) | −8·2 [−21·5, 7·5] | −1·9 [−29·1, 35·7] | 0·03 [−0·12, 0·18] | 0·70 |
| hs-CRP (μg/mL) | 11·6 [−14·4, 45·3] | 24·6 [−14·7, 82·1] | 0·07 [−0·13, 0·25] | 0·53 |
study primary endpoint
Linear regression for change (log10) modelled against treatment and baseline outcome variable.
Key secondary outcomes measured levels of inflammation and immune activation. Vorapaxar treatment had no significant impact on levels on plasma hs-CRP or IL-6 during the study (Figs 2B, C, Table 2). The change in hs-CRP from baseline to the average level 8 and 12 weeks after treatment with vorapaxar compared to placebo was −0·02 log10 ng/mL, 95% CI −0·20 to 0·24, p = 0.84. The change in IL-6 from baseline to the average level 8 and 12 weeks after treatment with vorapaxar compared to placebo was −0·08 log10 ng/mL, 95% CI −0·06 to 0·22, p = 0·29. Additional exploratory outcomes were studied to further probe any effect of vorapaxar. Soluble plasma CD14 and CD163 levels, markers of inflammation/microbial translocation and monocyte activation respectively, were also not changed by vorapaxar treatment (Appendix page 6).
Vorapaxar is a PAR-1 antagonist and a rationale for studying vorapaxar in the context of HIV was the observation that surface PAR-1 levels are elevated on CD4+ and CD8+ T cells in treated HIV infection.14 A subset of 39 participants (19 placebo, 20 vorapaxar) in this study had flow cytometric analysis of PAR-1 levels on CD4+ and CD8+ T cells on fresh blood samples. These 39 participants were selected based on being recruited at sites in Australia with ready access to the flow cytometry assay. PAR-1 expression on the total populations of CD4+ or CD8+ T cells was not changed by vorapaxar treatment (Appendix page 6).
Vorapaxar was generally well tolerated with only one participant ceasing vorapaxar because of an adverse event. Since vorapaxar is an anticoagulant we were particularly interested in bleeding events. There were 25 bleeding events (13 in placebo, 12 in vorapaxar arms) in 18 subjects. Most (23 events) were mild such as easy bruising or bleeding at the venepuncture site (BARC 1 grade) with no treatment required. One event (in a participant taking vorapaxar) was graded as moderate (BARC 2 grade); this was related to a cut from a kitchen instrument and the subject continued vorapaxar. One event (in a participant taking vorapaxar) was graded as severe (BARC 3 grade); this was related to a spinal hematoma developing after an emergency operation for spinal canal stenosis which required surgical treatment and the participant ceased vorapaxar.
Vorapaxar had no adverse effect on control of HIV viremia or maintenance of CD4 T cells. A plasma HIV RNA level of <50 copies per mL was maintained at week 18 in 29/30 in the placebo arm and 31/33 participants in the vorapaxar arm (p = 0·40). There was no significant difference in total CD4+ or CD8+ T cell levels between the placebo and vorapaxar arms during the course of the trial (Appendix page 7).
There were five protocol defined serious adverse events requiring hospitalisation for more than 24 hours, two in the placebo arm (pneumonia and colitis) and three in the vorapaxar arm (spinal canal stenosis requiring surgery, a spinal canal hematoma after surgery in the same subject, and gout). No subject experienced a serious non-AIDS related event, AIDS, pregnancy or death. There was a total of 161 adverse events, 84 in the placebo arm and 77 in the vorapaxar arm. There was no difference in the proportion of participants in each arm experiencing adverse events of any grade (Table 3) and no individual adverse event was markedly more common in one group (Appendix pages 8–10). No standard laboratory measures were different between the vorapaxar and placebo groups (data not shown).
Table 3.
Numbers of Adverse Events including Bleeding events^
| Grade 1 (mild) | Grade 2 (moderate) | Grade 3 (severe) | Grade 4 (potentially life threatening) | Total Adverse eventst | BARC bleeding events | |
|---|---|---|---|---|---|---|
| Placebo | ||||||
| n events, | 52 | 16 | 3 | 0 | 84* | 13 |
| n subjects#, % | 14, 45.2% | 10, 32.3% | 2, 6·5% | 0, 0·0% | 31 | 10, 32·3% |
| Vorapaxar | ||||||
| n events, | 37 | 24 | 3 | 1 | 77 | 12 |
| n subjects#, % | 13, 39·4% | 12, 36.4% | 2, 6·1% | 1, 3.0% | 33 | 8, 24·2% |
Fisher’s exact test for (a) difference in proportion of subjects having an adverse event Grade 1–4 by treatment arm (P=0·99) and (b) difference in proportion of subjects having any bleeding event by treatment arm (P= 0·58).
Subjects were classified according to the highest severity adverse event; any BARC event (yes/no) was compared. There were n=3 subjects (placebo) and n=5 (vorapaxar) with no adverse event.
Defined through the NH Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Paediatric Adverse Events (Version 2.0, November 2014) and the Bleeding Academic Research Consortium (BARC) Definitions for Bleeding Events (2011)
Discussion
We found vorapaxar was safe in people with well treated HIV infection but did not influence d-dimer levels nor a series of other inflammatory biomarkers associated with adverse outcomes. Our multisite double-blind randomised placebo-controlled study was powered to detect a clinically meaningful change in d-dimer levels, however no effect was observed across a series of time points during the trial and there was no rebound change after vorapaxar was ceased.
The participants studied were at high risk of future cardiovascular events, with median 11·4% 10-year cardiovascular disease risk. The high baseline levels of d-dimer in this recruited cohort suggest that their cardiovascular risk was even higher than that calculated by standard algorithms.4 The lack of effect of vorapaxar on biomarkers associated with cardiovascular risk emphasises the importance of standard cardiovascular risk reduction measures (including reducing cholesterol, controlling hypertension, stopping smoking) in this high-risk group. Our study excluded subjects with known cardiovascular disease. We cannot exclude that vorapaxar may have influenced d-dimer in subjects with known cardiovascular disease. However, we note that vorapaxar is already licensed for secondary cardiovascular disease prophylaxis. Further, a post hoc subgroup analysis (Appendix page 11) did not show evidence of d-dimer changes is subjects with high cardiovascular disease risk.
We studied vorapaxar since it acts via PAR-1 and this molecule was shown to be upregulated on T cells in the setting of HIV infection.14 The effect of vorapaxar on PAR-1 expression on T cell subsets was not known prior to this study. We found no effect of vorapaxar on PAR-1 expression levels in T cells in the subset of 39 subjects where we studied this repeatedly on fresh blood samples. This suggests PAR-1 levels on T cells is not central to d-dimer elevations, at least in the context of HIV infection. We did not study PAR-1 levels on platelets which may have been influenced by vorapaxar. Better targets susceptible to pharmacological interventions along the pathway of d-dimer and IL-6 production and cardiovascular disease are needed.
Vorapaxar had an acceptable safety profile in this HIV infected subject group. Two participants taking vorapaxar had significant bleeding episodes provoked by injury (one after a cut with a kitchen appliance and one after emergency back surgery). Future clinical trials of anticoagulant therapies in HIV infection are justified given the high rates of cardiovascular disease and expected safety profile of this level of anticoagulation in this subject group.
We acknowledge several limitations of our study. We did not use a loading dose of vorapaxar as some cardiovascular studies have done19, but we observed no trend over time in d-dimer changes. The dose studied, 2·5mg daily, is a relatively safe dose used in current practice for secondary prophylaxis.18 Although larger doses for a longer duration could have been studied, this would have placed subjects at a higher risk of bleeding complications. Our use of biomarkers as primary and secondary endpoints may miss biologic effects with the potential for clinical importance, but is much more efficient than devoting the resources for a clinical endpoint study at this stage of investigation. The size of our study also means we cannot exclude a modest effect of vorapaxar on d-dimer levels. In our study we saw an 8·5% reduction in mean week 8–12 d-dimer in the placebo arm, and a 10·8% reduction in the vorapaxar arm. In formal adjusted analyses this corresponded to a difference in log10 ddimer of −0·2 (95% CI −0·10 to 0·05). The 95% confidence limit for percent reduction in week 8–12 d-dimer level, given our sample size, rules out a reduction in d-dimer in the vorapaxar arm to a magnitude greater than 27%. This is much smaller than the 45% reduction in ddimer as a marker endpoint that our study was powered to detect. We reasoned that a large effect on this marker would be required to justify future larger studies.4 Since we found no significant effect on d-dimer or the other multiple surrogate markers studied, even those at high risk of future cardiovascular disease, we believe clinical endpoint trials with vorapaxar are not justified in this subject group.
In conclusion, vorapaxar had no significant effect on d-dimer or markers of inflammation in the 64 people we studied with treated HIV infection at high risk for future cardiovascular disease. Improved therapies and targets are needed to reduce cardiovascular disease in this vulnerable population.
Supplementary Material
Panel: Research in context
Evidence before this study
Cardiovascular disease is approximately 50% more common in HIV infected people despite ART. Standard cardiovascular risk factors remain important in the context of HIV infection. In addition, biomarkers in blood corresponding to increased coagulation and immune activation, particularly d-dimer, but also Interleukin-6 (IL-6) and high-sensitivity C-reactive protein (hs-CRP), appear to be important markers of HIV-related cardiovascular diseaserisk. It is not clear if the associated biomarker changes are causal or consequential. The physiological mechanisms underpinning poor clinical outcomes remain unclear. Vorapaxar is a novel oral anticoagulant recently licensed for secondary prevention of cardiovascular disease, which in HIV infection has potential to have an additional benefit through the reduction of immune activation. We searched PubMed for articles published between Jan 1 2000 and July 1 2015 using “vorapaxar” and “HIV”, reporting a “study” or “trial”. We found no reports of vorapaxar in the context of HIV infection.
Added value of this study
To the best of our knowledge, this multicentre, double-blind, randomised, placebo-controlled trial is the first to study vorapaxar in people with HIV infection at risk of future cardiovascular disease. This study found no effect of 12 weeks of treatment with vorapaxar on several biomarkers of cardiovascular risk, including no effect on d-dimer, IL-6, or hs-CRP levels.
Implications of all the available evidence
The results of our study suggest that vorapaxar should not be studied further as a treatment to reduce cardiovascular risk in people with HIV infection. Careful attention to existing proven interventions in HIV negative populations to modify cardiovascular disease risk remain the best available method to reduce cardiovascular disease in people with HIV infection.
Acknowledgements
We thank all the individuals who participated in this trial and the doctors, nurses and scientists associated with the trial. This work was supported by the Australian National Health and Medical Research Council (NHMRC; grant number 1052979) and National Cancer Institute, National Institutes of Health (NCI; contract number HHSN261200800001E). The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Funding Australian National Health and Medical Research Council, U.S. National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Kent reports grants and personal fees from ViiV HealthCare, grants and personal fees from Gilead Sciences, grants from Johnson and Johnson, grants from Sanofi-Pasteur, outside the submitted work; Dr. Law reports grants from Boehringer Ingelheim, Gilead Sciences, Merck Sharp & Dohme, Bristol-Myers Squibb, Janssen-Cilag, ViiV HealthCare, personal fees from Gilead Sciences, Sirtex Pty Ltd, outside the submitted work; Dr. Emery reports non-financial support from Merck, during the conduct of the study. Dr. Kelleher reports non-financial support from Merck, during the conduct of the study; non-financial support from Cytognos, grants from Calimmune, grants and other from Celgene, outside the submitted work. All other members of the writing committee report no disclosures.
References
- 1.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5: e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7: e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borges AH, Silverberg MJ, Wentworth D, et al. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS 2013; 27: 1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grund B, Baker JV, Deeks SG, et al. Relevance of Interleukin-6 and D-Dimer for Serious Non-AIDS Morbidity and Death among HIV-Positive Adults on Suppressive Antiretroviral Therapy. PLoS One 2016; 11: e0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jong E, Meijers JC, van Gorp EC, Spek CA, Mulder JW. Markers of inflammation and coagulation indicate a prothrombotic state in HIV-infected patients with long-term use of antiretroviral therapy with or without abacavir. AIDS Res Ther 2010; 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JV, Neuhaus J, Duprez D, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr 2011; 56: 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart BB, Nordell AD, Okulicz JF, et al. Inflammation-Related Morbidity and Mortality Among HIV-Positive Adults: How Extensive Is It? J Acquir Immune Defic Syndr 2018; 77: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arildsen H, Sorensen KE, Ingerslev JM, Ostergaard LJ, Laursen AL. Endothelial dysfunction, increased inflammation, and activated coagulation in HIV-infected patients improve after initiation of highly active antiretroviral therapy. HIV Med 2013; 14: 1–9. [DOI] [PubMed] [Google Scholar]
- 9.Freiberg MS, Bebu I, Tracy R, et al. D-Dimer Levels before HIV Seroconversion Remain Elevated Even after Viral Suppression and Are Associated with an Increased Risk of Non-AIDS Events. PLoS One 2016; 11: e0152588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuhaus J, Jacobs DR Jr., Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201: 1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delvaeye M, Conway EM. Coagulation and innate immune responses: can we view them separately? Blood 2009; 114: 2367–74. [DOI] [PubMed] [Google Scholar]
- 12.Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost 2011; 9 Suppl 1: 182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoniak S, Owens AP 3rd, Baunacke M, et al. PAR-1 contributes to the innate immune response during viral infection. J Clin Invest 2013; 123: 1310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurley A, Smith M, Karpova T, et al. Enhanced effector function of CD8(+) T cells from healthy controls and HIV-infected patients occurs through thrombin activation of protease-activated receptor 1. J Infect Dis 2013; 207: 638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funderburg NT, Mayne E, Sieg SF, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood 2010; 115: 161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker JV, Brummel-Ziedins K, Neuhaus J, et al. HIV replication alters the composition of extrinsic pathway coagulation factors and increases thrombin generation. J Am Heart Assoc 2013; 2: e000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green SA, Smith M, Hasley RB, et al. Activated platelet-T-cell conjugates in peripheral blood of patients with HIV infection: coupling coagulation/inflammation and T cells. AIDS 2015; 29: 1297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012; 366: 1404–13. [DOI] [PubMed] [Google Scholar]
- 19.Tricoci P, Huang Z, Held C, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med 2012; 366: 20–33. [DOI] [PubMed] [Google Scholar]
- 20.Martin A, Bloch M, Amin J, et al. Simplification of antiretroviral therapy with tenofoviremtricitabine or abacavir-Lamivudine: a randomized, 96-week trial. Clin Infect Dis 2009; 49: 1591–601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.