Abstract
Domoic acid (DA) is a neurotoxin produced during harmful algal blooms that accumulates in marine organisms that serve as food resources for humans. While acute DA neurotoxicity can cause seizures and hippocampal lesions, less is known regarding how chronic, subacute DA exposure in adulthood impacts the hippocampus. With more frequent occurrences of harmful algal blooms, it is important to understand the potential impact of repeated, low-level DA exposure on human health. To model repeated, low-dose DA exposure, adult mice received a single low-dose (0.75 ± 0.05 μg/g) of DA or vehicle weekly for 22 consecutive weeks. Quantitative immunohistochemistry was performed to assess the effects of repeated, low-level DA exposure on hippocampal cells and synapses. Vesicular glutamate transporter 1 (VGluT1) immunoreactivity within excitatory boutons in CA1 of DA-exposed mice was increased. Levels of other vesicular transporter proteins (i.e., VGluT2 and the vesicular GABA transporter (VGAT)) within boutons, and corresponding bouton densities, were not significantly altered in CA1, CA3, or dentate gyrus. There were no significant changes in neuron density or glial fibrillary acidic protein (GFAP) immunoreactivity following chronic, low-dose exposure. This suggests that repeated low doses of DA, unlike high doses of DA, do not cause neuronal loss or astrocyte activation in hippocampus in adult mice. Instead, these findings demonstrate that repeated exposure to low levels of DA leads to subtle changes in VGluT1 expression within CA1 excitatory boutons, which may alter glutamatergic transmission in CA1 and disrupt behaviors dependent on spatial memory.
Keywords: Domoic acid, Hippocampus, VGluT1, Parvalbumin, GFAP
1. Introduction
Domoic acid (DA) is a neurotoxin produced by several species of marine algae, including diatoms of the genus Pseudo-nitzschia (Lefebvre and Robertson, 2010; Nijjar et al., 1991; Takemoto, 1978). Organisms such as anchovies, mussels, razor clams, and Dungeness crabs can accumulate high levels of DA during harmful algal blooms (Kumar et al., 2009; Lefebvre et al., 2001; Perl et al., 1990), thus posing a risk to humans, marine mammals, and seabirds that feed on these animals (Scholin et al., 2000; Sierra Beltrán et al., 1997; Work et al., 1993). Acute DA exposure in humans can cause amnesic shellfish poisoning, which is associated with gastrointestinal illness, seizures, confusion, memory loss, coma, and death (Perl et al., 1990).
Because the incidence of the harmful algal blooms that lead to high levels of DA production is predicted to increase with ongoing climate change (Van Dolah, 2000), DA toxicity represents a significant threat to the health of humans and marine organisms. Although monitoring regulations are in place to reduce the risk of acute exposure in humans by prohibiting shellfish harvesting when DA levels are high (≥ 20 μg DA/gram)(Mariën, 1996), the effects of chronic, low-level DA exposure (i.e. levels that do not cause overt signs of toxicity) (< 20 μg/g) on human health are not well studied (Lefebvre and Robertson, 2010), and there are currently no guidelines for repetitive, low-level exposure. This is a concern because DA can persist in seafood; for example, razor clams retain low levels of DA (1–20 μg DA/gram) for up to one year after an algal bloom (Wekell et al., 1994). Thus, a better understanding of the potential subclinical impacts of chronic, DA exposure is necessary to determine the possible risks of repeated, low-level exposure in humans.
Studies suggest that the hippocampus is particularly susceptible to the toxic effects of acute DA exposure. Acute DA toxicosis leads to both acute and latent seizure activity in humans and sea lions (Cendes et al., 1995; Goldstein et al., 2008; Muha and Ramsdell, 2011). The seizure activity is associated with gross hippocampal alterations and changes in connectivity, neurological dysfunction, memory loss, and deficits in spatial task performance in humans, rodents, and sea lions following acute DA exposure (Cook et al., 2015; Perl et al., 1990; Petrie et al., 1992; Scholin et al., 2000; Strain and Tasker, 1991; Todd, 1993). At the cellular level, acute DA toxicity affects both neurons (Buckmaster et al., 2014; Cendes et al., 1995; Pulido, 2008; Teitelbaum et al., 1990), and glia (Chandrasekaran et al., 2004; Gill et al., 2008) of the hippocampus. DA can induce neuronal damage such as neuron degeneration, vacuolization, and swelling of dendritic processes (Ananth et al., 2003a, 2003b, 2001; Appel et al., 1997a; Strain and Tasker, 1991; Tryphonas et al., 1990b), and DA-induced glial alterations include microglial activation, astrocyte swelling, necrosis, incidence of reactive astrocytes, and increased glial fibrillary acidic protein (GFAP) immunoreactivity in the hippocampus (Ananth et al., 2003a, 2003b, 2001; Appel et al., 1997b; Pulido, 2008; Stewart et al., 1990; Strain and Tasker, 1991). Taken together, these reports illustrate the hippocampal neuropathology that follows acute exposure to high doses of DA, and raise the possibility that hippocampal cellular alterations may also occur following repeated exposure to lower levels of DA.
In addition to causing neuronal and glial alterations, DA may also impact excitatory and inhibitory neurotransmission in the hippocampus. DA is a tricarboxylic amino acid similar in structure to kainic acid, and is a potent agonist of both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainic acid subtypes of ionotropic glutamate receptors (Hampson et al., 1992; Larm et al., 1997; Sciancalepore et al., 1990; Zaczek and Coyle, 1982). Activation of AMPA receptors promotes fast excitatory neurotransmission (Seeburg, 1993), and, in addition to promoting excitatory neurotransmission, activation of kainic acid receptors is known to modulate glutamate and γ-aminobutyric acid (GABA) release at hippocampal excitatory and inhibitory synapses (Frerking et al., 2001; Sihra and Rodríguez-Moreno, 2013, 2011). Both kainic acid and DA directly inhibit GABA release in the hippocampus (Cunha et al., 1997), which could decrease inhibitory drive and further contribute to the observed DA-induced increase in hippocampal neuronal firing (Debonnel et al., 1989). Prolonged exposure of hippocampal cultures to DA leads to increased neuronal burst spiking and electrophysiological silencing of fast-spiking interneurons (Hiolski et al., 2016), further suggesting that DA exposure impacts both hippocampal excitatory and inhibitory neural activity.
Chronic DA exposure may also lead to changes in the responsiveness of postsynaptic neurons to glutamate release, as gria2a, encoding a subunit of the AMPA receptor, is downregulated in zebrafish following 2 weeks of low-level DA exposure, and may be a compensatory response to increased neural activity (Hiolski et al., 2014). In addition, exposure to DA leads to changes that are suggestive of synaptogenesis, such as mossy fiber sprouting and upregulation of synaptophysin and PSD-95 expression (Pérez-Gómez and Tasker, 2014, 2013). Greater numbers of excitatory synapses could contribute to increased excitability and elevated neuron activity (Sutula and Dudek, 2007). Thus, exposure to DA may induce changes in hippocampal synaptic connectivity and disrupt mechanisms regulating neurotransmitter release.
While the neurotoxic effects of acute exposure to high levels of DA have been extensively studied, comparatively little is known about the effects of chronic, low-level DA exposure in adult rodents. A recent study has demonstrated spatial learning and memory deficits following repetitive low-dose DA administration in adult mice (Lefebvre et al., 2017). It is possible, but not known, that there are cellular changes in the hippocampus that accompany these behavioral alterations. To address this, adult mice were exposed to low, asymptomatic doses of DA over 22 weeks, and the hippocampus was examined for glial, neuronal, and synaptic alterations. This chronic low-dose exposure regimen did not cause visible seizures, or lead to gross changes in hippocampal neuron density or astrocyte reactivity. Further, densities of excitatory and inhibitory synapses were not significantly altered following chronic, low-dose DA exposure. However, levels of vesicular glutamate transporter (VGluT1) immunoreactivity were selectively increased within glutamatergic boutons in the CA1 region of the hippocampus, suggesting a potential for increased glutamate release and elevated excitability.
2. Materials and Methods
2.1. Animals
Adult female mice (C57Bl/6NJ) were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed under standard caging conditions with ad libitum access to food and water. Mice included in the present study underwent repeated, low-dose DA exposure as described previously (Lefebvre et al., 2017). Briefly, mice received intraperitoneal injections of 0.75 +/− 0.05 μg DA/g total body weight or an equivalent volume of phosphate buffered saline (PBS) once per week for 22 weeks beginning at 12 weeks of age. No signs of acute toxicity (i.e. scratching, seizures, or convulsive activity) were observed immediately following DA injections. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington.
2.2. Tissue processing and immunohistochemistry
Following 22 weeks of DA exposure (Lefebvre et al., 2017), mice were anesthetized with 2.5% Avertin and transcardially perfused with PBS followed by 4% paraformaldehyde. Brains were extracted and post-fixed for 3–4 hours in 4% paraformaldehyde at 4oC. After post-fixation, brains were equilibrated in 30% sucrose at 4oC before coronal sectioning on a cryostat at a section thickness of 40 μm. Sections were stored at 4oC in tris-buffered saline (TBS) with 0.05% NaN3 until use in immunohistochemistry experiments.
Two hippocampus-containing sections per animal (n=6 control and n=7 DA-exposed), spaced 200 μm apart, approximately 1.7 mm posterior to Bregma, were selected for freefloating immunohistochemistry for NeuN, GFAP, or parvalbumin (PV), and cell nuclei were identified with 4’,6-diamidino-2-phenylindole (DAPI). Two hippocampus-containing sections adjacent to those sections selected for cellular marker immunohistochemistry (n=4 control and n=5 DA-exposed animals) were used for free-floating immunohistochemistry for the vesicular glutamate transporters (VGluT1 and VGluT2) and the vesicular GABA transporter (VGAT) to label excitatory and inhibitory synapses. Free-floating sections were rinsed in PBS or TBS for three 10 minute intervals at room temperature, and then blocked with 10% normal goat serum and 0.5% Triton-X in PBS or TBS for 2 hours (cellular markers) or 1 hour (synaptic markers) at room temperature. Sections were incubated at 4oC overnight with a primary antibody against GFAP (rabbit anti-GFAP, polyclonal, Abcam (ab7260), 1:1000) or for 48 hours with primary antibodies against NeuN (rabbit anti-NeuN, monoclonal, D3S3I, Cell Signaling (12943), 1:1000) or PV (mouse anti-PV, monoclonal, Millipore (MAB1572), 1:1000) or for 96 hours in primary antibodies against VGluT1 (guinea pig anti-VGluT1 (1:3000, Millipore, Billerica, MA)), VGluT2 (rabbit anti-VGluT2 (1:1000, Synaptic Systems, Goettingen, Germany)), and VGAT (mouse anti-VGAT (1:500, Synaptic Systems)). After incubation with primary antibodies, sections were rinsed three times for 10 minutes with PBS or TBS and incubated with secondary antibodies for 2 hours at room temperature (goat anti-rabbit AlexaFluor 488 for GFAP, goat anti-rabbit AlexaFluor 647 for NeuN, and goat anti-mouse AlexaFluor 555 for PV, goat anti-guinea pig Alexa Fluor 647 for VGluT1, goat anti-rabbit Alexa Fluor 488 for VGluT2, and goat anti-mouse Alexa Fluor 568 for VGAT (all 1:1000, Thermo Fisher Scientific). Finally, sections were washed three times for 10 minutes with PBS or TBS. Sections for cellular marker immunohistochemistry were incubated with DAPI for 10 minutes (300 nM, Invitrogen D21490/DAPI-Fluor-Pure Grade) and then washed three times for 10 minutes with TBS. All incubations noted were carried out on an orbital shaker. Sections were mounted on Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA) and coverslipped (Slip-Rite #1.5 coverglass, Thermo Fisher Scientific, Waltham, MA) using Fluoromount-G (Southern Biotech, Birmingham, AL). Slides were left to dry overnight prior to imaging. Sections were coded such that the experimenter was blind to exposure condition. Details of the methods and results of DAPI staining are reported in Lefebvre et al. (2017).
2.3. Microscopy
Sections immunostained for GFAP or NeuN were imaged on a Keyence Biorevo BZ-9000 digital widefield microscope using a 40X 0.95 N.A. objective. For quantification of PVimmunoreactive(-IR) cells, and to measure overall hippocampal area within sections, 20X (0.75 N.A.) stacks with 2 μm spacing between Z-planes were collected capturing both hippocampal hemispheres per section and tiled to generate a map of each hippocampal hemisphere. For GFAP staining, image stacks with 1 μm steps between Z axis planes were taken at two randomly selected locations per hippocampal subregion (CA1, CA3, dentate gyrus (DG)) within each section. For NeuN and PV immunostaining, image stacks were captured at three randomly selected locations per hippocampal subregion from the more rostral of the two sections per animal. For all image stacks, imaging parameters were consistent across all stacks and sections for each antigen.
Sections stained for VGluT1, VGluT2, and VGAT immunoreactivity were imaged using a Zeiss AxioImager.M2 widefield microscope (Carl Zeiss, AG, Oberkochen, Germany), with a 63X 1.4 N.A. Plan-Apochromat oil immersion objective, an X-Cite Series 120 mercury arc lamp (Excelitas Technologies, Waltham, MA), and a Hammamatsu Orca-ER CCD camera (Hamamatsu Photonics, Japan). Z stack collection (0.25 μm spacing between Z planes) was controlled using Stereo Investigator software (MBF Bioscience, Williston, VT). In Stereo Investigator, contours were drawn to outline the CA1, CA3 and DG regions of the hippocampus, bilaterally. The CA1, CA3, and DG regions of the hippocampus were identified using a mouse atlas (Paxinos and Franklin, 2004) and by the pattern of VGluT1 and VGluT2 immunoreactivity (Kaneko et al., 2002; Kaneko and Fujiyama, 2002). The total sampled area for each hippocampal region was not significantly different between DA-exposed and control mice (CA1: t7=0.334, p=0.748; CA3: t7=−1.252, p=0.251; DG: t7=−0.328, p=0.752). To generate random sampling sites within each region, the fractionator function was used and a square grid was randomly oriented over each region, with the X-Y spacing of the grid determined for each bilateral hippocampal region within a section according to the following formula:
where X is the length of the grid spacing, A denotes the contour areas of the hippocampal region of interest for the left and right hemispheres of each section, and 5 is the approximate desired number of collected stacks for each region within each section. A random rotation was applied to each grid, and a counting frame was generated by Stereo Investigator at each grid intersection. At each instance where the center point of the counting frame fell within the boundary of a contour, a stack was collected. Exposure times and image collection parameters for each marker were kept constant within each hippocampal region.
2.4. Image processing and quantification
2.4.1. Cell density and immunoreactivity measurements
Image stacks collected with the 40X objective were cropped to 10 μm-thick in the Z axis, and deconvolved using AutoQuant (Media Cybernetics). The surfaces function in Imaris (v. 8.0, Bitplane, Zurich, Switzerland) was used to quantify the numbers of NeuN-IR and PV-IR cells within each image stack for each region, which were then used to calculate average cell density. GFAP- and PV-IR cell relative fluorescence intensity levels were quantified as a proxy for levels of GFAP and PV protein. To quantify GFAP labeling, GFAP-immunoreactivity for each image stack was masked using the surfaces function in Imaris, and the total sum of GFAP immunoreactivity intensity levels for each stack was determined and added across all collected stacks for each hippocampal region for each animal. The volume of GFAP immunoreactivity was similarly determined as the total sum of all GFAP-IR voxels selected by the surfaces function for each stack, added for each region within each animal. For each PV-IR cell, the sum and mean voxel intensity values and the cell volume were determined and averaged across image stacks for each region for each animal.
2.4.2. Synaptic marker density and fluorescence intensity measurements
Image stacks underwent 3D deconvolution using AutoQuant, with an adaptive PSF, medium noise level, and maximum of 10 iterations. Stacks were exported to Imaris and cropped to 512 × 512 × 20 pixels (approximately 50 × 50 × 5 μm3). The surfaces function was used to identify individual VGluT1-, VGluT2-, and VGAT-IR puncta independently within each stack. It was then possible to determine the number and relative fluorescence intensity (voxel gray scale value) of immunoreactive puncta within each stack. For VGluT1 immunoreactivity, stacks were not included in the dataset if greater than 50% of the stack area encompassed the pyramidal neuron cell body layer of any hippocampal region, as there are few VGluT1-IR presynaptic boutons located within this layer. This reduced between-site, within-animal variability for the density measurements. Immunoreactive puncta densities were calculated for each animal based on the numbers of immunoreactive puncta within the sampled site volume, corrected for tissue shrinkage, using the following formula:
2.5. Statistical analyses
Differences in neuron densities, GFAP- and PV-immunoreactivity, immunoreactive synaptic puncta intensities and densities in each hippocampal region were analyzed using t-tests. For all tests where treatment group size was less than six, non-parametric Mann-Whitney U tests were also performed. The outcomes of the non-parametric tests did not lead to any changes of statistical significance; therefore, only results from t-tests are reported. All statistical tests were carried out at α=0.05 using SPSS software (IBM, version 23).
3. Results
3.1. Chronic DA exposure does not induce overt neuronal pathology in the hippocampus
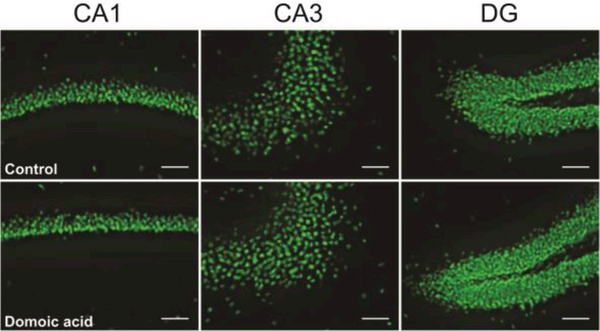
Acute DA exposure is associated with lesions and hippocampal atrophy (Ananth et al., 2001; Perl et al., 1990; Silvagni et al., 2005; Teitelbaum et al., 1990; Tryphonas et al., 1990b); however, less is known regarding the effects of chronic, low-dose exposure on the hippocampus. A previous examination of hippocampal tissue from mice included in the present study did not reveal any significant alterations in hippocampal regional areas or DAPI-labeled cell density following chronic low dose DA exposure (Lefebvre et al., 2017). We measured densities of NeuN-IR cells in hippocampus to determine if chronic, low dose exposure to DA causes alterations in hippocampal neuron density. Consistent with the overall cell density observations, the densities of NeuN-IR cells were not significantly different between DAexposed and control mice overall in the hippocampus or in any hippocampal subregion (Figure 1, Table 1). Together, these results demonstrate that repeated exposure to low doses of DA does not induce loss of cells, or neurons, in the hippocampus of adult mice.
Figure 1.
Examples of 20X images of NeuN-IR neurons in CA1 (left), CA3 (middle), and DG (right) in control (top row) and DA-exposed (bottom row) mice. Scale bar = 50 μm.
Table 1.
NeuN-immunoreactive neuron densities for individual and combined hippocampal subregions for control and DA-exposed mice. N=6 control and 7 DA-exposed mice per group.
| Measure | Control Mean (SEM) | Domoic Acid Mean (SEM) | t Statistic | p-value |
|---|---|---|---|---|
| NeuN | ||||
| Combined | 1.85 × 105 (2.86 × 103) | 1.84 × 105 (9.59 × 103) | t(11)=0.090 | 0.930 |
| CA1 | 1.38 × 105 (3.44 × 103) | 1.26 × 105 (6.40 × 103) | t(11)=1.471 | 0.169 |
| CA3 | 1.70 × 105 (5.36 × 103) | 1.77 × 105 (1.27 × 104) | t(11)=−0.503 | 0.625 |
| DG | 2.47 × 105 (7.86 × 103) | 2.48 × 105 (1.79 × 104) | t(11)=−0.460 | 0.964 |
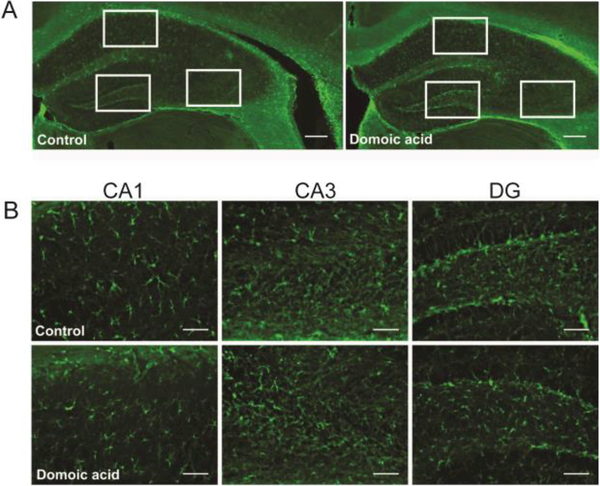
Several studies have reported that acute DA exposure leads to alterations in the brain consistent with increased neuroinflammation, such as increased reactivity of astrocytes (Ananth et al., 2003b, 2001; Appel et al., 1997b; Kirkley et al., 2014; Pulido, 2008; Strain and Tasker, 1991; Tryphonas et al., 1990b). To assess astrocyte reactivity in a chronic, low-dose exposure model, GFAP immunoreactivity was examined (Figure 2A and 2B). There were no significant changes in GFAP immunoreactivity in the hippocampus of DA-exposed mice as measured by both total intensity levels (Table 2) and average total volume of GFAP immunoreactivity within each sampled field of view (Table 2). Thus, chronic low-level DA exposure did not increase this marker of glial reactivity, in contrast to what is observed following acute exposure. Taken together, the lack of significant changes in hippocampal area, cell and neuron densities, and lack of any significant alteration in a marker for reactive astrocytes suggests that chronic, lowdose DA exposure during adulthood does not lead to extreme neurotoxicity as might occur following an acute, symptomatic exposure event. This indicates that this repetitive, low-dose DA exposure model allows for the examination of hippocampal changes that occur independently of the neuronal loss and astrocytosis that can be caused by acute exposure.
Figure 2.
A. Examples of GFAP-IR labeling in control (left) and DA-exposed (right) hippocampus. B. Examples of 20X images of GFAP immunoreactivity in CA1 (left), CA3 (middle), and DG (right) in control (top row) and DA-exposed (bottom row). Scale bars: 200 μm (A), 50 μm (B).
Table 2.
Mean fluorescence intensity and volume of GFAP immunoreactivity in hippocampal subregions of control and DA-exposed mice. N=6 control and 7 DA-exposed mice per group.
| Measure | Control Mean (SEM) | Domoic Acid Mean (SEM) | Statistic | p-value |
|---|---|---|---|---|
| GFAP intensity (gray scale units) | ||||
| CA1 | 4.44 × 107 (5.24 × 106) | 3.98 × 107 (4.97 × 106) | t(11)=0.622 | 0.546 |
| CA3 | 2.37 × 107 (2.58 × 106) | 2.70 × 107 (5.38 × 106) | t(11)=−0.526 | 0.609 |
| DG | 3.95 × 107 (3.75 × 106) | 5.63 × 107 (1.03 × 107) | t(11)=−1.442 | 0.177 |
| GFAP volume (um3) | ||||
| CA1 | 5.17 × 104 (5.07 × 103) | 4.82 × 104 (5.18 × 103) | t(11)=0.483 | 0.638 |
| CA3 | 3.17 × 104 (3.37 × 103) | 3.56 × 104 (6.41 × 103) | t(11)=−0.523 | 0.611 |
| DG | 5.18 × 104 (4.17 × 103) | 6.64 × 104 (9.66 × 103) | t(11)=−1.306 | 0.218 |
3.2. Chronic DA exposure leads to increased VGluT1 immunoreactivity in area CA1
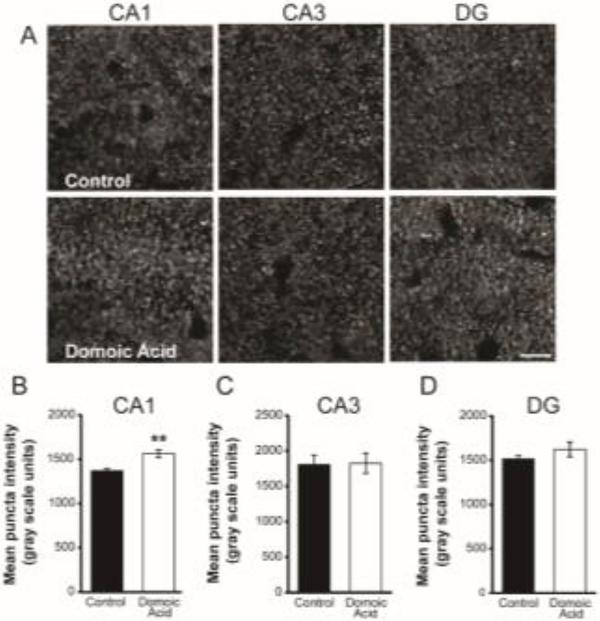
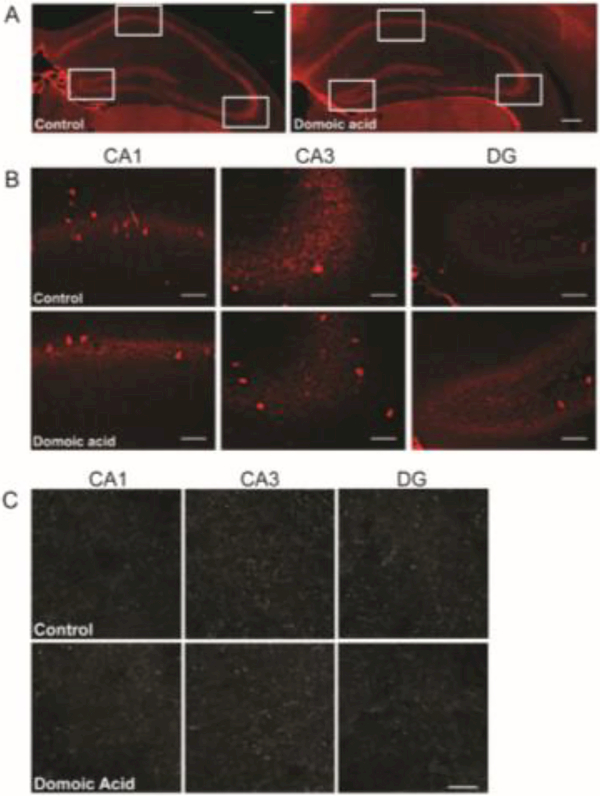
VGluT1 is the vesicular glutamate transporter expressed within a population of excitatory presynaptic boutons in the brain, including the hippocampus (Fremeau et al., 2004b, 2001), and changes in levels of vesicular transporters can alter the amount of neurotransmitter released from the bouton (Daniels et al., 2006, 2004; Wilson et al., 2005; Wojcik et al., 2004). Given this, if low-level DA exposure affects the levels of VGluT1 within hippocampal excitatory boutons, then glutamate release could be affected. As a proxy for VGluT1 protein content within individual VGluT1-expressing boutons, quantitative fluorescent immunohistochemistry was used to detect VGluT1 protein in hippocampal sections (Figure 3A) and the average and total pixel fluorescence intensities within VGluT1-IR puncta of control and DA-exposed mice were measured. The mean puncta fluorescence intensity of VGluT1-IR puncta (corresponding to the average voxel gray scale value of each VGluT1-IR surface object, averaged across VGluT1-IR surface objects) was significantly increased by 14% in the CA1 region of mice exposed to DA (control: 1371.45 gray scale units, DA: 1563.47 gray scale units; t7=−3.516, p= 0.010) (Figure 3B). In contrast to CA1, there were no significant differences between DA-exposed and control mice in measures of mean puncta intensity in CA3 (t7=−0.087, p=0.933) or in DG (t7=−1.071, p=0.320) (Figure 3C and D). Consistent with the puncta mean fluorescence intensity analysis, the average sum puncta VGluT1 fluorescence intensity, which is indicative of the total amount of VGluT1 per bouton (corresponding to the sum of all voxel gray scale values for each VGluT1-IR surface object, averaged across VGluT1-IR surface objects), was significantly increased by 15% in CA1 of DA-exposed mice (Table 3). However, there were no significant differences in the average sum puncta fluorescence intensity between DA-exposed and control mice in CA3 or DG.
Figure 3.
A. Examples of VGluT1-IR puncta in CA1 (left), CA3 (middle), and DG (right) of control (top row) and DA-exposed (bottom row) mice. B-D. Mean fluorescence intensity of VGluT1-IR puncta was significantly increased in CA1 in DA-exposed mice relative to controls (p=0.01), but not in CA3 or DG (p>0.05). Graphs are +/− SEM, n = 4 control and n = 5 DA-exposed mice. Scale bar = 10 μm.
Table 3.
Excitatory bouton measures for control and DA-exposed mice in hippocampal subregions.
| Measure | Control Mean (SEM) | Domoic Acid Mean (SEM) | Statistic | p-value |
|---|---|---|---|---|
| VGluT1-IR puncta sum intensity (gray scale units) | ||||
| CA1 | 3.66 × 105 (1.42 × 104) | 4.21 × 105 (9.56 × 103) | t(7)= −3.389 | 0.012* |
| CA3 | 4.81 × 105 (3.35 × 104) | 5.04 × 105 (3.65 × 104) | t(7)=−0.439 | 0.674 |
| DG | 3.90 × 105 (1.53 × 104) | 4.11 × 105 (2.44 × 104) | t(7)=−0.696 | 0.509 |
| VGluT1-IR puncta density (μm−3) | ||||
| CA1 | 0.084 (0.002) | 0.086 (0.004) | t(7)=−0.422 | 0.686 |
| CA3 | 0.068 (0.001) | 0.067 (0.003) | t(7)=0.377 | 0.746 |
| DG | 0.087 (0.001) | 0.091 (0.004) | t(7)=−0.760 | 0.472 |
| VGluT2-IR puncta mean intensity (gray scale units) | ||||
| DG granule cell layer | 2495 (215) | 3147 (505) | t(7)=−1.473 | 0.184 |
| DG molecular layer | 1697 (83) | 1716 (156) | t(7)=−0.104 | 0.920 |
| VGluT2-IR puncta sum intensity (gray scale units) | ||||
| DG granule cell layer | 5.14 × 105 (4.95 × 104) | 6.50 × 105 (9.76 × 104) | t(7)=−1.533 | 0.169 |
| DG molecular layer | 2.39 × 105 (1.13 × 104) | 2.49 × 105 (2.34 × 104) | t(7)=−0.371 | 0.742 |
| VGluT2-IR puncta density (μm−3) | ||||
| DG granule cell layer | 0.074 (0.004) | 0.069 (0.002) | t(7)=0.699 | 0.507 |
| DG molecular layer | 0.173 (0.005) | 0.168 (0.009) | t(7)=0.442 | 0.671 |
p<0.05. N= 4 control and 5 DA-exposed mice per group.
Increases in markers for synaptogenesis have been reported following DA exposure (Pérez-Gómez and Tasker, 2014, 2013); therefore, the density of VGluT1-expressing boutons could be altered in the hippocampus of mice chronically exposed to DA. However, there were no significant differences in average VGluT1-IR bouton density (puncta number per μm3 tissue) in CA1, CA3 or in DG (Table 3).
3.3. Chronic DA exposure does not affect VGluT2 immunoreactivity in DG
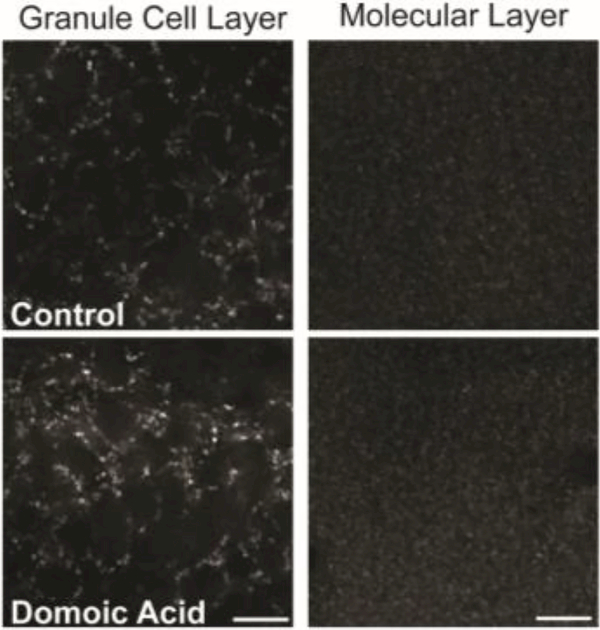
Excitatory boutons in the hippocampus may contain either the VGluT1 or VGluT2 isoform of the vesicular glutamate transporter, and expression of the different isoforms may reflect glutamatergic presynaptic boutons that arise from different origins and/or have different release properties (Fremeau et al., 2004a, 2004b; Halasy et al., 2004). Thus, bouton density and within-bouton vesicular transporter levels corresponding to a separate and complementary population of excitatory boutons identified by the expression of VGluT2 as a vesicular glutamate transporter were also assessed. The majority of glutamatergic boutons in the hippocampus express VGluT1 rather than VGluT2, and in fact, immunoreactivity for VGluT2 is relatively low in the CA1 and CA3 regions (Fremeau et al., 2001), but is present in both the granule cell and molecular layers of the DG (Fremeau et al., 2001; Halasy et al., 2004; Kaneko et al., 2002; Kaneko and Fujiyama, 2002; van der Hel et al., 2009). Therefore, VGluT2 immunoreactivity was quantified only in the DG granule cell body layer and molecular layer (Figure 4). There were no significant effect of DA exposure on VGluT2-IR puncta mean fluorescence intensity in either the granule cell body layer or the molecular layer (Table 3). Similarly, there were no significant differences in average puncta sum fluorescence intensity in either the granule cell layer or the molecular layer between DA-exposed and control mice (Table 3), nor were there significant treatment differences in puncta density in the granule cell body layer of the DG or in the molecular layer (Table 3).
Figure 4.
Examples of VGluT2-IR puncta in the granule cell body layer (left) and the molecular layer (right) of the DG in control (top row) and DA-exposed (bottom row) mice. Scale bar = 10 μm.
3.4. Chronic DA exposure does not affect PV or VGAT immunoreactivity in hippocampus
Studies have shown that GABAergic neurons are present in all hippocampal regions, dispersed throughout pyramidal cell layers and dendritic regions, and mainly form local intrinsic connections (Ribak et al., 1978; Storm-Mathisen et al., 1983). Subtle disruptions in GABAergic signaling can lead to hyperexcitability and hippocampal dysfunction (Thompson, 1994). In a rat model of early postnatal DA-induced seizures, PV immunoreactivity in hippocampus is decreased (Gill et al., 2010), raising the possibility that the PV subtype of GABAergic interneurons are affected by DA exposure. Further, it has recently been shown that prolonged exposure of hippocampal slice cultures to DA leads to electrophysiological silencing of fastspiking neurons, likely corresponding to the PV-expressing population of inhibitory interneurons (Hiolski et al., 2016). Thus, it is possible that chronic, low-dose exposure to DA leads to alterations in the PV-expressing population of inhibitory neurons in the hippocampus (Figure 5A-B). The densities of PV-IR neurons were not significantly different between DA-exposed and control mice in the combined regions of the hippocampus, or within any hippocampal subregion (Table 4). Further, levels of PV immunoreactivity within PV-IR cells were examined, as PV functions as a calcium binding protein and PV levels may impact cell excitability (Caillard et al., 2000). The average PV-IR neuron intensities were not significantly different between DA-exposed and control mice within any hippocampal subregion examined (Table 4). In addition, the average PV-IR neuron sum fluorescence intensity was not significantly different between DA-exposed and control mice, nor was the volume of PV-IR neurons different between DA-exposed and control mice (Table 4). Taken together, these results suggest that densities, size, and somal PV content of the PV-expressing population of inhibitory interneurons in the hippocampus were not impacted by chronic, low-dose exposure to DA.
Figure 5.
A. Example of PV immunoreactivity in the hippocampus of control (left) and DAexposed (right) mice. B. Example images of PV immunoreactivity in CA1 (left), CA3 (middle), and DG (right) in control (top row) and DA-exposed mice (bottom row). C. Examples of VGATIR puncta in CA1 (left), CA3 (middle), DG (right) in control (top row) and DA-exposed mice (bottom row). Scale bar: 200 μm (A), 50 μm (B), 10 μm (C).
Table 4.
PV-immunoreactive neuron and VGAT-immunoreactive bouton measures in hippocampal subregions of control and DA-exposed mice. PV-IR measures: n=6 control and 7 DA-exposed per group; VGAT-IR measures: n=4 control and 5 DA-exposed per group.
| Measure | Control Mean (SEM) | Domoic Acid Mean (SEM) | Statistic | p-value |
|---|---|---|---|---|
| PV-IR cell density (mm−3) | ||||
| Combined | 5395 (375) | 5572 (330) | t(11)=−0.355 | 0.729 |
| CA1 | 6969 (716) | 6359 (656) | t(11)=0.629 | 0.542 |
| CA3 | 6632 (834) | 7515 (606) | t(11)=−0.874 | 0.401 |
| DG | 2585 (355) | 2842 (145) | t(11)=−0.709 | 0.493 |
| PV-IR mean intensity (gray scale units) | ||||
| Combined | 16.90 (1.41) | 16.40 (1.43) | t(11)=0.247 | 0.810 |
| CA1 | 16.17 (1.71) | 18.53 (1.96) | t(11)=−0.893 | 0.391 |
| CA3 | 13.01 (1.09) | 12.02 (0.94) | t(11)=0.692 | 0.503 |
| DG | 21.51 (2.10) | 18.63 (2.11) | t(11)=0.961 | 0.357 |
| PV-IR sum intensity (gray scale units) | ||||
| Combined | 2.74 × 105 (3.29 × 104) | 2.64 × 105 (2.58 × 104) | t(11)=0.240 | 0.814 |
| CA1 | 2.28 × 105 (2.66 × 104) | 2.66 × 105 (3.71 × 104) | t(11)=−0.812 | 0.434 |
| CA3 | 2.13 × 105 (2.34 × 104) | 2.04 × 105 (2.18 × 104) | t(11)=0.270 | 0.792 |
| DG | 3.80 × 105 (6.11 × 104) | 3.21 × 105 (4.29 × 104) | t(11)=0.813 | 0.433 |
| PV-IR cell volume (μm3) | ||||
| Combined | 1128.86 (50.06) | 1158.71 (53.39) | t(11)=−0.403 | 0.695 |
| CA1 | 1008.66 (44.70) | 997.62 (50.299) | t(11)0.161 | 0.875 |
| CA3 | 1150.78 (55.29) | 1223.90 (69.91) | t(11)=−0.8 | 0.441 |
| DG | 1227.16 (83.81) | 1254.60 (117.59) | t(11)=−0.184 | 0.857 |
| VGAT-IR puncta mean intensity (gray scale units) | ||||
| CA1 | 1421 (83) | 1457 (93) | t(7)=−0.280 | 0.788 |
| CA3 | 1753 (130) | 1783 (149) | t(7)=−0.151 | 0.884 |
| DG | 1286 (56) | 1374 (83) | t(7)=−0.825 | 0.437 |
| VGAT-IR puncta sum intensity (gray scale units) | ||||
| CA1 | 2.47 × 105 (3.06 × 104) | 2.66 × 105 (2.03 × 104) | t(7)=0.539 | 0.606 |
| CA3 | 3.54 × 105 (3.41 × 104) | 3.48 × 105 (3.30 × 104) | t(7)=0.119 | 0.909 |
| DG | 2.46 × 105 (1.38 × 104) | 2.57 × 105 (1.83 × 104) | t(7)=−0.438 | 0.677 |
| VGAT-IR puncta density (μm−3) | ||||
| CA1 | 0.171 (0.019) | 0.174 (0.011) | t(7)=−0.153 | 0.882 |
| CA3 | 0.139 (0.015) | 0.138 (0.010) | t(7)=0.056 | 0.957 |
| DG | 0.221 (0.015) | 0.193 (0.012) | t(7)=1.46 | 0.187 |
Increases in neural activity have been shown to decrease levels of vesicular inhibitory amino acid transporter, suggesting that changes in excitatory and inhibitory vesicular neurotransmitter transporters may be regulated in opposite directions (De Gois et al., 2005). As DA exposure can impact excitatory activity, it is possible that homeostatic changes in the levels of the vesicular GABA transporter within inhibitory boutons, and the density of inhibitory boutons, (Fig. 5C) are present in the hippocampus of mice chronically exposed to DA. However, there were no significant differences in VGAT-IR puncta mean intensity levels, sum intensity levels, or densities between DA-exposed and control mice in CA1 (Table 4). Together with the PV-IR neuron results, this suggests that chronic, low-level DA exposure does not impact these features of inhibitory circuits in the hippocampus.
4. Discussion
As climate change and other factors lead to more frequent occurrences of conditions favorable for harmful algal blooms, it is increasingly necessary to understand the structural and functional impacts of long-term exposure to neurotoxins produced by harmful algal bloom species. This is especially important given that these toxins persist at low levels for extended periods in food sources such as razor clams, increasing the risk for periodic sub-clinical exposure in humans. Recent reports suggest a link between potential exposures to chronic sub-clinical DA and learning and memory in human and mouse models (Grattan et al., 2016; Lefebvre et al., 2017). In order to determine the effects of repeated, low-dose DA exposure on cells and synapses of the hippocampus, a brain structure critical for learning and memory, cell and synaptic markers were quantified in hippocampal tissue from adult mice exposed to DA on a weekly basis for 22 weeks. VGluT1-expressing presynaptic boutons in CA1 exhibited increased VGluT1 immunoreactivity in DA-exposed mice, while densities of neurons and synaptic boutons were not altered. This effect was specific for the CA1 region, and also specific for VGluT1-IR excitatory boutons, as VGluT2-IR boutons in DG, and VGAT-IR inhibitory boutons in CA1, CA3, and DG were not significantly altered. The increase in VGluT1 levels within excitatory boutons in CA1 suggests there may be increased excitatory neurotransmission in the CA1 region of the hippocampus following chronic, low-level DA exposure. Moreover, there was no significant neuronal loss or increased GFAP expression identified in any hippocampal region. Together, these findings suggest that this model of repeated low-level DA exposure appears to induce selective changes in VGluT1 levels in CA1, in the absence of the severe neuropathology associated with acute DA exposure. In a complimentary study using a similar dosing regimen, significant learning deficits were observed in mice following 25 weeks of chronic, low-dose DA exposure, but learning returned to control levels after a nine week recovery period of no exposure (Lefebvre et al., 2017). Changes in vesicular glutamate transporter expression with chronic low-level exposure as opposed to the permanent hippocampal damage and cell loss associated with acute high level DA exposure may provide a mechanistic explanation for the reversible learning deficits observed. Whether or not the increase in VGluT1 levels is reversible upon discontinuation of chronic DA exposure will be tested in future studies.
4.1. Repeated, low-dose DA exposure does not cause the same hippocampal deficits as acute, symptomatic DA exposure
These results suggest that, despite previously reported learning deficits, chronic exposure to low levels of DA does not induce severe pathological changes in the hippocampus, in contrast to acute symptomatic DA exposure. A recent study demonstrated that this DA exposure paradigm is not associated with overall hippocampal atrophy or cell loss (Lefebvre et al., 2017), consistent with the findings from other studies of low-dose DA exposure in early postnatal and adult rats (Bernard et al., 2007; Schwarz et al., 2014). Additionally, we found no significant increase in GFAP immunoreactivity, suggesting that chronic, low level DA exposure does not induce activation of astrocytes in the hippocampus. Similar to these findings, in both rats and monkeys, repeated low-dose oral exposure does not induce gross alterations in hippocampus, or changes in GFAP immunoreactivity (Truelove et al., 1997, 1996). In contrast with the findings from the chronic low-dose model used in the present study, acute doses of DA are known to elicit an increase in astrocytosis markers, including increased GFAP immunoreactivity and morphological evidence of reactive gliosis (Appel et al., 1997b; Chandrasekaran et al., 2004; Ross et al., 2000; Stewart et al., 1990; Tryphonas et al., 1990a, 1990b). Further, acute exposure of rodents to high levels of domoic acid that elicit neurological symptoms is associated with hippocampal neuronal loss and degeneration, particularly in regions CA1-CA3 (Iverson et al., 1989; Peng et al., 1994). Taken together, results from this study support the idea that effects of repeated, low-dose DA exposure on hippocampal neurons and astrocytes differ from those effects induced by acute exposure to higher doses of DA. Future work should be done to assess the effects of repeated low-dose domoic acid exposure specifically on activation of microglia, glial cells which also contribute to neuroinflammatory responses, and have been suggested to play a role in the neurotoxic effects of DA (Ananth et al., 2001; Appel et al., 1997b; Chandrasekaran et al., 2004).
A number of studies of acute DA exposure have reported a range of effects on the GABA system. GABAergic neurons participate in feedback and feedfoward inhibitory circuits in the hippocampus (Kullmann, 2011), and changes in the number or function of GABAergic synapses are likely to impact pyramidal neuron firing and hippocampal function (Hájos and Paulsen, 2009; Mann et al., 2005). In chick retina cells, application of DA stimulates GABA release (Alfonso et al., 1994), while it inhibits GABA release from hippocampal synaptosomes via actions at presynaptic kainic acid receptors (Cunha et al., 1997). In adolescent mice that received an acute in utero exposure to DA, levels of GABA in the hippocampus and cortex are significantly reduced (Dakshinamurti et al., 1993). However, another study found that an acute dose of DA given to adult rats increases GABA levels in the hippocampus, cortex, and amygdala, which could indicate increased GABA production and/or release following acute DA exposure (Durán et al., 1995). In contrast with reports of acute DA exposure-induced effects on GABAergic neurons, there was no significant effect of chronic, low-level DA exposure on the density or VGAT content of hippocampal GABAergic boutons, or the densities or PV content of PV-IR neurons. The findings of the present study differ from a low-dose, early postnatal DA-induced seizure model in rat, where PV immunoreactivity was decreased in the DG region of the dorsal hippocampus (Gill et al., 2010). It is possible that this discrepancy could indicate that the developmental timing of low-dose DA exposure is crucial, and earlier (postnatal) exposure may cause alterations in PV-IR hippocampal interneurons that are not observed following adult lowdose DA exposure. A study previously found that prolonged in vitro DA exposure leads to electrophysiological silencing of fast-spiking inhibitory interneurons in organotypic hippocampal culture (Hiolski et al., 2016). Taken together with results of the current study, this could suggest that chronic DA exposure does not lead to physical loss of PV-expressing interneurons, but may reduce their activity. It is also possible that repeated low-dose DA exposure may impact other interneuron populations, such as somatostatin-expressing interneurons. For example, chronically-exposed sea lions have fewer hilar somatostatin-positive neurons (Buckmaster et al., 2014), although a prior study reported no significant alterations in somatostatin-positive neuron counts in hippocampus following postnatal low-dose DA exposure (Gill et al., 2010). Future studies will be needed to gain insight into chronic domoic acid-induced alterations in other hippocampal interneuron populations. However, VGAT is a marker for GABAergic boutons from all populations of GABAergic interneurons, and the results of the present study do not suggest any change in density of inhibitory boutons in hippocampus, although it is possible that there are changes in inhibitory boutons attributable to specific interneuron subpopulations. Taken together with the lack of significant changes in hippocampal cell and neuronal counts, and astrocyte reactivity, the absence of alterations in markers of GABAergic synapses further differentiates the effects of low-level chronic DA exposure during adulthood from those effects caused by acute exposure to high doses of DA.
4.2. Chronic DA exposure may affect vesicular regulation of glutamatergic neurotransmission
The results of the present study suggest that chronic, low-dose DA exposure increases the level of VGluT1 protein within presynaptic boutons in CA1 of adult mice. VGluT1 is responsible for packaging glutamate into synaptic vesicles, and the extent of vesicle filling depends in part on the number of active vesicular transporters (Wilson et al., 2005). Under normal conditions, synaptic vesicles within glutamatergic and GABAergic boutons are not consistently filled with neurotransmitter to maximum capacity (Engel et al., 2001; Yamashita et al., 2003), suggesting that increasing or decreasing the amount of neurotransmitter transported into vesicles by adjusting numbers of available vesicular transporters should subsequently increase or decrease quantal size. In support of this, studies have demonstrated that the level of VGluTs regulates quantal release of glutamate, and alters postsynaptic responses (Daniels et al., 2006, 2004; Wilson et al., 2005; Wojcik et al., 2004). For example, VGluT1 deletion impairs glutamatergic transmission and overexpression of VGluT1 increases quantal size (Fremeau et al., 2004a; Wojcik et al., 2004). In drosophila, VGluT overexpression increases mini excitatory postsynaptic potential amplitude, and increases synaptic vesicle size, suggesting that increasing VGluT expression increases the total glutamate content of each vesicle (Daniels et al., 2004). VGluT overexpression also decreases synaptic transmission failure rate, which could indicate an increase in the number of vesicle release events large enough to elicit an AMPA receptormediated response, or an increase in the probability that a vesicle will be released after an action potential (Herman et al., 2014; Wilson et al., 2005). Taken together, these studies indicate that increasing and decreasing levels of vesicular neurotransmitter transporters is a mechanism by which neurotransmitter release, and thereby synaptic activity, may be altered. Thus, the finding of increased within-bouton VGluT1 immunoreactivity following DA exposure may be associated with increased glutamate release in the hippocampus CA1 region. This could contribute to increased hippocampal excitability, and/or memory impairment, given that aged rats with memory deficits exhibit increased VGluT expression (Ménard et al., 2015). Interestingly, adult female mice that underwent a similar repeated, low-level DA exposure paradigm exhibit spatial memory impairment (Lefebvre et al., 2017), suggesting that this exposure paradigm may be associated with both changes in hippocampal VGluT1 levels and memory impairments.
The increase in within-bouton VGluT1 levels was observed in boutons within the CA1 region of the hippocampus, but not CA3 or DG. VGluT1-immunoreactive boutons located in CA1 represent glutamatergic excitatory inputs to the CA1 region. One source of glutamatergic input to CA1 is the Schaffer collateral projections that arise from pyramidal neurons in CA3. In vivo evidence demonstrates that DA is 20 fold more potent in activating the CA3 region compared to the CA1 region (Debonnel et al., 1989) and the CA3 region of the hippocampus has a high density of kainic acid receptor binding sites (Monaghan and Cotman, 1982), as well as a lower threshold for kainic acid- induced seizures (Schwartzkroin and Prince, 1978). In addition, acute contaminated mussel extract exposure or DA exposure in rats revealed alterations in the CA3 region of the hippocampus (Tryphonas et al., 1990b, 1990c). These reports are consistent with idea that the increased VGluT1 levels in CA1 observed following repeated low-level DA exposure may reflect changes in the glutamatergic Schaffer collateral projections from CA3 pyramidal neurons to CA1. On the other hand, it is important to note that DA also induces potent excitation of CA1 in vivo (Debonnel et al., 1989), and increased excitability of CA1 has been observed after DA exposure in hippocampal slices with CA3 removed (Sari and Kerr, 2001), suggesting that DA exerts effects on excitatory neurotransmission in CA1 independently of its effects on CA3. Additionally, low dose DA applied to hippocampal slice cultures induces injury to cells in CA1 to a greater extent than areas CA3 or DG (Pérez-Gómez and Tasker, 2012). It will be informative to elucidate in future studies whether the increased CA1 VGluT1 levels following repeated low-dose DA exposure are caused by direct effects on area CA1, or whether increased VGlut1 in CA1 occurs downstream of effects on other regions such as CA3.
Further studies will be necessary to investigate the behavioral implications of altered within-bouton VGluT1 levels in the CA1 region. However, neuronal activity in the CA1 region is important for spatial memory (Jo et al., 2007; Moser and Moser, 1998; Tsien et al., 1996), and output from CA3 is important for rapid acquisition of memories in novel environments and for spatial tuning in CA1 pyramidal neurons (Nakashiba et al., 2008). Therefore, if repeated DA exposure increases glutamate release at CA3 to CA1 synapses, then this may impact spatial learning and memory in DA-exposed animals. In support of this, a previous study demonstrated that a similar repeated low dose DA exposure regimen caused spatial learning and memory impairment in mice (Lefebvre et al., 2017). These behavioral impairments were no longer present two months after the cessation of DA exposure, suggesting that the DA-induced spatial behavior impairments are reversible. Future studies will be necessary to determine if increased CA1 VGluT1 levels are similarly reversible, and to determine if there is a causal relationship between excitatory synapse alterations and spatial deficits in chronic, low-level DA-exposed mice.
4.3. Effects of chronic DA may be selective for changes in within-hippocampus glutamatergic boutons, rather than glutamatergic hippocampal afferents
Protein expression patterns of VGluT1 and VGluT2 are complementary in the hippocampus (Kaneko et al., 2002; Kaneko and Fujiyama, 2002), with VGluT2 immunoreactivity being much greater in DG than in CA1 or CA3. There were no significant changes in VGluT2-IR bouton fluorescence intensities or densities in DG, suggesting that the VGluT2-expressing population of excitatory boutons in DG is not affected by low-level, chronic DA exposure, and VGluT1-expressing boutons may be selectively affected. These boutons represent a subpopulation of glutamatergic boutons separate from the VGluT1-expressing bouton population in hippocampus. It has been reported that VGluT2 mRNA and protein are not expressed within hippocampal cells (Kaneko et al., 2002; Kaneko and Fujiyama, 2002; Ziegler et al., 2002), which suggests that VGluT2-IR boutons quantified in the present study may not originate within the hippocampus. Multiple studies have shown that VGluT2-expressing boutons originate outside the hippocampus (Fremeau et al., 2001; Halasy et al., 2004; Herzog et al., 2001; Hisano et al., 2000), and could represent, for example, projections to the DG from the medial septum diagonal band complex and the supramammillary nucleus (Boulland et al., 2009; Fremeau et al., 2001; Halasy et al., 2004; Hajszan et al., 2004; Lin et al., 2003; Ziegler et al., 2002). The fact that no alterations in VGluT2-expressing boutons were observed in the current study, while alterations in some VGluT1-expressing boutons were found, may suggest that chronic, low dose DA exposure primarily affects the hippocampus, rather than indirectly inducing changes in the hippocampus that are downstream of disruptions in other brain regions.
4.4. Limitations
There are several possible limitations to the present study, including the relatively small treatment group size, and that only female animals were studied. The small treatment group size and reduced statistical power may have affected our ability to detect a DA treatment effect in some outcomes, and suggests our negative findings should be interpreted with caution. Previous work has reported effects of domoic acid in female adult animals; for example, acute doses of domoic acid lower than those necessary to elicit neurological symptoms (e.g. seizures) alters the expression of c-fos immunoreactivity in hippocampus (Peng and Ramsdell, 1996). However, during the 1987 human DA exposure event, toxic effects were reportedly more severe in males (Perl et al., 1990). In animals, evidence suggests that males could be more susceptible to severe neurotoxic effects of DA following an acute high dose (Baron et al., 2013), and male rats exhibit increased lateral septal area cell loss compared to females after acute local DA infusion (Wetmore and Nance, 1991). Thus, it is important to consider that the effects reported here are not necessarily generalizable to males, and it will be important to determine the effects of chronic, low-level domoic acid exposure in adult male mice in future studies.
4.5. Conclusion
In summary, these results demonstrate that repeated, low level DA exposure in adult mice is associated with a selective increase in vesicular glutamate transporter levels within VGluT1-expressing boutons in the CA1 region of the hippocampus that occurs in the absence of markers of gross morphologic and neuroinflammatory alterations that follow acute exposure to high DA levels. This suggests there may be increased glutamate release from presynaptic excitatory boutons in the CA1 region that could lead to increased hippocampal excitability that may contribute to the hyperactivity and spatial learning and memory deficits reported previously following a similar repeated, low-level DA exposure regime (Lefebvre et al., 2017). Future studies will determine the cellular and molecular mechanisms that contribute to increased VGluT1 expression in the CA1 region of the hippocampus following repeated low-level DA exposure, and further elucidate the impacts of increased CA1 VGluT1 levels on neurobehavioral performance.
Highlights:
Chronic, low-dose domoic acid increased CA1 vesicular glutamate transporter levels
No changes in neuron or bouton density, or evidence of astrocytosis were identified
Changes in hippocampal inhibitory boutons and parvalbumin neurons were not observed
Acknowledgements
The authors thank Dr. Benjamin Abrams of the UCSC Life Sciences Microscopy Center for technical support, and Dr. Eric Chen for critical comments on the manuscript. This work was supported by NIH grants R01 ES021930, R01 MH104227, R01 MH109475, F32 ES026872, and NSF grants OCE-1314088 and DGE-0809125.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- Alfonso M, Duran R, Duarte CB, Ferreira IL, Carvalho AP, 1994. Domoic acid induced release of [3H]GABA in cultured chick retina cells. Neurochem. Int 24, 267–74. [DOI] [PubMed] [Google Scholar]
- Ananth C, Dheen ST, Gopalakrishnakone P, Kaur C, 2003a. Distribution of NADPHdiaphorase and expression of nNOS, N-methyl-D-aspartate receptor (NMDAR1) and non-NMDA glutamate receptor (GlutR2) genes in the neurons of the hippocampus after domoic acid-induced lesions in adult rats. Hippocampus 13, 260–72. doi: 10.1002/hipo.10060 [DOI] [PubMed] [Google Scholar]
- Ananth C, Gopalakrishnakone P, Kaur C, 2003b. Protective role of melatonin in domoic acid-induced neuronal damage in the hippocampus of adult rats. Hippocampus 13, 375–87. doi: 10.1002/hipo.10090 [DOI] [PubMed] [Google Scholar]
- Ananth C, Thameem Dheen, S., Gopalakrishnakone P, Kaur C, 2001. Domoic acid-induced neuronal damage in the rat hippocampus: changes in apoptosis related genes (bcl-2, bax, caspase-3) and microglial response. J. Neurosci. Res 66, 177–90. [DOI] [PubMed] [Google Scholar]
- Appel NM, Rapoport SI, O’Callaghan JP, 1997. Sequelae of parenteral domoic acid administration in rats: Comparison of effects on different anatomical markers in brain. Synapse 25, 350–358. doi: [DOI] [PubMed] [Google Scholar]
- Appel NM, Rapoport SI, O’Callaghan JP, Bell JM, Freed LM, 1997. Sequelae of parenteral domoic acid administration in rats: comparison of effects on different metabolic markers in brain. Brain Res. 754, 55–64. [DOI] [PubMed] [Google Scholar]
- Baron AW, Rushton SP, Rens N, Morris CM, Blain PG, Judge SJ, 2013. Sex differences in effects of low level domoic acid exposure. Neurotoxicology 34, 1–8. doi: 10.1016/j.neuro.2012.10.010 [DOI] [PubMed] [Google Scholar]
- Bernard PB, Macdonald DS, Gill DA, Ryan CL, Tasker RA, 2007. Hippocampal mossy fiber sprouting and elevated trkB receptor expression following systemic administration of low dose domoic acid during neonatal development. Hippocampus 17, 1121–33. doi: 10.1002/hipo.20342 [DOI] [PubMed] [Google Scholar]
- Boulland J-L, Jenstad M, Boekel AJ, Wouterlood FG, Edwards RH, Storm-Mathisen J, Chaudhry FA, 2009. Vesicular glutamate and GABA transporters sort to distinct sets of vesicles in a population of presynaptic terminals. Cereb. Cortex 19, 241–8. doi: 10.1093/cercor/bhn077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Wen X, Toyoda I, Gulland FMD, Van Bonn W, 2014. Hippocampal neuropathology of domoic acid-induced epilepsy in California sea lions (Zalophus californianus). J. Comp. Neurol 522, 1691–706. doi: 10.1002/cne.23509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A, 2000. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc. Natl. Acad. Sci. U. S. A 97, 13372–7. doi: 10.1073/pnas.230362997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Carpenter S, Zatorre RJ, Cashman NR, 1995. Temporal lobe epilepsy caused by domoic acid intoxication: evidence for glutamate receptor-mediated excitotoxicity in humans. Ann. Neurol. 37, 123–6. doi: 10.1002/ana.410370125 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran A, Ponnambalam G, Kaur C, 2004. Domoic acid-induced neurotoxicity in the hippocampus of adult rats. Neurotox. Res 6, 105–17. [DOI] [PubMed] [Google Scholar]
- Cook PF, Reichmuth C, Rouse AA, Libby LA, Dennison SE, Carmichael OT, Kruse-Elliott KT, Bloom J, Singh B, Fravel VA, Barbosa L, Stuppino JJ, Van Bonn WG, Gulland FMD, Ranganath C, 2015. Algal toxin impairs sea lion memory and hippocampal connectivity, with implications for strandings. Science 350, 1545–7. doi: 10.1126/science.aac5675 [DOI] [PubMed] [Google Scholar]
- Cunha RA, Constantino MD, Ribeiro JA, 1997. Inhibition of [3H] gamma-aminobutyric acid release by kainate receptor activation in rat hippocampal synaptosomes. Eur. J. Pharmacol 323, 167–72. [DOI] [PubMed] [Google Scholar]
- Dakshinamurti K, Sharma SK, Sundaram M, Watanabe T, 1993. Hippocampal changes in developing postnatal mice following intrauterine exposure to domoic acid. J. Neurosci 13, 4486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Chen K, Gelfand MV, Featherstone DE, DiAntonio A, 2006. A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle. Neuron 49, 11–6. doi: 10.1016/j.neuron.2005.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Gelfand MV, Dant J, Brooks ES, Krantz DE, DiAntonio A, 2004. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J. Neurosci 24, 10466–74. doi: 10.1523/JNEUROSCI.3001-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gois S, Schäfer MK-H, Defamie N, Chen C, Ricci A, Weihe E, Varoqui H, Erickson JD, 2005. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J. Neurosci. 25, 7121–33. doi: 10.1523/JNEUROSCI.5221-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debonnel G, Beauchesne L, de Montigny C, 1989. Domoic acid, the alleged “mussel toxin” might produce its neurotoxic effect through kainate receptor activation: an electrophysiological study in the rat dorsal hippocampus. Can. J. Physiol. Pharmacol 67, 29–33. [DOI] [PubMed] [Google Scholar]
- Durán R, Arufe MC, Arias B, Alfonso M, 1995. Effect of domoic acid on brain amino acid levels. Rev. española Fisiol 51, 23–7. [PubMed] [Google Scholar]
- Engel D, Pahner I, Schulze K, Frahm C, Jarry H, Ahnert-Hilger G, Draguhn A, 2001. Plasticity of rat central inhibitory synapses through GABA metabolism. J. Physiol 535, 473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH, 2004a. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science 304, 1815–9. doi: 10.1126/science.1097468 [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH, 2001. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 31, 247–60. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Voglmaier S, Seal RP, Edwards RH, 2004b. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 27, 98–103. doi: 10.1016/j.tins.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Frerking M, Schmitz D, Zhou Q, Johansen J, Nicoll RA, 2001. Kainate receptors depress excitatory synaptic transmission at CA3-->CA1 synapses in the hippocampus via a direct presynaptic action. J. Neurosci 21, 2958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DA, Ramsay SL, Tasker RA, 2010. Selective reductions in subpopulations of GABAergic neurons in a developmental rat model of epilepsy. Brain Res. 1331, 114–23. doi: 10.1016/j.brainres.2010.03.054 [DOI] [PubMed] [Google Scholar]
- Gill SS, Hou Y, Ghane T, Pulido OM, 2008. Regional susceptibility to domoic acid in primary astrocyte cells cultured from the brain stem and hippocampus. Mar. Drugs 6, 25–38. [PMC free article] [PubMed] [Google Scholar]
- Goldstein T, Mazet JAK, Zabka TS, Langlois G, Colegrove KM, Silver M, Bargu S, Van Dolah F, Leighfield T, Conrad PA, Barakos J, Williams DC, Dennison S, Haulena M, Gulland FMD, 2008. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. Proc. Biol. Sci 275, 267–76. doi: 10.1098/rspb.2007.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan LM, Boushey C, Tracy K, Trainer V, Roberts SM, Schluterman N, Morris JG, 2016. The association between razor clam consumption and memory in the CoASTAL Cohort. Harmful Algae 57, 20–25. doi: 10.1016/j.hal.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Paulsen O, 2009. Network mechanisms of gamma oscillations in the CA3 region of the hippocampus. Neural Netw. 22, 1113–9. doi: 10.1016/j.neunet.2009.07.024 [DOI] [PubMed] [Google Scholar]
- Hajszan T, Alreja M, Leranth C, 2004. Intrinsic vesicular glutamate transporter 2-immunoreactive input to septohippocampal parvalbumin-containing neurons: novel glutamatergic local circuit cells. Hippocampus 14, 499–509. doi: 10.1002/hipo.10195 [DOI] [PubMed] [Google Scholar]
- Halasy K, Hajszan T, Kovács EG, Lam T-T, Leranth C, 2004. Distribution and origin of vesicular glutamate transporter 2-immunoreactive fibers in the rat hippocampus. Hippocampus 14, 908–18. doi: 10.1002/hipo.20006 [DOI] [PubMed] [Google Scholar]
- Hampson DR, Huang XP, Wells JW, Walter JA, Wright JL, 1992. Interaction of domoic acid and several derivatives with kainic acid and AMPA binding sites in rat brain. Eur. J. Pharmacol 218, 1–8. [DOI] [PubMed] [Google Scholar]
- Herman MA, Ackermann F, Trimbuch T, Rosenmund C, 2014. Vesicular glutamate transporter expression level affects synaptic vesicle release probability at hippocampal synapses in culture. J. Neurosci 34, 11781–91. doi: 10.1523/JNEUROSCI.1444-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S, 2001. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J. Neurosci 21, RC181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiolski EM, Ito S, Beggs JM, Lefebvre KA, Litke AM, Smith DR, 2016. Domoic acid disrupts the activity and connectivity of neuronal networks in organotypic brain slice cultures. Neurotoxicology 56, 215–224. doi: 10.1016/j.neuro.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiolski EM, Kendrick PS, Frame ER, Myers MS, Bammler TK, Beyer RP, Farin FM, Wilkerson H-W, Smith DR, Marcinek DJ, Lefebvre KA, 2014. Chronic lowlevel domoic acid exposure alters gene transcription and impairs mitochondrial function in the CNS. Aquat. Toxicol 155, 151–9. doi: 10.1016/j.aquatox.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisano S, Hoshi K, Ikeda Y, Maruyama D, Kanemoto M, Ichijo H, Kojima I, Takeda J, Nogami H, 2000. Regional expression of a gene encoding a neuron-specific Na(+)-dependent inorganic phosphate cotransporter (DNPI) in the rat forebrain. Brain Res. Mol. Brain Res 83, 34–43. [DOI] [PubMed] [Google Scholar]
- Iverson F, Truelove J, Nera E, Tryphonas L, Campbell J, Lok E, 1989. Domoic acid poisoning and mussel-associated intoxication: preliminary investigations into the response of mice and rats to toxic mussel extract. Food Chem. Toxicol 27, 377–84. [DOI] [PubMed] [Google Scholar]
- Jo YS, Park EH, Kim IH, Park SK, Kim H, Kim HT, Choi J-S, 2007. The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J. Neurosci 27, 13567–78. doi: 10.1523/JNEUROSCI.3589-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, 2002. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci. Res 42, 243–50. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H, 2002. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J. Comp. Neurol 444, 39–62. [DOI] [PubMed] [Google Scholar]
- Kirkley KS, Madl JE, Duncan C, Gulland FM, Tjalkens RB, 2014. Domoic acid-induced seizures in California sea lions (Zalophus californianus) are associated with neuroinflammatory brain injury. Aquat. Toxicol 156, 259–68. doi: 10.1016/j.aquatox.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Kullmann DM, 2011. Interneuron networks in the hippocampus. Curr. Opin. Neurobiol 21, 709–16. doi: 10.1016/j.conb.2011.05.006 [DOI] [PubMed] [Google Scholar]
- Kumar KP, Kumar SP, Nair GA, 2009. Risk assessment of the amnesic shellfish poison, domoic acid, on animals and humans. J. Environ. Biol 30, 319–25. [PubMed] [Google Scholar]
- Larm JA, Beart PM, Cheung NS, 1997. Neurotoxin domoic acid produces cytotoxicity via kainate- and AMPA-sensitive receptors in cultured cortical neurones. Neurochem. Int 31, 677–82. [DOI] [PubMed] [Google Scholar]
- Lefebvre AK, Dovel LS, Silver WM, 2001. Tissue distribution and neurotoxic effects of domoic acid in a prominent vector species, the northern anchovy Engraulis mordax. Mar. Biol 138, 693–700. doi: 10.1007/s002270000509 [DOI] [Google Scholar]
- Lefebvre KA, Kendrick PS, Ladiges W, Hiolski EM, Ferriss BE, Smith DR, Marcinek DJ, 2017. Chronic low-level exposure to the common seafood toxin domoic acid causes cognitive deficits in mice. Harmful Algae 64, 20–29. doi: 10.1016/j.hal.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre KA, Robertson A, 2010. Domoic acid and human exposure risks: a review. Toxicon 56, 218–30. doi: 10.1016/j.toxicon.2009.05.034 [DOI] [PubMed] [Google Scholar]
- Lin W, McKinney K, Liu L, Lakhlani S, Jennes L, 2003. Distribution of vesicular glutamate transporter-2 messenger ribonucleic Acid and protein in the septum-hypothalamus of the rat. Endocrinology 144, 662–70. doi: 10.1210/en.2002-220908 [DOI] [PubMed] [Google Scholar]
- Mann EO, Radcliffe CA, Paulsen O, 2005. Hippocampal gamma-frequency oscillations: from interneurones to pyramidal cells, and back. J. Physiol 562, 55–63. doi: 10.1113/jphysiol.2004.078758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën K, 1996. Establishing tolerable dungeness crab (Cancer magister) and razor clam (Siliqua patula) domoic acid contaminant levels. Environ. Health Perspect 104, 1230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard C, Quirion R, Vigneault E, Bouchard S, Ferland G, El Mestikawy S, Gaudreau P, 2015. Glutamate presynaptic vesicular transporter and postsynaptic receptor levels correlate with spatial memory status in aging rat models. Neurobiol. Aging 36, 1471–82. doi: 10.1016/j.neurobiolaging.2014.11.013 [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW, 1982. The distribution of [3H]kainic acid binding sites in rat CNS as determined by autoradiography. Brain Res. 252, 91–100. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, 1998. Distributed encoding and retrieval of spatial memory in the hippocampus. J. Neurosci 18, 7535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muha N, Ramsdell JS, 2011. Domoic acid induced seizures progress to a chronic state of epilepsy in rats. Toxicon 57, 168–71. doi: 10.1016/j.toxicon.2010.07.018 [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S, 2008. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 319, 1260–4. doi: 10.1126/science.1151120 [DOI] [PubMed] [Google Scholar]
- Nijjar MS, Grimmelt B, Brown J, 1991. Purification of domoic acid from toxic blue mussels (Mytilus edulis) and phytoplankton. J. Chromatogr. B Biomed. Sci. Appl 568, 393–406. doi: 10.1016/0378-4347(91)80177-E [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K, 2004. The mouse brain in stereotaxic coordinates, 2nd ed Academic Press. [Google Scholar]
- Peng YG, Ramsdell JS, 1996. Brain Fos induction is a sensitive biomarker for the lowest observed neuroexcitatory effects of domoic acid. Fundam. Appl. Toxicol 31, 162–8. [DOI] [PubMed] [Google Scholar]
- Peng YG, Taylor TB, Finch RE, Switzer RC, Ramsdell JS, 1994. Neuroexcitatory and neurotoxic actions of the amnesic shellfish poison, domoic acid. Neuroreport 5, 981–5. [DOI] [PubMed] [Google Scholar]
- Pérez-Gómez A, Tasker RA, 2014. Enhanced mossy fiber sprouting and synapse formation in organotypic hippocampal cultures following transient domoic acid excitotoxicity. Neurotox. Res 25, 402–10. doi: 10.1007/s12640-013-9450-z [DOI] [PubMed] [Google Scholar]
- Pérez-Gómez A, Tasker RA, 2013. Transient domoic acid excitotoxicity increases BDNF expression and activates both MEK- and PKA-dependent neurogenesis in organotypic hippocampal slices. BMC Neurosci. 14, 72. doi: 10.1186/1471-2202-14-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Gómez A, Tasker RA, 2012. Enhanced neurogenesis in organotypic cultures of rat hippocampus after transient subfield-selective excitotoxic insult induced by domoic acid. Neuroscience 208, 97–108. doi: 10.1016/j.neuroscience.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Perl TM, Bédard L, Kosatsky T, Hockin JC, Todd EC, Remis RS, 1990. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N. Engl. J. Med 322, 1775–80. doi: 10.1056/NEJM199006213222504 [DOI] [PubMed] [Google Scholar]
- Petrie BF, Pinsky C, Standish NM, Bose R, Glavin GB, 1992. Parenteral domoic acid impairs spatial learning in mice. Pharmacol. Biochem. Behav 41, 211–4. [DOI] [PubMed] [Google Scholar]
- Pulido OM, 2008. Domoic acid toxicologic pathology: a review. Mar. Drugs 6, 180–219. doi: 10.3390/md20080010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Vaughn JE, Saito K, 1978. Immunocytochemical localization of glutamic acid decarboxylase in neuronal somata following colchicine inhibition of axonal transport. Brain Res. 140, 315–32. [DOI] [PubMed] [Google Scholar]
- Ross IA, Johnson W, Sapienza PP, Kim CS, 2000. Effects of the seafood toxin domoic acid on glutamate uptake by rat astrocytes. Food Chem. Toxicol 38, 1005–11. [DOI] [PubMed] [Google Scholar]
- Sari P, Kerr DS, 2001. Domoic acid-induced hippocampal CA1 hyperexcitability independent of region CA3 activity. Epilepsy Res. 47, 65–76. [DOI] [PubMed] [Google Scholar]
- Scholin CA, Gulland F, Doucette GJ, Benson S, Busman M, Chavez FP, Cordaro J, DeLong R, De Vogelaere A, Harvey J, Haulena M, Lefebvre K, Lipscomb T, Loscutoff S, Lowenstine LJ, Marin R, Miller PE, McLellan WA, Moeller PD, Powell CL, Rowles T, Silvagni P, Silver M, Spraker T, Trainer V, Van Dolah FM, 2000. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 403, 80–4. doi: 10.1038/47481 [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Prince DA, 1978. Cellular and field potential properties of epileptogenic hippocampal slices. Brain Res. 147, 117–30. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Jandová K, Struk I, Marešová D, Pokorný J, Riljak V, 2014. Low dose domoic acid influences spontaneous behavior in adult rats. Physiol. Res 63, 369–76. [DOI] [PubMed] [Google Scholar]
- Sciancalepore M, Gałdzicki Z, Zheng X, Moran O, 1990. Kainate activated single channel currents as revealed by domoic acid. Eur. Biophys. J 19, 63–8. [DOI] [PubMed] [Google Scholar]
- Seeburg PH, 1993. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 16, 359–65. [DOI] [PubMed] [Google Scholar]
- Sierra Beltrán A, Palafox-Uribe M, Grajales-Montiel J, Cruz-Villacorta A, Ochoa JL, 1997. Sea bird mortality at Cabo San Lucas, Mexico: evidence that toxic diatom blooms are spreading. Toxicon 35, 447–53. [DOI] [PubMed] [Google Scholar]
- Sihra TS, Rodríguez-Moreno A, 2013. Presynaptic kainate receptor-mediated bidirectional modulatory actions: mechanisms. Neurochem. Int 62, 982–7. doi: 10.1016/j.neuint.2013.03.012 [DOI] [PubMed] [Google Scholar]
- Sihra TS, Rodríguez-Moreno A, 2011. Metabotropic actions of kainate receptors in the control of GABA release. Adv. Exp. Med. Biol 717, 1–10. doi: 10.1007/978-1-4419-9557-5_1 [DOI] [PubMed] [Google Scholar]
- Silvagni PA, Lowenstine LJ, Spraker T, Lipscomb TP, Gulland FMD, 2005. Pathology of domoic acid toxicity in California sea lions (Zalophus californianus). Vet. Pathol 42, 184–91. doi: 10.1354/vp.42-2-184 [DOI] [PubMed] [Google Scholar]
- Stewart GR, Zorumski CF, Price MT, Olney JW, 1990. Domoic acid: A dementiainducing excitotoxic food poison with kainic acid receptor specificity. Exp. Neurol 110, 127–138. doi: 10.1016/0014-4886(90)90057-Y [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug FM, Ottersen OP, 1983. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature 301, 517–20. [DOI] [PubMed] [Google Scholar]
- Strain SM, Tasker RA, 1991. Hippocampal damage produced by systemic injections of domoic acid in mice. Neuroscience 44, 343–52. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Dudek FE, 2007. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog. Brain Res 163, 541–63. doi: 10.1016/S0079-6123(07)63029-5 [DOI] [PubMed] [Google Scholar]
- Takemoto T, 1978. Isolation and structural identification of naturally occurring excitatory amino acids, in: Kainic Acid as a Tool in Neurobiology. Raven Press, New York, pp. 1–15. [Google Scholar]
- Teitelbaum JS, Zatorre RJ, Carpenter S, Gendron D, Evans AC, Gjedde A, Cashman NR, 1990. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N. Engl. J. Med 322, 1781–7. doi: 10.1056/NEJM199006213222505 [DOI] [PubMed] [Google Scholar]
- Thompson SM, 1994. Modulation of inhibitory synaptic transmission in the hippocampus. Prog. Neurobiol 42, 575–609. [DOI] [PubMed] [Google Scholar]
- Todd EC, 1993. Domoic acid and amnesic shellfish poisonin-a review. J. Food Prot 56, 69–83. [DOI] [PubMed] [Google Scholar]
- Truelove J, Mueller R, Pulido O, Iverson F, 1996. Subchronic toxicity study of domoic acid in the rat. Food Chem. Toxicol 34, 525–9. [DOI] [PubMed] [Google Scholar]
- Truelove J, Mueller R, Pulido O, Martin L, Fernie S, Iverson F, 1997. 30-day oral toxicity study of domoic acid in cynomolgus monkeys: lack of overt toxicity at doses approaching the acute toxic dose. Nat. Toxins 5, 111–4. doi: [DOI] [PubMed] [Google Scholar]
- Tryphonas L, Truelove J, Iverson F, 1990a. Acute parenteral neurotoxicity of domoic acid in cynomolgus monkeys (M. fascicularis). Toxicol. Pathol 18, 297–303. [DOI] [PubMed] [Google Scholar]
- Tryphonas L, Truelove J, Nera E, Iverson F, 1990b. Acute neurotoxicity of domoic acid in the rat. Toxicol. Pathol 18, 1–9. [DOI] [PubMed] [Google Scholar]
- Tryphonas L, Truelove J, Todd E, Nera E, Iverson F, 1990c. Experimental oral toxicity of domoic acid in cynomolgus monkeys (Macaca fascicularis) and rats. Preliminary investigations. Food Chem. Toxicol 28, 707–15. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S, 1996. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–38. [DOI] [PubMed] [Google Scholar]
- van der Hel WS, Verlinde SAMW, Meijer DHM, de Wit M, Rensen MG, van Gassen KLI, van Rijen PC, van Veelen CWM, de Graan PNE, 2009. Hippocampal distribution of vesicular glutamate transporter 1 in patients with temporal lobe epilepsy. Epilepsia 50, 1717–28. doi: 10.1111/j.1528-1167.2009.02054.x [DOI] [PubMed] [Google Scholar]
- Van Dolah FM, 2000. Marine algal toxins: origins, health effects, and their increased occurrence. Environ. Health Perspect. 108 Suppl, 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekell JC, Gauglitz EJ, Barnett HJ, Hatfield CL, Simons D, Ayres D, 1994. Occurrence of domoic acid in Washington state razor clams (Siliqua patula) during 1991–1993. Nat. Toxins 2, 197–205. [DOI] [PubMed] [Google Scholar]
- Wetmore L, Nance DM, 1991. Differential and sex-specific effects of kainic acid and domoic acid lesions in the lateral septal area of rats on immune function and body weight regulation. Exp. Neurol 113, 226–36. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G, 2005. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J. Neurosci 25, 6221–34. doi: 10.1523/JNEUROSCI.3003-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C, 2004. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc. Natl. Acad. Sci. U. S. A. 101, 7158–63. doi: 10.1073/pnas.0401764101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work TM, Barr B, Beale AM, Fritz L, Quilliam MA, Wright JLC, 1993. Epidemiology of Domoic Acid Poisoning in Brown Pelicans ( Pelecanus occidentalis ) and Brandt’ s Cormorants (Phalacrocorax penicillatus ) in California. J. Zoo Wildl. Med 24, 54–62. [Google Scholar]
- Yamashita T, Ishikawa T, Takahashi T, 2003. Developmental increase in vesicular glutamate content does not cause saturation of AMPA receptors at the calyx of Held synapse. J. Neurosci 23, 3633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaczek R, Coyle JT, 1982. Excitatory amino acid analogues: neurotoxicity and seizures. Neuropharmacology 21, 15–26. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP, 2002. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J. Comp. Neurol 448, 217–29. doi: 10.1002/cne.10257 [DOI] [PubMed] [Google Scholar]