Abstract
We report the case of a 65-year-old patient with pseudolymphoma who developed acute toxoplasmosis following 6 cycles of rituximab and bendamustine therapy. Acute toxoplasmosis in the setting of biological response modifiers, rather than reactivation, is a unique unreported infection. The patient developed severe disease with multi-organ involvement, including retinitis, myocarditis, and myositis. We discuss the clinical findings, epidemiology, and laboratory diagnosis.
Keywords: toxoplasmosis, Toxoplasma gondii, encephalitis, biological therapy, rituximab, wild boar
Toxoplasma gondii remains a major global public health concern, infecting approximately one-third of the world’s population [1]. T. gondii exists in 3 different forms: the oocyst, the tachyzoite, and the bradyzoite. When mammals ingest oocytes, they give rise to tachyzoites, the rapidly dividing form of the organism. After acute infection, T. gondii persists within its mammalian host either as intramuscular cysts or inside neurons. The infection is usually asymptomatic in immunocompetent hosts. When symptoms occur, lymphadenopathy is the most common clinical presentation of Toxoplasma infection. However, encephalitis, a more severe form of the disease, becomes important in immunosuppressed individuals [2, 3]. T. gondii encephalitis typically occurs as a result of reactivation of the chronic stage of infection (bradyzoite) among immunocompromised hosts [1, 4], particularly in those with HIV/AIDS [2]. In addition, this organism is an important pathogen in patients receiving biological therapies [5]. Recently, Toxoplasma encephalitis may have occurred in patients receiving rituximab therapy [6–8]. Contrary to the widely held view that Toxoplasma encephalitis occurs exclusively from reactivation of latent infection, herein, we report the case of a 65-year-old male who presented with acute Toxoplasma encephalitis after recent acquisition of the infection while receiving biological therapies for the treatment of pseudolymphoma.
CASE PRESENTATION
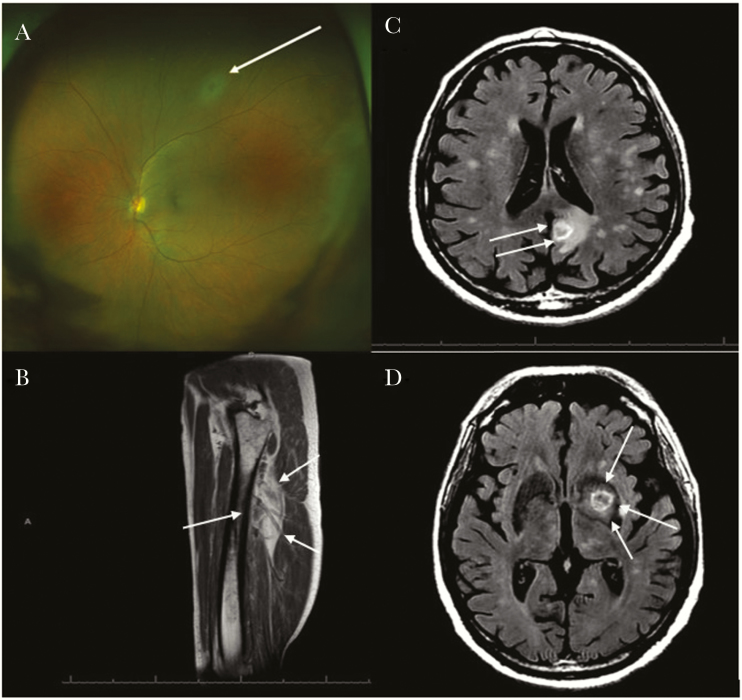
A 65-year-old male with a long-standing history of rheumatoid arthritis who was receiving Abatacept (Orencia) underwent emergent radiation therapy to the right orbit (1600 Cgy) due to the rapid expansion of ocular pseudolymphoma. He also received 6 cycles of bendamustine (Bendeka) and rituximab (Rituxan) over a 6-month period. Subsequently, due to progression of the disease, identified by magnetic resonance imaging (MRI) of the orbit concomitantly with rapid vision loss and the presence of a KRAS+ mutation, a course of trametinib (Mekinist)—a drug blocking the RAS-RAF-MEK-ERK signaling pathway involved in cell proliferation and differentiation—was administered, resulting in improvement of the orbital lesion. However, ~5 weeks after initiating therapy with trametinib, the patient presented to the hospital with gait incoordination and fine motor skill difficulties. An MRI of the brain (Figure 1C and D) demonstrated multiple supratentorial and infratentorial ring-enhancing lesions. Analysis of his cerebrospinal fluid demonstrated mild lymphocytic pleocytosis and the presence of T. gondii by polymerase chain reaction (PCR). Initial serological testing for toxoplasmosis conducted at our institution demonstrated an IgM titer of 11.4 IgM (reference range for positive ≥7.9) but negative IgG titers for T. gondii. A second serological assessment obtained only 2 days later was submitted to the National Reference Laboratory for the Study and Diagnosis of Toxoplasmosis (Palo Alto, CA). This laboratory confirmed recent acquisition of Toxoplasma infection by our patient, as demonstrated by a positive IgG (Dye test) at 1:512 titers (reference range for positive ≥1:16), IgM enzyme-linked immunosorbent assay (ELISA) at 2.9 (reference range for positive >2.0), low IgG avidity, and IgA ELISA antibody testing at 3.3 (reference range for positive ≥2.1). The patient also demonstrated findings consistent with disseminated toxoplasmosis: confirmed retinitis by ophthalmological evaluation (Figure 1A), myocarditis (the patient reported chest discomfort, dyspnea, and orthopnea associated with diffuse global hypokinesis demonstrated by echocardiogram and elevated serum troponin levels), and right thigh myositis (identified by MRI of the right thigh requested due to report of localized right leg pain) (Figure 1B). Approximately 3–4 weeks before presenting to the hospital, he had traveled to rural Mississippi to visit friends and family, where he reported ingestion of wild boar sausages while he was still receiving oral daily trametinib. Assuming the boar sausages were the source of the patient’s recent infection, we submitted a batch of sausages obtained from the same vendor in Mississippi for Toxoplasma PCR testing at Palo Alto. However, the results came back negative. After receiving a 16-week course of high-dose trimethoprim-sulfamethoxazole (TMP-SMX), the patient experienced substantial clinical improvement and a reduction in the size and number of central nervous system lesions.
Figure 1.
A, Fundus photo of a left eye. Superior area of retinal whitening consistent with retinitis (white arrow) without overlying vitritis noted on exam. B, T1: magnetic resonance image (MRI) of the right thigh showing right thigh myositis (white arrows, increased fluid signal within the vastus intermedialis muscle). C and D, Brain T2 fluid-attenuated inversion recovery (FLAIR) MRI showing postcontrast multiple ring-enhancing lesions of toxoplasmosis (white arrows).
DISCUSSION
Toxoplasmosis is a parasitic infection caused by the obligate intracellular parasite T. gondii [2]. Toxoplasmosis in immunocompromised individuals is usually the result of reactivation of a dormant infection [3]. In immunocompetent individuals, the organism can persist asymptomatically in the central nervous system [2]. In immunocompromised hosts, tissue cyst reactivation occurs, resulting in uncontrolled tachyzoite replication and potentially life-threatening disease [1]. Our patient’s new-onset neurological deficits, along with a history of receiving biological therapy and recent ingestion of wild boar sausage, led us to suspect Toxoplasma encephalitis. We report here the uncommon presentation of the acute form of the disease in an immunocompromised patient likely linked to the recent use of biological therapy. We propose that the use of biological response modifiers—rituximab and bendamustine—resulted in profound immunosuppression favoring the widespread dissemination of the tachyzoite form during primary Toxoplasma infection.
Serological techniques play a key role in differentiating between an acute and a chronic infection [9]. Our patient had positive Toxoplasma IgM initially but negative IgG. This was difficult to interpret as IgM alone is not an accurate marker of acute infection. It can remain elevated for several months and even years, resulting in a false-positive result, rendering it difficult to distinguish between an acute and a chronic infection. Toxoplasma IgG in our patient became positive at a later stage. In fact, the appearance of IgG after IgM points to the definite diagnosis of an acute (primary) infection. This was further confirmed by the low IgG avidity [9].
From an epidemiological perspective, our patient probably acquired toxoplasmosis from ingesting wild boar sausage during his recent trip to Mississippi, despite the negative Toxoplasma PCR testing of the sausage. PCR-based methods are less sensitive than bioassays because of the unequal distribution of tissue cysts and the small sample size [10]. The seroprevalence of T. gondii among feral swine in the United States is approximately 20%, and its ingestion is considered a risk factor for acquiring toxoplasmosis [11]. Other factors associated with the acquisition of T. gondii include the ingestion of raw ground beef, rare lamb, locally produced cured, dried, or smoked meat, raw oysters, clams, or mussels, drinking unpasteurized goat’s milk, working with meat, and having 3 or more kittens [4].
Biological therapy has improved the lives of many patients with debilitating immune-mediated inflammatory diseases. However, better quality of life has come with the added risk of infection by opportunistic pathogens. T. gondii has been identified as a potential opportunistic pathogen in the setting of biological therapy [5].
Rituximab is a chimeric monoclonal antibody that targets the protein CD-20, a B-cell-specific marker. It is used in the treatment of both autoimmune and malignant hematological disorders. Previously published studies showed an increased risk of opportunistic infections in patients with hematological malignancies receiving rituximab, though the association between rituximab use and the development of parasitic infections has not yet been clearly established [12]. Safa et al. [6] reported the case of a 71-year-old woman with reactivation of cerebral toxoplasmosis following rituximab therapy, highlighting the need to consider cerebral toxoplasmosis whenever patients receiving rituximab present with neurological deficits. Similarly, Holland et al. [7] described the case of a 77-year-old woman who developed cerebral toxoplasmosis while on rituximab therapy. Morjaria et al. [8] reported 3 cases of reactivation of Toxoplasma encephalitis, 2 of whom were receiving rituximab therapy. To our knowledge, we report here the first case describing acute cerebral toxoplasmosis, rather than reactivation, in a patient who received 6 cycles of rituximab and bendamustine therapy followed by a short course of trametinib.
We believe that the use of rituximab played a key role in the occurrence of toxoplasmosis in our patient. Indeed, opportunistic infections following completion of rituximab therapy may take place within the first months after its administration [6, 7]. Although rituximab’s main mechanism of action is B-cell suppression, it is also hypothesized that it exerts its effects on T cells, as manifested by the occurrence of CD4+ T-cell driven opportunistic infections under rituximab therapy. Recent studies have shown that there is a decline in inflammatory cytokines, including interferon-gamma and interleukin-12, following rituximab administration [13]. In vitro studies showed that both interferon-gamma and interleukin-12 are essential in host resistance against T. gondii [14]. This could account for the enhanced susceptibility to T. gondii infection following rituximab therapy. The use of bendamustine may have also contributed to the occurrence of toxoplasmosis [15, 16]. Nonetheless, our patient developed toxoplasma encephalitis during the acute phase of the infection compared with the cases of toxoplasma encephalitis caused by reactivation. Additionally, we were unable to identify any reported potential association between trametinib and toxoplasmosis.
In summary, with the growing number of individuals receiving potent biological therapies for the treatment of immune-mediated inflammatory diseases and malignancies, clinicians must be aware of the potential occurrence of Toxoplasma encephalitis not only during reactivation of latent infection but also presenting as a primary infection. This infection risk is not exclusive to patients on biological therapies, but it involves other immunocompromised patients such as HIV-positive and solid organ transplant patients. A key historical clue to the diagnosis of acute toxoplasmosis is recent travel to highly endemic areas, eating undercooked meat, or interacting with cats [17]. Finally, prophylactic treatment in patients on rituximab or bendamustine who test positive for Toxoplasma-specific IgG antibodies is not yet warranted. As the association between rituximab therapy and Toxoplasma encephalitis is only based on case reports [6–8], there is not yet enough evidence for screening or prophylactic therapy recommendations before rituximab monotherapy or when it is combined with other potent immunosuppressive agents such as bendamustine. Nevertheless, immunocompromised hosts, particularly those receiving rituximab-bendamustine regimens who do not qualify for prophylactic therapy due to negative Toxoplasma screening, should avoid ingestion of and interaction with sources of Toxoplasma, as mentioned previously, given the risk of developing an acute disseminated Toxoplasma infection.
Acknowledgments
Financial support. None.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wohlfert EA, Blader IJ, Wilson EH. Brains and brawn: toxoplasma infections of the central nervous system and skeletal muscle. Trends Parasitol 2017; 33:519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mendez OA, Koshy AA. Toxoplasma gondii: entry, association, and physiological influence on the central nervous system. PLoS Pathog 2017; 13:e1006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004; 363:1965–76. [DOI] [PubMed] [Google Scholar]
- 4. Jones JL, Dargelas V, Roberts J, et al. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis 2009; 49:878–84. [DOI] [PubMed] [Google Scholar]
- 5. Winthrop KL, Novosad SA, Baddley JW, et al. Opportunistic infections and biologic therapies in immune-mediated inflammatory diseases: consensus recommendations for infection reporting during clinical trials and postmarketing surveillance. Ann Rheum Dis 2015; 74:2107–16. [DOI] [PubMed] [Google Scholar]
- 6. Safa G, Darrieux L. Cerebral toxoplasmosis after rituximab therapy. JAMA Intern Med 2013; 173:924–6. [DOI] [PubMed] [Google Scholar]
- 7. Holland MS, Sharma K, Lee BC. Cerebral toxoplasmosis after rituximab therapy for splenic marginal zone lymphoma: a case report and review of the literature. JMM Case Reports 2015; 2:1–4. [Google Scholar]
- 8. Morjaria S, Epstein DJ, Romero FA, et al. Toxoplasma encephalitis in atypical hosts at an academic cancer center.Open ForumInfect Dis. 2016; XXX(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dard C, Fricker-Hidalgo H, Brenier-Pinchart MP, Pelloux H. Relevance of and new developments in serology for toxoplasmosis. Trends Parasitol 2016; 32:492–506. [DOI] [PubMed] [Google Scholar]
- 10. Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 2012; 25:264–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hill DE, Dubey JP, Baroch JA, et al. Surveillance of feral swine for Trichinella spp. and Toxoplasma gondii in the USA and host-related factors associated with infection. Vet Parasitol 2014; 205:653–65. [DOI] [PubMed] [Google Scholar]
- 12. Kelesidis T, Daikos G, Boumpas D, Tsiodras S. Does rituximab increase the incidence of infectious complications? A narrative review. Int J Infect Dis 2011; 15:e2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Avivi I, Stroopinsky D, Katz T. Anti-CD20 monoclonal antibodies: beyond B-cells. Blood Rev 2013; 27:217–23. [DOI] [PubMed] [Google Scholar]
- 14. Yarovinsky F. Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol 2014; 14:109–21. [DOI] [PubMed] [Google Scholar]
- 15. Fung M, Jacobsen E, Freedman A, et al. Increased risk of infectious complications in older patients with indolent non-Hodgkin lymphoma exposed to bendamustine. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chintakuntlawar A, Kidd M, Al-Kali A, et al. Toxoplasmosis in patients with hematologic malignancies. Leuk Lymphoma 2015; 56:536–8. [DOI] [PubMed] [Google Scholar]
- 17. Henao-Martínez AF, Franco-Paredes C, Palestine AG, Montoya JG. Symptomatic acute toxoplasmosis in returning travelers. Open ForumInfect Dis. 2018; XXX(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]