Abstract
Bovine respiratory syncytial virus (BRSV) is the leading cause of viral pneumonia in calves, making young passively immune calves candidates for vaccination, and raising issues concerning boosting of neonatally primed responses. To address this, 18, 2-month-old Angus-cross passively immune beef heifer calves that had been primed at birth with a combination viral intranasal vaccine were administered either a parenteral combination vaccine containing modified-live (MLV) BRSV or a similar vaccine containing inactivated BRSV. At 6 months of age, these calves and 2 controls that received only the MLV at 2 months of age were challenged with BRSV via aerosol. Two calves, 1 control, and 1 MLV-boosted, developed severe respiratory disease and required euthanasia; the remaining calves developed no or mild respiratory disease and recovered. Calves that received the inactivated booster had significantly higher arterial oxygen concentrations on Day 7 after challenge and had anamnestic BRSV-specific IgG and neutralizing antibodies after challenge; the MLV-boosted calves did not. These data suggest that adjuvanted inactivated parenteral BRSV vaccines administered at 2 months of age may provide better boosting for neonatally mucosally primed calves.
Résumé
Efficacité comparée des vaccins vivants modifiés et des vaccins inactivés pour améliorer la réponse au virus respiratoire syncytial bovin après la sensibilisation active néonatale des muqueuses chez les veaux de boucherie. Le virus respiratoire syncytial bovin (VRS) est la cause principale de pneumonie virale chez les veaux, ce qui rend des jeunes veaux à immunité passive des candidats pour la vaccination et soulève des enjeux liés à l’amélioration de la réponse des nouveau-nés sensibilisés. Dans le but d’aborder cette situation, 18 veaux de boucherie de race croisée Angus âgés de 2 mois ayant une immunité passive, qui avaient été sensibilisés activement à la naissance à l’aide d’une combinaison de vaccins intranasaux viraux, ont reçu soit un vaccin combiné parentéral contenant le VRS modifié vivant (VMV) ou un vaccin semblable contenant le VRS inactivé. À l’âge de 6 mois, ces veaux et deux témoins qui avaient reçu seulement le VNV à l’âge de 2 mois, ont été exposés au VRS par voie aérosol. Deux veaux, un témoin et un animal ayant reçu le rappel VMV, ont développé une maladie respiratoire grave et ont dû être euthanasiés; les autres animaux ont développé une maladie respiratoire légère et se sont rétablis ou n’ont manifesté aucun symptôme. Les veaux qui avaient reçu le rappel inactivé affichaient des concentrations d’oxygène significativement supérieures dans le sang artériel le jour 7 après le test et présentaient des anticorps neutralisants et anamnestiques spécifiques aux VRS après le test, contrairement aux veaux ayant reçu le rapport VNV. Ces données suggèrent que les vaccins VRS parentéraux inactivés avec adjuvants administrés à l’âge de 2 mois peuvent offrir une meilleure protection pour les veaux sensibilisés activement à la naissance sur les muqueuses.
(Traduit par Isabelle Vallières)
Introduction
Bovine respiratory syncytial virus (BRSV) is an important paramyxoviral pathogen of cattle, causing primary pneumonia in animals of all ages, and predisposing to secondary bacterial infections in the bovine respiratory disease complex (BRDC) (1). Vaccine development for BRSV commenced shortly after the nearly simultaneous isolation of the virus from afflicted cattle in Asia, Europe, and North America in the early 1970’s (2). By the late 1970’s both modified-live and inactivated vaccines were commercially available (2). Intranasal vaccines containing BRSV were launched first in Europe and then in North America (2). Representative commercial vaccines of all these types confer disease-sparing responses in a challenge model that closely mimics naturally occurring BRSV-associated respiratory disease (2). Nevertheless, ever since the first availability of BRSV vaccines, there has been controversy concerning efficacy and disease-enhancing potential of BRSV vaccines, especially inactivated BRSV vaccines (2). Despite proven efficacy, and the fact that uncommon instances of disease enhancement have been reported with both inactivated and modified live virus (MLV) BRSV vaccines, it remains in the lore of the veterinary profession that inactivated BRSV vaccines are generically ineffective and harmful (2). Much of the theoretical concern regarding inactivated BRSV vaccines derives from an extrapolation from the unfortunate history of a formalin-inactivated vaccine for human RSV, a significant human pathogen for which there is still no vaccine (2).
Given that BRSV is an especially significant pathogen in calves, one of the problems in application of BRSV vaccines has been the theoretical (3) and documented (4) issue of inhibition of priming by maternal antibodies (MatAb) in passively immune calves, a generic feature of immune response induction in young animals (3). Although it has been demonstrated that neonatal intranasal administration of MLV vaccines can override MatAb, there remains unanswered questions concerning the duration of immunity (DOI) of neonatal priming and the timing and choice of immunogen that can best boost that response (5). One approach to this issue in comparative vaccinology has been the heterologous prime-boost method of immunization, which utilizes different forms of an antigen, administered by different routes to broaden and extend immune responses (6). It was the purpose of this study to address these issues related to induction and duration of immunity to BRSV in cattle that were reared under conditions that were essentially those of a commercial beef operation.
An obvious null hypothesis in approaching this experiment was that there would be no difference in the ability of parenteral MLV and inactivated combination vaccines to boost primed responses of calves at approximately 2 months of age. However, our working hypothesis, given the increased antigenic mass and presence of an adjuvant that stimulates both adaptive and innate immune response, was that the inactivated BRSV vaccine would be a superior booster vaccine. This putative superiority would be demonstrable in superior BRSV-specific immune responses and/or, more importantly, superior disease-sparing clinical responses subsequent to BRSV challenge.
Materials and methods
Experimental design
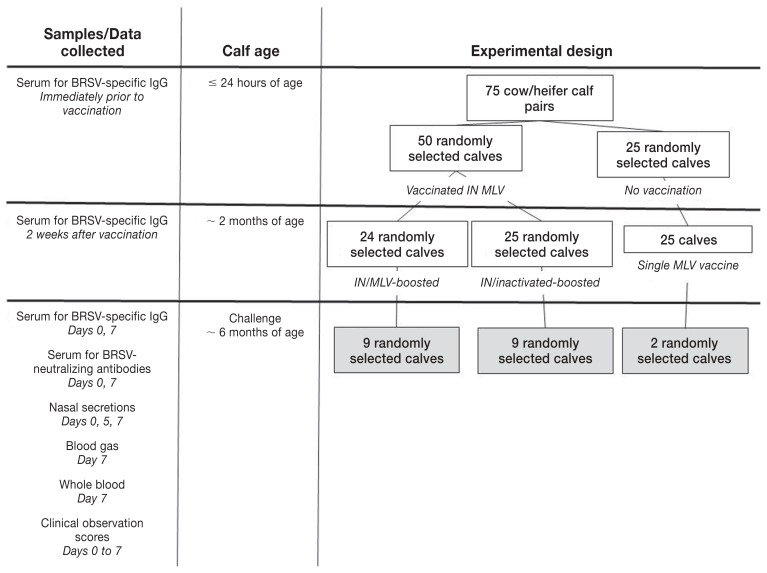
Cattle used in this experiment were part of a larger study to investigate the comparative efficacy of immune response induction by commercial vaccines (Figure 1). In brief, 75 heifer calves born to multiparous Angus-cross beef cows were randomized into 3 groups of 25 each. The calves were numbered sequentially as they were born, and the calf tag numbers were randomly allocated to a vaccination group before the beginning of calving season. Within 24 h of birth, 50 calves received a combination intranasal (IN) vaccine containing modified live BRSV, bovine parainfluenza-3 virus (BPIV-3), and bovine herpesvirus-1, (BHV-1) (Inforce-3; Zoetis Canada, Kirkland, Quebec). The remaining 25 calves were not vaccinated. At this time, serum was collected via jugular venipuncture for measurement of BRSV-specific IgG (Figure 1).
Figure 1.
Experimental design and sampling.
At approximately 2 mo of age, 24 (1 calf died within a few days of birth and had no signs of respiratory disease or other disease at gross postmortem) of the 50 neonatally IN-vaccinated calves were boosted with a parenteral combination modified-live virus (MLV) vaccine containing BRSV, bovine parainfluenza virus-3 (BPIV-3), bovine herpes virus-1 (BHV-1), and bovine virial diarrhea virus (BVDV) types 1 and 2 (Bovi-Shield Gold FP; Zoetis). These calves are identified as “IN/MLV-boosted” calves. The other 25 calves that were boosted at 2 mo of age received a parenteral combination inactivated vaccine containing BRSV, BPIV-3, BHV-1 and BVDV types 1 and 2 (Triangle 5; Boehringer Ingelheim Canada, Burlington, Ontario). Calves in this group are identified as “IN/Inactivated-boosted” calves. The 25 calves that were not neonatally vaccinated received the recommended single dose of the parenteral combination MLV vaccine (Bovi-Shield Gold FP; Zoetis) that had been historically used in this herd. This group was the source of “control” calves (Figure 1).
All vaccines were purchased from the manufacturers. Iterations of each of these vaccines containing the respective forms of the BRSV antigen have been shown to confer disease-sparing immunity to BRSV when used as standalone products in young BRSV-seronegative calves (5,7–9). Serum samples were obtained 2 wk after this vaccination for measurement of BRSV-specific IgG (Figure 1).
All 75 cow-calf pairs were maintained on pasture until the time of weaning. Calves were weaned at approximately 4 mo after the parenteral vaccination when they were 6 to 7 mo old. At this time, 9 IN/MLV-boosted calves [group mean weight (wt): 242 kg ± 26 kg standard deviation (SD)] and 9 IN/inactivated boosted calves (group mean wt: 232 kg ± 21 kg) were randomly selected from their respective treatment groups. Two control calves (229 kg and 247 kg) were randomly chosen from the calves that received only the combination MLV vaccine at 2 mo of age (controls; Figure 1).
The 20 calves were challenged on the day of weaning as previously described (4,5). Briefly, all calves were loaded into an enclosed transport stock trailer measuring approximately 7.3 × 2.4 × 2.4 m (29 m3 of air space). Calves were exposed by aerosol delivery of BRSV. The challenge inoculum consisted of a lung wash which was obtained from a newborn calf infected with BRSV (Asquith strain 9). The lung wash was confirmed negative for bacterial contamination, Mycoplasma spp., by culture, and for BHV-1, BPIV-3, bovine coronavirus, and BVDV by polymerase chain reaction (PCR). For aerosol delivery, 40 mL of the in vivo-passaged BRSV inoculum [103.4 plaque-forming units (pfu)/mL] was placed in each of 2 ultrasonic nebulizers (Ultra-Neb 99; Devilbiss, Somerset, Pennsylvania, USA) that were placed contralaterally ~ 1.8 m off the floor of the trailer. After 40 min in the sealed trailer, calves were removed and maintained as a single group in 1 large pen (4,5).
Clinical assessment and sample collection
Daily observations began during the challenge phase of the study. Calves were assessed on Day 0 immediately before BRSV inoculum challenge and on Days 1 through 14 after challenge. All observations were done by an experienced cattle veterinarian who was unaware of the treatment groups. The calves were observed in their paddock at the same time each morning for clinical signs using a previously described protocol (Appendix) (4,5).
In order to avert the potential confounding variable of stress associated with handling and confinement, calves were not confined in a chute system during observation except on Days 0, 5, and 7 when sampling was required. Samples collected during the challenge phase included: nasal mucus by deep nasal swabbing of both nares (4,5) on the day of challenge (Day 0) and on Days 5 and 7 after challenge for measurements of BRSV-specific IgA and shedding of BRSV; jugular venous blood for serum on Days 0 and 7 for measurement of BRSV-specific IgG and BRSV-neutralizing antibodies; unclotted jugular venous blood collected in acid citrate dextrose on Day 7 for measurement of BRSV-stimulated interferon-gamma; and arterial blood from the caudal thoracic aorta (10) on Day 7 for measurements of arterial oxygen (PaO2 in mmHg) which were corrected for rectal temperature (10) (Figure 1).
Euthanasia was performed if 2 clinical signs indicative of substantial respiratory tract disease, including, moderate signs of depression, dull eyes, droopy ears, rough coat, gauntness and moderate respiratory distress or dyspnea (> 100 breaths/min) were observed for 2 consecutive days (4,5). Calves were euthanized immediately if they were observed at any time with severe respiratory distress, for example, pronounced open-mouthed, labored breathing (as evidenced by an expiratory grunt), if they were severely depressed and recumbent with reluctance to rise, or if they had PaO2 < 60 mmHg [normal range: 80 to 100 mmHg in cattle; (10)]. These criteria were consistent with Canadian Council of Animal Care guidelines and were approved by the Committee on Animal Care and Supply at the University of Saskatchewan (4,5).
At the end of the trial, on Day 7, calves were treated with 6 mL of tulathromycin (Draxxin; Zoetis Canada) and observed for another 7 d to ensure recovery prior to return to the herd.
Quantitative virus isolation
Virus shedding in nasal secretions was quantitatively determined by use of a microisolation plaque assay with bovine embryonic lung fibroblasts (7).
Postmortem analysis
On necropsy the respiratory tract was harvested and percent pneumonic lung for each lung lobe was assessed visually and scored. Scores were weighted using the following ratios of individual lung lobes to total lung mass: left cranial 5%, left posterior cranial 6%, left caudal 32%, right cranial 6%, right posterior cranial 5%, right middle 7%, right caudal 35%, and intermediate 4%. The weighted lung lobe values were summed across lobes to yield the percentage of pneumonic lung for each calf necropsied.
Interferon gamma assay
Interferon gamma (IFNγ) was measured in plasma from 24 h BRSV-stimulated whole blood cultures (7) using an antigen capture enzyme-linked immunosorbent assay (ELISA) (Pierce Biotechnology, Rockford, Illinois, USA).
Antibody assays
Tests for BRSV neutralizing (VN) antibodies and total BRSV-specific IgG (ELISA) for serum samples, and BRSV-specific IgA (ELISA) for nasal secretions were performed and analyzed as previously described (7,11) in a single set of assays performed at the end of the study to reduce inter-assay variation.
Data analysis
Clinical and laboratory outcome variables were stratified by treatment group (i.e., IN/MLV-boosted and IN/inactivated-boosted calves) (Table 1). Descriptive statistics were performed and data were assessed for normality (12). Only PaO2 values were normally distributed and were analyzed using a 1-way analysis of variance (ANOVA), with the results presented as the mean and standard deviation. Statistically significant differences were considered at P ≤ 0.05.
Table 1.
Descriptive statistics for differences between IN/MLV-boosted and IN/inactivated-boosted calves.
| Outcome | Sample source | Time frame | Treatment group | Median | Range |
|---|---|---|---|---|---|
| Sum of clinical scores | Observation sheets | Challenge | IN/MLV-boosted | 1 | 0 to 4 |
| Day 0 to 7 | IN/inactivated-boosted | 0 | 0 to 1 | ||
| Sum of coughing scores | Observation sheets | Challenge | IN/MLV-boosted | 1 | 0 to 4 |
| Day 0 to 7 | IN/inactivated-boosted | 2 | 0 to 3 | ||
| BRSV virus isolation (PFU/mL) | Nasal swabs | Challenge Day 0 | IN/MLV-boosted | < 10 | 10 to 10 |
| IN/inactivated-boosted | < 10 | 10 to 10 | |||
| Challenge Day 5 | IN/MLV-boosted | 30 | 10 to 380 | ||
| IN/inactivated-boosted | < 10 | 10 to 250 | |||
| Challenge Day 7 | IN/MLV-boosted | < 10 | 10 to 30 | ||
| IN/inactivated-boosted | < 10 | 10 to 20 | |||
| BRSV specific IgG (ELISA units) | Serum | ≤ 24 h old | IN/MLV-boosted | 118.0 | 104.0 to 150.0 |
| IN/inactivated-boosted | 101.0 | 73.0 to 138.0 | |||
| 2 mo old | IN/MLV-boosted | 40.9 | 38.7 to 51.1 | ||
| IN/inactivated-boosted | 42.0 | 34.6 to 51.8 | |||
| Challenge Day 0 | IN/MLV-boosted | 0.0 | 0.0 to 2.5 | ||
| IN/inactivated-boosted | 0.0 | 0.0 to 11.5 | |||
| Challenge Day 7 | IN/MLV-boosted | 3.4 | 0.0 to 32.4 | ||
| IN/inactivated-boosted | 47.2 | 13.1 to 67.8 | |||
| BRSV specfic IgA (ELISA units) | Nasal swabs | Challenge Day 0 | IN/MLV-boosted | 1.8 | 0.0 to 4.7 |
| IN/inactivated-boosted | 3.3 | 0.0 to 5.2 | |||
| Challenge Day 5 | IN/MLV-boosted | 3.5 | −0.37 to 12.9 | ||
| IN/inactivated-boosted | 4.1 | 0.0 to 20.2 | |||
| Challenge Day 7 | IN/MLV-boosted | 1.9 | −0.05 to 12.9 | ||
| IN/inactivated-boosted | 5.0 | −0.11 to 14.2 | |||
| BRSV serum neutralization titers (geometic means) | Serum | Challenge Day 0 | IN/MLV-boosted | 0 | 0 to 2 |
| IN/inactivated-boosted | 0 | 0 to 0 | |||
| Challenge Day 7 | IN/MLV-boosted | 1 | 0 to 5 | ||
| IN/inactivated-boosted | 5 | 3 to 7 |
Measurements with repeated observations were summarized to reflect clinically important outcomes. For the variables listed in Table 1, it was the change in values between sampling days that was analyzed to determine if there were differences between the 2 treatment groups. Whenever samples were taken on several different days, this same approach was taken for each laboratory outcome variable of interest. For example, data were analyzed to determine if there was a difference between the 2 treatment groups in the change of BRSV-specific IgG from baseline (Day 0, just prior to challenge) to Day 5 after challenge.
For the daily clinical scores, data were summarized and analyzed for Days 0 through 7 after challenge as this is the time frame which has previously been analyzed using this model (2). Clinical scores were summed to derive a total clinical score for each calf during this phase of the trial. Total incidents of observed coughing were also summed in the same way for a total cough score. These parameters were used as an indicator of overall calf illness. The higher the score, the more clinical signs of disease, or the more coughing that was present.
The Mann-Whitney U-test for non-parameteric data was used to compare the difference between treatment groups for the summarized non-normally distributed data. These results are presented as medians and ranges. A Bonferroni correction was applied for multiple comparisons therefore differences between treatment groups were considered statistically significant where P ≤ 0.01 (12). All statistical analyses were performed using IBM SPSS Statistics for Windows V. 23 (IBM, Armonk, New York, USA).
Results
Clinical signs and mortality
Eighteen of the 20 calves developed signs of mild respiratory disease after aerosol challenge with BRSV that were reflected in low total clinical scores on Day 7 after challenge (Table 1). There were no statistically significant differences between the boosted groups with regard to total clinical scores (P = 0.76). Two calves, 1 of the controls, and 1 of the IN/MLV-boosted calves developed severe respiratory disease (total clinical scores of 8 and 4, respectively) and required euthanasia on Day 7 after challenge. The other control calf had a total clinical score of 2. There was a variable amount of coughing over the entire observation period after challenge that was not significantly different between the 2 boosted groups (Table 1; P = 0.80). Coughing was not observed by Day 14 after challenge. The 18 remaining calves were considered clinically normal at this time and were returned to the herd.
BRSV in nasal secretions
None of the calves had detectable BRSV on nasal swabs on the day of weaning/challenge (< 10 PFU/mL) (Table 1). On Day 5, the number of calves shedding BRSV was similar in both groups, with 6 IN/MLV-boosted calves and 5 IN/inactivated-boosted calves shedding ≥ 10 PFU/mL of virus. The 2 control calves shed 360 and 340 PFU of virus per mL on Day 5. On Day 7, only 1 IN/MLV-boosted calf (30 PFU/mL) and 1 IN/inactivated-boosted calf (20 PFU/mL) shed BRSV, and the amount of BRSV shed by those calves was close to the Day 0 values. The 2 control calves shed 40 and 30 PFU/mL of virus on Day 7. There were no statistically significant differences between the boosted groups in number of calves shedding or amount of BRSV shed (P > 0.67)
PaO2 and pneumonic lesions
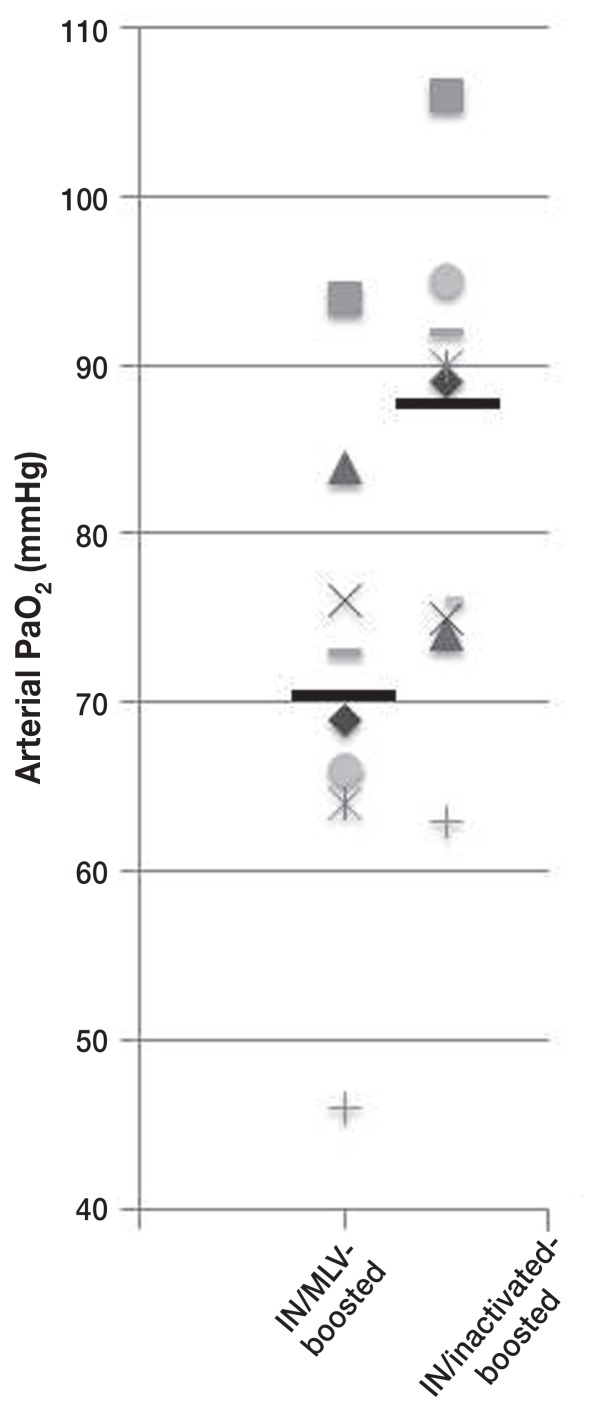
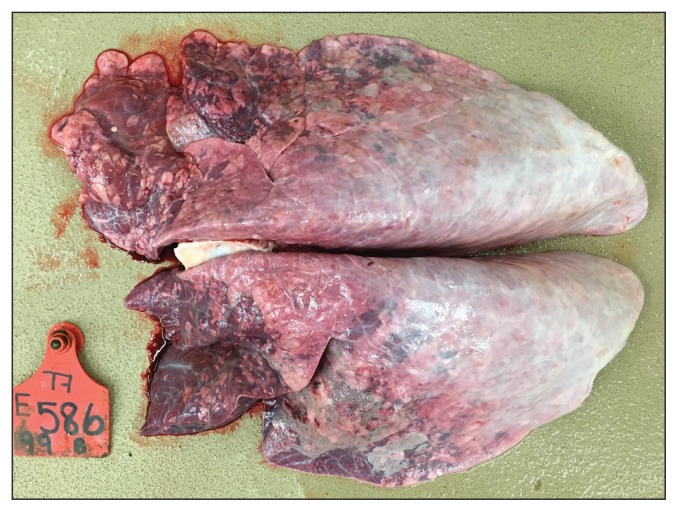
The IN/inactivated-boosted calves had significantly (P = 0.05) higher concentrations of arterial blood oxygen on Day 7 (mean: 87.1 ± 11.3 mmHg), compared to IN/MLV-boosted calves (mean: 70.4 ± 13.4 mmHg; Figure 2). The 2 calves that required euthanasia, a control and an IN/MLV-boosted calf, had PaO2 concentrations of 47 mmHg and 46 mmHg, respectively, and pneumonic lung involvement of 60.8% and 44.7%, respectively. The lungs in these calves had lesions characteristic of acute BRSV infection, comprising atelectasis of anterior ventral regions of lung lobes and hyperinflation and interstitial emphysema of dorsal aspects of the caudal lobe (Figure 3).
Figure 2.
Scatter plot of arterial PaO2 concentrations on Day 7 after BRSV infection in neonatally primed calves that received either modified-live or inactivated combination BRSV vaccines as booster immunizations at 2 mo of age. Lines indicate group mean values.
Figure 3.
Lungs from a passively immune calf that was neonatally primed and boosted at 2 mo of age with a combination modified-live BRSV vaccine and developed severe respiratory disease when challenged with BRSV at 6 mo of age. Note characteristic gross lesions of acute BRSV infection comprising atelectasis of anterior ventral aspects of the lung and hyperinflation of dorsal aspects.
Interferon gamma responses
Secretion of IFN-gamma by BRSV-stimulated blood leukocytes was variable and not significantly (P = 1.0) different between treatment groups (IN/inactivated-boosted calves median: 2.9 pg/mL, range: 0 to 51 pg/mL; IN/MLV-boosted calves median: 2.2 pg/mL, range: 0 to 51 pg/mL).
Antibody responses
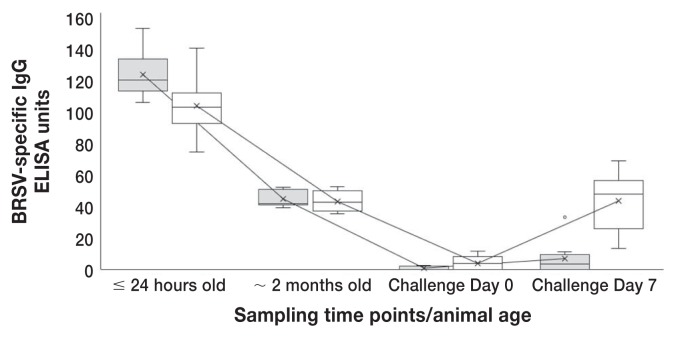
All 20 calves had high concentrations of BRSV-specific maternal (IgG) antibodies (as determined by ELISA post-suckling (≤ 24 h old) (Table 1; Figure 2). The 2 control calves had values of 127 and 93 units. By 2 mo of age, when the calves were boosted (or for the controls, vaccinated for the first time) with a MLV or inactivated parenteral vaccine, median values were nearly identical between the 2 boosted groups (Table 1; Figure 2) and there was no significant difference between the IN/inactivated-boosted group and the IN/MLV-boosted group in the change in BRSV-specific IgG antibodies between birth (≤ 24 h old) and 2 mo of age (Table 2). At weaning (6 to 7 months of age/Day 0 challenge) there was a further expected decay of maternal antibodies to low concentrations (Table 1; Figure 4). By Day 7 after challenge, all IN/inactivated-boosted calves had increases in BRSV-specific IgG in serum that were higher than the IN/MLV-boosted calves (Table 1). Only 1 calf in the IN/MLV-boosted group had a biologically significant increase (2 to 32 ELISA units; Figure 4) after challenge exposure to BRSV. The change in BRSV-specific IgG antibodies between challenge Day 0 (weaning/6 to 7 mo of age) and Day 7 after challenge were significantly (P < 0.0001) higher in the IN/inactivated-boosted group than the IN/MLV-boosted group (Table 2; Figure 4).
Table 2.
Summary of differences between IN/MLV-boosted and IN/inactivated-boosted calves before and during challenge.
| Sample (units) | Outcome | Treatment group | Median | Range | P-value* |
|---|---|---|---|---|---|
| BRSV specific IgG (ELISA units) | Change in BRSV serum specific | IN/MLV-boosted | 76.7 | 56.0 to 99.7 | 0.04 |
| IgG ≤ 24 h old (1st vaccination) to 2 mo of age (post boost) | IN/inactivated-boosted | 51.9 | 36.1 to 99.1 | ||
| Change in BRSV serum specific | IN/MLV-boosted | 3.3 | 0.0 to 29.9 | < 0.0001 | |
| IgG from baseline (Day 0) to Day 7 | IN/inactivated-boosted | 46.9 | 13.1 to 56.2 | ||
| BRSV specific IgA (ELISA units) | Change in BRSV specific IgA from baseline (Day 0) to Day 5 | IN/MLV-boosted | 1.4 | −2.0 to 12.9 | 0.73 |
| IN/inactivated-boosted | 1.0 | −5.2 to 16.8 | |||
| Change in BRSV specific IgA from baseline (Day 0) to Day 7 | IN/MLV-boosted | 1.5 | −4.5 to 11.5 | ||
| IN/inactivated-boosted | 2.2 | −14.5 | 0.73 | ||
| Change in BRSV specific IgA from Day 5 to Day 7 | IN/MLV-boosted | −0.27 | −9.41 to 13.0 | 0.83 | |
| IN/inactivated-boosted | −2.4 | −11.3 to 7.0 | |||
| BRSV neutralization titers (NT) (geometic means) | Change in BRSV NT from baseline (Day 0) to Day 7 | IN/MLV-boosted | 1 | −1 to 6 | < 0.0001 |
| IN/inactivated-boosted | 5 | 3 to 7 |
Statistical significance adjusted to P ≤ 0.01 due to multiple comparisons.
Figure 4.
Kinetics of BRSV-specific IgG response in neonatally-primed calves that received either modified-live (gray boxes) or inactivated (white boxes) combination BRSV vaccines as booster injections at 2 mo of age and were challenged at 6 mo of age. Box and whiskers plot interpretation: Top of box is the upper quartile, the line in the middle is the median, the bottom of the box is the lower quartile and the upper and lower whiskers represent scores outside the middle 50%. X represents the mean and the line connecting the dates represents the mean line.
Bovine respiratory syncytial virus-specific (IgA) nasal antibodies (as determined by ELISA) were low (≤ 14 units) and were not significantly different between the 2 boosted groups among time points (Tables 1, 2).
Bovine respiratory syncytial virus neutralizing antibody titres were similar between the 2 boosted groups on challenge Day 0. There was, however, a significant difference between the change in BRSV-neutralizing antibody titers between Days 0 and 7 (Table 2).
Discussion
To our knowledge this is the first study to examine the comparative efficacy of commercially available vaccines used in a heterologous prime-boost protocol of immunization under field-like conditions, including a challenge of immunity, in domestic animals. Two previous studies (13,14) examined antibody responses in neonatally primed commercially reared beef calves that were boosted homologously with the same MLV form of BRSV; but, importantly, there was no comparison with an inactivated booster vaccine, and no challenge of immunity was performed in those studies, limiting conclusions that could be drawn. Although none of the BRSV-challenged cattle herein were naïve to iatrogenic exposure to vaccinal BRSV, and clinical disease in the group was mild overall, there were significant differences in a key clinical correlate of pulmonary compromise, arterial PaO2 (10) and relevant immune responses measured between the 2 groups of boosted calves. In a direct comparison, the responses engendered by the inactivated boost of neonatal priming were apparently superior.
Consistent with the “3-Rs” (replacement, reduction, refinement in experimental animal use), we included only 2 control calves in this study to validate that the challenge caused disease in non-immune/control animals. This precluded inclusion of these 2 calves in any statistical analyses. As well, we used arterial PaO2 as a surrogate for postmortem evaluation (10) to assess pulmonary damage, sparing the lives of most calves in the study. Arguably, including 2 control calves was not necessary, as this challenge method, including use of the same dose of the same lot of BRSV inoculum as used in the current study, has consistently produced significant respiratory disease in non-immune cattle [2; J. Ellis unpublished data on file with United States of Agriculture Center for Veterinary Biologics (USDA-CVB), 2001–2017]. In this study, the 2 control calves had received a single dose of the combination MLV vaccine. Given that the goal was to conduct these studies under conditions that mimicked commercial beef operations in which these and other similar vaccines are used, from both logistical and biological standpoints, it would have been difficult to identify and include truly naïve animals without adding confounding variables. Moreover, since these calves were in essentially a commercial operation (a large provincially owned beef herd), they were treated as had been traditionally done; they received a single dose of a combination MLV vaccine at 2 mo of age in the face of decayed but not absent passive immunity (maternal antibodies to BRSV), but without having been neonatally primed. They, therefore, represented a more relevant control than no vaccination at all.
Generally, in studies using this infection model we have observed anamnestic BRSV-specific IgG responses in sera after challenge in calves that had been primed mucosally or parenterally (2, and J. Ellis unpublished data on file with the USDA-CVB, 2001–2017). The calves in the IN/inactivated-boosted group, in the face of maternal antibodies (IFOMA), had this response; whereas the calves in the IN/MLV-boosted group did not. Importantly, as in a previous study with this inactivated vaccine (8), the IgG response determined by ELISA was also reflected in an increase in VN antibodies, indicating that the inactivated vaccine formulation preserved epitopes that stimulate functionally important antibody responses. This has been a previous and ongoing concern with inactivated vaccines for pneumoviruses (2). Effective boosting of mucosally primed neonatal responses by this inactivated vaccine could be attributable to the increased antigen mass (generally 2 to 3 log10 higher in inactivated versus MLV vaccines; 2) and/or the presence of the saponin adjuvant which can induce both Th1 and Th2 responses as well as CD8+ cytotoxic lymphocyte responses (15). As in this study, we previously obtained inconsistent results when measuring IgA in nasal secretions of BRSV-vaccinated and/or exposed cattle (2). In this case it may be attributable to the relative brevity of mucosal memory for IgA production in the absence of repeated mucosal exposure to the virus. Nevertheless, it is well-recognized that IgG (1), as measured anamnestically in IN/inactivated-boosted calves after BRSV infection, is the primary antibody in the lower respiratory tract of cattle (16) where BRSV replicates most extensively and has its most important clinical effects.
Inhibition or blocking of primary antibody responses by maternal antibodies is a well-documented phenomenon of neonatal immunology and is a major consideration for vaccine choice and administration to young animals (3,17). Compared with other species, there is a relatively large amount of literature on vaccination of cattle IFOMA (reviewed in 18). It is recognized that the blocking effect of MatAb is not “all or none” and varies with the concentration of MatAb at the time of vaccination, the age of the calf, the type of vaccine, and the route of vaccine administration (18). Indeed, we previously demonstrated that induction of clinical immunity to BRSV by a representative combination MLV parenteral vaccine is effectively blocked by a concentration of MatAb consistent with good passive transfer (4), but, importantly, that this blocking effect can be overridden by mucosal administration of MLV vaccine (5). As indicated, all the calves in this study had high concentrations of BRSV-specific MatAb post-suckling of their routinely vaccinated damns. Although by virtue of pre hoc randomization there was an apparent numerical difference between the 2 groups of neonatally IN-vaccinated calves in the high concentrations of MatAb, this difference was not considered biologically significant to neonatal mucosal priming (5) and, importantly, there was no significant difference in the change from baseline concentrations of BRSV-specific antibodies between the 2 groups at the time of boosting, approximately 2 mo later. Therefore, the results of this study suggest that boosting of already primed (secondary) responses by parenteral MLV vaccines used at a time when commonly administered in the field, at approximately 2 mo of age, can also be at least partially blocked by residual maternal antibodies in calves with good passive transfer, probably simply by virtue of neutralization of vaccinal BRSV by the maternal antibodies. Less well-documented and more controversial is the effect of MatAb on the induction of T-cell immunity by vaccination of young cattle. A few studies have reported various T-cell responses to BRSV following vaccination IFOMA (2,18); however, there are scant data associating these mostly in vitro responses with clinical immunity in virus-challenged animals (7). In the absence of detectable antibody at the time of challenge and anamnestically after infection with BRSV, it is likely that the disease-sparing we observed in the MLV-boosted cattle in this study was attributable primarily to T-cell mediated immunity. The detection of BRSV-stimulated INF-gamma from peripheral blood leukocytes and previous work examining responses to parenterally administered MLV vaccines (7) support this hypothesis. It would be instructive in future work to comparatively examine both antibody and cell-mediated immunity in the lung (7) during BRSV infection to better understand the local and most relevant mechanisms of immunity differentially stimulated by different boosting protocols.
Bovine respiratory syncytial virus is not the only pathogen of concern in calves and older cattle; combination vaccines usually contain at least BPIV-3, BHV-1, and BVDVs in addition to BRSV, begging the question whether the responses to those viruses would be similar to those we documented to the latter. Given the biological relatedness of the respiratory paramyxoviruses (1,19), we would predict that the responses to BPIV-3 would be similar to those to BRSV. However, BHV-1 and BVDVs, whether vaccinal MLV or field viruses, have very different lifestyles in the host and perhaps different requirements for immunity, as well as safety. We are currently examining antibody responses to BHV-1 in a larger cohort of calves that was vaccinated as were those tested herein. Although, currently, there are no commercial intranasal vaccines for mucosal priming of young calves for BVDV [i.e., capable of overriding the blocking of those responses by MatAb (20)], there is precedent for their efficacy (21), and they will most likely be available in the future. Even if the responses to BHV-1 and BVDVs are only equivalent in mucosally primed and differentially boosted cattle, that equivalence would have significant impact on safe use of combination vaccines around and during pregnancy. Avoiding the use of vaccinal MLV fetotropic agents (BHV-1 and BVDV) by efficient boosting of neonatally primed responses with inactivated vaccines would avert concerns regarding the possibility of fetal damage and abortion (22), the possibility of recombination between vaccinal and field viruses (23) and, as well, allow for more efficient boosting of passive immunity by immunizing dams closer to term (rather than prior to breeding), justifying their use from those perspectives alone.
In conclusion, although recently there has been increased use of neonatal intranasal vaccination to prime the immune system of calves at an early age IFOMA, there is scant comparative information regarding the boosting of these responses which have a relatively short duration of immunity [2 to 3 mo in the case of BRSV (5)]. These results support the heterologous prime-boost approach to vaccination (6). When representative, commonly used parenteral combination viral MLV and inactivated vaccines were administered as boosters at the traditional time of handling of calves for a first vaccination, around 2 months of age in a commercial beef operation, overall, the inactivated immunogen engendered better clinical immunity, including relevant anamnestic antibody responses, subsequent to virulent challenge with BRSV. These data provide a basis for further rational, evidence-based vaccine protocol development in cattle.
Acknowledgments/Disclosures
This study was supported by Saskatchewan Agricultural Development Fund grant #20160055 (NE), University of Saskatchewan discretionary funds to study bovine medicine (NE) and viral immunology (JE), and the Saskatoon Colostrum Company, Saskatoon, Saskatchewan. The first author (JE) has conducted and conducts similar studies for Pfizer Animal Health, Zoetis, Merial, Merck Animal Health, Fort Dodge Animal Health, Boehringer Ingelheim Vetmedica, and Elanco. CVJ
Appendix
| Clinical scoring |
|---|
| Rectal temperatures |
| 0 = < 103°F |
| 1 = ≥ 103°F |
| Depression |
| 0 = normal |
| 1 = mild; moves slowly, head down |
| 2 = moderate; tends to lie down, staggers |
| 3 = severe; recumbent or stands with difficulty |
| Estimated respiratory rate |
| 0 = ≤ 44 breaths per minute (BPM) |
| 1 = 45 to 64 (BPM) |
| 2 = 65 to 80 (BPM) |
| 3 = ≥ 81 (BPM) |
| Dyspnea |
| 0 = normal |
| 1 = mild; short and rapid |
| 2 = moderate; labored, abdominal |
| 3 = severe; very labored, grunting |
| Cough (cough scores were assigned to calves with spontaneous coughing during the clinical examination observation period; approximately 1 h per day) |
| 0 = < 3 episodes |
| 1 = 3+ episodes |
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Ellis JA. Bovine respiratory syncytial virus. In: Munir M, editor. Mononegaviruses of Veterinary Importance. Vol. 1. Wallingford, UK: CAB International; 2013. pp. 170–184. [Google Scholar]
- 2.Ellis J. How efficacious are vaccines against bovine respiratory syncytial virus in cattle? Vet Microbiol. 2017;206:59–68. doi: 10.1016/j.vetmic.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Tizard I. Veterinary Immunology: An Introduction. 9th ed. Philadelphia, Pennsylvania: WB Saunders; 2013. Regulation of adaptive immunity; p. 215. [Google Scholar]
- 4.Ellis J, Gow S, Bolton M, Burdett W, Nordstrom S. Inhibition of priming for bovine respiratory syncytial virus–Specific protective immune responses following parenteral vaccination of passively immune calves. Can Vet J. 2014;55:1180–1185. [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis JA, Gow SP, Mahan S, Leyh R. Duration of immunity to experimental infection with bovine respiratory syncycial virus following intranasal vaccination of young passively immune calves. J Am Vet Med Assoc. 2013;243:1602–1608. doi: 10.2460/javma.243.11.1602. [DOI] [PubMed] [Google Scholar]
- 6.Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West KH, Petrie L, Konoby C, Haines DM, Cortese V, Ellis JA. The efficacy of modified-live respiratory syncytial virus vaccines in experimentally infected calves. Vaccine. 2000;18:907–919. doi: 10.1016/s0264-410x(99)00324-2. [DOI] [PubMed] [Google Scholar]
- 8.Ellis J, West KH, Waldner C, Rhodes C. Efficacy of a saponin-adjuvanted inactivated respiratory syncytial virus vaccine in calves. Can Vet J. 2005;46:155–162. [PMC free article] [PubMed] [Google Scholar]
- 9.West KH, Petrie L, Haines DM, et al. The effect of formalin-inactivated vaccine on respiratory disease associated with bovine respiratory syncytial virus infection in calves. Vaccine. 1999;17:809–820. doi: 10.1016/s0264-410x(98)00265-5. [DOI] [PubMed] [Google Scholar]
- 10.Ellis J, Waldner C, Gow S, Jackson M. Relationship of the extent of pulmonary lesions to the partial pressure of oxygen and lactate concentration in arterial blood in calves experimentally infected with bovine respiratory syncytial virus. Can J Vet Res. 2013;77:205–210. [PMC free article] [PubMed] [Google Scholar]
- 11.West K, Ellis JA. Functional analysis of antibody responses of feedlot cattle to bovine respiratory syncytial virus following vaccination with mixed vaccines. Can J Vet Res. 1997;61:28–33. [PMC free article] [PubMed] [Google Scholar]
- 12.Dohoo I, Martin W, Henrik S. Veterinary Epidemiologic Research. Charlottetown, Prince Edward Island: VER; 2009. [Google Scholar]
- 13.Stoltenow D, Cortese VS, Seeger JT, Stokka GS, Weigel D. Immunologic responses of beef calves to concurrent application of modified-live viral vaccine and systemic administered Manheimia haemolytica bacterin-leukotoxoid. Bov Pract. 2011;45:132–138. [Google Scholar]
- 14.Stokka GS, Neville B, Seeger JT, Cortese VS, Gaspers JJ. Serological effect of two concurrent IBRV, BVDV, BRSV, PI3V, and Manheimia haemolytica vaccination protocols and time interval between the first and second dose on the subsequent serological response to the BRSV and M. haemolytica fractions in suckling beef calves. Bov Pract. 2016;50:21–27. [Google Scholar]
- 15.Spickler AR, Roth JA. Adjuvants in veterinary vaccines: Modes of action and adverse effects. J Vet Intern Med. 2003;17:273–281. doi: 10.1111/j.1939-1676.2003.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 16.Morgan KL, Bourne FJ, Newby TJ, Bradley PA. Humoral factors in the secretory immune system of ruminants. Adv Exp Med Biol. 1981;137:391–412. [PubMed] [Google Scholar]
- 17.Tizard I. Veterinary Immunology: An Introduction. 9th ed. Philadelphia, Pennsylvania: WB Saunders; 2013. Immunity in the fetus and newborn; pp. 225–239. [Google Scholar]
- 18.Chamorro MF, Woolums A, Walz P. Vaccination of calves against common respiratory viruses in the face of maternally derived antibodies (IFOMA) Anim Health Res Rev. 2016;17:79–84. doi: 10.1017/S1466252316000013. [DOI] [PubMed] [Google Scholar]
- 19.Ellis JA. Bovine parainfluenza virus-3. Vet Clin Food Anim Pract. 2010;26:575–593. doi: 10.1016/j.cvfa.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Ellis J, West K, Cortese V, Konoby C, Weigel D. Effect of maternal antibodies on induction and persistence of vaccine-induced immune responses against bovine viral diarrhea virus type II in young calves. J Am Vet Med Assoc. 2001;21:351–356. doi: 10.2460/javma.2001.219.351. [DOI] [PubMed] [Google Scholar]
- 21.Xue W, Ellis J, Mattick D, Smith L, Brady R, Trigo E. Immunogenicity of a modified-live virus vaccine against bovine viral diarrhea virus types 1 and 2, infectious rhinotracheitis virus, bovine parainfluenza-3 virus, and bovine respiratory syncytial virus when administered intranasally to young calves. Vaccine. 2010;28:3784–3792. doi: 10.1016/j.vaccine.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 22.O’Toole D, Chase CCL, Miller MM, Van Campen H. Kennedy, the early sixties, and visitation by the angel of death. Vet Pathol. 2014;16:1051–1062. doi: 10.1177/0300985814548515. [DOI] [PubMed] [Google Scholar]
- 23.Thiry E, Muylkens B, Meurens F, Gogev S, Vanderplasschen A, Schynts F. Recombination in the alphaherpesvirus bovine herpesvirus 1. Vet Microbiol. 2006;113:171–177. doi: 10.1016/j.vetmic.2005.11.012. [DOI] [PubMed] [Google Scholar]