Abstract
This retrospective cohort study reports the observation of magnetic resonance imaging (MRI) epaxial muscle hyperintensity in dogs diagnosed with presumptive fibrocartilaginous embolic myelopathy (FCEM) (n = 61). It further reports the observation of vertebral column hyperesthesia lasting > 12 hours. The hypothesis tested was that the finding of MRI epaxial muscle hyperintensity correlated with dogs presenting with hyperesthesia. Client-owned dogs diagnosed with presumptive FCEM by specific MRI criteria were included. Statistical analysis was performed using Fisher’s exact test. Twenty-three percent (14/61) of MRIs displayed abnormal muscle hyperintensity and 43% (26/61) exhibited vertebral column hyperesthesia. No relationship was found between muscle hyperintensity and pain persisting beyond 12 hours. The muscle hyperintensity remains of unknown significance. That 43% of presumptive FCEM cases have prolonged signs of pain is a higher prevalence than previously reported, and may affect clinical differential diagnoses. This is especially significant in cases in which MRI is not possible and a presumptive diagnosis must be based on the clinical signs.
Résumé
Imagerie par résonance magnétique des lésions des muscles dans la myélopathie embolique fibrocartilagineuse canine présumée. Cette étude rétrospective de cohorte signale les observations de l’imagerie par résonance magnétique (IRM) pour l’hyperintensité du muscle épaxial chez les chiens diagnostiqués avec une myélopathie embolique fibrocartilagineuse (MEFC) présumée (n = 61). Elle signale aussi l’observation de l’hyperesthésie de la colonne vertébrale durant > 12 heures. L’hypothèse qui a été testée était qu’il y avait une corrélation entre l’observation de l’hyperintensité du muscle épaxial par IRM et les chiens présentés avec de l’hyperesthésie. Les chiens appartenant à des clients pour lesquels un diagnostic présomptif de MEFC avait été posé à l’aide du critère spécifique de l’IRM ont été inclus. L’analyse statistique a été réalisée en utilisant le test exact de Fisher. Vingt-trois pour cent (14/61) des IRM affichaient une hyperintensité anormale du muscle et 43 % (26/61) présentaient de l’hypersthésie de la colonne vertébrale. Aucun lien n’a été trouvé entre l’hyperintensité musculaire et la douleur persistant au-delà de 12 heures. La signification de l’hyperintensité musculaire est toujours inconnue. Le taux de 43 % de cas présomptifs de MEFC affichant des signes de douleur prolongée représente une prévalence supérieure aux données déjà signalées et pourrait affecter les diagnostics cliniques différentiels. Ce fait revêt une importance particulière lorsque l’IRM n’est pas possible et qu’un diagnostic présomptif doit se baser sur les signes cliniques.
(Traduit par Isabelle Vallières)
Introduction
Fibrocartilaginous embolic myelopathy (FCEM) is characterized by peracute onset of neurological symptoms of variable severity and symmetry, which stabilize after 24 h and either remain static or begin to improve (1–3). The affected areas correspond to necrotic damage of the spinal cord due to ischemia. Histopathological analysis of the affected areas of the spinal cord indicates cartilaginous material, histologically identical to nucleus pulposus, in the vasculature surrounding the ischemic area (1,4–6). Large or giant breed and non-chondrodystrophic breed dogs are most commonly affected, although there have been at least 2 cases reported in chondrodystrophic breed dogs (7) and perhaps a greater prevalence than once thought in small- and medium-sized dogs (8). The true incidence of FCEM may be misrepresented in the literature due to the likelihood of many cases going undiagnosed, not being referred in a timely manner, or as a result of the time-dependent nature of detection and diagnosis (1,6,9).
Up to 24% of FCEM cases are reported to have vertebral column hyperesthesia on examination, thus FCEM is typically considered a nonpainful condition after the vocalization reported at onset of signs (3,10–12). However, vertebral column hyperesthesia had been anecdotally observed more than 12 h after onset of clinical signs in a number of canine patients presenting to a referral hospital and diagnosed with presumptive FCEM. As previous reports did not specify the time from onset of signs to examination, the purpose of this study was to determine the frequency of canine FCEM patients in the referral hospital study population that exhibited signs of prolonged vertebral column hyperesthesia, defined as pain lasting longer than 12 h after onset of neurological signs (1,6,7,11,13,14). Anecdotally, abnormal epaxial muscle hyperintensity had also been observed in certain canine FCEM patients on MRI. This abnormal signal intensity had been noted commonly in the region of spinal cord ischemic injury. The origin, prevalence, and consequence of this abnormal muscle hyperintensity are unknown. A secondary objective of this study was to determine the frequency at which epaxial muscle hyperintensity occurs in canine FCEM patients and to determine if there is a possible association between this finding and the presence of prolonged vertebral column hyperesthesia. The study hypothesis was that the finding of MRI epaxial muscle hyperintensity correlated with dogs presenting with prolonged signs of pain.
Materials and methods
Medical records at the Ontario Veterinary College Health Sciences Centre were searched for client-owned dogs diagnosed with presumptive FCEM by magnetic resonance imaging (MRI) during the period from November 2004 to June 2013. To be included, dogs must have presented with a peracute onset of a spinal cord myelopathy of variable severity and symmetry that stabilized after 24 h and that either remained static or began to improve. Other inclusion criteria were: complete history, complete documentation of physical and neurological examination findings, and magnetic resonance imaging consistent with a diagnosis of FCEM. Any additional diagnostics performed on a case-by-case basis were also required to be consistent with a diagnosis of FCEM (e.g., negative infectious disease titers). Patients were excluded if there was evidence of concurrent vertebral column disease (intervertebral disk material within the vertebral canal, narrowed/collapsed intervertebral disks, bone fracture, luxation, skin or subcutaneous tissue injury, hemorrhage) or if there was insufficient evidence to support a diagnosis of FCEM.
Dogs were considered to be showing prolonged signs of pain if records indicated that vertebral column hyperesthesia was elicited upon physical examination, at or around the site of ischemic injury, > 12 h after the onset of clinical signs. The neurological or physical examinations in the medical record were examined for descriptions such as “discomfort elicited with palpation over the thoracolumbar region” or reports of changes in behavior, muscle tone, posture, vocalizations, resulting from manipulation versus “no pain on spinal palpation” or similar statements. The location of the pain was categorized as being elicited at the site of presumptive FCEM or at an alternate distant location. Two dogs were evaluated and imaged before 12 h after the onset of clinical signs. Both dogs were evaluated as non-painful; therefore, they were included in the non-painful group. However, had the dogs shown signs of hyperesthesia, they would have been excluded from the study as they would not have been able to be appropriately categorized.
Retrospectively, MR images were reviewed to confirm the diagnosis and location of presumptive FCEM in patients included in the study pool and also to determine which patients exhibited abnormal epaxial muscle hyperintensity. All magnetic resonance imaging had been performed under general anesthesia using a 1.5-T magnetic field strength on a Signa 1.5 Excite II MRI Scanner (General Electric Medical Systems, Florence, South Carolina, USA). An 8-channel spine coil by USA Instruments (Aurora, Ohio, USA) was used for all sequences. Sagittal, dorsal, and transverse imaging planes were scanned and 2D acquisitions were used. The complete MRI study was reviewed independently by 2 authors (FJ and SN), a Board-certified veterinary neurologist, and a Board-certified veterinary radiologist, blinded to patient history. In the case of discrepancies, a consensus opinion was reached. Variables included MRI sequences, the timing between onset of clinical signs and MRI, and the presence, site, and signal characteristics of spinal cord and/or epaxial muscle lesions. Magnetic resonance imaging characteristics used to establish a diagnosis of presumptive FCEM included a focal to regional, well-demarcated, T2-hyperintense intramedullary lesion primarily involving the gray matter, with no other visible abnormalities (15). Characteristics of MRI considered suggestive of an acute noncompressive nucleus pulposus extrusion (ANNPE), the main differential diagnosis for FCEM, resulted in exclusion from the study. These included: a focal T2-hyperintense intramedullary lesion directly above a narrowed intervertebral disk, nucleus pulposus with decreased signal intensity, and minimal to no spinal cord compression (15,16).
Patients were categorized as having a fat suppressed MRI sequence if a T1-weighted fat saturated sequence with contrast (Omniscan; GE Healthcare, Mississauga, Ontario), T2-weighted sequence with fat suppression, and/or a short tau inversion recovery (STIR) sequence was available. Abnormal muscle hyperintensity was defined as a region of muscle showing different signal characteristics from adjacent muscle; these differences included enhancement on the post-contrast T1-weighted sequence, hyperintensity on fat-suppressed sequences, and hyperintensity on T2-weighted sequences.
The relationship between the anatomical region of presumptive FCEM with respect to the region of muscle hyperintensity was also considered. The muscle lesion and FCEM were considered to be in the same location if they were within 1 vertebral body of each other and to be in different locations if they were separated by more than 1 vertebral body. Dogs diagnosed with presumptive FCEM but not exhibiting vertebral column hyperesthesia were considered the control group, while those exhibiting vertebral column hyperesthesia were the comparison group. Fisher’s exact tests (SAS OnlineDoc 9.2; SAS Institute, Cary, North Carolina, USA) were performed with a significance level of P ≤ 0.05.
Results
Sixty-one client-owned dogs were included in the study population: ages ranged from 7 mo to 11 y and 9 mo (median: 6 y) and weights ranged from 1.5 kg to 80 kg (median: 27.7 kg). There was no significant difference in age or weight between the 2 groups. For the group of dogs presenting with prolonged signs of vertebral column hyperesthesia, the age range was 2 y to 11 y and 9 mo (median: 6 y), and weights ranging from 3.1 kg to 57.5 kg (median: 26.6 kg). The control group (dogs without hyperesthesia) had ages ranging from 7 mo to 10 y and 6 mo (median: 5 y), and weights ranging from 1.5 kg to 80 kg (median: 30.75 kg). The gender distribution in the study pool was as follows: 3 intact females, 29 spayed females, 5 intact males, and 24 neutered males, for a total of 32 females and 29 males, with no apparent gender bias observed in the dogs presenting for presumptive FCEM. The gender distribution was similarly split between those presenting with hyperesthesia and the control group. The time elapsed between the onset of neurological signs and MRI ranged from 6 to 384 h. The breeds included in the study are shown in Table 1.
Table 1.
Breed distribution in the study population (N = 61).
| Breed | Number in study | Vertebral column hyperesthesia | Muscle abnormality |
|---|---|---|---|
| Labrador retriever | 19 | 6 | 3 |
| Miniature schnauzer (or mix) | 4 | 3 | 2 |
| Boxer | 4 | 2 | 2 |
| German shepherd (or mix) | 4 | 2 | 1 |
| Shetland sheepdog | 3 | 1 | 0 |
| Chihuahua | 3 | 1 | 0 |
| Poodle (or mix) | 3 | 2 | 1 |
| Border collie (or mix) | 3 | 1 | 1 |
| Beagle (or mix) | 2 | 2 | 0 |
| Alaskan malamute (or mix) | 2 | 1 | 1 |
| Boston terrier | 2 | 0 | 2 |
| Golden retriever | 2 | 1 | 0 |
| Other (American pit bull, Bernese mountain dog*+, Brussels griffon*, Chow-x, dalmatian, doberman, English bulldog, mixed breed*, Pomeranian, pug*, Rottweiler, Siberian husky*) | 12 (1 of each) | 4 | 1 |
| * in list | + in list |
Prolonged vertebral column hyperesthesia was reported in 26 dogs (43%) and epaxial muscle hyperintensity was observed in 14 dogs (23%) (Figure 1) among the 61 FCEM patients in the study population. The muscles affected included the longissimus, iliocostalis thoracis, and transversospinalis muscles. There were no instances of observed muscle hyperintensity in the 15 cases without a fat suppressed sequence available, whereas muscle hyperintensity was observed in 14 cases out of 46 cases with a fat suppressed sequence available. Fat suppressed sequences highlight muscle abnormality (P = 0.0137). In 10/14 cases the muscle lesions were noted on only T1 fat-suppressed post-contrast images, 0/14 on only T2 fat-suppressed images, and 4/14 on both T1 post-contrast and T2 fat-suppressed images. The lesions varied in shape and were noted to be linear and wedge in 11 and 3 patients, respectively (Figure 2).
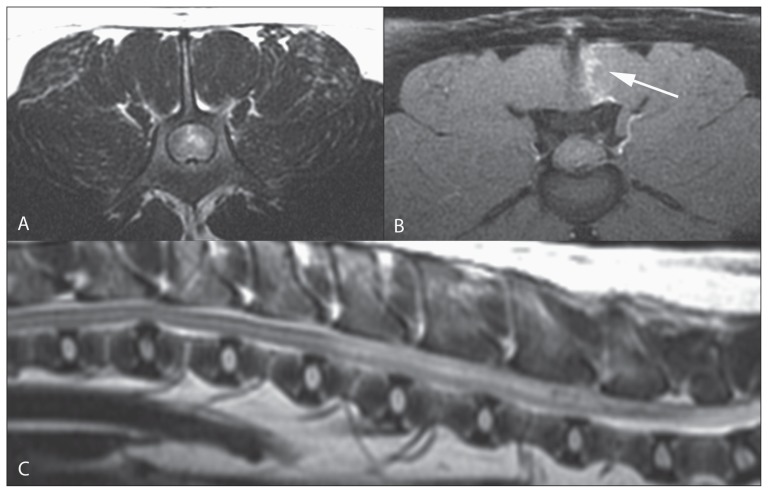
Figure 1.
Transverse T2 (A), T1 post-contrast (B) images at the level of L4 (patient’s right is on the left of the image) and sagittal T2 image (C) of the lumbar vertebral column. In the left spinal cord there is a focal high T2 signal intensity. In the left multifidus muscles there is a linear lesion (arrow) that was only evident on the T1 post-contrast fat-suppressed image.
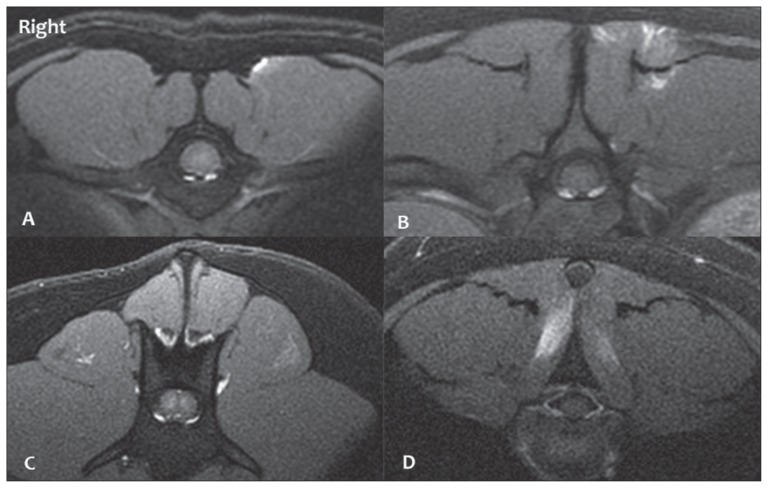
Figure 2.
Transverse T1 Fat Sat post-contrast images of 4 cases with contrast enhancing lesion in the epaxial muscles with varied distribution (patient’s right is on the left of the image): peripheral lesion on the left (A), patchy lesion on the left (B), central poorly enhancing lesion on the left (C), bilateral lesions (D).
In 71% (10/14) of the cases with epaxial muscle hyperintensity, the muscle abnormality was observed in the immediate region of FCEM. In some cases, the muscle hyperintensity and/or site of FCEM were observed to be primarily unilateral. Unilateral muscle hyperintensity was found in 10/14 patients exhibiting muscle hyperintensity and was ipsilateral to unilateral FCEM in 1/10 patients, while the remaining unilateral incidences of muscle hyperintensity were either contralateral to the site of FCEM (1/10) or alongside bilateral FCEM (8/10). Since only 1/10 patients displayed unilateral muscle hyperintensity along with ipsilateral unilateral FCEM, no further statistical analysis was performed.
The number of patients with hyperesthesia and/or muscle hyperintensity is given in Table 2. There was no significant association between epaxial muscle hyperintensity and prolonged signs of vertebral column hyperesthesia. Out of the 47 dogs without muscle hyperintensity, 18 (38%) had prolonged signs of vertebral column hyperesthesia and 29 (62%) did not. Out of the 14 patients with muscle hyperintensity, 8 (57%) had prolonged signs of vertebral column hyperesthesia and 6 (43%) did not, resulting in no determined association between the two (P = 0.5447).
Table 2.
Distribution of epaxial muscle hyperintensity and/or prolonged vertebral column hyperesthesia within the study population (N = 61).
| Muscle abnormality | No muscle abnormality | Total | |
|---|---|---|---|
| Hyperesthesia | 8 | 18 | 26 |
| No hyperesthesia | 6 | 29 | 35 |
| Total | 14 | 47 | 61 |
Discussion
Canine FCEM has not historically been associated with lasting vertebral column hyperesthesia (1,3,6,7,11–15). However, in almost half (43%) of the patients in this study, prolonged signs of vertebral column hyperesthesia were elicited in the region of ischemic injury. Therefore, vertebral column hyperesthesia appears to be much more prevalent in our study population than previously reported in the literature. Fibrocartilaginous embolic myelopathy has been documented in several other species as well, in which prolonged signs of vertebral column hyperesthesia are not reported as a common presentation, although in some species it may be more difficult to determine (17–23). In human medicine, there is little mention of hyperesthesia associated with FCEM and if present, it is typically only a sharp, transient pain associated with the onset of clinical signs. It is therefore unique that vertebral column hyperesthesia appears to frequently be recorded in these canine patients. It is speculated that hyperesthesia in these patients could be due to meningeal stretch at the time of the vascular incident, a result of vasospasm, or due to pain receptors in the annulus fibrosus of the implicated intervertebral disk (24). Multiple clinicians were involved in the assessment of hyperesthesia for the dogs retrospectively included in this study and, while the assessment is generally subjective, any instances in which hyperesthesia was not recorded as certain or was unable to be differentiated from anxiety were categorized as non-painful.
Several cases originally diagnosed as FCEM in the medical record were not found to meet the inclusion criteria for this study because they were diagnosed upon review to be an acute noncompressive nucleus pulposus extrusion (ANNPE), also known as high-velocity-low-volume disk extrusion or traumatic disk extrusion (16,25). Dogs with ANNPE are often presented with signs of asymmetric myelopathy that is non-progressive after the first 24 h, similar to FCEM. Patients with ANNPE are more likely to have a history of vocalization at the time of onset and exhibit vertebral column hyperesthesia at the time of examination (10). The 2 conditions share MRI characteristics, with the key differences being the presence of extraneous material or signal changes within the epidural space, characteristics of the hyperintensity (direction, length), as well as possible meningeal contrast enhancement in cases of ANNPE. Interobserver agreement based on MRI assessment of patients with suspected ANNPE or FCEM has been reported to be 100% based on these characteristics (26). Patients with these ANNPE MRI characteristics did not meet the inclusion criteria for the present study; however, if imaging occurred after dissipation of the extruded nucleus pulposus, the 2 conditions would only be distinguishable on histopathology. If this were the case, then some patients diagnosed with FCEM may simply be the ANNPE cases in which disk material is not visible due either to the small amount herniated, the disk material dissipating within the canal prior to imaging, or inappropriate MRI slice thickness being used and the extruded disk material being missed. A subsequent study would be required to properly assess these variables since our study criteria excluded patients diagnosed with ANNPE.
Abnormal muscle hyperintensity was displayed on MRI in over one-fifth (23%) of canines diagnosed with presumptive FCEM. High signal intensity in the epaxial muscles of patients was primarily seen in fat-suppressed MRI sequences. Fat-suppressed sequences can be generated by 2 techniques (inversion recovery, chemical fat suppression) but both result in nulling of the high signal intensity of fat, increasing the sensitivity to detect lesions in areas that contain both soft tissue and fat (e.g., muscle) (27). In all cases, muscle hyperintensity was seen on fat-suppressed sequences and, in patients in which these sequences were not available, muscle abnormality was either not present or not able to be observed. In 14 of the 61 cases in this study, fat-suppressed sequences were not obtained; therefore, it cannot be determined definitively whether these cases had unobserved muscle abnormalities. This further supports the proposal that fat-suppressed sequences should be included when imaging suspected FCEM patients in order to determine if muscle abnormality is present (28). The origin of the observed muscle hyperintensity has not been determined and other possibilities must be considered such as trauma, intra-muscular injections, and infarction of the muscle along with the spinal cord during FCEM. For the patients included in this study, there was no known trauma. All intra-muscular injections prior to MRI were recorded, with 25/61 dogs having received intra-muscular injections and 7 of the 14 patients with observed muscle hyperintensity not having received an intra-muscular injection. The exact site of injection was unknown; however, the focal distribution of the observed muscle hyperintensity was frequently found within 1 muscle belly or 1 head of the multifidus muscle near the site of presumptive FCEM, further suggesting that intra-muscular injections were unlikely to be responsible. Abnormal epaxial muscle hyperintensity has also been associated with other myelopathies, e.g., approximately 40% of dogs with meningoencephalomyelitis of unknown etiology (MUE); however, the clinical presentation in these cases was suspicious for inflammatory spinal cord disease (28).
In summary, abnormal muscle hyperintensity was found in 23% of canine patients diagnosed with presumptive FCEM by MRI in the study population. This muscle hyperintensity is either unilateral or bilateral and often found in the epaxial muscles immediately surrounding the region of presumptive FCEM. In this study, muscle hyperintensity was exclusively observed in the fat-suppressed MRI sequences. Several (6/14) cases without fat-suppressed MRI sequences were reported to have prolonged signs of hyperesthesia, and because of this, undetected muscle abnormalities are a limitation of the present study that could have statistically affected the results. In subsequent studies, it would be valuable to gather a larger pool of subjects and ensure that fat-suppressed sequences are taken, especially for those patients presenting with prolonged signs of hyperesthesia. In addition to having fat-suppressed sequences included, clinicians should be aware that muscle abnormality may be present. Carefully recording the site of intra-muscular injections prior to MRI and ensuring that they are not in the area of suspected injury would be beneficial in ruling out any relation to the presence of muscle abnormality. Further research is required to determine the origin and consequence of MRI muscle hyperintensity in these patients. A prospective study would be recommended in order to have specific detail in medical records especially pertaining to hyperesthesia and location of intra-muscular injections, and to ensure that fat-suppressed MRI sequences are included.
The purpose of this study was to report the observation that vertebral column hyperesthesia is present in cases of presumptive canine FCEM more often than currently described in literature, to evaluate the occurrence and frequency of epaxial muscle hyperintensity observed on MRI of some presumptive canine FCEM patients, and to determine if the latter is related to the presence of prolonged vertebral column hyperesthesia. The presence of abnormal muscle hyperintensity could not be correlated to those patients presenting with prolonged vertebral column hyperesthesia. Therefore, further research is indicated in order to assess the origin and pathology. Overall, patient records indicate that prolonged vertebral column hyperesthesia is repeatedly being recorded in presumptive canine FCEM patients, which has not been previously reported. This finding is clinically significant as FCEM is characteristically considered to be a non-painful condition, and the presence of pain may direct clinicians towards an alternate disease process, particularly in cases in which MRI is not available. CVJ
Acknowledgments
The authors thank Gabrielle Monteith for assistance with statistical analyses, Brandon Reid for assistance with initial data collection, and Alice Daw for assistance in obtaining MR images and specifications.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
Supported in part by the Dr. Robert W. Woolner Summer Student Research Assistantship, Ontario Veterinary College, University of Guelph.
References
- 1.De Risio L, Platt SR. Fibrocartilaginous embolic myelopathy in small animals. Vet Clin North Am Small Anim Pract. 2010;40:859–869. doi: 10.1016/j.cvsm.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Nakamoto Y, Ozawa T, Katakabe K, et al. Usefulness of an early diagnosis for the favorable prognosis of fibrocartilaginous embolism diagnosed by magnetic resonance imaging in 10 small- to middle-sized dogs. Vet Res Commun. 2008;32:609–617. doi: 10.1007/s11259-008-9061-y. [DOI] [PubMed] [Google Scholar]
- 3.Bartholomew KA, Stover KE, Olby NJ, Moore SA. Clinical characteristics of canine fibrocartilaginous embolic myelopathy (FCE): A systematic review of 393 cases (1973–2013) Vet Rec. 2016;179:650–650. doi: 10.1136/vr.103863. [DOI] [PubMed] [Google Scholar]
- 4.Neer TM. Fibrocartilaginous emboli. Vet Clin North Am Small Anim Pract. 1992;22:1017–1026. doi: 10.1016/s0195-5616(92)50090-0. [DOI] [PubMed] [Google Scholar]
- 5.Zaki FA, Prata RG. Necrotizing myelopathy secondary to embolization of herniated intervertebral disk material in the dog. J Am Vet Med Assoc. 1976;169:222–228. [PubMed] [Google Scholar]
- 6.Junker K, van den Ingh TS, Bossard MM, van Nes JJ. Fibrocartilaginous embolism of the spinal cord (FCE) in juvenile Irish Wolfhounds. Vet Q. 2000;22:54–156. doi: 10.1080/01652176.2000.9695046. [DOI] [PubMed] [Google Scholar]
- 7.Ueno H, Shimizu J, Uzuka Y, et al. Fibrocartilaginous embolism in a chondrodystrophoid breed dog. Austr Vet J. 2005;83:142–144. doi: 10.1111/j.1751-0813.2005.tb11620.x. [DOI] [PubMed] [Google Scholar]
- 8.Grunenfelder FI, Weishaupt D, Green R, Steffen F. Magnetic resonance imaging findings in spinal cord infarction in three small breed dogs. Vet Radiol Ultrasound. 2005;46:91–96. doi: 10.1111/j.1740-8261.2005.00019.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakamoto Y, Ozawa T, Katakabe K, et al. Fibrocartilaginous embolism of the spinal cord diagnosed by characteristic clinical findings and magnetic resonance imaging in 26 dogs. J Vet Med Sci. 2009;71:171–176. doi: 10.1292/jvms.71.171. [DOI] [PubMed] [Google Scholar]
- 10.Fenn J, Drees R, Volk HA, De Decker S. Comparison of clinical signs and outcomes between dogs with presumptive ischemic myelopathy and dogs with acute noncompressive nucleus pulposus extrusion. J Am Vet Med Assoc. 2016;249:767–775. doi: 10.2460/javma.249.7.767. [DOI] [PubMed] [Google Scholar]
- 11.Dyce J, Houlton JEF. Fibrocartilaginous embolism in the dog. J Small Anim Pract. 1993;34:332–336. [Google Scholar]
- 12.Cauzinille L, Kornegay JN. Fibrocartilaginous embolism of the spinal cord in dogs: Review of 36 histologically confirmed cases and retrospective study of 26 suspected cases. J Vet Int Med. 1996;10:241–245. doi: 10.1111/j.1939-1676.1996.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 13.Gandini G, Cizinauskas S, Lang J, Fatzer R, Jaggy A. Fibrocartilaginous embolism in 75 dogs: Clinical findings and factors influencing the recovery rate. J Small Anim Pract. 2003;44:76–80. doi: 10.1111/j.1748-5827.2003.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 14.Axlund TW, Isaacs AM, Holland M, O’Brien DP. Fibrocartilaginous embolic encephalomyelopathy of the brainstem and midcervical spinal cord in a dog. J Vet Intern Med. 2004;18:765–767. doi: 10.1892/0891-6640(2004)18<765:feeotb>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.De Risio L. A Review of fibrocartilaginous embolic myelopathy and different types of peracute non-compressive intervertebral disk extrusions in dogs and cats. Frontiers Vet Sci. 2015;2:24. doi: 10.3389/fvets.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Decker S, Fenn J. Acute herniation of nondegenerate nucleus pulposus: Acute noncompressive nucleus pulposus extrusion and compressive hydrated nucleus pulposus extrusion. Vet Clin North Am Small Anim Pract. 2018;48:95–109. doi: 10.1016/j.cvsm.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Mikszewski JS, Winkle TJV, Troxel MT. Fibrocartilaginous embolic myelopathy in five cats. J Am Anim Hosp Assoc. 2006;42:226–233. doi: 10.5326/0420226. [DOI] [PubMed] [Google Scholar]
- 18.Landolfi JA, Saunders GK, Swecker WS. Fibrocartilaginous embolic myelopathy in a calf. J Vet Diagn Investig. 2004;16:360–362. doi: 10.1177/104063870401600421. [DOI] [PubMed] [Google Scholar]
- 19.Turner PV, Percy DH, Allyson K. Fibrocartilaginous embolic myelopathy in a cat. Can Vet J. 1995;36:712–713. [PMC free article] [PubMed] [Google Scholar]
- 20.Tessaro SV, Doige CE, Rhodes CS. Posterior paralysis due to fibrocartilaginous embolism in two weaner pigs. Can J Comp Med. 1983;47:124–126. [PMC free article] [PubMed] [Google Scholar]
- 21.Stedman NL, Brown TP, Rowland GN. Intravascular cartilaginous emboli in the spinal cord of turkeys. Avian Dis. 1998;42:423–428. [PubMed] [Google Scholar]
- 22.Adaska JM, Lynch S. Fibrocartilaginous embolic myelopathy in a Sumatran tiger (Panthera tigris sumatrae) J Zoo Wildl Med. 2004;35:242–244. doi: 10.1638/02-028. [DOI] [PubMed] [Google Scholar]
- 23.Ricci E, Cavicchio P, Cantile C. Fibrocartilaginous embolic myelopathy in a lion (Panthera leo) J Zoo Wildl Med. 2010;41:334–337. doi: 10.1638/2009-0101R.1. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton T, Glass E, Drobatz K, Agnello KA. Severity of spinal cord dysfunction and pain associated with hydrated nucleus pulposus extrusion in dogs. Vet Comp Ortho Traumatol. 2014;27:313–318. doi: 10.3415/VCOT-13-06-0076. [DOI] [PubMed] [Google Scholar]
- 25.De Risio L, Adams V, Dennis R, McConnell FJ. Association of clinical and magnetic resonance imaging findings with outcome in dogs with presumptive acute noncompressive nucleus pulposus extrusion: 42 cases (2000–2007) J Am Vet Med Assoc. 2009;234:495–504. doi: 10.2460/javma.234.4.495. [DOI] [PubMed] [Google Scholar]
- 26.Specchi S, Johnson P, Beauchamp G, Masseau I, Pey P. Assessment of interobserver agreement and use of selected magnetic resonance imaging variables for differentiation of acute noncompressive nucleus pulposus extrusion and ischemic myelopathy in dogs. J Am Vet Med Assoc. 2016;248:1013–1021. doi: 10.2460/javma.248.9.1013. [DOI] [PubMed] [Google Scholar]
- 27.D’Anjou MA, Carmel EN, Tidwell AS. Value of fat suppression in gadolinium-enhanced magnetic resonance neuroimaging. Vet Radiol Ultrasound. 2011;52( 1 Suppl 1):S85–90. doi: 10.1111/j.1740-8261.2010.01789.x. [DOI] [PubMed] [Google Scholar]
- 28.Eminaga S, Cherubini GB, Villiers E, Targett M, Caine A. STIR muscle hyperintensity in the cervical muscles associated with inflammatory spinal cord disease of unknown origin. J Small Anim Pract. 2013;54:137–142. doi: 10.1111/jsap.12035. [DOI] [PubMed] [Google Scholar]