Abstract
Glucagon-like peptide-1 (GLP-1) and serotonin play critical roles in energy balance regulation. Both systems are exploited clinically as anti-obesity strategies. Surprisingly whether they interact in order to regulate energy balance is poorly understood. Here we investigated mechanisms by which GLP-1 and serotonin interact at the level of the CNS. Serotonin depletion impaired the ability of exendin-4, a clinically utilized GLP-1 analogue, to reduce body weight in rats, suggesting serotonin is a critical mediator of the energy balance impact of GLP-1R activation. Serotonin turnover and expression of 5HT2A and 5HT2C serotonin receptors in the hypothalamus were altered by GLP-1R activation. We demonstrate that 5HT2A, but surprisingly not 5HT2C, receptor is critical for weight-loss, anorexia and fat mass reduction induced by central GLP-1R activation. Importantly, central 5HT2A receptors are also required for peripherally injected liraglutide to reduce feeding and weight. Dorsal raphe (DR) harbors cell bodies of serotonin producing neurons that supply serotonin to the hypothalamic nuclei. We show that GLP-1R stimulation in DR is sufficient to induce hypophagia and increase electrical activity of the DR serotonin neurons. Finally our results disassociate brain metabolic and emotionality pathways impacted by GLP-1R activation. This study identifies serotonin as new critical neural substrate for GLP-1 impact on energy homeostasis, and expands the current map of brain areas impacted by GLP-1R activation.
Keywords: GLP-1, Exendin-4, food intake, obesity, serotonin
Introduction
Glucagon-like peptide-1 (GLP-1), a peptide produced in the brain and in the intestine, is a critical regulator of energy balance; its glucoregulatory and anti-obesity properties are currently successfully utilized in the clinic (1–3). While the ability of GLP-1 and its stable analogues, exendin-4 (EX4) for example, to reduce food intake is well established, the brain mechanisms governing GLP-1 receptor (GLP-1R)-induced anorexia are still poorly understood.
Serotonin has long been explored for its potential as an anti-obesity treatment, with a mixed success history, primarily due to troublesome side effects of drugs broadly affecting the serotonin system (4). Lorcaserin (Belviq ©) is currently the only serotonin receptor activating anti-obesity pharmaceutical which is still approved for clinical use; it acts as a 5HT2C agonist while to a lesser extent also activating 5HT2A receptors. In line with its anorexic and weight-reducing role, central injections of serotonin or its precursor reduce food intake (5, 6). Conversely, brain serotonin depletion leads to hyperphagia and obesity (7, 8), though this effect is not always replicated (9, 10). Serotonin signals through at least 18 receptors; thus far the 5HT2C receptor received most attention for its anorexic and weight loss impact (4, 11, 12).
Surprisingly little is known about the interaction of the central GLP-1 and serotonin systems. However existing data suggest that an interaction is possible: 1. GLP-1 and EX4 have been shown to dose dependently release serotonin from rat hypothalamic synaptosomes (13); 2. GLP-1 receptors have been identified in the dorsal raphe nucleus (DR), a nucleus harboring cell bodies of serotonergic neurons supplying serotonin to many forebrain sites including the hypothalamus (14–16); 3. Molecular mechanism for the induction of serotonin by GLP-1R activation is suggested by a recent study showing that, at the level of the colon, EX4 attenuates hyperalgesia by increasing colonic serotonin production (17). At the level of the colon the GLP-1-serotonin interaction may be reciprocal since serotonin or serotonin 5HT1B receptor agonists also increase GLP-1 secretion from the enteroendocrine cells (18, 19).
Here we employed several methodological approaches to determine whether the two clinically relevant anti-obesity systems interact and to identify the neuroanatomical mechanism of this interaction. Behavioral, neuropharmacological, electrophysiological and neuroanatomical results obtained here support a direct impact of central GLP-1 activation to increase central serotonin neurotransmission, a relationship discovered to be critical for GLP-1-induced weight-loss or weight-loss maintenance and hypophagia.
Research Design and Methods
Animals
Adult male Sprague-Dawley rats, weighing 200-250g (Charles River, Germany) were housed in individual plastic cages under 12/12 hours dark/light cycle, at 20°C and 50% humidity. Adult female and male mGLU-124 Venus yellow fluorescent protein transgenic mice (YFP-PPG mice; University of Cambridge, United Kingdom (20)) were housed in plastic cages. Water and standard chow were available ad libitum. All studies were carried out with ethical permissions from the Animal Welfare Committee of the Göteborg University (permission 195-13), in accordance with legal requirements of the European Community (Decree 86/609/EEC).
Drugs
GLP-1 (7-36), Exendin-4 (EX4; GLP-1R agonist), Exendin 9-39 (Ex9-39; GLP-1R antagonist), para-chlorophenylalanine (PCPA), R-96544 (selective 5HT2A antagonist (21)), SB242084 (5HT2C antagonist (22)), and angiotensin II were purchased from Tocris (Bristol, UK). All substances, except for SB242084, liraglutide, and PCPA, were dissolved in artificial cerebrospinal fluid (aCSF), vehicle for central injections. Liraglutide (Bachem) was dissolved in 0.9% saline. PCPA was dissolved in 0.9% saline by gentle warming and sonication to a concentration of 100 mg/ml (23, 24). SB242084 was dissolved in 16% DMSO. 5HT2C receptor antagonist SB242084 displays 158- and 100-fold selectivity over 5HT2A and 5HT2B receptors respectively and it also displays selectivity over a range of other 5-HT, dopamine and adrenergic receptors. R-96544 is a potent, selective 5HT2A receptor antagonist; R-96544 shows 100-fold higher affinity for the human 5HT2A receptors than 5HT1A, 5HT1B, 5HT1D, 5HT5A, 5HT6, 5HT7 receptors and 5-HT transporter, although R-96544 has relatively high affinity for 5HT2C receptors (fourfold less compared to 5-HT2A) (21).
Brain cannulation
Rats were implanted with a guide cannula (26 gauge cannula; Plastics One, Roanoke, VA) as described previously (25) to allow drug injections into lateral ventricle (LV) or DR. The following injection coordinates were used: ±1.6mm/-0.9mm/-4.0mm for LV and 0.0mm/-7.7mm/-6.8mm for DR (given from midline/bregma/skull). The LV placement was verified with the angiotensin II drinking test (26). The microinjection site for the DR guide cannula was verified post mortem by microinjection of India ink at the same microinjection volume (0.3μl) used throughout the study.
RNA isolation and mRNA expression
Hypothalamic gene expression levels were measured after chronic (daily, for 10 days) LV injections of EX4 (0.2µg) or vehicle (aCSF). A third group of rats was included to determine whether chronic GLP-1R activation interacts with weight loss-induced changes in serotonin receptors. These rats were pair-fed daily to the amount of chow eaten by the EX4-treated rats. The following serotonin receptor genes were examined: Htr1a, Htr2a, Htr2c, Htr3a. These genes were chosen based on their previously shown connection to GLP-1R activation or their well-established role in feeding regulation (see discussion section for details). Brains were rapidly removed 24h after the last EX4 injection and the hypothalamus was dissected. Gene expression was determined using TaqMan RT-PCR and primer/probe sets as described previously (26–28) (for reference numbers see table 1 SI). Gene expression values were calculated based on the ΔΔCt method (29), with vehicle-injected group set as the calibrator. PPIA (peptidylprolyl isomerase A) was used as a reference gene.
Immunohistochemistry
Mice were anaesthetized and perfused transcardially with heparinized saline followed by buffered fixative solution. The GLP-1 fibers and TPH positive neurons were visualized with a confocal microscope (LSM 700; Carl Zeiss AG). Antibodies, manufacturers, and dilutions are listed in Supplementary Table 2.
Whole-cell electrophysiology
Coronal slices of rat brainstem, 200μm thick, containing DR were cut in ice-cold extracellular solution using a vibratome and maintained in incubation chamber at room temperature. Whole-cell recordings where obtained from visually-identified neurons using glass pipettes containing (in mM): 130 K-gluconate, 2 NaCl, 1 MgCl2, 10 Hepes, 0.1 EGTA, 10 Na phosphocreatine, 4 MgATP, 0.5 Na2GTP, biocytin 0.1%, pH 7.3. Continuous recordings of the membrane potential (Vm) were used to monitor the effect of EX4. The input resistance (Rinput) was calculated from the response to hyperpolarizing current pulses injected through the recording pipette. EX4 or Ex9-39 were added to the superfusion buffer. Electrophysiological data were analyzed with pClamp10 (Molecular Devices). Pooled data were presented as mean±SEM; a repeated measures ANOVA was used to determine significance since vehicle baseline and EX4 or Ex9-39 and EX4 effects were measured in the same neuron. DR slices were fixed with PFA 4% after recording. Fluorescence images of labeled sections were acquired with a confocal microscope (TCS SP5, Leica Microsystems) and reconstructed in 3 dimensions to assess TPH expression in biocytin-containing cells.
Serotonin turnover
Brains were dissected 24h after the last EX4 injection (injections were given daily for 8 days). Brains were rapidly removed and the hypothalamus was dissected using a brain matrix. Tissue concentrations of serotonin and its metabolite (5-HIAA) were determined via high performance liquid chromatography (30).
Forced swim test (FST)
The FST was originally developed by Porsolt and colleagues (31) to screen for the anti-depressive effect of pharmacotherapeutics. Here we used the modified version of the FST shown to provide a greater reliability for detection of depressant or antidepressant-like effects for compounds that affect the serotonergic system (32). The test was performed as previously described (26).
Experimental design
Effect of serotonin depletion on weight-loss impact of GLP-1R activation
To assess the impact of serotonin depletion on the weight-loss effect of GLP-1R activation, serotonin depleted rats were injected with EX4 and their body weight was followed for five consecutive days of central EX4 injections. Serotonin depletion was performed by three intraperitoneal injections of PCPA, an irreversible inactivator of TPH, an enzyme required for the synthesis of serotonin. Rats received one daily injection of 300mg/kg for 3 days. This treatment regimen was previously shown to decrease serotonin content to 5–10% of original levels (24, 33), in our hands serotonin turnover in the hypothalamus was still reduced by over 40% one week after the PCPA treatment (2.53±0.2 vs. 1.57±0.2: serotonin turnover 5-HIAA/5-HT for vehicle- and PCPA-treated rats respectively; p<0.01). Control rats received saline injections. 24h after the last PCPA injection, daily EX4 (0.2µg) or vehicle (1µl of aCSF) injections commenced. Dose of EX4 was chosen based on (25, 34, 35).
Effect of 5HT2A or 5HT2C receptor blockade on feeding and weight-loss impact of GLP-1R activation
Rats were divided into the following four groups, matched for body weight: vehicle (1µl aCSF)/vehicle (1µl aCSF), vehicle/EX4 (0.2µg), R-96544 (10µg)/EX4, R-96544/vehicle to test the impact of 5HT2A blockade. To test the contribution of 5HT2C receptors rats were divided into the following four groups, matched for body weight at the start of the experiment: vehicle (1µl of 16%DMSO in aCSF)/vehicle (1µl aCSF), vehicle/EX4 (0.2µg), SB242084 (10µg)/EX4, SB242084/vehicle. The injection of the antagonist was performed 10 minutes before the injection of the agonist or the respective vehicle. All injections were performed once a day, during mid light cycle, and intra-LV (total of 8 days of injections). Gonadal white adipose tissue (GWAT) pads were dissected 24h after the last injection. In an additional group of rats we tested whether central blockade of the 5HT2A receptor attenuates the weight loss induced by peripherally (ip) injected liraglutide (75 µg/kg).
Intra-DR GLP-1R activation
Vehicle (0.3µL/aCSF), EX4 (0.05µg), or GLP-1 (2µg) were injected into the DR of rats that were mildly food restricted (50% of their normal overnight chow) to promote reliable consumption of chow after the DR-injections. The intake of chow was measured at 1 and 24h; body weight change was measured at 24h after intra-DR injection. These doses were chosen to be below LV threshold for effect to avoid potential confounding effect of potential drug leakage into the cerebral aqueduct located just above the DR.
Statistics
All statistical analysis was performed in Graph Pad Prism software (San Diego, CA). Statistical significance was analyzed using ANOVA followed by Holm-Sidak´s multiple comparison test or students t-test as appropriate. All data are presented as mean±SEM.
Results
Serotonin depletion attenuated EX4-induced weight loss maintenance
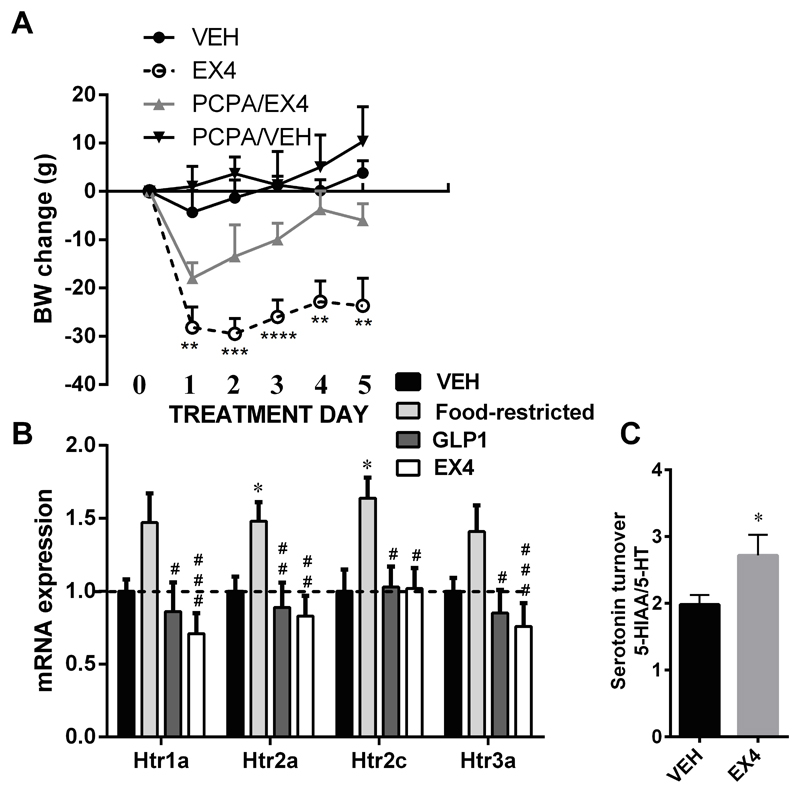
Serotonin synthesis is initiated by hydroxylation of tryptophan, a rate-limiting reaction performed by the enzyme tryptophan hydroxylase (TPH) in the brain. PCPA acts as a selective and irreversible inhibitor of TPH. To evaluate whether intact serotonin synthesis is necessary for the energy balance impact of GLP-1R activation, serotonin was depleted by a well-established treatment regimen of three-day intraperitoneal PCPA injections (24, 33). Consistently with a critical downstream role of serotonin in the weight loss effect of GLP-1R stimulation, serotonin depletion resulted in an attenuation of EX4-induced weight loss maintenance (Figure 1A). Two-way repeated measures ANOVA on body weight data indicated significant effect of drug treatment (F(3, 15)=18.69; P<0.0001) and treatment day (F(4, 60)=4.655; P<0.005), but no significant interaction of treatment and time (F(12, 60)=0.517; P=0.896). Holm-Sidak's multiple comparisons test indicated a significant weight loss effect induced by EX4 for all testing days. In contrast EX4 did not exert any significant effect on body weight in the rat group that has been depleted of serotonin by PCPA as compared to vehicle-injected rats. A significant difference in weight loss between EX4 vs. PCPA/EX4 rats was however detected 48h after the first EX4 injection (Holm-Sidak's test: day 1 t=1.7, P=ns; day 2 t=2.7, P<0.05; day 3 t=2.7, P<0.05; day 4 t=3.2, P<0.01; day 5 t=3.0, P<0.05). A significant difference in weight loss was also detected between PCPA/VEH vs. PCPA/EX4 rats on day 1, 2, and 5 (Holm-Sidak's test: day 1 t=3.0, P<0.05; day 2 t=2.7, P<0.05; day 3 t=1.8, P=ns; day 4 t=1.4, P=ns; day 5 t=2.5, P<0.05). This indicates that serotonin signaling may not be essential for the weight loss effect of EX4 acutely, but is crucial in a sub-chronic setting. PCPA/VEH animals did not increase in weight; this lack of effect of PCPA alone for male rats is consistent with a previous report (9).
Fig 1.
Serotonin depletion interferes with the weight-loss maintenance after central GLP-1R activation (A). While daily central EX4 injections in control rats led to a potent reduction of body weight, the weight loss impact of EX4 was markedly attenuated in rats depleted of serotonin by PCPA suggesting that intact serotonin synthesis is needed for a full expression of EX4-induced weight loss. PCPA/VEH animals did not increase in weight. n = 3–6 per each treatment group. Activation of central GLP-1R ameliorated changes in serotonin receptor genes in the hypothalamus induced by food restriction (B) and also increased serotonin turnover in the hypothalamus (C). *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001 compared to vehicle-treated rats. #p < 0.05, ##p < 0.01, ###p < 0.005 compared to pair-fed rats.
Chronic central GLP-1R stimulation affected hypothalamic serotonin receptor expression
The hypothalamus is a major target site for the energy balance effect of serotonin. In order to determine whether GLP-1R activation changes hypothalamic serotonin signaling we measured changes in hypothalamic serotonin receptors and serotonin turnover after central chronic EX4 or GLP-1 treatment. Central injection of EX4 or GLP-1 abolished the increase of hypothalamic 5-HT1A, 5-HT2A, 5-HT2C and 5-HT3A induced by the restricted feeding (F(3, 41)=6.85, p<0.001; F(3, 399)=6.35, p<0.001; F(3, 41)=5.14, p<0.005; F(3, 41)=6.34, p<0.005, respectively for each receptor, Figure 1B). Feeding was restricted to the daily amount voluntarily eaten by EX4-treated rats. While food restriction elevated the mRNA of all serotonin receptors tested, this change reached significance only for Htr2a and Htr2c mRNA (Figure 1B). Of note the food restriction promoted increased expression of these two receptors also when both control and food-restricted groups did not receive cannulation or injection (Figure S1). Consistent with the idea that GLP-1R activation increases serotonin neurotransmission in order to produce hypophagia we found that serotonin turnover was increased in the hypothalami of EX4-treated rats (Figure 1C).
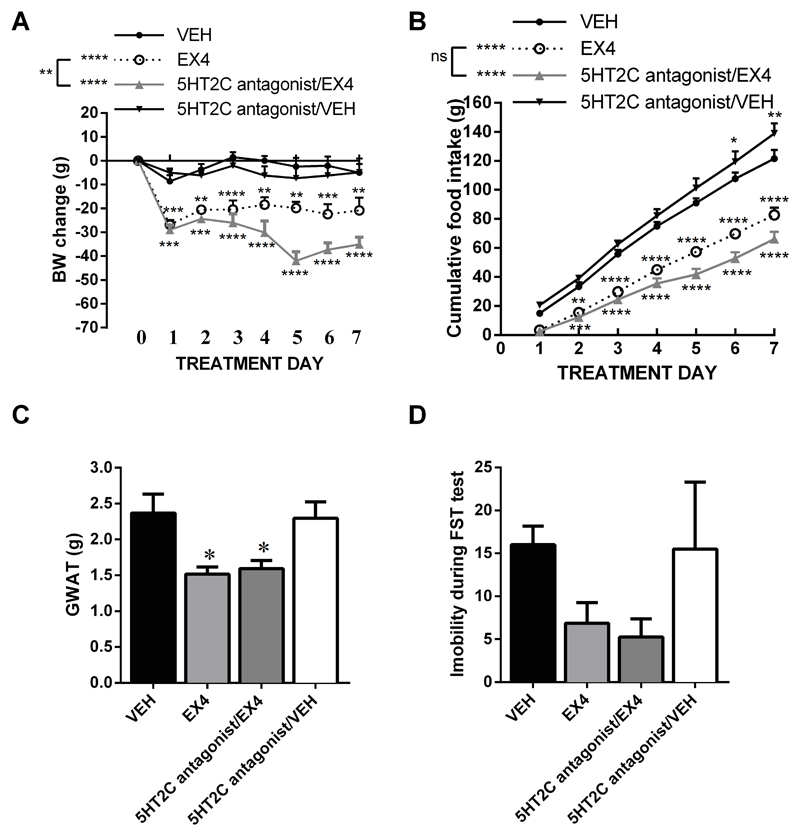
5HT2C receptor blockade does not attenuate EX4-induced weight loss or anorexia
The antagonist used to block 5HT2A receptors (below), R-96544, offers a very high selectivity for all but one serotonin receptor – it only offers four-fold selectivity for the 5HT2C receptor. Due to the possibility of some contribution of 5HT2C blockade by the R-96544 to the results obtained, along with the well-established role of 5HT2C stimulation in weight-regulation, we tested whether a selective 5HT2C receptor antagonist was sufficient to attenuate the impact of EX4 on body weight, fat deposition and food intake. Selective blockade of 5HT2C with SB242084 did not attenuate EX4-induced weight-loss (Figure 2A). Surprisingly blockade of the 5HT2C receptor slightly and significantly exacerbated the EX4-induced weight loss. Two-way repeated measures ANOVA on body weight data indicated a significant interaction (F(18, 162)=1.998; P<0.05), significant effect of drug treatment (F(3, 27)=34.12; P<0.0001) and treatment day (F(6, 162)=3,099; P<0.01). While the reduction of food intake by EX4 seemed exacerbated by the SB242084, this effect was not significant (Figure 2B). Also for food intake a significant interaction (F18, 162)=15.38; P<0.0001), effect of drug treatment (F(3, 27)=49.85; P<0.0001) and treatment day (F(6, 162)=748.7; P<0.0001) were found. The weight of GWAT was clearly reduced by the sub-chronic central EX4 treatment but this effect was not altered by coapplication of the 5HT2C antagonist (Figure 2C; F(3, 27)=5.329; P<0.01). It is unlikely that the lack of attenuation of the weight loss and anorexia induced by EX4 are due to insufficient dose of the drug, since previous reports have reported even lower doses of the drug applied into the brain (intracerebroventricular) to be effective in changing food intake in mice or rats (36, 37). Trends in reduced FST immobility after EX4 treatment were detected (Figure 2D), although these results did not receive statistical significance, irrespective of the antagonist coapplication, due to large variability in the antagonist administered group.
Fig 2.
Blockade of central 5HT2C receptors does not reduce the anorexic or weight-loss effect of EX4. Week long daily central EX4 treatment produced a profound and sustained weight loss; blockade of 5HT2C receptor concurrent with the EX4 treatment did not affect the acute EX4-induced weight loss but potentiated the EX4-induced weight loss on the remaining days of the treatment (A). In line with the body weight results, EX4 reduced food intake, this reduction was not significantly altered by the 5HT2C antagonist co-treatment (B) Similarly the reduction in the weight of the gonadal adipose fat pad (GWAT) induced by EX4 was spared by the concurrent blockade of the 5HT2C receptor (C). The impact of EX4 on depression-like behavior was unaltered by the chronic 5HT2C receptor blockade (D). n = 7–16 per each treatment group. Data are presented as mean ± SEM.*p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001.
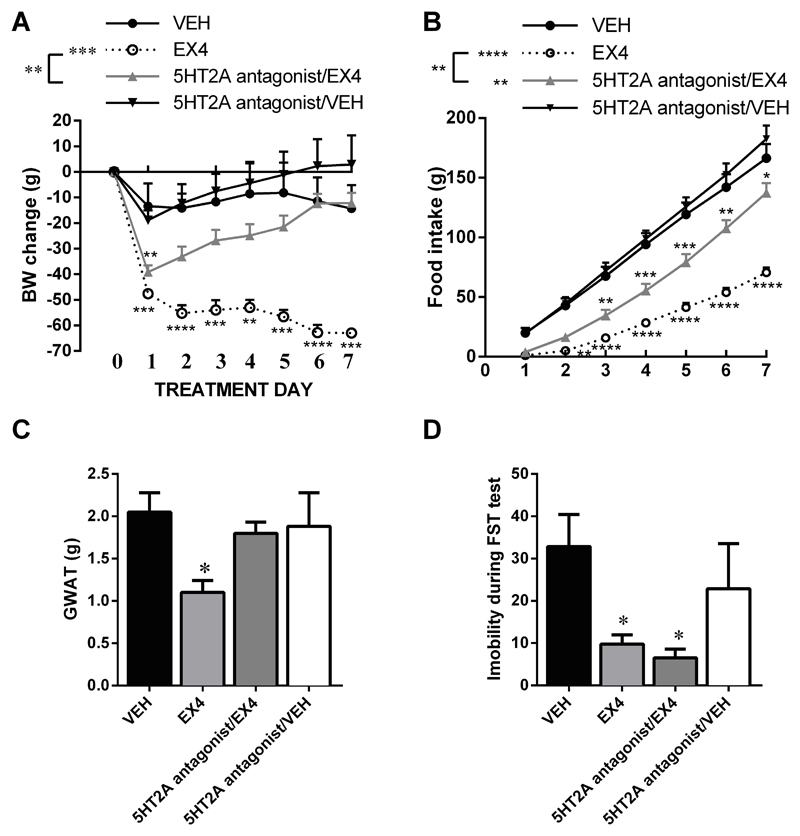
5HT2A receptor blockade attenuated EX4-induced weight loss and anorexia
Because 5HT2A and 5HT2C were two hypothalamic receptors we found to be responsive to food restriction, and central chronic EX4 treatment, we hypothesized that their pharmacological blockade should impair the metabolic impact of EX4. In line with this idea, central blockade of 5HT2A receptor, with the selective antagonist R-96544, significantly attenuated the weight loss induced by EX4 (Figure 3A). Interestingly, the onset of attenuation was delayed to 48h after the injections of EX4 suggesting that the 5HT2A receptor becomes essential to the weight-loss effect only after two days of treatment, while it seems dispensable for the acutely induced weight loss. Two-way repeated measures ANOVA on body weight data indicated a significant interaction (F(18, 156)=6.104; P<0.0001), significant effect of drug treatment (F(3, 26)=13.87; P<0.0001) and a significant effect of treatment day (F(6, 156)=5,893; P<0.0001). An identical pattern of effect of treatment impact was detected for food intake (Figure 3B), with a significant interaction (F(18, 156)=25.68; P<0.0001), significant effect of drug treatment (F(3, 26) = 985.6; P<0.0001), and a significant effect of treatment day (F(6, 156)=29.21; P<0.0001). In line with the food intake and body weight results the GWAT weight was potently reduced by the sub-chronic central EX4 treatment, an effect completely abolished by the co-treatment with the 5HT2A antagonist (Figure 3C). One-way ANOVA indicated a significant effect of the treatment on the GWAT weight (F(3, 23)=3.328; P<0.05). Since sub-chronic (but not acute) central EX4 treatment was recently shown to reduce depression-like behavior (26) in rat FST and serotonin has a well-established role in regulation of emotionality we hypothesized that the anti-depression action of EX4 may be mediated by the central 5-HT2A receptors. Data obtained did not support this idea, and clearly indicate that 5-HT2A blockade did not affect the EX4-induced reduction of immobility in the FST (Figure 3D).
Fig 3.
Blockade of the central 5HT2A receptors attenuates the anorexic and weight-loss effect of EX4. Subchronic, daily central EX4 treatment produced profound and sustained weight loss; blockade of 5HT2A receptors concurrent with the EX4 treatment did not affect the acute EX4-induced weight loss but abolished EX4-induced weight loss after one week (A). In line with the body weight results, EX4 produced a profound food intake reduction which was abolished after 5HT2A antagonist treatment, but only after three days of co-treatment (B) Similarly the reduction in the weight of the gonadal adipose fat pad (GWAT) induced by EX4 was completely abolished by the concurrent blockade of the 5HT2A receptor (C). In contrast, the impact of EX4 on depression-like behavior was unaltered by chronic 5HT2A receptor blockade (D). n = 6–8 per each treatment group. Data are presented as mean ± SEM.*p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001 compared to vehicle-treated rats.
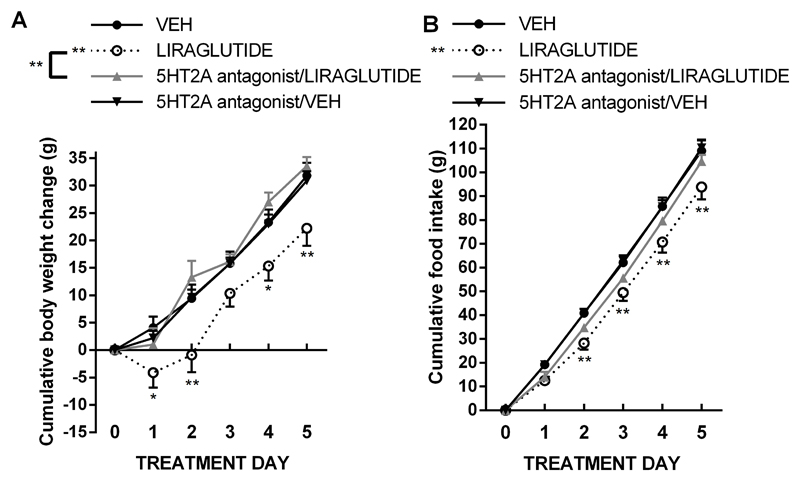
Central blockade of 5HT2A receptor also significantly attenuated the weight loss induced by peripherally injected liraglutide (Figure 4A). The pattern of results was somewhat different from that obtained with EX4, since the intraperitoneally-injected liraglutide was much less potent at reducing body weight, and, interestingly, the 5HT2A blockade was effective already at 24h. Two-way repeated measures ANOVA on body weight data indicated a significant interaction, significant effect of drug treatment (F(3,32)=6.282; P<0.005) and a significant effect of treatment day (F(4, 128)=184.5; P<0.0001). A similar pattern of effect was detected for food intake (Figure 4B): significant interaction, significant effect of drug treatment (F(3, 32)=5.82; P<0.005), and significant effect of treatment day (F(6, 156)=29.21; P<0.0001) were found. The amount of weight loss differed among the four GLP-1 analogue-treated rat groups (Figure 1A, 2A, 3A, 4A), this is likely because each of these groups received different vehicle treatments for the accompanying serotonin signaling targeting drug, or different GLP-1 analogues displaying different potency of effect, or different route of administration (peripheral vs. icv) resulting in different effect strength. Importantly, however, the interaction with serotonin discovered here persisted irrespective of the degree of weight loss induced by the GLP-1 analogues. Thus, the interaction is not restricted to cases where GLP-1 analogues result in largest weight loss.
Fig 4.
Blockade of the central 5HT2A receptors attenuates the anorexic and weight-loss effect of peripherally injected liraglutide. Daily peripheral (ip) injections of liraglutide (75 µg/kg) promoted weight loss; blockade of 5HT2A receptors concurrent with the liraglutide treatment abolished liraglutide-induced weight loss (A). In line with the body weight results, liraglutide reduced food intake, an effect that was attenuated by 5HT2A receptor antagonist treatment (B) n = 7–10 per each treatment group. Data are presented as mean ± SEM.*p < 0.05, **p < 0.01 compared to vehicle-treated rats.
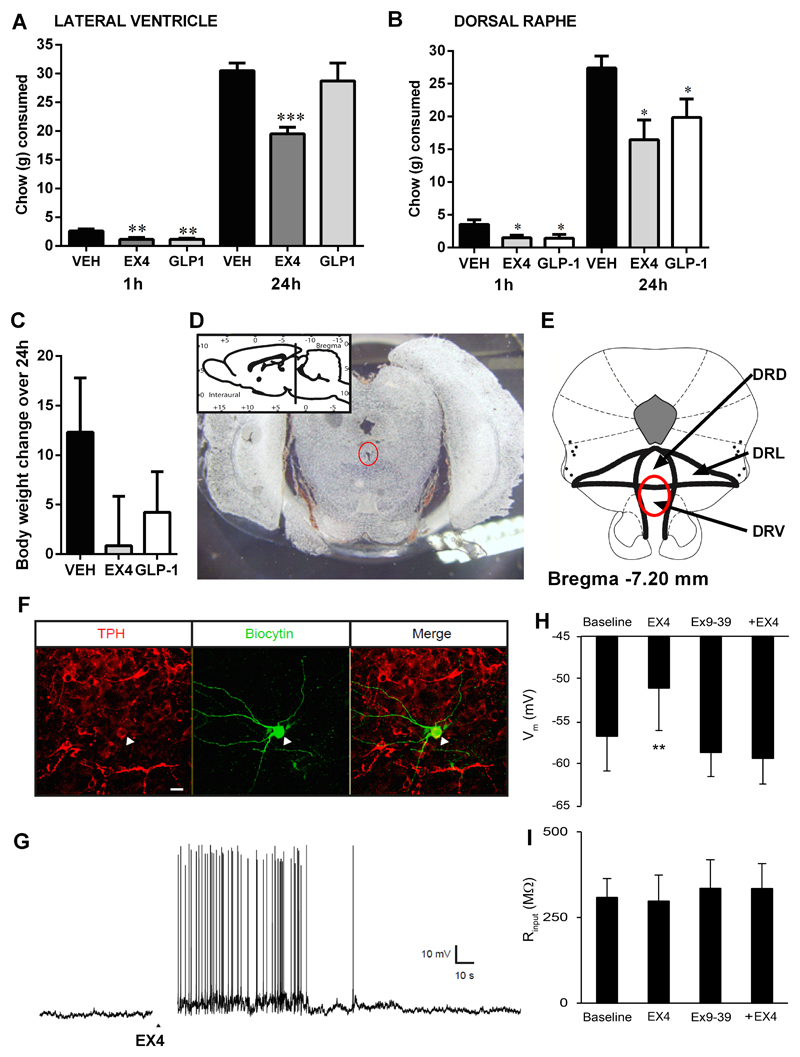
Acute intra-DR administration of GLP-1 or EX4 is sufficient to reduce food intake
Acute injections of EX4 (0.2µg) or GLP-1 (10µg) into the brain ventricles (here intra-LV) reduced food intake at 1h (ANOVA; F(2, 30)=9.37, p<0.001) for both EX4 and GLP-1 and at 24h (F(2, 30)=8.34, p<0.005) for EX4 (Figure 5A). This application route likely delivers the GLP-1R agonists to many brain sites from which a potential anorexic effect can be elicited. Since both serotonin depletion and the 5HT2A receptor blockade suggested an interaction of GLP-1R activation with serotonin, we hypothesized that GLP-1 may be exerting a part of its anorexigenic and weight-loss effects by acting directly on the DR. Rats microinjected with GLP-1 or EX4 into the DR ate significantly less chow both at 1h (ANOVA; F(2, 23)=4.17, p<0.05) and 24h (F(2, 23)=5.01, p<0.05, Figure 5B). The weight loss of rats injected with either EX4 or GLP-1 did not, however, reach statistical significance (F(2,23)=1.39, p=0.26, Figure 5C). Intra-DR GLP-2 microinjections did not change food intake or body weight (Figure S2).
Fig 5.
Dorsal raphe GLP-1R activation is sufficient to reduce food intake and body weight and to increase electrical activity of DR 5-HT neurons. GLP-1 or EX4 potently reduced the amount of chow eaten at 1h when applied to the lateral ventricle, but only EX4 remains effective at reducing intake at 24h (A). n = 9–14 per each treatment group. When applied directly to the DR both EX4 and GLP1 reduce food intake at 1h and 24h (B). The weight loss at 24h did not reach significance for either of the two compounds (C). n = 7–10 per each treatment group. Data are expressed as mean ± SEM. Photomicrograph of a 40μm coronal section of rat brain illustrating the injection site and schematic representation of the DR according to the rat brain atlas (D-E). The red circle illustrates the detected diffusion of ink. Immunohistochemical identification of TPH expression (red) in a biocytin-filled (green) DR 5-HT neuron (arrowhead) (F). Scale bar: 20 µm. Voltage trace obtained from whole-cell recordings from DR 5-HT neurons showing the response of a DR 5-HT neuron to EX4 (1 µM, arrowhead) (G). Bar graphs summarizing EX4 effect on Vm (H) and Rinput (I) on DR 5-HT neurons in baseline condition or after pre-incubation with Ex9-39 1 µM (n=6). DRD, DRL, and DRL: dorsal, lateral and ventral subdivisions of the dorsal raphe, respectively. Data are presented as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.005.
GLP-1R activation increased the electrical activity of DR 5-HT neurons
To investigate the effect of EX4 on excitability of the DR 5-HT neurons we carried out whole cell electrophysiology. Neurons recovered after electrical recordings were identified as serotoninergic by their TPH expression (Figure 5F). EX4 (1μM) applied to the superfusion buffer induced a rapid and reversible depolarization of the membrane potential which, in some cells, was sufficient to trigger action potential firing (Figure 5G). Mean depolarization was 5.7±1.0 mV (p<0.002, Figure 5H). There was no change in Rinput (Figure 5I). The effect of EX4 was then tested after pre-incubation with Ex9-39 (1μM) to verify the specificity of its effect on 5-HT neurons. In this condition, EX4 did not change membrane potential (mean depolarization: -0.7±0.4mV; p<0.12, Figure 5H), confirming that EX4 effect is mediated by the GLP-1R. These results are in agreement with an excitatory effect of GLP-1R activation on DR 5-HT neurons.
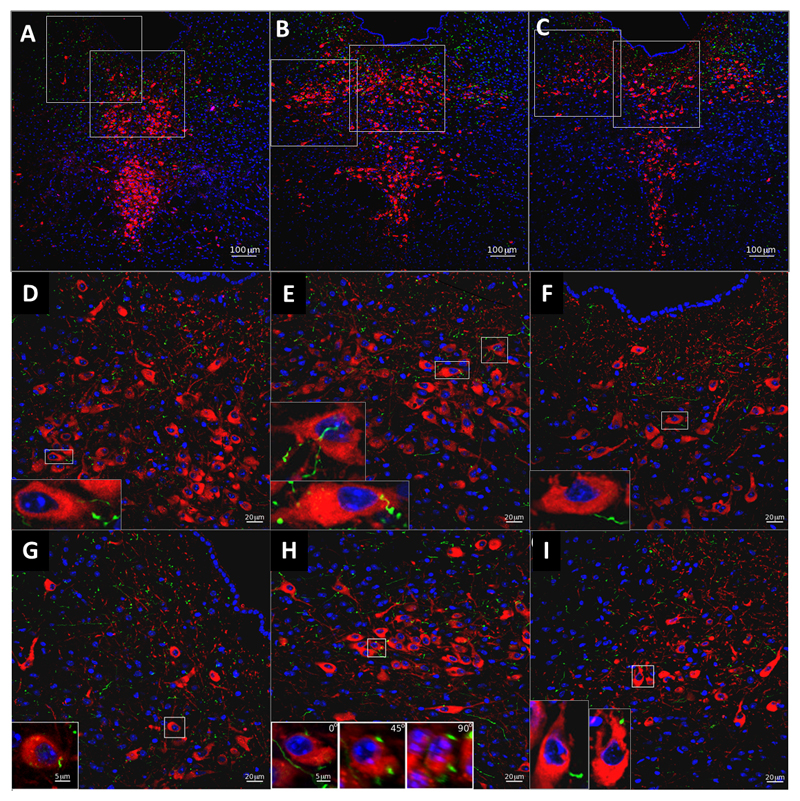
Fibers of GLP-1-producing neurons innervate the DR
To determine whether GLP-1-producing neurons innervate the DR, thereby providing a route via which the endogenous peptide could reach DR serotonin neurons, mice engineered to express yellow fluorescent protein (YFP) in GLP-1-producing preproglucagon (PPG) neurons were used (38) along with immunohistochemical detection of TPH to identify serotonin neurons. YFP-immunoreactive innervation was assessed in coronal brain slices taken throughout the rostro-caudal extent of the DR in YFP-PPG mice (38). YFP-immunoreactive fibers were found throughout the DR (Figure 6); however the dorsal and dorsolateral divisions of the DR contained the highest density of the YFP-immunoreactive axons. In this area many TPH-positive neurons were found to receive innervation from GLP-1-producing neurons (Figure 6, Figure S3: video file). Z-line three-dimensional reconstruction of a confocal image stack focused on a single TPH-positive YFP-innervated neuron was used to confirm the YFP innervation, and to differentiate fibers terminating on the TPH-positive neuron from passing fibers. Only very sparse PPG neuron innervation was detected at the level of the ventral DR (Figure S4).
Fig 6.
Immunohistochemical labeling for YFP in YFP–PPG neurons (green) and 5-HT in serotonin neurons (red) in coronal sections through the DR nucleus of YFP–PPG mice. Low magnification micrographs showing the rostro-caudal extent of the DR examined (A-C). Groups of red 5-HT-immmunoreactive cell bodies are shown at higher magnification in D-I. Higher magnification of the dorsal subregion of the DR is displayed in D-E, and of the lateral subregion in G-I. For each of these subregions examples of individual TPH-positive neurons receiving YFP-labeled innervation from GLP-1-producing neurons are shown in the bottom left corner of each panel. Innervation of chosen individual neurons was confirmed by creating a three dimensional reconstruction of Z-stack images (Fig S3). High levels of immunoreactive YFP fibers are found in both the dorsolateral and dorsal section of the DR but not in the ventral section (Fig S4).
Discussion
The results presented here demonstrate that intact brain serotonin signaling is critical for maintenance of weight loss induced by GLP-1R activation. The time course and specific serotonin receptor contribution discovered here are unexpected but consistent with the recently emerging literature (39). Moreover, congruent results from electrophysiological, immunohistochemical and neuropharmacological studies place the DR on the brain map of sites directly impacted by GLP-1 to regulate energy homeostasis.
Considering the well-established role of the 5HT2C receptor in energy balance along with several studies indicating an opposing effect of 5HT2A and 5HT2C receptors in behavioral inhibition (40, 41), the critical role of the 5HT2A receptor in GLP-1-induced weight loss is unexpected. However 5HT2A and 5HT2C receptors may have similar molecular structures (42, 43). Furthermore careful literature investigation does suggest a perhaps overlooked role of the 2A receptor in energy balance regulation. Hypermethylation of 5HT2A receptor is associated with elevated baseline insulin levels and reduced sensitivity to the weight-loss effect of dietary interventions in human patients (44). 5HT2A activation in the hypothalamus attenuates hunger induced by food deprivation or NPY administration (45). Stimulation of 5HT2A, but not 5HT2C or 5HT1 receptors, in the paraventricular nucleus reduced NPY-induced hyperphagia, possibly through activation of CRF release (45). Finally a recent study suggests that the 2A receptor, like the 5HT2C receptor, is expressed on hypothalamic POMC neurons (39). In this study pharmacological activation of the brain 5HT2A receptors resulted in reduced food intake. Thus current results are consistent with several published reports that show an anorexic potential of 5HT2A receptors at the level of the brain. However, one study reported that peripheral injections of a 5HT2A receptor antagonist decrease body weight in mice (46). Thus, it seems that the anorexic effect may be driven by the central 5HT2A receptors, while manipulations that include peripheral receptors may have other effects; especially since the 5HT2A receptors are expressed on vagal afferents and other tissues, for examples platelets. Also 5HT2A receptor knockout mice do not show altered body weight or feeding when maintained on laboratory chow, suggesting that some degree of compensation may be present in the knockout mice (47). Data showing that pharmacological, but not genetic, blockade of 5HT2C receptors attenuates LPS anorexia suggest that the manipulation of serotonin receptors is prone to compensatory reorganization (48).
The 5HT2C receptor is the most extensively studied serotonin receptor in relation to food intake regulation (4, 11, 12). It is widely expressed in the CNS and it is also a target of a clinically approved anti-obesity drug. Therefore it was plausible that blockade of this receptor should interfere with the weight-loss and anorexic response to EX4. Our results, however, did not support this hypothesis. The pharmacological blockade of the 5HT2C receptor did not alter EX4-induced reduction in food intake or fat mass. Furthermore, the body weight loss produced by chronic EX4 treatment seemed to be potentiated rather than attenuated by the 5HT2C receptor blockade. This may seem to contrast with one previous report showing that 30-minute food intake reduction, induced by peripheral GLP-1 injection in mice, is attenuated in 5HT2C KO mice (49). However, the same report states that the attenuation of anorexia was no longer significant at subsequent 1 and 2h measurements. Another report shows that 5HT2C KO mice display an attenuated anorexic response to low dose of GLP-1, but not liraglutide, at 2h (50). Thus, we may speculate that if 5HT2C has a contribution to anorexia resulting from GLP-1R stimulation it may only be relevant in a very acute setting (≤2h). On the other hand acute, 1 or 2h, anorexic response to liraglutide was not attenuated in mice treated with PCPA (51). These findings together with the current results suggest that chronic, but not acute, anorexic effect of liraglutide may require serotonin. Furthermore, in line with our results, the activation of neurons indicated by cFos induction at 90min, after peripheral GLP-1 injection in mice, is actually potentiated in two hypothalamic nuclei: paraventricular and arcuate nuclei (49). It is also important to note that 5HT2C and 5HT2A are only two, out of at least 18 serotonin receptors, thus future studies should evaluate the contribution of the remaining receptors to energy balance regulation, especially since the mRNA of the two other receptors measured in this study also seems to be affected by GLP-1R activation.
Furthermore, even though blockade of 5HT2A or 5HT2C receptors did not significantly alter feeding and weight loss responses acutely (<24h), this may only reflect the redundancy of the systems activated by GLP-1R signaling acutely. The redundancy hypothesis is also favored by data demonstrating 1) EX4 or GLP-1 activates DR serotonin neurons, 2) intra-DR injections of these agonists effectively reduce intake already at 1h, 3) they also release serotonin from hypothalamic synaptosomes in a matter of minutes (13).
Dorsal raphe is a major serotonin source for the hypothalamus (15, 16). Indeed, our data suggest that the hypothalamus could be one of the downstream targets of GLP-1R-activated serotonergic neurons. These results are consistent with the critical role of the hypothalamus in energy balance regulation. That chronic treatment with EX4 increased serotonin turnover in the hypothalamus suggests that increased activity of central serotonin does not display tolerance to the daily GLP-1R agonist treatment. Moreover, the increased expression of 5HT2A and 5HT2C receptors in the hypothalamus induced by food restriction was ameliorated by either central EX4 or GLP1 treatment. These results suggest that GLP-1R activation counteracts the reduced serotonergic activity associated with negative energy balance (52).
Given the importance of serotonin in emotional homeostasis and the clinical application of serotonin-acting pharmaceuticals in the treatment of depression it was plausible that the anti-depression impact of GLP-1 (26, 53, 54) utilizes central serotonin signaling. Surprisingly, blockade of either 5HT2A or 5HT2C receptors did not influence the potent anti-depression impact of chronic GLP-1 activation. Therefore, while serotonin signaling through the 5HT2A receptor proved to be critical for the energy balance effect of EX4, the impact of the drug on emotionality was spared by the 5HT2A receptor blockade suggesting that the interaction is critical for metabolic effects of GLP-1 but not necessary for emotionality regulation. Thus these findings disassociate the metabolic and emotionality pathways impacted by GLP-1R activation. These results are also consistent with the lack of a depression-like phenotype in the 5HT2A receptor knockout mouse (47).
With serotonin and GLP-1 recently becoming the most relevant two brain systems for development of anti-obesity therapies, it is increasingly important to understand the neural circuitry underlying their metabolically beneficial effects. Surprisingly, little is known on how these two systems may interact. Current data indicate that GLP-1 avails of serotonin signaling in order to achieve its metabolic impact. Unexpectedly the most widely researched serotonin receptor, 5HT2C, was not necessary for this interaction; instead a receptor much less explored with respect to energy balance, 5HT2A, was shown to be critical for the hypophagic and weight loss impact of a clinically approved GLP-1 analogue, EX4. Our data also suggest that the effect of GLP-1 on serotonin is direct since GLP-1R activation increased serotonin neuron activity, at the level of the DR, a key brain nucleus supplying serotonin to many forebrain areas, and caudal brainstem GLP-1-producing neurons innervate the DR. Our results also disassociate the brain metabolic and emotionality pathways impacted by GLP-1R activation. Finally few studies look at the long-term impact of GLP-1 analogues on the brain; this is somewhat surprising since it is the long-term impact that is likely the most relevant for the anti-obesity impact of these substances. Our data indicate that the critical neural substrates activated acutely by GLP-1 analogues may differ from those activated with longer-term repeated drug administration. In summary, the present results contribute to our understanding of neural substrates regulating food intake, body fat and body weight; knowledge of considerable clinical potential. Our data are preclinical, and thus provide an interesting avenue to explore in humans, or larger animal models where the data may translate more readily to humans, but currently can only be considered preliminary in nature.
Supplementary Material
Acknowledgements
This research was funded by the Swedish Research Council (2014-2945 and 2013-7107), Novo Nordisk Foundation Excellence project grant, Ragnar Söderberg Foundation, Harald Jeanssons Stiftelse and Greta Jeanssons Stiftelse, and Magnus Bergvalls Stiftelse. We thank Fredrik Anesten for lending his expertise in immunohistochemistry.
Footnotes
All authors declare no conflict of interest.
Authors have nothing to disclose.
Author Contributions: KPS designed and performed experiments, supervised experimentation, analyzed data, coordinated the project, and wrote the manuscript. CH, HN, FB performed the HPLC; CL performed all electrophysiology and associated immunohistochemistry; EB and IWA performed fat dissections; FG, FR generated the YFP mice; JR performed the FST scoring; JR and KE performed dorsal raphe immunohistochemistry; RA, JR, and LLF performed the brain surgery, injections, food and body weight measurements. KPS is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Dailey MJ, Moran TH. Glucagon-like peptide 1 and appetite. Trends in endocrinology and metabolism: TEM. 2013;24(2):85–91. doi: 10.1016/j.tem.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holst JJ. Incretin hormones and the satiation signal. Int J Obes (Lond) 2013;37(9):1161–1168. doi: 10.1038/ijo.2012.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iepsen EW, Torekov SS, Holst JJ. Liraglutide for Type 2 diabetes and obesity: a 2015 update. Expert review of cardiovascular therapy. 2015;13(7):753–767. doi: 10.1586/14779072.2015.1054810. [DOI] [PubMed] [Google Scholar]
- 4.Burke LK, Heisler LK. 5-hydroxytryptamine medications for the treatment of obesity. Journal of neuroendocrinology. 2015;27(6):389–398. doi: 10.1111/jne.12287. [DOI] [PubMed] [Google Scholar]
- 5.Blundell JE, Latham CJ. Serotonergic influences on food intake: effect of 5-hydroxytryptophan on parameters of feeding behaviour in deprived and free-feeding rats. Pharmacol Biochem Behav. 1979;11(4):431–437. doi: 10.1016/0091-3057(79)90120-5. [DOI] [PubMed] [Google Scholar]
- 6.Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behavioural brain research. 1996;73(1–2):37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 7.Breisch ST, Zemlan FP, Hoebel BG. Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine. Science. 1976;192(4237):382–385. doi: 10.1126/science.130678. [DOI] [PubMed] [Google Scholar]
- 8.Saller CF, Stricker EM. Hyperphagia and increased growth in rats after intraventricular injection of 5,7-dihydroxytryptamine. Science. 1976;192(4237):385–387. doi: 10.1126/science.1257774. [DOI] [PubMed] [Google Scholar]
- 9.Hoebel BG, et al. Differential effects of p-chlorophenylalanine and 5,7-dihydroxytryptamine on feeding in rats. Annals of the New York Academy of Sciences. 1978;305:590–594. doi: 10.1111/j.1749-6632.1978.tb31550.x. [DOI] [PubMed] [Google Scholar]
- 10.Harsing LG, Jr, Yang HY, Costa E. Accumulation of hypothalamic endorphins after repeated injections of anorectics which release serotonin. The Journal of pharmacology and experimental therapeutics. 1982;223(3):689–694. [PubMed] [Google Scholar]
- 11.Marston OJ, Heisler LK. Targeting the serotonin 2C receptor for the treatment of obesity and type 2 diabetes. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34(1):252–253. doi: 10.1038/npp.2008.169. [DOI] [PubMed] [Google Scholar]
- 12.Smith SR, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. The New England journal of medicine. 2010;363(3):245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 13.Brunetti L, et al. Glucagon-like peptide 1 (7-36) amide (GLP-1) and exendin-4 stimulate serotonin release in rat hypothalamus. Peptides. 2008;29(8):1377–1381. doi: 10.1016/j.peptides.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403(2):261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Willoughby JO, Blessing WW. Origin of serotonin innervation of the arcuate and ventromedial hypothalamic region. Brain Res. 1987;418(1):170–173. doi: 10.1016/0006-8993(87)90975-9. [DOI] [PubMed] [Google Scholar]
- 16.Sawchenko PE, Swanson LW, Steinbusch HW, Verhofstad AA. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983;277(2):355–360. doi: 10.1016/0006-8993(83)90945-9. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, et al. Exendin-4, an analogue of glucagon-like peptide-1, attenuates hyperalgesia through serotonergic pathways in rats with neonatal colonic sensitivity. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2014;65(3):349–357. [PubMed] [Google Scholar]
- 18.Ripken D, et al. Nutrient-induced glucagon like peptide-1 release is modulated by serotonin. The Journal of nutritional biochemistry. 2016;32:142–150. doi: 10.1016/j.jnutbio.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Nonogaki K, Kaji T. Pharmacological stimulation of serotonin 5-HT1B receptors enhances increases in plasma active glucagon-like peptide-1 levels induced by dipeptidyl peptidase-4 inhibition independently of feeding in mice. Diabetes & metabolism. 2015;41(5):425–428. doi: 10.1016/j.diabet.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Reimann F, et al. Glucose sensing in L cells: a primary cell study. Cell metabolism. 2008;8(6):532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa T, Sugidachi A, Tanaka N, Fujimoto K, Asai F. Pharmacological profiles of R-96544, the active form of a novel 5-HT2A receptor antagonist R-102444. European journal of pharmacology. 2002;457(2–3):107–114. doi: 10.1016/s0014-2999(02)02654-7. [DOI] [PubMed] [Google Scholar]
- 22.Kennett GA, et al. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36(4–5):609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- 23.Alves SE, et al. Serotonin mediates CA1 spine density but is not crucial for ovarian steroid regulation of synaptic plasticity in the adult rat dorsal hippocampus. Synapse. 2002;45(2):143–151. doi: 10.1002/syn.10093. [DOI] [PubMed] [Google Scholar]
- 24.Naslund J, Studer E, Nilsson K, Westberg L, Eriksson E. Serotonin depletion counteracts sex differences in anxiety-related behaviour in rat. Psychopharmacology. 2013;230(1):29–35. doi: 10.1007/s00213-013-3133-6. [DOI] [PubMed] [Google Scholar]
- 25.Dickson SL, et al. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(14):4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderberg RH, et al. GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology. 2015;65:54–66. doi: 10.1016/j.psyneuen.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Shirazi R, et al. Glucagon-like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL-1 and IL-6. Proc Natl Acad Sci U S A. 2013;110(40):16199–16204. doi: 10.1073/pnas.1306799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderberg RH, et al. The Stomach-Derived Hormone Ghrelin Increases Impulsive Behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Hansson C, et al. Influence of ghrelin on the central serotonergic signaling system in mice. Neuropharmacology. 2014;79:498–505. doi: 10.1016/j.neuropharm.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 32.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nature protocols. 2012;7(6):1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 33.Miczek KA, Altman JL, Appel JB, Boggan WO. Para-chlorophenylalanine, serotonin and killing behavior. Pharmacol Biochem Behav. 1975;3(3):355–361. doi: 10.1016/0091-3057(75)90043-x. [DOI] [PubMed] [Google Scholar]
- 34.Turton MD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 35.Tang-Christensen M, Larsen PJ, Thulesen J, Romer J, Vrang N. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat Med. 2000;6(7):802–807. doi: 10.1038/77535. [DOI] [PubMed] [Google Scholar]
- 36.Yakabi K, et al. Reduced ghrelin secretion in the hypothalamus of rats due to cisplatin-induced anorexia. Endocrinology. 2010;151(8):3773–3782. doi: 10.1210/en.2010-0061. [DOI] [PubMed] [Google Scholar]
- 37.Saegusa Y, et al. Decreased plasma ghrelin contributes to anorexia following novelty stress. American journal of physiology. Endocrinology and metabolism. 2011;301(4):E685–696. doi: 10.1152/ajpendo.00121.2011. [DOI] [PubMed] [Google Scholar]
- 38.Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin-Gronert MS, et al. 5-HT2A and 5-HT2C receptors as hypothalamic targets of developmental programming in male rats. Disease models & mechanisms. 2016 doi: 10.1242/dmm.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson ES, et al. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(10):2398–2406. doi: 10.1038/sj.npp.1301636. [DOI] [PubMed] [Google Scholar]
- 41.Winstanley CA, Theobald DEH, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology. 2004;176(3–4):376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- 42.Julius D, Huang KN, Livelli TJ, Axel R, Jessell TM. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci U S A. 1990;87(3):928–932. doi: 10.1073/pnas.87.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pritchett DB, et al. Structure and functional expression of cloned rat serotonin 5HT-2 receptor. The EMBO journal. 1988;7(13):4135–4140. doi: 10.1002/j.1460-2075.1988.tb03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez-Cornago A, Mansego ML, Zulet MA, Martinez JA. DNA hypermethylation of the serotonin receptor type-2A gene is associated with a worse response to a weight loss intervention in subjects with metabolic syndrome. Nutrients. 2014;6(6):2387–2403. doi: 10.3390/nu6062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grignaschi G, Sironi F, Samanin R. Stimulation of 5-HT2A receptors in the paraventricular hypothalamus attenuates neuropeptide Y-induced hyperphagia through activation of corticotropin releasing factor. Brain Res. 1996;708(1–2):173–176. doi: 10.1016/0006-8993(95)01373-3. [DOI] [PubMed] [Google Scholar]
- 46.Nonogaki K, Nozue K, Oka Y. Increased hypothalamic 5-HT2A receptor gene expression and effects of pharmacologic 5-HT2A receptor inactivation in obese Ay mice. Biochemical and biophysical research communications. 2006;351(4):1078–1082. doi: 10.1016/j.bbrc.2006.10.173. [DOI] [PubMed] [Google Scholar]
- 47.Weisstaub NV, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313(5786):536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- 48.Asarian L, Kopf BS, Geary N, Langhans W. Pharmacological, but not genetic, disruptions in 5-HT(2C) receptor function attenuate LPS anorexia in mice. Pharmacol Biochem Behav. 2007;86(3):493–498. doi: 10.1016/j.pbb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Asarian L. Loss of cholecystokinin and glucagon-like peptide-1-induced satiation in mice lacking serotonin 2C receptors. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R51–56. doi: 10.1152/ajpregu.90655.2008. [DOI] [PubMed] [Google Scholar]
- 50.Nonogaki K, Suzuki M, Sanuki M, Wakameda M, Tamari T. The contribution of serotonin 5-HT2C and melanocortin-4 receptors to the satiety signaling of glucagon-like peptide 1 and liraglutide, a glucagon-like peptide 1 receptor agonist, in mice. Biochemical and biophysical research communications. 2011;411(2):445–448. doi: 10.1016/j.bbrc.2011.06.175. [DOI] [PubMed] [Google Scholar]
- 51.Nonogaki K, Kaji T. The acute anorexic effect of liraglutide, a GLP-1 receptor agonist, does not require functional leptin receptor, serotonin, and hypothalamic POMC and CART activities in mice. Diabetes research and clinical practice. 2016;120:186–189. doi: 10.1016/j.diabres.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Bubenik GA, Ball RO, Pang SF. The effect of food deprivation on brain and gastrointestinal tissue levels of tryptophan, serotonin, 5-hydroxyindoleacetic acid, and melatonin. Journal of pineal research. 1992;12(1):7–16. doi: 10.1111/j.1600-079x.1992.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 53.Komsuoglu Celikyurt I, et al. Exenatide treatment exerts anxiolytic- and antidepressant-like effects and reverses neuropathy in a mouse model of type-2 diabetes. Medical science monitor basic research. 2014;20:112–117. doi: 10.12659/MSMBR.891168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isacson R, et al. The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. European journal of pharmacology. 2011;650(1):249–255. doi: 10.1016/j.ejphar.2010.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.