Abstract
Objective:
It is a well-known fact that there is significant heterogeneity in the origins of asthma in adults and children. This article examines the roots of asthma across the ages including atopy, the role of the microbiome and viral infections, along with comorbidities/confounders such as obesity, aspirin-exacerbated respiratory disease (AERD), neutrophilic asthma, cigarette smoking and the possibility of an asthma-chronic obstructive pulmonary disease (COPD) overlap syndrome.
Data Sources:
Data was taken from various scientific search engines including PubMed and Science Direct databases.
Study Selections:
Articles that reviewed information on the origins of asthma in persons of all ages including different phenotypes and genotypes of asthma were used.
Results:
Asthma is a common and complex disease whose origins are likely a combination of both genetic predisposition and environmental exposures. Factors such as the microbiome, other atopic disease, viral infections in young children, and other diagnoses such as obesity or AERD are important to consider when creating a treatment plan for patients.
Conclusion:
Asthma is a disease that is often diagnosed in childhood but can present at any age. There is debate in the field as to whether asthma is one disease or several different diseases that include airway inflammation as a key finding. There are risk factors for disease in the environment and thru co-morbidities that likely play significant roles in both the origins of asthma, the development of symptoms, and the response to treatment. These factors are even more important as we look towards the future with the goal of personalized medicine.
Keywords: Asthma, phenotype, genotype, origin of disease
Introduction
Asthma is a common diagnosis, which afflicts an estimated 300 million people worldwide according to the Global Initiative for Asthma.1 The prevalence of asthma is estimated between 1% to 16% depending on location and population, and asthma is known as the most chronic disease in childhood.2,3 Asthma is a complex and heterogeneous disease that includes various phenotypical disorders sharing a common pathway of airway obstruction from bronchial smooth muscle constriction and inflammation of airway mucosa. The diagnosis of asthma is made by obtaining a history of respiratory symptoms such as wheeze, shortness of breath, chest tightness and cough that vary over time and in intensity, together with evidence of variable expiratory airflow limitation.1 Asthma often begins in childhood but can be diagnosed at any age. Children diagnosed with asthma may or may not continue to have symptoms as they move through childhood into adulthood; even patients whose symptoms appear to have remitted at a young age can have them recur later in life. Severity and frequency of the disease varies from person to person, but nonetheless, asthma is a cause of significant morbidity and mortality in people of all ages. The diagnosis of asthma goes beyond the physical health burden, as it also is a costly disease with significant impact on quality of life for both patients and their families.
Asthma is a multifaceted disease whose symptoms likely are caused by a combination of both genetic and environmental factors. In fact, both “nature” and “nurture” appear to play a significant role in not only the origins of the disease, but also in the acute and chronic symptoms that patients experience. There has been an increase in the prevalence of atopic disease over the past few decades in different parts of the world, which has led to the hypothesis that changes in the environment are underlying the increased prevalence.4 Much debate has occurred as to whether asthma is a single disease with differing presentations or is in fact a variety of diseases in which airflow obstruction is a key feature.5,6 Around two thirds of patients with asthma have an allergic component to their disease and are felt to have allergic asthma. These patients present earlier in life and have concomitant allergic disease and elevated IgE. The other third of asthma patients have non-allergic asthma, which presents more often later in life.7
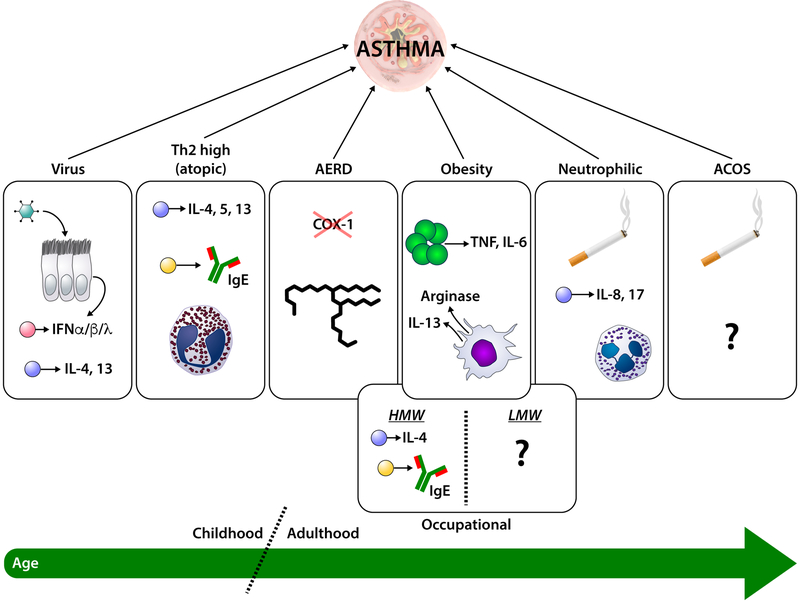
This difference in atopic status and age of onset of asthma suggests that there may be different etiologies for asthma. Supporting this idea is the fact that patients with asthma can be classified based on their endotype or phenotype. Endotypes are based upon underlying pathophysiology of the disease, which is the inflammatory disease that is a hallmark of both allergic and non-allergic asthma. Many inflammatory cells play a role in asthma including lymphocytes, neutrophils, eosinophils, and macrophages. Patients with asthma can be classified based on their endotype into TH2-high or TH2-low subcatagories.8,9 Patients with a TH2-high (T2-high) based disease often have eosinophils in their sputum and can have increased production of IL-4, IL-5, and IL-13 in bronchoalveolar lavage (BAL) fluid and lung tissue. Airway and peripheral eosinophilia are often seen with T2-high disease. These patients often are noted to be atopic with specific allergens that can trigger their disease.10 Steroid responsiveness is an important feature of the T2-high endotype. Patients with a more TH-2 low (T2-low) endotype are noted to have a different cytokine profile in BAL fluid and lung tissue. They also tend to respond less well with corticosteroid treatment compared to patients with T2-high asthma.8,9 It is not clear if patients maintain the same endotype over the course of their life or if changes possibly due to environmental exposures and aging. Phenotypes are another way to classify asthma and are based on a cluster of characteristics that can be used to define the disease. There are specific phenotypes seen more often in children and others that are more common in adults (see Figure 1).
Figure 1. Phenotypic origins of asthma in children and adults.
Specific phenotypes of asthma are more often associated with childhood onset of disease, while others are more commonly found in adults. The various phenotypic origins of asthma are depicted as individual circles with potential representative mechanisms (i.e., Virus, Th2 high, AERD, Occupational, Obesity, Neutrophilic, and ACOS). The bottom line indicates the age at which each of these phenotypes are most predominant. See text for details. ACOS = Asthma-COPD Overlap Syndrome; COX = cyclooxygenase 1; IFN = interferon; IL = interleukin; TNF = tumor necrosis factor. T cells are shown as blue circles, B cells are yellow circles, plasmacytoid dendritic cells are pink circles, eosinophils are pink circles with red inclusions, neutrophils are blue circles with polymorphic nuclei, macrophages are orange, adipocytes are dark green, and exposure to cigarette smoke is indicated by a cigarette.
Origins of Pediatric Asthma
There are distinct phenotypes of asthma seen in patients of all ages. Asthma that begins in childhood may be mechanistically different from disease that starts in adulthood. It is important to recognize the phenotypes of asthma in children not only to determine the best treatment options, but also to allow clinicians to work with families of children with chronic inflammatory pulmonary symptoms to determine risk factors for disease. Atopic disease including the influence of genetics, the microbiome, and the role of viral infections are the most important categories playing a role in childhood asthma.
Atopic Predisposition
Atopic March
It is well known that children of parents with atopic disease are at risk of allergic disease themselves and the risk is even more significant if both parents have an atopic history. This underlying genetic predisposition is important to consider in a child with inflammatory pulmonary symptoms, as the majority of asthma in children is associated with allergic or atopic disease. In fact, the development of a series of atopic diagnoses through the first decade or two of life is often called the “atopic march”. The “march” frequently begins with atopic dermatitis and in the subsequent years progresses through food allergy, allergic rhinitis, and culminates with asthma. One hypothesis is that allergen exposure via eczematous skin leads to sensitization and the start of the “march” through different atopic diagnoses.11 Asthma associated with allergic disease, therefore, often appears near the end of the atopic march, rather than in the first several years of life. Interestingly, while approximately 20% of children with mild atopic dermatitis develop asthma, over 60% of children with severe skin symptoms eventually develop the disease.12,13 A child with atopic dermatitis is also at risk for increased severity and persistence of asthma beyond the childhood years.14,15 Even the presence of food allergy can increase the risk for development of asthma; in a recent study, allergy to peanut, cow’s milk, or egg was associated with future development of asthma.11,16 This important factor and clinical tool allows both clinicians and families to more strongly consider asthma in a young child with food allergy and possible wheezing. As one continues down the atopic march, there is a well-studied association between asthma and allergic rhinitis with a significant number of patients with asthma reporting allergic nasal symptoms.17 Given the tight correlation between other atopic diseases and asthma, it would strongly support the idea that asthma in childhood may simply be an extension of the Th2 mediated (T2-high) response to the airways. However, there are data suggesting other mechanisms might be at play in the development of asthma, and these are discussed in more detail below.
Aeroallergens
It is thought that there could be a more severe prognosis in children with asthma associated with IgE mediated pulmonary disease.18 There may even be a more significant decline in lung function in children and adults with atopic asthma.19 In a recent study, it was found that aeroallergen sensitization by the age of one year old increased the risk of asthma persisting into adolescent years.20 Allergy testing for common environmental allergens is a frequent occurrence in children with suspected atopic asthma. Takkouche, et al, completed a review of cohort studies published between 1996–2007 and found that exposure to cat might have a small preventative effect on asthma while exposure to dog only slightly increased the risk of disease.21 Dust mite hypersensitivity also is believed to play a role in children with atopic disease such as asthma. Children exposed to high levels of house dust mite during the first year of life demonstrated a higher risk of allergic sensitization and asthma in a study by Sporik, et al.22 Cockroach sensitization also is associated with allergic disease and is a significant risk factor for the development of asthma in low-income urban children.23,24
Microbiome and Microbial Exposures
One interesting observation in asthma (and allergic disease) has been that children who grow up on farms or close to livestock have a low prevalence of asthma.25 This observation, plus the fact that vaccination and increased use of antimicrobials in Westernized countries, has reduced the frequency of childhood infections during the same time period as the increase in prevalence of asthma and allergic disease, has led to the so-called “hygiene hypothesis.” This hypothesis posits that by reducing exposure to bacteria, the immune system is being skewed towards T2-high type responses.26
The microbiome refers to the collection of all bacteria that colonize mucosal surfaces. Studies have shown that the developing immune system can be profoundly affected by changes in the microbial communities on the skin and gastrointestinal tract. As a result, changes in the microbiome early in life are thought to play a role in susceptibility to allergic disease and asthma. Many factors such as both pre and postnatal exposure to antibiotics, method of delivery, and early diet have the ability to influence microbial colonization.4
The microbiome can be classified by the number of different species represented (richness) as well as how evenly distributed those species are distributed (evenness). The combination of richness and evenness gives the diversity of a microbial community. Reduced diversity of stool flora at one month of age has been shown to be predictive of atopic disease in children at both 2 and 6 years4,27 Specific microbial species found in the infant gut may play a role in the risk of allergic sensitization, and, in fact, reduced early life colonization with Lactobacillus species and Bacteroides species and increased colonization of Clostridia species and yeast were found in atopic children.28,29
In addition to atopy, differences in the microbiome have been associated with asthma risk. For example, as mentioned the route of delivery can have a profound effect on the microbiome, and children born via cesarean and vaginal delivery have very different microbial colonization patterns.30 Since the risk for developing asthma is greater in children born by cesarean section31, it is intriguing to hypothesize that this risk of asthma is due to how a cesarean section alters the microbial communities of the infant, and the effect on the developing immune system.32 Arrieta, et al, in the Canadian Healthy Infant Longitudinal Development (CHILD) study found that the first 100 days of life represent a crucial time frame in which altered gut microbial communities, also known as microbial dysbiosis, are linked to the risk of asthma and allergic disease.33 The CHILD study found that reductions in four bacterial genera, Lachnospira, Veillonella, Faecalibacterium, and Rothia, were associated with increased risk of developing asthma.33 The study also found that these microbial changes were less apparent by the age of one year old, suggesting that any effect of the microbiome would be limited to ages under 1 year.33
Exposures in the child’s indoor and outdoor environment may increase or decrease likelihood of future atopic disease. Studies have revealed that endotoxin (a component of gram-negative bacterial cell walls, also known as lipopolysaccharide or LPS) in the environment can modulate the risk for allergic disease. One study found that higher levels of endotoxin in a child’s mattress were associated with decreased risk of allergic sensitization and atopic asthma.34 The exact mechanism for this reduced atopic response is unknown but it fits with the understanding of the hygiene hypothesis, and in mouse studies, exposure to LPS reduces allergic disease (and asthma).34
Viral Infections
While essentially all children become infected with Respiratory Syncytial Virus (RSV) in the first two years of life only a small percentage develop severe bronchiolitis and require hospitalization.35,36 These children tend to be infected in the first 2–6 months of life and have been shown to have a markedly elevated risk for developing asthma (and allergic disease).35,36 Other studies have shown a similar risk with Metapneumovirus (MPV) and Rhinovirus (RV).37,38 RV, in fact, is a common respiratory virus, and the Childhood Origins of Asthma (COAST) study demonstrated that infection with RV imparts an increased risk of developing asthma.39 In fact, the risk of more significant symptoms correlated most with the specific clade (RV-C) of RV.40 The COAST study also suggested that the children who went on to develop asthma had become atopic before the viral infection.39 This is in contrast to the RSV studies, where most children developed allergic sensitization after the viral infection.36 As a result, it suggests that the mechanisms underlying development of asthma after the two different viral etiologies may be different.
The mechanism(s) through which these RNA respiratory viral infections early in life translate to asthma are not fully known. Interestingly, part of the antiviral immune response in humans is the production of IgE against the virus.36,41 Whether this IgE leads to exacerbation of airway disease during a viral infection is unknown (although, in at least one study, viral exacerbations of asthma were reduced when treating with anti-IgE).36,41 However, it does indicate that in humans there is a link between the Th2 mediated allergic response and the Th1 mediated antiviral immune response.36 This is an area of intensive research, and a recent review discusses possible immunologic mechanisms that may underlie the risk of asthma from early viral infections.36,42
Origins of Adult Asthma
Although patients with childhood asthma may have symptoms into adulthood, there are distinct phenotypes more common in adult onset disease (see Figure 1). These phenotypes may have implications for therapeutic interventions, so it is important to consider them when symptoms begin later in childhood or as an adult. Similar to children, adult patients with asthma often have increased atopy; however, obesity, Aspirin-exacerbated respiratory disease, occupational asthma, neutrophilic asthma, and COPD or other effects of exposure to cigarette smoke are more commonly phenotypes of asthma (or related diseases) that present in adults.
Obesity
Obesity is a significant public health problem in both adults and children. In a study by Schatz, et al, that included patients with severe or difficult to treat asthma, almost 60% of the adolescent and adult subjects were obese compared to approximately only 30% obesity in unaffected subjects.43 In fact, obesity-related asthma appears to have significant sex bias, as it is primarily seen in women. One meta-analysis of the relationship between obesity and asthma found a direct relationship between the odds of having asthma and weight status. The odds ratio for asthma in overweight subjects was 1.38, while obese patients had an even higher risk of disease with an odds ratio of 1.92.44 In addition to obese patients being at a higher risk for asthma and more severe disease, obese patients also demonstrate decreased responses to inhaled corticosteroids, alone or in combination with long-acting β-agonists.45,46 It is not surprising that these patients have a lower quality of life than non-obese asthmatics.47 Even the risk of hospitalization for asthma is 4-to 6-fold increased in obese patients.45,48 What underlies this relationship between obesity and asthma is not well understood. Obesity can be associated with an inflammatory state (usually related to TNF and IL6), and there is a relationship between alternatively activated macrophages and obesity (likely through leptin and adipose tissue).44,49 Alternatively activated macrophages also have been implicated in asthma; however, it is not clear if these immune alterations are the link between obesity and asthma. However, weight loss and antioxidants appear to have beneficial effects in this form of asthma.45
Aspirin-Exacerbated Respiratory Disease
Aspirin-exacerbated respiratory disease (AERD) consists of a constellation of diseases and symptoms that include asthma, recurrent sinus disease with nasal polyps, and sensitivity to aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) that inhibit cyclooxygenase-1. The pathogenesis of AERD is thought to be secondary to a dysregulation of leukotriene synthesis.50 In patients with AERD, respiratory symptoms occur when they ingest or inhale the offending medication. In a large meta-analysis performed Rajan, et al, the combined prevalence rate of AERD in asthmatics was 7.2% (95% CI, 5.26% to 9.03%).51 Patients with severe asthma had a combined prevalence rate nearly double that of all asthmatics (14.89% (95% CI, 6.48% to 23.29%)).51 The role of IgE mediated allergic sensitivity is not clear in AERD, but 30% of patients with AERD also have IgE to environmental allergens; clearly AERD can exist with or without underlying atopy.50 Unlike the childhood onset asthma phenotypes, AERD does not appear to be due to type 2 cytokine mediated disease – instead, as mentioned, it is dysregulation in lipid mediators. This is important, because aspirin desensitization can be curative for AERD. Why AERD is not more prevalent in children is unknown.
Occupational Asthma
A major difference between children and adults is the work environment. A significant portion of adult-onset asthma, up to 1 in 6 cases, can be considered job related.52 Occupational asthma may often be defined as asthma caused by a specific exposure at work. This may be due to workplace sensitizers or irritants in the specific environment.53 Symptoms can occur in people who have previously had a history of inflammatory lung disease or in those with new onset pulmonary disease. Though many patients find symptoms improve when they are away from work, the pulmonary inflammatory response may continue even when a person is not acutely exposed to the inciting agent. Many causes of occupational asthma have been described depending on the different types of workplaces. Inciting agents of occupational asthma are classified into high-molecular-weight (HMW) and low-molecular-weight (LMW) compounds. HMW compounds frequently are from biological sources such as wheat allergens or fungal enzymes, and often cause pulmonary symptoms through an IgE-dependent mechanism. LMW compounds such as diiocyanates, however, are likely to cause asthma symptoms through non–IgE-dependent pathways.53,54 So, unlike most of the other forms of asthma discussed in this review, occupational asthma contains both and atopic and nonatopic mechanisms of disease.
Neutrophil predominant asthma
One of the hallmarks of allergic asthma is the presence of eosinophils in the sputum and/or bronchoalveolar lavage. However, in adult patients with more severe disease, eosinophils are often lacking and there is an abundance of neutrophils.55 This severely obstructed disease is often referred to as neutrophilic asthma. What role the neutrophils play in the pathophysiology of the disease is not known. This form of asthma does not appear to be the same as “sudden death” asthma, where the presence of neutrophils at death is likely due to an acute infectious process. In animal models (and some human studies), the presence of neutrophils is often associated with IL-8 and IL-17 (often from Th17 cells) over-production.56 However, blockade of these cytokines (or their receptors) for asthma treatment in humans have not been very successful in clinical trials. In fact, due to the severity of their disease and relative lack of steroid responsiveness, neutrophilic asthma can be difficult to treat and is in need of new therapeutic approaches.55
Smoking and Asthma
According to the CDC and U.S. Department of Health and Human Services, cigarette smoking is the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths every year, or about 1 in 5 deaths.57 It is estimated that 37.8 million adults in the United States currently smoke cigarettes58 and more than 16 million Americans live with a smoking-related disease.57 Though there has been much research on the topic of the development of asthma in persons who smoke cigarettes, not all studies have shown a direct association.59 Regardless of whether there is a causative association, it is clear that more severe symptoms and increased morbidity and mortality are present in patients with asthma who smoke.60,61
Asthma and Chronic Obstructive Pulmonary Disease (COPD)
Over the past decade there have been more data suggesting that asthma and COPD might be two ends of a continuum of the same disease. These two illnesses are the most common obstructive airway diseases.62 As a result, a new term or diagnosis, “asthma–COPD overlap syndrome” (ACOS), has been proposed to classify people who have clinical features of both diseases.63 Given an unclear definition of ACOS, it is not surprising that the prevalence of this “overlap” syndrome is unknown. More studies are necessary to better define this possible diagnosis, as it will likely have important clinical ramifications on treatment and prognosis.64
Conclusion
Though at this time the diagnosis of asthma conjures a specific illness script to many clinicians, it is more likely a complex set of diseases in which airway inflammation is the key finding. The origins of the diseases are varied across age, environmental exposures, and co-morbidities of the patient. Endotypes and phenotypes (Figure 1) have begun to be used to explain the physical symptoms, pathophysiology, and response to treatment found in different patients. However, the mechanisms that underlie these different forms of asthma are not clear, and that has hindered the ability to target specific therapies to patients. This is especially an issue in terms of adult neutrophilic asthma, where no clearly efficacious therapy options currently exist. By further defining the “sub-types” of asthma, clinicians should be able to work with patients and their families to determine best treatment options. As we look towards an era of personalized precision medicine, further understanding of the origins of asthma and how the underlying mechanisms effect treatment and prognosis will become even more critical.
Acknowledgments
Funding: This work was funded in part by the NIH HL087778 and AI120655, and the Research Institute at Nationwide Children’s Hospital (all to MHG).
References
- 1.Global Initiative for Asthma. A Pocket Guide for Health Professionals Updated 2018. Available from: http://ginasthma.org/gina-reports/.
- 2.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120 (5 suppl): S94–S138. [DOI] [PubMed] [Google Scholar]
- 3.Green RH, Brightling CE, Bradding P. The reclassification of asthma based on subphenotypes. Curr Opin Allergy Clin Immunol. 2007;7:43–45. [DOI] [PubMed] [Google Scholar]
- 4.Burbank AJ, Sood AK, Kesic MJ, Peden DB, Hernandez ML . Environmental determinants of allergy and asthma in early life. J Allergy Clin Immunol. 2017. July;140(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest 2001;119:1329–36. [DOI] [PubMed] [Google Scholar]
- 6.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008;178: 218–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holgate S and Thomas M. Asthma In: O’Hehir R, Holgate S, Sheikh A eds. Middleton’s Allergy Essentials. 1st ed. Philadelphia: Elsevier; 2016. [Google Scholar]
- 8.Stokes JR and Casale TB. Characteristics of Asthma Endotypes: Implications for Therapy. Ann Allergy Asthma Immunol. 2016;117:121–25. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J Allergy Clin Immunol. 2015;136:531–545. [DOI] [PubMed] [Google Scholar]
- 10.Gelfand EW. et al. The other side of asthma: Steroid-refractory disease in the absence of TH2-mediated inflammation. J Allergy Clin Immunol 2015;135(5):1196–1198. [DOI] [PubMed] [Google Scholar]
- 11.Hill DA, Spergel JM. The atopic march: Critical evidence and clinical relevance. Ann Allergy Asthma Immunol. 2018. February;120(2):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gustafsson D, Sjoberg O, Foucard T Development of allergies and asthma in infants and young children with atopic dermatitis: a prospective follow-up to 7 years of age. Allergy. 2000;55:240–245. [DOI] [PubMed] [Google Scholar]
- 13.Pearce N, Ait-Khaled N, Beasley R et al. , Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC).Thorax. 2007;62:758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strachan DP, Butland BK, Anderson HR Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ. 1996;312:1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. [DOI] [PubMed] [Google Scholar]
- 16.Hill DA, Grundmeier RW, Ram G, Spergel JM The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leynaert B, Neukirch C, Kony S et al. , Association between asthma and rhinitis according to atopic sensitization in a population-based study. J Allergy Clin Immunol. 2004;113:86–93. [DOI] [PubMed] [Google Scholar]
- 18.Carroll WD, Lenney W, Child F, Strange RC, Jones PW, Whyte MK et al. , Asthma severity and atopy: how clear is the relationship?. Arch Dis Child. 2006;91:405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai TR, Vonk JM, Postma DS, Boezen HM Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30:452–456. [DOI] [PubMed] [Google Scholar]
- 20.Rubner FJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE et al. , Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol. 2017;139:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takkouche B, Gonzalez-Barcala FJ, Etminan M, Fitzgerald M Exposure to furry pets and the risk of asthma and allergic rhinitis: a meta-analysis. Allergy. 2008;63:857–864. [DOI] [PubMed] [Google Scholar]
- 22.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–507 [DOI] [PubMed] [Google Scholar]
- 23.Do DC, Zhao Y, Gao P. Cockroach Allergen Exposure and Risk of Asthma. Allergy. 2016;71(4):463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunol. 2010;125(3):540–544. [DOI] [PubMed] [Google Scholar]
- 25.von Mutius E The microbial environment and its influence on asthma prevention in early life J Allergy Clin Immuno 2016; 137 (3):680–689. [DOI] [PubMed] [Google Scholar]
- 26.Liu AH. Hygiene theory and allergy and asthma prevention Paediatr Perinat Epidemiol. 2007. November;21 Suppl 3:2–7. [DOI] [PubMed] [Google Scholar]
- 27.Abrahamsson TR, Jakobsson HE, Anderson AF, Bjorksten B, Enstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clini Immnol 2012;129:434–440,el-2. [DOI] [PubMed] [Google Scholar]
- 28.Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001. January;107(1):129–34. [DOI] [PubMed] [Google Scholar]
- 29.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW, Wegienka G, Boushey HA, Ownby DR, Zoratti EM, Levin AM, Johnson CC, Lynch SV. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. 2016;22(10):1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(26):11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kero J, Gissler M, Grönlund MM, Kero P, Koskinen P, Hemminki E, Isolauri E.. Mode of delivery and asthma -- is there a connection?. Pediatr Res. 2002. July;52(1):6–11. [DOI] [PubMed] [Google Scholar]
- 32.Neu J, Rushing J. Cesarean versus Vaginal Delivery: Long term infant outcomes and the Hygiene Hypothesis. Clinics in perinatology. 2011;38(2):321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arrieta MC, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Science Translational Medicine 2015; 7 (30); 307ra152. [DOI] [PubMed] [Google Scholar]
- 34.Braun-Fahrländer C,Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Exposure to Endotoxin and Its Relation to Asthma in School-Age Children. N Engl J Med 2002; 347:869–77. [DOI] [PubMed] [Google Scholar]
- 35.Psarras S, Papadopoulos NG and Johnston SL, Pathogenesis of respiratory syncytial virus bronchiolitis-related wheezing. Paediatr. Respir. Rev 2004. 5: S179–S184 [DOI] [PubMed] [Google Scholar]
- 36.Martorano LM, Grayson MH. Respiratory viral infections and atopic development: From possible mechanisms to advances in treatment. Eur J Immunol. 2018. March;48(3):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coverstone AM. Wilson B, Burgdorf D, et al. Recurrent wheezing in children following human metapneumovirus infection. J Allergy Clin Immunol. 2018. March 2 pii: S0091–6749(18)30293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 2008;178:667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson DJ, Gern JE, Lemanske RF. The Contributions of Allergic Sensitization and Respiratory Pathogens to Asthma Inception. The Journal of allergy and clinical immunology. 2016;137(3):659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bizzintino J, Lee W-M, Laing I, et al. Association between human rhinovirus C and severity of acute asthma in children. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2011;37(5):1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tam JS, Jackson WT, Hunter D, Proud D, Grayson MH. Rhinovirus Specific IgE Can Be Detected in Human Sera. The Journal of allergy and clinical immunology. 2013;132(5):1241–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheung DS, Siqua JA, Simpson PM. Cysteinyl leukotriene receptor 1 expression identifies a subset of neutrophils during the antiviral response that contributes to postviral atopic airway disease. J Allergy Clin Immunol. 2017. December 18 pii: S0091–6749(17)32913–5. doi: 10.1016/j.jaci.2017.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE et al. , Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133:1549–1556. [DOI] [PubMed] [Google Scholar]
- 44.Beuther DA, Sutherland ER Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol 2018;141(4): 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boulet LP, Franssen E Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med. 2007;101:2240–2247. [DOI] [PubMed] [Google Scholar]
- 47.Vortmann M, Eisner MD BMI and health status among adults with asthma. Obesity (Silver Spring). 2008;16:146–152. [DOI] [PubMed] [Google Scholar]
- 48.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC et al. , Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127:1486–1493. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med 2016;4:574–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ledford DK, Wenzel SE, Lockey RF. Aspirin or Other Nonsteroidal Inflammatory Agent Exacerbated Asthma. J Allergy Clin Immunol Pract, Volume 2, Issue 6, 653–657.e1 [DOI] [PubMed] [Google Scholar]
- 51.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol 135:676–681.e1, 2015. [DOI] [PubMed] [Google Scholar]
- 52.Malo JL, Vandenplas O. Definitions and classification of work-related asthma. Immunol Allergy Clin North Am, 2011. 31(4): p. 645–62. [DOI] [PubMed] [Google Scholar]
- 53.Doa A, Bernstein DI. Occupational exposure and asthma. Ann Allergy Asthma Immunol. 2018. March 23 pii: S1081–1206(18)30224–2. doi: 10.1016/j.anai.2018.03.026. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 54.Maestrelli P, Boschetto P, Fabbri LM, Mapp CE. Mechanisms of occupational asthma. J Allergy Clin Immunol 2009;123 (3):531–542 [DOI] [PubMed] [Google Scholar]
- 55.Kamath AV, Pavord ID, Ruparelia PR, et al. Is the neutrophil the key effector cell in severe asthma? Thorax 2005;60:529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y-H, Wills-Karp M. The Potential Role of IL-17 in Severe Asthma. Current allergy and asthma reports. 2011;11(5):388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014 [Google Scholar]
- 58.Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults—United States, 2016. Morbidity and Mortality Weekly Report 2018;67(2):53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomson NC, Chaudhuri R, Livingston E. Asthma and cigarette smoking. European Respiratory Journal 2004: 24: 822–833. [DOI] [PubMed] [Google Scholar]
- 60.Althuis M, Sexton M, Prybylski D. Cigarette smoking and asthma symptom severity among adult asthmatics.J Asthma 1999;36:257–264 [DOI] [PubMed] [Google Scholar]
- 61.Siroux V, Pin I, Oryszcyn MP, Le Moual N, Kauffmann F. Relationships of active smoking to asthma and asthma severity in the EGEA study. Eur Respir J 2000;15:470–47. [DOI] [PubMed] [Google Scholar]
- 62.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 2009;64:728–35. [DOI] [PubMed] [Google Scholar]
- 63.Global Initiative for Asthma. Asthma, COPD and asthma-COPD overlap syndrome (ACOS). Global Initiative for Asthma website. http://www.ginasthma.org/local/uploads/files/ACOS_2015.pdf.
- 64.Barnes P Asthma-COPD Overlap. CHEST 2016; 149(1):7–8 [DOI] [PubMed] [Google Scholar]