Abstract
Malaria parasites remodel their host erythrocytes to gain nutrients and avoid the immune system. Host erythrocytes are modified by hundreds of effector proteins exported from the parasite into the host cell. Protein export is mediated by the PTEX translocon comprising five core components of which EXP2 is considered to form the putative pore that spans the vacuole membrane enveloping the parasite within its erythrocyte. To explore the function and importance of EXP2 for parasite survival in the asexual blood stage of Plasmodium falciparum we inducibly knocked down the expression of EXP2. Reduction in EXP2 expression strongly reduced parasite growth proportional to the degree of protein knockdown and tended to stall development about half way through the asexual cell cycle. Once the knockdown inducer was removed and EXP2 expression restored, parasite growth recovered dependent upon the length and degree of knockdown. To establish EXP2 function and hence the basis for growth reduction, the trafficking of an exported protein was monitored following EXP2 knockdown. This resulted in severe attenuation of protein export and is consistent with EXP2, and PTEX in general, being the conduit for export of proteins into the host compartment.
Introduction
Almost half the world’s population is at risk of malaria, the disease caused by infection with Plasmodium spp. parasites. In 2016 there was an estimated 216 million cases reported, resulting in 445,000 deaths, mostly of children under 5 [1]. Plasmodium parasites invade erythrocytes and remodel them to obtain supplementary nutrition from the blood plasma and to evade the immune system. Symptomatic malaria disease is caused by intracellular blood stage parasites which are enveloped in a parasitophorous vacuole membrane (PVM) within the erythrocyte. Residing on the PVM is an essential protein translocon called PTEX (Plasmodium translocon for exported proteins) [2]. PTEX appears to be responsible for exporting hundreds of parasite effector proteins across the PVM into the host erythrocyte where they perform their functions [3, 4].
PTEX consists of five core components, HSP101, PTEX150, EXP2, TRX2 and PTEX88 [2]. Of the core PTEX components only two have homology to other known proteins outside the Plasmodium genus. The first is HSP101, a AAA+ heat shock protein chaperone which is predicted to form a hexameric structure to unfold proteins for export [2, 5]. The second is TRX2, a thioredoxin-like protein possibly involved in regulating PTEX or reducing the disulfide bonds in cargo proteins prior to export. TRX2 is not essential for blood stage growth in the murine malaria species Plasmodium berghei since its gene can be deleted, however its loss reduces export efficiency and virulence [4, 6, 7]. PTEX150 bears no obvious homology to other proteins, and deletion mutants indicate it is essential and probably responsible for maintaining the structural integrity of PTEX [8]. PTEX88 is a predicted β-propeller protein and appears to be involved in parasite sequestration as knockout or knockdown of PTEX88 in P. berghei resulted in reduced sequestration and virulence [9, 10], and an inducible knockdown in P. falciparum resulted in reduced binding to the endothelial receptor CD36 [10].
The final core PTEX protein is EXP2 which lacks predicted transmembrane spanning domains typical of membrane pores, yet it is the most membrane associated protein of the PTEX complex [2, 11, 12]. Very recently a partial structure of the PTEX complex was solved based on cryo-EM images derived from purified parasites complexes [13]. This indicated seven EXP2 protomers form a funnel-shaped channel in the PVM projecting into the parasitophorous vacuole (PV) lumen. A HSP101 hexamer is anchored via its C-terminus to the EXP2 funnel with seven PTEX150 protomers nestled between the EXP2 protomers helping to form a protein-translocating channel through the center of the structure. Cycles of HSP101 allosteric movements powered by ATP hydrolysis appear to push unfolded proteins through the channel into the erythrocyte via a ratchet mechanism [13].
In addition to a full size PTEX complex of >1200 kDa, we have shown EXP2 forms homo-oligomers of approximately 600 and 700 kDa in size by blue native polyacrylamide gel electrophoresis raising the question of what the smaller forms could be doing [12]. EXP2 could form both a large protein exporting pore, and a smaller nutrient importing pore. Small solutes <1400 Daltons can cross the PVM, and EXP2 can complement a knockdown of another protein, GRA17, predicted to form a nutrient import pore in the related apicomplexan Toxoplamsa gondii [14, 15, 16, 17]. Strongly supporting EXP2’s role as both a protein translocon and as a nutrient channel are recently published patch-clamp experiments with EXP2 knockdown parasites indicating there is reduced conductance of the PVM upon EXP2 knockdown [14].
Export of effector proteins can be inhibited by disrupting the PTEX complex by inducibly knocking down expression of HSP101, PTEX150 and EXP2 [4, 14] or by disrupting the oligomerisation of HSP101 [3]. Loss of PTEX function leads to rapid parasite arrest within a cell cycle. Prior to export, proteins require unfolding and inhibiting this appears to impede PTEX by preventing the export of other essential effector proteins [5, 15, 16].
To explore the role of EXP2 in the asexual blood stages of P. falciparum we have inducibly knocked down EXP2 expression. We show that EXP2 knockdown is detrimental for parasite growth and the extent of growth arrest is correlated with the degree of knockdown. Upon restoration of EXP2 expression parasite growth can partially recover dependent on the length and strength of knockdown. Upon EXP2 knockdown, growth arrest was probably due to the inhibition of export of essential effector proteins since the export of a protein marker was also curtailed.
Materials and methods
Plasmid construction
From 100 ng of 3D7 P. falciparum genomic DNA, the 3’ end of the EXP2 coding region was amplified with forward primer EXP2_1F: AGGAGATCTGGTCACGTATGTGGTGGGTA and reverse primer EXP2_2R A’: CTTATACTGCAGCTTCTTTATTTTCATCTTTTTTTTCATTTTTAAATAAATCTCCACTGGCA. After digestion with BglII and PstI the exp2 DNA fragment was ligated into pPTEX150_HAglmS after the ptex150 flank was removed by digestion with the same enzymes to produce pEXP2-HAglmS.
Parasite culture and transfection
P. falciparum was cultured in human RBCs (Australian Red Cross Blood Bank, blood-group O+) at 4% haematocrit (HCT) in AlbuMaxII media (RPMI-HEPES, 0.5% AlbuMaxII [GIBCO], 0.2% NaHCO3, 0.37 mM hypoxanthine) at 37°C as described previously [17]. 100 μg of pEXP2-HAglmS was electroporated into erythrocytes in which P. falciparum strain 3D7 was subsequently cultured [18]. Transfected parasites were selected with 2.5 nM WR99210 and were cycled on and off the drug to select for integration into the exp2 locus. Once western blot validation with anti-HA IgG indicated the exp2 locus had been appended with the HAglmS construct, clonal parasite lines were isolated by limiting dilution. Successful integration was validated by performing diagnostic PCRs with genomic DNA isolated from each clonal parasite line using the primers: A TGCAGAAACAACTTTGCCACA; A’ TGGCATCTTCTTCTTCAACGGT; B’ TCCTTACGGCTGTGATCTGC.
EXP2 knockdown and western blotting
To knockdown protein expression in EXP2-HAglmS parasites 1M glucosamine (Sigma) was added at the desired concentration to 5 mL of 1% synchronous ring stage parasites in 4% hematocrit. Parasites were grown for 1 cell cycle (~48 h) and then harvested by centrifugation. The cell pellet was treated with 0.09% saponin in PBS with Complete Protease Cocktail Inhibitors (Roche) to remove the haemoglobin. Cell proteins were solubilised in reducing SDS sample buffer and fractionated on 4–12% acrylamide Bis-Tris gels (Invitrogen) and transferred via iBlot to nitrocellulose membranes (Invitrogen). Membranes were blocked in 1% casein and probed with mouse EXP2 monoclonal, rabbit anti-ERC IgG and chicken anti-HA IgY (Abcam) as per [4]. The membranes were then probed with 700 or 800 nm goat anti-rabbit or goat anti-mouse secondary IgGs (Rockland) and goat anti-chicken IgG-HRP conjugate (Abcam). Probed blots were imaged with a Li-Cor Odyssey InfraRed system and densitometry was performed with Li-Cor Image Studio software.
EXP2-HA knockdown growth and recovery assays
For parasite growth and recovery assays 5 mL ring stage parasites at 4% hematocrit and 3% parasitemia were treated with the desired GlcN concentration for 1 day. Thin blood smears were then made and 3 x 100 μL aliquots of culture removed for LDH assays. The remaining culture was treated with 0.09% saponin with Complete Protease Cocktail Inhibitors (Roche) to remove the haemoglobin. Parasites to be sampled 2 and 3 days after GlcN treatment were set up as above but diluted 1/5 in 4% hematocrit and those to be sampled after 5 days were diluted 1/25. At days 2 and 3 post GlcN treatment samples were removed for analysis as above. Parasites that were to be grown for 5 days were fed on days 2 and 3 with fresh media and washed to remove GlcN or fresh GlcN was added back. After completion of the time course lactate dehydrogenase (LDH) assays that correlate with parasitaemia were performed as described previously [19] and absorbance measured at 650 nm on a Multiskan GO (Thermo Scientific). The absorbance values were multiplied by culture dilution factors to derive cumulative growth levels at OD 650 nm. Thin blood smears were stained with Giemsa to visualize parasite cell cycle stages. Western blots of EXP2 and ERC levels were performed as described above.
Microscopy
IFAs were performed as described previously [4]. Briefly, parasites were synchronized with sorbitol and heparin to within a 4 h window and plated into 6 well plates at 0.5% parasitaemia and 2% HCT. GlcN was added to the ring stage and one cell cycle later the parasites were collected at approximately 8 hpi and were attached to poly-L-lysine coated slides. The cells were fixed in 4% paraformaldehyde and 0.0075% glutaraldehyde [20], followed by quenching and permeabilisation with 125 mM glycine pH7, 0.05% TX-100 in PBS. Primary antibodies were added at 1:100 in 3% BSA for 4 h. Secondary antibodies (AlexaFluor 488 and 594) were added at 1:2000 for 2 h. Imaging was performed on a Zeiss Axio Observer ZV1 and with the same exposure times used for all parasites for each channel. The cells selected for counting were acquired based solely on their nuclear staining and not their SBP1 staining so as to not bias the sampling. To accurately quantify the degree of export, only SBP1 foci that were located within the erythrocyte and not within the parasite as delineated by nuclear and PVM staining were counted. To do this, FIJI software was used to draw a circle around the erythrocyte to select it and then a second circle was drawn inside and around the parasite to exclude it. In the SBP1 channel, positive staining foci in the erythrocyte above a fixed noise tolerance were automatically counted using the ‘Find Maxima’ function. For counting the ratio of parasite stages, GlcN was added as above and at the appropriate times post GlcN addition, dried blood smears were fixed in 100% methanol for 5 min and stained in 10% Giemsa in water for 30 min. The parasite stages were then counted and photographed.
Results
Inducible reduction of EXP2 expression reduces parasite growth
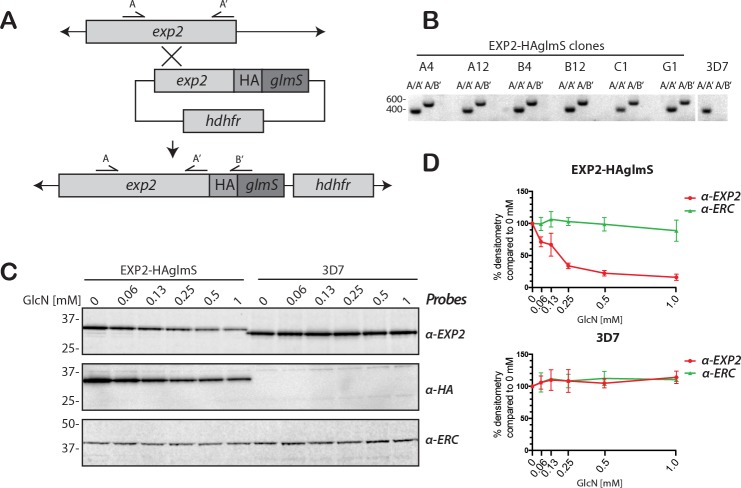
EXP2 is essential for the survival of P. falciparum in in vitro culture [2, 14, 21, 22]. Given it was not possible to infer EXP2 function in exp2 null parasites, an alternative approach was adopted where an inducible knockdown system based on the glmS ribozyme was employed [4, 23]. In this system the 3’ UTR of exp2 was substituted with a heterologous 3' UTR containing glmS (Fig 1A). A haemagglutinin (HA) epitope tag was also appended to the 3' end of the exp2 coding sequence for antibody-based detection of the protein. In this knockdown system the glmS ribozyme self cleaves the mRNA upon addition of glucosamine (GlcN) to the parasite culture, leading to removal of the poly-A tail, destabilization of the mRNA and subsequent reduction in protein production. The EXP2-HAglmS construct was introduced into the exp2 locus of the wildtype 3D7 parasite line by homologous recombination (Fig 1A) [24]. Clonal populations of the EXP2-HAglmS parasites were selected by limiting dilution and integration was confirmed by PCR in six clones (Fig 1B). Protein knockdown and growth was assessed in clones A12, B12 and G1 and no difference was observed between the lines so all subsequent assays were performed with clone B12.
Fig 1. Modification of the exp2 locus with a glmS ribozyme permits knockdown of EXP2 expression levels.
(A) Schematic showing HAglmS tag integrated into exp2 locus. (B) Using the indicated primers and genomic DNA template from wildtype 3D7 and six transfected clonal populations, correct 3' recombination leading to the HA-glmS tagging of exp2 locus was confirmed. See methods for primer sequences. (C) GlcN dependent knockdown of EXP2 in EXP2-HAglmS ring stage parasites but not in 3D7 parasites after one cell cycle of GlcN treatment. Size shift in EXP2 indicates HA tagging confirmed with a HA-specific antibody. (D) Densitometry of EXP2, and ERC loading control from three western blot replicates of GlcN treated EXP2-HAglmS and 3D7 ring stage parasites. Protein densitometry is shown relative to 0 mM GlcN treated parasites normalised to 100%.
To determine the effect of GlcN addition on EXP2 expression, GlcN was added to ring stage parasites at approximately 12–16 hours post invasion (hpi), before expression of EXP2 peaks at approximately 20–30 hpi [25, 26]. Parasite samples were harvested at early ring stage of the next cycle approximately 48 h later, to allow time for the level of EXP2-HA to become depleted [12]. Subsequent western blots were first probed with a mouse monoclonal for EXP2 that labeled a single band in wildtype 3D7 parasites of about 30.5 kDa predicted for the mature EXP2 protein (Fig 1C) [2]. A slightly larger band was labeled in EXP2-HAglmS parasites consistent with the addition of the small HA epitope tag (Fig 1C). This was confirmed with anti-HA chicken IgY that labeled a single similarly sized band in the EXP2-HAglmS parasites but not 3D7 (Fig 1C). A cross-reactive band of 40 kDa was observed in both parasites that was not therefore specific for the HA epitope (S1 Fig). The blots were also probed for ERC (endoplasmic reticulum-located, calcium-binding protein) to ensure equal protein loading [27]. Examination of the EXP2 and HA blots indicated that GlcN treatment led to a concentration dependent reduction in EXP2 expression in EXP2-HAglmS parasites but not in wildtype 3D7 parasites (Fig 1D). Quantification of three western blots showed a mean reduction of 84.0% (SD, 4.8%) in EXP2 expression with 1 mM GlcN, similar to that observed for PTEX150-HAglmS [4]. Concentrations above 1 mM GlcN did not further reduce protein expression and with a lower concentration of 0.5 mM GlcN, EXP2 knockdown showed a mean reduction of 77.7% (SD, 4.2%).
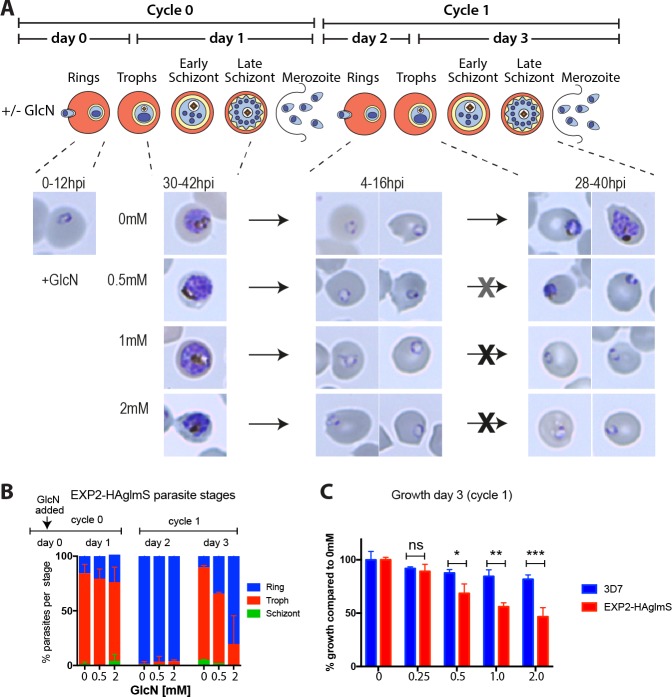
It was expected that a reduction in EXP2 levels would have major effects on the growth and protein export of the parasites on the basis that reducing the function of the other two main PTEX components, PTEX150 and HSP101, had a significant effect [3, 4]. To investigate this, GlcN was added to ring stage EXP2-HAglmS parasites (cycle 0, Fig 2A). Giemsa stained images of the parasites indicated that maturation into trophozoites in cycle 0 (day 1) was not adversely affected (Fig 2A and 2B). Invasion and the subsequent presence of rings in the following cycle 1, (day 2) was also not greatly reduced presumably because sufficient EXP2-HA remained to satisfy parasite requirements (Fig 2A and 2B). However by the trophozoite stage of cycle 1 (day 3), 2 mM GlcN had caused parasite growth to arrest at rings. Treatment with 0.5 mM GlcN appeared to have an intermediate effect (Fig 2A and 2B). As previous counts of GlcN treated 3D7 parasites indicated no deleterious effects after one cell cycle they were not included here [4].
Fig 2. EXP2 knockdown results in reduced capacity of parasites to progress past the ring stage in the cycle after glucosamine addition.
(A) Schematic showing glucosamine (GlcN) addition and EXP2 synthesis. After addition of GlcN in cycle 0 rings, Giemsa smears show that in cycle 1 parasites enter ring stage but appear to stall at the early trophozoite stage (representative data of n = 2). (B) Percent of each parasite stage following addition of GlcN to EXP2-HAglmS ring stage parasites at day 0, cycle 0. Giemsa smears of parasite stages were quantified every day for three days (bars indicate mean, SD, representative data of n = 2). (C) Growth assay at day 3 (cycle 1) by lactate dehydrogenase activity (LDH) with increasing GlcN concentrations (n = 4). GlcN was added to rings in cycle 0 and LDH activity was measured in trophozoites in cycle 1. Mean, SD are indicated. Two-step analysis using an unpaired T-test with False Discovery Rate approach set to 1% method of Benjamini, Krieger and Yekutieli *p<0.01, **p<0.001, ***p<0.0001.
Next, parasite proliferation using lactate dehydrogenase (LDH) activity as a marker of biomass was measured and indicated that GlcN-induced knockdown of EXP2 proportionally reduced proliferation after 3 days (cycle 1) (Fig 2C). Growth inhibition in EXP2-HAglmS parasites was significantly reduced compared to control 3D7 parasites at all GlcN concentrations except for 0.25 mM GlcN. Importantly, GlcN did not significantly reduce the growth of wildtype 3D7 relative to untreated parasites up to 1mM GlcN concentration, indicating it was the knockdown of EXP2 expression that was restricting parasite growth (Fig 2C). There was some toxicity associated with 2 mM GlcN treatment as the growth of 3D7 parasites was slightly but significantly reduced by 18.2% (SD 4.4%), T-test p = 0.006. This was quite low however compared to 2 mM GlcN treated EXP2-HAglmS parasites whose growth was reduced by 53.3% (SD 4.4%), T-test p < 0.0001.
Restoration of EXP2-HA expression and parasite growth recovery depends on the degree and length of knockdown
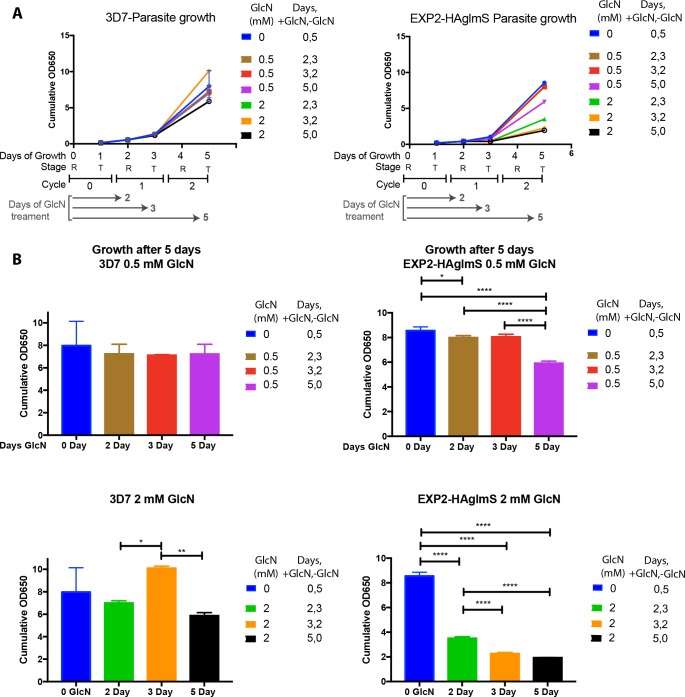
To further explore the relationship between EXP2 levels and parasite growth, 0.5 and 2 mM GlcN that had been added to EXP2-HAglmS and 3D7 rings in cycle 0 was washed out in cycle 1 at rings (2 day GlcN treatment) or trophozoites (3 day GlcN treatment). The parasites were then further grown until cycle 2 (day 5) and compared with untreated parasites (0 mM GlcN) and with parasites continuously treated with GlcN for 5 days. Parasite samples from days 1, 2, 3 and 5 were retained for analysis and LDH assays indicated that in parasites treated with 0.5 mM GlcN for 5 days significant growth inhibition was only observed for EXP2-HAglmS. For parasites in which 0.5 mM GlcN was removed after 2 and 3 days, growth almost fully recovered by day 5 (Fig 3A and 3B). For 2 mM GlcN, the 5 day treatment strongly reduced the growth of EXP2-HAglmS and weakly reduced growth of 3D7 (Fig 3A and 3B). In EXP2-HAglmS parasites, removal of 2 mM GlcN after 2 and 3 days allowed partial growth recovery especially after 2 days (Fig 3A and 3B). For 3D7, the toxicity of 2 mM GlcN was less apparent if it was removed after 2 and especially 3 days (Fig 3A and 3B). Whether the enhancement of growth observed in 3D7 after 3 days GlcN treatment was genuine or due to experimental variability was not investigated further because in comparison, the effects of GlcN treatment on EXP2-HAglmS were much stronger and significant.
Fig 3. Glucosamine treatment greatly reduces the growth of EXP2-HAglmS parasites relative to 3D7 control parasites.
(A) GlcN was added to 3% ring stage parasites to 0, 0.5 and 2 mM on day 0, cell cycle 0. To prevent the parasites from over-growing those that were to be harvested at cycle 1 and 2 were diluted 1/5 and 1/25, respectively. The parasites were grown for 5 days (2 cell cycles) with samples removed for growth and western blot analysis on day 1, cycle 0; day 2 and day 3, cycle 1; and day 5, cycle 2. To assess recovery of growth after EXP2 knockdown, some of the parasites were only treated with GlcN for 2 and 3 days after which the GlcN was removed and growth continued until day 5. ‘Days, +GlcN,-GlcN’ indicates the number of days ‘on’ GlcN and ‘off’ GlcN. To quantify growth, parasite lactate dehydrogenase (LDH) activity of 3 technical replicates was measured at each time point. The LDH values (OD 650 nm) were multiplied by the parasite dilution factor to produce a cumulative measure of growth. (B) Cumulative OD650 values taken from (A) at day 5 indicate that growth of 3D7 is not significantly reduced by 0.5 mM GlcN treatment. 3D7 growth in 2 mM GlcN is reduced but not significantly so from untreated 3D7. Conversely, continuous treatment of EXP2-HAglmS for 5 days with 0.5 and 2 mM GlcN significantly reduces growth. Treating parasites with GlcN for only 2 and 3 days allowed full recovery and partial recovery for 0.5 and 2 mM GlcN, respectively. Tukey’s multiple comparison, *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
The EXP2-HAglmS parasites were also monitored by microscopy throughout the knockdown and recovery period. In 0.5 mM GlcN the parasites appeared to lag behind the untreated parasites so that by day 5 the trophozoite stage parasites were smaller than the untreated trophozoites and younger rings stages were also present (S2A Fig). By day 5 the growth lag was less obvious if the GlcN had been removed at earlier cycles. Microscopy of the 2 mM GlcN treated parasites indicated their progression through the cell cycle was greatly delayed and treatment for 3 days probably lead to some parasite death due to the appearance of small dark pyknotic forms (S2A Fig). It has recently been noted that knockdown of EXP2 and HSP101 causes the PVM to develop protrusions which extend into the erythrocyte [14]. We also observed these protrusions in EXP2-HAglmS trophozoites after 3 and 4 days treatment with 2 mM GlcN treatment but not in rings or in 0.5 mM GlcN treated or in untreated parasites (S2B Fig).
Western blot analysis was next performed on EXP2-HAglmS to determine how protein levels responded during knockdown and recovery. A second 5 day knockdown and recovery assay was performed and the LDH growth results were similar as before (S3A and S3B Fig). Western blots of parasites collected after GlcN treatment indicated there was strong GlcN-dependent knockdown of EXP2-HA from 1 to 5 days of treatment (S3C and S3D Fig). In contrast, the levels of the loading control ERC remained relatively constant each day in 0.5 mM GlcN treated parasites. The removal of 0.5 mM GlcN after 2 and 3 days treatment permitted strong recovery of the expression of EXP2-HA. In parasites treated with 2 mM GlcN, EXP2-HA expression was strongly knocked down after one day. ERC expression however, did not decline until day 3 when both LDH and microscopy of parasites indicated growth arrest began to occur (S3C and S3D Fig). Removal of 2 mM GlcN on Day 2 allowed partial recovery of EXP2-HA and ERC expression. However, when removal of 2 mM GlcN was delayed until day 3, there was poor recovery of protein expression coinciding with microscopy observations that parasites had begun to die by this stage (S2A Fig and S3C and S3D Fig).
Knockdown of EXP2-HA reduced export of the skeleton binding protein 1 marker protein
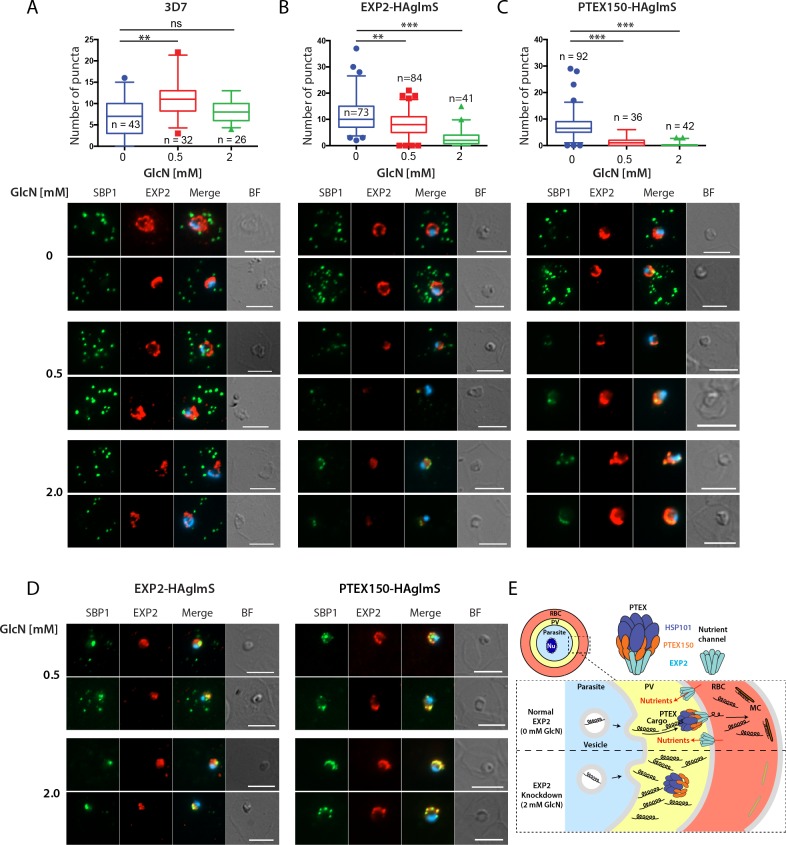
In light of EXP2's putative PTEX pore function we expected that the growth arrest observed upon reduction of EXP2 levels would be due to the parasite's inability to export critical proteins into the erythrocyte. To determine if protein export in the EXP2 knockdown parasites was curtailed we performed microscopy on skeleton binding protein 1 (SBP1), an early exported protein, in ring stage parasites before growth arrested. Once exported, SBP1 accumulates at Maurer's clefts which are parasite-formed vesicular structures that play a role in PfEMP1 export [28, 29]. Immunofluorescence microscopy of 3D7 parasites indicated there were several bright, punctate SBP-labeled structures in the erythrocyte cytoplasm of each cell (Fig 4A). These were automatically counted using FIJI software and it was found that GlcN treatment did not reduce the average number of SBP1 puncta although their numbers did vary from one treatment to the next possibly due to the numbers of puncta in focus during image acquisition.
Fig 4. Knockdown of PTEX proteins EXP2 and PTEX150 reduces protein export into host erythrocytes.
Export of Maurer’s cleft SBP1 protein after one cycle of GlcN treatment at 8 hpi rings by widefield microscopy in (A) 3D7, (B) EXP2-HAglmS and (C) PTEX150-HAglmS parasites. (Top) Quantitation of the number of SBP1 puncta in the parasites from n = number of individual cells counted. Kruskall-Wallis test used *p<0.01, **p<0.005, *** p < 0.0001. (Bottom) Representative immunofluorescence images showing reduced export of SBP1 structures after treatment of parasites with 0, 0.5 and 2 mM GlcN. Parasites were probed with rabbit anti-SBP1 serum (green), EXP2 mouse monoclonal IgG (red) and merged with DAPI DNA stain (blue). BF, brightfield. The brightness and contrast for SBP1 and EXP2 were equally adjusted for each image based on 3D7, 0 mM GlcN. (D) Images of GlcN-treated EXP2-HAglmS and PTEX150-HAglmS parasites in which the SBP1 and EXP2 signal has been enhanced. All size bars = 5 μm. (E) Diagram showing how knockdown of EXP2 blocks protein export. PTEX structure derived from Ho et al. (2018)[13]. RBC, red blood cell; PV, parasitophorous vacuole; Nu, nucleus; MC, Maurer’s cleft.
The exposure times required to produce satisfactory images of the untreated 3D7 parasites were used for all subsequent imaging and likewise all brightness and contrast settings were identically adjusted for the SBP1 and EXP2 fluorescence channels. Imaging of EXP2-HAglmS parasites indicated that EXP2 levels were markedly reduced following GlcN treatment (Fig 4B). Furthermore the number and brightness of SBP1 puncta was also reduced particularly in the 2 mM GlcN treatment. The observed puncta tended to be clustered around the periphery of the parasite and were not exported (Fig 4B). To determine if SBP1 might still be exported but not visible due to reduced expression the brightness and contrast were increased for SBP1 and EXP2 and this revealed strong trapping of SBP1 at the parasite periphery overlapping with residual EXP2 in 2 mM GlcN (Fig 4D). In 0.5 mM GlcN there was also SBP1 trapping but some export was observed.
Although we have previously reported GlcN mediated export trapping in PTEX150-HAglmS parasites we repeated this here for comparison with EXP2-HAglmS [4]. GlcN treatment of PTEX150-HAglmS parasites did not reduce EXP2 expression as anticipated but did decrease the intensity of SBP1 puncta and caused them to overlap with EXP2 around the parasite periphery (Fig 4C). Enhancement of the SBP1 signal revealed that export of virtually all of this protein was blocked. Casual observation of the both parasite lines suggested that export was more efficiently blocked in PTEX150-HAglmS compared to EXP2-HAglmS particularly in 0.5 mM GlcN. This was supported by the SBP1 puncta counts that indicated a stronger reduction in export in PTEX150-HAglmS (Fig 4B and 4C).
Discussion
To investigate the role of EXP2 in the PTEX protein export complex we attempted to knockdown EXP2 and examine subsequent phenotypes. Our knockdown strategy appended a HA tag and glmS ribozyme onto EXP2 and reduced its expression by up to 84% following treatment with 1 mM GlcN. This appeared to arrest parasite growth in late rings one cycle after adding GlcN to degrade the protein’s mRNA. The EXP2-HA knockdown parasites export SBP1 less efficiently than parasites with normal levels of EXP2 suggesting growth arrest could be due to the failure to export functionally crucial proteins other than SBP1, which itself is not essential [28, 29]. During preparation of this work another study was published in which EXP2 was knocked down by 90% using a TetR-DOZI-aptamer system [14]. Both this study and ours similarly confirm EXP2 knockdown greatly reduced parasite growth as well as protein export. Although our study does not shed light on the pore forming role of EXP2, two recent studies confirm that it serves both as a protein pore for the PTEX complex and as a potential nutrient pore for transfer of low molecular weight solutes across the PVM [13, 14].
That the glmS mediated knockdown of EXP2-HA was responsible for growth reduction is provided by the fact that the HAglmS tag only appeared to append to EXP2 and no other genes since no other HA bands apart from EXP2 were present in western blots. The degree of knockdown of EXP2-HA protein was critically dependent on the concentration of GlcN added to the parasite culture and GlcN had no substantial effect on 3D7 parasites except at high concentrations (≥ 2 mM) for 3 or more days. Furthermore, when GlcN was removed EXP2 expression and parasite growth were partially restored. Specifically, when 0.5 mM GlcN was washed out after 2 and 3 days treatment, EXP2 protein levels and parasite proliferation almost fully recovered by day 5. At a higher dose of 2 mM GlcN for 2 days, only partial recovery occurred by day 5. GlcN mediated knockdown of EXP2 also greatly reduced export of SBP1 consistent with EXP2 being part of the PTEX complex [28, 29]. Confirmation that EXP2 is part of the PTEX complex was also provided by a recent separate study, where immunoprecipitation of EXP2-HA pulled down all four other proteins of the PTEX complex as well as a cargo reporter protein [16].
Our initial data suggested that growth of EXP2-HAglmS parasites was arrested by 0.5 mM GlcN treatment after 2–3 days, however closer investigation revealed that growth was more likely just slowed given that the parasites recovered well once GlcN was removed and EXP2 expression resumed. These parasites had about a half to a third the normal amount of EXP2-HA after 2–3 days treatment and could probably still export enough proteins and obtain sufficient nutrients to survive and hence recovered following GlcN removal. Whether slowed growth also resulted in fewer merozoites being formed was not examined, but nutrient restriction via knockdown of the new permeability pathway protein RhopH2 has been noted to reduce merozoite numbers per schizont [30]. This could also be one of the factors underpinning reduced proliferation in GlcN-treated EXP2-HAglmS parasites.
Parasites seem to be only able to tolerate the strong knockdown of EXP2 caused by 2 mM GlcN treatment for a few days since after this, growth recovery was poor and dead parasites began to be observed after 3 days of 2 mM GlcN treatment. After 2 days of 2 mM GlcN treatment EXP2 levels had fallen to 20% of normal levels and this enabled many parasites to survive since they partially recovered by day 5 if the GlcN was washed out after 2 days. After 3 days 2 mM GlcN treatment when levels of EXP2 had fallen to ~10%, recovery was poor probably because most parasites had died by then. The ability of ring stage arrested parasites to partially recover growth as long as 2 days after arrest has been previously detected for another PTEX protein HSP101, and is consistent with our EXP2 observations here [3].
Both our study and Garten et al (2018) [14] showed that the PEXEL negative exported protein (PNEP) SBP1 export is reduced when EXP2 is knocked down [14]. We are confident that the trafficking of other exported proteins, in particular PEXEL proteins, would also be similarly inhibited since we have seen this previously following the knockdown of PTEX150 [4]. Knockdown of HSP101 expression in P. berghei also reduced export of several different PEXEL proteins and in P. falciparum knockdown of HSP101 function reduced the export of several PEXEL and PNEP proteins [3, 4]. A knockout study of ~50 exported proteins has indicated that most PEXEL proteins (~75%) are probably non-essential [29, 31–33]. This raises the question of what essential functions are carried out by exported proteins whose export blockage results in parasite growth arrest? Although the intra-erythrocytic locations of many exported proteins are known along with their effects on erythrocyte rigidity, knob formation, PfEMP1 display and the trafficking of other exported proteins, the precise functional role for most exported proteins is not understood, with a few exceptions [34]. A recent attempt to carry out saturation mutagenesis of the P. falciparum genome with piggyBac transposons has helped determine which genes might be unmutable and essential and are therefore a priority for future study [22]. Although the essentiality of PTEX might depend on the critical functions of a small number of essential exported proteins it could also be equally dependent on minor functions of dozens of non-essential proteins that collectively have a huge effect on parasite viability when their trafficking is blocked.
In conclusion, we have shown that EXP2 is crucial for survival of P. falciparum blood stages probably because it is required for the efficient export of functionally important proteins into the erythrocyte compartment. Although this finding has been recently published [14] our study utilizes a different methodological approach and we also investigate the capacity of parasites to recover when EXP2 knockdown was reversed. It is interesting that given EXP2’s dual role in protein export and nutrient import, the knockdown of this protein and subsequent growth arrest and parasite death does not appear to be more severe than for other PTEX proteins which presumably only function in protein export. We can only conclude that EXP2’s higher overall level of expression level compared to other PTEX proteins [14], still leaves enough residual EXP2 functionality to allow parasites to survive in the short term.
Supporting information
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank the Australian Red Cross Blood Bank for the provision of human blood, Jacobus Pharmaceuticals for providing WR99210 and Monash Micro Imaging. We are grateful to Leann Tilley and Brian Cooke for providing ERC and SBP1 antibodies and Eva Pesce and Thorey Jonsdottir for technical assistance. The authors gratefully acknowledge funding from the Victorian Operational Infrastructure Support Program received by the Burnet Institute and for grants from the National Health and Medical Research Council of Australia (1068287, 1128198 and 1092789).
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
These authors received the following grant funding from the National Health and Medical Research Council of Australia (https://www.nhmrc.gov.au). BSC and PRG [1068287 and 1128198] and BSC [1092789]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Malaria Report 2017 [Internet]. 2017. Available from: http://www.who.int/malaria/publications/world-malaria-report-2017/en/.
- 2.de Koning-Ward TF, Gilson PR, Boddey JA, Rug M, Smith BJ, Papenfuss AT, et al. A newly discovered protein export machine in malaria parasites. Nature. 2009;459(7249):945–9. 10.1038/nature08104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck JR, Muralidharan V, Oksman A, Goldberg DE. PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature. 2014;511(7511):592–5. 10.1038/nature13574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elsworth B, Matthews K, Nie CQ, Kalanon M, Charnaud SC, Sanders PR, et al. PTEX is an essential nexus for protein export in malaria parasites. Nature. 2014;511(7511):587–91. 10.1038/nature13555 [DOI] [PubMed] [Google Scholar]
- 5.Gehde N, Hinrichs C, Montilla I, Charpian S, Lingelbach K, Przyborski JM. Protein unfolding is an essential requirement for transport across the parasitophorous vacuolar membrane of Plasmodium falciparum. Molecular Microbiology. 2009;71(3):613–28. 10.1111/j.1365-2958.2008.06552.x [DOI] [PubMed] [Google Scholar]
- 6.Matthews K, Kalanon M, Chisholm SA, Sturm A, Goodman CD, Dixon MW, et al. The Plasmodium translocon of exported proteins (PTEX) component thioredoxin-2 is important for maintaining normal blood-stage growth. Mol Microbiol. 2013;89(6):1167–86. 10.1111/mmi.12334 [DOI] [PubMed] [Google Scholar]
- 7.Matz JM, Matuschewski K, Kooij TW. Two putative protein export regulators promote Plasmodium blood stage development in vivo. Molecular and Biochemical Parasitology. 2013;191(1):44–52. 10.1016/j.molbiopara.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 8.Elsworth B, Sanders PR, Nebl T, Batinovic S, Kalanon M, Nie CQ, et al. Proteomic analysis reveals novel proteins associated with the Plasmodium protein exporter PTEX and a loss of complex stability upon truncation of the core PTEX component, PTEX150. Cellular Microbiology. 2016;18:1551–69. 10.1111/cmi.12596 [DOI] [PubMed] [Google Scholar]
- 9.Matz JM, Ingmundson A, Costa Nunes J, Stenzel W, Matuschewski K, Kooij TWA. In Vivo Function of PTEX88 in Malaria Parasite Sequestration and Virulence. Eukaryotic Cell. 2015;14(6):528–34. 10.1128/EC.00276-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chisholm SA, McHugh E, Lundie R, Dixon MW, Ghosh S, O'Keefe M, et al. Contrasting Inducible Knockdown of the Auxiliary PTEX Component PTEX88 in P. falciparum and P. berghei Unmasks a Role in Parasite Virulence. PLoS One. 2016;11(2):e0149296 10.1371/journal.pone.0149296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hakamada K, Watanabe H, Kawano R, Noguchi K, Yohda M. Expression and characterization of the Plasmodium translocon of the exported proteins component EXP2. Biochem Biophys Res Commun. 2017;482(4):700–5. 10.1016/j.bbrc.2016.11.097 [DOI] [PubMed] [Google Scholar]
- 12.Bullen HE, Charnaud SC, Kalanon M, Riglar DT, Dekiwadia C, Kangwanrangsan N, et al. Biosynthesis, Localization, and Macromolecular Arrangement of the Plasmodium falciparum Translocon of Exported Proteins (PTEX). J Biol Chem. 2012;287(11):7871–84. 10.1074/jbc.M111.328591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho C-M, Beck JR, Lai M, Cui Y, Goldberg DE, Egea PF, et al. Malaria parasite translocon structure and mechanism of effector export. Nature. 2018. 10.1038/s41586-018-0469-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garten M, Nasamu AS, Niles JC, Zimmerberg J, Goldberg DE, Beck JR. EXP2 is a nutrient-permeable channel in the vacuolar membrane of Plasmodium and is essential for protein export via PTEX. Nature Microbiology. 2018. 10.1038/s41564-018-0222-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesen-Ramirez P, Reinsch F, Blancke Soares A, Bergmann B, Ullrich AK, Tenzer S, et al. Stable Translocation Intermediates Jam Global Protein Export in Plasmodium falciparum Parasites and Link the PTEX Component EXP2 with Translocation Activity. PLoS Pathog. 2016;12(5):e1005618 10.1371/journal.ppat.1005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charnaud SC, Jonsdottir TK, Sanders PR, Bullen HE, Dickerman BK, Kouskousis B, et al. Spatial organisation of protein export in malaria parasite blood stages. Traffic. 2018; 10.1111/tra.12577 [DOI] [PubMed] [Google Scholar]
- 17.Trager W, Jensen J. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–5. 10.1126/science.781840 [DOI] [PubMed] [Google Scholar]
- 18.Hasenkamp S, Russell KT, Horrocks P. Comparison of the absolute and relative efficiencies of electroporation-based transfection protocols for Plasmodium falciparum. Malar J. 2012;11:210 10.1186/1475-2875-11-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makler MT, Hinrichs DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48(2):205–10. [DOI] [PubMed] [Google Scholar]
- 20.Tonkin CJ, van Dooren GG, Spurck TP, Struck NS, Good RT, Handman E, et al. Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol Biochem Parasitol. 2004;137(1):13–21. 10.1016/j.molbiopara.2004.05.009 [DOI] [PubMed] [Google Scholar]
- 21.Kalanon M, Bargieri D, Sturm A, Matthews K, Ghosh S, Goodman CD, et al. The Plasmodium translocon of exported proteins component EXP2 is critical for establishing a patent malaria infection in mice. Cellular Microbiology. 2016;18(3):399–412. 10.1111/cmi.12520 [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Wang C, Otto TD, Oberstaller J, Liao X, Adapa SR, et al. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science. 2018;360(6388). 10.1126/science.aap7847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prommana P, Uthaipibull C, Wongsombat C, Kamchonwongpaisan S, Yuthavong Y, Knuepfer E, et al. Inducible Knockdown of Plasmodium Gene Expression Using the glmS Ribozyme. PLoS ONE. 2013;8(8):e73783 10.1371/journal.pone.0073783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fidock DA, Wellems TE. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proceedings of the National Academy of Sciences. 1997;94(20):10931–6. 10.1073/pnas.94.20.10931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llinas M, Bozdech Z, Wong ED, Adai AT, DeRisi JL. Comparative whole genome transcriptome analysis of three Plasmodium falciparum strains. Nucleic Acids Res. 2006;34(4):1166–73. Epub 2006/02/24. 10.1093/nar/gkj517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozdech Z, Llinás M, Pulliam BL, Wong ED, Zhu J, DeRisi JL. The Transcriptome of the Intraerythrocytic Developmental Cycle of Plasmodium falciparum. PLoS Biol. 2003;1(1):e5 10.1371/journal.pbio.0000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Greca N, Hibbs AR, Riffkin C, Foley M, Tilley L. Identification of an endoplasmic reticulum-resident calcium-binding protein with multiple EF-hand motifs in asexual stages of Plasmodium falciparum. Mol Biochem Parasitol. 1997;89(2):283–93. [DOI] [PubMed] [Google Scholar]
- 28.Cooke BM, Buckingham DW, Glenister FK, Fernandez KM, Bannister LH, Marti M, et al. A Maurer's cleft–associated protein is essential for expression of the major malaria virulence antigen on the surface of infected red blood cells. The Journal of Cell Biology. 2006;172(6):899–908. 10.1083/jcb.200509122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier AG, Rug M, O'Neill MT, Beeson JG, Marti M, Reeder J, et al. Skeleton-binding protein 1 functions at the parasitophorous vacuole membrane to traffic PfEMP1 to the Plasmodium falciparum-infected erythrocyte surface. Blood. 2007;109(3):1289–97. 10.1182/blood-2006-08-043364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Counihan N, Chisholm S, Bullen H, Srivastava A, Sanders P, Jonsdottir T, et al. Plasmodium parasites deploy RhopH2 into the host erythrocyte to obtain nutrients, grow and replicate. eLife. 2017;e23217 10.7554/eLife.23217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maier AG, Rug M, O'Neill MT, Brown M, Chakravorty S, Szestak T, et al. Exported Proteins Required for Virulence and Rigidity of Plasmodium falciparum-Infected Human Erythrocytes. Cell. 2008;134(1):48–61. 10.1016/j.cell.2008.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crabb BS, Cooke BM, Reeder JC, Waller RF, Caruana SR, Davern KM, et al. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89(2):287–96. [DOI] [PubMed] [Google Scholar]
- 33.Pei X, Guo X, Coppel R, Bhattacharjee S, Haldar K, Gratzer W, et al. The ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum stabilizes spectrin tetramers and suppresses further invasion. Blood. 2007;110(3):1036–42. 10.1182/blood-2007-02-076919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill RJ, Ringel A, Knuepfer E, Moon RW, Blackman MJ, van Ooij C. Regulation and Essentiality of the StAR-related Lipid Transfer (START) Domain-containing Phospholipid Transfer Protein PFA0210c in Malaria Parasites. J Biol Chem. 2016;291(46):24280–92. 10.1074/jbc.M116.740506 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.