ABSTRACT
The study aimed to investigate the mechanism and biological roles of long noncoding RNAKCNQ1OT1 in adipogenic and osteogenic differentiation of tendon stem cell. In this study, tendon injury mice model was established to detect the expression of lncRNA KCNQ1OT1, miR-138, peroxisome proliferator-activated receptor gamma (PPARγ) and runt-related gene 2 (RUNX2) using quantitative real-time PCR (qRT-PCR) and western blot. Mechanical testing was carried out to assess tendon function. Adiponectin and Osterix were used to evaluate the adipogenic and osteogenic differentiation of tendon stem cells (TSCs). The interaction between lncRNA KCNQ1OT1 and miR-138 was detected by RNA immunoprecipitation (RIP) assay and RNA pull-down assay. We found that lncRNA KCNQ1OT1, PPARγ and RUNX2 expression were significantly upregulated, while miR-138 was suppressed in tendon tissue of injured group and the separated TSCs. lncRNA KCNQ1OT1 knockdown inhibited the adipogenic and osteogenic differentiation of TSCs. Further studies indicated that lncRNA KCNQ1OT1 functioned as a competing endogenous RNA (ceRNA) by sponging miR-138 in TSCs. Further investigations confirmed that lncRNA KCNQ1OT1 knockdown exerted anti-adipogenic and anti-osteogenic function via miR-138/PPARγ and miR-138/RUNX2 axis. Therefore, the lncRNA KCNQ1OT1/miR-138/PPARγ or RUNX2 axis modulated adipogenic and osteogenic differentiation of tendon stem cell, which might be a promising therapeutic target for tendon injuries.
KEYWORDS: Tendon injuries, lncRNA KCNQ1OT1, miR-138, adipogenic, osteogenic
Introduction
Tendons, as an integral part of the musculoskeletal system, are connective tissues that transmit force from muscle to bone. Tendon injures, such as tendon rupture or tendinopathy, are frequently occurred in both sports and the workplace [1]. Due the low metabolic rate, the healing of tendon injures can take more than one year [2]. Despite great efforts that have been made to improve the functional outcome of patients with tendon injures, clinical therapeutic options are still limited to conservative and surgical treatments, which bring considerable pain with a long recovery period [2]. Tendon stem cells (TSCs) with multilineage differentiation potential, have been demonstrated in human beings and other animals [3–5]. TSCs can differentiate into non-tenocyte lineages such as adipocytes, chondrocytes and osteocytes under suitable conditions, providing a possible mechanism for the osteogenic and adipogenic changes associated with tendon injuries [6–8]. Tendon stem cell can form tendon-like, cartilage-like, and bone-like tissues [9,10]. Rui et al found that erroneous tendon-derived stem cell differentiation was involved in the pathogenesis of calcifying tendinopathy [11]. Hence, the differentiation of tendon stem cells into tenocytes, tendon-like, and bone-like tissues can lead a new direction for the treatment and regeneration of tendon diseases. Thus, exploring mechanism underlying the adipogenic and osteogenic differentiation of TSCs was useful in clinical diagnosis and treatment of tendon injuries.
Peroxisome proliferator-activated receptor-γ (PPARγ) is a transcription factor which belongs to the ligand-activated nuclear receptor superfamily [12]. Eevated cellular levels of PPARγ promoted the adipogenicdifferentiation of human bone marrow mesenchymal stem cells (hBMSCs) and inhibited their osteogenic differentiation, which increased cellular lipid levels and decreased bone formation [13]. On the other hand, runt-related transcription factor-2 (RUNX2), as a member of the Runt domain family, binds specific DNA sequences to regulate transcription of numerous genes, thereby controlling osteoblast development from mesenchymal stem cells and maturation into osteocytes [14].
Recent microRNA (miRNA) microarray analysis revealed a cohort of miRNAs that functioned in regulating adipogenic and osteogenic differentiation. One of these miRNAs, endogenous miR-138, was expressed at a low expression level during the induction of adipogenic differentiation and resulted in the downregulation of PPARγ, which led to adipogenesis inhibition of human adipose tissue-derived mesenchymal stem cells (hAD-MSCs) [15]. Besides, Deng et al showed that the antimiR-138 delivery remarkably enhanced the in vitro osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs), and up-regulated osteogenesis related genes including Runx2, osterix, alkaline phosphatase, osteocalc in and bone morphogenetic protein-2 at both mRNA and protein levels [16]. Long noncoding RNAs (lncRNAs) were another novel class of RNA transcripts longer than 200 nucleotides with narrow protein coding functions. Emerging data have revealed that lncRNAs were involved in regulating various cell biological processes, such as cell growth, differentiation and apoptosis [17]. lncRNA KCNQ1OT1 was overexpressed in mesenchymal stem cells, and KCNQ1OT1 knockdown protected against myocardial ischemia/reperfusion injury [18,19]. However, the function of lncRNA KCNQ1OT1 on the tendon injuries is till unclear. At the post-transcriptional level, lncRNAs could serve as miRNA sponges and modulate the occurrence and development of tendon injuries. Therefore, we focused on the interaction between lncRNA KCNQ1OT1 and miR-138 in TSCs.
Our study aims to explore the underlying mechanisms of lncRNA KCNQ1OT1 knockdown in the attenuation of the adipogenic and osteogenic differentiation of TSCs via miR-138/PPARγ or miR-138/RUNX2 axis in tendon injured rats.
Materials and methods
Tendon injury model of mouse
Six-week old C57BL/6 mice were used to establish tendon injury model. C57BL/6 mice were purchased from the Experimental Animal Center of Shandong University (Jinan, Shandong, China). Prior to surgery, mice were anesthetized by intraperitoneal injection of a ketamine (80 mg/kg) and xylazine (5 mg/mg) mixture. Anesthesia was maintained using 1% isoflurane. A longitudinal incision was made on the surface of the Achilles tendon. Stacked sharp blades were used to separate the soft tissue, free the Achilles tendon and cut off the stop point. At the end of the operation, a 6–0 non-absorbable material needle was used to suture the tendon. To examine the effect of tendon injury on lncRNA KCNQ1OT1 and related molecular expression in vivo. Eighteen mice were divided into three groups, Control (sham group), Injured group and Injured+TSCs group. Proximal calcaneus of tendon injured mice performed a drilling with diameter of 0.8 mm and injected with 100 μL Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA; Injured group) or TSCs (5 × 106 cells, Injured+TSCs group). At 2 wk after the surgery, all mice were allocated for biomechanical testing. The tendon tissues were harvested for further use. All experiments were approved by the Animal Experimentation Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University.
TSCs isolation and induction of tenogenic differentiation
After the mice were killed, they were immersed in 75% alcohol for 5 min, then they were moved to clean bench and fixed in the supine position. The skin of the leg was cut off, the tendons on both sides of the thigh were collected, the tendon sheath and peritendon tissue were carefully removed, and the tendons were cut into small pieces (1 mm3), and digested with a sterile phosphate-buffered saline (PBS) solution containing type I collagenase (Sigma-Aldrich, St. Louis, MO, USA) and dispase (StemCell technologies Inc., Vancouver, BC, Canada) for 2.5 h at 37°C. The digested tissue was blown for 20 times and filtered through 200 mesh cell sieve to collect filtrate. The supernatant was discarded after centrifuging the suspension at 1,500 × g for 15 min and the pellet was resuspended in DMEM containing 10% fetal bovine serum, 100 U/ml penicillin, 100 mg/ml streptomycin and 2 mM L-glutamine (Invitrogen, Carlsbad, CA, USA), and seeded in Poly-L-lysine coated culture dish. Cells were cultured at 37°C in 5% CO2 following dilution to different densities, washed with PBS for twice, and removed non-adherent cells 2 d after plating. On day 7, cells were trypsinized and mixed together as primary TSCs. Three generations of TSCs were used for subsequent experiments.
Adipogenic differentiation was induced by incubating TSCs in adipogenic inductionmedium (Lonza, Walkersville, MD, USA). After 21 d, cells were washed with PBS and fixed in 10% neutral buffered formalin, while osteogenic differentiation was performed by incubating in osteogenic induction medium for 28 d.
RNA interference
To determine if lncRNA KCNQ1OT1 induced tenogenic differentiation, si-control or si-KCNQ1OT1 transfected TSCs were maintained in adipogenic or osteogenic induction medium for 21 or 28 d, respectively.
RNA immunoprecipitation and RNA pull down
DIANA tools (http://carolina.imis.athena-innovation.gr/) were used to identify the underlying binding sites between lncRNA KCNQ1OT1 and miR-138, which were verified by RNA immunoprecipitation (RIP) and RNA pull down assays. Briefly, RIP assay was conducted using Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. HEK293T cells were rinsed with cold PBS and lysed in radioimmunoprecipitation buffer at 4℃ for 30 min. Cell extracts were incubated with protein A/G sepharose beads conjugated to antibodies against Ago2 (Millipore) or normal mouse immunoglobulin G (IgG; Millipore). Immunoprecipitated RNA and total RNA from the whole cell lysates (input controls) were extracted for western blotting and qRT-PCR analysis.
The interaction between lncRNA KCNQ1OT1 and miR-138 was further examined by RNA pull-down using a Pierce Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Protein extracts from HEK293T cells were mixed with 50 pmol of biotinylated lncRNA KCNQ1OT1 and incubated with 50 µL of streptavidin agarose magnetic beads (Life Technologies, Carlsbad, CA, USA) at 4℃ for 1 h. The associated RNA-protein complex was eluted with Biotin Elution Buffer and then boiled in SDS buffer for another 10 min. The retrieved Ago2 protein levels were detected using western blot, while miR-138 mRNA expression levels were measured by qRT-PCR using IgG as the negative control (NC).
Cell transfection
The biological functions and molecular mechanism of lncRNA KCNQ1OT1 on adipogenic and osteogenic differentiation of mouse TSCs were determined by transfection with si-control or si-KCNQ1OT1(3’-GGGAAUCUGGUCUAAUGAATTUUCAUUAGACCAGAUUCCCTT-5’) alone, or with si-KCNQ1OT1 in combination with inhibitor negative control (NC) or miR-138 inhibitor using Lipofectamine 2000 (Invitrogen) before culturing in adipogenic or osteogenic induction medium.
Injection of KCNQ1OT1-overexpressing stable cells
pcDNA-KCNQ1OT1was constructed by inserting KCNQ1OT1 cDNA into pcDNA3.1 (Invitrogen, USA). TSCs infected with empty vector (Injured+pcDNA-TSCs) or TSCs infected with lentviruses of overexpressing lncRNA KCNQ1OT1 (Injured+pcDNA-KCNQ1OT1-TSCs) were injected locally into the injury site after the surgery. All the mice were euthanized two weeks after the operation and the tendon tissues were harvested for further in vivo examination.
Biomechanical testing
Each specimen obtained from mice was preloaded to 0.1 N and loaded to failure at a rate of 14 mm/s, corresponding to approximately 0.4% strain. The ultimate load to failure was recorded. The displacement was measured using a 1-mm-resolution micrometer system attached to a linear stage. The linear region of the load-displacement curve was used to calculate the stiffness.
Total RNA isolation and quantitative real-time qPCR
Total RNA was extracted from tendon tissues or TSCs using Trizol reagent (Invitrogen, Carlsbad, CA, USA), and then reverse-transcribed to cDNA using a lnRcute lncRNA cDNA synthesis Kit (Tiangen Biotech, Beijing, China)according to the manufacturer’s protocol. Then, the cDNA was mixed with FastFire qPCR PreMix (Tiangen Biotech, Beijing, China) and determined using the Quant One Step qRT-PCR Kit (Tiangen Biotech, Beijing, China) performing on an ABI 7500 Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA) and calculated by the 2−ΔΔCt method. The relative expression of KCNQ1OT1, adiponectin and miR-138 are normalized to GAPDH and U6.
Western blot analysis
Total protein was obtained from tendon tissues or TSCs using Radio-Immunoprecipitation Assay buffer (Beyotime, Shanghai, China), separated with 6% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes with 350 mA for 4 h. Afterwards, PVDF membranes were blocked with tris buffered saline tween (TBST) containing 5% skim milk at room temperature for 2.5 h, followed by incubation with rabbit polyclonal anti-RUNX2 (1:200 dilution), anti-PPARγ (1:1000 dilution), anti-Osterix (1:200dilution) and anti-β-actin at 1:500 dilution(all from Abcam, Cambridge, MA, USA) at 4°C overnight. Blots were incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibodies at room temperature for 2 h and visualized using enhanced chemiluminescence (Thermo Scientific, Shanghai, China) by a Molecular Imager ChemiDoc XRS System (Bio-Rad Laboratories, Hercules, CA, USA).
Flow cytometry
Tendon stem cells were collected, centrifuged at 1500 × g for 5 min, and washed with PBS for three times, and made a final concentration of 2 × 105 /mL. Antibodies for CD90, CD44, CD34 and CD106 were purchased from Abcam (USA). Tendon stem cell surface markers CD90, CD44, CD34 and CD106 were detected by Flow cytometry (FACS 420, BD Biosciences, USA).
Alkaline phosphatase (ALP) activity detection
TSCs lysate was prepared by Radio-Immunoprecipitation Assay buffer (Beyotime, Shanghai, China), and centrifuged for supernatant. The ALP activity was detected by Alkaline phosphatase detection kit (Beyotime, Shanghai, China) according to the manufacturer’s instruction.
Statistical analysis
All analyses were performed using SPSS version 22.0 (IBM, CA, USA). All data were presented as the mean±standard deviation (SD). The results were analyzed by Student’s t test or one way analysis of variance (ANOVA) with P-value < 0.05 considered statistically significant.
Results
lncRNA KCNQ1OT1 was up-regulated in the tendon injury model of mouse
As shown in Figure 1(a), the decreased ultimate load to failure and stiffness were observed in a mice model of tendon injury, while TSCs significantly elevated the levels of ultimate load to failure and stiffness in specimens of injured tendon at 2 wk, indicating that the tendon injured mouse model was well established. Histopathological sections of tendon tissue for control, Injury, Injury+TSCs groups were shown in Supplement 1A. We detected the expression levels of lncRNA KCNQ1OT1, miR-138, RUNX2 and PPARγ in tendon tissues of control group, tendon injured group and TSCs treatment group. Our result showed that the relative lncRNA KCNQ1OT1 level was significantly up-regulated in tendon tissues of Injured group and the mRNA expression of miR-138 was down-regulated in tendon tissues of Injured group (Figure 1(b), P < 0.05), whereas, the protein levels of RUNX2 and PPARγ were greatly elevated (Figure 1(c)) in specimens of injured tendon at 2 wk, and TSCs treatment reversed these effects. Bone cell-specific markers OCN (osteocyte marker), Osterix, and ALP expression in tendon tissue were detected in control, Injury, and Injury+TSCs groups, which indicated that TSCs could relieve tendon injury (Supplement 1B)
Figure 1.
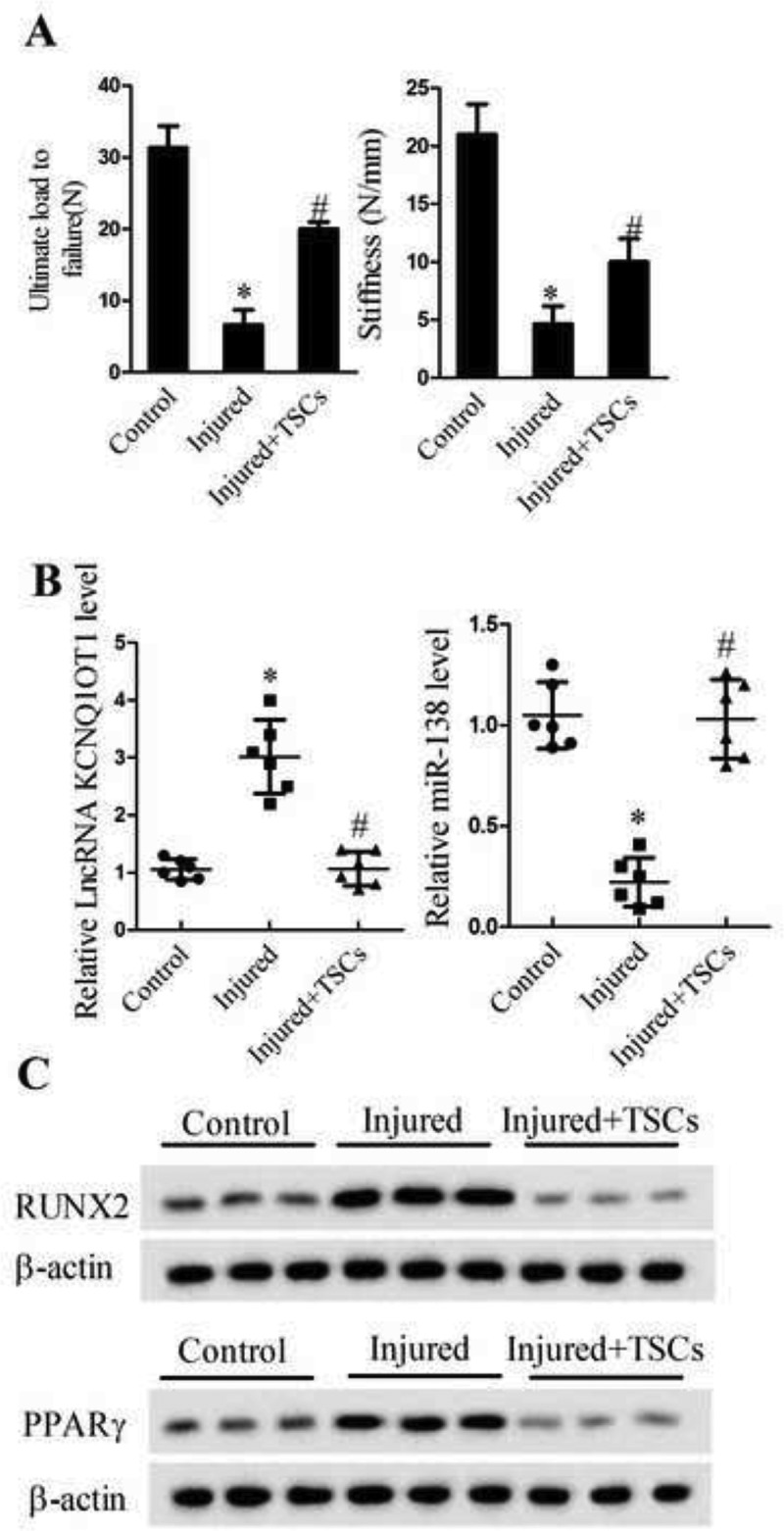
lncRNA KCNQ1OT1 was up-regulated in tendon tissue of the tendon injury model of mouse. Tendon injury mice model was established and divided into control (n = 6), Injured (n = 6), and Injured+TSCs groups (n = 6). (a) Biomechanical assessment of ultimate load to failure and stiffness of the tendon at the insertion site; (b) qRT-PCR assay detected lncRNA KCNQ1OT1 and miR-138 levels in the tendon tissues; (c) Western blot assay detected the protein expression levels of RUNX2 and PPARγ in the tendon tissues.
*p < 0.05, vs. Control group; #p < 0.05, vs. Injured group.
lncRNA KCNQ1OT1 was up-regulated during tenogenic differentiation
TSCs have been reported to be good cell source for tendon tissue engineering, and they have spontaneous tenogenic differentiation potentials in vitro. Thus, we used mTSCs as a cell model in the present study. TSCs were incubated in adipogenic induction medium for adipogenic differentiation for 21 d, and incubated in osteogenic induction medium for osteogenic differentiation for 28 d. Flow cytometry assay showed tendon stem cell surface markers CD90 and CD44 were highly expressed with a positive rate of 99.2% and 90.7%, whereas null expression of CD34 and CD106 (Figure 2(a)). The western blotting analysis showed that protein levels of osteogenic differentiation transcription factor RUNX2, adipogenic differentiation transcription factor PPARγ and osteogenic specific transcription factor Osterix were higher in osteogenic and adipogenic induction medium compared with normal culture medium treated TSCs (Figure 2(a), P < 0.05). Alizarin red and oil red O staining images of adipogenic and osteogenic differentiation of TSC were also shown in Figure 2(a). To further validate the lncRNA KCNQ1OT1 and miR-138 expression patterns in mTSCs, we used qPCR examination and confirmed that lncRNAKCNQ1OT1 was up-regulated, and miR-138 expression was suppressed during tenogenic differentiation (Figure 2(b), P < 0.05), which strongly support the possibility that lncRNA KCNQ1OT1 may serve as a biomarker for tendon injury.
Figure 2.
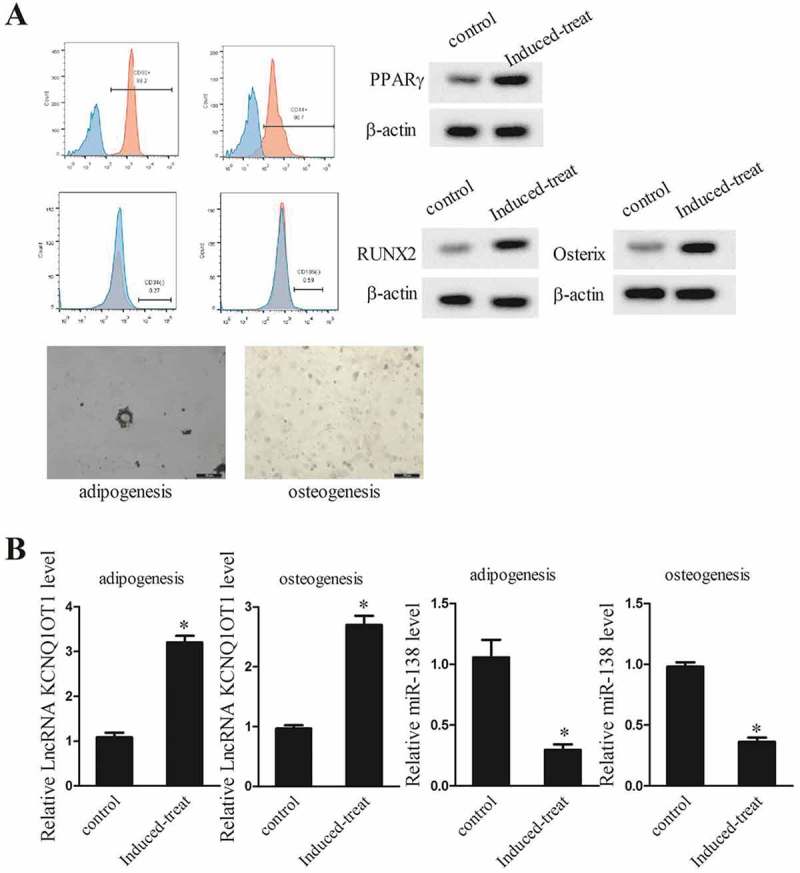
lncRNA KCNQ1OT1 was up-regulated during tenogenic differentiation. TSCs were incubated in adipogenic induction medium for adipogenic differentiation for 21 d, and TSCs were incubated in osteogenic induction medium for osteogenic differentiation for 28 d. TSCs were divided into control and Induced-treat groups. (a) Tendon stem cell surface markers CD90 and CD44 expression. Expressions of osteogenic differentiation transcription factor RUNX2 and adipogenic differentiation transcription factor PPARγ and osteogenic specific transcription factor Osterix. Alizarin red and oil red O staining images of adipogenic and osteogenic differentiation of TSC. (b) Relative lncRNA KCNQ1OT1 and miR-138 levels in TSCs after adipogenic induction and osteogenic induction.
*p < 0.05, vs. Control group.
Knockdown of lncRNA KCNQ1OT1 suppressed adipogenic and osteogenic differentiation of TSCs in vitro
To evaluate the function of lncRNA KCNQ1OT1 during adipogenic and osteogenic differentiation of TSCs, we used siRNA to downregulate KCNQ1OT1 gene expression in TSCs, and then treated with osteogenic and adipogenic induction medium. We employed the qRT-PCR and western blot assay to detect the expressions of lncRNA KCNQ1OT1 and adipocytokins adiponectin. Our results showed that the expressions of lncRNA KCNQ1OT1 (Figure 3(a)), adiponectin (Figure 3(b), P < 0.05) and osteoblast specific transcription factor Osterix (Figure 3(c)) were significantly decreased in si-KCNQ1OT1 group compared with si-control group. In addition, we found that si-KCNQ1OT1 inhibited alkaline phosphatase (ALP) activity (Figure 3(c)). These results indicated that lncRNA KCNQ1OT1 knockdown inhibited of adipogenic and osteogenic differentiation of TSCs.
Figure 3.
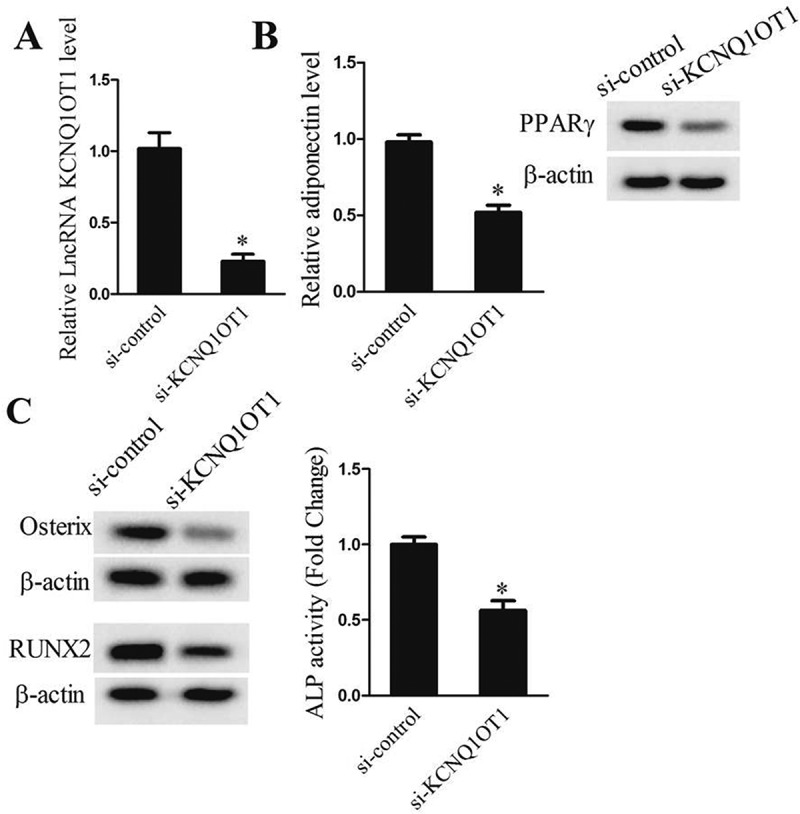
lncRNA KCNQ1OT1 knockdown suppressed adipogenic and osteogenic differentiation of TSCs in vitro. After adipogenic induction or osteogenic induction, TSCs were divided into si-control and si-KCNQ1OT1 groups. (a) qRT-PCR assay detected lncRNA KCNQ1OT1levels in si-control and si-KCNQ1OT1 groups. (b) qRT-PCR assay detected adipocytokine adiponectinlevel in si-control and si-KCNQ1OT1 groups. (c) Western blot assay detected osteogenic specific transcription factor Osterix expression in si-control and si-KCNQ1OT1 groups.
*p < 0.05, vs. si-control group.
lncRNA KCNQ1OT1 directly targeted mir-138 in HEK293T cells
We screened the candidate miRNAs by bioinformatics prediction and identified a putative binding site between miR-138 and lncRNA KCNQ1OT1 (Figure 4(a)). To further validate the interaction between lncRNA BCYRN and miR-138, we performed RNA immunoprecipitation assay using an antibody against AGO2 from RNA extracts of HEK293T cells. We found the significant enrichment of miR-138 (Figure 4(b)) and lncRNA KCNQ1OT1 (Figure 4(b)) using AGO2 antibody. We further confirmed the association between lncRNA KCNQ1OT1 and miR-138 using RNA pull down. Figure 4(c) showed a higher level of miR-138 in KCNQ1OT1 pulled down pellet than beads and negative control. All these results reveal that lncRNA KCNQ1OT1 interacted with miR-138.
Figure 4.
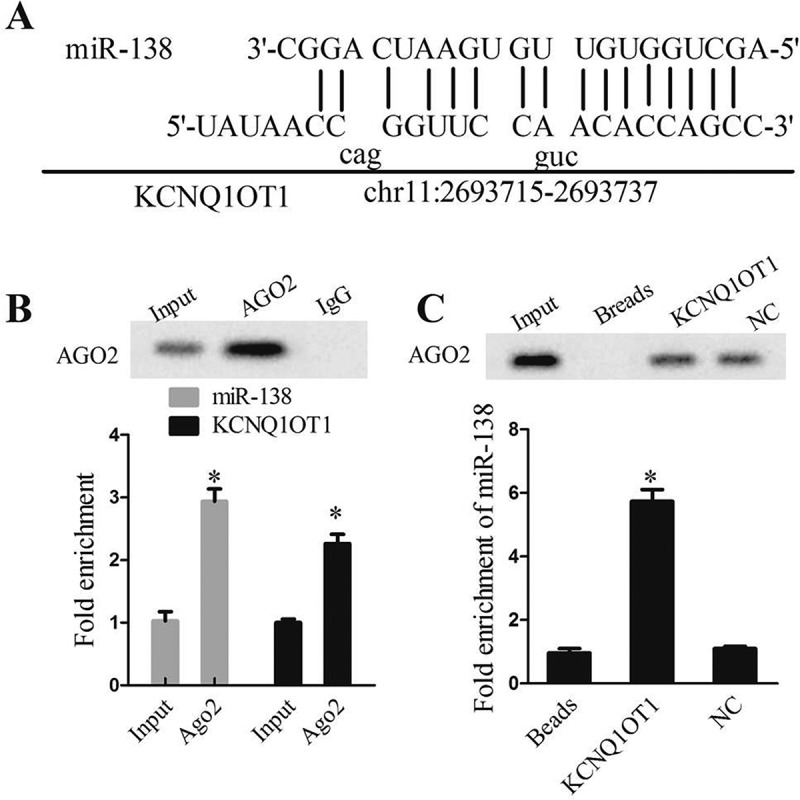
lncRNA KCNQ1OT1 interacted with miR-138. (a) DIANA tool spredicted the binding sites between lncRNA KCNQ1OT1 and miR-138. (b) RIP experiment with anti-Ago2, IgG as negative control or input as a positive control from HEK293T cells extracts using western blotting and qRT-PCR.*P < 0.05 compared with IgG group; (c) Ago2 expression levels and fold enrichment of miR-138 expression after RNA pull-down experiment with HEK293T cells extracts in different groups.*P < 0.05 compared with NC group.
lncRNA KCNQ1OT1 knockdown suppressed adipogenic and osteogenic differentiation of TSCs through mir-138
We next further identify the roles of lncRNA KCNQ1OT1 in tenogenic differentiation. miR-138 expressions were up-regulated in si-KCNQ1OT1 group (Figure 5(a)), whereas RUNX2, PPARγ (Figure 5(b)), adiponectin (Figure 5(c)) and Osterix (Figure 5(d)) were decreased. Nevertheless, co-silencing of lncRNA KCNQ1OT1 and miR-138 induced low expression of miR-138 (Figure 5(a)) and increased RUNX2, PPARγ (Figure 5(b)), adiponectin (Figure 5(c)) and Osterix (Figure 5(d)) levels. Taken together, lncRNA KCNQ1OT1 was regarded as a positive regulator of tenogenic differentiation via miR-138.
Figure 5.
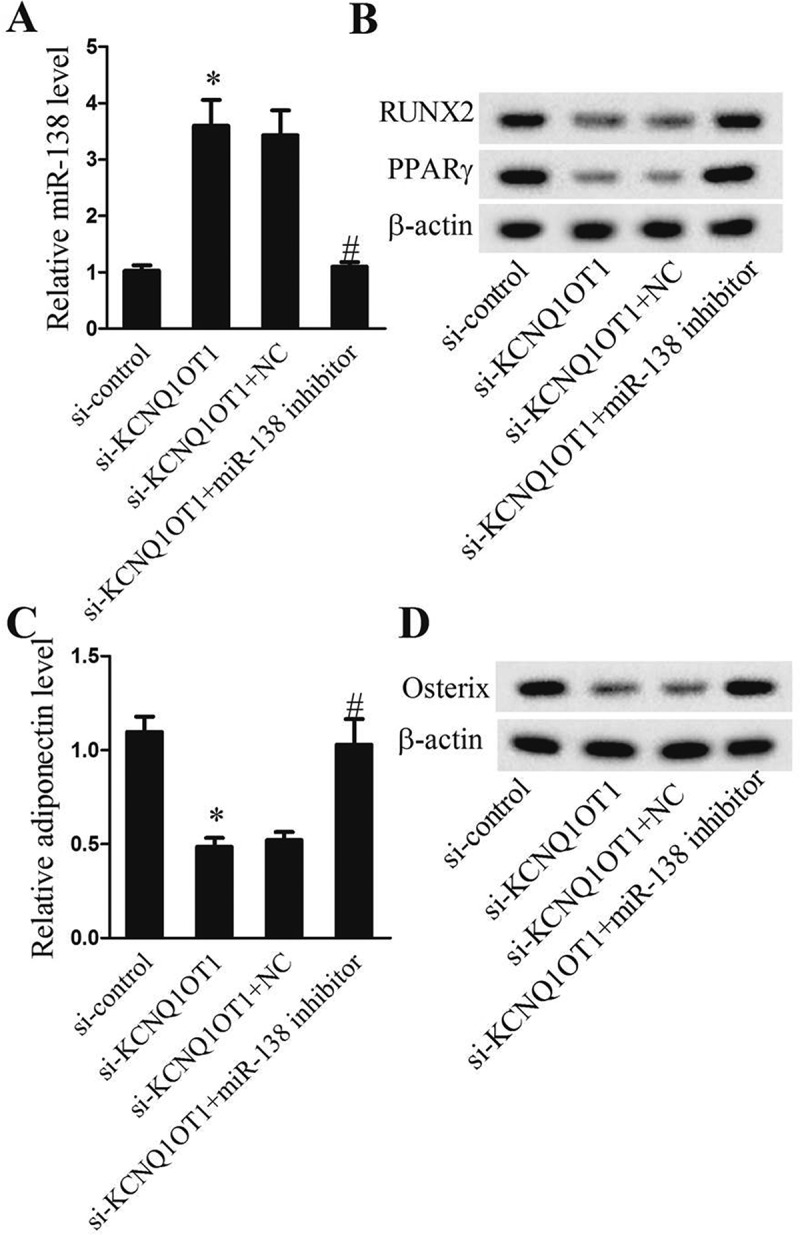
lncRNA KCNQ1OT1 knockdown suppressed adipogenic and osteogenic differentiation of TSCs through miR-138. After adipogenic induction or osteogenic induction, TSCs were divided intosi-control, si-KCNQ1OT1, si-KCNQ1OT1+NC, and si-KCNQ1OT1+miR-138 inhibitor groups. (a) Relative miR-138 levels. (b) Protein levels of RUNX2 and PPARγ. (c) Relative adiponectinlevel. (d) Protein levels of Osterix.
*p < 0.05, vs. si-control group; #p < 0.05, vs. si-KCNQ1OT1+ NC group.
lncRNA KCNQ1OT1 accelerated osteogenic and adipogenic differentiation of TSCs and depressed tendon healing through mir-138
Lastly, we examined the effects of lncRNA KCNQ1OT1 on tendon repair by using a mouse tendon defect model. As shown in Figure 6, TSCs significantly elevated the levels of ultimate load to failure and stiffness (Figure 6(a)) and miR-138 levels (Figure 6(b)), and declined the protein levels of RUNX2 and PPARγ (Figure 6(c)) in the injured tendon specimens at 2 wk. Infection with KCNQ1OT1-overexpressing TSCs reversed the effects of TSCs on all biomechanical markers, miR-138, RUNX2 and PPARγ expressions in the injured tendon specimens. The data confirmed that lncRNA KCNQ1OT1 overexpression aggravated tendon repair in vivo, which was consistent with the in vitro results.
Figure 6.
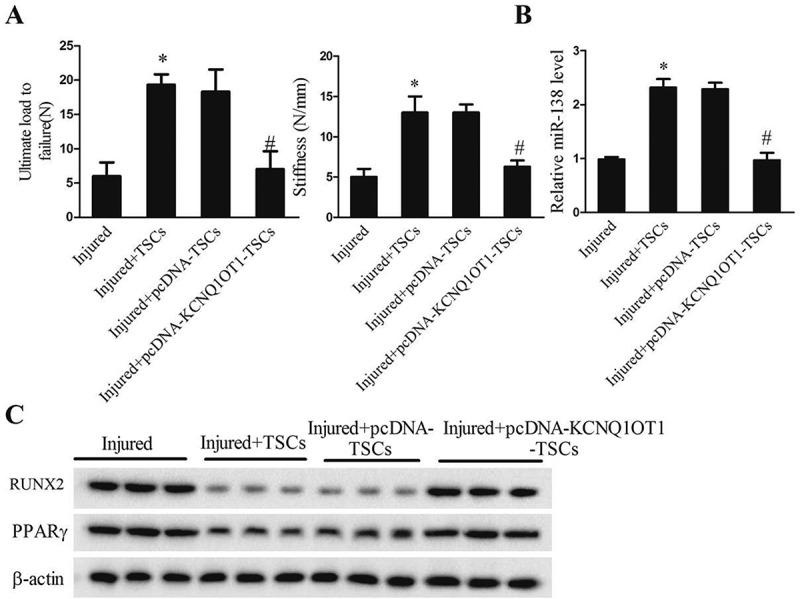
lncRNA KCNQ1OT1 accelerated osteogenic and adipogenic differentiation of TSCs and depressed tendon healing. After TSCs transfected with pcDNA-KCNQ1OT1, the TSCs were injected into the injured site in the tendon injury mice model. Mice were divided into injured, injured+TSCs, injured+pcDNA+TSCs, injured+pcDNA- KCNQ1OT1+TSCs groups. (a) Biomechanical assessment of ultimate load to failure and stiffness of the tendonat the insertion site in the four groups; (b) Relative miR-138level in the tendon tissues in the four groups; (c) The protein levels of RUNX2 and PPARγ in the tendon tissues in the four groups.
*p < 0.05, vs. Injured group; #p < 0.05, vs. Injured+pcDNA-TSCs group (injection with empty vector treated TSCs).
Discussion
In the present study, lncRNA KCNQ1OT1-silencing TSCs had better healing effect in tendon tear repair of the tendon injury mice model than TSCs implantation only or without implantation. lncRNA KCNQ1OT1 actedasamediatorfortenogenicdifferentiationviadirectlytargetingmiR-138 thus to increase the expressions of RUNX2 and PPARγ. These results were supported by our in vitro studies, which showed that lncRNA KCNQ1OT1 knockdown induced specific differentiation of TSCs into osteoblasts and adipocytes.
There is a growing interest for noncoding RNA (ncRNA)-mediated epigenetic regulation of transcription in diverse biological functions. Recent evidence suggests that a subset of long ncRNA epigenetically regulate the transcription of multiple genes in chromosomal domains via interaction with chromatin. As an imprinted gene, lncRNA KCNQ1OT1 is one such long chromatin-interacting ncRNA that silences multiple genes in the KCNQ1OT1 domain by establishing a repressive higher order chromatin structure [20]. Over the years, accumulating evidence exists with regard to the importance of lncRNA KCNQ1OT1in the development of musculoskeletal system. A recent study indicated that lncRNA KCNQ1OT1 ameliorated particle-induced osteolysis through inducing macrophage polarization by suppressing miR-21a-5p [21]. The present study showed that lncRNA KCNQ1OT1 was up-regulated in the injured tendon specimens of tendon injury mice model and during osteogenic and adipogenic differentiation of TSCs, which suggested that KCNQ1OT1 might involve in the osteogenic and adipogenic changes.
Increasingly evidences implied that lncRNA acts as a competing endogenous RNA (ceRNA) or miRNA sponge, thus to modulate a variety of cellular biological activities [22,23]. Therefore, we investigated whether lncRNA KCNQ1OT1 functioned as a ceRNA by interacting with miRNAs. RIP and RNA pull down showed that lncRNA KCNQ1OT1 served as a miRNA sponge for miR-138 in HEK293T cells. The miR-138 family has been reported to be involved in the promotion of osteogenesis in mesenchymal stem cell sheets. For instance, Hu et al showed that miR-138 is the upstream regulator of focal adhesion kinase signaling in the promotion of osteogenic differentiation induced by extracorporeal shockwave [24]. Furthermore, miR-138 is considered as a negative regulator of PPARγ and RUNX2 in mesenchymal stem cell sheets [15,16]. Besides, thetranscription factors RUNX2 and PPARγ are required for bone and fat formation, and the relative activity of RUNX2 versusPPARγ determines whether a mesenchymal progenitor undergoes osteoblastogenesis oradipogenesis [25]. This study also showed that lncRNA KCNQ1OT1 knockdown suppressed osteogenic and adipogenic differentiation through competing miR-138, which suggested the possibility of a ceRNA mechanism during TSCs differentiation.
In summary, our study demonstrates the interactions among the lncRNA KCNQ1OT1, miR-138 and PPARγ along with RUNX2 in tendon injuries. lncRNA KCNQ1OT1 knockdown protects TSCs from adipogenic and osteogenic differentiation via increasing the expression of miR-138 and downregulating the expressions of PPARγ and RUNX2. These results may provide new insight for the prevention and cure of tendon injury.
Funding Statement
This study was supported by Natural Science Foundation of ZheJiang Province (Y18H060051), Wenzhou Municipal Science and Technology Bureau (Y20170229) and Zhejiang Medical and Health Science and Technology Project (2019RC218).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Gulati V, Jaggard M, Al-Nammari SS, et al. Management of achilles tendon injury: a current concepts systematic review. World J Orthop. 2015;6:380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sharma P, Maffulli N.. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. [DOI] [PubMed] [Google Scholar]
- [3].Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. [DOI] [PubMed] [Google Scholar]
- [4].Tao X, Liu J, Chen L, et al. EGR1 induces tenogenic differentiation of tendon stem cells and promotes rabbit rotator cuff repair. Cell Physiol Biochem. 2015;35:699–709. [DOI] [PubMed] [Google Scholar]
- [5].Chen W, Tang H, Zhou M, et al. Dexamethasone inhibits the differentiation of rat tendon stem cells into tenocytes by targeting the scleraxis gene. J Steroid Biochem Mol Biol. 2015;152:16–24. [DOI] [PubMed] [Google Scholar]
- [6].Chen W, Tang H, Liu X, et al. Dickkopf1 up-regulation induced by a high concentration of dexamethasone promotes rat tendon stem cells to differentiate into adipocytes. Cell Physiol Biochem. 2015;37:1738–1749. [DOI] [PubMed] [Google Scholar]
- [7].Augustyniak E, Trzeciak T, Richter M, et al. The role of growth factors in stem cell-directed chondrogenesis: a real hope for damaged cartilage regeneration. Int Orthop. 2015;39:995–1003. [DOI] [PubMed] [Google Scholar]
- [8].MacLean S, Khan WS, Malik AA, et al. Tendon regeneration and repair with stem cells. Stem Cells Int. 2012;2012:316281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rui YF, Lui PPY, Yin MW, et al. Altered fate of tendon-derived stem cells isolated from a failed tendon-healing animal model of tendinopathy. Stem Cells Dev. 2013;22:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang J, Wang JH.. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rui Y, Lui P, Chan L, et al. Does erroneous differentiation of tendon-derived stem cells contribute to the pathogenesis of calcifying tendinopathy? Chin Med J. 2011;124:606–610. [PubMed] [Google Scholar]
- [12].Li H, Li T, Wang S, et al. miR-17-5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Res. 2013;10:313–324. [DOI] [PubMed] [Google Scholar]
- [13].Sun J, Wang Y, Li Y, et al. Downregulation of PPARgamma by miR-548d-5p suppresses the adipogenic differentiation of human bone marrow mesenchymal stem cells and enhances their osteogenic potential. J Transl Med. 2014;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schroeder TM, Jensen ED, Westendorf JJ. RUNX2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today. 2005;75:213–225. [DOI] [PubMed] [Google Scholar]
- [15].Yang Z, Bian C, Zhou H, et al. MicroRNA hsa-miR-138 inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells through adenovirus EID-1. Stem Cells Dev. 2011;20:259–267. [DOI] [PubMed] [Google Scholar]
- [16].Yan J, Zhang C, Zhao Y, et al. Non-viral oligonucleotide antimiR-138 delivery to mesenchymal stem cell sheets and the effect on osteogenesis. Biomaterials. 2014;35:7734–7749. [DOI] [PubMed] [Google Scholar]
- [17].Chen LL, Zhao JC. Functional analysis of long noncoding RNAs in development and disease. Adv Exp Med Biol. 2014;825:129–158. [DOI] [PubMed] [Google Scholar]
- [18].Li X, Dai Y, Yan S, et al. Down-regulation of lncRNA KCNQ1OT1 protects against myocardial ischemia/reperfusion injury following acute myocardial infarction. Biochem Biophys Res Commun. 2017;491:1026–1033. [DOI] [PubMed] [Google Scholar]
- [19].Golding MC, Magri LS, Zhang L, et al. Depletion of KCNQ1OT1 non-coding RNA does not affect imprinting maintenance in stem cells. Development. 2011;138:3667–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kanduri C. KCNQ1OT1: a chromatin regulatory RNA. Semin Cell Dev Biol. 2011;22:343–350. [DOI] [PubMed] [Google Scholar]
- [21].Gao X, Ge J, Li W, et al. lncRNA KCNQ1OT1 ameliorates particle-induced osteolysis through inducing macrophage polarization by inhibiting miR-21a-5p. Biol Chem. 2018;399:375–386. [DOI] [PubMed] [Google Scholar]
- [22].Poliseno L, Salmena L, Zhang J, et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hu J, Liao H, Ma Z, et al. Focal adhesion kinase signaling mediated the enhancement of osteogenesis of human mesenchymal stem cells induced by extracorporeal shockwave. Sci Rep. 2016;6:20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ge C, Cawthorn WP, Li Y, et al. Reciprocal control of osteogenic and adipogenic differentiation by ERK/MAP kinase phosphorylation of RUNX2 and PPARgamma transcription factors. J Cell Physiol. 2016;231:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


