Abstract
Sensitization to Aspergillus species is associated with allergic respiratory diseases. Allergen immunotherapy with nonstandardized Aspergillus extracts is commonly used as therapy in these patients. Unfortunately, no method exists to measure the relevant allergen protein content in diagnostic and therapeutic extracts. Thus, there is a critical need for Aspergillus extract standardization. We hypothesized that development of Aspergillus-specific human IgE mAbs would allow for the characterization of the relevant human allergenic epitopes among currently available commercial Aspergillus fumigatus extracts. Patients with allergic bronchopulmonary mycosis were recruited from Vanderbilt University Medical Center. IgE antibody–secreting B cells were grown and immortalized using human hybridoma techniques first described here. Twenty-six human Aspergillus-reactive IgE mAbs were used as capture and detection reagents to characterize the Aspergillus allergen content of commercial extracts. We found extreme variability in the specificity and quantity of their protein targets. Just 4 mAbs reacted with all available extracts, and only 1 of 4 extracts contained the major allergen Asp f 1. This degree of variability will almost certainly affect the efficacy of these reagents when used in diagnosis and treatment. Human IgE mAbs represent an innovative tool for the evaluation of relevant human allergenic epitopes, which may assist in future development and long-term standardization of mold extracts.
Keywords: Immunology, Pulmonology
Keywords: Allergy, Asthma, Immunotherapy
A method to produce human Aspergillus-specific IgE mAbs allows the relevant allergen content of commercial extracts used for immunotherapy to be probed.
Introduction
At the center of the pathogenesis of type I hypersensitivity reactions lies the IgE molecule (1). In sensitized (IgE antibody–positive) individuals, reexposure to an allergen leads to IgE engagement by the allergen, resulting in FcεRI cross-linking and activation of mast cells and basophils (2). This causes the release of mediators initially into the local tissue with quick spreading to the systemic circulation, resulting in the vast array of symptoms associated with allergic disease, including anaphylactic shock. To date, studies of the human IgE molecule, and its targeted epitopes on allergens, have been very limited. Most of our knowledge of this process has come from studies using allergic patient sera (3). Because serum contains a mixture of many antibodies, having many specificities, directed toward many epitopes, and having many affinities, the ability to study and quantify the molecular interaction of IgE with its target allergen by serology is greatly limited. The ideal way to study this process is to use naturally occurring human IgE mAbs, isolated from allergic patients; however, no such mAbs have previously ever been created. Here we describe for the first time to our knowledge a novel method to grow and identify IgE-expressing B cells in the peripheral blood of individuals with allergic diseases, allowing for the generation of human hybridomas secreting IgE mAbs (Supplemental Figure 1). This technology was used to generate a large, unbiased panel of Aspergillus-specific human IgE mAbs from subjects with allergic bronchopulmonary mycosis (ABPM) and use these mAbs to probe the relevant allergen content of commercial extracts.
The clinical evaluation of sensitization to mold occurs by one of two methodologies: direct testing of serum for the presence of fungal-specific IgE or evaluation of the skin for a wheal and flare reaction following percutaneous or intradermal administration of a crude mold-specific allergen extract. Testing for serum-specific IgE is most commonly accomplished using a modified sandwich ELISA where an allergen, derived from allergen extracts, recombinant protein sources, or both, is irreversibly bound to a high-binding-capacity solid phase. This is followed by binding of the patients’ endogenous IgE to the allergosorbent and subsequent detection with an enzyme- or fluorophore-labeled secondary anti-IgE antibody. A positive percutaneous and intradermal test results from cross-linking of specific IgE on mast cell surfaces following introduction of the allergen, with subsequent degranulation. Both methods of evaluation require a commercially available fungal allergen extract, which is assumed to contain the relevant allergen proteins recognized by the disease, causing human IgE antibody response.
Identifying a patient’s unique pattern of allergic sensitization to fungi is important both for counseling the patient regarding potential avoidance of allergic triggers and also in formulating allergen immunotherapy using similar extracts to those used in diagnostic skin testing. There is some evidence that specific allergen immunotherapy to mold is effective in decreasing associated symptoms of mold allergy (4, 5). At present, the commercially available mold allergen extracts used in skin testing and immunotherapy have not been standardized for their allergen content. The Aspergillus species used in the production of allergen extracts is developed from curated strains that are maintained for that purpose with the hope of maintaining genetic stability and production of a consistent extract. There are, however, marked differences among mold extract manufacturers in the method of cultivation that they use. Variables include the substrate on which the mold is grown, the relative proportion of extract material derived from hyphae and spore material, and the inclusion of proteins secreted in the growth media, some of which, including Asp f 1, have been shown to be important allergens (6). Extensive variability among the commercial mold extracts has been demonstrated using protein staining of the crude extract following SDS-PAGE (7). Specific evaluation of Alt a 1 and Asp f 1 content in a number of commercially produced mold allergen extracts using mouse-derived mAbs has also shown extensive variability involving several logs of potency between the preparations (8).
ABPM occurs predominantly in the setting of mold colonization in the airway of patients with asthma or cystic fibrosis. The chronic antigenic stimulation leads to an exuberant allergic inflammatory reaction characterized by high total IgE levels, which is viewed as evidence of type I hypersensitivity to the colonizing mold. There is often peripheral and airway eosinophilia, as well as progressive airway remodeling with the development of bronchiectasis, and in some cases progression to respiratory failure if left untreated. Therapy for ABPM is primarily antiinflammatory with the use of systemic corticosteroids. The addition of antifungal agents can be steroid sparing, but they are not consistently useful in inducing remission (9). There is recent evidence that treatment with omalizumab can decrease the risk of recurrent exacerbations. This argues for IgE’s central role in the pathology of the disease process (10, 11). Although a number of molds have been implicated as causes of ABPM, Aspergillus species are by far the most common — of which A. fumigatus is seen most commonly (12). Sensitization to A. fumigatus is not uncommonly found in asthmatics, though it is not the most common mold sensitization associated with severe asthma. Routine surveillance of sputum samples of cystic fibrosis patients has shown an association between chronic airway colonization with Aspergillus and the eventual development of ABPM. During that period of colonization, patients who develop ABPM often display a broadening of the specificity of the IgE antibody response to increasing numbers of Aspergillus allergens. This diversification of the patient’s IgE response appears to be associated with an increased risk for the development of ABPM, although it remains unclear whether a specific evolution of sensitization reliably predicts the transition to frank ABPM (3, 13).
Because of the robust allergic response present in patients with ABPM, their high circulating IgE levels, and the diversity of mold proteins recognized by their serum IgE, these patients represent an ideal population from which to develop panels of IgE mAbs directed toward Aspergillus. Human IgE mAbs are used as valuable tools to evaluate the specificity and quantity of relevant allergenic proteins in commercially available A. fumigatus extracts.
Results
Aspergillus-reactive IgE–secreting human hybridomas can be generated from peripheral blood mononuclear cells of subjects with ABPM.
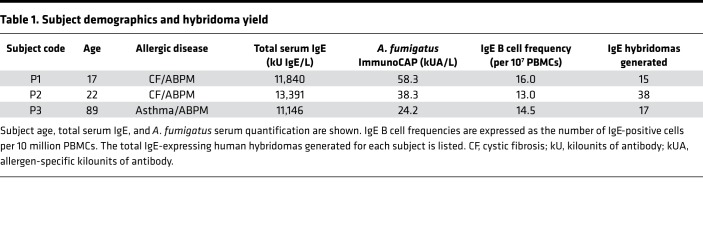
We selected 3 subjects with ABPM who had high levels of circulating Aspergillus-specific IgE. Our hope was that their mold hypersensitivity would be reflected in their memory B cell population from which we developed panels of IgE mAbs directed toward Aspergillus. We expanded B cells in culture as described in the Methods section and calculated minimum IgE B cell frequencies based on isotype-specific ELISA results (see Table 1). We observed a minimum IgE-encoding B cell frequency of 16.0, 13.0, and 14.5 per 10 million peripheral blood mononuclear cells (PBMCs) from each subject, respectively, and established 70 stable human hybridomas from B cell lines secreting IgE antibodies. Of note, none of the human IgE mAbs were screened for reactivity toward Aspergillus, or any other specificity, before their generation. Thus, no antigen-specific bias was introduced. All mAbs were selected only based on the B cell’s production of the IgE isotype. The purified human IgE mAb used for characterizations was produced by large-scale hybridoma growth in serum-free culture medium.
Table 1. Subject demographics and hybridoma yield.
Allergen extracts and IgE binding analysis.
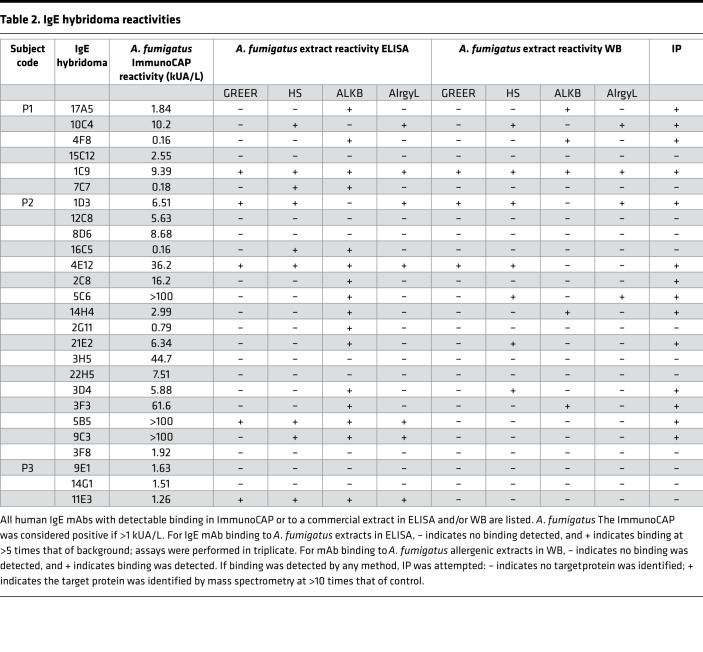
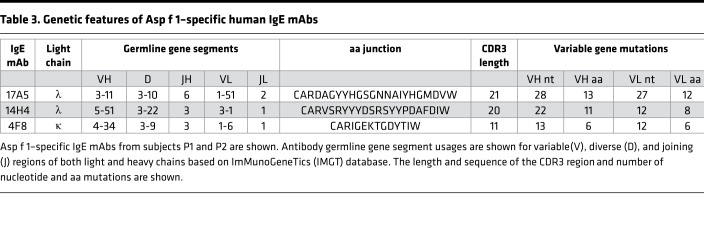
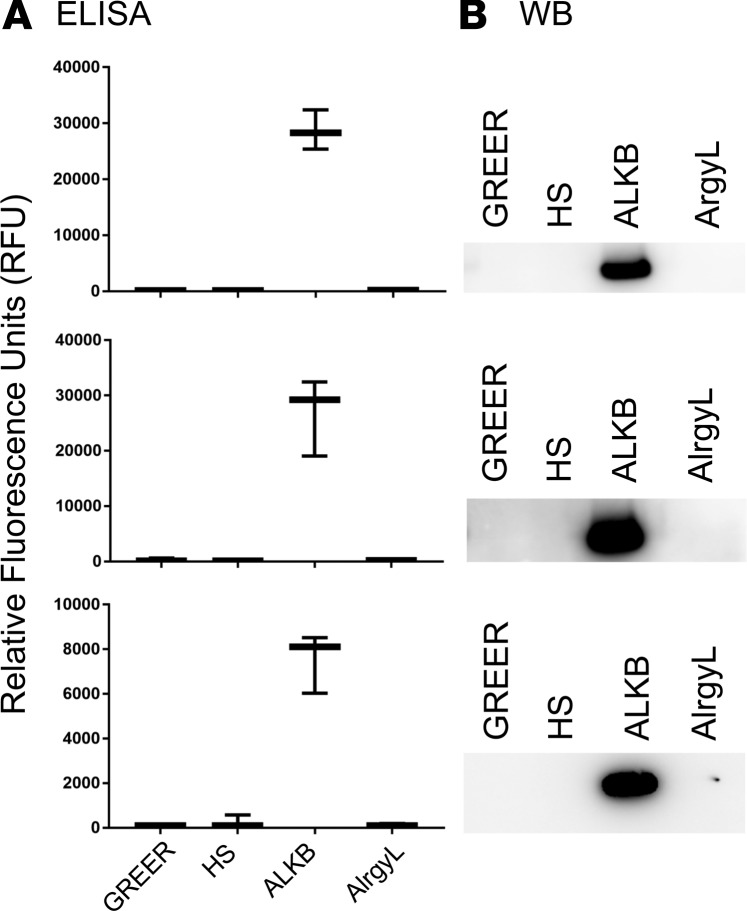
Aspergillus reactivity was identified by the presence of specific activity to Aspergillus using the ImmunoCAP autoanalyzer (Thermo Fisher Scientific) or by specific binding to one or more of the A. fumigatus extracts commercially available for human use in the US (GREER, Jubilant HollisterStier [HS], ALK-Abelló [ALKB], and Allergy Laboratories [AlrgyL]). Specific binding to A. fumigatus extract was evaluated both by indirect fluorescence ELISA and Western blot analysis — all 70 human mAbs were screened. It should be noted that some of these antibodies may be reactive toward Aspergillus components that are not present in any of the commercially available materials, resulting in an underestimation of the response. Of the 70 mAbs (see Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.123387DS1), 26 bound to the well-characterized Aspergillus allergosorbents in the ImmunoCAP and/or 1 or more allergenic extracts in ELISA (see Table 2). All 26 Aspergillus-reactive IgE mAbs were then tested for their ability to bind to purified recombinant Asp f 1 protein. Three mAbs, 2 from patient 1 (P1) (17A5 and 4F8) and 1 from patient 2 (P2) (14H4), were Asp f 1 specific — see Table 3 for unique genetic features of Asp f 1–specific mAbs. Notably, only 2 of the 3 Asp f 1 antibodies were detected by the ImmunoCAP, and those were weak reactors, while the third was undetected (Table 2). Figure 1 shows Asp f 1–specific IgE binding through ELISA and Western blot analysis using the 4 manufacturers’ extracts. Only ALKB extract possessed detectable levels of Asp f 1. Additionally, reactivity for ALKB’s extract was very high, in several cases reaching the upper maximum limit of our ELISA.
Table 2. IgE hybridoma reactivities.
Table 3. Genetic features of Asp f 1–specific human IgE mAbs.
Figure 1. Identification of Asp f 1 in commercial extracts using human IgE mAbs.
(A) Indirect fluorescence ELISA analysis of allergenic extracts. (B) Western blot (WB) analysis of allergenic extracts. Analyses were performed on commercially manufactured allergenic extracts from GREER, HS, ALKB, and AlrgyL using human IgE mAbs to the major allergen Asp f 1. Box-and-whisker plots show the median, first and fourth quartiles, and minimum and maximum values (whiskers).
As shown in Table 2, reactivity varied extensively among the mAbs and extracts. The ImmunoCAP was the most consistent in detecting binding of 22 of the 26 antibodies. Binding to the 4 manufacturers’ extracts was highly variable. The number of antibodies that reacted to the extracts, either in ELISA or Western blot analysis, was as follows: GREER, 6 mAbs; AlrgyL, 8 mAbs; HS, 12 mAbs; and ALKB, 16 mAbs. Of the 26 antibodies, only 8 reacted to more than 1 allergenic extract. Only 4 antibodies bound targets in all the manufacturers’ extracts. Supplemental Figures 2–6 show examples of the variability of binding to extracts in ELISA and Western blot analysis as seen using the human Aspergillus-reactive IgE mAbs.
IP and mass spectroscopy.
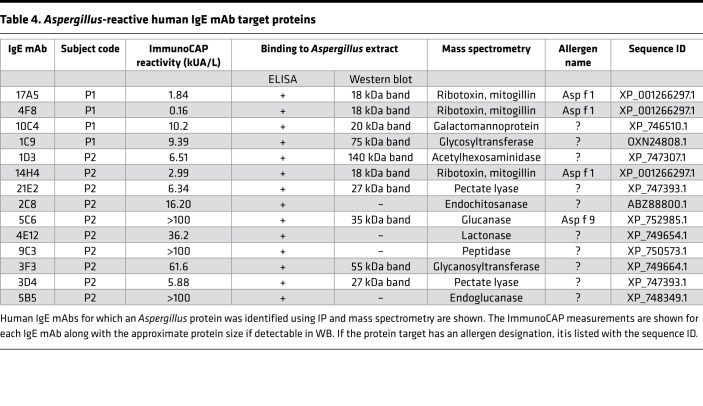
Because our panel of IgE mAbs was generated from subjects with ABPM without bias toward any given allergen, the targets of these antibodies likely represent a stochastic sampling of the mold proteins generating the immune response during the development of the disease. We next set out to define the target proteins of the 26 Aspergillus-reactive IgE mAbs using immunoaffinity purification and mass spectrometry and the commercially available allergen extracts as source material. As shown in Table 4, we were able to identify 14 IgE mAb target proteins. The majority of Aspergillus proteins identified contain intrinsic enzymatic activity associated with fungal metabolism. Interestingly, we identified an endochitosanase, which is secreted in high amounts by growing fungus. A previous study using proteomic screening with serum from patients with ABPM also suggested that this protein may be a target of IgE antibodies (14). The enzyme pectate lyase was the target protein of 2 mAbs, 3D4 and 21E2. Proteins with potential as virulence factors, such as Asp f 1 (the ribotoxin mitogillin), were identified by 3 antibodies that bound recombinant Asp f 1 (17A5, 4F8, and 14H4). The target of 10C4 is a mannoprotein, whose ortholog in Talaromyces marneffei is a virulence factor associated with sequestration of host arachidonic acid (15). Although both Asp f 1 and Asp f 9 were targets of our mAbs, the majority of the characterized targets are not proteins with recognized allergenic potential.
Table 4. Aspergillus-reactive human IgE mAb target proteins.
Human FcεRIα–transgenic mouse anaphylaxis.
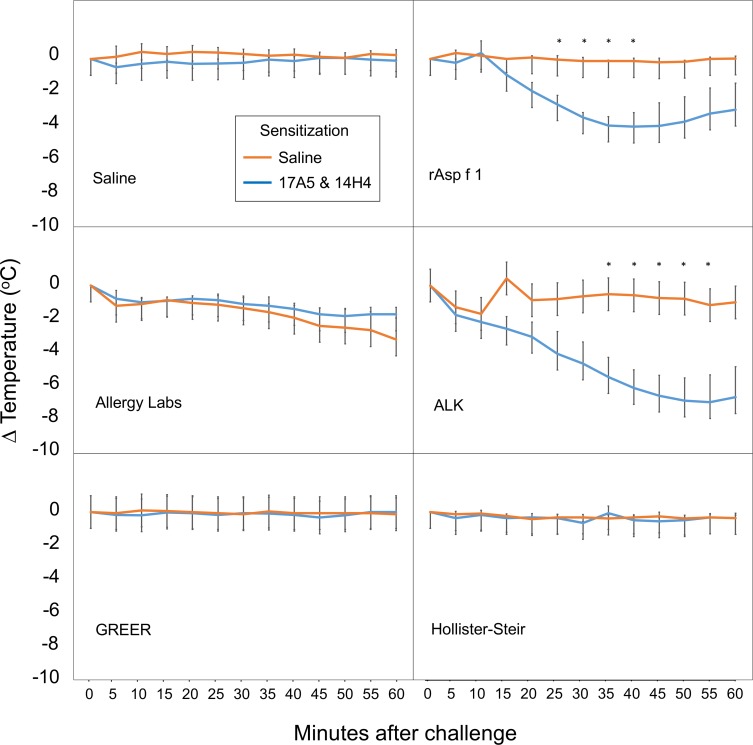
To evaluate commercial extracts for functional levels of the major Aspergillus allergen Asp f 1, we employed a transgenic mouse model of anaphylaxis (see Figure 2). Mice expressing the human FcεRIα were sensitized with injections of 17A5 and 14H4, 2 Asp f 1–specific IgE mAbs, or sham sensitized with saline injections. Each group had 3 mice. Human mAbs 17A5 and 14H4 were selected because they bind distinct, noncompeting epitopes on Asp f 1 and are able to function together to trigger mast cell degranulation (data not shown). Mice sensitized with mAbs and sham-sensitized mice were then challenged via an intraperitoneal injection with each of the commercially prepared extracts, recombinant Asp f 1, or a saline control. Mice undergoing anaphylaxis develop marked cutaneous vasodilation, which produces a fall in their core body temperature. Only those mice injected with the recombinant Asp f 1 and the ALKB A. fumigatus extract containing Asp f 1 elicited a significant fall in temperature when compared with the sham-sensitized animals.
Figure 2. Asp f 1–induced anaphylaxis in human FcεRIα–transgenic mice.
Human FcεRIα–transgenic mice were sensitized with human Asp f 1–specific IgE mAbs 17A5 and 14H4 or saline. Animals were challenged with 5 μg of recombinant Asp f 1 or the maximum tolerated dose of extract manufactured by GREER, HS, ALKB, or AlrgyL. Each group had 3 mice. Error bars represent the SEM, with temperature differences between control and allergen-challenged animals assessed by paired 2-tailed t test with a calculated P value of less than 0.05 deemed significant and highlighted by an asterisk. rAsp f 1, recombinant Asp f 1.
Discussion
This study provides the first description to our knowledge of a method to generate naturally occurring human allergen–specific IgE mAbs. Until now, attempts to clone human IgE antibodies from peripheral blood cells by flow cytometry has proved difficult to impossible, due to the cells’ low frequency and poor B cell receptor expression. Human IgE mAbs act as tools to evaluate both the quantity and specificity of allergenic components in A. fumigatus extracts. Only allergen proteins that are present in the manufacturing process, appropriately folded, and not proteolytically degraded can be detected by these antibodies. We have used a large panel of human IgE mAbs, generated in an unbiased manner from patients with ABPM, to identify the presence of relevant allergenic proteins in both the 4 commercial allergenic extracts and the A. fumigatus allergosorbent used for IgE antibody serology worldwide in the state-of-the-art ImmunoCAP autoanalyzer. Significant variability in allergen content was found among extracts, which in the case of Asp f 1 was shown to be functionally significant. Also, 9 potential new allergens were identified that are produced by Aspergillus and that elicit a human IgE antibody response.
Respiratory disease involving type I hypersensitivity following mold exposure is common and associated with significant morbidity. Evaluation for sensitization to mold is largely dependent on either skin testing with commercially available extracts or in vitro testing for allergen-specific IgE. Additionally, mold allergen extracts are used in routine clinical practice for immunotherapy. Despite their common usage in clinical practice, no mechanism for standardizing mold extracts currently exists, and available commercial mold allergen extracts are not standardized for their allergen content or even their starting source materials used in their manufacture. Production of panels of monoclonal IgE from patients with type I hypersensitivity as shown in this study provides a unique tool, with de facto biological relevance to human disease. It allows for more accurate assessment of the allergen content, which is vital in better understanding the results of monoclonal IgE’s diagnostic and therapeutic use. Previous attempts to standardize mold extracts using heterogeneous, polyclonal IgE antibodies derived from human serum have been complicated because of the inherent variability of the allergen-specific IgE among humans. Moreover, the absolute and relative levels of allergen-specific IgE in individuals are constantly changing. The polyclonal nature of serum-derived IgE as a mixture of many antibodies with distinct specificities and affinities, directed toward many epitopes, limits the ability to identify specific protein targets that elicit humoral IgE responses to these allergens. The ability to study the molecular interaction of IgE with its target allergen proteins by this method is thus greatly limited. The development of naturally occurring human monoclonal IgE from patients with Aspergillus allergy represents a significant new tool for evaluating the biological relevance of the components of allergen extracts and for understanding the development of allergic pathology.
We selected 3 subjects with ABPM, having high circulating Aspergillus-specific IgE levels, with the hope that their mold hypersensitivity would be reflected in their memory B cell population from which we would develop panels of monoclonal IgE antibodies directed toward Aspergillus. Our subjects possessed high circulating frequencies of B cells encoding IgE antibodies, averaging 14.5 cells per 10 million PBMCs. In our experience, this is 2- to 5-fold higher than subjects with allergies to common aeroallergens or foods. We generated a total of 70 IgE mAbs. Consistent with the primacy of Aspergillus sensitization in the development of ABPM, one-third of the mAbs showed detectable activity toward A. fumigatus. Because these monoclonal IgE antibodies were isolated in an unbiased manner, these antibodies represent a stochastic sampling of the patients’ total IgE repertoire. These data confirm that patients with ABPM mount a dramatic allergic response directed toward the mold. It should be noted that some of these antibodies may be reactive toward Aspergillus components that are not present in any of the commercially available materials, resulting in an underestimation of the response.
One question that often comes to mind when one hears of our method to grow, identify, and make stable cell lines (human hybridomas) secreting naturally occurring allergen-specific IgE mAbs from peripheral blood cells is whether artificial class-switching occurs during this process. We have not seen any evidence of this occurring and have now made hundreds of allergen-specific human IgE mAbs. Using the hybridomas presented here, one can make simple calculations to prove this point. We created, in an antigen-independent manner, 15 IgE-secreting hybridomas from subject P1 and 38 from P2. Of those, 6 from P1 and 17 from P2 were found to be reactive to Aspergillus. That means if class-switching occurred randomly within our culture system, a minimum of 40% of P1’s and 45% of P2’s entire B cell repertoire would be dedicated to fighting Aspergillus. This is at minimum an order of magnitude higher than anything ever described in the literature for any infectious process and is not possible. However, to think that a person with ABPM might have 40% to 45% of his or her IgE-encoding B cell repertoire devoted to Aspergillus seems highly plausible. Moreover, the minimum IgE-encoding B cell frequencies seen in these subjects were 13 and 16 cells per 10 million PBMCs for P1 and P2, respectively. If we assume that approximately 10% of PBMCs are B cells, then there is approximately 1 IgE-encoding B cell per 70,000 total B cells. It would therefore be impossible to obtain so very few IgE B cells, of which 40% to 45% are specific for the most clinically relevant antigens of that subject, if class-switching occurred while growing and screening B cell cultures without antigen.
Of the 26 Aspergillus-reactive human IgE mAbs generated, only 4 (1 each from P1 and P3, 2 from P2) showed evidence of binding to all 4 manufacturers’ extracts and the Aspergillus antigens on the ImmunoCAP allergosorbent. This demonstrates the tremendous variability of relevant Aspergillus antigens in these extracts. The absence of secreted proteins, such as Asp f 1, glycanosyltransferase, and endochitosanase, in all the tested extracts except ALKB suggests that some of the heterogeneity seen is likely due to the inclusion of secreted proteins in the final preparation of the extract that varies with the media used to grow the fungus. The target protein of 1D3, β-N-acetylhexosaminidase, however, is present in all extracts except for ALKB. A significant number of antibodies bind the allergen extracts but do not have target proteins that could be detected by mass spectrometry. These antibodies may be directed toward glycan derivatives from the fungal cell wall or other nonproteinaceous components of the allergen extract. The World Health Organization and International Union of Immunological Societies Allergen Nomenclature Database lists 23 A. fumigatus allergens (http://www.allergen.org/), from which only 2 (Asp f 1 and Asp f 9) were recognized by 4 of the 14 IgE mAbs with identifiable targets. The remaining 10 IgE mAbs allowed for the identification of potentially new Aspergillus allergens. Although additional characterization of the IgE antibody response to the newly identified proteins remains to be undertaken, this study suggests the response to Aspergillus in ABPM is broader than previously realized, and this study raises the question of whether a similarly unremarked diversity of allergenic response to mold in asthma or atopic dermatitis may also be present.
This study raises significant questions about the reliability of diagnostic and therapeutic use of the currently available commercial Aspergillus allergen extracts. The biological relevance of the variability in extract content is highlighted by the inability of 75% of the commercially produced allergen extracts to induce anaphylaxis in a humanized mouse model that is ideally sensitized to Asp f 1. Development of human monoclonal IgE provides a new and biologically relevant tool to investigate these widely used extracts for their content of allergenic proteins. Given the diversity of potentially novel allergens seen in this study, before attempting standardization of the existing mold extracts, significant additional work needs to be undertaken to clarify the patterns of allergic sensitization in patients with diverse expressions of mold allergy, including ABPM, asthma with fungal sensitization, and atopic dermatitis. Understanding the patterns of specific IgE responses to these allergenic proteins in these diverse disease states may allow us to move forward toward effective and reliable standardized Aspergillus extracts.
Methods
Research subjects.
The protocol for recruiting and collecting blood samples from allergic subjects was approved by the Vanderbilt University Medical Center Institutional Review Board (IRB 141330 and 142030). We identified a panel of allergic subjects in Tennessee who were diagnosed with ABPM. The relevant clinical information is summarized in Table 1 and in Supplemental Methods. Diagnosis was based on clinical history and testing serum for the presence and quantity of IgE antibody to molds and specifically A. fumigatus. One hundred milliliters of blood was collected and processed to isolate PBMCs by density gradient separation on Ficoll. The cells were immediately cryopreserved and stored in liquid nitrogen.
Human hybridoma generation.
IgE-secreting human hybridomas were generated using methodology that has for the most part not been described previously; see Supplemental materials for a detailed description. Cryopreserved PBMC samples obtained from the above research subjects were grown in culture for 6 days before screening for IgE secretion using an ELISA. A nonsecreting myeloma cell then was used to immortalize IgE-secreting B cells by electrical cytofusion (16, 17). Human hybridomas secreting IgE antibody were selected using hypoxanthine-aminopterin-thymidine medium. Hybridomas were cloned biologically by indexed single-cell flow cytometric sorting. Each hybridoma was expanded, with IgE mAb then expressed in serum-free medium and purified by immunoaffinity chromatography.
Human hybridoma antibody gene analysis.
Total RNA was extracted from 1 million clonal IgE-expressing human hybridoma cells (RNeasy kit, Qiagen: 74104). Reverse transcription PCR (RT-PCR) then was performed for 30 cycles with a 5′ primer set described previously (18) and a 3′ primer specific to the IgE constant using the OneStep RT-PCR kit (Qiagen: 210210). Following gel purification, the cDNA product was cloned into pCR2.1 using a TA cloning kit (Invitrogen: 45-0046). Antibody genes were Sanger sequenced and analyzed using the IMGT database.
Allergen extracts and IgE analysis.
A. fumigatus extracts were obtained from GREER (M3A15 lot: 322265), HS (5021JV lot: U1601190), ALKB (ASFU lot: 0002193123), and AlrgyL (182 lot: 182053116) and stored at 4°C until use. Recombinant Asp f 1 protein was provided by INDOOR Biotechnologies Inc. (RP-AF1-1). For indirect ELISA, the allergen extract was mixed with an equal volume of carbonate buffer and bound to 384-well black ELISA plates at 37°C for 3 hours. After blocking overnight at 4°C, monoclonal IgE containing supernatant from hybridoma cultures was added for 1 hour at room temperature. IgE binding was assayed using HRP-conjugated mouse anti–human IgE and a fluorogenic substrate.
IP and mass spectroscopy.
Human IgE mAbs that demonstrated binding to 1 or more extracts in ELISA and/or Western blot analysis were used for IP. Each purified mAb was covalently coupled per the manufacturer’s instructions to magnetic microbeads (Invitrogen Dynabeads: 14311D). Using the extract that had the most protein target for that given mAb, IP was done in parallel with an irrelevant IgE mAb as a control. Eluted target protein was then identified using mass spectrometry proteomics analysis. The target protein was confirmed if there was an A. fumigatus protein present in the elution of the unknown IgE mAb that was ≥10 times the total spectrum count of the same protein from the elution of the control mAb.
Human FcεRI–transgenic mouse anaphylaxis.
Human FcεRI–transgenic mice [B6.Cg-Fcεr1atm1Knt Tg(FCER1A) 1Bhk/J] were purchased from The Jackson Laboratory (stock 010506), brought out of cryogenic storage, bred, and genotyped. Mice were maintained under specific pathogen–free conditions and used in compliance with the revised Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011). These mice with 2 gene mutations express the human Fc of IgE, high affinity I, receptor for α polypeptide (FCER1A), under the control of the human FCER1A promoter and carry the mutation targeted for Fcεr1atm1Knt (19). Mice that are hemizygous for the transgene and homozygous for the targeted deletion of the mouse FcεRI respond to experimental induction of anaphylaxis with human IgE. Transgenic mice were sensitized by serial IP injections of 100 μg total IgE over 3 days and challenged by IP injection with predetermined maximal tolerated doses of allergen extract. Change in temperature from baseline was monitored using implanted temperature probes.
Statistics.
For ELISA studies, mean relative fluorescence units were calculated using independently performed assays; error bars represent SEM. Temperatures of sensitized and sham-sensitized mice following allergen challenge were compared independently for each allergen challenge solution and at each time point using paired 2-tailed t test assuming unequal variance (Microsoft Excel Office Professional Plus 2016). Time points with calculated P values less than 0.05 were labeled and considered significant. Error bars for the mouse temperature measurements represent SEM.
Study approval.
The protocol for recruiting and collecting blood samples from allergic subjects was approved by the Vanderbilt University Medical Center Institutional Review Board (IRB 141330 and 142030). All human study participants provided written informed consent. The protocols for animal care and use for these experiments have been approved by the Institutional Animal Care and Use Committee (IACUC M1700156-00). All personnel are trained on the topic of responsible conduct of research and certified to conduct animal experiments by the institutional IACUC and veterinarian.
Author contributions
SAS designed and supervised the project, generated the human hybridomas, and assisted in writing the manuscript. MAW prepared the analyzed data, figures, and tables and wrote the manuscript. AH, DJH, JD, and OB conducted binding experiments. AP contributed to the identification of Asp f 1–specific hybridomas and edited the manuscript. RGH performed ImmunoCAP evaluations. KG and RSP performed murine anaphylaxis studies.
Supplementary Material
Acknowledgments
We would like to thank Sayeh Agah, Sabina Wuenschmann, and Martin D. Chapman from INDOOR Biotechnologies Inc., for providing purified recombinant Asp f 1. We also thank all the study volunteers at Vanderbilt University Medical Center. This work was supported by NIH/National Institute of Allergy and Infectious Disease grants K08AI103038, R01AI130459, and R21AI123307 (to SAS); R01AI124456, R01AI111820, and U19AI095227 (to RSP); and R01AI077653 (to AP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Version 1. 10/18/2018
Electronic publication
Footnotes
Conflict of interest: SAS is an inventor on a pending patent (PCT/US2018/015870) describing a method to make IgE mAbs using human hybridoma technology.
License: Copyright 2018, American Society for Clinical Investigation.
Reference information: JCI Insight. 2018;3(20):e123387. https://doi.org/10.1172/jci.insight.123387.
Contributor Information
Azadeh Hadadianpour, Email: azadeh.hadadianpour@vanderbilt.edu.
Kasia Goleniewska, Email: kasia.goleniewska@vanderbilt.edu.
Scott A. Smith, Email: scott.smith.1@vumc.org.
References
- 1.Johansson SG, Bennich H. Immunological studies of an atypical (myeloma) immunoglobulin. Immunology. 1967;13(4):381–394. [PMC free article] [PubMed] [Google Scholar]
- 2.Oettgen HC, Burton OT. IgE receptor signaling in food allergy pathogenesis. Curr Opin Immunol. 2015;36:109–114. doi: 10.1016/j.coi.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casaulta C, Flückiger S, Crameri R, Blaser K, Schoeni MH. Time course of antibody response to recombinant Aspergillus fumigatus antigens in cystic fibrosis with and without ABPA. Pediatr Allergy Immunol. 2005;16(3):217–225. doi: 10.1111/j.1399-3038.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 4.Dreborg S, Agrell B, Foucard T, Kjellman NI, Koivikko A, Nilsson S. A double-blind, multicenter immunotherapy trial in children, using a purified and standardized Cladosporium herbarum preparation. I. Clinical results. Allergy. 1986;41(2):131–140. doi: 10.1111/j.1398-9995.1986.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 5.Horst M, Hejjaoui A, Horst V, Michel FB, Bousquet J. Double-blind, placebo-controlled rush immunotherapy with a standardized Alternaria extract. J Allergy Clin Immunol. 1990;85(2):460–472. doi: 10.1016/0091-6749(90)90156-X. [DOI] [PubMed] [Google Scholar]
- 6.Esch RE, Codina R. Fungal raw materials used to produce allergen extracts. Ann Allergy Asthma Immunol. 2017;118(4):399–405. doi: 10.1016/j.anai.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Esch RE. Manufacturing and standardizing fungal allergen products. J Allergy Clin Immunol. 2004;113(2):210–215. doi: 10.1016/j.jaci.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Vailes L, Sridhara S, Cromwell O, Weber B, Breitenbach M, Chapman M. Quantitation of the major fungal allergens, Alt a 1 and Asp f 1, in commercial allergenic products. J Allergy Clin Immunol. 2001;107(4):641–646. doi: 10.1067/mai.2001.114118. [DOI] [PubMed] [Google Scholar]
- 9.Reddy A, Greenberger PA. Allergic Bronchopulmonary Aspergillosis. J Allergy Clin Immunol Pract. 2017;5(3):866–867. doi: 10.1016/j.jaip.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Voskamp AL, et al. Clinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2015;3(2):192–199. doi: 10.1016/j.jaip.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Emiralioglu N, Dogru D, Tugcu GD, Yalcin E, Kiper N, Ozcelik U. Omalizumab Treatment for Allergic Bronchopulmonary Aspergillosis in Cystic Fibrosis. Ann Pharmacother. 2016;50(3):188–193. doi: 10.1177/1060028015624204. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhary A, Agarwal K, Kathuria S, Gaur SN, Randhawa HS, Meis JF. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview. Crit Rev Microbiol. 2014;40(1):30–48. doi: 10.3109/1040841X.2012.754401. [DOI] [PubMed] [Google Scholar]
- 13.Nikolaizik WH, Weichel M, Blaser K, Crameri R. Intracutaneous tests with recombinant allergens in cystic fibrosis patients with allergic bronchopulmonary aspergillosis and Aspergillus allergy. Am J Respir Crit Care Med. 2002;165(7):916–921. doi: 10.1164/ajrccm.165.7.2109008. [DOI] [PubMed] [Google Scholar]
- 14.Gautam P, et al. Identification of novel allergens of Aspergillus fumigatus using immunoproteomics approach. Clin Exp Allergy. 2007;37(8):1239–1249. doi: 10.1111/j.1365-2222.2007.02765.x. [DOI] [PubMed] [Google Scholar]
- 15.Sze KH, et al. Talaromyces marneffei Mp1p Is a Virulence Factor that Binds and Sequesters a Key Proinflammatory Lipid to Dampen Host Innate Immune Response. Cell Chem Biol. 2017;24(2):182–194. doi: 10.1016/j.chembiol.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe JE. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J Virol. 2012;86(5):2665–2675. doi: 10.1128/JVI.06335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SA, Crowe JE. Use of Human Hybridoma Technology To Isolate Human Monoclonal Antibodies. Microbiol Spectr. 2015;3(1):AID–0027. doi: 10.1128/microbiolspec.AID-0027-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith K, et al. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protoc. 2009;4(3):372–384. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dombrowicz D, et al. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157(4):1645–1651. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.