Abstract
Background:
The 4Kscore is a new commercially available blood-based diagnostic test which predicts risk for aggressive, clinically significant prostate cancer on prostate biopsy. The 4Kscore is currently restricted to patients who have not had a digital rectal exam (DRE) in the previous 96 hours, owing to prior mixed data suggesting that prostate specific antigen (PSA) isoforms may increase by a statistically significant—if not necessarily clinically significant—amount shortly after DRE. Our primary objective was to determine if 4Kscore test results are affected by a preceding DRE.
Methods:
Participants at a Prostate Cancer Awareness Week screening event sponsored by the Prostate Conditions Education Council filled out clinical history questionnaires and had blood samples for 4Kscore testing drawn prior to DRE, then 15–45 minutes following DRE. Patients with prior cancer diagnosis, 5-alpha reductase inhibitor medication use, or lower urinary tract procedures in the prior six months were excluded, resulting in a population of 162 participants for analysis. Values were then compared to determine if there was a significant difference in 4Kscore following DRE.
Results:
A statistically significant increase was seen in levels of 3 kallikreins measured (total PSA, free PSA, and intact PSA; median <0.03 ng/mL for all). This resulted in a small but statistically significant decrease in post-DRE 4Kscore (median absolute score decrease 0.43%). Using a 4Kscore cutoff of 7.5% resulted in reclassification of 10 patients (6.2%), nine of whom were “downgraded” from above the cutoff to below.
Conclusions:
If the blood draw for the 4K score is performed after a screening DRE, there is a statistically significant difference in the 4K score results, but in the vast majority of cases it would not affect clinical decision making.
Introduction
The 4Kscore® Test (OPKO BioReference Lab, Elmwood Park, NJ) is a new blood-based diagnostic test used to predict risk of Gleason score 7 or greater prostate cancer on prostate needle biopsy. The 4Kscore is assessed by a computer algorithm using levels of four serum human kallikrein biomarkers including total PSA (tPSA), free PSA (fPSA), intact PSA (iPSA), and human kallikrein 2 (hK2) along with clinical information including age, digital rectal exam (DRE) findings, and prior biopsy status. The 4Kscore has shown high specificity for Gleason score ≥ 7 prostate cancer in both retrospective1–5 and prospective6 studies and has also demonstrated potential for reducing unnecessary prostate needle biopsies1, 7 making it a valuable decision-making tool for urologists.
Currently OPKO recommends against 4Kscore testing in patients who have undergone a DRE within the previous 96 hours8. This is presumably based on mixed past data examining the effect of DRE and other prostatic manipulations (cystoscopic procedures and prostate biopsies) on total and/or free PSA levels and serum kinetics studies demonstrating a half-life of 14–18 hours for free PSA9, 10. In a study of 82 patients, Collins et al. found that DRE increased total PSA levels on average by 0.3 ng/mL and free PSA by 0.25 ng/mL, resulting in an average absolute increase in free/total PSA ratio of 0.0711. Based on this, the authors recommended phlebotomy precedes any prostatic manipulation. Lechevallier, et al. reported significant increases in PSA and free PSA of 50% and 20%, respectively, and thus also recommended blood draw prior to DRE12. Conversely, there have been multiple other studies showing either no statistically significant change in total PSA and/or free PSA, or statistically significant but not clinically significant changes after DRE and other prostatic manipulations13–18.
In most cases, it is feasible and only a minor inconvenience to wait a few days for a blood draw to obtain a 4Kscore. However, in a tertiary care setting or other practices with a large referral area, it can be a significant burden for patients from far away to wait a few days and return for a blood draw. As there are no studies in the literature examining the effect of DRE prior to 4Kscore blood draw, we investigated whether performing a DRE prior to a blood draw results in statistically and/or clinically significant changes in the 4Kscore.
Materials and Methods
Study Population
This study was approved by the Colorado Multiple Institutional Review Board (COMIRB) Protocol #00–840. We obtained consent from participants at the annual Prostate Cancer Awareness Week (PCAW) event sponsored by the Prostate Conditions Education Council on September 21, 2015. We excluded patients with 5-alpha reductase inhibitor use, prior cancer diagnosis, has received a DRE in the previous 96 hours before phlebotomy, or therapy to treat symptomatic benign prostatic hyperplasia (BPH) or any invasive urologic procedure within the prior 6 months from this study but they were eligible to participate in the other screening and educational activities of the event.
Data Collection
Patients filled out a clinical history questionnaire including demographic and clinical information, then underwent a blood draw prior to DRE and screening discussion with one of seven physicians volunteering at the event. Fifteen to 45 minutes after the DRE, the patients underwent a second blood draw. Time interval from DRE to this second blood draw was recorded.
Sample Preparation
Venous blood samples were collected in a K2EDTA tube (Lavender top) with approximately 7.5 mL of blood (at least 5 mL of blood). To ensure good mixing with the EDTA, the tube of blood was inverted eight times shortly after sample collection. These quantities of blood ensure that enough plasma can be obtained. The single determination of the four kallikrein markers requires approximately 1 mL of plasma, and the Laboratory requires 2 mL of plasma to allow re-run of the sample, as needed. Blood samples were stored at 2 to 8°C and centrifuged within 60 hours of collection at 1,000 x g for 10 minutes. Plasma samples were sent to OPKO Lab for analysis.
Hypothesis
Our hypothesis was that there would not be a difference between pre- and post-DRE 4Kscore values.
Data Analysis
Pre- and post-DRE 4Kscore values were compared using Wilcoxon signed-rank test and Passing-Bablok regression analysis. Multivariate regression analysis was also performed to examine potential association between patient clinical factors (age, DRE findings, and prior biopsy status) and significant changes in 4Kscore. We further examined changes in a subgroup of patients with initial 4Kscore between 5% and 10%, as these values are close to the 7.5% threshold for proceeding with prostate biopsy described in the literature and would potentially be most affected by changes after DRE. We used McNemar’s test to compare proportions of men upgraded/downgraded based on pre- and post-DRE 4Kscore. Results were considered statistically significant at P ≤ 0.05.
Results
A total of 162 PCAW patients met inclusion criteria and consented to participate in our study. Study population baseline characteristics are reported in Table 1. A small but statistically significant increase (P≤0.05) in the medians and interquartile ranges (IQR) were seen in levels of three kallikreins after DRE – tPSA (median = +0.027 ng/mL, IQR = −0.03–0.10), fPSA (median = +0.024, IQR = −0.001–0.066), and iPSA (median = +0.012, IQR = −0.003–0.033) as reported in the Table 2. Variations in the levels of hk2 were not significantly different (median change = 0 ng/mL, IQR = −0.002–0.001). The net effect of these changes was a median absolute decrease in 4Kscore of 0.43% (IQR = −0.91% to −0.04%). Figure 1 illustrates changes in the four kallikreins and 4Kscore pre- and post-DRE.
Table 1:
Study Population Characteristics (N = 162)
| Characteristic | Value |
|---|---|
| Median Age (IQR) | 67.13 yrs (60.93 - 72.51 yrs) |
| Median Time between DRE and 2nd Blood Draw (IQR) | 20 mins (18 – 26 min) |
| Abnormal DRE | 18 patients (11%) |
| Prior Negative Biopsy | 28 patients (17%) |
| Mean tPSA before DRE | 2.37 ng/mL |
| Mean tPSA after DRE | 2.41 ng/mL |
| Caucasian race | 123 (75.9%) |
| African American race | 24 (14.8%) |
Table 2:
4Kscore and Kallikrein values before and after DRE
| Pre-DRE | Post-DRE | Delta, Median (IQR) | p value | |
|---|---|---|---|---|
| 4Kscore (%) | −0.43% (−0.91%, −0.04%) | <0.01 | ||
| 10th percentile | 1.4 | 1.2 | ||
| 25th percentile | 2.9 | 2.7 | ||
| 50th percentile | 4.8 | 4.5 | ||
| 75th percentile | 8.7 | 7.6 | ||
| 90th percentile | 16.8 | 15.3 | ||
| tPSA (ng/ml) | 0.027 (−0.03, 0.10) | <0.01 | ||
| 10th percentile | 0.51 | 0.52 | ||
| 25th percentile | 0.93 | 0.90 | ||
| 50th percentile | 1.61 | 1.72 | ||
| 75th percentile | 3.19 | 3.38 | ||
| 90th percentile | 4.84 | 4.92 | ||
| fPSA (ng/ml) | 0.024 (−0.001, 0.066) | <0.01 | ||
| 10th percentile | 0.16 | 0.17 | ||
| 25th percentile | 0.26 | 0.26 | ||
| 50th percentile | 0.43 | 0.47 | ||
| 75th percentile | 0.73 | 0.81 | ||
| 90th percentile | 1.24 | 1.26 | ||
| iPSA (ng/ml) | 0.012 (−0.003, 0.033) | <0.01 | ||
| 10th percentile | 0.11 | 0.13 | ||
| 25th percentile | 0.17 | 0.17 | ||
| 50th percentile | 0.28 | 0.29 | ||
| 75th percentile | 0.44 | 0.50 | ||
| 90th percentile | 0.70 | 0.72 | ||
| hK2 (ng/ml) | 0 (−0.002, 0.001) | NS | ||
| 10th percentile | 0.013 | 0.014 | ||
| 25th percentile | 0.025 | 0.025 | ||
| 50th percentile | 0.048 | 0.049 | ||
| 75th percentile | 0.074 | 0.074 | ||
| 90th percentile | 0.114 | 0.115 | ||
| f/tPSA ratio | 0.009 0.003, 0.026) | <0.01 | ||
| 10th percentile | 0.16 | 0.17 | ||
| 25th percentile | 0.20 | 0.23 | ||
| 50th percentile | 0.26 | 0.27 | ||
| 75th percentile | 0.35 | 0.37 | ||
| 90th percentile | 0.42 | 0.44 |
FIGURE 1.
Changes in 4 individual Kallikreins and 4Kscore After Digital Rectal Exam. p-value <0.01 for all except hk2, which was >0.05
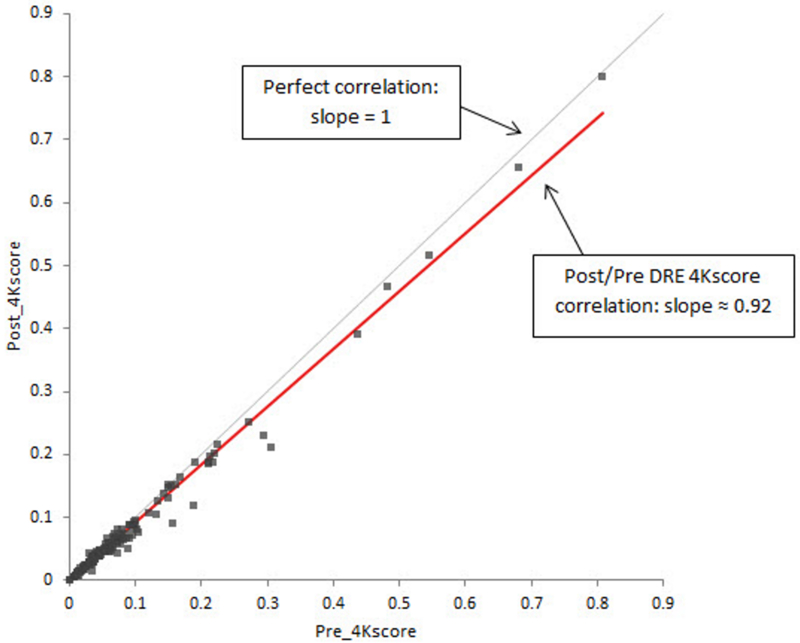
Passing-Bablok analysis demonstrated generally good agreement between pre- and post-DRE 4Kscore values (Figure 2). The slope of this comparison fit line was 0.92, while a comparison line showing no effect of DRE would have an expected slope of 1.0.
FIGURE 2.
Passing-Bablok Regression Graph for Pre- and Post-DRE 4Kscore Values.
Multivariate logistic regression analysis demonstrated that positive DRE and increasing age were independently associated with increase in post-DRE fPSA (p = 0.025 and p = 0.017, respectively) and decrease in post-DRE 4Kscore (p = 0.017 and p = 0.006, respectively). Other factors examined in the multivariate regression which did not demonstrate any independent effect on the degree of change in 4Kscore included patient race (Caucasian versus African American), prior negative biopsy history, and time to second blood draw.
Using a 4Kscore of 7.5% as a theoretical trigger for prostate biopsy, we found that 10 patients (6.2% of participants) would be reclassified based on change in 4Kscore; 9 of these patients had a 4Kscore greater than 7.5% prior to DRE and 4Kscore less than 7.5% after DRE while one patient had an initial 4Kscore less than 7.5% prior to DRE and greater than 7.5% after DRE. Thus, a statistically significant proportion of men at risk for high grade prostate cancer with pre-DRE 4Kscore ≥ 7.5% were downgraded by post-DRE 4Kscore < 7.5% compared to those upgraded (McNemar’s test P ≤ 0.022). These results are reported in Table 3. All ten of these patients had an initial 4Kscore between 5% and 10% and post-DRE 4Kscores remained between 5% and 10%. The absolute change in results from pre- to post-DRE 4Kscore among patients with initial 4Kscore between 5% and 10% ranged from −3.7% to +1.2%.
Table 3:
Reclassification of 4Kscore Above and Below DRE Cutoff of 7.5%
| Post-DRE 4Kscore < 7.5% | Post-DRE 4Kscore ≥ 7.5% | Total | |
|---|---|---|---|
| Pre-DRE 4Kscore ≥ 7.5% | 112 | 1 | 113 |
| Pre-DRE 4Kscore ≥ 7.5% | 9 | 40 | 49 |
| Total | 121 | 41 | 162 |
| Reclassified patients* | 9 | 1 | 10 |
McNemar’s test P = 0.022
Discussion
Overall changes in kallikrein levels measured in our study were small, with median change less than 0.03 ng/mL for all four measured. The majority of change was seen in free PSA levels. These changes resulted in a median decrease in the 4Kscore of less than 0.5%. With a median pre-DRE 4Kscore of 4.8%, this represents a small change. Our data demonstrate that while a DRE prior to blood draw appears to result in a statistically significant change in 4Kscore, this small change is clinically insignificant in the overwhelming majority of cases (about 94%). As a result, a significantly higher proportion of men will be downgraded from high-risk to low-risk for high-grade prostate cancer than those upgraded. Due to lack of biopsy information for this cohort (limitations discussed further below), we are unable to assess the clinical impact. However, the overall range of change in 4Kscore was −9.5% to +1.5%, which is enough to potentially impact clinical decision-making, particularly in patients with 4Kscore values near the biopsy threshold of 7.5%.
Our study does have several limitations. First, the study population is at risk of selection bias, given that these are men who voluntarily chose to seek annual prostate cancer screening and education at our PCAW event. Second, DRE technique could potentially have some effect on the degree of change in serum kallikreins. Having multiple providers performing DREs should hopefully have limited this effect to some degree. We did retrospectively look at the providers who performed DREs on patients with absolute change in 4Kscore greater than 1.5%, and all providers were represented in this group in similar numbers. Third, although we did this study in a prospective fashion, one could argue that including controls undergoing two 4Kscore blood draws without an intervening DRE could help by providing comparison to the effect of variation between sequential blood draws alone. Finally, the nature of this study at an informational event certainly introduces a limitation due to the fact that while patients undergoing screening receive advice to seek follow up to discuss abnormal test results, we do not have access to information about how many patients actually proceeded to biopsy nor to pathology results from those who subsequently did so, as patients who did seek further evaluation likely did so across multiple locations and providers, many outside of our health system. In an ideal study all patients undergoing blood testing would also have undergone prostate biopsy, but this would have exposed many patients to potential unnecessary harm and thus was ethically not feasible.
Conclusions
We conclude that while a DRE does appear to have a minor effect on 4Kscore results, the impact of the change is small and is unlikely to affect clinical decision making in the overwhelming majority of cases. Ideally, it is preferable for a blood draw to be performed prior to DRE, however a DRE should not necessarily preclude a patient from undergoing a subsequent 4Kscore blood draw at the same visit. If the 4Kscore result is less than 5% or greater than 10%, we feel the clinician can be confident that the result is reliable even after a recent DRE. However, physicians and their patients with 4Kscore results between 5% and 10% should consider the possibility that DRE caused the result to change relative to the 7.5% threshold and consider if such a change would alter management decisions. It is important to also keep in mind that while a value of 7.5% is often used as a trigger for prostate biopsy, this is not an absolute cutoff; rather, the 4Kscore is meant to be considered in conjunction with other patient information to aid the physician and patient in making management decisions.
Acknowledgments
Funding: Prostate Cancer Awareness Week event was sponsored and promoted by the Prostate Conditions Education Council. OPKO Lab provided 4Kscore testing and assisted with statistical analysis.
Footnotes
Disclosures: Dr. Mitchell Steiner is a consultant for OPKO Lab and Dr. Jay Newmark is Senior Director of Medical Affairs at OPKO Health. All other authors report no relevant disclosures.
Note: Results of this study in abstract form have been presented during 2016 American Urological Association (AUA) meeting in San Diego, CA and 2016 AUA South Central Section meeting in Colorado Springs, CO.
Reference List
- 1.Vickers AJ, Cronin AM, Aus G, Pihl CG, Becker C, Pettersson K, Scardino PT, Hugosson J, Lilja H. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC.Med 2008; 6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vickers AJ, Cronin AM, Roobol MJ, Savage CJ, Peltola M, Pettersson K, Scardino PT, Schroder FH, Lilja H. A four-kallikrein panel predicts prostate cancer in men with recent screening: data from the European Randomized Study of Screening for Prostate Cancer, Rotterdam. Clinical Cancer Research 2010; 16(12):3232–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Roobol MJ, Savage CJ, Peltola M, Pettersson K, Scardino PT, Vickers AJ, Schroder FH, Lilja H. A four-kallikrein panel for the prediction of repeat prostate biopsy: data from the European Randomized Study of Prostate Cancer screening in Rotterdam, Netherlands. British Journal of Cancer 2010; 103(5):708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vickers AJ, Gupta A, Savage CJ, Pettersson K, Dahlin A, Bjartell A, Manjer J, Scardino PT, Ulmert D, Lilja H. A panel of kallikrein marker predicts prostate cancer in a large, population-based cohort followed for 15 years without screening. Cancer Epidemiology, Biomarkers and Prevention 2011; 20(2):255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsson S, Maschino A, Schroder F, Bangma C, Steyerberg EW, Van der Kwast T, van LG, Vickers A, Lilja H, Roobol MJ. Predictive value of four kallikrein markers for pathologically insignificant compared with aggressive prostate cancer in radical prostatectomy specimens: results from the European Randomized Study of Screening for Prostate Cancer section Rotterdam. Eur.Urol 2013; 64(5):693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parekh DJ, Punnen S, Sjoberg DD, Asroff SW, Bailen JL, Cochran JS, Concepcion R, David RD, Deck KB, Dumbadze I, Gambla M, Grable MS, Henderson RJ, Karsh L, Krisch EB, Langford TD, Lin DW, McGee SM, Munoz JJ, Pieczonka CM, Rieger-Christ K, Saltzstein DR, Scott JW, Shore ND, Sieber PR, Waldmann TM, Wolk FN, Zappala SM. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur.Urol 2015; 68(3):464–470. [DOI] [PubMed] [Google Scholar]
- 7.Vickers A, Cronin A, Roobol M, Savage C, Peltola M, Pettersson K, Scardino PT, Schroder F, Lilja H. Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: an independent replication. Journal of Clinical Oncology 2010; 28(15):2493–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.OPKO Lab, Nashville TN 4Kscore Clinical Kit, Revision 2. 2014. Retrieved at https://opkodd.files.wordpress.com/2014/12/opko_4Kscore_test_package_insert_v2.pdf
- 9.Bjork T, Ljungberg B, Piironen T, Abrahamsson PA, Pettersson K, Cockett AT, Lilja H. Rapid exponential elimination of free prostate-specific antigen contrasts the slow, capacity-limited elimination of PSA complexed to alpha 1-antichymotrypsin from serum. Urology 1998; 51(1):57–62. [DOI] [PubMed] [Google Scholar]
- 10.Lilja H, Haese A, Bjork T, Friedrich MG, Piironen T, Pettersson K, Huland E, Huland H. Significance and metabolism of complexed and noncomplexed prostate specific antigen forms, and human glandular kallikrein 2 in clinically localized prostate cancer before and after radical prostatectomy. J.Urol 1999; 162(6):2029–2034. [DOI] [PubMed] [Google Scholar]
- 11.Collins GN, Martin PJ, Wynn-Davies A, Brooman PJ, O’Reilly PH. The effect of digital rectal examination, flexible cystoscopy and prostatic biopsy on free and total prostate specific antigen, and the free-to-total prostate specific antigen ratio in clinical practice. J.Urol 1997; 157(5):1744–1747. [PubMed] [Google Scholar]
- 12.Lechevallier E, Eghazarian C, Ortega JC, Roux F, Coulange C. Effect of digital rectal examination on serum complexed and free prostate-specific antigen and percentage of free prostate-specific antigen. Urology 1999; 54(5):857–861. [DOI] [PubMed] [Google Scholar]
- 13.Yuan JJ, Coplen DE, Petros JA, Figenshau RS, Ratliff TL, Smith DS, Catalona WJ. Effects of rectal examination, prostatic massage, ultrasonography and needle biopsy on serum prostate specific antigen levels. J.Urol 1992; 147(3 Pt 2):810–814. [DOI] [PubMed] [Google Scholar]
- 14.Deliveliotis C, Alivizatos G, Stavropoulos NJ, Makrychoritis K, Koutsokalis G, Kiriakakis Z, Kostakopoulos A, Dimopoulos C. Influence of digital examination, cystoscopy, transrectal ultrasonography and needle biopsy on the concentration of prostate-specific antigen. Urol Int 1994; 53(4):186–190. [DOI] [PubMed] [Google Scholar]
- 15.Crawford ED, Schutz MJ, Clejan S, Drago J, Resnick MI, Chodak GW, Gomella LG, Austenfeld M, Stone NN, Miles BJ, . The effect of digital rectal examination on prostate-specific antigen levels. JAMA 1992; 267(16):2227–2228. [PubMed] [Google Scholar]
- 16.Ornstein DK, Rao GS, Smith DS, Ratliff TL, Basler JW, Catalona WJ. Effect of digital rectal examination and needle biopsy on serum total and percentage of free prostate specific antigen levels. J.Urol 1997; 157(1):195–198. [PubMed] [Google Scholar]
- 17.Cevik I, Turkeri LN, Ozveri H, Ilker Y, Akdas A. Short-term effect of digital rectal examination on serum prostate-specific antigen levels. A prospective study. Eur.Urol 1996; 29(4):403–406. [DOI] [PubMed] [Google Scholar]
- 18.Chybowski FM, Bergstralh EJ, Oesterling JE. The effect of digital rectal examination on the serum prostate specific antigen concentration: results of a randomized study. J.Urol 1992; 148(1):83–86. [DOI] [PubMed] [Google Scholar]