Abstract
The blood platelets are multifunctional blood cells which are involved in the initiation of atheroma, endothelial dysfunction, and modulation of inflammatory and immune responses in the pathophysiology of many diseases. Because of their multifaceted pro‐inflammatory activity, platelets may be involved in the pathogenesis of autoimmune thyroid diseases (AITDs), such as Hashimoto's thyroiditis and Graves' disease. The aim of this study was to assess the level of activation and response ability of platelets in AITDs. We used the flow cytometry technique and kinetic measurement of aggregation to analyse platelet function immediately after blood collection and to demonstrate their activation in the circulation of patients with AITDs. We noted reorganization of platelet subpopulations (normal platelets, microparticles and aggregates) in AITDs, dependent on the degree of cell activation. We proved the elevated expression of the active form of integrin receptor GPIIb/IIIa, responsible for platelet aggregation, and in the kinetic test we confirmed the increased aggregation of platelets in different intracellular signal pathways (dependent on ADP, collagen, arachidonic acid). Our study demonstrates the high platelet activation level found in AITDs.
Keywords: aggregation, autoimmune thyroid diseases, blood platelets, platelet‐derived microparticles
1. INTRODUCTION
The involvement of blood platelets in autoimmune diseases is well documented, and disturbance in their functioning is a hallmark of idiopathic thrombocytopenic purpura (ITP),1 rheumatoid arthritis (RA),2 systemic lupus erythematosus (SLE),3 antiphospholipid syndrome 4 and multiple sclerosis.5 However, there is no data about platelet activity in autoimmune thyroid diseases (AITDs): Hashimoto's thyroiditis (HT) and Graves' disease (GD). The pro‐inflammatory activity of platelets leads to disturbance of the haemostatic balance and can increase the risk of cardiovascular disease.6, 7, 8, 9 Studies indicate that people with AITDs are prone to the development of other autoimmune diseases and cardiovascular diseases. These include ITP and thrombocytopenia associated with disorders in the structure and function of blood platelets.10 The potential mechanism that may be associated with AITDs is the immunogenic destruction of platelets by circulating autoantibodies, which react with both target thyroid antigens and epitopes located on the platelets' surface, mainly the typical glycoprotein receptors.10
In view of the proven significant contribution of platelets to the mechanisms of inflammation and to autoimmune processes, the aim of this current study was to answer the simple question of whether platelets present in the circulation of people with autoimmune hyperthyroidism (GD) or hypothyroidism (HT) exhibit the characteristics of increased activity.
2. MATERIALS AND METHODS
2.1. Demographic and clinical characteristics
The study population included 25 HT patients positive for both TPO‐Ab, Tg‐Ab and with elevated level of TSH and 50 GD patients with elevated concentration of T4 and/or T3, and suppressed TSH level, diffusely increased thyroidal uptake of iodine‐131, presence of TSHR antibodies and/or antimicrosomal antibodies. All subjects were without other autoimmune or acute and chronic inflammatory diseases. The healthy control (HC) consisted of 40 donors without any autoimmune or chronic inflammatory disease. All subjects were characterized by the correct number of platelets and did not use antiplatelet or immunomodulate drugs for at least 14 days prior to blood collection.
All procedures were carried out according to the Helsinki Declaration and were approved by the Bioethics Committee of the Faculty of Biology and Environmental Protection of the University of Lodz, Poland, with Resolution No. 12/KBBN‐UŁ/II/2014.
2.2. Platelet aggregation
Platelet aggregation determined in response to physiological agonists: ADP (10 μmol/L), collagen (2 μg/mL) or arachidonic acid (0.5 mmol/L) was measured in platelet‐rich plasma using the turbidimetric method on the optical Chrono‐Log Aggregometer.
2.3. Flow cytometry analysis
The resting or agonist‐stimulated (ADP 20 μmol/L, collagen 20 μg/mL) platelets were analysed using a flow cytometer ‐ LSR II Flow Cytometer (Becton Dickinson, San Diego, CA, USA). After fixation (1% Cellfix solution) blood samples were stained with saturating concentrations of murine monoclonal IgG1 antibodies: peridinin–chlorophyllprotein complex (PerCp)—a conjugated antibody against CD61 (constitutive platelets' surface receptor, that distinguishes platelets from other cells), and fluorescein isothiocyanate (FITC)—a conjugated PAC‐1 antibody binds to the activated conformation of GPIIb/IIIa receptor. The fluorescence of 10 000 platelets (CD61/PerCP‐positive objects) was measured each time. In each sample, FITC fluorescence was detected and the percentage of PAC‐1‐positive platelets was determined relative to the total number of platelets (10,000 CD61/PerCP‐positive cells). Based on size and granularity, we determined forward light scatter (FSC) vs. side light scatter plots (SSC) in CD61/PerCP‐positive objects, formation of platelet subpopulations dependent on the degree of cell activation: aggregates (PAs) and platelet‐derived microparticles (PMPs). Using reference beads, we estimated FSC gates. CD61PerCP‐positive objects with an FSC lower than 102.3 were characterized as PMPs, while objects with FSC higher than 104 were considered PAs. All data analysis was performed in FACSDiva version 6.1.2.
2.4. Statistical analysis
The results were analysed for normality with a Shapiro‐Wilk test. The significance of the differences between the values was determined by normality using a U‐Mann‐Whitney test (for data deviating from normal distribution).
3. RESULTS
In blood with non‐stimulated platelets, we observed an augmented basal level of PAs in the both GD and HT groups (about 2‐fold vs HC; P < 0.001) as well as an increased level of PMPs in GD (2‐fold vs HC; P < 0.001) and HT (2.5‐fold vs HC; P < 0.001). Platelet activation was also measured through surface binding of PAC‐1 antibodies complementary only to the active form of GPIIb/IIIa responsible for platelet aggregation. Objects with a level of FITC fluorescence greater than 103.05 were characterized as platelets with PAC‐1 antibody binding. Their number was 2.5‐fold higher in GD patients (P < 0.001), and 2‐fold higher in HT patients (P < 0.001), than in HC. The analysis of blood platelet responsiveness to the action of physiological agonists: ADP (20 μmol/L) or 20 μg/mL of collagen, showed the elevated PAC‐1 binding (almost 1.5‐fold increase for GD, P < 0.001; and 2‐fold for HT, P < 0.001), relative to HC. The pool of PAs in GD patients was 1.5‐fold greater vs HC; P < 0.001, and in the HT patients 1.2‐fold greater vs HC; P < 0.001. Similarly, the proportion of PMPs in GD patients was about 2.5‐fold larger vs HC; P < 0.001 and in HT patients was almost 2‐fold larger vs HC; P < 0.01 (Figure 1).
Figure 1.

Cytometry analysis of nonstimulated and agonist‐stimulated platelets (ADP and collagen) in whole blood samples from GD and HT patients vs healthy controls. The data represents the median ± interquartile range Q1‐Q3 (box), and range—minimum and maximum (whisker) for each group. In each sample, 10 000 CD61‐positive objects (platelets) were measured. The subpopulations of platelets were distinguished based on their size and granularity on the forward light scatter (FSC) vs side light scatter (SSC) plots. CD61‐positive objects with FSC higher than 104 were characterized as platelet aggregates (A), with FSC lower than 102.3 were characterized as PMPs (B). Expression of the active form of GPIIb/IIIa was determined based on fluorescence of PAC‐1‐FITC monoclonal antibody (C). Statistical analysis was performed using a Mann‐Whitney U test for GD and HT patients vs HC; ∗ P < 0.01, ∗∗ P < 0.001
We also monitored the kinetic course of the aggregation process. Examples of aggregation curves recorded in an optical aggregate are shown in Figure 2. The platelet aggregation upon ADP stimulation was 10% higher for GD (P < 0.005), and 8% for HT (P < 0.05), compared to HC. Collagen caused 10% growth of control for GD and 11% for HT (P < 0.005), while aggregation induced by arachidonic acid was 11% greater for GD (P < 0.05) and 20% for HT (P < 0.005), than in HC (Figure 2).
Figure 2.
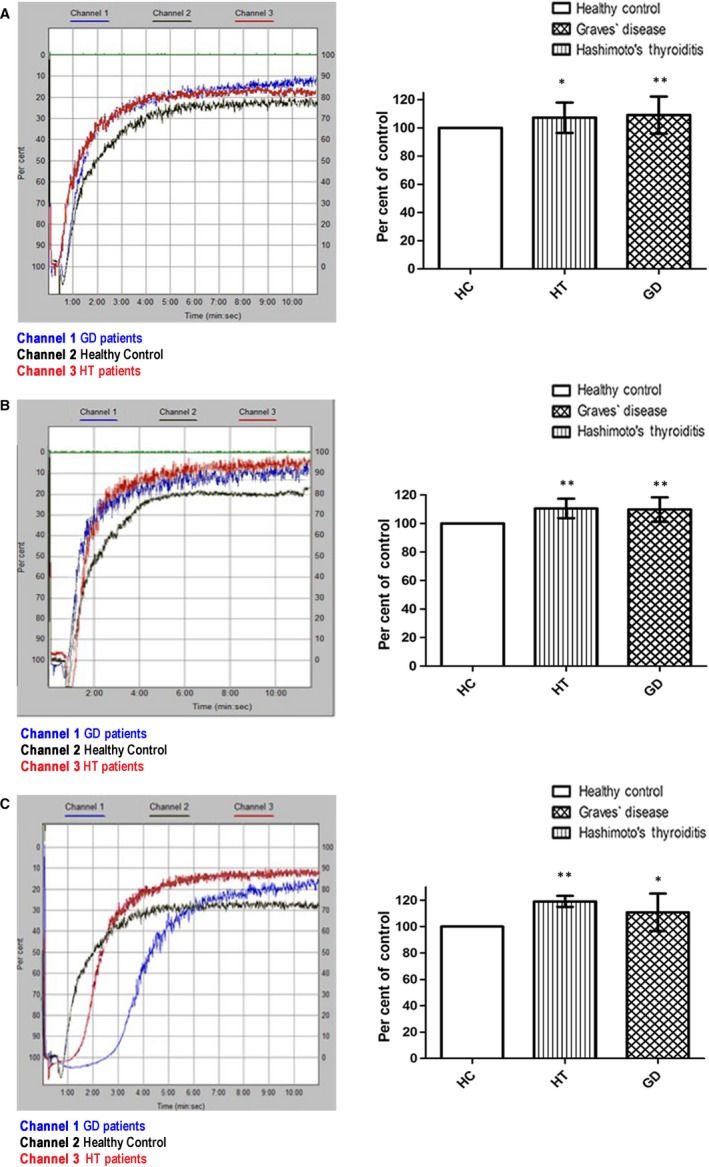
Blood platelet aggregation measured in platelet‐rich‐plasma. The typical curves of platelet aggregation after stimulation of platelets by ADP (A), collagen (B), arachidonic acid (C), were recorded with the optical Chrono‐Log aggregometer. The data are also presented as means ± SD for HT and GD platelets vs HC, when the value of the control was taken as 100%;. ∗ P < 0.05, ∗∗ P < 0.005 (by Mann‐Whitney U test)
4. DISCUSSION
Our studies, for the first time demonstrated changes in the haemostatic function of platelets in HT and GD. We proved the elevated levels of platelet aggregation and generation of PMPs (vesicular structures mainly produced during activation and cell death) as well a greater sensitivity to agonists, which is crucial for platelet haemostatic function. The excessive production of microparticles induced by permanent cell activation may contribute to chronic inflammatory processes11 and predispose to autoimmune diseases.12 Our findings are in line with results indicating an increased level of PMPs in autoimmune diseases, such as RA and SLE, which have been associated with disease activity.12, 13 It has been proposed that PMPs can interact with circulating autoantibodies and C1q, participating in the formation of immune complexes, which could trigger immune responses in autoimmune diseases.14
GPIIb/IIIa receptors function as constituent antigens, but after platelet activation the number of GPIIb/IIIa copies grows and receptors change their conformation. Therefore, as surface antigens, they are a good marker for platelet activation. The conformational changes in the GPIIb/IIIa complex upon the platelets' activation allow binding of fibrinogen, and in consequence platelet aggregation.15 We showed the increased surface expression of the active form of GPIIb/IIIa on platelets in AITDs. Aggregation is the final stage of platelet activation applicable to the cellular processes of haemostasis. We demonstrated significantly higher platelet aggregation in AITDs.
5. CONCLUSIONS
Our analysis in whole blood samples without isolation of platelets significantly reduces the risk of creating artefacts and may illustrate the activation state of platelets in circulation. Therefore, we can conclude that the platelet hyperactivity is a phenomenon occurring in the vascular system of patients with AITDs. Because of the lack of differences in the studied groups (HT vs GD), we postulate that in AITDs, platelet abnormalities result from inflammation and autoimmune processes, more than hormone disorders.
AUTHOR CONTRIBUTIONS
Małgorzata Tomczyńska and Michał Bijak conceived and designed the experiments; Małgorzata Tomczyńska performed the experiments; Małgorzata Tomczyńska and Joanna Saluk‐Bijak analysed the data; Ireneusz Salata contributed reagents/materials/analysis tools; Małgorzata Tomczyńska, Joanna Saluk‐Bijak and Michał Bijak wrote the manuscript. All authors approved the final version of the manuscript.
CONFLICTS OF INTEREST
The authors confirm that there are no conflicts of interest.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Polish National Science Centre (no. 2014/13/N/NZ5/01389).
Tomczyńska M, Salata I, Bijak M, Saluk‐Bijak J. The potential contribution and role of a blood platelets in autoimmune thyroid diseases. J Cell Mol Med. 2018;22:6386–6390. 10.1111/jcmm.13862
REFERENCES
- 1. Sugimoto K, Sasaki M, Isobe Y, et al. Improvement of idiopathic thrombocytopenic purpura by antithyroid therapy. Eur J Haematol. 2005;74:73‐74. [DOI] [PubMed] [Google Scholar]
- 2. Wang F, Wang NS, Yan CG, et al. The significance of platelet activation in rheumatoid arthritis. Clin Rheumatol. 2007;26:768‐771. [DOI] [PubMed] [Google Scholar]
- 3. Yee NS, Schuster SJ. Clinical remission of acquired thrombasthenia with low‐dose methotrexate in a patient with systemic lupus erythematosus. Mayo Clin Proc. 2006;81:566‐567. [DOI] [PubMed] [Google Scholar]
- 4. Baroni G, Banzato A, Bison E, et al. The role of platelets in antiphospholipid syndrome. Platelets. 2017;7:1‐5. [DOI] [PubMed] [Google Scholar]
- 5. Morel A, Rywaniak J, Bijak M, et al. Flow cytometric analysis reveals the high levels of platelet activation parameters in circulation of multiple sclerosis patients. Mol Cell Biochem. 2017;430:69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gremmel T, Ay C, Riedl J, et al. Platelet‐specific markers are associated with monocyte‐platelet aggregate formation and thrombin generation potential in advanced atherosclerosis. Thromb Haemost. 2016;115:615‐621. [DOI] [PubMed] [Google Scholar]
- 7. Pfluecke C, Berndt K, Wydra SN, et al. Atrial fibrillation is associated with high levels of monocyte‐platelet‐aggregates and increased CD11b expression in patients with aortic stenosis. Thromb Haemost. 2016;115:1‐8. [DOI] [PubMed] [Google Scholar]
- 8. Andrews RK, Arthur JF, Gardiner EE. Neutrophil extracellular traps (NETs) and the role of platelets in infection. Thromb Haemost. 2014;4:659‐665. [DOI] [PubMed] [Google Scholar]
- 9. Gowert NS, Donner L, Chatterjee M, et al. Blood platelets in the progression of Alzheimer's disease. PLoS ONE. 2014;9:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cordiano I, Betterle C, Spadaccino CA, et al. Autoimmune thrombocytopenia (AITP) and thyroid autoimmune disease (TAD), overlapping syndromes? Clin Exp Immunol. 1998;113:373‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cloutier N, Tan S, Boudreau LH, et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle‐associated immune complexes. EMBO Mol Med. 2013;5:235‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dye JR, Ullal AJ, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Scand J Immunol. 2013;78:140‐148. [DOI] [PubMed] [Google Scholar]
- 13. Sellam J, Proulle V, Jüngel A, et al. Increased levels of circulating microparticles in primary Sjögren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2009;11:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nielsen CT, Østergaard O, Stener L, et al. Increased IgG on cell‐derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 2012;64:1227‐1236. [DOI] [PubMed] [Google Scholar]
- 15. Nagy JR, Debreceni IB, Kappelmayer J. Flow cytometric investigation of classical and alternative platelet activation markers. EJIFCC. 2012;4:124‐134. [PMC free article] [PubMed] [Google Scholar]


