Abstract
Colon crypts are recognized as a mechanical and biochemical Turing patterning model. Colon epithelial Caco‐2 cell monolayer demonstrated 2D Turing patterns via force analysis of apical tight junction live cell imaging which illuminated actomyosin meshwork linking the actomyosin network of individual cells. Actomyosin forces act in a mechanobiological manner that alters cell/nucleus/tissue morphology. We observed the rotational motion of the nucleus in Caco‐2 cells that appears to be driven by actomyosin during the formation of a differentiated confluent epithelium. Single‐ to multi‐cell ring/torus‐shaped genomes were observed prior to complex fractal Turing patterns extending from a rotating torus centre in a spiral pattern consistent with a gene morphogen motif. These features may contribute to the well‐described differentiation from stem cells at the crypt base to the luminal colon epithelium along the crypt axis. This observation may be useful to study the role of mechanogenomic processes and the underlying molecular mechanisms as determinants of cellular and tissue architecture in space and time, which is the focal point of the 4D nucleome initiative. Mathematical and bioengineer modelling of gene circuits and cell shapes may provide a powerful algorithm that will contribute to future precision medicine relevant to a number of common medical disorders.
Keywords: 4D nucleome, colon crypt, colorectal cancer, functional bowel disorders, glucocorticoid, HES1, mechanobiology, Notch, tight junction, turing pattern
1. GENERAL HYPOTHESIS
Alan Turing made the initial proposal that it is feasible to mathematically model specific patterns observed in multicell systems in his 1952 seminal paper “The Chemical Basis of Morphogenesis”.1 He mentioned that “The purpose of this paper is to discuss a possible mechanism by which the genes of a zygote may determine the anatomical structure of the resulting organism.”, he also suggested that “The genes themselves may also be considered to be morphogens.”.1 Turing was limited by the lack of availability of appropriate data: “There are probably many biological examples of this metabolic oscillation, but no really satisfactory one is known to the author.”.1 The NIH launched the 4D Nucleome program to understand the principles behind the 3D organization of the nucleus in space and time (the 4th dimension), the role nuclear organization plays in gene expression and cellular function, and how changes in the nuclear organization affect normal development as well as various diseases. Following Turing's inspiration, 3D‐FISH has been employed to track movements of oscillatory genes in synchronized cells to generate data required for producing 4D Nucelome algorithms of the genome.2, 3 Smale and Rajapakse expanded the Turing “gene morphogen” proposal in publications that updated mathematical models able to handle extra dimensions in addition to 2D Turing pattering and proposed implications relevant to the 4D Nucleome initiative.2, 4, 5 Colon crypts are viewed as a 2D Turing patterning multicell system based on cell‐cell junctional forces or transcription factors HES1 and MATH1 mathematical analysis.6, 7 The neuroectoderm Turing patterning Delta‐Notch lateral inhibition mechanism has been mathematically verified in colon crypts.6 Relevant to this article, overexpression of colon epithelial cell tight junction protein CLDN1 (claudin 1) increases Notch activity measured by HES1 and MATH1 expression.8 Notch signalling target HES1 transcription can be triggered by mechanical forces that pull cells apart which activate Notch cleavage.9 CLDN1 holds cells together which counteracts the pulling force. These features provide a potential linkage between oscillatory actomyosin forces and oscillatory HES1 expression.10, 11 Gene regulatory circuits containing cell and developmental stage‐specific transcription factors forming “a two‐gene network with two repressors” that bind each other's promoters have been proposed as “hardwiring” required for stable equilibrium of cell types in “Mathematics of the Genome” involving “n‐Dimensional Dynamical Systems”.5 We observed potential “toggle gene circuits” as proposed involving the transcription factors HES1 and NR3C1 (glucocorticoid receptor, GR) that regulate CLDN1 along the human colon crypt axis, and in vitro Caco‐2 cell differentiation.5, 12, 13, 14 We propose that modelling the 4D Nucleome dynamics, 4D mRNA distribution and actomyosin forces that regulate tight junction protein expression and function will predict the self‐organizing of epithelial cells in a cell type‐, developmental stage‐specific manner. This information will be useful in generating a precise mathematical model of human colon crypts, which could be employed as a powerful algorithm to help design precision medicine approaches for targeted, disease‐specific treatments in a variety of medical ailments, including functional bowel disorders (FBD) and colorectal cancer (CRC).5, 12, 14, 15, 16
To generate “proof of concept” data, we tracked the formation of a coordinated epithelial cell sheet during Caco‐2 cell differentiation on a smooth, flat and hard glass surface that recapitulates known gene expression patterns that occur along the colon crypt axis. Detailed in‐depth description and discussion of the rotational 3D mechanogenomic Turing patterns observed during differentiation are included in the supplementary and online material (Figure 1 and S1) (http://www.socr.umich.edu/projects/3d-cell-morphometry/data.html).17, 18, 19, 20
Figure 1.
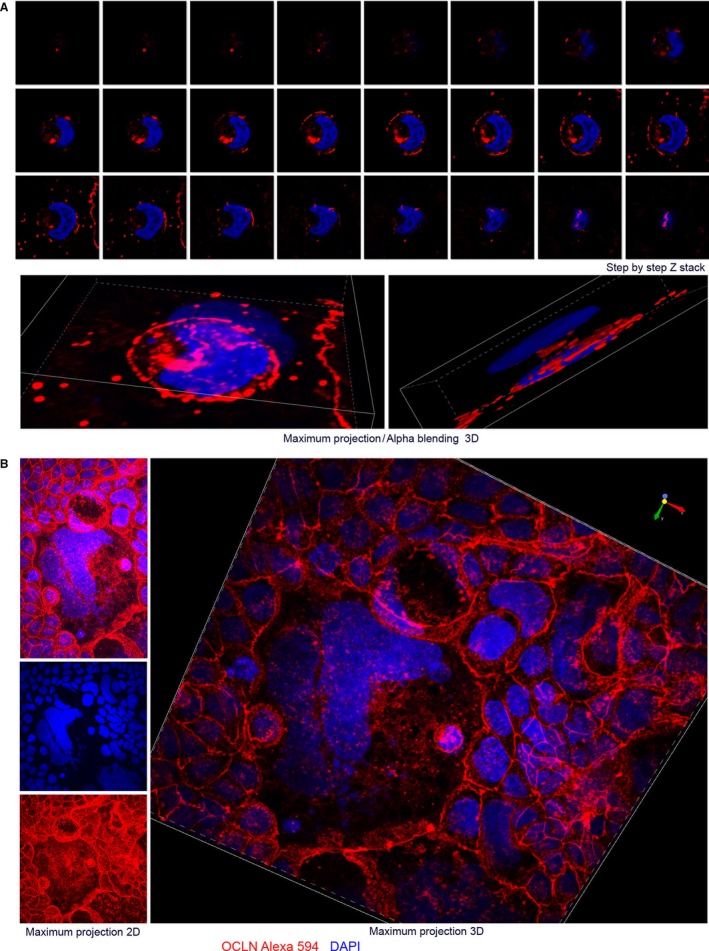
Fractal rotational patterns of tight junctions and nucleus DNA. Caco‐2 BBe cells on day 6 cover clips are labelled with OCLN protein and DNA. Yin‐yang (YY) shapes, small YY (A) in big YY (B). YY shape may correlate with rotational motion and symmetry breaking.
2. SOME POTENTIAL APPLICATIONS OF TURING PATTERN ANALYSIS IN GASTROINTESTINAL DISORDERS
2.1. Functional bowel disorders (FBD) and colorectal cancer
Functional bowel disorders including irritable bowel syndrome (IBS) represent dysfunction in the bidirectional brain‐gut axis, intestinal barrier integrity and interactions with the microbiota and dietary factors.21 Clinical colonoscopy biopsies harvested from diarrhoea‐predominant IBS (IBS‐D) patients demonstrated decreased CLDN1 levels, while CLDN1 was increased in constipation‐predominant IBS (IBS‐C) patients.22 The CLDN1 promoter is under the dual reciprocal regulation by HES1 and NR3C1 in Caco‐2 cells and a validated chronic, intermittent water avoidance (WA) stress rat model of stress‐induced enhanced abdominal pain that mimics several clinical features observed in IBS‐D patients.12 We observed down‐regulation of both HES1 and NR3C1 via a glucocorticoid negative feedback pathway in WA‐stressed rat colon crypts, and similar trends were observed in the hippocampus in a validated restraint‐stress mouse model demonstrating anxiety and depression‐like behaviours.12, 23 Deletion of the Notch signalling ligand Delta‐like 2 (DLK2) increased anxiety and depressive‐like behaviours and altered the vulnerability to restraint stress, and reversed stress‐induced down‐regulation of NR3C1 and HES1.23 HES1 is responsible for maintaining gut homeostasis via preventing microbial dysbiosis in the mouse, and HES1‐knockout altered colon crypt morphology.24 The probiotic combination of Lactobacillus helveticus and Bifidobacterium longum helped reverse WA‐stress‐induced changes in the mouse hypothalamic‐pituitary‐adrenal axis and WA stress‐induced visceral hyperalgesia by blocking decrease of NR3C1 in the hypothalamus, hippocampus and prefrontal cortex.25 These reports support the potential of HES1‐CLDN1 and NR3C1 acting as equilibrium maintaining gene circuits consists of three genes that regulate each other in a cyclical manner and their potential roles in homeostasis of the Microbiota‐Gut‐Brain Axis. Future advances in personalized probiotics based, in part, on 4D Nucleome algorithms represent a potentially promising therapeutic area.21, 25
Colorectal cancer is the second most common cancer in women and the third most common in men, CLDN1 is recognized as a potential biomarker.26 Overexpression of CLDN1 induced elevated levels of Wnt and Notch signalling, promoted colon tumorigenesis in mice, and altered goblet cell differentiation, which conforms to 2D colon crypt Delta‐Notch lateral inhibition Turing patterning.6, 8 Turing models of metabolism in colon cancer link Wnt signalling and gene circuits. We propose that these gene circuits represent potentially promising novel colon cancer targets to model mathematically.12, 27 A preclinical confocal colonoscopy study demonstrated that a CLDN1‐binding peptide can visualize overexpressed CLDN1 in colonic adenomas in vivo, and actomyosin meshwork measurements have been used to demonstrate morphogenesis. Therefore, it is feasible to combine these techniques to assess tight junction geometric patterns potentially useful to monitor the transformation of normal to abnormal (e.g. cancer) actomyosin meshwork pattern(s).3, 28, 29 Human colon epithelium demonstrates various patterns during colonoscopy that correlate with different physiological and pathological conditions, which can be recognized using recently developed artificial intelligence algorithms.16, 29 2D/3D patterns consistent with Turing's hypothesis appear to be present in our clinical data set (Figure 2A).30, 31 We propose that these distinctive colon mucosa patterns support the presence of morphogenetic feedback mechanisms which are hypothesized to regulate the topological architecture of tissues.3, 32 Integrating our knowledge about gene circuits and tissue topology mathematically may help to guide the development of novel interventions targeting the glucocorticoid/Notch/Wnt pathways.8, 13, 27, 33 Dynamic 4D Nucleome algorithms may provide a more powerful predictive model of colon cancer with the applications of machine learning methods.16
Figure 2.
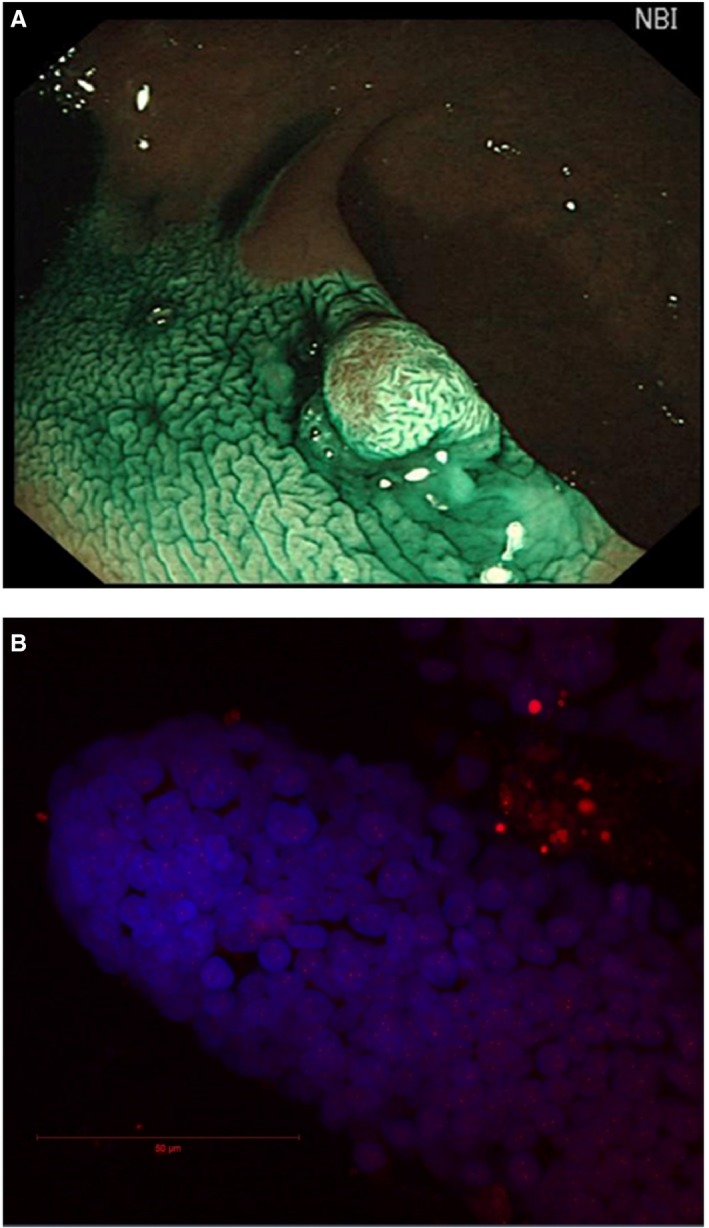
Human colon crypts could be isolated form Turing patterning epithelium for 4D‐nucelome analysis. A, An example of colonoscopy image containing 2D/3D Turing patterns.30, 31 B, An example of BAC‐FISH analysis of 3D human colon crypts isolated from clinical biopsy
2.2. Analysis and bioengineering of human colon crypts
In addition to robust‐omics analysis including RNA‐seq, emerging 4D Nucleome algorithms demonstrate the potential application to pharmacogenomics.34 Clinical colonoscopy biopsies can be used to isolate intact crypts with the structure similar to gastrulation as mentioned by Turing.1 Given biopsy samples, clumps of normal/cancer cells can be fixed and imaged using the same confocal microscopy methods used for the coverslip. Then, the same protocol for automated cell and subnuclear structure labelling can be applied to generate solid volumetric masks of nuclear components. Our pipeline workflow protocol may need to be tailored to each new imaging data format; however, the overall design of the protocol is not expected to drastically change.17, 18, 19, 20, 35 Application of BAC‐FISH methods to study colon crypts is feasible in our pilot study (Figure 2B). Correlating morphogenesis with oscillatory gene movement and expression including clarification of the biophysical mechanisms that determine DNA‐histone interactions and their regulation by cooperative transcription factors will be required to generate convincing proof of Turing's “gene morphogenesis hypothesis” in primary cells and tissue models.1, 2, 3, 14, 36
We anticipate that application of in vitro mechanobiology culture methods to isolated human colon crypts and stem cells will replace the Caco‐2 cell model in testing the gene morphogen hypothesis with programmable morphogenesis parameters.37, 38 Readily available biopsy specimens demonstrating distinct epithelial lumen surface Turing patterns can be used to isolate crypts to study stem cells present at the crypt base from healthy normal and abnormal epithelium using in vitro organoid preparations (Figure 2A).39 We anticipate the programmable “gut‐on‐a‐chip” methodology will demonstrate epithelium morphology similar to the colon lumen surface. This system can be used to study the topology of colon epithelium in response to morphogens including FGF2/glucocorticoids/microbiome, etc.25, 40, 41, 42 High‐throughput “gut‐on‐a‐chip” array methods allow automated experimental designs incorporating various chemical, medicinal and mechanical morphogen conditions. These approaches will generate large datasets requiring the concurrent development of machine learning algorithms to expedite analysis, and potentially confirmation of the “gene morphogen hypothesis”.16, 43, 44
2.3. Mathematical/computational problems unresolved by Alan Turing's Model
Testing the hypothesis presented in this communication will require advanced analytical techniques to generate the relevant information regarding the determinants of cell and tissue morphology patterns under physiologic and pathophysiologic conditions. Prior computational models suggested considering the nucleus as a “cellular decision‐making unit” that is a pivotal component in “cellular decision‐making networks”.45 Computational modelling of cellular “decisions” in response to multiple biochemical and biophysical cues will require a level of mathematics capable of “handling” multiple dimensions.45 Advanced mathematical methods including chaos theory and fractal geometry, in addition to the “relatively elementary” linear models and differential equations used by Alan Turing will be required to explore “The Secret Life of Chaos” of gut homeostasis.1, 4, 5, 46 Fractal geometry was developed to understand self‐similar structure at multiple scales. It provides the powerful strategies for analysing self‐similar shapes using efficient computational algorithms. Growth spiral (Logarithmic Spiral) is a self‐similar spiral curve which often appears in nature, it is frequently used to demonstrate fractal geometry which was known as known as expanding symmetry or evolving symmetry. Self‐similar spirals with various parameters are used to illustrate bifurcation of two‐gene networks responsible for cellular decision‐making and “tissue homeostasis”.4, 5 Self‐similar genome (DNA) and tight junction shapes are observed in our preliminary study, rotational motion of the nucleus, HES1 mRNA/protein distribution, tight junction shapes labelled with occludin antibody seems contributed to those shapes (Figures 1, S1‐S5 and S9). Correlating visionary mathematical idealization and real subjects is very intriguing and challenging, we hope our observation and thoughts could benefit future studies.
CONFLICT OF INTERESTS
The authors declare that declare that they have no significant competing financial, professional or personal interests that might have influenced the performance or presentation of the work described in this manuscript.
AUTHORS’ CONTRIBUTIONS
G.Z. conceived the experiments shown in Figures 1 and 2B. S.Z. acquired image shown in Figure 2A. W.M. acquired image shown in Figure 2B. A.K., I.D.,W.M., S.Z. and J.W. participated discussion and editing. All authors reviewed the manuscript.
Supporting information
ACKNOWLEDGEMENTS
We acknowledge Microscopy & Image Analysis Laboratory (MIL), the University of Michigan Medical School for providing the core service. This study was supported by NIH RO1DK098205 (JWW), NIH R21AT009253 (JWW/SH), NSF grants 17348531 and 1636840, and NIH grants P20 NR015331 and P50 NS091856.
Zheng G, Kalinin AA, Dinov ID, Meixner W, Zhu S, Wiley JW. Hypothesis: Caco‐2 cell rotational 3D mechanogenomic turing patterns have clinical implications to colon crypts. J Cell Mol Med. 2018;22:6380–6385. 10.1111/jcmm.13853
REFERENCES
- 1. Turing AM. The chemical basis of morphogenesis. Philos T Roy Soc B. 1952;237:37‐72. [Google Scholar]
- 2. Chen H, Chen J, Muir LA, et al. Functional organization of the human 4D Nucleome. Proc Natl Acad Sci USA. 2015;112:8002‐8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilmour D, Rembold M, Leptin M. From morphogen to morphogenesis and back. Nature. 2017;541:311‐320. [DOI] [PubMed] [Google Scholar]
- 4. Rajapakse I, Smale S. Emergence of function from coordinated cells in a tissue. Proc Natl Acad Sci USA. 2017;114:1462‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rajapakse I, Smale S. Mathematics of the genome. Found Comput Math. 2017;17:1195‐1217. [Google Scholar]
- 6. Toth B, Ben‐Moshe S, Gavish A, Barkai N, Itzkovitz S. Early commitment and robust differentiation in colonic crypts. Mol Syst Biol. 2017;13:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dunn SJ, Appleton PL, Nelson SA, Nathke IS, Gavaghan DJ, Osborne JM. A two‐dimensional model of the colonic crypt accounting for the role of the basement membrane and pericryptal fibroblast sheath. PLoS Comput Biol. 2012;8:e1002515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pope JL, Ahmad R, Bhat AA, Washington MK, Singh AB, Dhawan P. Claudin‐1 overexpression in intestinal epithelial cells enhances susceptibility to adenamatous polyposis coli‐mediated colon tumorigenesis. Mol Cancer. 2014;13:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gordon WR, Zimmerman B, He L, et al. Mechanical allostery: evidence for a force requirement in the proteolytic activation of notch. Dev Cell. 2015;33:729‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore T, Wu SK, Michael M, Yap AS, Gomez GA, Neufeld Z. Self‐organizing actomyosin patterns on the cell cortex at epithelial cell‐cell junctions. Biophys J . 2014;107:2652‐2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shimojo H, Kageyama R. Oscillatory control of Delta‐like1 in somitogenesis and neurogenesis: A unified model for different oscillatory dynamics. Semin Cell Dev Biol. 2016;49:76‐82. [DOI] [PubMed] [Google Scholar]
- 12. Zheng G, Victor Fon G, Meixner W, et al. Chronic stress and intestinal barrier dysfunction: glucocorticoid receptor and transcription repressor HES1 regulate tight junction protein Claudin‐1 promoter. Sci Rep. 2017;7:4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Real PJ, Tosello V, Palomero T, et al. Gamma‐secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2009;15:50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Revollo JR, Oakley RH, Lu NZ, Kadmiel M, Gandhavadi M, Cidlowski JA. HES1 is a master regulator of glucocorticoid receptor‐dependent gene expression. Sci Signal. 2013;6:ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalinin AA, Higgins GA, Reamaroon N, et al. Deep learning in pharmacogenomics: from gene regulation to patient stratification. arXiv. 2018;19:629‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ching T, Himmelstein DS, Beaulieu‐Jones BK, et al. Opportunities and obstacles for deep learning in biology and medicine. bioRxiv. 2017. This preprint is published as PMID: 29618526 PMCID: PMC5938574; http://10.1098/rsif.2017.0387. (J R Soc Interface. 2018 Apr;15(141). pii: 20170387. do10.1098/rsif.2017.0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalinin AA, Allyn‐Feuer A, Ade A, et al. 3D cell nuclear morphology: microscopy imaging dataset and voxel‐based morphometry classification results. bioRxiv. 2017. 10.1101/208207 [DOI] [Google Scholar]
- 18. Kalinin AA, Feuer AA, Ade A, et al. 3D cell nuclear morphology: microscopy imaging dataset and voxel‐based morphometry classification results. 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops, Salt Lake City, UT, June 18‐22, 2018; 2018;2272‐2280.
- 19. Kalinin AA, Athey BD, Dinov ID. Evaluation of methods for cell nuclear structure analysis from microscopy data. Supplementary Proceedings of the 7th International Conference on Analysis of Images, Social Networks and Texts (AIST‐SUP 2018), Moscow, Russia, July 5‐7, 2018; 2018.
- 20. Kalinin AA, Allyn‐Feuer A, Ade A, et al. 3D shape modeling for cell nuclear morphological analysis and classification. bioRxiv. 2018; 10.1038/s41598-018-31924-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang L, Di Lorenzo C, Farrugia G, et al. Functional bowel disorders: a roadmap to guide the next generation of research. Gastroenterology. 2018;154:723‐735. [DOI] [PubMed] [Google Scholar]
- 22. Cheng P, Yao J, Wang C, Zhang L, Kong W. Molecular and cellular mechanisms of tight junction dysfunction in the irritable bowel syndrome. Mol Med Rep. 2015;12:3257‐3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navarrete F, Garcia‐Gutierrez MS, Laborda J, Manzanares J. Deletion of Dlk2 increases the vulnerability to anxiety‐like behaviors and impairs the anxiolytic action of alprazolam. Psychoneuroendocrinology. 2017;85:134‐141. [DOI] [PubMed] [Google Scholar]
- 24. Guo XK, Ou J, Liang S, Zhou X, Hu X. Epithelial Hes1 maintains gut homeostasis by preventing microbial dysbiosis. Mucosal Immunol. 2018;11:716‐726. [DOI] [PubMed] [Google Scholar]
- 25. Ait‐Belgnaoui A, Payard I, Rolland C, et al. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress‐related visceral hypersensitivity through hypothalamic‐pituitary‐adrenal axis modulation. J Neurogastroenterol Motil. 2018;24:138‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ouban A. Claudin‐1 role in colon cancer: an update and a review. Histol Histopathol. 2018: 10.14670/HH-11-980 [DOI] [PubMed] [Google Scholar]
- 27. Lee M, Chen GT, Puttock E, et al. Mathematical modeling links Wnt signaling to emergent patterns of metabolism in colon cancer. Mol Syst Biol. 2017;13:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chanet S, Miller CJ, Vaishnav ED, Ermentrout B, Davidson LA, Martin AC. Actomyosin meshwork mechanosensing enables tissue shape to orient cell force. Nat Commun. 2017;8:15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mesejo P, Pizarro D, Abergel A, et al. Computer‐aided classification of gastrointestinal lesions in regular colonoscopy. IEEE Trans Med Imaging. 2016;35:2051‐2063. [DOI] [PubMed] [Google Scholar]
- 30. Painter KJ, Hunt GS, Wells KL, Johansson JA, Headon DJ. Towards an integrated experimental‐theoretical approach for assessing the mechanistic basis of hair and feather morphogenesis. Interface Focus. 2012;2:433‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miyazawa S, Okamoto M, Kondo S. Blending of animal colour patterns by hybridization. Nat Commun. 2010;1:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dalle Nogare D, Chitnis AB. Self‐organizing spots get under your skin. PLoS Biol. 2017;15:e2004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin KT, Wang LH. New dimension of glucocorticoids in cancer treatment. Steroids. 2016;111:84‐88. [DOI] [PubMed] [Google Scholar]
- 34. Seaman L, Rajapakse I. 4D nucleome analysis toolbox: analysis of Hi‐C data with abnormal karyotype and time series capabilities. Bioinformatics. 2018;34:104‐106. [DOI] [PubMed] [Google Scholar]
- 35. Kalinin AA, Athey BD, Dinov ID. Evaluation of methods for cell nuclear structure analysis from microscopy data. bioRxiv. 2018; 10.1101/254219 [DOI] [Google Scholar]
- 36. Morgunova E, Taipale J. Structural perspective of cooperative transcription factor binding. Curr Opin Struct Biol. 2017;47:1‐8. [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Kim R, Gunasekara DB, et al. Formation of human colonic crypt array by application of chemical gradients across a shaped epithelial monolayer. Cell Mol Gastroenterol Hepatol. 2018;5:113‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Richmond CA, Breault DT. Move over Caco‐2 cells: human‐induced organoids meet gut‐on‐a‐chip. Cell Mol Gastroenterol Hepatol. 2018;5:634‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dame MK, Attili D, McClintock SD, et al. Identification, isolation, and characterization of human LGR5‐positive colon adenoma cells. Development. 2018;145:dev153049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim HJ, Li H, Collins JJ, Ingber DE. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut‐on‐a‐chip. Proc Natl Acad Sci USA. 2016;113:E7‐E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deng X, Szabo S, Khomenko T, et al. Novel pharmacologic approaches to the prevention and treatment of ulcerative colitis. Curr Pharm Des. 2013;19:17‐28. [DOI] [PubMed] [Google Scholar]
- 42. Taha Y, Raab Y, Carlson M, et al. Steroids reduce local inflammatory mediator secretion and mucosal permeability in collagenous colitis patients. World J Gastroenterol. 2006;12:7012‐7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ashammakhi N, Elmusrati M. An Array of gut‐on‐a‐chips for drug development. bioRxiv. 2018; 10.1101/273847 [DOI] [Google Scholar]
- 44. Probst C, Schneider S, Loskill P. High‐throughput organ‐on‐a‐chip systems: current status and remaining challenges. Curr Opin Biomed Eng. 2018;6:33‐41. [Google Scholar]
- 45. Kreeger PK, Strong LE, Masters KS. Engineering approaches to study cellular decision‐making. Annu Rev Biomed Eng. 2018;20:49‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Al‐Khalili J. The secret life of chaos. BBC; 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


