Abstract
Accumulating evidence indicates that cancer cells spread much earlier than was previously believed. Recent technological advances have greatly improved the detection methods of circulating tumour cells (CTCs), suggesting that the dissemination of cancer cells into the circulation occurs randomly. Most CTCs die in circulation as a result of shear stress and/or anoikis. However, the persistence of disseminated tumour cells (DTCs) in the bone marrow is the result of interaction of DTCs with bone marrow microenvironment. DTCs in the bone marrow undergo successive clonal expansions and a parallel progression that leads to new variants. Compared to the CTCs, DTCs in the bone marrow have a unique signature, which displayed dormant, mesenchymal phenotype and osteoblast‐like or osteoclast‐like phenotype. The persistence of DTCs in the bone marrow is always related to minimal residual diseases (MRDs). This review outlines the difference between CTCs and DTCs in the bone marrow and describes how this difference affects the clinical values of CTCs and DTCs, such as metastasis and recurrence. We suggest that DTCs remaining in the bone marrow after therapy can be used as a superior marker in comparison with CTCs to define patients with an unfavourable prognosis and may therefore be a potential prognostic factor and therapeutic target for cancer therapy.
Keywords: cancer relapse, circulating tumour cells, disseminated tumour cell
1. BACKGROUND
Metastasis is a major reason for the poor prognosis of patients with cancer and is responsible for over 90% of cancer‐related deaths.1, 2, 3, 4 Metastases occur when cancer cells dissociate from the primary cancer and enter into the circulation.5 Circulating tumour cells (CTCs) spread out through circulation and may subsequently reside in the permissive target tissues,6 in which case the cells are called disseminated tumour cells (DTCs). Disseminated tumour cells from various types of cancers are often found in specific organs, including bone marrow and lymph nodes.1, 2, 7 Research on the roles of CTCs and DTCs in bone marrow in the evaluation of cancer prognosis has grown exponentially. Significant evolution often occurs during cancer progression, generating variability between the primary cancer, CTCs and DTCs in the bone marrow. In this review, we summarize the difference between CTCs and DTCs and describe how this difference affects the clinical values of CTCs and DTCs, such as metastasis and recurrence. We suggest that DTCs in the bone marrow are the origin of cancer relapse and may therefore be a potential prognostic factor and therapeutic target for cancer therapy.
2. CANCER CELL DISSEMINATION IS AN EARLY EVENT
Cancer cell dissemination has long been considered to be a late event in tumour development. However, accumulating evidence indicates that cancer cells spread much earlier than was previously believed,8 even before the primary tumour is detected.9 Tumour cells are frequently detected in the blood and bone marrow of cancer patients who have no clinical or even histopathologic signs of metastasis.10 The variability in detection rates is likely due to differences in selection criteria and methodologies (Table 1). Recent technological advances have greatly improved CTC detection methods. An advanced unique microfluidic platform (CTC‐Chip) was found to identify CTCs in the peripheral blood of more than 90% of patients with metastatic lung, prostate, pancreatic, breast cancer and colon cancer and did not detect CTCs in the healthy control. In addition, CTCs were isolated in 100% of patients with early‐stage prostate cancer using the same platform,11, 12 indicating that the dissemination of cancer cells into the circulation may occur randomly. CTCs that home to the bone marrow are also detected in patients with pre‐invasive lesions, suggesting that bloodborne dissemination is also an early event.12 Given the much lower incidences of metastasis, the correlation between CTCs, DTCs and metastasis remains elusive. To date, the detection of CTCs and DTCs remains a challenging diagnostic approach and prognostic biomarker, not only as a result of methodological limitations but also because the heterogeneity among CTCs and DTCs in bone marrow compromises their ability to predict the metastatic behaviours. Neither CTC status nor DTC status has been included in routine clinical analysis.13
Table 1.
Clinical relevance of different detection of CTCs or DTCs
| Type | n | CTC/DTC | Measurement | Positive (%) | References |
|---|---|---|---|---|---|
| Gastric cancer | 81 | CTC | A45‐B/B3, vimentin, CD45 | 63 | 131 |
| Circulating tumour microemboli (CTM) | 18.6 | ||||
| Colon cancer | 299 | CTC | CK20,RT‐PCR | 37.4 | 132 |
| 227 | DTC | CK20 | 35.7 | ||
| 61 | BER‐EP4 | 19.7 | |||
| 134 | A45‐B/B3 | 22.4 | |||
| Breast cancer | 83 | CTC | A45‐B/B3, CD45 | 52 (≥5 CTCs) | 133 |
| 83 (underwent therapy) | 25 (≥5 CTCs) | ||||
| Breast cancer | 431 | CTC | A45‐B/B3 | 13 | 134 |
| 414 | DTC | A45‐B/B3 | 24 | ||
| Breast cancer | 350 | DTC | EMA | 25 | 119 |
| Various cancers | 116 | CTC | Microfluidic platform (the “CTC‐chip”) | 99 | 11 |
| Prostate cancer | 7 | CTC | Microfluidic platform (the “CTC‐chip”) | 100 | 11 |
A45B/B3 detects cytokeratins 8,18,19; AE1 detects cytokeratins 10,14,15,16 and 19; AE3 detects cytokeratins 1,2,3,4,5,6,7 and 8; BER‐EP4 detects EpCAM; EMA detects epithelial membrane antigen; Microfluidic platform (CTC‐chip):antibody (EpCAM)‐coated microposts.
3. BONE MARROW IS A RESERVOIR OF DISSEMINATED TUMOUR CELLS
Bone marrow is a critical site of immune cell development and erythropoiesis. The bone marrow parenchyma includes hematopoietic stem cells and hematopoietic progenitor cells. The stroma, which is composed of stromal stem cells, extracellular matrix and several types of secreted cytokines, is highly vascular and enriched with numerous blood vessels and capillaries.14 Bone marrow is also a niche for mature plasma cells and memory T cells.15 Bone marrow displays structural and functional features resembling a secondary lymphoid organ, providing appropriate support for T cells. Accumulated evidence demonstrates that, in addition to the hematopoietic progenitor cells, bone marrow contains various immune cells, including regulatory T cells, conventional T cells, B cells, dendritic cells, natural killer T (NKT) cells, neutrophils, myeloid‐derived suppressor cells and mesenchymal stem cells.14
Bone marrow diseases, including leukaemia, lymphoma, multiple myeloma anaemia and other life‐threatening diseases, lead to an abnormality in the production of mature blood cells. Bone marrow is a preferred metastatic site for several solid tumours, such as breast cancer, lung cancer, prostate cancer and others.16 Bone marrow also represents a sanctuary site for DTCs derived from various additional types of epithelial tumours.16 The presence of DTCs in the bone marrow is associated with not only bone metastasis but also the development of distant tumours. DTCs in the bone marrow may re‐enter the vasculature and disseminate secondarily throughout the body.17, 18
Although the dissemination of cancer cells into the circulation and the bone marrow is an efficient process, cancer metastasis is an inefficient process. The persistence of DTCs in the bone marrow is always related to minimal residual diseases (MRDs). The mechanism underlying the process of DTCs localizing to and colonizing the bone marrow is complex, and the homing and survival of DTCs are more like a selective process. In one study, intracardiac injection of tumour cells was performed to study late stage of metastasis.19 These DTCs may transform into more aggressive variants and grow out to overt metastasis.20 Disseminated tumour cells were found to gather in the bone marrow and display unique gene expression, including the significant enrichment of genes known to regulate interleukin‐6 (IL‐6) signalling, cell adhesion and angiogenesis. Bone marrow contains unique anatomic regions defined by specialized endothelium. The specialized vasculature expresses the adhesion molecule E‐selection and the chemoattractant SDF‐1.21 Silencing CXCR4, VLA4 and FAK can effectively decrease the homing phenomenon.19 Disseminated hormone receptor–positive breast cancer cells hunt for specific blood vessels in bone marrow that contain the molecule E‐selectin. Due to the key molecules on their surface that bind to E‐selectin, the cancer cells enter the spongy tissue inside bones, often lying dormant for years.22
4. BONE MARROW FORMS A PREMETASTATIC NICHE FOR DISSEMINATED TUMOUR CELLS
Secondary cancer growths do not occur randomly.23 Stephen Paget's “seed and soil” hypothesis proposed that the homing and settlement of cancer cells depend on the fertile “soil” provided by a given microenvironment. Bone marrow is a major target organ for metastasis, providing a fertile “soil” for circulating tumour cells to settle and repopulate.24 The fertile “soil” is sometimes formed before the arrival of circulating tumour cells, in which case it is termed a “premetastatic niche.” Cells in this favourable microenvironment or “premetastatic niche” secrete many cytokines and chemokines, attracting primary tumour cells to the niche as well as supporting their subsequent colonization. The formation of the premetastatic niche is influenced by the primary cancer.25 Recent studies suggest that primary tumour cell–derived extracellular vesicles (EV) contribute to distant metastasis effectively. EV carried miR‐122 suppress glucose uptake of microenvironmental cells by downregulating the glycolytic enzyme pyruvate kinase.26 Tumour exosomal small nuclear RNAs remodel the lung metastatic niche by activating alveolar epithelial TLR3 to recruit neutrophil.27
The premetastatic niche is defined as a microenvironment that is highly proinflammatory, adhesive, vascular, proproliferative and chemotactic. Integrins on both tumour cells and the supporting stromal cells in the bone marrow, such as osteoclasts, inflammatory cells and bone marrow stromal cells, play key roles in enhancing the localization of cancer cells to the bone marrow.28 The interaction of integrins and their ligands allows for the mobilization and adhesion of cancer cells to the bone marrow stromal cells (αvβ3, α5β1, α4β1),29, 30, 31 osteoclasts (αvβ3),32, 33 platelets (αvβ3, α1β3),34 bone marrow hematopoietic progenitor cells (α4β1)28, 35 and endothelial cells (α1β1, α2β1).36 Chemokines such as SDF‐1,37, 38 S100A8 and S100A9,39 which are produced by bone marrow–derived cells (BMDCs) or primary cancer cells, elicit the accumulation of cancer cells, endothelial cells and macrophages in the bone marrow.39
Cancer‐induced stress elicits BMDCs, such as bone marrow–derived hematopoietic progenitor cells, and the mobilization of stromal stem cells from the bone marrow into the primary cancer microenvironment.40 Bone marrow–derived mesenchymal stem cells have the potential to develop into cancer‐associated fibroblasts (CAFs).41 Il‐6 and GM‐CSF were identified as the key factors released from CAFs that promote tumour‐associated macrophages infiltration in the premetastatic niche.42 Cancer‐educated BMDCs move back into the circulation and home to tumour‐specific premetastatic sites or reprogramme the microenvironment in distant sites by secreting chemokines41 or cytokines before the arrival of tumour cells.37, 39 Interestingly, a subpopulation of bone marrow–derived stromal cells (MSCs), which express endothelial and pericytic cells surface markers (CD31 and CD146), has been reported to reduce cancer cell homing to the bone marrow43. Bone marrow–derived CD11b+ Jagged2+ cells infiltrate primary tumours and accelerate cancer cell EMT. Moreover, circulating CD11b+ Jagged2+ cells offer an indicator for metastasis of colorectal cancer cells.44 Cancer cells preferentially home to the osteoblastic niche in bone marrow, competing with normal hematopoietic stem cells.45, 46
5. CHARACTERIZATION OF CTCS AND DTCS IN BONE MARROW
5.1. Intratumour genetic heterogeneity and parallel tumour evolution
CTCs are derived from primary tumour sites and secondarily disseminated from metastatic tumours. DTCs in various organs have a unique signature that reveals their origin.12 The adaption of CTCs occurs upon contact with the specific environment, indicating that DTCs undergo successive clonal expansions and a parallel progression that leads to new variants.12
Next‐generation sequencing (NGS) and single‐cell sequencing have facilitated the detection of genome variation among CTCs and DTCs. Fluorescence in situ hybridization (FISH) analysis can be used to detect gene translocation.47 Multiregion genetic analysis of renal carcinomas revealed intratumour heterogeneity.48 The phylogenetic reconstruction of those renal carcinomas also revealed branched evolutionary tumour growth, with 63%‐69% of unique somatic mutations present in various tumour regions.48 Nonmutagenic treatments, such as everolimus, did not cause more mutations compared to pretreatment; on the contrary, certain mutations in the primary cancer disappeared after treatment with everolimus, indicating that the treatment resulted in clonal selection. Prostate cancer, which is histologically multifocal, is an example. The primary tumour foci from similar physical regions were found to be more closely related, suggesting that cancer arises from an ancestor and undergoes divergent evolution thereafter.49 The heterogeneity of CTCs reflects the heterogeneity of the primary cancer. Single‐cell deep sequencing studies have shown that mutations or somatic single nucleotide variants (sSNVs) in CTCs could be found in subclones of the primary tumour and metastases, indicating the cell origin of the CTCs.49 The identical genomic profiles for CTCs and the primary tumour reflect the linear model by which tumour cells are disseminated into the circulation. However, the colonization of CTCs in distant sites reflects the branching evolution model. CTCs overlap with more than 90% of primary cancer mutations but only 73% of metastatic mutations.49 When the DNA copy aberration of the cells (CNA) was compared, the DTCs in bone marrow showed 53% CNA similarity with that of the primary tumour, whereas the majority of the DTCs in the bone marrow displayed genomic profiles unrelated to the primary tumour, indicating that the parallel evolution model applies to the disseminated cells in the bone marrow (Figure 1).50, 51
Figure 1.
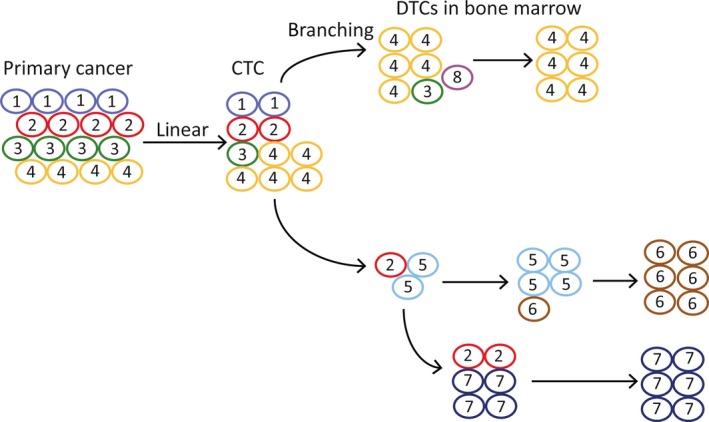
Linear and parallel clonal evolution in CTCs and DTCs in bone marrow. Left: Linear evolution. The final CTCs carry all mutations arising during the evolution. Right: Parallel evolution. CTCs circulated or arrived in bone marrow, the final DTCs in bone marrow may be dominated by a single done. Clones arise through divergent evolution. Numerals and circles colour indicate the subclones of the cancer cells
5.2. Bone marrow is a dormant niche for DTCs
Metastasis is an inefficient process. Although the dissemination of cancer cells occurs at an early stage of cancer, many cancer cells delivered into the circulation either die or go into a dormant state.52 The following three types of gene expression signatures have been identified in CTCs: low‐proliferative signatures, biphenotypic signatures, in which both epithelial and mesenchymal markers are expressed, and epithelial‐stromal interface signatures, in which igfbp5 is expressed.53 Due to the short half‐life of CTCs in circulation, which persist for only 1‐2 hours, and due to apoptotic CTCs, the detection and prognostic value of CTCs in circulation has been unclear. Most CTCs die in circulation as a result of shear stress and/or anoikis.54, 55 Contact with integrins and exposure to bone‐derived cytokines in the bone marrow can reduce proapoptotic signalling.56, 57 The persistence of DTCs in the bone marrow during follow‐up is an indicator of the prognosis.58 Up to 40% of patients diagnosed with nonmetastatic breast cancer still have a significant risk of relapse, even after the complete surgical removal of the tumour, most likely because of the existence of DTCs.51, 59 DTCs in the bone marrow are usually considered to be nonproliferative and are believed to be the source of metastasis, independent of the primary tumour site and the pattern of overt metastases.10 More than 80% of patients with solid tumours harbour Ki67‐negative DTCs in the bone marrow.60 DTCs in the bone marrow exhibit very low or even no detectable pAKT levels.61 Cancer cells invade the bone marrow, where they remain dormant and are protected from chemotherapy or hormonal therapies that could otherwise eradicate them.
The quiescence of DTCs depends on the cross‐talk between the cancer cells and the bone marrow microenvironment. Bone marrow seems to be a particularly dormancy‐inducing environment for DTCs. Stable microvasculature constitutes a dormant niche for DTCs.62 Thrombospondin‐1 (TSP‐1) secretion by mature endothelial cells induces the sustained quiescence of breast cancer cells, whereas sprouting microvasculature secretes TGF‐β1 and periostin (POSTN) to promote tumour growth,62 indicating that the dormancy and re‐activation of DTCs are closely associated with the vascular basement membrane. Intravital microscopy images have shown that adhesion to the abluminal surface of the vasculature is one of the prerequisites for disseminated tumour cell survival.63 However, TGFβ2, which is present in bone marrow, induces dormancy through TGFβ receptor I, TGFβ receptor III and SMAD1/5 activation.64 Osteoclasts are specialized cells for the resorption of mineralized bone matrix. It has been reported that the proliferation of leukaemic cells is significantly suppressed when the cells are cocultured with osteoclasts.65 TGF‐β derived from osteoclasts was also shown to play roles in maintaining the quiescent state of cancer cells in the bone marrow.65
The HSC niche may act as a permissive site for DTCs to escape from source of stress. DTCs may reside in close proximity to osteoblasts while expressing high levels of Axl, one of the tyrosine kinase receptors for growth arrest–specific 6 (GAS6). The secretion of osteoblast‐derived GAS6 ligands by this niche can induce tumour cells to increase the ratio of GAS6 receptor Axl expression to Tyro3 expression which leads to a more dormant phenotype.66 DTCs recovered from the bone marrow are regulated by GAS6 through the MER/mTOR pathway, exhibiting a stem cell–like phenotype.67
5.3. Immuno‐mediated dormancy in bone marrow
The process of tumour immuno‐editing includes the following three key phases: elimination, equilibrium and escape.68 During the equilibrium phase, the immune system and the tumour cells experience mutual dynamic and constant selective pressure, and equilibrium between the immune response and the tumour cells results in a long‐term latency or relative dormancy.69, 70, 71 Cancer cells will resist the selective pressure from the immune system by acquiring mutations or undergoing other changes that allow for tumour progression in the face of an ongoing immune response.72, 73, 74 DTCs are characterized by a reduced expression of MHC class I molecules, which could result in these cells escaping the immune system.75, 76 Bone marrow aspirates from breast cancer patients with higher rates of overt bone metastasis more frequently revealed the absence of MHC I expression.77 Cell surface cytokeratin 8, 18, and 19 are trypsin‐sensitive factors that mask HLA class I molecules.78 FasL expressed on disseminated cancer cells mediates immune evasion by eliminating infiltrating lymphocytes.79, 80 Natural killer (NK) cells eliminate target cells with low or absent expression of MHC‐I. DTCs coated with platelets are conferred a false pretence phenotype, which helps them to evade NK cell–mediated cytotoxicity.81 In the interplay between DTCs and NK cells, the secretion of LDH5 and the ADAM10‑mediated shedding of the NKG2D ligand MICA/MICB from CTCs prevents the recognition and elimination of the cells via NK cell–mediated lysis.82, 83
On the other hand, cancer cells are regulated in the bone marrow by the immune system. The proportion of memory T cells among the CD4 and CD8 T cells was found to be much higher in the bone marrow of cancer patients compared with healthy donors (P < 0.001), suggesting that the immune equilibrium between DTCs and memory T cells is involved in the balance between dormant cancer cells and tumour escape.84 Only some of these immunological changes were also detectable in peripheral blood samples.84, 85 Functional immunosuppressive cells, such as MDSCs, Tregs and Bregs, are involved in the maintenance of DTC dormancy in the bone marrow.86, 87 This balanced state may be disturbed by both changes in the DTCs (eg, additional mutations or epigenetic modifications in genes controlling cell proliferation and apoptosis) and changes in the surrounding microenvironment (eg, the release of growth factors, angiogenic factors and cytokines).17, 88
5.4. DTCs in bone marrow showed mesenchymal phenotype
Most DTCs in the bone marrow display properties of epithelial‐mesenchymal transition,89 during which they gain expression of the mesenchymal marker N‐cadherin and lose expression of the epithelial marker E‐cadherin (Figure 2).90, 91, 92, 93, 94 The synergistic effect of Snail and β‐catenin confers cancer cells the ability to survive during dissemination and invasion.95 Additionally, DTCs often display attributes of a stem cell–like phenotype (CD44high/CD24low) related to self‐renewal and angiogenesis.96, 97, 98, 99 This more plastic phenotype may be endowed by the primary tumour and clonally selected for during the process of homing, or promoted by the bone marrow niche as a stem cell niche to induce terminally differentiated cells to become stemlike.46 The mesenchymal phenotype may be a transient state of DTCs in the bone marrow, keeping them dormant and resistant to radiotherapy/chemotherapy.100 The overexpression of unfolded protein response (UPR) proteins and protein disulphide‐isomerase was observed in DTCs from bone marrow, suggesting that DTCs may resist the stress attributed to UPR phenotype.96
Figure 2.
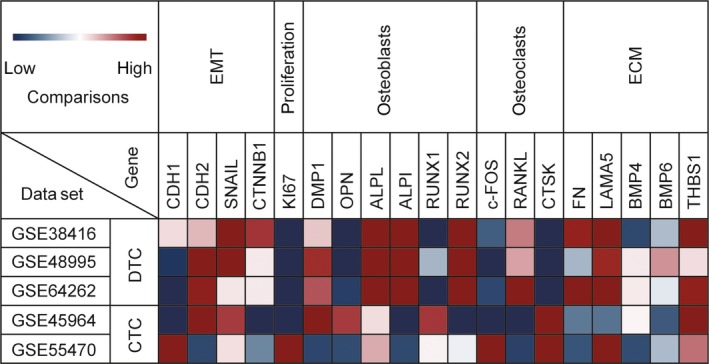
Heatmap representing expression profile of representative differentially expressed genes in DTCs and CTCs using Mev. The cDNA array data sets (GSE38416, GSE48995, GSE64262, GSE45964, GSE55470) were collected from the National Center for Biotechnology Information's Gene Expression Omnibus (GEO, NCBI)
5.5. DTCs in the bone marrow displayed an osteoblast‐like or osteoclast‐like phenotype
Several distinct niches in the bone marrow, including the endosteal niche (osteoblasts) and the vascular niche, can protect DTCs from adjuvant therapies.57, 101 Transcriptome analyses of DTCs in the bone marrow have identified an osteoblast‐like or osteoclast‐like phenotype in which cancer cells in the bone marrow undergo an osteomimetism, expressing a pool of genes normally expressed by osteoblasts or osteoclasts.12 RANK‐L/RANK/OPG axis which regulates the process of bone turnover is activated in DTCs in bone marrow. Besides, the expression of genes participating in osteomimicry or osteolysis such as OPN, ALP and RUNX2 is also different from that of CTCs (Figure 2). Cancer cells have been shown to adopt an osteoblast‐like phenotype that may help them survive in the bone marrow.102 Cancer cells with an osteoblast‐like phenotype have been shown to exhibit an enhanced invasive ability.103 The dormant DTCs may be reactivated by the osteoclast‐mediated release of bone‐derived growth factors.57 The interaction between the bone marrow niche and the cancer cells was involved cancer‐derived E‐cadherin and osteogenic N‐cadherin.104
5.6. Human epidermal growth factor receptor 2 (HER2) and the estrogen receptor (ER)
Primary tumours and DTCs display a discordant ERα and HER2 status.105 This discordance may be important for determining which patients will benefit from endocrine and/or HER2 targeted therapy. Patients lacking ER or HER2 expression on the primary cancer but showing ER‐positive or HER2‐positive DTCs may benefit from an endocrine and/or HER2‐targeted therapy. This discordance may explain the failure rates seen in conventional endocrine adjuvant therapy for patients with DTCs that were ERα negative despite the presence of ERα positive primary tumours.106 Approximately 90% of analysed breast cancer patients with HER3 activation were found to exhibit very low or even no detectable levels of pAKT S473, suggesting that these cells might have fallen into dormancy.61
6. DTC IN BONE MARROW MAY BE A PROGNOSTIC BIOMARKER FOR RELAPSE AND A THERAPEUTIC TARGET FOR CANCER
Lymphatic metastasis has long been considered to be the primary route of metastasis, partly due to the accessibility of the lymph nodes. However, haematogenous metastasis is becoming increasingly recognized and confirmed because of the detection of CTCs. Lymph‐node involvement does not accurately predict the haematogenous dissemination of cancer cells, nor is haematogenous dissemination necessarily associated with lymph‐node involvement.59 Although the routine detection of CTCs has not been recommended as a prognostic method, numerous studies have demonstrated that the number of CTCs per mL is associated with the median overall survival.107 Hundreds of clinical trials incorporating CTC count as a biomarker in patients with various types of solid tumours are ongoing.108 CTCs thresholds range from 1 to 5 per mL for prognostic value in various cancer types, and the range for DTCs in the bone marrow is 1‐2 per 2 × 106 cells.109 Sensitive immunocytochemical methods (ICCs), RT‐PCR, epithelial immunospot (EPISPOT), flow cytometry and a series of other strategies have overcome the difficulties in cell filtration, enrichment and identification. Equipment such as the FDA‐approved CellSearch® system is already applied in tracking the CTCs in cancer patients.110 Clinically, liquid bioscopy helps to find DTCs in the bone marrow of patients with various types of solid tumours.16
CTCs and DTCs have been reported as independent prognostic markers that impact the progression‐free survival (PFS) and overall survival (OS).13 The number of CTCs is also correlated with serum levels of other markers, such as the prostate cancer biomarker, PSA.49 However, the inconsistency of these markers and the short half‐life of CTCs limit their application as diagnostic and prognostic biomarkers in the clinical setting. It has been considered that CTCs are singly suspended in the circulation, but the amount of CTC clusters can be underestimated due to the lack of appropriate detection methods.111 The survival rates of CTCs are highly variable, and EpCAM‐negative and CD44‐positive subsets exhibit quiescence properties and can initiate relapse or metastasis.112 The high heterogeneity of CTCs dilutes the gene signatures that are indicative of the phenotypic characteristics that allow CTCs to survive in distant organs. CTCs also include the DTCs in bone marrow that recirculate to other secondary organs or even back to the primary tumour site.108, 113 The detection of dormant DTCs enriched in the bone marrow may offer an ideal way to detect the minimal residue diseases. The detection rates at the same time in individual patients have always shown discordance of CTCs and DTCs, especially in patients after therapy.114 Some studies have compared the detection rates in the same patient. CTCs were detected in 10% of the patients, and DTCs were detected in 14% of the same patients.115 As DTCs in the bone marrow display a more malignant transformed phenotype, and the colonization of CTCs in the bone marrow is more like a selective process, DTCs in bone marrow are expected to be a superior marker for predicting overall survival, compared to CTCs. However,this conclusion is controversial.114, 115, 116, 117 Some studies have demonstrated a superior performance of DTCs in predicting overall survival in patients with cancer.118 In contrast, other studies reported that DTCs were associated with bone metastasis (P = 0.0001) but not with a poorer overall survival.116 At this time, DTCs have not been recommended as a substitute for CTCs as the prognostic biomarker, because bone marrow aspiration is invasive and uncomfortable for patients.
However, the presence of bone marrow DTCs in patients with primary breast cancer is associated with a shorter relapse‐free survival and locoregional relapse, although it is not an independent prognostic factor.58, 119 The persistence of DTCs during follow‐up has been shown to significantly predict the increased risk for subsequent relapse and death.120 The importance of histologic or genetic subclassification of cancer lies principally in the tumour biology, especially the response to therapy and the outcome. DTC status has been included in the new American Joint Committee on cancer classification.13 The presence of DTCs identified (at median 85 months follow‐up) a subgroup of luminal A patients characterized by high expression levels of ER‐related genes and low expression of the HER2 cluster and proliferation‐associated genes. This subgroup was found to have particularly poor outcomes (P = 0.008); however, this finding was not apparent for other tumour subtypes.121 DTCs, but not CTCs, are associated with DFS in node‐negative patients.115 Currently, no diagnostic tools are available to monitor the response after the completion of adjuvant treatment to identify patients who need secondary adjuvant therapy due to persistent tumour cell load. The persistence of DTCs in the bone marrow after a disease‐free follow‐up interval indicates that DTCs in the bone marrow may be used as a surrogate marker to predict the risk of relapse, especially to identify patients with a poor response to therapy. The reevaluation of bone marrow status may be a promising tool because the presence of DTCs is a possible surrogate marker for persistent MRD. Studies have shown that the persistence of DTCs in the bone marrow of patients with primary breast cancer after conventional adjuvant therapy is associated with a poor prognosis.106 The existence of dormant DTCs in the bone marrow increases the risk of late relapse. When patients were followed for years, DTCs in the bone marrow were found to be a superior marker for predicting relapse. The persistence of DTCs in the bone marrow during follow‐up significantly predicted the increased risk for subsequent relapse and could be used as an indicator for secondary treatment intervention.120 Combining the data from various laboratories, it is estimated that before treatment, the positivity of DTCs in the bone marrow is approximately 30%.122, 123 Chemotherapy or bisphosphonates can reduce the positivity of DTCs.123 Six months after treatment, DTCs remaining in the bone marrow after therapy define patients with an unfavourable prognosis.122 Several studies have connected the presence of occult metastases in the bone marrow with a higher risk of recurrence.124, 125 DTC status can be used to identify high‐risk patients after chemotherapy and guide treatment decisions. However, DTC status after surgery was not associated with overall survival (Table 2).126
Table 2.
Prognostic relevance of DTCs or CTCs
| n | DTC/CTC | Marker | Treatment | OS (pre) | OS (post) | DFS (pre) | DFS (post) | References |
|---|---|---|---|---|---|---|---|---|
| 83 | CTC | A45B/B3 | ACT | 0.0048 | 0.0029 | 0.0014 | 0.007 | 133 |
| 213 | CTC | EpCAM, CK | NACT | 0.0057(CTC≥1) | ns | 0.031(CTC≥1) | 0.43(CTC≥1) | 135 |
| <0.0001(CTC≥2) | ns | <0.0001(CTC≥2) | 0.69(CTC≥2) | |||||
| 394 | DTC | A45B/B3 | ACT | 0.156 | 136 | |||
| 47 | DTC | A45B/B3 | ACT | 0.009 | 0.004 | 137 | ||
| 236 | DTC | AE1/E3 | NACT | 0.671 | <0.001 | 138 | ||
| CTC | NACT | 0.318 | ||||||
| DTC | Surgery | 0.715 | ||||||
| CTC | Surgery | 0.631 | ||||||
| 211 | DTC | NACT | 0.602 | 0.003 | ||||
| CTC | NACT | 0.146 | 0.434 | |||||
| DTC | Surgery | 0.48 | ||||||
| CTC | Surgery | 0.551 | ||||||
| 1489 | CTC | EpCAM | ACT | 0.023 | 0.154 | <0.0001 | 0.054 | 139 |
| 100 | DTC | AE1/E3 | ACT | <0.0001 | 140 | |||
| 129 | 0.92 | |||||||
| 60 | DTC | AE1/E3 | Radiotherapy | ns | 0.02 | 141 | ||
| 193 | DTC | A45B/B3 | NACT | 0.0035 | 142 | |||
| 60 | CTC | pan‐CK | ACT | 0.002 | <0.001 | 143 | ||
| DTC | 0.0005 | 0.003 | ||||||
| 103 | DTC | CK20 | NACT | 0.04 | 0.03 | 144 | ||
| 117 | CTC | ns | ns |
A45B/B3 detects cytokeratins 8,18,19; AE1 detects cytokeratins 10,14,15,16 and 19; AE3 detects cytokeratins 1,2,3,4,5,6,7 and 8; pre: DTC/CTC detection performed before treatment; post: DTC/CTC detection performed after treatment; ACT: adjuvant therapy; NACT: neoadjuvant therapy.
Analysis of bone marrow DTCs offers the possibility of developing targeted therapies to eliminate the residue of dormant cells.127 The eradication of DTCs by bisphosphonates has already been demonstrated, and it was recently demonstrated that clodronate is effective in DTC elimination even after years of first diagnosis.13 Treatment with clodronate has made a significant improvement in OS and significantly reduced the incidence of bone metastasis.128 Preventing dormant DTCs in the bone marrow from reactivating cell cycling or kicking dormant cancer cells back into circulation may be strategies for preventing the relapse of metastatic disease. β‐adrenergic receptor antagonists, which interfere with proliferative re‐activation norepinephrine (NE) signalling, may reduce cancer relapse or slow disease progression.129 One potential strategy is finding a way to inhibit E‐selectin, which could limit the ability of the cancer cells to travel into the bone and resurge as metastatic cancer. A combination of the E‐selectin inhibitor GMI‐1271, daunorubicin and cytarabine, was shown to result in a greater depletion of AML from the bone marrow.130 Moreover, GMI‐1271 was reported to enhance the response to chemotherapy. Bone marrow transplantation could also be a good choice after the surgery or chemotherapy.
7. CONCLUSION
Dissemination of cancer cells is considered to be an early and random event in the process of cancer progression as detection methods have been greatly improved. DTCs in the bone marrow may be endowed with particular characteristics that are different from CTCs in the circulation by the special environment. These dormant, mesenchymal, osteoblast‐like or osteoclast‐like signatures may provide superior markers to CTCs for predicting metastasis or relapse of cancer and provide potential therapeutic targets for therapy.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interests.
Sai B, Xiang J. Disseminated tumour cells in bone marrow are the source of cancer relapse after therapy . J Cell Mol Med. 2018;22:5776–5786. 10.1111/jcmm.13867
Funding information
This work was supported by National Natural Science Foundation, China (grant number 81472695, 81773147, 81272255); Natural Science Foundation, Hunan (grant number : 2015JJ2181);National Training and Research Base for Talents of principles of carcinogenesis foundation (111 project: 111‐2‐12).
REFERENCES
- 1. Effenberger KE, Schroeder C, Eulenburg C, et al. Disseminated tumor cells in pancreatic cancer‐an independent prognosticator of disease progression and survival. Int J Cancer. 2012;131:E475‐E483. [DOI] [PubMed] [Google Scholar]
- 2. Vashist YK, Effenberger KE, Vettorazzi E, et al. Disseminated tumor cells in bone marrow and the natural course of resected esophageal cancer. Ann Surg. 2012;255:1105‐1112. [DOI] [PubMed] [Google Scholar]
- 3. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559‐1564. [DOI] [PubMed] [Google Scholar]
- 4. Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18:43‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel LR, Camacho DF, Shiozawa Y, Pienta KJ, Taichman RS. Mechanisms of cancer cell metastasis to the bone: a multistep process. Future Oncol. 2011;7:1285‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679‐695. [DOI] [PubMed] [Google Scholar]
- 7. Rack B, Schindlbeck C, Juckstock J, et al. Circulating tumor cells predict survival in early average‐to‐high risk breast cancer patients. J Natl Cancer Inst. 2014;106:2504‐2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rocken M. Early tumor dissemination, but late metastasis: insights into tumor dormancy. J Clin Invest. 2010;120:1800‐1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dasgupta A, Lim AR, Ghajar CM. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol Oncol. 2017;11:40‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alix‐Panabieres C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res. 2008;14:5013‐5021. [DOI] [PubMed] [Google Scholar]
- 11. Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alix‐Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623‐631. [DOI] [PubMed] [Google Scholar]
- 13. Hoffmann O, Schroer‐Zuendorf IA, Kasimir‐Bauer S, Oberhoff C, Kimmig R, Heubner M. Evaluation of the prognostic significance of disseminated tumor cells in the bone marrow of primary, non‐metastatic breast cancer patients after a 7‐year follow‐up. Arch Gynecol Obstet. 2015;292:1117‐1125. [DOI] [PubMed] [Google Scholar]
- 14. Zhao E, Xu H, Wang L, et al. Bone marrow and the control of immunity. Cell Mol Immunol. 2012;9:11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacNamara KC, Oduro K, Martin O, et al. Infection‐induced myelopoiesis during intracellular bacterial infection is critically dependent upon IFN‐gamma signaling. J Immunol. 2011;186:1032‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pantel K, Alix‐Panabieres C. Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. Bonekey Rep. 2014;3:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895‐904. [DOI] [PubMed] [Google Scholar]
- 18. Venkateshwar K, Douglas A, Gregory K. Lung cancer metastasis: novel biological mechanisms and impact on clinical practice. Int J Radiat Oncol Biol Phys. 2006;66:117‐125.16765528 [Google Scholar]
- 19. Sacco A, Roccaro AM, Ma D, et al. Cancer cell dissemination and homing to the bone marrow in a Zebrafish model. Cancer Res. 2016;76:463‐471. [DOI] [PubMed] [Google Scholar]
- 20. Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563‐572. [DOI] [PubMed] [Google Scholar]
- 21. Sipkins DA, Wei X, Wu JW, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969‐973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Price TT, Sipkins DA. E‐Selectin and SDF‐1 regulate metastatic trafficking of breast cancer cells within the bone. Mol Cell Oncol. 2017;4:e1214771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peinado H, Zhang H, Matei IR, et al. Pre‐metastatic niches: organ‐specific homes for metastases. Nat Rev Cancer. 2017;17:302‐317. [DOI] [PubMed] [Google Scholar]
- 24. Morris EV, Edwards CM. Bone marrow adipose tissue: a new player in cancer metastasis to bone. Front Endocrinol (Lausanne). 2016;7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 2017;77:3655‐3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fong MY, Zhou W, Liu L, et al. Breast‐cancer‐secreted miR‐122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17(2):183‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Y, Gu Y, Han Y, et al. Tumor Exosomal RNAs Promote Lung Pre‐metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell. 2016;30(2):243‐256. [DOI] [PubMed] [Google Scholar]
- 28. Schneider JG, Amend SR, Weilbaecher KN. Integrins and bone metastasis: integrating tumor cell and stromal cell interactions. Bone. 2011;48:54‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van der Velde‐Zimmermann D, Verdaasdonk MA, Rademakers LH, De Weger RA, Van den Tweel JG, Joling P. Fibronectin distribution in human bone marrow stroma: matrix assembly and tumor cell adhesion via alpha5 beta1 integrin. Exp Cell Res. 1997;230:111‐120. [DOI] [PubMed] [Google Scholar]
- 30. Korah R, Boots M, Wieder R. Integrin alpha5beta1 promotes survival of growth‐arrested breast cancer cells: an in vitro paradigm for breast cancer dormancy in bone marrow. Cancer Res. 2004;64:4514‐4522. [DOI] [PubMed] [Google Scholar]
- 31. Mori Y, Shimizu N, Dallas M, et al. Anti‐alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood. 2004;104:2149‐2154. [DOI] [PubMed] [Google Scholar]
- 32. Takayama S, Ishii S, Ikeda T, Masamura S, Doi M, Kitajima M. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res 2005;25:79‐83. [PubMed] [Google Scholar]
- 33. Nakamura I, Duong LT, Rodan SB, Rodan GA. Involvement of alpha(v)beta3 integrins in osteoclast function. J Bone Miner Metab. 2007;25:337‐344. [DOI] [PubMed] [Google Scholar]
- 34. Bakewell SJ, Nestor P, Prasad S, et al. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci U S A. 2003;100:14205‐14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shiozawa Y, Havens AM, Jung Y, et al. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105:370‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanjore H, Zeisberg EM, Gerami‐Naini B, Kalluri R. Beta1 integrin expression on endothelial cells is required for angiogenesis but not for vasculogenesis. Dev Dyn. 2008;237:75‐82. [DOI] [PubMed] [Google Scholar]
- 37. Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1‐positive haematopoietic bone marrow progenitors initiate the pre‐metastatic niche. Nature. 2005;438:820‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell‐derived factor‐1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832‐1837. [PubMed] [Google Scholar]
- 39. Hiratsuka S, Watanabe A, Sakurai Y, et al. The S100A8‐serum amyloid A3‐TLR4 paracrine cascade establishes a pre‐metastatic phase. Nat Cell Biol. 2008;10:1349‐1355. [DOI] [PubMed] [Google Scholar]
- 40. Wels J, Kaplan RN, Rafii S, Lyden D. Migratory neighbors and distant invaders: tumor‐associated niche cells. Genes Dev. 2008;22:559‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jung Y, Kim JK, Shiozawa Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cho H, Seo Y, Loke KM, et al. Cancer‐stimulated CAFs enhance monocyte differentiation and pro‐tumoral TAM activation via IL‐6 and GM‐CSF secretion. Clin Cancer Res. 2018. 10.1158/1078-0432.CCR-18-0125 [DOI] [PubMed] [Google Scholar]
- 43. Rossnagl S, Ghura H, Groth C, et al. A subpopulation of stromal cells controls cancer cell homing to the bone marrow. Cancer Res. 2018;78(1):129‐142. [DOI] [PubMed] [Google Scholar]
- 44. Caiado F, Carvalho T, Rosa I, et al. Bone marrow‐derived CD11b+Jagged2 + cells promote epithelial‐to‐mesenchymal transition and metastasization in colorectal cancer. Cancer Res. 2013;73(14):4233‐4246. [DOI] [PubMed] [Google Scholar]
- 45. Schuettpelz LG, Link DC. Niche competition and cancer metastasis to bone. J Clin Invest. 2011;121:1253‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shiozawa Y, Pedersen EA, Havens AM, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Calabuig‐Farinas S, Jantus‐Lewintre E, Herreros‐Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer‐which one will win? Transl Lung Cancer Res. 2016;5:466‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lohr JG, Adalsteinsson VA, Cibulskis K, et al. Whole‐exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol. 2014;32:479‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schmidt‐Kittler O, Ragg T, Daskalakis A, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci U S A. 2003;100:7737‐7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Demeulemeester J, Kumar P, Moller EK, et al. Tracing the origin of disseminated tumor cells in breast cancer using single‐cell sequencing. Genome Biol. 2016;17:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cote RJ, Datar R. Circulating Tumor Cells. New York, NY: Springer; 2016. [Google Scholar]
- 53. Ting DT, Wittner BS, Ligorio M, et al. Single‐cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 2014;8:1905‐1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell. 2013;24:410‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meng S, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152‐8162. [DOI] [PubMed] [Google Scholar]
- 56. Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14:2519‐2526. [DOI] [PubMed] [Google Scholar]
- 57. Gnant M, Hadji P. Prevention of bone metastases and management of bone health in early breast cancer. Breast Cancer Res. 2010;12:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hartkopf AD, Wallwiener M, Fehm TN, et al. Disseminated tumor cells from the bone marrow of patients with nonmetastatic primary breast cancer are predictive of locoregional relapse. Ann Oncol. 2015;26:1155‐1160. [DOI] [PubMed] [Google Scholar]
- 59. Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793‐802. [DOI] [PubMed] [Google Scholar]
- 60. Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448‐456. [DOI] [PubMed] [Google Scholar]
- 61. Balz LM, Bartkowiak K, Andreas A, et al. The interplay of HER2/HER3/PI3K and EGFR/HER2/PLC‐gamma1 signalling in breast cancer cell migration and dissemination. J Pathol. 2012;227:234‐244. [DOI] [PubMed] [Google Scholar]
- 62. Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kienast Y, von Baumgarten L, Fuhrmann M, et al. Real‐time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116‐122. [DOI] [PubMed] [Google Scholar]
- 64. Bragado P, Estrada Y, Parikh F, et al. TGF‐beta2 dictates disseminated tumour cell fate in target organs through TGF‐beta‐RIII and p38alpha/beta signalling. Nat Cell Biol. 2013;15:1351‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yokota A, Kimura S, Tanaka R, et al. Osteoclasts are involved in the maintenance of dormant leukemic cells. Leuk Res. 2010;34:793‐799. [DOI] [PubMed] [Google Scholar]
- 66. Taichman RS, Patel LR, Bedenis R, et al. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS ONE. 2013;8:e61873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shiozawa Y, Berry JE, Eber MR, et al. The marrow niche controls the cancer stem cell phenotype of disseminated prostate cancer. Oncotarget. 2016;7:41217‐41232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329‐360. [DOI] [PubMed] [Google Scholar]
- 69. Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903‐907. [DOI] [PubMed] [Google Scholar]
- 71. Teng MW, Swann JB, Koebel CM, Schreiber RD, Smyth MJ. Immune‐mediated dormancy: an equilibrium with cancer. J Leukoc Biol. 2008;84:988‐993. [DOI] [PubMed] [Google Scholar]
- 72. Baxevanis CN, Perez SA. Cancer dormancy: a regulatory role for endogenous immunity in establishing and maintaining the tumor dormant state. Vaccines (Basel). 2015;3:597‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Marcus A, Gowen BG, Thompson TW, et al. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol. 2014;122:91‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565‐1570. [DOI] [PubMed] [Google Scholar]
- 75. Banys M, Krawczyk N, Fehm T. The role and clinical relevance of disseminated tumor cells in breast cancer. Cancers (Basel). 2014;6:143‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells ‐ mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2017;14:155‐167. [DOI] [PubMed] [Google Scholar]
- 77. Pantel K, Schlimok G, Kutter D, et al. Frequent down‐regulation of major histocompatibility class I antigen expression on individual micrometastatic carcinoma cells. Cancer Res. 1991;51:4712‐4715. [PubMed] [Google Scholar]
- 78. Wu MS, Li CH, Ruppert JG, Chang CC. Cytokeratin 8‐MHC class I interactions: a potential novel immune escape phenotype by a lymph node metastatic carcinoma cell line. Biochem Biophys Res Commun. 2013;441:618‐623. [DOI] [PubMed] [Google Scholar]
- 79. Hall CL, Yao M, Hill LL, Owen‐Schaub LB. Essential role for hematopoietic Fas ligand (FasL) in the suppression of melanoma lung metastasis revealed in bone marrow chimeric mice. Clin Exp Metastasis. 2004;21:251‐256. [DOI] [PubMed] [Google Scholar]
- 80. Mitsiades N, Poulaki V, Mastorakos G, et al. Fas ligand expression in thyroid carcinomas: a potential mechanism of immune evasion. J Clin Endocrinol Metab. 1999;84:2924‐2932. [DOI] [PubMed] [Google Scholar]
- 81. Placke T, Orgel M, Schaller M, et al. Platelet‐derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012;72:440‐448. [DOI] [PubMed] [Google Scholar]
- 82. Wang B, Wang Q, Wang Z, et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014;74:5746‐5757. [DOI] [PubMed] [Google Scholar]
- 83. Barsoum IB, Hamilton TK, Li X, et al. Hypoxia induces escape from innate immunity in cancer cells via increased expression of ADAM10: role of nitric oxide. Cancer Res. 2011;71:7433‐7441. [DOI] [PubMed] [Google Scholar]
- 84. Feuerer M, Rocha M, Bai L, et al. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int J Cancer. 2001;92:96‐105. [PubMed] [Google Scholar]
- 85. Mahnke YD, Schwendemann J, Beckhove P, Schirrmacher V. Maintenance of long‐term tumour‐specific T‐cell memory by residual dormant tumour cells. Immunology. 2005;115:325‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Di Mitri D, Toso A, Chen JJ, et al. Tumour‐infiltrating Gr‐1 + myeloid cells antagonize senescence in cancer. Nature. 2014;515:134‐137. [DOI] [PubMed] [Google Scholar]
- 87. Saudemont A, Quesnel B. In a model of tumor dormancy, long‐term persistent leukemic cells have increased B7‐H1 and B7.1 expression and resist CTL‐mediated lysis. Blood. 2004;104:2124‐2133. [DOI] [PubMed] [Google Scholar]
- 88. Semesiuk NI, Zhylchuk A, Bezdenezhnykh N, et al. Disseminated tumor cells and enhanced level of some cytokines in bone marrow and peripheral blood of breast cancer patients as predictive factors of tumor progression. Exp Oncol. 2013;35:295‐302. [PubMed] [Google Scholar]
- 89. Alix‐Panabieres C, Bartkowiak K, Pantel K. Functional studies on circulating and disseminated tumor cells in carcinoma patients. Mol Oncol. 2016;10:443‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Welty CJ, Coleman I, Coleman R, et al. Single cell transcriptomic analysis of prostate cancer cells. BMC Mol Biol. 2013;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chery L, Lam HM, Coleman I, et al. Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget. 2014;5(20):9939‐9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ruppender N, Larson S, Lakely B, et al. Cellular adhesion promotes prostate cancer cells escape from dormancy. PLoS ONE. 2015;10(6):e130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lang JE, Scott JH, Wolf DM, et al. Expression profiling of circulating tumor cells in metastatic breast cancer. Breast Cancer Res Treat. 2015;149(1):121‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fina E, Callari M, Reduzzi C, et al. Gene expression profiling of circulating tumor cells in breast cancer. Clin Chem. 2015;61(1):278‐289. [DOI] [PubMed] [Google Scholar]
- 95. Wang Y, Shi J, Chai K, Ying X, Zhou BP. The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets. 2013;13:963‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bartkowiak K, Effenberger KE, Harder S, et al. Discovery of a novel unfolded protein response phenotype of cancer stem/progenitor cells from the bone marrow of breast cancer patients. J Proteome Res. 2010;9:3158‐3168. [DOI] [PubMed] [Google Scholar]
- 97. Willipinski‐Stapelfeldt B, Riethdorf S, Assmann V, et al. Changes in cytoskeletal protein composition indicative of an epithelial‐mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005;11:8006‐8014. [DOI] [PubMed] [Google Scholar]
- 98. Baccelli I, Schneeweiss A, Riethdorf S, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539‐544. [DOI] [PubMed] [Google Scholar]
- 99. Cayrefourcq L, Mazard T, Joosse S, et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 2015;75:892‐901. [DOI] [PubMed] [Google Scholar]
- 100. Zheng H, Kang Y. Cradle of evil: osteogenic niche for early bone metastasis. Cancer Cell. 2015;27:153‐155. [DOI] [PubMed] [Google Scholar]
- 101. Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22:941‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bellahcene A, Bachelier R, Detry C, Lidereau R, Clezardin P, Castronovo V. Transcriptome analysis reveals an osteoblast‐like phenotype for human osteotropic breast cancer cells. Breast Cancer Res Treat. 2007;101:135‐148. [DOI] [PubMed] [Google Scholar]
- 103. Zhang L, Sun J, Liu Z, et al. Mesenchymal stem cells regulate cytoskeletal dynamics and promote cancer cell invasion through low dose nitric oxide. Curr Mol Med. 2014;14:749‐761. [DOI] [PubMed] [Google Scholar]
- 104. Wang H, Yu C, Gao X, et al. The osteogenic niche promotes early‐stage bone colonization of disseminated breast cancer cells. Cancer Cell. 2015;27:193‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jager BA, Finkenzeller C, Bock C, et al. Estrogen receptor and HER2 status on disseminated tumor cells and primary tumor in patients with early breast cancer. Transl Oncol. 2015;8:509‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fehm T, Krawczyk N, Solomayer EF, et al. ERalpha‐status of disseminated tumour cells in bone marrow of primary breast cancer patients. Breast Cancer Res. 2008;10:R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kulemann B, Rosch S, Seifert S, et al. Pancreatic cancer: circulating tumor cells and primary tumors show heterogeneous KRAS mutations. Sci Rep. 2017;7:4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bednarz‐Knoll N, Alix‐Panabieres C, Pantel K. Clinical relevance and biology of circulating tumor cells. Breast Cancer Res. 2011;13:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Szatanek R, Drabik G, Baran J, et al. Detection of isolated tumour cells in the blood and bone marrow of patients with gastric cancer by combined sorting, isolation and determination of MAGE‐1, ‐2 mRNA expression. Oncol Rep. 2008;19:1055‐1060. [PubMed] [Google Scholar]
- 110. Schindlbeck C, Andergassen U, Jueckstock J, Rack B, Janni W, Jeschke U. Disseminated and circulating tumor cells in bone marrow and blood of breast cancer patients: properties, enrichment, and potential targets. J Cancer Res Clin Oncol. 2016;142:1883‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hong Y, Fang F, Zhang Q. Circulating tumor cell clusters: what we know and what we expect (Review). Int J Oncol. 2016;49:2206‐2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vishnoi M, Peddibhotla S, Yin W, et al. The isolation and characterization of CTC subsets related to breast cancer dormancy. Sci Rep. 2015;5:17533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Comen E, Norton L, Massague J. Clinical implications of cancer self‐seeding. Nat Rev Clin Oncol. 2011;8:369‐377. [DOI] [PubMed] [Google Scholar]
- 114. Daskalaki A, Agelaki S, Perraki M, et al. Detection of cytokeratin‐19 mRNA‐positive cells in the peripheral blood and bone marrow of patients with operable breast cancer. Br J Cancer. 2009;101:589‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wiedswang G, Borgen E, Schirmer C, et al. Comparison of the clinical significance of occult tumor cells in blood and bone marrow in breast cancer. Int J Cancer. 2006;118:2013‐2019. [DOI] [PubMed] [Google Scholar]
- 116. Bidard FC, Vincent‐Salomon A, Sigal‐Zafrani B, et al. Prognosis of women with stage IV breast cancer depends on detection of circulating tumor cells rather than disseminated tumor cells. Ann Oncol. 2008;19:496‐500. [DOI] [PubMed] [Google Scholar]
- 117. Hinz S, Bockhorst J, Roder C, et al. Disseminated tumor cells in the bone marrow negatively influence survival after resection of colorectal liver metastases. Ann Surg Oncol. 2012;19:2539‐2546. [DOI] [PubMed] [Google Scholar]
- 118. Benoy IH, Elst H, Philips M, et al. Real‐time RT‐PCR detection of disseminated tumour cells in bone marrow has superior prognostic significance in comparison with circulating tumour cells in patients with breast cancer. Br J Cancer. 2006;94:672‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mansi JL, Gogas H, Bliss JM, Gazet JC, Berger U, Coombes RC. Outcome of primary‐breast‐cancer patients with micrometastases: a long‐term follow‐up study. Lancet. 1999;354:197‐202. [DOI] [PubMed] [Google Scholar]
- 120. Janni W, Vogl FD, Wiedswang G, et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse–a European pooled analysis. Clin Cancer Res. 2011;17:2967‐2976. [DOI] [PubMed] [Google Scholar]
- 121. Naume B, Zhao X, Synnestvedt M, et al. Presence of bone marrow micrometastasis is associated with different recurrence risk within molecular subtypes of breast cancer. Mol Oncol. 2007;1:160‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Drageset V, Nesland JM, Erikstein B, et al. Monitoring of disseminated tumor cells in bone marrow in high‐risk breast cancer patients treated with high‐dose chemotherapy. Int J Cancer. 2006;118:2877‐2881. [DOI] [PubMed] [Google Scholar]
- 123. Hoffmann O, Aktas B, Goldnau C, et al. Effect of ibandronate on disseminated tumor cells in the bone marrow of patients with primary breast cancer: a pilot study. Anticancer Res. 2011;31:3623‐3628. [PubMed] [Google Scholar]
- 124. Slade MJ, Payne R, Riethdorf S, et al. Comparison of bone marrow, disseminated tumour cells and blood‐circulating tumour cells in breast cancer patients after primary treatment. Br J Cancer. 2009;100:160‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329‐340. [DOI] [PubMed] [Google Scholar]
- 126. Naume B, Synnestvedt M, Falk RS, et al. Clinical outcome with correlation to disseminated tumor cell (DTC) status after DTC‐guided secondary adjuvant treatment with docetaxel in early breast cancer. J Clin Oncol. 2014;32:3848‐3857. [DOI] [PubMed] [Google Scholar]
- 127. Pillai SG, Zhu P, Siddappa CM, et al. Enrichment and molecular analysis of breast cancer disseminated tumor cells from bone marrow using microfiltration. PLoS ONE. 2017;12:e0170761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Diel IJ, Jaschke A, Solomayer EF, et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long‐term follow‐up. Ann Oncol. 2008;19:2007‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Decker AM, Jung Y, Cackowski FC, Yumoto K, Wang J, Taichman RS. Sympathetic signaling reactivates quiescent disseminated prostate cancer cells in the bone marrow. Mol Cancer Res. 2017;15:1644‐1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chien S, Zhao X, Brown M, et al. A novel small molecule e‐selectin inhibitor GMI‐1271 blocks adhesion of AML Blasts to E‐Selectin and mobilizes blood cells in Nodscid IL2Rgc−/− mice engrafted with human AML. Blood. 2012;120:4092. [Google Scholar]
- 131. Zheng X, Fan L, Zhou P, et al. Detection of circulating tumor cells and circulating tumor microemboli in gastric cancer. Transl Oncol. 2017;10:431‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hinz S, Hendricks A, Wittig A, et al. Detection of circulating tumor cells with CK20 RT‐PCR is an independent negative prognostic marker in colon cancer patients ‐ a prospective study. BMC Cancer. 2017;17:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420‐1430. [DOI] [PubMed] [Google Scholar]
- 134. Fehm T, Hoffmann O, Aktas B, et al. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT‐PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res. 2009;11:R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Riethdorf S, Muller V, Loibl S, et al. Prognostic impact of circulating tumor cells for breast cancer patients treated in the Neoadjuvant “Geparquattro” trial. Clin Cancer Res. 2017;23(18):5384‐5393. [DOI] [PubMed] [Google Scholar]
- 136. Hoffmann O, Schroer‐Zuendorf IA, Kasimir‐Bauer S, et al. Evaluation of the prognostic significance of disseminated tumor cells in the bone marrow of primary, non‐metastatic breast cancer patients after a 7‐year follow‐up. Arch Gynecol Obstet. 2015;292(5):1117‐1125. [DOI] [PubMed] [Google Scholar]
- 137. Sola M, Margeli M, Castella E, et al. Detection of disseminated tumor cells in locally advanced breast cancer patients before primary systemic therapy. Breast. 2013;22(5):908‐913. [DOI] [PubMed] [Google Scholar]
- 138. Mathiesen RR, Borgen E, Renolen A, et al. Persistence of disseminated tumor cells after neoadjuvant treatment for locally advanced breast cancer predicts poor survival. Breast Cancer Res. 2012;14(4):R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rack BK, Schindlbeck C, Andergassen U, et al. Use of circulating tumor cells(CTC)in peripheral blood of breast cancer patients before and after adjuvant chemotherapy to predict risk of relapse: the success trial. Anticancer Res. 2010;28(6B):4048‐4049. [Google Scholar]
- 140. Lilleby W, Stensvold A, Mills IG, et al. Disseminated tumor cells and their prognostic significance in nonmetastatic prostate cancer patients. Int J Cancer. 2013;133(1):149‐155. [DOI] [PubMed] [Google Scholar]
- 141. Lilleby W, Nesland JM, Fossa SD, et al. The prognostic impact of cytokeratin‐positive cells in bone marrow of patients with localized prostate cancer. Int J Cancer. 2003;103(1):91‐96. [DOI] [PubMed] [Google Scholar]
- 142. Kollermann J, Weikert S, Schostak M, et al. Prognostic significance of disseminated tumor cells in the bone marrow of prostate cancer patients treated with neoadjuvant hormone treatment. J Clin Oncol. 2008;26(30):4928‐4933. [DOI] [PubMed] [Google Scholar]
- 143. Carlsson A, Kuhn P, Luttgen MS, et al. Paired high‐content analysis of prostate cancer cells in bone marrow and blood characterizes increased androgen receptor expression in tumor cell clusters. Clin Cancer Res. 2017;23(7):1722‐1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kienle P, Koch M, Autschbach F, et al. Decreased detection rate of disseminated tumor cells of rectal cancer patients after preoperative chemoradiation: a first step towards a molecular surrogate marker for neoadjuvant treatment in colorectal cancer. Ann Surg. 2003;238(3):324‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]


