Abstract
Inter–individual differences in pain sensitivity vary as a function of interactions between sensory, cognitive–affective and dispositional factors. Trait mindfulness, characterized as the innate capacity to non–reactively sustain attention to the present moment, is a psychological construct that is associated with lower clinical pain outcomes. Yet, the neural mechanisms supporting dispositional mindfulness are unknown. In an exploratory data analysis obtained during a study comparing mindfulness to placebo–analgesia, we sought to determine if dispositional mindfulness is associated with lower pain sensitivity. We also aimed to identify the brain mechanisms supporting the postulated inverse relationship between trait mindfulness and pain in response to noxious stimulation. We hypothesized that trait mindfulness would be associated with lower pain and greater deactivation of the default mode network. Seventy–six, meditation–naïve and healthy volunteers completed the Freiburg Mindfulness Inventory (FMI) and were administered innocuous (35°C) and noxious stimulation (49°C) during perfusion–based functional magnetic resonance imaging. Higher FMI ratings were associated with lower pain intensity (p =.005) and pain unpleasantness ratings (p =.005). Whole brain analyses revealed that higher dispositional mindfulness was associated with greater deactivation of a brain region extending from the precuneus to posterior cingulate cortex (PCC) during noxious heat. These novel findings demonstrate that mindful individuals feel less pain and evoke greater deactivation of brain regions supporting the engagement sensory, cognitive and affective appraisals. We propose that mindfulness and the PCC should be considered as important mechanistic targets for pain therapies.
INTRODUCTION
Pain is a highly subjective and dynamic experience constructed and modulated by ascending nociceptive signals and a myriad of interactions between psychological, cognitive and contextual factors [8; 56]. Inter–individual differences in pain sensitivity are also shaped by fluctuating shifts in attention between nociceptive–driven salience and self–referential processes [4; 53; 77]. Resting brain states can reliably predict if individuals will report magnified sensory and affective responses or if they can effectively cope with real–time pain [34; 35]. Further, individuals with a greater propensity to decouple affective appraisals from sensory discriminative processes report less pain [24]. Interestingly, mindfulness meditation, a technique premised on sustaining non–reactive awareness to the present moment, reduces pain through a non–evaluative attentional stance towards painful sensations [62; 85; 90], evidenced by greater somatosensory processing and reduced prefrontal cortical activation during painful heat [19; 24].
Trait mindfulness is defined as the innate propensity to be aware of the present moment in a non–reactive manner [11]. Long–term meditation practitioners express exceptionally high levels of mindfulness likely due to extensive bouts of mental training. Yet, there is wide–ranging trait–level variability in mindfulness that is independent of mindfulness meditation practice. Individuals exhibiting low levels of dispositional mindfulness have a higher susceptibility to ruminate [49] and catastrophize about pain [59]. In contrast, mindful individuals a) demonstrate a greater ability to self–regulate, b) engage acceptance–based coping strategies during pain and, c) report lower pain across a number of chronic pain populations [42; 51; 63]. However, the neural mechanisms supporting the relationship between dispositional mindfulness and pain are unknown.
There has been a significant amount of attention directed towards identifying the impact of mindfulness practices on resting network–based brain mechanisms such as the default mode network (DMN). In brief, the DMN is defined by oscillating activity within a group of distinct brain regions [medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC)/precuneus, inferior and lateral temporal cortices] and is associated with facilitating self–referential processes [12; 39; 54; 68]. The PCC is the central node of the DMN and has been characterized as a prominent mechanistic marker uniquely impacted by mindfulness [5; 6; 23; 65; 89] and pain [17; 34; 35; 40]. Mindfulness meditation reduces PCC activation after brief [83; 88] and extensive [6; 70; 71] training. Further, mindfulness–induced PCC alterations are associated with lower anxiety [89] and inflammatory IL–6 circulation [10] corresponding to a decrease in self–referential cognitive distortions [41]. Yet, we have not identified if inter–individual differences in trait mindfulness co–vary with pain sensitivity in meditation naïve individuals and the brain mechanisms supporting the hypothesized relationship between trait mindfulness and pain. The present analysis examined cerebral blood flow (CBF) using perfusion MRI and a relatively large sample size to determine if higher trait mindfulness is associated with lower pain sensitivity reports and greater deactivation of the default mode network.
METHODS
Participants
The present data are derived from portions of a previously published neuroimaging experiment [83] examining the neural mechanisms supporting mindfulness–based analgesia when compared to placebo. Participants underwent two separate fMRI sessions before and after separate 4–session interventions. This study examined data obtained in the pre–intervention MRI session only. Eighty–five healthy, pain–free, right–handed volunteers successfully completed the pre–intervention MRI session. Nine subjects were excluded from the final analysis due to MRI–related artifacts (defined below in the CBF Artifact Detection Procedures section). Thus, a total of 76 participants (mean age = 27 ± 5; age range 22–52 years; 40 females) are included in this study. The distribution of ethnicities included 57 Whites, 8 Blacks, 5 Asians, 5 “mixed”, and one Hispanic. Individuals were excluded from participating in the study if they were: depressed, taking psychotropic and/or pain medication, pregnant, experiencing ongoing/chronic pain, and reported prior meditation experience. Wake Forest School of Medicine’s Institutional Review Board approved all study procedures.
Stimuli
As in our previous studies [82; 83; 88], a TSA–II device (Medoc Inc.) was used to deliver all thermal stimuli using a 16 mm2 surface area thermal probe. The thermal probe was moved to a new stimulation site after each experimental series to reduce habituation/sensitization and all stimulus temperatures were ≤ 49°C.
Psychophysical Assessment of Pain
As previously [8; 82], pain intensity and unpleasantness ratings were assessed using a 15 cm plastic sliding visual analog scale (VAS) [57]. Operational distinctions between pain intensity and unpleasantness are delineated in greater detail in our previous study [83].
Dispositional Mindfulness Assessment
The Freiburg Mindfulness Inventory short form (FMI) is a 14–item assessment designed to assess dispositional mindfulness because it’s validity is not predicated upon prior meditative experience, does not require experience with Buddhism or meditation, and has been utilized as a validated measure of trait mindfulness [48; 75; 91]. The FMI is a psychometrically valid instrument that exhibited high internal consistency (Cronbach alpha = 0.86) in this study and previous work [75]. Higher scores indicate higher mindfulness.
Anatomical MRI Acquisition
Participants were scanned on a 3T Siemens Skyra scanner with a 32–channel head coil (Siemens AG, Erlangen, Germany). High–resolution T1–weighted images were obtained using a MP–RAGE sequence: Flip Angle = 9°, TI = 900 ms, TE = 2.95, TR = 2300 ms, pixel bandwidth = 240 Hz/pix, FOV = 25.6 × 24 cm, 192 number of slices, 1 mm isotropic spatial resolution, GRAPPA factor of 2, scan time = 5 min 12s.
Functional MRI Acquisition
Pseudo–continuous arterial spin labeling (PCASL)[66] was performed to acquire whole–brain CBF images: tagging duration = 1.8 s, TI = 3 s, TE = 12 ms, TR = 4 s, reps = 66, FOV = 22 × 22 cm, in–plane matrix size = 64 × 64, 26, 5 mm axial slices with 1 mm slice gap, scan time = 4 min 24 s. A single shot EPI acquisition with GRAPPA factor of 2 was used.
Study Design
There were a total of seven, experimental sessions including a pre and post intervention MRI session in the previous study [83]. In the present study, only data collected from the first two experimental sessions will be presented.
Experimental Session 1 (Psychophysical Training):
After providing written consent, subjects completed the FMI. During psychophysical training, subjects were familiarized with 32, 5s duration stimuli (ventral aspect of the left forearm; 35 – 49°C) to better train usage with the VAS. We then administered a 4min and 24s series of alternating 49°C (12s plateau) and 35°C (12s plateau) stimulation to the back of the left calf, identical to the “heat” paradigm used in subsequent MRI experimental sessions.
Experimental Session 2 (MRI session):
Participants reported to the Wake Forest Center for Biomolecular Imaging, were positioned in the MRI scanner, and placed their right leg on the thermal probe using custom–made probe holder. A structural MRI scan was first acquired followed by four total series of PCASL images. Two “neutral” and two “heat” series were administered in an alternating fashion and counterbalanced across subjects during PCASL acquisition. The neutral series consisted of sustained innocuous 35°C stimulation. The heat series included ten, 12 second plateaus of 49°C interleaved between eleven, 8 second periods of 35°C stimulation [83]. We obtained VAS pain intensity and unpleasantness ratings after each PCASL series. The thermal probe was moved to a new location on the right calf after each series.
Analysis of Behavioral Data
Behavioral data were analyzed with SPSS 19.0 software (IBM, Armonk, New York, USA).
Pain and Freiburg Mindfulness Inventory Ratings
Pain intensity and unpleasantness ratings from the two respective heat series were averaged to produce a mean intensity and a mean unpleasantness rating, respectively. In two separate regression analyses, pain intensity and unpleasantness ratings were entered as the dependent variable, respectively while controlling for age and sex. FMI ratings were then entered to examine the relationship between FMI on pain intensity and unpleasantness ratings, respectively.
Analysis of Neuroimaging Data
Calculation of Cerebral Blood Flow
Each 4D series of PCASL images was converted into a single CBF volume. The alternating tag and control images were subtracted to generate a perfusion–weighted series (see [83] for more details).
CBF Artifact Detection Procedures
Careful visual inspections were first performed on perfusion–weighted images to identify gross MRI–related artifacts. Next, regional masks were created to sample CBF in the territories of the carotid and vertebral arteries to identify potential tagging failures. Additionally, global CBF values were extracted to further characterize potential CBF artifacts. CBF images exhibiting low (≤ 20ml/100g tissue/min) global/regional CBF values were subsequently characterized as anomalous [83]. In sum, nine subjects exhibited artifacts and/or anomalous CBF and their data were subsequently removed from the present analyses (76 participants are included in the present study).
Statistical Analyses of Regional Signal Changes within the Brain
Brain activation was inferred via significant changes in CBF. The functional image analysis package FSL [Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (Center for FMRIB, University of Oxford, Oxford, UK) was used for image processing and analyses. Functional data were spatially smoothed with a 6mm full–width at half–maximum three–dimensional isotropic Gaussian kernel prior to standard processing within the FEAT module of FSL (see [83] for more details).
Each subject’s functional images were registered to their structural data using a six–parameter linear 3–D transformation. Brain extracted structural data (using BET)[67] were transformed into standard stereotaxic space (as defined by Montreal Neurologic Institute) using a 12–parameter affine transformation followed by a non–linear transformation [1; 2; 30]. This non–linear transform was then applied to CBF data.
Statistical analysis of regional signal changes was performed on 4D concatenated CBF data (first–level analyses) using a fixed–effects general linear modeling approach [78]. Random effects analyses were employed to assess activation across individuals. T/F statistic images were Gaussianized and thresholded using clusters determined by a z > 2.3. Corrected cluster significance threshold was set at p < 0.05 [80]. This procedure ensures that the probability of false positive findings is corrected for multiple comparisons across all brain voxels [79].
A first level one factor ANOVA was performed for each individual in order to identify a main effect of stimulation (heat vs. neutral). A second level analysis was conducted across individuals to identify significant mean effects associated with stimulation level and whole–brain regression analysis examined the neural correlates supporting individual differences in FMI scores. The first regressor modeled the mean effect of noxious thermal stimulation (heat vs. neutral) on regional brain activation. The second regressor modeled the effect of mean centered FMI ratings on regional brain activation.
To better visualize the results corresponding to our primary hypothesis that higher trait mindfulness would be associated with greater deactivation of the default mode network in response to noxious heat stimulation, binary masks were created to extract CBF values from brain regions of interest that significantly covaried with FMI ratings. Global and region of interest (ROI) CBF values were calculated by performing the following procedures. First, whole–brain and ROI binary masks were created and registered to each individual’s anatomical space [25; 30; 31]. ROI–based binary masks were generated around the significant thresholded clusters corresponding to our specific hypotheses using the Harvard–Oxford Probabilistic Atlas (defined as contiguous voxels with ≥ 50% probability of being labeled as the PCC) [14; 55]. We then calculated (FSL’s fslstats command) each individual’s mean CBF value from their respective perfusion–weighed images corresponding to whole–brain (whole–brain CBF) and ROI mask (mask CBF) during each heat and neutral series. Next, the mean CBF ratio (mask CBF/whole–brain CBF) corresponding to heat and neutral series was calculated, respectively. Finally, separate bivariate correlation analyses were conducted to determine if mean CBF ratio values in the PCC during heat were associated with a) pain ratings, and b) to confirm the relationship with FMI values. Conjunction analyses were also conducted to examine if there was significant overlapping activation between brain regions supporting the main effect of pain and FMI scores (z threshold = 2.3, p value threshold = 0.05) [46].
RESULTS
Behavioral Findings
Dispositional Mindfulness is Inversely Associated with Self–Reported Pain
Pain Intensity
When controlling for age and sex, higher dispositional mindfulness ratings (M = 40.37, SEM = .79) were significantly associated [semipartial coefficient (sr) = −.32, p = .005] with lower pain intensity ratings (Figure 1a; Table 1). There was no significant relationship between age and sex and a) pain intensity ratings and b) FMI values (ps > .05).
Figure 1.
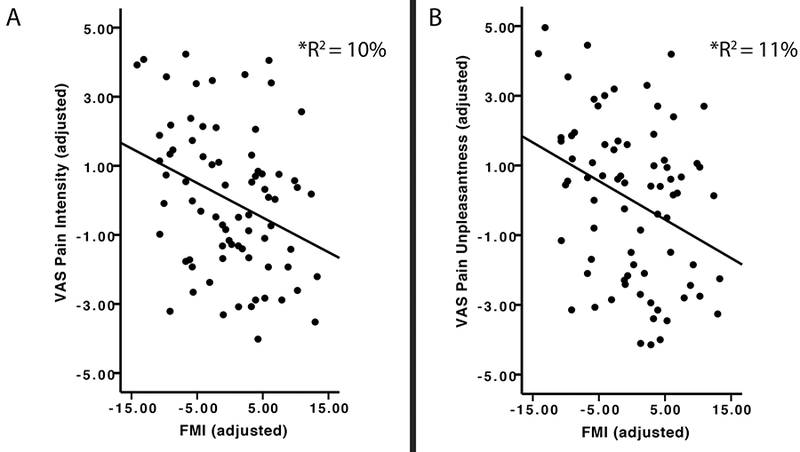
Note. N=76. Left Graph (A): The relationship between mean adjusted visual analog scale (VAS) pain intensity ratings (Mean = 4.75; SEM = .24) and mean adjusted Freiburg Mindfulness Inventory (FMI; Mean = 40.39; SEM =.79, FMI), after accounting for age and sex. After controlling for age and sex, FMI scores uniquely accounted for 10% (p< .001) of the shared variance with VAS pain intensity ratings. Right Graph (B): The relationship between mean centered VAS pain unpleasantness ratings (Mean = 5.02; SEM = .27) and mean centered FMI ratings (Mean = 40.39; SEM =.79 , FMI), after accounting for age and sex. After controlling for age and sex, mean centered FMI scores uniquely accounted for 11% (p< .001) of the shared variance with mean centered VAS pain unpleasantness ratings.
Table 1.
Multiple regression analysis assessing the relationship among pain intensity ratings, age, gender, and Freiburg Mindfulness Inventory ratings (n=76)
| Variable | B | SE B | β | sr2 | Model R2 | F |
|---|---|---|---|---|---|---|
| .12 | 3.16* | |||||
| Age | −.01 | .05 | −.02 | .00 | ||
| Sex | .37 | .47 | .09 | .00 | ||
| FMI | −.10 | .03 | −.32** | .10 |
Dependent variable = pain intensity ratings; independent variables = age, sex, FMI ratings; B =unstandardized beta coefficient; SE B= standard error of unstandardized beta coefficient, β = standardized beta coefficient; sr2= semi–partial coefficient squared; FMI = Freiburg Mindfulness Inventory ratings.
p = 0.03.
p= 0.005.
Pain Unpleasantness
Higher dispositional mindfulness scores were significantly associated (sr = −.32, p = .005) with lower pain unpleasantness ratings (Figure 1b) when controlling for age and sex (Figure 1b; Table 2). There was no significant relationship between age and sex and a) pain unpleasantness ratings and b) FMI values (ps > .05).
Table 2.
Multiple regression analysis assessing the relationship among pain unpleasantness ratings, age, gender, and Freiburg Mindfulness Inventory ratings (n=76)
| Variable | B | SE B | β | sr2 | Model R2 | F |
|---|---|---|---|---|---|---|
| .13 | 3.67* | |||||
| Age | −.001 | .05 | −.001 | .00 | ||
| Sex | .65 | .52 | .14 | .02 | ||
| FMI | −.11 | .04 | −.32** | .10 |
Dependent variable = pain unpleasantness ratings; independent variables = age, sex, FMI ratings; B =unstandardized beta coefficient; SE B= standard error of unstandardized beta coefficient, β = standardized beta coefficient; sr2= semi–partial coefficient squared; FMI = Freiburg Mindfulness Inventory ratings.
p = 0.02.
p= 0.005.
Neuroimaging Findings
Noxious Heat–Related Brain Activation
When compared to neutral (35°C) stimulation, noxious (49°C) stimulation produced activation in the primary somatosensory cortex (SI) corresponding to the stimulation site, periaqueductal gray matter (PAG), bilateral thalamus, bilateral secondary somatosensory cortices (SII), bilateral parietal operculum, posterior/anterior insula, mid–cingulate cortex, dorsal anterior cingulate cortex (dACC), cerebellum, supplementary motor area (SMA) and deactivation of the PCC/precuneus, bilateral dorsolateral prefrontal cortex (dlPFC), and mPFC (Figure 2; Table 3).
Figure 2.
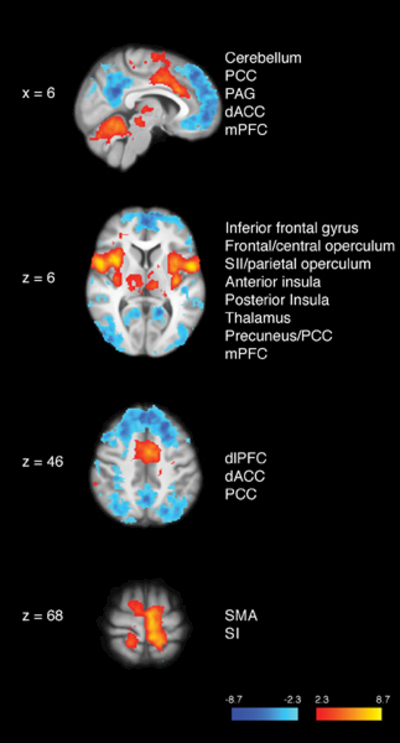
Brain activations and deactivations during the main effect of pain. Greater activation in the bilateral thalamus, primary somatosensory cortex (SI) corresponding to the stimulation site (i.e., right calf), bilateral secondary somatosensory cortices (SII), bilateral posterior insula, bilateral anterior insula, dorsal anterior cingulate cortex (dACC), supplementary motor area (SMA), cerebellum, and periaqueductal gray matter (PAG) was detected in the presence of noxious heat when compared to innocuous neutral series. Greater deactivation of the precuneus/posterior cingulate cortex (PCC), medial prefrontal cortex (mPFC), and dorsolateral PFC (dlPFC) was revealed during heat when compared to neutral stimuli. R= subject right, slice locations correspond to standard stereotaxic space.
Table 3.
Supplementary brain coordinates
| Region | Z–score | (x, y, z) | P value | ||
|---|---|---|---|---|---|
| Figure 2: Main Effect of Pain 49°C vs. 35°C | |||||
| Activations | |||||
| L. Cerebellum | 5.86 | −46, −56, −36 | < .001 | ||
| R. Cerebellum | 5.61 | 38, −62, −38 | < .001 | ||
| PAG | 3.14 | −4, −28, −6 | < .001 | ||
| Dorsal ACC | 6.62 | −4, 0, 46 | < .001 | ||
| R. Interior Frontal Gyrus | 6.42 | 58, 10, 8 | < .001 | ||
| L. Interior Frontal Gyrus | 3.89 | −58, 12, 4 | < .001 | ||
| L. Frontal/Central Operculum | 5.22 | −38, 10, 6 | < .001 | ||
| R. Frontal/Central Operculum | 7.24 | 40, 14, 6 | < .001 | ||
| R. SII/Posterior Insula | 5.97 | 36, −16, 14 | < .001 | ||
| L. SII/Posterior Insula | 7.62 | −36, −20, 14 | <. 001 | ||
| R. Anterior Insula | 6.94 | 40, 10, −6 | < .001 | ||
| L. Anterior Insula | 6.05 | −36, 6, 4 | < .001 | ||
| L. Thalamus | 4.97 | −10, −20, 4 | < .001 | ||
| R. Thalamus | 4.15 | 8, −20, 4 | < .001 | ||
| L. SI | 6.63 | −6, −40, 68 | < .001 | ||
| SMA | 7.02 | −6, −10, 68 | < .001 | ||
| Deactivations | |||||
| mPFC | 5.74 | −6, 54, 0 | < .001 | ||
| PCC | 6.23 | −6, −52, 22 | < .001 | ||
| Precuneus | 6.00 | −6, −56, 38 | < .001 | ||
| Figure 3: Neural correlates of trait mindfulness during noxious heat stimulation | |||||
| Deactivations | |||||
| dPCC | 3.52 | 0, −40, 44 | .002 | ||
| Precuneus | 4.40 | −2, −48, 48 | .002 | ||
| SI | 3.39 | 4, −44, 72 | .002 | ||
| Figure 5: Conjunction analysis between the main effect of pain and FMI correlates | |||||
| Precuneus | 3.69 | 0, −48, 48 | .001 | ||
| PCC | 3.52 | 0, −40, 44 | .001 | ||
Higher Trait Mindfulness is associated with greater PCC Deactivation during Noxious Heat
Higher FMI ratings were associated with greater deactivation of a brain region extending from the precuneus to the dorsal PCC (dPCC) and the ipsilateral SI during noxious heat (Figure 3; Table 3). During the 49°C heat series, greater CBF in the dPCC was significantly associated with higher pain unpleasantness (r = .23, p = .04; Figure 4) and lower FMI ratings (r = −.23, p = .04; Figure 4). There was a trend towards significance in the relationship between dPCC activation and pain intensity ratings (r = .20, p = .08) during heat series. Dorsal PCC activation during neutral series was significantly correlated with pain unpleasantness ratings (r = .23, p < .05) but not with FMI (r = −.17, p = .15) or pain intensity ratings (r = .19, p = .10).
Figure 3.
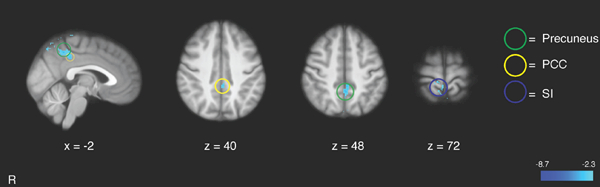
Relationship between Freiburg Mindfulness Inventory (FMI) ratings and brain activation during noxious heat series. Higher FMI scores were associated with greater deactivation of the precuneus and posterior cingulate cortex (PCC), and primary somatosensory cortex (SI). R= subject right, slice locations correspond to standard stereotaxic space.
Figure 4.
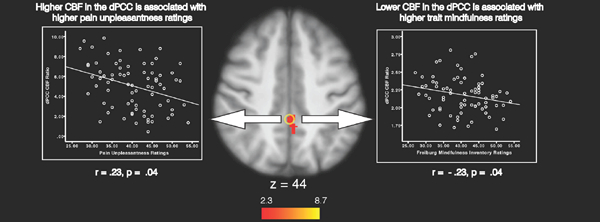
Relationship between dorsal posterior cingulate cortex (dPCC) activation, pain unpleasantness and Freiburg Mindfulness Inventory (FMI) ratings. Bivariate correlation analyses were conducted on the mean CBF ratio (mask/whole–brain) in peak dPCC activation (z–score = 3.52; Tailarach coordinates: 0, – 40, 44) during heat series between pain unpleasantness (left) and Freiburg Mindfulness Inventory (right) ratings. Higher CBF in the dPCC was associated with higher pain unpleasantness and lower mindfulness ratings in the presence of noxious heat stimulation. Slice locations correspond to standard stereotaxic space.
Significant overlap between pain and FMI–related brain deactivation during noxious heat
Conjunction analyses revealed significant (p = .001) overlap between deactivation corresponding to the main effect of pain and higher FMI rating–related deactivation in the precuneus/PCC (Figure 5; Table 3).
Figure 5.
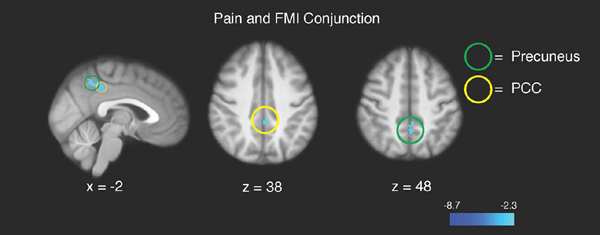
Conjunction analyses revealed significant overlap between brain regions supporting pain–related deactivation and higher FMI rating–related deactivation in the precuneus and posterior cingulate cortex (PCC). R= subject right, slice locations correspond to standard stereotaxic space.
Global Cerebral Blood Flow
Consistent with prior work [9; 83], paired samples t–tests revealed that global CBF values were significantly lower during noxious heat when compared to innocuous neutral series (Table 4; p < .001).
Table 4.
Cerebral blood flow across conditions
| Mean | SEM | |
|---|---|---|
| Whole brain CBF: Heat | 36.88* | .86 |
| Whole brain CBF: Neutral | 40.09 | .91 |
Note. N = 76. Cerebral blood flow (CBF) was significantly lower during noxious heat series (Heat) when compared to neutral series (Neutral),
p < .001.
DISCUSSION
The present findings demonstrate that higher trait mindfulness is associated with lower pain ratings (Figure 1; Table 1 & 2). Higher trait mindfulness ratings were significantly associated with lower pain sensitivity and greater deactivation of the posterior, midline nodes of the default mode network. Specifically, greater deactivation of the dorsal PCC (dPCC) and precuneus was associated with higher trait mindfulness during noxious heat when compared to innocuous stimulation (Figure 3). Post–hoc, confirmatory analyses revealed that greater CBF values in the dPCC were associated with higher pain unpleasantness and lower trait mindfulness ratings (Figure 4). These novel findings demonstrate that meditation naïve individuals exhibiting higher levels of trait mindfulness feel less pain in response to frankly noxious stimulation and evoke greater deactivation of brain regions supporting internal and external engagement of attention and corresponding affective appraisals to arising sensory events [3; 36; 37; 50].
This is the first study to demonstrate that individual differences in pain sensitivity are inversely associated with trait mindfulness levels in individuals without prior meditation experience. The mindfulness inventory (FMI) used in this study was designed to measure trait mindfulness, as characterized by the day to day ability to experience sensations and emotions without reaction [75]. It is then fitting that the association between the dPCC, precuneus and trait mindfulness was associated with the affective dimension, in contrast to the sensory aspect, of self–reported pain [58]. Further, there was significant overlap PCC and precuneus between pain and FMI–related deactivation during painful heat. These findings provide a potential neural substrate for the how trait mindfulness influences pain processing. That is, the precuneus/PCC are implicated in processing self–referential and ruminative cognitions [41; 73]. The present evidence suggest that highly mindful individuals may be more resilient to pain–related ruminations [43; 44; 60; 81] and potentially less likely to magnify evaluations that normally impart significance to salient sensory events, mechanisms also associated with mindfulness meditation–induced anxiety [89] and pain reductions [83; 88].
Greater dPCC activity is associated with lower trait mindfulness and higher pain unpleasantness ratings
Based on prior work [6; 10; 71; 83; 88; 89], we expected that mindful individuals would exhibit greater deactivation of the ventral aspect of the PCC (vPCC). Yet, the dPCC, not the vPCC, was inversely associated with trait mindfulness. The dPCC has a high metabolic rate [61], exhibits high CBF values relative to all other brain regions [52], and is anatomically and functionally hetereogeous [37; 74]. The dPCC is significantly integrated with other neural networks and has multiple, significant roles in facilitating internally directed attention and sensory awareness [28; 38]. It is also associated with monitoring and signaling affective changes in the sensory environment and allocating attentional resources to address said environmental alterations [29; 36; 37]. These characterizations are remarkably fitting with respect to the present findings.
Individuals with low dispositional mindfulness exhibited higher pain intensity and unpleasantness ratings, suggesting that they have heightened attentional sensitivity and affective engagement to noxious stimuli [36]. A recent study revealed that lower trait mindfulness ratings, in older adults, were associated with weaker functional connectivity within the dPCC, possibly indicating higher dysfunctional cognitive and affective control processes [65]. The dPCC has also been repeatedly implicated in processing the sense of self [26; 76] because it is anatomically organized and connected to five functional subsystems including attentional, motor, sensory, salience, and DMN [27; 36; 38] and facilitates broad attentional monitoring and affective reactions to initiate behavior change [37; 52]. Thus, in the context of the present data, we propose that greater precuneus/PCC–related activation uniquely corresponds to greater propensity to attach to [32], ruminate on [34] and personalize [3] pain appraisals. While non–mindful individuals may be more likely to “get caught up” [5] in pain, individuals higher in trait mindfulness may have a greater ability to decouple noxious sensory discriminations from affective appraisals [90]. In fact, mindful individuals exhibit an increased tendency to experience pleasure [72], reduced pain catastrophizing [13; 51; 63], and recover from stress more effectively than non mindful individuals [11]. Taking into consideration the present findings and previous work [7; 49; 69], greater precuneus/PCC deactivation may be associated with the meta–cognitive ability to let go of maladaptive, self–referential evaluations.
Recent work has shown that chronic pain and alcohol dependent patients exhibiting low levels of trait mindfulness have a greater proclivity to misuse opioids and alcohol during treatment and crave alcohol/opioids during recovery, respectively [20–22]. Thus, mindfulness could be an important psychological target for health promotion since it can be increased even after only brief bouts of meditation training. For instance, we found that mindfulness significantly increases on average by 13% after only three to four, 20 minutes sessions of mindfulness–based mental training [83; 84; 86–88]. Increases in mindfulness levels, in response to brief meditation training, were also associated with greater meditation–based analgesia [84] and meditation practice frequency is positively associated with dispositional mindfulness [75]. Similarly, converging lines of evidence demonstrate that a robust association between greater meditation experience and stabilized, neuroplastic induced deactivation of the PCC [6; 70]. Thus, the relationship between PCC deactivation and trait mindfulness likely reflects a broader characterization of mindfulness–based cognitive disposition.
The present study shows that mindful individuals are less sensitive to pain and exhibit significantly lower activation in the precuneus/dPCC, central nodes of self–referential [12; 61] and nociceptive processing [17; 64]. It is possible that a more conservative multiple comparison threshold would lower family wise error rates (FWE) [16]. However, the results from the present study may be less susceptible to inflated FWE rates because we employed perfusion–based fMRI, FSL’s conservative FMRIB’s Local Analysis of Mixed Effects (FLAME 1+2) Bayesian estimation method and larger voxel sizes than conventional slice parameters. Together, these approaches are associated with significantly lower FWE rates [15; 18; 45]. We postulate that future pain studies may consider including trait mindfulness measures as covariates of inter–individual variability due to the potential influence of mindfulness on the constellation of interactions between sensory, cognitive and affective factors that impact the subjective experience of pain [8; 33; 47]. These findings could be utilized to better develop adjunctive pain therapies (biofeedback; mindfulness meditation; dialectical behavioral therapy) to specifically target increases in trait mindfulness and reductions in PCC/precuneus activity to treat pain.
ACKNOWLEDGMENTS:
This work was supported by the National Center for Complementary and Integrative Health (R21–AT007247; F32–AT006949; K99/R00–AT008238; F30–AT009165), the National Institute of Neurological Disorders and Stroke (R01–NS239426), the Mind and Life Institute Francisco J. Varela Award, and the Wake Forest Center for Integrative Medicine.
References Cited
- [1].Andersson J, Jenkinson M, Smith SM. Non–linear optimisation. FMRIB Technical Report 2007.
- [2].Andersson J, Jenkinson M, Smith SM. Non–linear registration, aka Spatial normalisation. FMRIB Technical Report 2007.
- [3].Andrews–Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional–anatomic fractionation of the brain’s default network. Neuron 2010;65(4):550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proceedings of the National Academy of Sciences of the United States of America 2007;104(29):12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brewer JA, Garrison KA. The posterior cingulate cortex as a plausible mechanistic target of meditation: findings from neuroimaging. Annals of the New York Academy of Sciences 2013. [DOI] [PubMed]
- [6].Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America 2011;108(50):20254–20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brown KW, Weinstein N, Creswell JD. Trait mindfulness modulates neuroendocrine and affective responses to social evaluative threat. Psychoneuroendocrinology 2012;37(12):2037–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proceedings of the National Academy of Sciences of the United States of America 2003;100(14):8538–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Coghill RC, Sang CN, Berman KF, Bennett GJ, Iadarola MJ. Global cerebral blood flow decreases during pain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 1998;18(2):141–147. [DOI] [PubMed] [Google Scholar]
- [10].Creswell JD, Taren AA, Lindsay EK, Greco CM, Gianaros PJ, Fairgrieve A, Marsland AL, Brown KW, Way BM, Rosen RK, Ferris JL. Alterations in Resting–State Functional Connectivity Link Mindfulness Meditation With Reduced Interleukin–6: A Randomized Controlled Trial. Biological psychiatry 2016. [DOI] [PubMed]
- [11].Daubenmier J, Hayden D, Chang V, Epel E. It’s not what you think, it’s how you relate to it: dispositional mindfulness moderates the relationship between psychological distress and the cortisol awakening response. Psychoneuroendocrinology 2014;48:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Davey CG, Pujol J, Harrison BJ. Mapping the self in the brain’s default mode network. NeuroImage 2016;132:390–397. [DOI] [PubMed] [Google Scholar]
- [13].Day MA, Smitherman A, Ward LC, Thorn BE. An investigation of the associations between measures of mindfulness and pain catastrophizing. The Clinical journal of pain 2015;31(3):222–228. [DOI] [PubMed] [Google Scholar]
- [14].Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- [15].Detre JA, Wang J. Technical aspects and utility of fMRI using BOLD and ASL. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 2002;113(5):621–634. [DOI] [PubMed] [Google Scholar]
- [16].Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false–positive rates. Proceedings of the National Academy of Sciences of the United States of America 2016;113(28):7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Emerson NM, Zeidan F, Lobanov OV, Hadsel MS, Martucci KT, Quevedo AS, Starr CJ, Nahman–Averbuch H, Weissman–Fogel I, Granovsky Y, Yarnitsky D, Coghill RC. Pain sensitivity is inversely related to regional grey matter density in the brain. Pain 2014;155(3):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Flandin G, Friston KJ. Analysis of family–wise error rates in statistical parametric mapping using random field theory. Human brain mapping 2017. [DOI] [PMC free article] [PubMed]
- [19].Gard T, Holzel BK, Sack AT, Hempel H, Vaitl D, & Ott U Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cerebral cortex 2011;191(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Garland EL. Trait Mindfulness Predicts Attentional and Autonomic Regulation of Alcohol Cue–Reactivity. Journal of psychophysiology 2011;25(4):180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Garland EL, Hanley AW, Thomas EA, Knoll P, Ferraro J. Low dispositional mindfulness predicts self–medication of negative emotion with prescription opioids. Journal of addiction medicine 2015;9(1):61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Garland EL, Roberts–Lewis A, Kelley K, Tronnier C, Hanley A. Cognitive and affective mechanisms linking trait mindfulness to craving among individuals in addiction recovery. Substance use & misuse 2014;49(5):525–535. [DOI] [PubMed] [Google Scholar]
- [23].Garrison KA, Zeffiro TA, Scheinost D, Constable RT, Brewer JA. Meditation leads to reduced default mode network activity beyond an active task. Cognitive, affective & behavioral neuroscience 2015;15(3):712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Grant JA, Courtemanche J, Rainville P. A non–elaborative mental stance and decoupling of executive and pain–related cortices predicts low pain sensitivity in Zen meditators. Pain 2011;152(1):150–156. [DOI] [PubMed] [Google Scholar]
- [25].Greve DN, Fischl B. Accurate and robust brain image alignment using boundary–based registration. NeuroImage 2009;48(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Guterstam A, Bjornsdotter M, Gentile G, Ehrsson HH. Posterior cingulate cortex integrates the senses of self–location and body ownership. Current biology : CB 2015;25(11):1416–1425. [DOI] [PubMed] [Google Scholar]
- [27].Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS biology 2008;6(7):e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. The Journal of neuroscience : the official journal of the Society for Neuroscience 2006;26(51):13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Heilbronner SR, Platt ML. Causal evidence of performance monitoring by neurons in posterior cingulate cortex during learning. Neuron 2013;80(6):1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 2002;17(2):825–841. [DOI] [PubMed] [Google Scholar]
- [31].Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis 2001;5(2):143–156. [DOI] [PubMed] [Google Scholar]
- [32].Kim K, Johnson MK. Extended self: spontaneous activation of medial prefrontal cortex by objects that are ‘mine’. Social cognitive and affective neuroscience 2014;9(7):1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proceedings of the National Academy of Sciences of the United States of America 2005;102(36):12950–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kucyi A, Moayedi M, Weissman–Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Enhanced medial prefrontal–default mode network functional connectivity in chronic pain and its association with pain rumination. The Journal of neuroscience : the official journal of the Society for Neuroscience 2014;34(11):3969–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proceedings of the National Academy of Sciences of the United States of America 2013;110(46):18692–18697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 2012;32(1):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. The Journal of neuroscience : the official journal of the Society for Neuroscience 2011;31(9):3217–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain : a journal of neurology 2014;137(Pt 1): 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Maki–Marttunen V, Castro M, Olmos L, Leiguarda R, Villarreal M. Modulation of the default–mode network and the attentional network by self–referential processes in patients with disorder of consciousness. Neuropsychologia 2016;82:149–160. [DOI] [PubMed] [Google Scholar]
- [40].Martucci KT, Shirer WR, Bagarinao E, Johnson KA, Farmer MA, Labus JS, Apkarian AV, Deutsch G, Harris RE, Mayer EA, Clauw DJ, Greicius MD, Mackey SC. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network–a resting–state study from the MAPP Research Network. Pain 2015;156(9):1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus–independent thought. Science 2007;315(5810):393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McCracken LM, Gauntlett–Gilbert J, Vowles KE. The role of mindfulness in a contextual cognitive–behavioral analysis of chronic pain–related suffering and disability. Pain 2007;131(1–2): 63–69. [DOI] [PubMed] [Google Scholar]
- [43].Mrazek MD, Franklin MS, Phillips DT, Baird B, Schooler JW. Mindfulness training improves working memory capacity and GRE performance while reducing mind wandering. Psychological science 2013;24(5):776–781. [DOI] [PubMed] [Google Scholar]
- [44].Mrazek MD, Smallwood J, Schooler JW. Mindfulness and mind–wandering: finding convergence through opposing constructs. Emotion 2012;12(3):442–448. [DOI] [PubMed] [Google Scholar]
- [45].Mueller K, Lepsien J, Moller HE, Lohmann G. Commentary: Cluster failure: Why fMRI inferences for spatial extent have inflated false–positive rates. Frontiers in human neuroscience 2017;11:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage 2005;25(3):653–660. [DOI] [PubMed] [Google Scholar]
- [47].Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting discrimination of sensory features of pain: a new model. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009;29(47):14924–14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ouwens MA, Schiffer AA, Visser LI, Raeijmaekers NJ, Nyklicek I. Mindfulness and eating behaviour styles in morbidly obese males and females. Appetite 2015;87:62–67. [DOI] [PubMed] [Google Scholar]
- [49].Paul NA, Stanton SJ, Greeson JM, Smoski MJ, Wang L. Psychological and neural mechanisms of trait mindfulness in reducing depression vulnerability. Social cognitive and affective neuroscience 2013;8(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pearson JM, Hayden BY, Raghavachari S, Platt ML. Neurons in posterior cingulate cortex signal exploratory decisions in a dynamic multioption choice task. Current biology : CB 2009;19(18):1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Petter M, Chambers CT, McGrath PJ, Dick BD. The role of trait mindfulness in the pain experience of adolescents. The journal of pain : official journal of the American Pain Society 2013;14(12):1709–1718. [DOI] [PubMed] [Google Scholar]
- [52].Pfefferbaum A, Chanraud S, Pitel AL, Muller–Oehring E, Shankaranarayanan A, Alsop DC, Rohlfing T, Sullivan EV. Cerebral blood flow in posterior cortical nodes of the default mode network decreases with task engagement but remains higher than in most brain regions. Cerebral cortex 2011;21(1):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proceedings of the National Academy of Sciences of the United States of America 2010;107(1):355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Poerio GL, Sormaz M, Wang HT, Margulies D, Jefferies E, Smallwood J. The role of the default mode network in component processes underlying the wandering mind. Social cognitive and affective neuroscience 2017. [DOI] [PMC free article] [PubMed]
- [55].Poldrack RA. Region of interest analysis for fMRI. Social cognitive and affective neuroscience 2007;2(1):67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Price DD, Barell JJ. Inner Experience and Neuroscience. Cambridge, MA: MIT Press, 2012. [Google Scholar]
- [57].Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain 1994;56(2):217–226. [DOI] [PubMed] [Google Scholar]
- [58].Price DD, Harkins SW, Baker C. Sensory–affective relationships among different types of clinical and experimental pain. Pain 1987;28(3):297–307. [DOI] [PubMed] [Google Scholar]
- [59].Prins B, Decuypere A, Van Damme S. Effects of mindfulness and distraction on pain depend upon individual differences in pain catastrophizing: an experimental study. European journal of pain 2014;18(9):1307–1315. [DOI] [PubMed] [Google Scholar]
- [60].Rahl HA, Lindsay EK, Pacilio LE, Brown KW, Creswell JD. Brief mindfulness meditation training reduces mind wandering: The critical role of acceptance. Emotion 2017;17(2):224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America 2001;98(2):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Salomons TV, Kucyi A. Does Meditation Reduce Pain through a Unique Neural Mechanism? The Journal of neuroscience : the official journal of the Society for Neuroscience 2011;31(36):12705–12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schutze R, Rees C, Preece M, Schutze M. Low mindfulness predicts pain catastrophizing in a fear–avoidance model of chronic pain. Pain 2010;148(1):120–127. [DOI] [PubMed] [Google Scholar]
- [64].Seminowicz DA, Davis KD. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. Journal of neurophysiology 2007;97(5):3651–3659. [DOI] [PubMed] [Google Scholar]
- [65].Shaurya Prakash R, De Leon AA, Klatt M, Malarkey W, Patterson B. Mindfulness disposition and default–mode network connectivity in older adults. Social cognitive and affective neuroscience 2013;8(1):112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Shin DD, Liu TT, Wong EC, Shankaranarayanan A, Jung Y. Pseudocontinuous arterial spin labeling with optimized tagging efficiency. Magnetic resonance in medicine 2012;68(4):1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Smith SM. Fast robust automated brain extraction. Human brain mapping 2002;17(3):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of cognitive neuroscience 2010;22(6):1112–1123. [DOI] [PubMed] [Google Scholar]
- [69].Tamagawa R, Giese–Davis J, Speca M, Doll R, Stephen J, Carlson LE. Trait mindfulness, repression, suppression, and self–reported mood and stress symptoms among women with breast cancer. Journal of clinical psychology 2013;69(3):264–277. [DOI] [PubMed] [Google Scholar]
- [70].Taylor VA, Daneault V, Grant J, Scavone G, Breton E, Roffe–Vidal S, Courtemanche J, Lavarenne AS, Marrelec G, Benali H, Beauregard M. Impact of meditation training on the default mode network during a restful state. Social cognitive and affective neuroscience 2013;8(1):4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Taylor VA, Grant J, Daneault V, Scavone G, Breton E, Roffe–Vidal S, Courtemanche J, Lavarenne AS, Beauregard M. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. NeuroImage 2011;57(4):1524–1533. [DOI] [PubMed] [Google Scholar]
- [72].Thomas EA, Garland EL. Mindfulness is Associated With Increased Hedonic Capacity Among Chronic Pain Patients Receiving Extended Opioid Pharmacotherapy. The Clinical journal of pain 2017;33(2):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].van Buuren M, Gladwin TE, Zandbelt BB, Kahn RS, Vink M. Reduced functional coupling in the default–mode network during self–referential processing. Human brain mapping 2010;31(8):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage 2006;29(2):452–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Walach H, Buchheld N, Buttenmuller V, Kleinknecht N, Schmidt S. Measuring mindfulness—the Freiburg Mindfulness Inventory (FMI). Personality and individual differences 2006;40:1543–1555. [Google Scholar]
- [76].Whitfield–Gabrieli S, Moran JM, Nieto–Castanon A, Triantafyllou C, Saxe R, Gabrieli JD. Associations and dissociations between default and self–reference networks in the human brain. NeuroImage 2011;55(1):225–232. [DOI] [PubMed] [Google Scholar]
- [77].Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I. Anterior insula integrates information about salience into perceptual decisions about pain. The Journal of neuroscience : the official journal of the Society for Neuroscience 2010;30(48):16324–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage 2001;14(6):1370–1386. [DOI] [PubMed] [Google Scholar]
- [79].Worsley KJ. Statistical analysis of activation images In: Jezzard P, Matthews PM, Smith SM (eds). Functional MRI: An Introduction to Methods. Oxford University Press Inc. New York, New York: Oxford University Press Inc., 2001. [Google Scholar]
- [80].Worsley KJ, Evans AC, Marrett S, Neelin P. A three–dimensional statistical analysis for CBF activation studies in human brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 1992;12(6):900–918. [DOI] [PubMed] [Google Scholar]
- [81].Xu M, Purdon C, Seli P, Smilek D. Mindfulness and mind wandering: The protective effects of brief meditation in anxious individuals. Consciousness and cognition 2017;51:157–165. [DOI] [PubMed] [Google Scholar]
- [82].Zeidan F, Adler–Neal AL, Wells RE, Stagnaro E, May LM, Eisenach JC, McHaffie JG, Coghill RC. Mindfulness–Meditation–Based Pain Relief Is Not Mediated by Endogenous Opioids. The Journal of neuroscience : the official journal of the Society for Neuroscience 2016;36(11):3391–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, Coghill RC. Mindfulness Meditation–Based Pain Relief Employs Different Neural Mechanisms Than Placebo and Sham Mindfulness Meditation–Induced Analgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience 2015;35(46):15307–15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zeidan F, Gordon NS, Merchant J, Goolkasian P. The effects of brief mindfulness meditation training on experimentally induced pain. The journal of pain : official journal of the American Pain Society 2010;11(3):199–209. [DOI] [PubMed] [Google Scholar]
- [85].Zeidan F, Grant JA, Brown CA, McHaffie JG, Coghill RC. Mindfulness meditation–related pain relief: evidence for unique brain mechanisms in the regulation of pain. Neuroscience letters 2012;520(2):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zeidan F, Johnson SK, Diamond BJ, David Z, Goolkasian P. Mindfulness meditation improves cognition: evidence of brief mental training. Consciousness and cognition 2010;19(2):597–605. [DOI] [PubMed] [Google Scholar]
- [87].Zeidan F, Johnson SK, Gordon NS, Goolkasian P. Effects of brief and sham mindfulness meditation on mood and cardiovascular variables. Journal of alternative and complementary medicine 2010;16(8):867–873. [DOI] [PubMed] [Google Scholar]
- [88].Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. The Journal of neuroscience : the official journal of the Society for Neuroscience 2011;31(14):5540–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zeidan F, Martucci KT, Kraft RA, McHaffie JG, Coghill RC. Neural correlates of mindfulness meditation–related anxiety relief. Social cognitive and affective neuroscience 2014;9(6):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zeidan F, Vago DR. Mindfulness meditation–based pain relief: a mechanistic account. Annals of the New York Academy of Sciences 2016;1373(1):114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhang J, Wu C. The influence of dispositional mindfulness on safety behaviors: a dual process perspective. Accident; analysis and prevention 2014;70:24–32. [DOI] [PubMed] [Google Scholar]


