Abstract
While nonsteroidal inflammatory drugs (NSAIDs) are the first line of therapeutics for the treatment of mild to moderate somatic pain, they are not generally considered to be effective for neuropathic pain. In the current study, direct activation of spinal Toll-like 4 receptors (TLR4) by the intrathecal (IT) administration of KDO2 lipid A (KLA), the active component of lipopolysaccharide (LPS), elicits a robust tactile allodynia that is unresponsive to cyclooxygenase (COX) inhibition, despite elevated expression of COX metabolites in the spinal cord. IT KLA increases 12-Lipoxygenase-mediated hepoxilin production in the lumbar spinal cord, concurrent with expression of the tactile allodynia. The TLR4-induced hepoxilin production also was observed in primary spinal microglia, but not in astrocytes, and was accompanied by increased microglial expression of the 12/15-lipoxygenase enzyme 15-LOX-1. IT KLA-induced tactile allodynia was completely prevented by spinal pretreatment with the 12/15-Lipoxygenase inhibitor CDC or a selective antibody targeting rat 15-LOX-1. Similarly, pretreatment with the selective inhibitors ML127 or ML351 both reduced activity of the rat homolog of 15-LOX-1 heterologously expressed in HEK-293T cells and completely abrogated NSAID-unresponsive allodynia in vivo following IT KLA. Finally, spinal 12/15-Lipoxygenase inhibition by NDGA both prevents Phase II Formalin flinching and reverses Formalin-induced persistent tactile allodynia. Taken together, these findings suggest that spinal TLR4-mediated hyperpathic states are mediated at least in part through activation of microglial 15-LOX-1.
Keywords: 12-lipoxygenase, glia, pain, toll receptors, lipids
1. Introduction.
Persistent nociceptive primary afferent neuronal activation leads to central sensitization, which underlies the development of pain hypersensitivity observed following tissue injury and inflammation. It is commonly appreciated that after tissue inflammation or injury, non-neuronal cells (particularly astrocytes and microglia) contribute to nociceptive signaling cascades in the CNS [8; 32; 43; 48; 61]and can be stimulated by a variety of mediators such as glutamate, substance P, ATP, cytokines or chemokines and Toll-like receptor (TLR) ligands [10; 35; 52; 53; 74]. TLRs are expressed in the CNS, with TLR1–9 found in microglia and TLR1, 3, 4, 5 and 9 located in astrocytes [31]. Activated spinal glia contribute directly to nociceptive processing [22; 41; 56; 72] by releasing a variety of neuroactive substances including glutamate, ATP, cytokines and prostaglandins (PG) such as PGE2, which in turn trigger adjacent glia and neurons to drive maintenance of these facilitated states [43; 63]. Several investigators have shown that spinal delivery of agonists of TLR4, such as LPS and KDO2-Lipid A (KLA, the active component of LPS), elicits tactile allodynia in rats [41; 59; 60]. We found that either systemic or intrathecal (IT) administration of nonsteroidal inflammatory drugs (NSAIDs) such as ibuprofen and ketorolac failed to attenuate nociceptive behavior despite complete inhibition of TLR4-induced spinal PGE2 release [60]. Surprisingly, IT pretreatment with the glial cell-preferring inhibitor pentoxifylline significantly reduced both spinal prostaglandin release and tactile allodynia in this model [60], indicating that activated glial cells contribute to spinal TLR4-mediated allodynia.
Spinal TLRs, in particular TLR4, also have been implicated in the transition from acute to chronic pain syndromes [1; 14; 36; 50; 69; 71; 77]. For example, subcutaneous (SC) Formalin Phase II flinching exhibits significant correlation with anti-hyperalgesic drug responsiveness in models of nerve injury such as CCI [75], suggesting at least some translatability of molecular mechanisms underlying formalin-induced pain hypersensitivity to those contributing to peripheral neuropathy. Phase II flinching is sensitive to inhibition by anti-inflammatory drugs at high (2.5–5%) but not low (1%) concentrations of Formalin [38; 45; 82]. Furthermore, Formalin (5%) in the dorsal surface of the paw also produces persistent secondary tactile allodynia for up to 3 weeks [21] that is initiated by stimulation of spinal TLR4 [77] and is mediated in part by sustained spinal microglial activation [20; 22].
Previously, we and others have demonstrated that increased synthesis of arachidonic acid (AA)-derived metabolites of spinal 12-lipoxygenases (12-LOX), specifically hepoxilins (HXA3 and HXB3) and 12-hydroxyeicosatetraenoic acid (12-HETE), contributes significantly to hyperalgesia following peripheral inflammation via activation of TRPA1 and TRPV1 receptors and spinal substance P release [7; 24; 25; 66]. While it is widely accepted that activation of TLR4 by lipopolysaccharide (LPS) releases eicosanoids from cyclooxygenase (COX) and/or 5-lipoxygenase (5-LOX) pathways [17], the upstream activators as well as the cellular source(s) of 12-LOX metabolites following injury remain largely undefined [6; 27; 40; 46; 49; 60]. In the current report, we demonstrate that spinal TLR4 activation also increases microglial production of 12-lipoxygenase metabolites (particularly HXB3) via 15-LOX-1, which contributes directly to NSAID-unresponsive allodynia in rats.
2. Methods.
Drugs.
Arachidonic Acid, AA (Cayman); KDO2-Lipid A, KLA [55] (Avanti Polar Lipids); Ketorolac, KETO (Allergan); ML127 and ML351 (NIH Chemical Genomics); NDGA (Cayman Chemical) and Formalin (Fisher Scientific) were prepared in vehicles (VEH) as follows. For in vivo IT delivery: KLA 1 μg/10 μl in 1% DMSO; Ketorolac 50 μg/10 μl in saline; ML127 or ML351 0.1–10 μg/10 μl in 5% PEG-400/5% Cremaphor-EL/saline; NDGA (10 or 60μg/10 μl in 20% β-cyclodextrin in saline). For in vivo SC delivery: Formalin (2.5% or 5% in saline, 50 μl). For cell culture: AA (70 μM) in serum-free DMEM as described [25]; KLA (100 ng/ml), ML127 or ML351 (0.1 nM-10 μM) in serum-free DMEM to a final maximum concentration of 0.1% (v/v) DMSO.
Animals.
Holtzman Sprague-Dawley rats (male, 300–350g for behavioral analyses; neonatal male and female P1-P3 for primary cultures; Harlan) were used in accordance with protocols approved by the IACUC of UCSD. IT catheter implantation of adult rats and drug delivery was performed as described previously [81] with modifications [37]. Animals exhibiting motor or postural deficits after surgery (<5%) were immediately sacrificed. All behavioral testing was performed by the same observer blinded to the treatment conditions, with animals randomized by another investigator.
Cell culture and transfection.
Primary cultures of rat spinal microglia or astrocytes were prepared as described previously with modifications. Briefly, spinal cords from neonatal rats were hydroextruded with saline, followed by trituration in complete DMEM (containing 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin G sodium and 100 μg/ml streptomycin). The cell suspension was passed sequentially through 100 μM and 70 μM filters and plated at a density of 3–4 cords/flask. Mixed cultures were maintained for 2 weeks with regular media replacement in a humidified incubator at 37oC/5% CO2 prior to sequential separation of glial cell types. Microglia were detached by shaking the flasks for 2h at 37oC and then cultured in serum-starved DMEM for 1d prior to experiments. Astrocytes were obtained by trypsinization of the remaining cells and subculturing in complete DMEM. For experiments, cells were plated at a density of 50,000 cells/well or 250,000 cells/well for 24-well or 6-well plates, respectively, and serum-starved for 24h. Some cultures were grown on glass slides coated with poly-D-lysine 0.5 mg/ml and then subjected to immunofluorescence to verify >98% purity as we and others have reported [16; 28; 60; 64]. HEK-293T cells were cultured and transfected with one of each of the six recombinant pcDNA3.1/rat 12/15-LOX constructs (3 μg) using Lipofectamine 2000 (Life Technologies) as we described previously [25].
Immunofluorescence.
Primary cultures were fixed with 3% formaldehyde and then labeled with markers of astrocytes (GFAP, vimentin), microglia (Iba-1) and DAPI to demonstrate >98% purity as described (data not shown) [28; 60; 64].
LC-MS/MS.
LC-MS/MS of eicosanoids (Supplementary Table 1) was conducted using a tandem quadrupole mass spectrometer (ABI 4000 Q-Trap®) as described previously [7; 25; 26]. Eicosanoid levels were measured in rat L4/L5 lumbar spinal cord tissue after IT KLA (2 h) or SC Formalin (day 7), in media from primary cultures of spinal cells following treatment with KLA, as well as in media from HEK-293T cells supplemented with AA as substrate.
Quantitative real-time PCR.
Total RNA was isolated from spinal cords using RNeasy Lipid Tissue Mini Kit or from primary cultures using RNeasy Mini Kit and RNase-free DNase kit (Qiagen) and samples were prepared for real-time qPCR with RT2 SYBR green/ROX kit as directed using prevalidated rat primer sets (SA Biosciences) for Alox12 (12-LOX-p), Alox12b (12R-LOX), Alox12e (12-LOX-e), Aloxe3 (eLOX3), Alox15 (15-LOX-1), Alox15b (15-LOX-2) and Actb (β-actin). The comparative CT method was used for relative quantification, and 2-ΔΔCT values were calculated and averaged for each target as described in detail previously [25]. We confirmed specificity of these primer sets against their respective target genes without cross-reactivity to other isozymes in HEK-293T overexpression systems [25]. For purposes of clarity, in this paper we refer primarily to the protein nomenclature, with the enzyme family as 12/15-LOX and enzyme activities as 12-lipoxygenase (12-LOX), hepoxilin synthase (HXS), or 15-lipoxygenase (15-LOX).
Immunoblot.
Total protein was extracted from primary cells and processed for western blot of 12/15-LOX enzymes with primary antibodies generated in mouse targeting leukocyte type 15-LOX-1 (Abnova H00000246-M04), which we verified for specificity in a 15-LOX-1 HEK-293T overexpression system [25].
Behavioral testing.
Tactile thresholds.
Tactile allodynia was assessed using von Frey filaments by the up-down method of Dixon as described [12] at baseline and after IT delivery of drugs (ML127, ML351 and CDC or corresponding VEH) 30 min prior to IT administration of KLA [60]. For measurement of long-term hyperalgesia in a TLR4-dependent model of chronic pain [77], rats were given SC formalin (5%) on the dorsal surface of the hindpaw and then tactile thresholds were measured on days 1, 3, 5 and 7 as described previously [21]. Rats allodynic on day 7 were post-treated IT with drugs (NDGA or VEH) and then tactile thresholds recorded at 30 min and 1, 2, and 4 hours after treatment. Data were expressed as 50% gram thresholds v. time % Maximum Possible Effect (MPE) and as area under the curve (AUC) or hyperalgesic index (% change from baseline × min). Any rat with a basal 50% paw withdrawal threshold below 10g was excluded from the study (<5% of animals).
Acute Formalin flinching.
An automated system was used to quantify Formalin-induced flinching (University Anesthesia Research and Development Group, Department of Anesthesiology, University of California, San Diego, La Jolla, CA) as we have described previously [80]. Briefly, drugs (NDGA or VEH) were administered IT as a pretreatment 30 min prior to SC administration of Formalin (2.5%) to the dorsal surface of the hindpaw, and then rats were immediately placed in the recording chambers. Flinches were counted in 1 min intervals for 60 min. Data were expressed as total number of flinches observed during phase I (0–9 min) and phase II (10–60 min).
Statistics.
Normalized raw data of spinal eicosanoids were filtered using the Grubbs’ test as previously described [7]. P values were determined using SPSS software (PASW version 18, SPSS Inc.) as follows: t-test for two group analysis and repeated measures or standard ANOVA for multiple group analysis with Bonferroni post-hoc for behavioral data, Dunnett’s post-hoc for biochemical data, or Benjamini-Hochberg Procedure (false discovery rate=0.1, see [5] for details) for lipid array data. Normalized metabolite, mRNA or protein levels and tactile thresholds were expressed as the mean ± standard error of mean (SEM). A value of P < 0.05 was considered significant.
3. Results.
Spinal TLR4-mediated tactile allodynia is unresponsive to NSAIDs.
As we and others have shown previously, IT KLA (1 μg), elicits significant tactile allodynia in rats after a single injection (Figure 1A, B), [41; 59; 60]. Despite significant inhibition of KLA-induced PGE2 release, pretreatment with the COX-1/2 inhibitor ketorolac (50 μg, IT) failed to reduce IT KLA-induced tactile allodynia (Figure 1A, B and [60]).
Figure 1. Intrathecal delivery of KLA induces tactile allodynia that is unresponsive to NSAIDs.
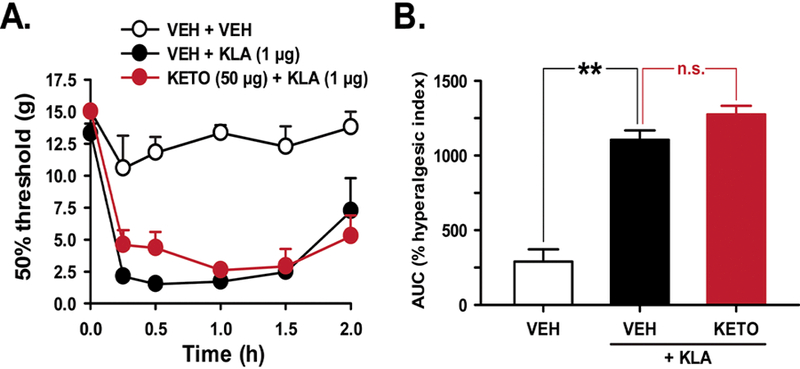
(A). Time course and (B) percent hyperalgesic index or AUC values reveal that spinal pretreatment with the COX-1/2 inhibitor ketorolac (50 μg IT) has no significant effect on IT KLA-induced pain hypersensitivity. **P<0.01 v. VEH; n.s., not significantly different from VEH. VEH, vehicle; KETO, ketorolac; KLA, KDO2-Lipid A.
TLR4 activation increases levels of hepoxilins in both in lumbar spinal tissue and in primary spinal microglia
Next, we conducted lipidomic analysis of lumbar spinal cord tissue at 2h after treatment with IT KLA to determine which eicosanoids were altered concurrent with the allodynia. As predicted, levels of COX metabolites of arachidonic acid (AA) including PGE2, prostaglandin D2, 6-keto prostaglandin F1α, and thromboxane B2 were elevated in lumbar spinal cord tissue at 2h after IT KLA (Fig. 2A, Supplementary Table 1). However, we reasoned that since NSAIDs do not block TLR4-mediated allodynia regardless of inhibiting synthesis of COX products (Figure 1 and [60]), other factors must contribute to this pain hypersensitivity. Previously, we observed that IT pentoxifylline, an inhibitor targeting microglia and astrocytes, significantly attenuated KLA-mediated allodynia as well as the increase in PGE2 release. Thus, we also examined lipid metabolites released from cultured spinal microglia and astrocytes at 2, 4, and 24h after KLA treatment. The remaining metabolites were examined to identify non-COX-derived lipids altered by KLA in lumbar spinal tissue and in primary glial cells that may underlie KLA-induced allodynia (Figure 2B). Of the 62 eicosanoids identified in this study, only the 12-LOX product Hepoxilin B3 was significantly increased both in lumbar spinal tissue (Figure 2C) and in microglia (Figure 2D). It is important to note that while Hepoxilin A3 (HXA3) and Hepoxilin B3 (HXB3) are synthesized concurrently, and HXA3 has stronger bioactive effects in vivo [4], HXB3 is more stable and serves as a marker of HXS activity.
Figure 2. Direct activation of spinal TLR4 by KLA in vivo significantly increases hepoxilin production in lumbar spinal cord tissue and microglial cultures.
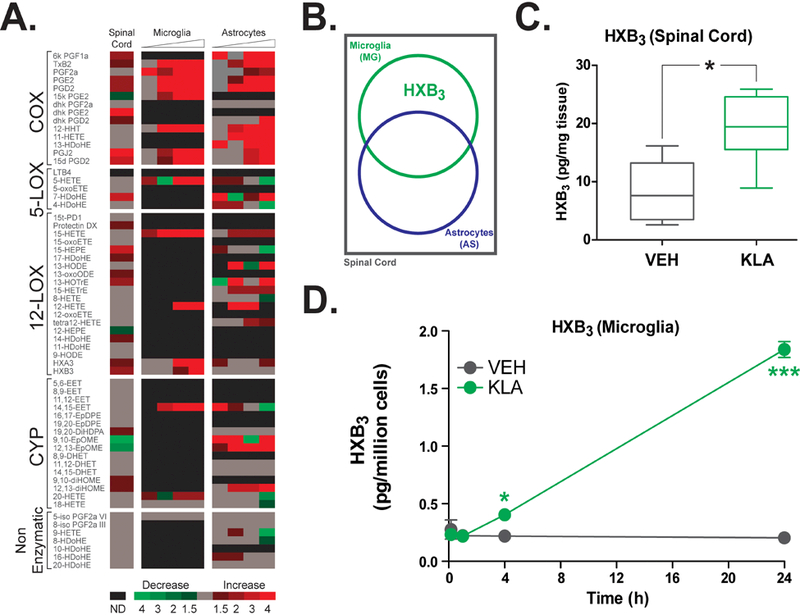
(A) Timecourse array of changes in levels of COX, nonenzymatic, LOX and CYP metabolites measured in lumbar spinal cord tissue at 2h and released from cultures of spinal microglia and astrocytes at baseline, 2h and 8h after treatment with VEH (IT saline or 0.1% DMSO, respectively) or KLA (1 μg/10 μl IT or 100 ng/ml, respectively). Fold differences are indicated on the heat map as follows: green, decreased; red, increased; gray, no change; black, not detected. (B) Metabolite changes following KLA treatment were analyzed using the following criteria (illustrated in diagram): not produced by COX (due to NSAID insensitivity), significantly (P<0.05) and robustly (≥ 2-fold) altered in both spinal cord tissue and cultured glial cells (microglia, astrocytes, or both). These criteria reveal a single lipid target (HXB3) that is increased in spinal cord (C) and microglia (D) following treatment with KLA. *P<0.05, ***P<0.001, n = 8–15 rats/group or n = 4 wells/treatment. VEH, vehicle; KLA, KDO2 Lipid A; COX, cyclooxygenase; LOX, lipoxygenase; CYP, cytochrome P450.
12/15-LOX enzymes are present in primary spinal glial cells and microglial 15-LOX-1 is induced by KLA
The rat 12/15-Lipoxygenases encompass a family of six enzymes encoded by six distinct genes (Table 1): 12-LOX-p (Alox12); 12(R)-LOX (Alox12b); 12-LOX-e (Alox12e, pseudogene); eLOX3 (Aloxe3); 15-LOX-1 (Alox15); and 15-LOX-2 (Alox15b) [4]. Of the four 12/15-LOX enzymes expressed in rat spinal cord under basal conditions, three of these possess HXS activity – 12-LOX-p, eLOX3 and 15-LOX-1 [25]. We detected mRNA for all three synthases in microglia (Figure 3A) and two in astrocytes (Fig. 3B). Given the lack of a commercially available, selective antibody for rat 12-LOX-p [25], we investigated the effect of KLA treatment on the expression of 15-LOX-1 protein in glial cells targeted by pentoxifylline, microglia and astrocytes. Following exposure to KLA, 15-LOX-1 protein was upregulated in microglia at 8h and 24h after exposure to KLA (Fig. 4A, C). Similar treatment in astrocytes produced no effects on 15-LOX-1 expression (Fig. 4B, D).
Table 1. The 12/15-Lipoxygenase family of enzymes.
| Pathway | Common Name |
Gene | Protein | Activity (rat) | Activity (human) | % ID |
|---|---|---|---|---|---|---|
| 12-LOX | platelet-type 12(R)-type epidermis-type |
Alox12
Alox12b Alox12e Aloxe3 |
12-LOX-p 12(R)-LOX 12-LOX-e eLOX3 |
12(S)-LOX; HXS 12(R)-LOX 12(R)-LOX(low) HXS |
12(S)-LOX; HXS, LXS 12(R)-LOX pseudogene HXS |
84 87 - 88 |
| 15-LOX | leukocyte-type |
Alox15 Alox15b |
15-LOX-1 15-LOX-2 |
12(S)-LOX; HXS 15-LOX |
15-LOX 15-LOX |
75 81 |
12-LOX = 12-lipoxygenase; HXS = hepoxilin synthase; 15-LOX = 15-lipoxygenase; LXS = lipoxin synthase; % ID = Percent identity of each rat enzyme to its human ortholog.
Figure 3. Cultured spinal microglia and astrocytes express mRNAs encoding 12/15-LOX with Hepoxilin synthase activity.
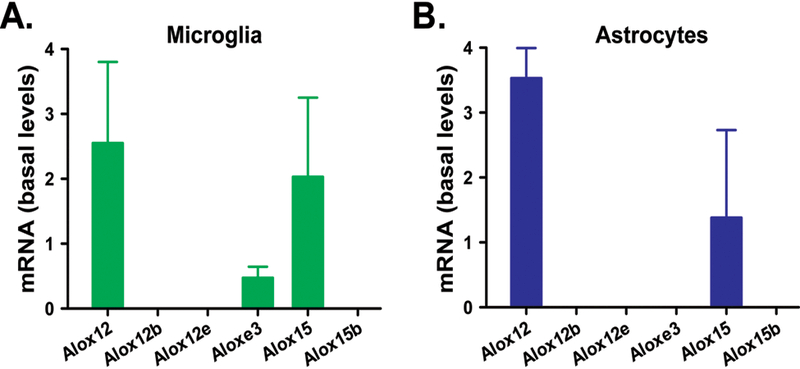
Primary rat spinal microglia (A) express mRNAs for 12-LOX-p (Alox12), eLOX3 (Aloxe3) and 15-LOX-1 (Alox15) while spinal astrocytes (B) express mRNAs for 12-LOX-p (Alox12) and 15-LOX-1 (Alox15) as measured by qPCR. Levels are expressed in arbitrary units, n = 5 samples/group.
Figure 4. KLA treatment upregulates 15-LOX-1 protein in primary spinal microglia.
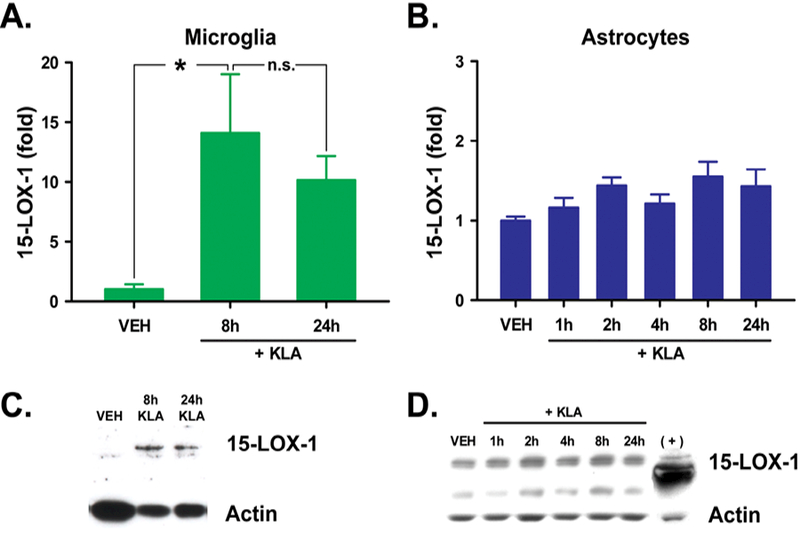
Expression of 15-LOX-1 protein is increased significantly in primary spinal (A,C) microglia at 8 and 24h but not in (B,D) astrocytes after treatment with KLA (100 ng/ml). VEH, vehicle. *P<0.05; n.s., not significantly different from VEH. n = 6 wells/group.
ML351 and ML127 inhibit rat 15-LOX-1
Previously, we examined the selectivity of commercially available inhibitors against rat 12/15-lipoxygenase enzymes heterologously expressed in HEK-293T cells to investigate their selectivity in this species. We found that CDC is specific for inhibition of rat 12-LOX and HXS activity from 15-LOX-1 and 12-LOX-p, while PD146176 preferentially targets 15-LOX activity from rat 15-LOX-2 [25]. While CDC lacks adequate selectivity for discriminating between rat 15-LOX-1 and 12-LOX-p [25], agents have been developed to selectively target the human orthologs 15-LOX-1 (ML351, [58] and 12-LOX-p (ML127, [57]). Thus, in order to investigate further the potential role(s) of these enzymes in the KLA-induced spinal facilitated state, we examined the effects of ML351 and ML127 as potential inhibitors of the rat 12/15-LOX enzymes overexpressed in HEK-293T cells using our previously described methods [25]. Using this approach, ML351 (Figure 5A) inhibited lipoxygenase activity from both rat 15-LOX-1 and rat 12-LOX-p, but did so at substantially lower concentrations (50% inhibition at 10 nM) than ML127 (Figure 5B). ML127 reduced rat 15-LOX-1 lipoxygenase activity by 50% at 1 μM, and exhibited very little effect on rat 12-LOX-p at concentrations up to 10 μM.
Figure 5. ML351 and ML127 inhibit rat 15-LOX-1 activity.
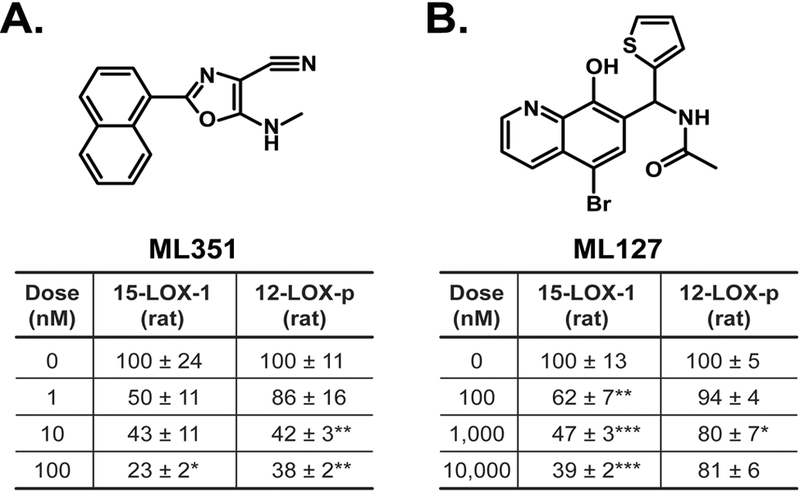
The inhibitors (A) ML351 and (B) ML127 were tested against rat 15-LOX-1 and 12-LOX-p. Enzymes were expressed in HEK-293T cells, incubated with vehicle or inhibitor (ML351 or ML127) for 30 min, then incubated with 70 μM arachidonic acid for 1h. The extracellular media was analyzed for 12-HETE production by LC-MS/MS. n = 4–8 wells/treatment. *P<0.05, **P<0.01, ***P<0.001.
Inhibition of spinal 15-LOX-1 attenuates TLR4-mediated pain hypersensitivity
As hepoxilins trigger calcium mobilization in sensory neurons and produce significant tactile allodynia after IT administration [24], we examined the ability of the selective inhibitors ML127 and ML351 to attenuate KLA-induced spinal facilitated states that are unresponsive to NSAIDs (Figure 6). Treatment with ML351 at all doses tested (0.1–10 μg, IT) attenuated spinal TLR4-mediated pain hypersensitivity such that tactile threshold levels were not different from control animals. The antihyperalgesic effect of ML127 was dose-dependent, with the highest dose (10 μg, IT) producing significant inhibition of IT KLA-induced allodynia. These data are consistent with in vitro results, where ML351 exhibited increased apparent potency for 15-LOX-1, while ML127 showed higher selectivity for 15-LOX-1. In addition, KLA-induced allodynia was attenuated by IT pretreatment with the specific 12/15-LOX inhibitor CDC or a prevalidated selective 15-LOX-1 antibody (Supplementary Figure 1) using methods we have described previously [25].
Figure 6. ML351 and ML127 attenuate KLA-induced tactile allodynia.
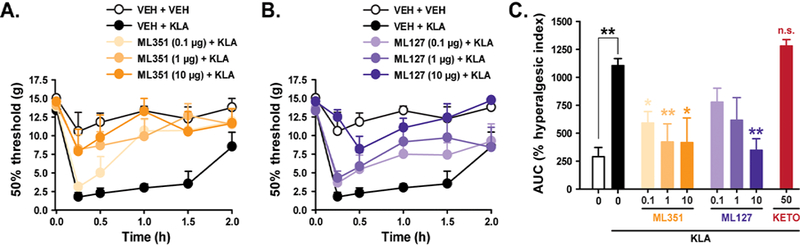
Timecourse curves reveal that spinal pretreatment with (A) ML351 or (B) ML127 significantly attenuate IT KLA-induced pain hypersensitivity in rats in a dose-dependent manner. (C) Percent hyperalgesic index or AUC values demonstrate greater apparent anti-hyperalgesic potency of ML351 in vivo, and a comparative lack of effect of ketorolac. n = 8–10 rats/treatment. *P<0.05, **P<0.01, n.s. (not significantly different from IT KLA).
Finally, we investigated if 12/15-LOX activation contributes to an established, clinically-relevant model of spinal TLR4-mediated chronic pain, SC formalin-induced long-term allodynia [21; 77]. Pretreatment with the general 12/15-Lipoxygenase inhibitor NDGA (10 μg, IT) significantly reduced Phase II, but not Phase I, Formalin (2.5%) flinching compared with vehicle, which was not different from saline (Figure 7A). In addition, Formalin (5%) produced a significant increase compared with saline in spinal cord HXB3 levels ipsilateral but not contralateral to the site of injury (Figure 7B) and significant tactile allodynia that was observed on days 1, 3, 5, and 7 after injection as described previously [21] (data not shown). Importantly, NDGA (60 μg, IT) administered as a post-treatment (single injection on day 7 after 5% Formalin) reversed established tactile allodynia as depicted by timecourse curves and % MPE (Figure 7C, D)
Figure 7. Spinal inhibition of 12/15-LOX attenuates formalin Phase II acute flinching and persistent allodynia.
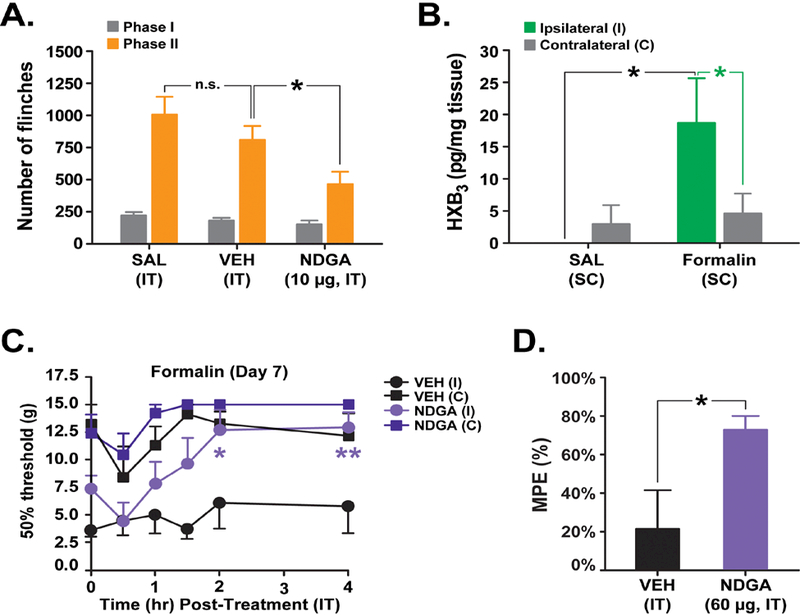
(A) Pretreatment with the general 12/15-LOX inhibitor NDGA (10 μg IT) significantly reduces Phase II acute flinching following SC formalin (2.5%, 50 μl) into the dorsal surface of the hindpaw. (B) HXB3 levels are increased significantly in ipsilateral (I) but not contralateral (C) lumbar spinal cord on day 7 following SC formalin (5%, 50 μl), concurrent with allodynia. Post-treatment with NDGA (60 μg IT) reverses established IPLT formalin-induced allodynia on day 7 as depicted by (C) timecourse and (D) %MPE values. n = 6 rats/treatment. *P<0.05, **P<0.01, v. VEH; n.s. not significantly different from SAL. SAL = saline; VEH = vehicle; SC = subcutaneous; MPE = maximum possible effect.
Discussion.
In the present study, we utilized a rat model of spinally-mediated, TLR4-dependent tactile allodynia that is unresponsive to NSAIDs to identify novel mechanisms of neuropathic-like pain behaviors in the absence of peripheral inflammation or injury. Using a comprehensive lipidomic approach for measuring eicosanoids from established pathways, the 12/15-lipoxygenase family of enzymes was identified as a target for facilitating nociceptive behavior induced by spinal TLR4 activation, comparable to our previous observations with a model of peripheral inflammation [7; 24]. We have shown that IT delivery of the 12/15-lipoxygenase metabolites 12(S)-HpETE, 12(S)-HETE, HXA3 and HXB3 directly produce profound tactile allodynia but modest transient thermal hyperalgesia in rats, and that spinal hepoxilins produced during peripheral inflammation contribute to allodynia via stimulation of spinal TRPA1 and TRPV1 receptors and NK1 receptor internalization [24]. Therefore, we reasoned that increased spinal 12-LOX/HXS activity may also underlie TLR4-dependent allodynia. While ML127 exhibited higher selectivity for rat 15-LOX-1, ML351 displayed a greater apparent potency than ML127 both in vivo and in vitro. Differential selectivity of lipoxygenase inhibitors between species has been observed previously; for example, PD146176 targets human 15-LOX-1 [65], but is highly selective for rat 15-LOX-2 without effect on rat 15-LOX-1 [26]. A similar phenomenon also has been observed for probes designed for monoacylglycerol lipase (MAGL), wherein JZL184 is equipotent against the human and mouse orthologs, yet exhibits significantly reduced potency against rat MAGL [11]. Our observations regarding antihyperalgesic effects of IT pretreatment with a selective antibody targeting rat 15-LOX-1 provides additional support for a significant contribution of this enzyme in spinal TLR4-mediated allodynia. Therefore, if interpreted with caution, inhibitors designed for the human 12/15-lipoxygenases also may be used as chemical probes for disease models in rats [30].
Herein, we extend previous findings utilizing a behavioral model of nociception with direct activation of spinal TLR4 [41; 59; 60] via IT delivery of KLA, the chemically defined component of LPS [55]. Others have shown that pain hypersensitivity produced by local delivery of LPS in the paw of mice and rats [34] is blocked by systemic delivery of NSAIDs [62], and this effect may be explained in part by inhibition of cyclooxygenases activated in the periphery, for example in circulating peripheral blood leukocytes expressing TLR4. However, we found that spinal administration of KLA elicited a profound tactile allodynia that, unexpectedly, was unresponsive to pretreatment with either systemic or intrathecal NSAIDs (ibuprofen and ketorolac) at doses that completely prevented TLR4-induced release of PGE2 both in lumbar spinal cerebrospinal fluid and from primary spinal astrocyte cultures [60]. Similarly, pretreatment either with systemic ibuprofen or with IT ketorolac (non-specific COX inhibitors) failed to block spinal TLR4-mediated allodynia at doses that exhibit antihyperalgesic effects in other nociceptive models in which CNS synthesis of prostaglandins is elevated [38; 39; 73]. The dose we selected (50 μg IT) in the current study is the maximum dose that can be delivered given the standard injection volume of 10 μl and available ketorolac concentration[79]. Importantly, this maximum soluble dose of IT ketorolac completely inhibited COX-mediated PGE2 release into spinal CSF, thus ruling out the possibility that the drug was not administered at the appropriate time to be effective[60]. This lack of response to treatment with NSAIDs also has been found in other paradigms of chronic pain, including diabetic neuropathy (PDN) [9], chemotherapy-induced peripheral neuropathy (CIPN) [51], and the post-inflammatory late phases of both K/BxN serum-transfer and Collagen Antibody-Induced (CAIA) models of rheumatoid arthritis [2; 13], indicating that cyclooxygenase metabolites likely do not contribute to the maintenance of these hyperpathic states. It should be noted that daily administration of COX inhibitors reduces peripheral inflammation severity in rheumatoid arthritis and PDN models [19; 54; 70]; however, NSAIDs may have limited efficacy in certain patient populations [33] and their long-term usage has been associated with serious GI, renal and cardiovascular side effects in humans [47; 67]. Importantly, TLR4 plays a critical role in the development of neuropathic pain [69; 71] and in the transition in rodents from an acute to chronic hyperalgesic state in experimental arthritis [1; 14]. It is possible that our observations regarding the involvement of 15-LOX-1 in nociceptive behavior following direct activation of spinal TLR4 may extend to paradigms of chronic pain with a neuropathic component that are less responsive or even refractory to NSAID therapy, as suggested by antihyperalgesic effects of 12/15-LOX inhibition in formalin-mediated sustained allodynia. As such, the involvement of spinal 15-LOX-1 in pain chronification warrants further study.
Since rat 12/15-lipoxygenases are encoded by six distinct enzymes that can stereospecifically insert O2 into polyunsaturated fatty acids such as arachidonic acid [4], we examined which of these were expressed in glial cells, were altered by TLR4 activation, and could be targeted by selective inhibitors. Of these six isozymes, four are expressed in rat spinal cord: 12-LOX-p, 12R-LOX, eLOX3 and 15-LOX-1 [25]. eLOX3 is a HXS that can form hepoxilins from 12-HpETE (or from AA when coexpressed with 12(R)-LOX), while 12-LOX-p and 15-LOX-1 exhibit both 12-LOX and HXS activity [4; 25; 29]. Of the three enzymes with HXS activity expressed in spinal microglia, there is no commercially available selective antibody for 12-LOX-p and we have already investigated the role of eLOX3 in inflammatory hyperalgesia [25]. Hence, we examined TLR4-mediated changes in 15-LOX-1 protein and found that IT KLA treatment produced a robust upregulation of 15-LOX-1 expression in spinal microglia but not in astrocytes. As changes in protein levels of enzymes do not necessarily correlate with their altered activity, we investigated the effects of selective inhibitors targeting human 12-LOX-p (ML127 [57]) and 15-LOX-1 (ML351 [58]). At low nanomolar concentrations, ML351 significantly decreased 12-LOX and HXS activity from the rat homologs of 15-LOX-1 and 12-LOX-p in the presence of AA substrate. In contrast, ML127 preferentially reduced 15-LOX-1 activity in a concentration-dependent manner with little effect on 12-LOX-p. Likewise, IT pretreatment with ML351 completely abrogated TLR4-dependent tactile allodynia, but at 100-fold lower dose than ML127, which we speculate is due to its greater relative potency at 15-LOX-1.
While some groups present a case for neuronal TLR4 activation in response to LPS [18; 42], others report a major role for TLR4 expressed in glial cells as the fundamental drivers of many inflammatory and neuropathic pain conditions [1; 14; 56; 71], and there are different views as to whether microglia or astrocytes play a more significant role in the chronification of these hyperpathic states which may be paradigm- and sex-specific [2; 69; 84]. Indeed, direct activation of spinal TLR4 by IT LPS or KLA is attenuated by agents that target glial cells such as minocycline and pentoxifylline [3; 60], suggesting the involvement of factors released from microglia and/or astrocytes. Thus, we sought to determine through lipidomic analysis as conducted previously [6; 7], which eicosanoids (other than cyclooxygenase metabolites) were increased significantly in lumbar spinal cord both concurrent with KLA-induced allodynia and in primary spinal microglia and/or astrocytes following exposure to KLA. In this study, we report that spinal 15-LOX-1 in microglia contributes to TLR4-dependent allodynia in male rats. Our findings regarding a critical role of microglia in spinal TLR4-mediated pain states in males is consistent with previous reports that IT LPS increases expression of pronociceptive factors in spinal microglia, and that suppression of microglial but not astrocytic activation attenuates the concomitant allodynia [15; 23; 76; 83]. It is important to note, however, that the current observations do not preclude a role for additional 12-LOX enzymes with HXS activity expressed in microglia (i.e., 12-LOX-p or eLOX3), other lipids or factors released from astrocytes, or sex differences in the contribution of TLR4 to pain behaviors [68; 77], and these avenues merit future investigation.
Collectively, these results demonstrate that microglial 15-LOX-1 contributes to the development of TLR4-mediated allodynia in male rats. Future studies should investigate potential receptor targets of hepoxilins in addition to TRPA1 and TRPV1[24], as clinical development of antagonists for these channels has been hampered by issues with bioavailability/kinetics[44] and serious side effects[78]. In addition, the importance of 12-LOX/HXS activity in models of chronic pain in females as well as in mice should be explored in more detail, so as to confirm translatability between both sexes and species. Accordingly, selective drugs impeding 12-LOX/HXS activity and/or that of downstream receptor targets may be useful in the treatment of hyperpathic pain states that are refractory to treatment with NSAIDs without the concomitant risk associated with chronic opioid use.
Supplementary Material
Acknowledgements:
We thank Dr. Jing Yang for use of equipment and Drs. Camilla I. Svensson, Xiao-Ying Hua and Ted Holman for helpful discussions. This work was supported by NIH DA035865 (MWB); GM020501, GM064611, and GM069338 (EAD); NS099338 and DA002110 (TLY) and in part by the intramural research program of the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH).
Footnotes
COI statement: AMG, MWB, DSD, PCN, DJM, QX, SCW, BLF, EAD and TLY have no conflict of interest to declare. GR, AS and AJ have patent US 20130096159 and WO 2011146618 issued for ML127, patent US 20160168137 and WO 2015027146 issued for ML531.
References
- [1].Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundback P, Palmblad K, Andersson U, Harris H, Svensson CI. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain 2014;155(9):1802–1813. [DOI] [PubMed] [Google Scholar]
- [2].Bas DB, Su J, Sandor K, Agalave NM, Lundberg J, Codeluppi S, Baharpoor A, Nandakumar KS, Holmdahl R, Svensson CI. Collagen antibody-induced arthritis evokes persistent pain with spinal glial involvement and transient prostaglandin dependency. Arthritis Rheum 2012;64(12):3886–3896. [DOI] [PubMed] [Google Scholar]
- [3].Bastos LF, Godin AM, Zhang Y, Jarussophon S, Ferreira BC, Machado RR, Maier SF, Konishi Y, de Freitas RP, Fiebich BL, Watkins LR, Coelho MM, Moraes MF. A minocycline derivative reduces nerve injury-induced allodynia, LPS-induced prostaglandin E2 microglial production and signaling via toll-like receptors 2 and 4. Neurosci Lett 2013;543:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res 2009;50(6):1015–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Buczynski MW, Polis IY, Parsons LH. The volitional nature of nicotine exposure alters anandamide and oleoylethanolamide levels in the ventral tegmental area. Neuropsychopharmacology 2013;38(4):574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Buczynski MW, Stephens DL, Bowers-Gentry RC, Grkovich A, Deems RA, Dennis EA. TLR-4 and sustained calcium agonists synergistically produce eicosanoids independent of protein synthesis in RAW264.7 cells. J Biol Chem 2007;282(31):22834–22847. [DOI] [PubMed] [Google Scholar]
- [7].Buczynski MW, Svensson CI, Dumlao DS, Fitzsimmons BL, Shim JH, Scherbart TJ, Jacobsen FE, Hua XY, Yaksh TL, Dennis EA. Inflammatory hyperalgesia induces essential bioactive lipid production in the spinal cord. J Neurochem 2010;114(4):981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Burke NN, Fan CY, Trang T. Microglia in health and pain: impact of noxious early life events. Exp Physiol 2016;101(8):1003–1021. [DOI] [PubMed] [Google Scholar]
- [9].Calcutt NA, Chaplan SR. Spinal pharmacology of tactile allodynia in diabetic rats. Br J Pharmacol 1997;122(7):1478–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Carpentier PA, Duncan DS, Miller SD. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav Immun 2008;22(2):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chang JW, Niphakis MJ, Lum KM, Cognetta AB 3rd, Wang C, Matthews ML, Niessen S, Buczynski MW, Parsons LH, Cravatt BF. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem Biol 2012;19(5):579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [13].Christianson CA, Corr M, Firestein GS, Mobargha A, Yaksh TL, Svensson CI. Characterization of the acute and persistent pain state present in K/BxN serum transfer arthritis. Pain 2010;151(2):394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, Yaksh TL. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain 2011;152(12):2881–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, Malcangio M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci 2010;30(2):573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Codeluppi S, Gregory EN, Kjell J, Wigerblad G, Olson L, Svensson CI. Influence of rat substrain and growth conditions on the characteristics of primary cultures of adult rat spinal cord astrocytes. J Neurosci Methods 2011;197(1):118–127. [DOI] [PubMed] [Google Scholar]
- [17].Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol 2015;15(8):511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res 2011;90(6):759–764. [DOI] [PubMed] [Google Scholar]
- [19].El-Lithy GM, El-Bakly WM, Matboli M, Abd-Alkhalek HA, Masoud SI, Hamza M. Prophylactic L-arginine and ibuprofen delay the development of tactile allodynia and suppress spinal miR-155 in a rat model of diabetic neuropathy. Transl Res 2016;177:85–97 e81. [DOI] [PubMed] [Google Scholar]
- [20].Fu KY, Light AR, Maixner W. Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience 2000;101(4):1127–1135. [DOI] [PubMed] [Google Scholar]
- [21].Fu KY, Light AR, Maixner W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J Pain 2001;2(1):2–11. [DOI] [PubMed] [Google Scholar]
- [22].Fu KY, Light AR, Matsushima GK, Maixner W. Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res 1999;825(1–2):59–67. [DOI] [PubMed] [Google Scholar]
- [23].Grace PM, Wang X, Strand KA, Baratta MV, Zhang Y, Galer EL, Yin H, Maier SF, Watkins LR. DREADDed microglia in pain: Implications for spinal inflammatory signaling in male rats. Exp Neurol 2018;304:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gregus AM, Doolen S, Dumlao DS, Buczynski MW, Takasusuki T, Fitzsimmons BL, Hua XY, Taylor BK, Dennis EA, Yaksh TL. Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc Natl Acad Sci U S A 2012;109(17):6721–6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gregus AM, Dumlao DS, Wei SC, Norris PC, Catella LC, Meyerstein FG, Buczynski MW, Steinauer JJ, Fitzsimmons BL, Yaksh TL, Dennis EA. Systematic analysis of rat 12/15-lipoxygenase enzymes reveals critical role for spinal eLOX3 hepoxilin synthase activity in inflammatory hyperalgesia. FASEB J 2013;27(5):1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Harmon GS, Dumlao DS, Ng DT, Barrett KE, Dennis EA, Dong H, Glass CK. Pharmacological correction of a defect in PPAR-gamma signaling ameliorates disease severity in Cftr-deficient mice. Nat Med 2010;16(3):313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hua XY, Chen P, Marsala M, Yaksh TL. Intrathecal substance P-induced thermal hyperalgesia and spinal release of prostaglandin E2 and amino acids. Neuroscience 1999;89(2):525–534. [DOI] [PubMed] [Google Scholar]
- [28].Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci 2005;22(10):2431–2440. [DOI] [PubMed] [Google Scholar]
- [29].Ivanov I, Heydeck D, Hofheinz K, Roffeis J, O’Donnell VB, Kuhn H, Walther M. Molecular enzymology of lipoxygenases. Arch Biochem Biophys 2010;503(2):161–174. [DOI] [PubMed] [Google Scholar]
- [30].Ivanov I, Kuhn H, Heydeck D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15). Gene 2015;573(1):1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 2005;175(7):4320–4330. [DOI] [PubMed] [Google Scholar]
- [32].Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain 2013;154 Suppl 1:S10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kaley TJ, Deangelis LM. Therapy of chemotherapy-induced peripheral neuropathy. Br J Haematol 2009;145(1):3–14. [DOI] [PubMed] [Google Scholar]
- [34].Kanaan SA, Saade NE, Haddad JJ, Abdelnoor AM, Atweh SF, Jabbur SJ, Safieh-Garabedian B. Endotoxin-induced local inflammation and hyperalgesia in rats and mice: a new model for inflammatory pain. Pain 1996;66(2–3):373–379. [DOI] [PubMed] [Google Scholar]
- [35].Lacagnina MJ, Watkins LR, Grace PM. Toll-like receptors and their role in persistent pain. Pharmacol Ther 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li X, Wang XW, Feng XM, Zhou WJ, Wang YQ, Mao-Ying QL. Stage-dependent anti-allodynic effects of intrathecal Toll-like receptor 4 antagonists in a rat model of cancer induced bone pain. J Physiol Sci 2013;63(3):203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Malkmus SA, Yaksh TL. Intrathecal catheterization and drug delivery in the rat. Methods Mol Med 2004;99:109–121. [DOI] [PubMed] [Google Scholar]
- [38].Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther 1992;263(1):136–146. [PubMed] [Google Scholar]
- [39].Martin TJ, Zhang Y, Buechler N, Conklin DR, Eisenach JC. Intrathecal morphine and ketorolac analgesia after surgery: comparison of spontaneous and elicited responses in rats. Pain 2005;113(3):376–385. [DOI] [PubMed] [Google Scholar]
- [40].Matsui T, Svensson CI, Hirata Y, Mizobata K, Hua XY, Yaksh TL. Release of prostaglandin E(2) and nitric oxide from spinal microglia is dependent on activation of p38 mitogen-activated protein kinase. Anesth Analg 2010;111(2):554–560. [DOI] [PubMed] [Google Scholar]
- [41].Meller ST, Dykstra C, Grzybycki D, Murphy S, Gebhart GF. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology 1994;33(11):1471–1478. [DOI] [PubMed] [Google Scholar]
- [42].Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernandez-Pena C, Talavera A, Kichko T, Navia B, Sanchez A, Senaris R, Reeh P, Perez-Garcia MT, Lopez-Lopez JR, Voets T, Belmonte C, Talavera K, Viana F. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun 2014;5:3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 2009;10(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moran MM, Szallasi A. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br J Pharmacol 2018. June;175(12):2185–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Munro G Pharmacological assessment of the rat formalin test utilizing the clinically used analgesic drugs gabapentin, lamotrigine, morphine, duloxetine, tramadol and ibuprofen: influence of low and high formalin concentrations. Eur J Pharmacol 2009;605(1–3):95–102. [DOI] [PubMed] [Google Scholar]
- [46].Navratil AR, Shchepinov MS, Dennis EA. Lipidomics Reveals Dramatic Physiological Kinetic Isotope Effects during the Enzymatic Oxygenation of Polyunsaturated Fatty Acids Ex Vivo. J Am Chem Soc 2018;140(1):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nissen SE, Yeomans ND, Solomon DH, Luscher TF, Libby P, Husni ME, Graham DY, Borer JS, Wisniewski LM, Wolski KE, Wang Q, Menon V, Ruschitzka F, Gaffney M, Beckerman B, Berger MF, Bao W, Lincoff AM, Investigators PT. Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis. N Engl J Med 2016;375(26):2519–2529. [DOI] [PubMed] [Google Scholar]
- [48].Old EA, Clark AK, Malcangio M. The role of glia in the spinal cord in neuropathic and inflammatory pain. Handb Exp Pharmacol 2015;227:145–170. [DOI] [PubMed] [Google Scholar]
- [49].Palma C, Minghetti L, Astolfi M, Ambrosini E, Silberstein FC, Manzini S, Levi G, Aloisi F. Functional characterization of substance P receptors on cultured human spinal cord astrocytes: synergism of substance P with cytokines in inducing interleukin-6 and prostaglandin E2 production. Glia 1997;21(2):183–193. [DOI] [PubMed] [Google Scholar]
- [50].Park HJ, Stokes JA, Corr M, Yaksh TL. Toll-like receptor signaling regulates cisplatin-induced mechanical allodynia in mice. Cancer Chemother Pharmacol 2014;73(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL. Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth Analg 2013;116(1):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci 2007;30(10):527–535. [DOI] [PubMed] [Google Scholar]
- [53].Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol 1997;51(4):439–455. [DOI] [PubMed] [Google Scholar]
- [54].Pulichino AM, Rowland S, Wu T, Clark P, Xu D, Mathieu MC, Riendeau D, Audoly LP. Prostacyclin antagonism reduces pain and inflammation in rodent models of hyperalgesia and chronic arthritis. J Pharmacol Exp Ther 2006;319(3):1043–1050. [DOI] [PubMed] [Google Scholar]
- [55].Raetz CR, Garrett TA, Reynolds CM, Shaw WA, Moore JD, Smith DC Jr., Ribeiro AA, Murphy RC, Ulevitch RJ, Fearns C, Reichart D, Glass CK, Benner C, Subramaniam S, Harkewicz R, Bowers-Gentry RC, Buczynski MW, Cooper JA, Deems RA, Dennis EA. Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4. J Lipid Res 2006;47(5):1097–1111. [DOI] [PubMed] [Google Scholar]
- [56].Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci 2004;20(2):467–473. [DOI] [PubMed] [Google Scholar]
- [57].Rai G, Jadhav A, Schultz L, Kenyon V, Leister W, Simeonov A, Holman TR, Maloney DJ. Selective small molecule inhibitors of 12-human lipoxygenase (12-hLO) Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010, 2009 November 30. [PubMed] [Google Scholar]
- [58].Rai G, Joshi N, Perry S, Yasgar A, Schultz L, Jung JE, Liu Y, Terasaki Y, Diaz G, Kenyon V, Jadhav A, Simeonov A, van Leyen K, Holman TR, Maloney DJ. Discovery of ML351, a potent and selective inhibitor of human 15-lipoxygenase-1 Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010, 2013 April 15. [PubMed] [Google Scholar]
- [59].Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 2000;4(3):247–257. [DOI] [PubMed] [Google Scholar]
- [60].Saito O, Svensson CI, Buczynski MW, Wegner K, Hua XY, Codeluppi S, Schaloske RH, Deems RA, Dennis EA, Yaksh TL. Spinal glial TLR4-mediated nociception and production of prostaglandin E(2) and TNF. Br J Pharmacol 2010;160(7):1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med 2017;23(9):1018–1027. [DOI] [PubMed] [Google Scholar]
- [62].Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, Hammock BD. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A 2006;103(37):13646–13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007;10(11):1361–1368. [DOI] [PubMed] [Google Scholar]
- [64].Schwartz JP, Wilson DJ. Preparation and characterization of type 1 astrocytes cultured from adult rat cortex, cerebellum, and striatum. Glia 1992;5(1):75–80. [DOI] [PubMed] [Google Scholar]
- [65].Sendobry SM, Cornicelli JA, Welch K, Bocan T, Tait B, Trivedi BK, Colbry N, Dyer RD, Feinmark SJ, Daugherty A. Attenuation of diet-induced atherosclerosis in rabbits with a highly selective 15-lipoxygenase inhibitor lacking significant antioxidant properties. Br J Pharmacol 1997;120(7):1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci U S A 2002;99(15):10150–10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Solomon DH, Husni ME, Libby PA, Yeomans ND, Lincoff AM, Lupsilonscher TF, Menon V, Brennan DM, Wisniewski LM, Nissen SE, Borer JS. The Risk of Major NSAID Toxicity with Celecoxib, Ibuprofen, or Naproxen: A Secondary Analysis of the PRECISION Trial. Am J Med 2017;130(12):1415–1422 e1414. [DOI] [PubMed] [Google Scholar]
- [68].Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015;18(8):1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Stokes JA, Cheung J, Eddinger K, Corr M, Yaksh TL. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J Neuroinflammation 2013;10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Suhail MS, Christianson C, Koehrn F, Malkmus SA, Mitchell W, Corr M, Yaksh TL. Effects of long term polyarthritis and subsequent NSAID treatment on activity with disassociation of tactile allodynia in the mouse. Neurocomputing 2012;84:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A 2005;102(16):5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int 2004;45(2–3):397–407. [DOI] [PubMed] [Google Scholar]
- [73].Trang T, Sutak M, Quirion R, Jhamandas K. The role of spinal neuropeptides and prostaglandins in opioid physical dependence. Br J Pharmacol 2002;136(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].van Rossum D, Hanisch UK. Microglia. Metab Brain Dis 2004;19(3–4):393–411. [DOI] [PubMed] [Google Scholar]
- [75].Vissers K, Hoffmann V, Geenen F, Biermans R, Meert T. Is the second phase of the formalin test useful to predict activity in chronic constriction injury models? A pharmacological comparison in different species. Pain Pract 2003;3(4):298–309. [DOI] [PubMed] [Google Scholar]
- [76].Woller SA, Choi SH, An EJ, Low H, Schneider DA, Ramachandran R, Kim J, Bae YS, Sviridov D, Corr M, Yaksh TL, Miller YI. Inhibition of Neuroinflammation by AIBP: Spinal Effects upon Facilitated Pain States. Cell Rep 2018;23(9):2667–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Woller SA, Ravula SB, Tucci FC, Beaton G, Corr M, Isseroff RR, Soulika AM, Chigbrow M, Eddinger KA, Yaksh TL. Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: The role of TLR4 in the evolution of a persistent pain state. Brain Behav Immun 2016;56:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res Rev 2009;60(1):267–277. [DOI] [PubMed] [Google Scholar]
- [79].Yaksh TL, Horais KA, Tozier N, Rathbun M, Richter P, Rossi S, Grafe M, Tong C, Meschter C, Cline JM, Eisenach J. Intrathecal ketorolac in dogs and rats. Toxicol Sci 2004;80(2):322–334. [DOI] [PubMed] [Google Scholar]
- [80].Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol (1985) 2001;90(6):2386–2402. [DOI] [PubMed] [Google Scholar]
- [81].Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav 1976;17(6):1031–1036. [DOI] [PubMed] [Google Scholar]
- [82].Yashpal K, Coderre TJ. Influence of formalin concentration on the antinociceptive effects of anti-inflammatory drugs in the formalin test in rats: separate mechanisms underlying the nociceptive effects of low- and high-concentration formalin. Eur J Pain 1998;2(1):63–68. [DOI] [PubMed] [Google Scholar]
- [83].Yoon SY, Patel D, Dougherty PM. Minocycline blocks lipopolysaccharide induced hyperalgesia by suppression of microglia but not astrocytes. Neuroscience 2012;221:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zheng FY, Xiao WH, Bennett GJ. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience 2011;176:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


